45 Treatment of Thoracic Vertebral Fractures
KEY POINTS
Introduction
Thoracic fractures account for approximately 16% of all spinal fractures.4 Multiple classification systems have been developed in an attempt to characterize thoracic fractures as stable or unstable. While it is important to realize that no classification is perfect, these classification systems aid in making sound clinical decisions. They range in simplicity from the Denis three-column classification to the complicated Magerl (AO) classification.7 Regardless of the type of classification system employed, the presence of neurologic deficits, ligamentous injury, and a significant loss of height, angulation, translation, distraction, and/or rotation at the level of the vertebral injury must always increase suspicion for spinal instability.5
Basic Science
The incidence of neurological deficits from thoracic fractures is about 10% or greater; this occurs for several reasons. First, the diameter of the thoracic spinal canal is smaller than the canal of the cervical or lumbar region, being narrowest at T3-T9.9 Second, the midthoracic cord is located in a watershed region between the blood supply to the cervicothoracic and thoracolumbar spines. Last, the high-energy mechanism of injury required for most thoracic fractures is transferred to the underlying cord and spinal nerve roots.
Clinical Practice Guidelines
Stable Thoracic Vertebral Fractures
A significant percentage of thoracic compression fractures fail to heal within 3 to 6 weeks. Such fractures are prone to a progression in the kyphotic deformity and may cause severe back pain. In some instances, the pain is so debilitating that patients remain sedentary, placing them at increased risk for deep vein thrombosis, pneumonia, and bone resorption. Initially developed to treat painful vertebral hemangiomas, vertebroplasty and kyphoplasty offer marked to complete pain relief in 63% to 90% of nonhealing thoracic compression fractures.8
While there are few absolute contraindications to vertebroplasty and kyphoplasty, these interventions are strongly discouraged in the presence of systemic infection, bleeding diathesis, and spinal canal or neural foraminal stenosis leading to myelopathy or radiculopathy, respectively. Patients with pathologic compression fracture resultant from an underlying neoplasm are also candidates for vertebroplasty or for kyphoplasty; however, surgery must be coordinated with chemotherapy and/or irradiation.
Once the cannulated needle is satisfactorily positioned in the vertebral body using radiographic guidance, polymethyl methacrylate (PMMA) cement is instilled. In the case of kyphoplasty, a balloon is first inflated through the cannulated needle to create a cavity for the cement. This maneuver enables a 50% restoration in vertebral body height and alignment in two thirds of patients undergoing kyphoplasty.6
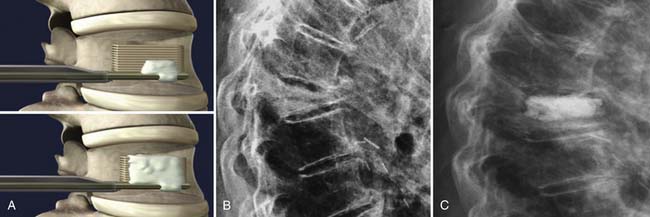
The StaXx kyphoplasty is a newer system that allows the firing of a series of PEEK wafers into the vertebral body through a device secured just inferior to the pedicle and at its lateral edge (Figure 45-1). This is performed under fluoroscopic guidance. The number of PEEK wafers required in the fractured vertebral body is determined once endplate reduction is obtained and appropriate vertebral body height correction is established. The wafers provide structural support to the vertebral body. A smaller amount of PMMA is then injected through the same channel around the PEEK construct, as compared to both vertebroplasty and kyphoplasty. The StaXx kyphoplasty system potentially offers an advantage over kyphoplasty alone, in which the space opened by the balloon may undergo some collapse before the PMMA is injected. In vertebroplasty, the PMMA simply flows to the spaces of least resistance, but does not offer height restoration.
Clinical Case Examples
Case 1: Kyphoplasty
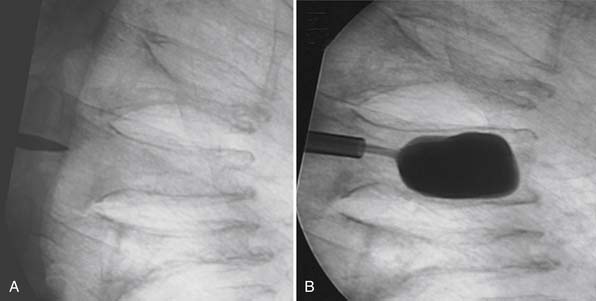
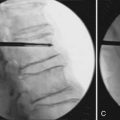
An 80-year-old male with steroid-induced osteoporosis presented with mid-back pain of 10 week’s duration. X-rays of the thoracic spine showed T7, T8, and T9 compression fractures with significant height loss and mild kyphotic deformity (Figure 45-2A). MRI showed a T2 hyperintensity at T8 consistent with acute fracture, while the other two fractures appeared chronic. Despite undergoing thorough conservative management, the patient continued to experience back pain that significantly limited both function and mobility.
He underwent a kyphoplasty of the T8 with satisfactory restoration of height and reduction of his kyphotic deformity (Figure 45-2B). Postoperatively, the patient had no significant residual back pain and returned to his premorbid function.
Case 2: Percutaneous Instrumentation
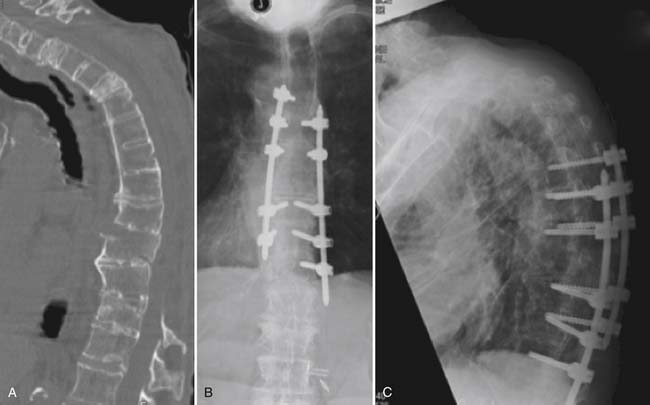
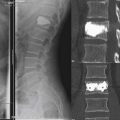
An 84-year-old female presented 1 month after she was involved in a motor vehicle accident in which she sustained a T9 Chance fracture (Figure 45-3A). Thoracic MRI performed hours after the accident showed a spinal epidural hematoma at the level of the fracture and an intact posterior ligamentous complex. The patient underwent multilevel thoracic laminectomies and evacuation of the epidural hematoma. Subsequent MRI showed no residual spinal cord compression; however, she had persistent spinal cord injury and was unable to lift the lower extremities against gravity. She also had severe back pain, which limited her mobility and rehabilitation. We opted to perform percutaneous T6-12 instrumentation (Figure 45-3B). Two months later, the patient was tolerating rehabilitation well, and her lower extremity strength was markedly improved.
Case 3: Pedicle Subtraction Osteotomy
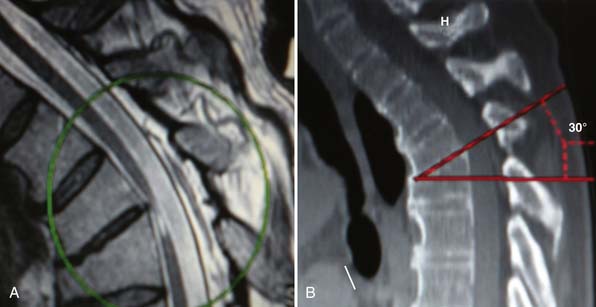
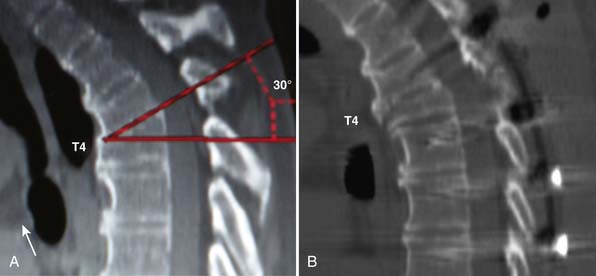
MRI and CT scans revealed severe compression of the ventral spinal cord due to a 30-degree focal kyphotic deformity at the level of T3-T4. A T2 signal change was detected within the spinal cord at the same level, consistent with advanced spinal cord injury (Figure 45-4A and B).
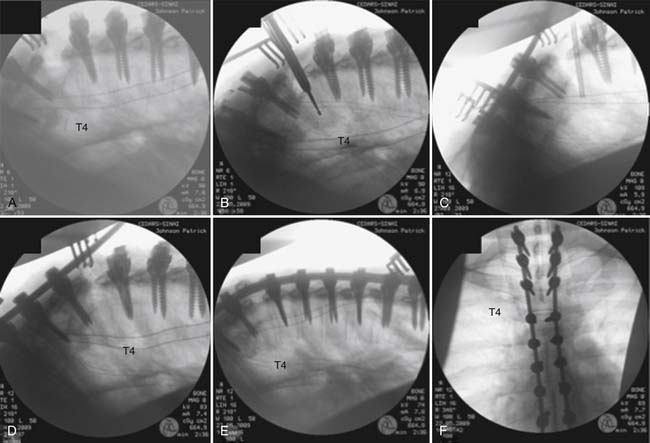
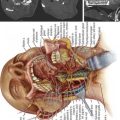
A posterior transpedicular osteotomy was performed using biplane fluoroscopy, which guided the resection of a wedge of the superior T4 vertebra (Figure 45-5A-C). Rib osteotomies were completed at the level of T4, and lateral wall osteotomies with preservation of the pleura were accomplished. After a spontaneous partial reduction of the osteotomy, the Mayfield head holder was elevated to reduce the gibbus and correct the alignment of the upper thoracic spine. Longitudinal rods were contoured and placed at the level of T1, T2, and T3 after reduction was completed manually, with biplane fluoroscopy and intraoperative evoked potentials, by closing the bony defect created by the vertebral osteotomy (Figure 45-5 C-D). The longitudinal rods were placed into a reduced position, achieving complete reduction of the kyphotic and sagittal plane deformity and annihilating the gibbus deformity (Figure 45-5E). Biplane fluoroscopy confirmed alignment of the sagittal and coronal plane at the instrumented levels with complete resolution of the gibbus, and evoked potentials remained unchanged (Figure 45-5E and F).
Two days after the surgery, the patient was transferred from the intensive care unit to the floor, where he quickly recuperated his baseline strength and was discharged home ambulating independently with the use of a cane. Postoperative CT scans confirmed the established correction of the sagittal plane deformity (Figure 45-6A and B).
Deformity Correction
Anterior approaches to treat a spinal deformity can also be performed. However, any multisegment anterior fusion construct is subject to sustaining increased mechanical stress, which can thereby result in the failure of the anterior spinal fusion.3 In addition, the upper thoracic spine is a difficult region to access anteriorly due to the presence of major vascular elements as well as vital structures ventral to the spine.2 Moreover, Boockvar et al reported that anterior reconstruction alone might not meet the biomechanical needs of the upper thoracic spine. Recent advances in anesthesia, neural monitoring, and posterior instrumentation have made a posterior approach to the upper thoracic spine possible and treatment of the upper thoracic spine achievable.1
1. Abumi K., Shono Y., Ito M., Taneichi H., Kotani Y., Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25:962-969.
2. Boockvar J.A., Philips M.F., Telfeian A.E., O’Rourke D.M., Marcotte P.J. Results and risk factors for anterior cervicothoracic junction surgery. J. Neurosurg. 2001;94:12-17.
3. Defino H.F., Rodriquez-Fuentes A.E., Piola F.P. Surgical treatment of pathological kyphosis [in Spanish]. Acta Ortoped. Bras.. 2002;10:10-16.
4. Gertzbein S.D. Scoliosis Research Society: multicenter spine fracture study. Spine. 1992;17:528.
5. Lee J.Y., Vacarro A.R., Lim M.R., et al. Thoracolumbar injury classification and severity score: a new paradigm for the treatment of thoracolumbar spine trauma. J. Orthop. Sci.. 2005;10:671-675.
6. Lieberman I.H., Dudeney S., Reinhardt M.K., et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631-1638.
7. Magerl F., Aebi M., Gertzbein S.D., et al. A comprehensive classificaton of thoracic and lumbar injuries,. Eur. Spine J.. 1994;3:184-201.
8. Martin J.B., Jean B., Sugui K., et al. Vertebroplasty: clinical experience and follow-up results. Bone. 1999;25:11S-15S.
9. Panjabi M.M., Takata K., Goal V., et al. Thoracic human vertebrae: quantitative three-dimensional anatomy. Spine. 1991;26:888-901.