Chapter 42 Treatment of the Stable Patient with Chronic Obstructive Pulmonary Disease
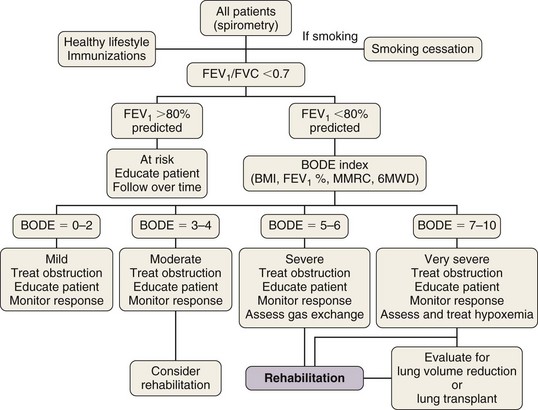
Box 42-1 summarizes the available therapeutic options for patients with COPD. This chapter reviews medical management of COPD that centers on three goals: (1) prevent deterioration in lung function, (2) alleviate symptoms, and (3) treat complications as they arise. Once diagnosed, patients with COPD should be encouraged to participate actively in their management; collaborative management improves their self-reliance and self-esteem. All patients should be encouraged to lead a healthy life and exercise regularly. Preventive care is extremely important, and all patients should receive immunizations, including pneumococcal vaccine every 5 years and yearly influenza vaccine. Figure 42-1 provides an algorithm detailing this comprehensive approach.
Box 42-1
Therapy of Patients with Symptomatic Stable Chronic Obstructive Pulmonary Disease
Multicomponent Disease
Based on the multidimensional nature of COPD and the availability of multiple effective therapies, the approach shown in Figure 42-1 may more accurately help clinicians evaluate patients and choose therapies than the current approach, using primarily the FEV1 percentage from reference values.
Respiratory Manifestations
Pharmacologic Therapy of Airflow Obstruction
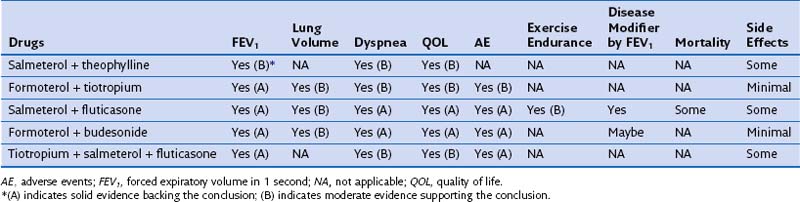
Most patients with COPD require pharmacologic therapy. This should be organized according to the severity of symptoms, the degree of lung dysfunction, and the tolerance of the patient to specific drugs. A stepwise approach similar to that developed for systemic hypertension may be helpful, because medications alleviate symptoms, improve exercise tolerance and QOL, and may decrease mortality. Tables 42-1 and 42-2 summarize the evidence supporting the effect of individual and combined therapies on outcomes of importance in patients with COPD. Because most patients with COPD are elderly, care must be taken when prescribing drugs for this population. Comorbidities are frequently present in patients with COPD, mandating caution to ensure that therapy takes these into account.
Table 42-1 Effect of Single Pharmacologic Agents on Important Outcomes in Chronic Obstructive Pulmonary Disease Patients
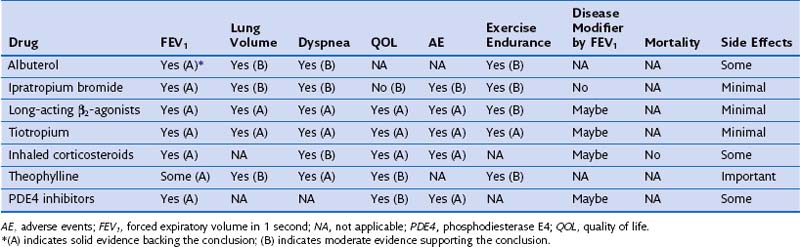
Table 42-2 Effect of Combined Pharmacologic Agents on Important Outcomes in Chronic Obstructive Pulmonary Disease Patients
Systemic Manifestations
Pulmonary Rehabilitation
Therapeutic Modalities That Improve Patient Performance
Physical Modalities of Ventilatory Therapy
Exacerbations, Hospitalization, and Discharge Criteria
Although acute exacerbations are difficult to define and their pathogenesis is poorly understood, impaired lung function can lead to respiratory failure, requiring intubation and mechanical ventilation. In addition, repeated exacerbations are associated with poor outcome. The purpose of acute treatment is to manage the patient’s decompensation and comorbid conditions to prevent further deterioration and readmission (see Chapter 43).
Controversies and Pitfalls
1. Should asymptomatic patients who are diagnosed with airflow obstruction by spirometry be treated?
2. Should symptomatic patients with mild obstruction be treated, and if so, what is the best initial choice for therapy?
3. Should most patients with persistent symptoms be treated with double- or triple-drug therapy?
4. If double-agent therapy is selected, is it better to treat with a β2-agonist plus inhaled corticosteroids or a long-acting β2-agonist (LABA) and long-acting muscarinic antagonist (LAMA)?
5. For which patients is pulmonary rehabilitation more effective?
6. Do maintenance programs of pulmonary rehabilitation need to be developed? Are they useful?
7. Is there a definitive role for chronic antibiotic therapy for patients with COPD, and if so, which agents in which schedule?
Agustí AG, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–360.
Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. COPD Clinical Research Network. N Engl J Med. 2011;365:689–698.
Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Lung Health Study Research Group. Ann Intern Med. 2005;142:233–239.
Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. TORCH Investigators. N Engl J Med. 2007;356:775–789.
Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. M2-124 and M2-125 study groups. Lancet. 2009;374:685–694.
Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:591–597.
Celli BR, MacNee W. Standards for the diagnosis and treatment of COPD. Eur Respir J. 2004;23:932–946.
Celli BR, Cote CG, Marin JM, et al. The body mass index, airflow obstruction, dyspnea and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012.
Global Initiative for Chronic Obstructive Lung Disease. www.GOLD.org, 2010. updated
National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073.
Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. ATS/ERS Pulmonary Rehabilitation Writing Committee. Am J Respir Crit Care Med. 2006;173:1390–1413.
Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340:1941–1947.
O’Donnell D, Lam M, Webb K. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:542–549.
Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. UPLIFT Study Investigators. N Engl J Med. 2008;359:1543–1554.
Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:741–750.

 hours daily) exhibited increased upper extremity endurance after 6 weeks; most importantly, their increase in maximal sustainable ventilatory capacity was similar to that obtained with ventilatory muscle training. This suggests that arm exercise training programs can train ventilatory muscles.
hours daily) exhibited increased upper extremity endurance after 6 weeks; most importantly, their increase in maximal sustainable ventilatory capacity was similar to that obtained with ventilatory muscle training. This suggests that arm exercise training programs can train ventilatory muscles.