CHAPTER 53 Treatment of Peptic Ulcer Disease
Chapter 52 reviews the epidemiology, clinical features, diagnosis, and complications of peptic ulcer disease. This chapter focuses on its treatment.
OVERVIEW
For more than a century, peptic ulcer was considered a chronic, incurable disorder characterized by frequent exacerbations and remissions. The discovery of the link between Helicobacter pylori and peptic ulcer by Marshall and Warren1 in the mid-1980s revolutionalized the concept and treatment of peptic ulcer. Now there is overwhelming evidence to support H. pylori infection as the most important cause of duodenal and gastric ulcers worldwide. Curing the infection not only heals peptic ulcer but also prevents ulcer relapse.2 Peptic ulcer is, in fact, a curable infectious disease.
Beside H. pylori–related peptic ulcers, use of nonsteroidal anti-inflammatory drugs (NSAIDs) and low-dose aspirin is another major cause of peptic ulcer complications particularly among older adults.3 Co-therapy with antiulcer drugs and the replacement of conventional nonselective NSAIDs with NSAIDs selective for cyclooxygenase-2 (COX-2 selective NSAIDs) have become alternative treatments for patients who are at risk for peptic ulcer disease. Data suggest that COX-2 selective NSAIDs and some nonselective NSAIDs increase the risk of serious cardiothrombotic events. Prescribing NSAIDs therefore requires a careful assessment of individual patients’ gastrointestinal (GI) and cardiovascular risks.
As discussed in the preceding chapter, due to the declining prevalence of H. pylori infection, the proportion of patients with H. pylori–negative, NSAID-negative idiopathic ulcers is growing. In the United States, the reported proportion of H. pylori–negative, NSAID-negative idiopathic ulcers is between 20% and 30%.4,5 It has been argued that as the incidence of H. pylori–related ulcers falls, a greater proportion of H. pylori–negative, NSAID-negative idiopathic ulcers will be seen.6 Of interest, a prospective cohort study has demonstrated a four-fold rise in the absolute incidence of idiopathic bleeding ulcers, and the risk of recurrent ulcer bleeding in these patients is high.7 Thus, long-term prophylaxis with antisecretory drugs for idiopathic bleeding ulcers is advisable (see later), although this recommendation is not evidence based.
ANTISECRETORY AND ACID-NEUTRALIZING AGENTS
Antacids
Mechanisms of Action
When Peterson and coworkers8 showed that a liquid antacid preparation of magnesium-aluminum hydroxide (approximately 1000 mmol HCl neutralizing capacity per day) was more effective than placebo for hastening the healing of duodenal ulcer, it was thought antacids promoted ulcer healing by neutralizing gastric acid. However, later studies showed that far smaller doses of antacids (neutralizing capacity 120 mmol HCl per day) had virtually identical efficacy for healing peptic ulcerations.9 The precise mechanisms by which antacids hasten the healing of peptic ulcerations are not clear. Although antacids have been shown to be superior to placebo in healing peptic ulcers, their efficacy in ulcer healing is limited. In one study, the healing rates for gastric ulcer after 6 weeks were 67% in the antacids group and 25% in the placebo group.9
Adverse Effects
For the magnesium-containing agents, the most common side effect is diarrhea. In contrast, antacids that contain aluminum hydroxide primarily, and those that contain calcium, may cause constipation. All antacids must be used with caution, if at all, in patients who have chronic kidney disease. In such patients, magnesium-containing agents can cause hypermagnesemia; the use of calcium carbonate can cause hypercalcemia and alkalosis and further renal impairment (milk-alkali syndrome), and aluminum hydroxide antacids can cause aluminum neurotoxicity.10
Histamine-2 Receptor Antagonists
Mechanisms of Action
Four histamine-2 (H2) receptor antagonists are available—cimetidine (Tagamet), ranitidine (Zantac), famotidine (Pepcid), and nizatidine (Axid). These compounds are competitive inhibitors of histamine-stimulated acid secretion, although famotidine appears to have some component of noncompetitive inhibition as well.11 All four agents suppress basal acid output as well as acid output stimulated by meals (see Chapter 49).
Pharmacokinetics
The H2 receptor antagonists are well absorbed after oral dosing, and their absorption is not affected by food. Peak blood levels are achieved within 1 to 3 hours after an oral dose. These drugs cross the blood-brain barrier and the placenta.12,13 After oral administration, cimetidine, ranitidine, and famotidine undergo first-pass hepatic metabolism, which reduces their bioavailability by 35% to 60%. In contrast, nizatidine does not undergo first-pass metabolism, and its bioavailability approaches 100% with oral dosing. When administered in the evening, the drugs are especially effective in suppressing nocturnal acid output.14
All four H2 receptor antagonists are eliminated by a combination of renal excretion and hepatic metabolism. After intravenous administration, in contrast, all four agents are eliminated principally through renal excretion. For cimetidine and famotidine it is recommended that the doses be cut in half in patients whose creatinine clearance is 15 to 30 mL/minute. For nizatidine and ranitidine, the dose should be halved if the creatinine clearance is less than 50 mL/minute. Dialysis does not remove substantial amounts of the H2 receptor antagonists, so dose adjustments for dialysis are not necessary. Liver failure has been found to prolong the half-life of cimetidine, but dose reductions are generally not needed for patients with hepatic failure unless it is accompanied by chronic kidney disease.11
Tolerance to the antisecretory effects of H2 receptor antagonists appears to develop quickly and frequently.15 The mechanisms that mediate tolerance to the antisecretory effects of H2 receptor antagonists are not entirely clear.
Adverse Effects
The H2 receptor antagonists are a remarkably safe and well-tolerated group of agents. The overall incidence of side effects is less than 4%, and serious side effects are decidedly uncommon. One meta-analysis of randomized clinical trials concluded that the overall rate of adverse effects reported for the H2 blockers did not differ significantly from that for placebo.16 Nevertheless, a number of untoward effects have been described, primarily in anecdotal reports and uncontrolled series. Cimetidine has weak antiandrogenic activity that occasionally can cause gynecomastia and impotence.17 Myelosuppression is an uncommon, presumably idiosyncratic side effect of the H2 receptor antagonists. In one large series of patients with bone marrow transplants, however, ranitidine was implicated as a possible cause of myelosuppression in 5%.18 The contribution of ranitidine to the bone marrow suppression in such patients is not clear, but pending further data, it seems prudent to avoid the use of H2 receptor antagonists in bone marrow transplant recipients.
Drug Interactions
Both cimetidine and ranitidine bind to the hepatic cytochrome P-450 (CYP) mixed-function oxidase system, and this binding can inhibit the elimination of other drugs that are metabolized through the same system, including theophylline, phenytoin, lidocaine, quinidine, and warfarin.19 Famotidine and nizatidine have no significant avidity for the CYP system, and these agents do not appear to have any important drug interactions.
Proton Pump Inhibitors
Mechanisms of Action
The proton pump inhibitors (PPIs) are a class of drugs that decrease gastric acid secretion through inhibition of H+,K+-ATPase, the proton pump of the parietal cell (see Chapter 49). Five PPIs are used widely as antisecretory agents—omeprazole (Prilosec), esomeprazole (Nexium; the S optical isomer of omeprazole), lansoprazole (Prevacid), pantoprazole (Protonix), and rabeprazole (Aciphex). These agents are prodrugs that must be activated by acid to inhibit the H+,K+-ATPase. However, PPIs as prodrugs are acid-labile compounds that must be protected from degradation by stomach acid after oral administration.20
Pharmacokinetics
All PPIs undergo significant hepatic metabolism. Because there is no direct toxicity from PPIs, dose adjustments are not required even in patients with significant renal or hepatic impairment. However, there are significant genetic polymorphisms for one of the CYP isoenzymes involved in PPI metabolism, CYP2C19. Approximately 3% of white persons and 15% of Asians are deficient in CYP2C19. This polymorphism has been shown to substantially raise plasma levels of omeprazole, lansoprazole, and pantoprazole but not those of rabeprazole.21,22
As a result of their requirement for concentration and activation in acidic compartments, the PPIs bind predominantly to those proton pumps that are actively secreting acid. Thus, the efficacy of the PPIs for inhibiting acid secretion is limited if they are administered during the fasting state, when only approximately 5% of the stomach’s proton pumps are active. With meal stimulation, in contrast, 60% to 70% of the proton pumps actively secrete acid. Thus, the PPIs are most effective if they are administered immediately before meals. For once-daily dosing, it is recommended that the PPIs be taken immediately before breakfast.23 Unlike H2 receptor antagonists, tolerance to the antisecretory effects of PPI therapy has not been seen during short-term investigations.
Adverse Effects
The PPIs are a remarkably safe and well-tolerated group of agents. The most commonly reported side effects are headache and diarrhea, yet the rate at which patients experience these symptoms does not differ significantly from that for patients treated with placebo.24
PPIs and other antisecretory agents cause hypergastrinemia by inhibiting gastric acid secretion (see Chapter 49). In addition to stimulating acid secretion, gastrin has been shown to have trophic effects on the GI enterochromaffin-like (ECL) cells. Female rats in which protracted hypergastrinemia has been induced by PPIs develop ECL cell hyperplasia and gastric carcinoid tumors.25 However, there are no reports of gastric carcinoid tumors attributable to PPIs in humans. Even in patients with Zollinger-Ellison syndrome who have severe hypergastrinemia, carcinoid tumors are uncommon and occur predominantly in patients with multiple endocrine neoplasia type I (MEN-I).26
Some data suggest that the long-term administration of PPIs to patients who are infected with H. pylori might accelerate the development of gastric atrophy.27 However, these early observations have not been conformed by subsequent studies.28 The U.S. Food and Drug Administration (FDA) advisory group concluded that the available data did not establish such an effect, and did not recommend routine testing for and treatment of H. pylori before initiation of PPI therapy.29
Data from observational studies have found that PPIs increase the risk of osteoporosis-related fracture. The strength of the association increases with increasing duration and dose of PPI therapy.30,31 The mechanisms underlying such an association are unknown.
Drug Interactions
The elevation of gastric pH induced by the PPIs can affect the absorption of a number of medications. However, this antisecretory action rarely has clinically important effects on drug pharmacokinetics, except when the PPIs are given with ketoconazole or digoxin.32 Ketoconazole requires stomach acid for absorption, and this drug may not be absorbed effectively after PPIs have inhibited gastric acid secretion. Conversely, an elevated gastric pH facilitates the absorption of digoxin, resulting in higher plasma levels of this agent. If a patient requires both PPI and antifungal therapy, it is recommended that an agent other than ketoconazole be chosen. For patients treated concomitantly with PPIs and digoxin, clinicians should consider monitoring plasma digoxin levels.
Because the PPIs are metabolized by the CYP system, there is potential for them to alter the metabolism of other drugs that are eliminated by CYP enzymes. Among the available PPIs, omeprazole appears to have the greatest potential for such drug interactions and has been shown to delay the clearance of warfarin, diazepam, and phenytoin.33 Lansoprazole, pantoprazole, and rabeprazole do not appear to interact significantly with drugs metabolized by the CYP system. Even with omeprazole, however, clinically important drug interactions are uncommon. Evidence is accumulating that several PPIs may inhibit the activation of clopidogrel to its active metabolite, thus impairing the antiplatelet effect of clopidogrel, with adverse cardiovascular outcomes.
MUCOSA-PROTECTIVE AGENTS
Sucralfate
Mechanisms of Action
Sucralfate (Carafate) has demonstrated efficacy (similar to that of the H2 receptor antagonists) in healing duodenal ulcer when given in a dose of 1 g four times daily.34 The drug has demonstrated efficacy in the treatment of gastric ulcer as well, but sucralfate has not been approved by the FDA for this indication. Sucralfate is a complex metal salt of sulfated sucrose. Although the sucralfate molecule contains aluminum hydroxide, the agent has little acid-neutralizing capacity. When exposed to gastric acid, the sulfate anions can bind electrostatically to positively charged proteins in damaged tissue, thereby forming a protective barrier that may prevent further acid-peptic attack. Other proposed beneficial effects of sucralfate are enhancement of mucosal prostaglandin levels, stimulation of mucus and bicarbonate secretion, binding of mucosa-irritating bile salts, binding of epidermal growth factors, and promotion of angiogenesis.34
Pharmacokinetics
Less than 5% of the sucralfate administered is absorbed owing to its poor solubility.34 The drug is excreted in the feces. The high aluminum content causes a small but significant rise in serum and urine aluminum levels within 2 days. In patients with normal renal function, the minor amounts of aluminum absorption with short-term therapy are of no clinical significance.
Toxicity and Drug Interactions
Because of the lack of systemic absorption, sucralfate appears to have no systemic toxicity. The effect on the disposition of aluminum in the body has not been adequately studied in patients with chronic kidney disease. Sucralfate is best avoided in this population. The drug can bind to a number of medications, including phenytoin and warfarin, reducing their absorption. Important drug interactions appear to be rare, however, and can be avoided entirely if sucralfate is administered at a time separate from other medications.34
Bismuth
Mechanisms of Action
Two colloidal preparations of bismuth have been most commonly used, colloidal bismuth subcitrate and bismuth subsalicylate (e.g., Pepto-Bismol). These agents have some efficacy in healing peptic ulcers, but the mechanisms underlying this therapeutic effect are not clear.35 The bismuth forms complexes with mucus that appear to coat ulcer craters. Effects on increasing mucosal prostaglandin synthesis and bicarbonate secretion also have been proposed, and bismuth has documented antimicrobial actions against H. pylori. Bismuth has been approved by the FDA for use in combination with other agents for the treatment of H. pylori infection (see Chapter 50).
Pharmacokinetics
Bismuth is largely unabsorbed and is excreted in the feces. Colonic bacteria convert bismuth subcitrate and bismuth subsalicylate to bismuth sulfide, which turns the stools black. Trace amounts of bismuth are absorbed in the upper GI tract. Absorbed bismuth is slowly excreted in the urine for three months or longer.35
Prostaglandin E Analogs
Mechanisms of Action
Endogenous prostaglandins, including prostaglandin E2 (PGE2), regulate mucosal blood flow, epithelial cell proliferation, epithelial restitution, mucosal immunocyte function, mucus and bicarbonate secretion, and basal acid secretion.36 There is substantial evidence that the ulcerogenic effect of an NSAID correlates well with its ability to suppress prostaglandin synthesis.37 Misoprostol, a prostaglandin E1 analog, is the only prostaglandin analog approved by the FDA for the prevention of NSAID-induced ulcer disease. The drug not only enhances mucosal defense mechanisms but also inhibits gastric acid secretion. After binding to the prostaglandin receptor on the parietal cell, misoprostol inhibits gastric acid secretion in a dose-dependent manner that is mediated through inhibition of histamine-stimulated cyclic adenosine monophosphate (cAMP) production.38 It has been shown that misoprostol significantly reduces nocturnal, basal, and meal-stimulated acid secretion at a standard therapeutic dose, although the effect is not as potent as that of other classes of antisecretory agents.39
Pharmacokinetics
Misoprostol is well absorbed after oral administration. The plasma concentration peaks at about 30 minutes, with a serum half-life of approximately 1.5 hours. The drug has no effect on hepatic cytochrome P-450. Misoprostol metabolites are excreted in the urine, but dose reduction is unnecessary in patients with chronic kidney disease.40
Toxicity
Dose-related diarrhea is the most common side effect, occurring in up to 30% of patients and limiting the usefulness of misoprostol.41 Diarrhea is related to prostaglandin-induced increases in intestinal water and electrolyte secretion or acceleration of intestinal transit time. Administration of misoprostol with food may reduce diarrhea. Prostaglandins stimulate uterine smooth muscle. Misoprostol is therefore contraindicated in women who may be pregnant.
ULCERS ASSOCIATED WITH HELICOBACTER PYLORI INFECTION
The discovery of H. pylori and its role in peptic ulcer disease has revolutionized the approach to management. Before this discovery, annual ulcer recurrence rates were as high as 80%, often requiring long-term maintenance therapy for ulcer prevention. Now it is well established that curing H. pylori infection not only heals peptic ulcer but also prevents relapse. The following sections outline the management of peptic ulcers associated with H. pylori infection. The choice of diagnostic tests and treatment regimens for H. pylori infection is discussed in Chapter 50.
DUODENAL ULCER
Because H. pylori infection accounts for 70% or more of duodenal ulcers, one must test for the infection using one of the noninvasive tests recommended in Chapter 50. If the diagnosis of ulcer disease is made endoscopically, gastric biopsy specimens should be taken to detect H. pylori infection. If H. pylori infection is documented, the patient should be treated with one of the regimens recommended in Chapter 50, irrespective of whether he or she has a history of NSAID use. There is good evidence that a course of H. pylori eradication therapy is sufficient to heal complicated and uncomplicated duodenal ulcers such that additional antisecretory therapy is usually not required. In a meta-analysis of 52 trials, it was found that the eradication of H. pylori alone was superior to use of an ulcer-healing drug (relative risk of ulcer, 0.66) and to no treatment (relative risk, 0.37).42 Follow-up endoscopic examination to ensure healing and testing to document H. pylori eradication after antibiotic therapy are not recommended routinely in patients with uncomplicated ulcers. However, noninvasive tests such as the urea breath test and fecal antigen test can be used to confirm H. pylori eradication in patients with ulcer complications.
GASTRIC ULCER
If H. pylori infection is documented, the patient should be treated with one of the regimens recommended in Chapter 50 regardless of whether he or she has a history of NSAID use. Whether antisecretory therapy is required after a course of H. pylori eradication therapy is controversial. It has been shown that 1 week of antibacterial therapy without acid suppression effectively heals gastric ulcers.42 In a meta-analysis of ulcer healing trials, treatment with H. pylori eradication therapy was not significantly different from treatment with an ulcer healing drug.43 For patients with large (>1.5 cm) or complicated gastric ulcers, however, additional antisecretory therapy has been shown to promote ulcer healing.44,45 Routine follow-up endoscopy is recommended to document ulcer healing, to exclude malignancy, and to confirm successful H. pylori eradication (see also Chapter 52).
ROLE OF MAINTENANCE THERAPY
After the eradication of H. pylori infection, there is little evidence that maintenance therapy with antisecretory agents is required, even for patients with complicated peptic ulcers.46,47 A meta-analysis showed that H. pylori eradication therapy was superior to no treatment in preventing recurrence of duodenal ulcer (relative risk, 0.19) or gastric ulcer (relative risk, 0.31).42 In another meta-analysis of H. pylori eradication therapy versus maintenance antisecretory therapy in prevention of recurrent ulcer bleeding, rebleeding occurred in 1.6% of the H. pylori eradication therapy group and 5.6% of the maintenance therapy group (odds ratio, 0.25; 95% confidence interval [CI], 0.08 to 0.76).48 Although some prospective trials reported that patients with duodenal ulcer had asymptomatic ulcer recurrences after eradication of H. pylori,49 these asymptomatic ulcers probably had little clinical significance.
ULCERS ASSOCIATED WITH NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
ACTIVE ULCERS
Histamine-2 Receptor Antagonists
There are limited data on the efficacy of H2 receptor antagonists in healing NSAID-associated ulcers. Current evidence suggests that conventional doses of H2 receptor antagonists effectively heal duodenal ulcers but are ineffective for gastric ulcers. In a multicenter study, the effects of ranitidine on ulcer healing were compared in a group of patients who had stopped NSAID therapy and another group who continued NSAID therapy. Gastric ulcers healed in 63% of those still taking NSAIDs compared with 95% of those who had stopped. At 12 weeks, 79% of gastric ulcers and 92% of duodenal ulcers were healed in the group continuing NSAIDs, whereas all ulcers healed in those who had stopped taking NSAIDs.50 The ability of H2 receptor antagonists given in conventional doses to heal NSAID-associated ulcers also depends on the size of the ulcers. One early study reported that when NSAIDs were continued, 90% of gastric ulcers smaller than 5 mm healed after 8 weeks of cimetidine, whereas only 25% of ulcers larger than 5 mm healed.51
Proton Pump Inhibitors
Several large-scale studies have investigated the efficacy of PPIs for healing of NSAID-associated ulcers.52–55 Current evidence indicates that PPIs are superior to standard-dose H2 receptor antagonist therapy in healing NSAID-associated ulcers. In a large-scale randomized comparison of two doses of esomeprazole, 20 and 40 mg, and ranitidine, 150 mg twice daily, in patients who continue to take NSAIDs, ulcer healing at eight weeks was found in 85.7% of patients given esomeprazole 40 mg daily, in 84.8% of those given esomeprazole 20 mg daily, and in 76.3% of those given ranitidine 150 mg twice daily.52 In another study of 350 patients with NSAID-associated gastric ulcers who continued to use NSAIDs, ulcer healing at eight weeks was found in 69% of patients given lansoprazole 15 mg daily, in 73% of those given lansoprazole 30 mg daily, but in only 53% of those given ranitidine 150 mg twice daily.55
Misoprostol
In a randomized placebo-controlled trial in which patients continued NSAID therapy, misoprostol resulted in healing of gastric and duodenal ulcers in 67% of patients at eight weeks, compared with 26% of patients treated with placebo.56 However, misoprostol is not as effective as PPIs in healing NSAID-associated ulcers. One large-scale, randomized trial compared misoprostol 200 µg four times daily with omeprazole 20 or 40 mg daily in patients who continued NSAID treatment.53 After eight weeks, duodenal ulcers healed in 89% of patients receiving either dose of omeprazole and in 77% of those receiving misoprostol. Gastric ulcers healed in 80% of those receiving 40 mg of omeprazole, in 87% of those receiving 20 mg of omeprazole, and in 73% of those receiving misoprostol.
Sucralfrate
In a single-blind endoscopic study, sucralfate was significantly less effective than omeprazole in healing NSAID-associated gastroduodenal ulcers.57
Adverse Role of Cyclooxygenase-2 Inhibitors in Ulcer Healing
There is good evidence that COX-2 inhibitors induce less gastric mucosal injury than conventional NSAIDs. However, animal experiments have consistently shown that COX-2, but not COX-1, is up-regulated in gastric ulcer.58,59 The administration of COX-2 inhibitors actually retards the healing of rodent gastric ulcers.60–62 These results suggest that prostaglandins generated by COX-2 contribute to restoring the integrity of gastric mucosa. A double-blind randomized trial of celecoxib on the healing of bleeding gastric ulcer found that after eight weeks, the ulcer healing rate was 65.7% in the celecoxib group and 80% in the placebo group.63 This finding indicates that treatment with COX-2 inhibitors such as celecoxib delays the healing of complicated gastric ulcers.
Recommendations
For patients in whom ulcers develop in association with the use of NSAIDs, it is recommended that NSAIDs should be discontinued if possible. Current evidence indicates that PPIs are more effective than H2 receptor antagonists, sucralfate, and misoprostol in healing NSAID-associated ulcers when continuous NSAID treatment is required. When NSAIDs can be discontinued, an H2 receptor antagonist is an effective alternative. Treatment with COX-2 inhibitors in patients with active ulcers who continue to require anti-inflammatory therapy is not recommended. In the Maastricht III Consensus Report, eradication of H. pylori is advisable in patients who plan to start long-term NSAID therapy.2 The influence of H. pylori infection on the healing and relapse of NSAID-associated ulcer is discussed later.
ULCER PROPHYLAXIS
For many years an ulcer visible at endoscopy has been extensively used as a surrogate endpoint to assess the efficacy of prophylactic agents in preventing complications of NSAID-induced ulcers. An “endoscopic ulcer” has been arbitrarily defined as a circumscribed mucosal defect having a diameter of 5 mm or more with a perceivable depth.63 However, many studies have loosened this criterion to include flat mucosal breaks with a diameter of 3 mm or more as ulcers. The distinction between small ulcers and erosions is arbitrary and is prone to interobserver bias. The clinical relevance of these minor endoscopic lesions is uncertain. Although endoscopic findings roughly correlate with clinical outcomes in subjects at low to average risk for complications, current evidence indicates that the results of endoscopic studies cannot be generalized to high-risk patients.64 Because there are few prospective outcome trials to evaluate the true efficacy of prophylactic agents, clinical judgment relies on data largely using endoscopic endpoints.
Antacids
Antacids have no proven efficacy in the prevention of NSAID-induced ulcers. However, many clinicians still prescribe antacids as co-therapy for patients taking NSAIDs, both for symptom relief and prevention of ulcers. A case-control study showed that NSAID users receiving prophylaxis with antacids and H2 receptor antagonists had a more than two-fold increased risk of ulcer complications compared with those not taking these prophylactic agents.65 This finding was attributed to the possibility that antacids might have masked the dyspeptic symptoms, thereby creating a false sense of protection and raising the risk of silent ulcer complications. Co-prescription of antacids in patients taking NSAIDs who are at risk for ulcer should be discouraged.
Histamine-2 Receptor Antagonists
Several endoscopic studies investigated the efficacy of standard-dose H2 receptor antagonist therapy for the prevention of NSAID-induced ulcers.66 A systematic review of randomized trials showed that H2 receptor antagonists significantly reduce the risk of endoscopic duodenal ulcers but not gastric ulcers.96 In contrast, it has been shown that double-dose famotidine significantly reduced the risk of both endoscopic duodenal and gastric ulcers. In one study, NSAID-related gastric ulcers developed in 20% of patients receiving placebo, in 13% of those receiving 40 mg of famotidine once daily, and in only 8% of those receiving 40 mg of famotidine twice daily.67 In another study, however, the gastric ulcer rates at 24 weeks were 41% and 19% in the groups receiving placebo and double-dose famotidine, respectively.68 The large discrepancy (8% vs. 19%) in the efficacy of double-dose famotidine between these two studies raises doubt about the true efficacy of this agent in preventing NSAID-induced gastric injury. To date there is no clinical outcome study to assess whether high-dose H2 receptor antagonist therapy prevents NSAID-induced ulcer complications.
Misoprostol
More than 20 randomized controlled trials assessed the efficacy of misoprostol in preventing NSAID-induced ulcers. A meta-analysis of the randomized trials indicated that all doses of misoprostol (400 to 800 µg per day) reduce the risk of NSAID-induced endoscopic ulcers.66 Only 800 µg per day of misoprostol is documented to reduce ulcer complications, however. In a large-scale, randomized double-blind trial in patients with rheumatoid arthritis who received NSAIDs, misoprostol (200 µg four times daily) significantly lowered the rate of GI complications, by 40% (0.95% in the placebo group vs. 0.57% in the misoprostol group). However, up to 30% of misoprostol-treated patients experienced GI upset, thus limiting its clinical use.69 Subsequent endoscopic studies suggested that lower doses of misoprostol, such as 200 µg two or three times per day, prevented NSAID-induced endoscopic ulcers with fewer side effects.70 However, there is evidence that low-dose misoprostol therapy fails to prevent ulcer complications.71
Misoprostol has been found to be superior to H2 receptor antagonists for the prevention of NSAID-induced gastric ulcers. In one study, ranitidine (150 mg twice daily) was compared with misoprostol (200 µg four times daily) in long-term NSAID users.72 After four to eight weeks, about 1% of patients in each group demonstrated endoscopic duodenal ulcers. In contrast, gastric ulcers occurred in 5.7% of patients receiving ranitidine, compared with 0.6% of those receiving misoprostol.
Proton Pump Inhibitors
A systematic review of randomized controlled trials of PPIs for prophylaxis against NSAID-induced endoscopic ulcers found that PPIs significantly reduce the risk of endoscopic duodenal and gastric ulcers.66 The efficacy of PPIs has been compared with that of H2 receptor antagonists and misoprostol in patients who continued to receive NSAIDs. Two studies compared omeprazole 20 mg once daily with standard-dose ranitidine (150 mg twice daily) and half-dose misoprostol (200 µg twice daily) for six months.53,54 Omeprazole was found to be more effective than standard-dose ranitidine but only comparable with half-dose misoprostol in preventing gastric ulcers. However, it should be noted that the superiority of omeprazole in preventing NSAID-related ulcer was due to a significant reduction of duodenal ulcers. A post hoc analysis revealed that most of the added protection attributable to omeprazole occurred among those with H. pylori infection. Another study compared high-dose misoprostol (200 µg four times daily) with two doses of lansoprazole (15 and 30 mg daily) for the prevention of ulcers in long-term NSAID users without H. pylori infection and with a history of gastric ulcer.73 Misoprostol was more effective than the two doses of lansoprazole in preventing gastric ulcer, but there was no practical advantage of misoprostol over lansoprazole because of the high withdrawal rate in the misoprostol group. In a head-to-head endoscopic ulcer prevention study comparing two doses of pantoprazole with 20 mg/day of omeprazole in patients with rheumatoid arthritis receiving NSAIDs, the six-month probabilities of remaining ulcer free were 91%, 95%, and 93% for pantoprazole 20 mg, pantoprazole 40 mg, and omeprazole 20 mg, respectively.74
Two identical multicenter randomized clinical trials (RCTs) have been reported together. They compared esomeprazole (20 or 40 mg) with placebo in the prevention of ulcers in patients taking NSAIDs or COX-2 inhibitors over a six-month period. Patients in both studies were H. pylori negative, older than 60, and had a history of gastric or duodenal ulcer. Overall, the rates of ulcers were 17.0%, 5.2%, and 4.6% in the groups receiving placebo, esomeprazole 20 mg, and esomeprazole 40 mg, respectively.75
Whether PPIs can reduce the risk of NSAID-associated ulcer bleeding is largely based on observational studies and one randomized trial in high-risk patients. A large-scale case control study found that PPI therapy was associated with a significant reduction in risk of upper GI bleeding among chronic NSAID users (relative risk, 0.13; 95% CI, 0.09 to 0.19).76 One randomized trial compared long-term (six-month) omeprazole therapy with one week of H. pylori eradication therapy for the prevention of recurrent ulcer bleeding in H. pylori–infected patients with a recent history of NSAID-related ulcer bleeding who continued to use naproxen.77 Recurrent ulcer bleeding was seen in 18.8% of patients undergoing eradication therapy, compared with 4.4% of patients receiving omeprazole. In a randomized comparison of diclofenac plus omeprazole versus celecoxib for secondary prevention of ulcer bleeding in patients who either were H. pylori negative or had undergone H. pylori eradication,78 a similar proportion had recurrent bleeding in six months (6.4% in the combination therapy group compared with 4.9% of patients in the celecoxib group). These results indicate that omeprazole reduces but does not eliminate the risk of ulcer bleeding associated with NSAID use in very-high-risk patients. However, the following two important issues remain unresolved: first, the actual risk reduction achieved by PPI is unknown because of the lack of a placebo group, and second, there are no data on the efficacy of PPIs in preventing ulcer complications in low- or moderate-risk users of NSAIDs.
Role of Cyclooxygenase-2 Inhibitors in Ulcer Prevention
Consistent with the notion that inhibition of COX-2 spares the gastric mucosa, clinical trials using endoscopic ulcer as the endpoint have consistently shown that COX-2 inhibitors induced fewer ulcers than do conventional NSAIDs. Five COX-2 inhibitors have been evaluated in clinical trials: the sulfonamides celecoxib [Celebrex] and valdecoxib [Bextra] (parecoxib is a prodrug of valdecoxib), the methylsulfones rofecoxib [Vioxx] and etoricoxib, and the phenylacetic acid derivative lumiracoxib. Four large-scale clinical outcome studies—the Celecoxib Long-Term Arthritis Safety Study (CLASS),79 the Vioxx Gastrointestinal Outcomes Research Study (VIGOR),80 the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET),81 and the Multinational Etoricoxib and Diclofenac Arthritis Long-term programme (MEDAL)82—evaluated the gastrointestinal safety of celecoxib, rofecoxib, lumiracoxib, and etoricoxib, respectively. In the CLASS trial, patients with osteoarthritis or rheumatoid arthritis were randomized to receive celecoxib versus diclofenac or ibuprofen in two substudies of identical design. On primary analysis, there was no significant difference in the incidence of ulcer complications between the celecoxib group and the nonselective NSAIDs group.79 Whether the failure of CLASS was due to flaws in the study design remains controversial. The VIGOR study compared rofecoxib with naproxen in patients with rheumatoid arthritis.80 Unlike CLASS, VIGOR demonstrated that treatment with rofecoxib significantly reduced clinical GI events (combined endpoint of ulcer complications and symptomatic ulcers) by about 50%, compared with treatment with naproxen. Patients requiring low-dose aspirin were excluded from the VIGOR study, whereas 20% of patients in CLASS received low-dose aspirin concomitantly. The TARGET study compared lumiracoxib with naproxen or ibuprofen in patients with osteoarthritis using two substudies of identical design.81 Randomization was stratified for low-dose aspirin use and age. TARGET showed that treatment with lumiracoxib significantly reduced the incidence of ulcer complications compared with nonselective NSAIDs in the subgroup of patients not taking low-dose aspirin. Current evidence indicates that low-dose aspirin negates the GI mucosa-sparing effect of COX-2 inhibitors. The MEDAL program was a prespecified pooled analysis of data from three prospective trials. A total of 34,701 arthritic patients were treated with 60 or 90 mg of etoricoxib or 150 mg of diclofenac daily. Unlike the previous large-scale studies, the primary endpoint of this study was cardiothrombotic events. Therefore, patients on low-dose aspirin were enrolled and encouraged to receive PPI co-therapy. There was no between-group difference in terms of complicated GI events including bleeding, perforation, or obstruction. However, the overall incidence of uncomplicated GI events was significantly less with etoricoxib than with diclofenac. The reduction in uncomplicated GI events with etoricoxib is maintained in patients treated with PPIs and is also observed with regular low-dose aspirin use.82 In a systematic review of randomized trials of COX-2 inhibitors, COX-2 inhibitors produced significantly fewer gastroduodenal ulcers (relative risk, 0.26; 95% CI, 0.23 to 0.30) and ulcer complications (relative risk, 0.39; 95% CI, 0.31 to 0.5), as well as fewer withdrawals caused by GI symptoms when compared with nonselective NSAIDs.83
Current evidence indicates that COX-2 inhibitors probably are as effective as a combination of nonselective NSAIDs combined with a PPI in patients at risk for ulcers. In a double-blind randomized outcome trial of celecoxib and the combination of omeprazole and diclofenac in patients with a recent history of ulcer bleeding, approximately 5% of patients in the two treatment groups still had recurrent ulcer bleeding at 6 months.78 Although the two treatments were comparable in terms of the incidence of ulcer bleeding, a subsequent follow-up endoscopic study showed that 20% to 25% of patients receiving either treatment developed recurrent ulcers at 6 months.84 These findings suggest that neither treatment could eliminate the risk of recurrent bleeding in very-high-risk patients. Recently, a double-blind randomized trial compared celecoxib alone with combination of celecoxib and esomeprazole in patients with a history of NSAID-associated ulcer bleeding. All patients had a negative test for H. pylori infection before randomization. After a median follow-up of 13 months, 8.9% of the celecoxib-alone group had recurrent ulcer bleeding compared with none of the combined therapy group (P = 0.0004).85
Cardiovascular Risk of COX-2 Inhibitors and Nonselective NSAIDs
Despite the improved gastric safety profile of COX-2 inhibitors, the cardiovascular risk associated with this new class of NSAIDs has been the subject of much concern. In the VIGOR study, the incidence of acute myocardial events, although low, was four times higher among patients receiving rofecoxib than among patients receiving naproxen.80 Whether the observed difference in infarction rates between the two treatments was related to an antiplatelet property of naproxen or to a thrombotic effect of rofecoxib was hotly debated. Further data regarding the cardiovascular hazard of COX-2 inhibitors were derived from two long-term studies of colon polyp prevention using rofecoxib (Adenomatous Polyp Prevention on Vioxx [APPROVE] study)86 and celecoxib (Adenoma Prevention with Celecoxib [APC] study).87 In the APPROVE study, interim data at 18 months indicated that patients who received 25 mg rofecoxib a day had double the risk of serious cardiovascular events compared with patients who received placebo.86 In September 2004, rofecoxib was voluntarily withdrawn from worldwide markets in light of this unexpected finding. In APC study, interim data at 33 months showed that the occurrence of serious cardiovascular events was significantly higher for celecoxib at the very high dose of 400 mg twice a day (hazard ratio, 1.9; 95% CI, 1 to 3.3).87 In addition, a randomized, placebo-controlled trial of parecoxib and valdecoxib in patients who had undergone coronary artery bypass surgery found an almost four-fold increased risk of myocardial infarction.88
Do COX-2 inhibitors as a class increase the risk of myocardial infarction? Results of these three placebo-controlled trials indicate that this is the case. Both polyp prevention trials, however, investigated by design supratherapeutic doses of rofecoxib and celecoxib for extended time periods.86,87 In the TARGET study, rates of myocardial infarction with lumiracoxib were lower than with ibuprofen but higher than with naproxen. Neither result was statistically significant because the trial was underpowered to detect a difference in cardiovascular outcomes between treatment groups.84 The MEDAL program was a prespecified pooled analysis of cardiothrombotic events from three trials in which patients with osteoarthritis or rheumatoid arthritis were randomly assigned to etoricoxib (60 mg or 90 mg daily) or diclofenac (150 mg daily). After an average treatment of 18 months, rates of cardiothrombotic events were similar between the two treatment groups.89
Emerging evidence suggests that not only COX-2 inhibitors but also nonselective NSAIDs, with the exception of full-dose naproxen (1000 mg a day), increase cardiothrombotic risk. In a meta-analysis of randomized trials of COX-2 inhibitors (data mostly derived from rofecoxib and celecoxib), all COX-2 inhibitors increased the cardiothrombotic risk compared with placebo (risk ratio, 1.42; 95% CI, 1.13 to 1.78). This was largely attributable to an increased risk of myocardial infarction, with little difference in other vascular outcomes. A dose-dependent increase in cardiothrombotic events was observed with celecoxib. Importantly, there was no significant difference in cardiothrombotic risk between COX-2 inhibitors and nonselective NSAIDs. Naproxen (500 mg twice daily) was the only exception.90,91 In a meta-analysis of observational studies, high-dose rofecoxib (>25 mg a day), diclofenac, and indomethacin were associated with an increase in cardiothrombotic events, whereas celecoxib did not significantly increase the cardiothrombotic risk, though an increased risk could not be excluded with doses greater than 200 mg a day.92
In February 2005, the FDA issued recommendations proposing new serious labeling warnings for valdecoxib and celecoxib with respect to increased cardiovascular risks. In April of the same year, the FDA took the further step of asking the manufacturer to remove valdecoxib from the market. All sponsors of marketed prescription NSAIDs have been asked to revise the labeling for their products to include a boxed warning highlighting the potential for increased risk of cardiovascular events in addition to the potential life-threatening GI bleeding associated with their use. Manufacturers of over-the-counter NSAIDs are also being asked to revise their labeling to provide more specific information about the potential cardiovascular and GI risks associated with their individual products and to remind patients of the importance of limited dose and duration of treatment of these products in accordance with the package instructions.93 A large-scale, randomized head-to-head comparison of COX-2 inhibitors and nonselective NSAIDs using predefined cardiothrombotic events as the primary endpoint is under way.
THE ROLE OF HELICOBACTER PYLORI INFECTION IN ULCER DISEASE ASSOCIATED WITH NSAID USE
Whether H. pylori infection influences the risk of ulcer in patients receiving NSAIDs is one of the controversial issues in peptic ulcer research. Factors such as the choice of H. pylori diagnostic test, a history of ulcer complication, concomitant use of antisecretory agents, prior exposure to NSAIDs, and the use of low-dose aspirin affect the outcome.94 A meta-analysis showed that H. pylori raised the risk of ulcer bleeding more than 6-fold in patients receiving long-term NSAIDs, whereas H. pylori and NSAIDs alone raised the risk 1.79-fold and 4.85-fold, respectively.95 An updated meta-analysis showed similar findings.96 Among patients who are about to start NSAID therapy, eradication of H. pylori reduces the subsequent risk of ulcer development.97,98 Two systematic reviews have consistently shown that eradication of H. pylori is superior to placebo in preventing peptic ulcers among NSAID users.96,99 However, eradication of H. pylori infection alone is not sufficient for the prevention of ulcer bleeding in NSAID users with high ulcer risk.77
It has been suggested that the eradication of H. pylori might retard healing of gastric ulcers.100 This was not confirmed by a prospective randomized trial using ulcer healing as the predefined endpoint.101 Currently, there is no evidence that curing H. pylori infection has any clinically important negative effect on the healing of NSAID-related ulcers.
There is growing evidence that H. pylori increases the ulcer risk in patients receiving low-dose aspirin. Among patients with H. pylori infection and a history of ulcer bleeding who continued to use low-dose aspirin, a randomized trial found that successful eradication of H. pylori alone substantially reduced the risk of recurrent bleeding in six months.77 However, a later low-dose aspirin study suggested that co-therapy with a PPI after eradication of H. pylori was still required because of a high failure rate of H. pylori eradication and because concomitant NSAID use is not uncommon in clinical practice.102
RECOMMENDATIONS FOR THE PREVENTION OF NSAID-INDUCED ULCER COMPLICATIONS
Assessment of Gastrointestinal Risk
Before the cardiovascular hazards of COX-2 inhibitors and nonselective NSAIDs was a concern, prevention of NSAID-induced ulcer complications had been based on assessment of individual patients’ GI risk factors (Table 53-1). In clinical practice, patients receiving NSAIDs can be stratified according to their levels of GI risk, as follows (Table 53-2):
Table 53-1 Risk Ratios for the Various Risk Factors for Ulcer Complications Induced by NSAIDs*
| RISK FACTOR | RISK RATIO |
|---|---|
| History of complicated ulcer | 13.5 |
| Use of multiple NSAIDs (including aspirin), cyclooxygenase-2 [COX-2] inhibitor) | 9 |
| High doses of NSAIDs | 7 |
| Use of an anticoagulant | 6.4 |
| History of uncomplicated ulcer | 6.1 |
| Age > 70 years | 5.6 |
| Helicobacter pylori infection | 3.5 |
| Use of a glucocorticoid | 2.2 |
NSAIDs, nonsteroidal anti-inflammatory drugs.
* Not all NSAIDs pose the same risk.
Table 53-2 Recommendations for Reducing the Risk of Ulcers Associated with Nonsteroidal Anti-inflammatory Drugs (NSAIDs) as a Function of Gastrointestinal and Cardiovascular Risk
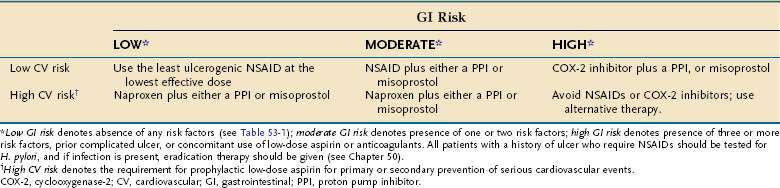
Because H. pylori infection raises the risk of ulcer complications in NSAID users, patients with a history of ulcer who require NSAIDs should be tested for H. pylori, and if present, the infection should be eradicated. The Maastricht III Consensus Guidelines also consider it advisable to test and treat for H. pylori infection in patients who are about to start regular NSAID therapy.2
High-Risk Patients
In general, NSAIDs should be avoided in these patients, not only because of the high risk of ulcer complications but also owing to the serious consequences of ulcer complications in the presence of comorbidities. Glucocorticoid therapy can be considered if short-term anti-inflammatory therapy is required for acute, self-limiting arthritis (e.g., gout), because glucocorticoids alone do not increase the risk of ulcer. If regular anti-inflammatory therapy is required for chronic arthritis, the combination of a COX-2 inhibitor and either misoprostol or a PPI probably offers the best GI protection, although this approach remains to be examined in prospective trials.85
Approach to Patients with High Cardiovascular Risk
After the withdrawal of rofecoxib, rational prescription of NSAIDs has become a clinical challenge. Not only GI but also cardiovascular risk factor must be assessed for the individual patient (see Table 53-2). For patients who do not have a history of coronary heart disease or ischemic stroke, identifying those with significant cardiovascular risk is not that straightforward. The American Heart Association (AHA) recommends that aspirin should be considered in all apparently healthy men and women whose 10-year risk for a cardiovascular event is 10% or above.103 We consider patients with arthritis to have significant cardiovascular risk if they are already on aspirin for secondary prophylaxis or if they require aspirin for primary prophylaxis according to the AHA guidelines.
Among patients with low cardiovascular risk, prescription of NSAIDs can be based on the presence of GI risk factors only. Patients known to have high cardiovascular risk should receive low-dose aspirin irrespective of NSAID use. Because of the potential cardiovascular hazards of COX-2 inhibitors and some nonselective NSAIDs, patients with high cardiovascular risk should avoid using these drugs if possible. Ibuprofen has been found to attenuate the cardioprotective effect of aspirin, possibly through competitive binding to platelet COX-1.104,105 Concomitant use of ibuprofen and low-dose aspirin therefore should be avoided. If anti-inflammatory analgesics are deemed necessary in patients at high cardiovascular risk, evidence suggests that full-dose naproxen (500 mg twice daily) does not increase the cardiothrombotic risk. However, it remains uncertain whether the cardioprotective effect of naproxen will persist at lower doses or when naproxen is co-prescribed with low-dose aspirin. Although naproxen has an antiplatelet effect, we do not recommend using naproxen as a substitute for low-dose aspirin in patients with high cardiovascular risk. This is because naproxen has a weak antiplatelet effect and patients take NSAIDs only intermittently for pain relief. One major drawback of concomitant use of NSAIDs and low-dose aspirin is that the combination will markedly increase the risk of ulcer complications over that incurred with NSAIDs alone (see Table 53-1; Chapter 52). Thus, co-therapy with a PPI or misoprostol is necessary even if patients do not have other GI risk factors (see Table 53-2).
REFRACTORY ULCERS
STRESS-RELATED MUCOSAL INJURY
Stress-related mucosal injury is an illness of the critically ill who are typically cared for in intensive care units. Fortunately, only a small proportion of patients with stress-related mucosal lesions have clinically overt bleeding. In a prospective study of more than 2000 patients admitted to intensive care units, only 1.5% experienced clinically important bleeding.106 Respiratory failure and coagulopathy were strong, independent risk factors for stress-related hemorrhage. Important bleeding occurred in 3.7% of the 847 patients who had one or both of these risk factors, whereas only 0.1% of 1405 patients without respiratory failure or coagulopathy experienced such bleeding. Thus, routine uses of stress ulcer prophylaxis in the intensive care unit is not recommended unless the patient has a coagulopathy or is receiving mechanical ventilation.
Although intravenous H2 receptor antagonists and oral or intragastric sucralfate are widely used to prevent stress ulcers in critically ill patients, the true efficacy of these treatments remains controversial. Moreover, few studies have evaluated PPIs for stress ulcer prophylaxis. The majority of studies have demonstrated that enteral or intravenous administration of PPIs to critically ill patients elevates intragastric pH and consistently maintains pH at 4 or higher.107 To date, no large-scale, clinical outcome study has been conducted to assess the efficacy of PPIs for this condition, despite their widespread use. An early, widely quoted meta-analysis conducted by Cook and associates108 concluded that H2 receptor antagonists were more effective than placebo in reducing the incidence of clinically important GI bleeding. However, in a later meta-analysis performed by Messori and colleagues,109 trials of ranitidine showed no difference from placebo; moreover, the data available on sucralfate did not allow any conclusion to be drawn. The Messori meta-analysis differed from the Cook meta-analysis in two important aspects. First, Cook and associates108 included trials that used either overt bleeding or clinically important GI bleeding as an endpoint, whereas Messori and colleagues included only trials that used clinically important GI bleeding as an endpoint. Second, in assessing the effectiveness of H2 receptor antagonists, Cook’s group included five trials that used cimetidine and three trials with negative results that used ranitidine, whereas Messori’s group included only the trials of ranitidine. A separate analysis showed that cimetidine but not ranitidine significantly reduced the rate of bleeding. The differences in outcome between cimetidine and ranitidine were probably due to chance rather than to a genuine difference between these two H2 receptor antagonists. Despite these discrepancies, there was either a rising trend108 or a significant increase109 in the incidence of nosocomial pneumonia with ranitidine compared with sucralfate. Overall, the findings are based on small numbers of patients with variable study design, and thus, firm conclusions cannot be drawn. Currently, neither the FDA nor the European Medicines Evaluation Agency has given approval to the routine use of H2 receptor antagonists, sucralfate, or PPIs for stress ulcer prophylaxis.
TREATMENT OF COMPLICATIONS OF PEPTIC ULCER DISEASE
HEMORRHAGE100,111
Initial Management (see Chapter 19)
Consensus groups have recommended a multidisciplinary approach to the care of patients presenting with upper GI bleeding.112,113 A team should include both medical and surgical gastroenterologists with access to skills in endoscopic hemostasis, which has become the mainstay of treatment. Patients identified as being at high risk of continued or recurrent bleeding should be admitted to an intensive care unit.
Patients with acute upper GI bleeding should be assessed promptly on presentation. Resuscitation and volume restoration should take priority and should precede endoscopy. Features of liver cirrhosis should call attention to the possibility of bleeding from esophagogastric varices rather than an ulcer. This distinction has prognostic as well as management implications. Variceal hemorrhage carries a higher death rate. The possibility of variceal hemorrhage calls for specific measures prior to endoscopy, such as the use of vasoactive drugs (e.g., octreotide) and antibiotics (e.g., cefotaxime) as prophylaxis against spontaneous bacterial peritonitis (see Chapters 90 and 91).
Risk Stratification
Bleeding stops in about 80% of patients presenting with acute upper GI bleeding. The remaining 20% constitute a high-risk group with substantial morbidity and mortality. It is therefore important to identify and direct appropriate care to patients at risk of continued or recurrent bleeding. For practical purposes, the management distinctions to be made are whether the patient is in need of urgent endoscopy and whether the patient is likely to have recurrent bleeding after initial endoscopic control. Some of the clinical predictors of increased risk for continued or recurrent bleeding are older age, shock, comorbid illnesses, low hemoglobin value, need for transfusion, and the finding of fresh blood in emesis or on rectal examination.106 Patients with signs of ongoing bleeding need urgent endoscopy with a view to securing hemostasis. Older adult patients tolerate blood loss poorly and are likely to have organ dysfunction consequent to bleeding; in such patients, the basis for early intervention should be more liberal. Other clinical predictors associated with higher mortality include the onset of bleeding in patients already hospitalized for other reasons.
Several derived risk scores have been developed to aid physicians in clinical decisions.114–116 The Rockall and Baylor scores are composite systems consisting of two components, the pre-endoscopy and postendoscopy scores.114,115 The Rockall scoring system (see Chapter 19) was derived from data gathered from the National United Kingdom Audit. A score of 0 to 2 indicates an excellent prognosis, whereas a score of 9 or more is associated with a high risk of death. The Blatchford score, on the other hand, uses clinical parameters only and is calculated from patients’ hemoglobin and blood urea concentrations, pulse and systolic blood pressure on admission, the presence or absence of melena or syncope, as well as of evidence of cardiac or hepatic failure.116
Endoscopic stigmata of bleeding not only pinpoint the source of bleeding but are themselves prognostic. The commonly used nomenclature is a version modified from Forrest and Finlayson’s117 original description, as follows (Table 53-3):
Table 53-3 Frequency and Prognosis of Various Endoscopic Stigmata of Hemorrhage in Patients with Bleeding Peptic Ulcer*
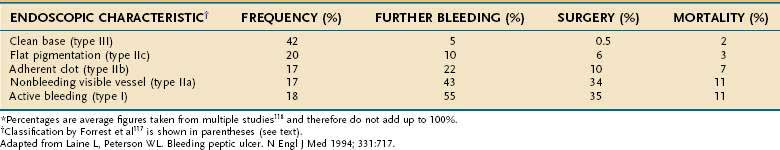
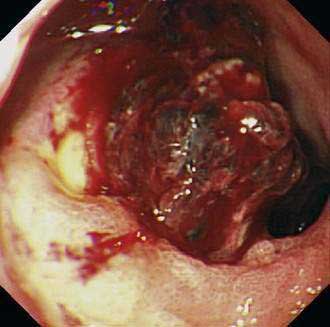
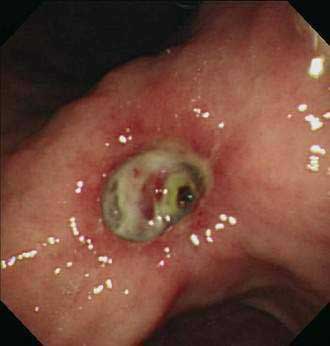
Reported prevalences of these endoscopic stigmata and their respective risks of recurrent bleeding have varied widely. This variation may be attributed to differences in visual interpretation among endoscopists and to varying definitions of recurrent bleeding. In one review,118 the rate of recurrent bleeding was less than 5% in patients with a clean ulcer base (type III) and increased to 10% in patients with a flat pigmentation (IIc), to 22% in those with an adherent clot (IIb), to 43% in those with a nonbleeding visible vessel (IIa), and to 55% in those with active bleeding, either spurting or oozing (type I) (see Table 53-3). Actively bleeding ulcers and ulcers with nonbleeding visible vessels (“protuberant discoloration”) warrant endoscopic therapy.119
Endoscopic therapy of ulcers with “adherent clots” has been controversial (Fig. 53-1). The definition of adherent clot varies with the vigor in endoscopic washing. Some endoscopists use targeted irrigation from a thermal probe. Some go to the extent of mechanical removal using a polypectomy snare. Two randomized controlled studies and a meta-analysis compared medical therapy with endoscopic treatment in patients with ulcers harboring “adherent clots” and concluded that clot removal followed by endoscopic treatment to the vessel underneath would reduce the risk of recurrent bleeding from around 30% to 5%.120–122 It is not often possible to distinguish a clot from a vessel. Indeed, it is logical to believe that for every clot there is an underlying artery. Johnston123 introduced the term sentinel clot, which is often used synonymously with visible vessel. An ulcer stops bleeding when an eroded artery is plugged by a clot, which varies in color. The “sentinel clot” can be contiguous with a larger overlying clot. With time, the ulcer heals, initially leaving a flat pigment to the ulcer base, and the vessel eventually disappears from the ulcer floor. This evolution of a bleeding vessel usually takes less than 72 hours. Ulcers with a flat spot or a clean base do not warrant endoscopic therapy (Fig. 53-2).
Endoscopic Therapy (see also Chapter 19)
Early endoscopy is generally defined as endoscopic examination performed within 24 hours of the patient’s admission. In patients with signs of active bleeding, it is common sense that urgent endoscopy establishes diagnosis and offers possible intervention. Such an approach in high-risk patients is generally believed to improve outcome. Randomized controlled trials demonstrated that early endoscopy in patients at low risk enabled their early hospital discharge and resource utilization or even management as outpatients.124–126 Many low-risk patients can be scheduled for endoscopy the morning after admission. A small but significant portion of patients with major bleeding require urgent endoscopy and therapy.
Two meta-analyses of endoscopic therapy showed significant reductions in the rates of further bleeding, surgery, and, importantly, mortality.127,128 Endoscopic therapy can be divided into injection, thermal, and mechanical methods.
Injection Methods
Endoscopic injection of diluted epinephrine into a bleeding peptic ulcer works by volume tamponade and local vasoconstriction, as blanching and edema of the mucosa are observed. It is an easy technique to learn and diluted epinephrine is non-tissue damaging and therefore safe to use. Diluted epinephrine, however, does not induce vessel thrombosis. Recurrent bleeding after injection with diluted epinephrine alone occurs in 20% to 30% of patients. In theory, the addition of a second agent to cause vessel thrombosis would further reduce the rate of recurrent bleeding. Various sclerosants (e.g., sodium tetradecyl sulfate, polidocanol, absolute alcohol) have been applied to the vessel after initial hemostasis with epinephrine. The addition of a sclerosant has not shown to further reduce rebleeding.129 Sclerosants damage tissue in a dose-dependent manner. Cases of gastric necrosis, some of them fatal, have been reported after sclerosant injection.130
Thrombin and fibrin, derived from both bovine and human sources, have been used as injection agents. A large-scale European multicenter trial demonstrated a statistically significant lower rate of recurrent bleeding associated with repeated injections of fibrin sealant (a mixture of fibrin and thrombin) at scheduled daily endoscopic examinations in comparison with a single injection of epinephrine plus polidocanol.131 The close surveillance rather than action of the fibrin sealant per se might have accounted for the difference. A single injection of fibrin sealant was not superior to epinephrine-polidocanol injection. There are also concerns about transmission of viral agents and anaphylaxis with the use of products derived from pooled plasma.
Thermal Methods
Thermal methods of endoscopic therapy are divided into contact and noncontact methods. Noncontact methods refer to the former use of laser photocoagulation and the current use of argon plasma coagulation. Laser therapy is no longer used because laser units are bulky and difficult to transport. In canine mesenteric artery models, Johnston and colleagues132 compared laser photocoagulation with contact thermal probes in hemostasis. The use of 3.2-mm contact probes consistently sealed arteries up to 2 mm in size. Laser probes were much less effective. The researchers introduced the term coaptive thermocoagulation and emphasized the need for compression of vessel walls. The two walls of an artery are pressed together by firm tamponade. This in itself stops blood flow and reduces the “heat-sink” effect. Heat energy is then generated, welding the arterial lumen. The commonly used contact thermal probes are the heater probe, which has a polytetrafluoroethylene (Teflon)-coated copper tip with three water ports for targeted irrigation, and bipolar probes. Firm tamponade is the key to successful application of contact probes. At least in animal experiments, thermal methods are superior to injection therapy in achieving hemostasis. Comparative clinical trials of injection and thermal methods did not show any difference in clinical outcomes.
Mechanical Methods
Because surgical plication of the bleeding artery is considered the most definitive treatment to achieve hemostasis, mechanical methods such as the endoscopic application of a hemoclip come closer to what would otherwise be done at surgery. The tangential application of hemoclips in treating posterior duodenal bulbar ulcerations or their use with the endoscope in a retroflexed position for treatment of high lesser curvature ulcers can be technically difficult. In a meta-analysis that included 15 randomized studies that compared injection, thermocoagulation, and hemoclipping, successful application of hemoclips was superior to injection alone but comparable to thermocoagulation in producing definitive hemostasis.133
Combination Methods
The benefit of combination therapy has been confirmed in two meta-analyses.134,135 In the first meta-analysis, the addition of a second modality significantly reduced the rate of recurrent bleeding from 18.4% to 10.6% and that of emergency surgery from 11.3% to 7.6%. The mortality rate decreased significantly from 5.1% to 2.6%. Eleven studies used injected substances such as a sclerosant, tissue adhesive, or thrombin; two added hemoclips; and three evaluated the added use of thermal devices. Findings of the meta-analysis suggest that a second modality should be added after injection of diluted epinephrine to bleeding peptic ulcers. The meta-analysis also confirmed that the rate of significant complications such as perforation and gastric wall necrosis was higher in the combined therapy group (6 of 558 patients) than in the epinephrine-alone group (1 of 560 patients). Furthermore, the improvement in prognosis seems to be more evident in ulcers with active bleeding (Forrest type I ulcers).
The critical determinant of the efficacy of endoscopic therapy is the size of the eroded artery in the ulcer base. Swain and associates136 studied gastrectomy specimens in patients who required emergency gastrectomy for bleeding gastric ulcers. The researchers suggested that bleeding from arteries larger than 1 mm could not be stopped by existing methods of hemostasis. Most studies on predictors of persistent or recurrent bleeding from ulcers find ulcer size of larger than 2 cm and ulcer location either high on the lesser curvature of the stomach or in the superior or posterior duodenal bulb to be associated with poorer outcomes. These are the classic locations for ulcers that erode into major artery complexes, such as the left gastric artery and the gastroduodenal artery, respectively. Surgery remains the only definitive method of securing bleeding in these patients.
Antisecretory Therapy
Labenz and associates137 studied gastric pH in patients with peptic ulcers receiving either a high dose of omeprazole (intravenous bolus 80 mg, followed by 8 mg/hr) or a high-dose ranitidine infusion (intravenous bolus 50 mg, followed by 0.25 mg/kg/hr). The gastric pH was less than 6 just 0.1% of the time in patients with either gastric or duodenal ulcers treated by high-dose omeprazole, much less than with ranitidine (20% in duodenal ulcers and 46% in gastric ulcers). In another study that measured gastric pH over three days, the use of histamine receptor antagonists given either in high-dose intravenous infusion or in bolus form led to progressive loss of antisecretory effect over days two and three because of tolerance. To achieve a gastric pH consistently above 6, a high-dose proton pump infusion is required.
The use of H2 receptor antagonists in the management of bleeding peptic ulcers has been evaluated in numerous clinical trials and summarized in meta-analyses. Patients with duodenal ulcer bleeding, who typically have a higher gastric acid output, do not benefit from the use of H2 receptor antagonists. A recent meta-analysis of 30 randomized trials concluded that the use of H2 receptor antagonists would be of benefit only in patients with gastric ulcers (absolute risk reductions of 7.2%, 6.7%, and 3.2% in the rates of recurrent bleeding, surgery, and death, respectively).138
Strong evidence for the use of PPIs in patients with bleeding peptic ulcers comes from a clinical trial reported by Lau and associates,139 in which early endoscopy was used to triage patients with bleeding peptic ulcers; only those at high risk of recurrent bleeding (i.e., those who had actively bleeding ulcers or ulcers with nonbleeding visible vessels) were enrolled. After endoscopic thermocoagulation of the ulcers, patients were randomly assigned to receive a high-dose omeprazole infusion or a placebo for 72 hours. The rate of recurrent bleeding in those who received the PPI infusion was 6.7% at day 30, compared with 22.5% in those who received placebo. In addition, the trial showed significant reductions in the need for further intervention, transfusion, and hospitalization as well as a trend in reducing the death rate in patients who received omeprazole. Similar benefits with intravenous esomeprazole given after successful endoscopic therapy in patients at high risk for recurrent ulcer bleeding have recently been reported.140
A Cochrane Systematic Review later concluded that the use of PPI therapy significantly reduces rates of recurrent bleeding and surgery but not mortality.141 In a subgroup analysis including studies that allowed initial endoscopic control, a significant reduction in mortality among Asians was seen in association with the use of a PPI. This supports the use of PPI as an adjunct to endoscopic therapy. The optimal dose to use and the routine of PPI administration continue to be controversial. The authors advocate the use of early endoscopic triage with a strategy to treat actively bleeding ulcers and ulcers with nonbleeding vessels, followed by adjunctive use of a high-dose intravenous infusion of a PPI.
Preemptive use of an intravenous PPI infusion prior to endoscopy was studied in a large-scale randomized study.142 Patients with overt signs of upper GI bleeding were randomized to receive either a high dose PPI infusion or placebo. In the cohort, 60% were found to be bleeding from a peptic ulcer during endoscopy. The study demonstrated that early PPI infusion downstaged bleeding stigmata in ulcers and thereby reduced the need for endoscopic therapy. In the PPI group there were fewer ulcers with active bleeding or with major stigmata observed the next morning during endoscopy. PPI infusion starts ulcer healing, and significantly more clean-based ulcers are seen the next day. The study has cost-saving implications with less endoscopic therapy required with the use of intravenous PPI. In patients awaiting endoscopy it is reasonable to start PPI therapy.
Emergency Surgery
Indications
Effective endoscopic intervention and improved pharmacotherapy have greatly reduced the need for emergency ulcer surgery. Not so long ago, surgery was the only reliable means of stopping bleeding. The National United Kingdom Audit performed more than a decade ago revealed an operative rate of 12% among 2071 patients with bleeding peptic ulcers and an associated mortality rate of 24%.143 Endoscopic intervention had not been used in 78% of these patients.
Timing
The timing of surgery for ulcer bleeding has been a subject of intense debate. In the 1980s, when endoscopic therapy was not available, Morris and colleagues144 published the only prospective randomized study that compared early surgery with delayed surgery, if needed, in 140 patients with bleeding ulcers. In patients younger than 60 years, there was no death in either group, but the more aggressive early surgery policy led to an unacceptably high operation rate (52% compared with only 5% for the delayed surgery group). For those older than 60 years, the operation rate was 62% in the early group and 27% in the delayed group. There were three deaths in 48 patients (6%) in the early surgery group and seven deaths in 52 patients (13%) in the delayed group. On intention-to-treat analysis, the difference did not reach statistical significance. According to treatment-received analysis, difference in mortality in patients with gastric ulcers was statistically significant (0 deaths in 19 of the early group vs. 5 in 21 of the delayed group, P < 0.01). The trial has been criticized for its small sample size and the use of subgroup analysis. In patients assigned to delayed surgery, ongoing bleeding was allowed before surgical intervention. Nevertheless, the study clearly demonstrated that early intervention reduced blood loss in older adult patients and improved outcome.
Choice of Operation
Two randomized studies that compared minimal with definitive surgery have been published.145,146 A United Kingdom multicenter study compared minimal surgery (oversewing the vessel or ulcer excision alone plus intravenous H2 receptor antagonist therapy) with a definitive ulcer surgery (vagotomy and pyloroplasty or partial gastrectomy) in patients with bleeding gastric or duodenal ulcerations. Of the 62 patients assigned to conservative treatment, 7 experienced rebleeding, of whom 6 died. Of the 67 patients who received conventional ulcer surgery, 4 had rebleeding and none died; in all rebleeding cases, vagotomy and oversewing of the ulcer had been performed. The overall mortality in this high-risk group of patients was similar in the two groups: 26% after minimal surgery and 19% after conventional surgery. The trial was terminated because of the high rate of fatal rebleeding in the conservative surgery group in comparison with the conventional surgery group.145
In the French Association of Surgical Research trial, patients with duodenal ulcers were randomly assigned to either oversewing plus vagotomy and drainage or partial gastrectomy.146 After oversewing and vagotomy, recurrent postoperative bleeding occurred in 10 of 60 patients (17%), in 6 of whom conversion to a Billroth II gastrectomy was required. Five of these 6 patients experienced duodenal stump dehiscence. In the group of 60 assigned to undergo partial gastrectomy, only 2 patients (3%) had rebleeding, both of whom recovered after conservative treatment. Of the 60 patients assigned to partial gastrectomy, Billroth I reconstruction was performed in 18, Billroth I reconstruction plus vagotomy in 6, Billroth II reconstruction in 20, and Billroth II reconstruction plus vagotomy in 16 patients. No duodenal leak occurred in 24 patients after Billroth I reconstruction. Among the 36 patients who received Billroth II reconstruction, duodenal stump leaks occurred in 8 (22%). The rate of duodenal stump leak in the overall gastrectomy group was therefore 8 in 60 (13%). When the results were analyzed on an intention-to-treat basis, and data from patients with duodenal leaks after reoperations for rebleeding in the oversewing and vagotomy groups were included, the duodenal leak rates were similar in the two groups (7 of 58 vs. 8 of 60, respectively). The researchers concluded that a more aggressive approach would be warranted in the surgical treatment of duodenal ulcers.
Difficult Ulcers
At surgery, a bulbar duodenal ulcer can be accessed via a longitudinal pylorotomy extending into the duodenum. Berne and Rosoff147 identified the confluence of several branches of the gastroduodenal artery in the vicinity of a bleeding posterior duodenal ulcer. Ligations above and below the bleeding artery are insufficient to ensure hemostasis. Berne and Rosoff suggested a U stitch in the center after ligations above and below. Many surgeons perform plications at four quadrants, and a few figure-of-eight stitches at varying angles along the course of the artery are often required. The longitudinal pylorotomy is then closed vertically as a Mikulicz-Heineke type of pyloroplasty.147 Whether the procedure should be completed with a truncal vagotomy is unclear, because powerful PPI therapy is now available. Recurrent bleeding occurs in 5% to 17% of cases after a vagotomy and pyloroplasty, often with a fatal outcome. To avoid this complication, many surgeons argue for excluding the duodenal ulcer by closing the duodenal stump distal to the ulcer. Some surgeons advocate end-to-end gastroduodenostomy (a Billroth I type reconstruction), in which the gastric remnant is advanced over the ulcer crater and sutured to the normal duodenal mucosa distal to the ulcer. No attempt is made to dissect the posterior duodenal wall distal to the inferior border of the ulcer. Often the duodenum retracts distally, and the stump can be closed by suturing of the divided anterior duodenal wall onto the distal lip of the ulcer (Nissen’s method). Other techniques in dealing with a difficult duodenal stump include a side catheter duodenostomy and the technique of Roux-en-Y jejunoduodenal anastomosis.
Surgery Versus Endoscopic Retreatment After Recurrent Bleeding
The choice between endoscopic re-treatment and surgery for recurrent bleeding after initial endoscopic control was addressed by Lau and colleagues148 in a randomized trial. In a cohort of 1169 patients with bleeding peptic ulcers treated by epinephrine injection followed by thermocoagulation, recurrent bleeding occurred in 8.7%. Ninety-two patients (mean age 65 years, 76% men) were randomly assigned to undergo either endoscopic re-treatment or surgery. Using intention-to-treat analysis, the endoscopic re-treatment and surgery groups did not significantly differ in mortality at 30 days (10% for re-treatment vs. 18% for surgery), duration of hospitalization (median 10 vs. 11 days, respectively), need for intensive care or length of stay in an intensive care unit (5 vs. 10 patients, respectively; median of 59 days for both), or units of blood transfused (median 8 vs. 7 units, respectively). Patients who underwent surgery were significantly more likely to have complications (16 vs. 7, respectively). Endoscopic re-treatment was able to control bleeding in three quarters of the patients. In those for whom endoscopic re-treatment failed, salvage surgery carried substantial mortality. In a regression analysis of a small subgroup of patients, ulcers 2 cm or larger and hypotension at rebleeding were two independent factors predicting failure with endoscopic re-treatment. Findings of this trial suggest that a selective approach can be adopted on the basis of the characteristics of the ulcer.148 Large chronic ulcers should probably be treated with expedited surgery at the time of rebleeding. Early elective surgery may have been more appropriate in these chronic ulcers after initial endoscopic control.
Angiographic embolization of bleeding arteries to peptic ulcer is a nonoperative option. In a nonrandomized comparison to surgery, angiographic embolization carried a similar rate of recurrent bleeding (29% vs. 23%), need for further intervention (16% vs. 31%), and death (26% vs. 21%).149
PERFORATION
A short period of resuscitation after ulcer perforation is diagnosed or suspected is often desirable, with restoration of fluids, electrolytes, and, if needed, blood. A nasogastric tube and a urinary catheter should be in place. Pain should be relieved with opiates after a presumptive diagnosis is made. As described in Chapter 37, intravenous broad-spectrum antibiotics should be administered parenterally, even though gastric juice contains few organisms. A majority of cultures of peritoneal fluid collected at the time of surgery for duodenal ulcer perforation are sterile; this is especially true when surgery is undertaken soon after perforation because the initial peritonitis is chemical in etiology. Nevertheless, acid-suppressive agents raise gastric pH and allow bacterial overgrowth in the stomach. The yield from culture increases with time as secondary peritonitis sets in. Coliforms, streptococci (usually anaerobic), staphylococci, and Candida species are the common organisms isolated. In late disease with abscess formations, anaerobes can often be isolated.
Medical Management
Nonoperative management of ulcer perforation has been advocated. The regimen involves nasogastric suctioning, parenteral antibiotics, and intravenous fluids. Crofts and associates150 randomly assigned patients with the presumptive diagnosis of perforated ulcers to either conservative treatment or surgery. Of 40 patients assigned to conservative treatment, 11 showed no improvement in 12 hours and underwent operation. Three of these 11 patients were found to have perforated carcinomas, 2 gastric and 1 sigmoid. Morbidity and mortality rates were similar in the medical and surgical groups. This trial may have included lower-risk patients or patients who presented early, because the 5% overall mortality is low. Findings of the study highlight common objections to the use of nonoperative management error in diagnosis, uncertainty of site of perforation, and the possibility of a perforated gastric tumor. Older adult patients should be operated on early for the following reasons: (1) malignancy is more likely; (2) atrophy of the greater omentum makes spontaneous sealing less likely; (3) such patients often withstand sepsis and organ dysfunction poorly; and (4) early surgery leads to a better outcome.150 In a patient in whom the perforation is considered confined on clinical assessment, it would be prudent to verify this judgment with a contrast radiographic study. If sealing is confirmed, conservative treatment may be reasonable.
Surgical Management
Whether to perform definitive ulcer surgery at the time of ulcer perforation was an argument that predated the era of H. pylori and PPIs. Relapse of ulcer disease with modern medical therapy is now uncommon and therefore is no longer a factor in the consideration as to the type of surgery to perform. There is now evidence that H. pylori eradication reduces relapse of ulceration after patch repair.151 In a randomized study enrolling patients with perforated duodenal ulcers after omental patch repair, patients received either a PPI alone for four weeks or quadruple anti–H. pylori therapy. After a year, ulcer relapse had occurred in 38% in the PPI group compared with only 5% in the H. pylori eradication group. Thus, ulcer relapse is uncommon after simple closure of a perforation and H. pylori eradication. These data would support the performance of simple closure alone in perforated ulcers.151
Although the role of H. pylori in perforated prepyloric or antral gastric ulcers after their patch closure is not known, it is probably similar to the organism’s role in perforated duodenal ulcer. The advocates for primary resection in perforated gastric ulcers argue that mortality rates after gastrectomy are not increased and that the rate of postoperative ulcer-related complications is reduced. The arguments for primary resection include the possibility that the ulcer is malignant. Malignancy is seen in approximately 6% of perforated gastric ulcers.152 In a retrospective series comprising 287 perforated gastric ulcers, death occurred in 21.5% of patients who underwent patch closure alone and in 24.3% of those who underwent gastrectomy.153 A patch closure is a far more popular operation for perforated gastric ulcers than resection.
Three randomized trials that compared laparoscopic repair with open repair in perforated ulcers favored the use of laparoscopic technique.154–156 In one study, Siu and associates155 randomly assigned 121 patients without other ulcer complications to undergo one of the two repairs. Laparoscopic repair was quicker to perform and led to less postoperative pain, smaller analgesic requirement, and shorter hospitalization. The same researchers later reported the routine use of laparoscopic repair in a cohort of 172 patients with perforated ulcers, about 80% of which were duodenal ulcers (Fig. 53-3).157 Conversion to open surgery occurred in 37 patients (around 1 in 5) because of perforations larger than 10 mm, non-juxtapyloric gastric ulcer, or perforations that could not be identified at laparoscopy. Persistent leaks after patch repair occurred in 2 patients who required laparotomy and gastrectomy. The overall mortality rate was 8.1%.
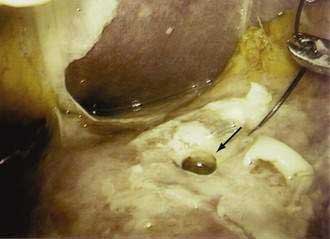
Figure 53-3. Laparoscopic view of a perforated duodenal ulcer (arrow) with fibrinous exudate on the adjacent peritoneum.
Several series studied risk factors for mortality in patients undergoing surgery for perforated ulcers. Boey and associates158 identified preoperative shock, major medical illnesses, and perforation for longer than 12 hours as important risk factors. Irvin159 identified two other risk factors—age older than 70 years and use of NSAIDs. Definitive surgery (vagotomy and gastrectomy) also led to a higher mortality in Irvin’s series. The deaths, however, occurred in older patients who had other, concomitant ulcer complications.
OBSTRUCTION
Endoscopic Management
Endoscopic balloon dilation has been used successfully in patients with benign gastric outlet obstruction (Fig. 53-4). During endoscopic examination, the stenosis is traversed by means of a biliary-type guidewire with a flexible hydrophilic tip. A low-compliance balloon is then passed over the guidewire. The use of a balloon is preferred because its inflation produces an even radial force, which has a theoretical advantage over the longitudinal shearing force associated with the use of conventional dilators. The availability of through-the-scope balloons that can be passed via the small channel of an endoscope enables the dilation to be seen and monitored. The procedure is typically performed with fluoroscopic guidance. A regimen of gradual dilation over two or three sessions seems sensible. The largest diameter of stenosis at which symptoms occur is unclear. Many authorities recommend dilation to 15 mm, which is often associated with relief of symptoms. The presence of gastric atony also contributes to symptoms. The risk of perforation rises with the size of balloon. Almost all of the perforations in one series occurred after dilation with a 20-mm balloon.160
Several series describing the use of endoscopic balloon dilation in the treatment of benign gastric outlet obstruction have been published.160–162 They varied in case mix, methods of dilation, and duration of follow-up. Most reported immediate relief of obstruction in 78% to 100% of cases. Recurrences, however, are common. In a series of 41 patients with a strong ulcer diathesis, half of the patients experienced relapse with recurrent obstruction, hemorrhage, or active ulceration after a median follow-up of 39 months.160 In a later series involving a handful of patients in whom H. pylori status was better defined, balloon dilation followed by H. pylori eradication in those shown to be infected led to more sustained symptom relief.163 Results of these studies suggest that endoscopic balloon dilation produces better results in patients with acute inflammatory edema than those with chronic scarring and fibrosis.
Surgical Management
Today, gastric outlet obstruction from peptic ulcer disease is uncommon, ulcer recurrence is unlikely once H. pylori and NSAIDs have been eliminated, excellent antisecretory therapy can be offered, there are a number of endoscopic techniques for dilating stenosis, and total parenteral nutrition is widely available (see Chapter 5). Therefore, an immediate decision regarding the need for surgery is generally not necessary for a patient who presents with gastric outlet obstruction. The problem can be managed with medical and endoscopic means in approximately 70% of cases, and only 30% eventually require one of the previously mentioned operations to bypass the gastric outlet obstruction.
Barkun A, Bardou M, Marshall JK. Nonvariceal Upper GI Bleeding Consensus Conference Group: Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843-57. (Ref 113.)
Chan FK, Hung LC, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104-10. (Ref 78.)
Chan FK, Wong VW, Suen BY, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: A double-blind randomized trial. Lancet. 2007;369:1621-6. (Ref 85.)
Kahi CJ, Jensen DM, Sung JJ, et al. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: A meta-analysis. Gastroenterology. 2005;129:855-62. (Ref 122.)
Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomized trials. BMJ. 2006;332:1302-8. (Ref 90.)
Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717-27. (Ref 118.)
Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-15. (Ref 76.)
Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631-40. (Ref 142.)
Leontiadis GI, Sreedharan A, Dorward S, et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007;11:1-164. (Ref 99.)
McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633-44. (Ref 92.)
Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995;311:222-6. (Ref 110.)
Saeed ZA, Winchester CB, Michaletz PA, et al. A scoring system to predict rebleeding after endoscopic therapy of nonvariceal upper gastrointestinal hemorrhage, with a comparison of heat probe and ethanol injection. Am J Gastroenterol. 1993;88:1842-9. (Ref 115.)
Sung JJY, Tsoi KK, Lai LH, et al. Endoscopic clipping versus injection and thermocoagulation in the treatment of non-variceal upper gastrointestinal bleeding: A meta-analysis. Gut. 2007;56:1364-73. (Ref 133.)
Sung JJY, Barkum A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding. Ann Int Med. 2009;150:455-64. (Ref 140.)
Vergara M, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev 2007; 2:CD005584. (Ref 135.)
Vergara M, Catalan M, Gisbert JP, Calvet X. Meta-analysis: Role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21:1411-18. (Ref 96.)
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-15.
2. Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut. 2007;56:772-81.
3. Higham J, Kang JY, Majeed A. Recent trends in admissions and mortality due to peptic ulcer in England: Increasing frequency of haemorrhage among older subjects. Gut. 2002;50:460-4.
4. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-15.
5. Ciociola AA, McSorley DJ, Turner K, et al. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834.
6. Graham DY. Large U.S. clinical trials report a high proportion of H. pylori negative duodenal ulcers at study entry as well as a high recurrence rate after cure of the infection: Have we all been wrong? Gastroenterology. 1998;114:A17.
7. Hung LC, Ching JY, Sung JJ, et al. Long-term outcome of Helicobacter pylori–negative idiopathic bleeding ulcers: A prospective cohort study. Gastroenterology. 2005;128:1845-50.
8. Peterson WL, Sturdevant RAL, Frankl HD, et al. Healing of duodenal ulcer with an antacid regimen. N Engl J Med. 1977;297:341-5.
9. Rydning A, Weberg R, Lange O, Berstad A. Healing of benign gastric ulcer with low-dose antacids and fiber diet. Gastroenterology. 1986;91:56-61.
10. Sherrard DJ. Aluminum—Much ado about something. N Engl J Med. 1991;324:558-9.
11. Feldman M, Burton ME. Histamine2-receptor antagonists: Standard therapy for acid-peptic diseases. N Engl J Med. 1990;323:1672-80.
12. Cantu TG, Korek JS. Central nervous system reactions to histamine2-receptor blockers. Ann Intern Med. 1991;114:1027-34.
13. Michaletz-Onody PA. Peptic ulcer disease in pregnancy. Gastroenterol Clin North Am. 1992;21:817-26.
14. Patel N, Ward U, Rogers MJ, Primrose JN. Night-time or morning dosing with H2-receptor antagonists: Studies on acid inhibition in normal subjects. Aliment Pharmacol Ther. 1992;6:381-7.
15. Wilder-Smith CH, Ernst T, Genonni M, et al. Tolerance to oral H2-receptor antagonists. Dig Dis Sci. 1990;8:976-83.
16. Richter JM, Colditz GA, Huse DM, et al. Cimetidine and adverse reactions: A meta-analysis of randomized clinical trials of short-term therapy. Am J Med. 1989;87:278-84.
17. Garcia Rodriguez LA, Jick H. Risk of gynaecomastia associated with cimetidine, omeprazole, and other anti-ulcer drugs. BMJ. 1994;308:503-6.
18. Agura ED, Vila E, Petersen FB, et al. The use of ranitidine in bone marrow transplantation: A review of 223 cases. Transplantation. 1988;46:53-6.
19. Hansten PD. Drug interactions with antisecretory agents. Aliment Pharmacol Ther. 1991;5:121-8.
20. Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: What the practising physician needs to know. Drugs. 2003;63:2739-54.
21. Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome p450 activities. Drug Metab Dispos. 2004;32:821-7.
22. Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol. 2004;95:2-8.
23. Wolfe MM, Sachs G. Acid suppression: Optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology. 2000;118:S9-S31.
24. Colin-Jones D. Safety of lansoprazole and omeprazole. Lancet. 1994;343:1369.
25. Havu N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion. 1986;35:42-55.
26. Solcia E, Capella C, Fiocca R, et al. Gastric argyrophil carcinoidosis in patients with Zollinger-Ellison syndrome due to type 1 multiple endocrine neoplasia: A newly recognized association. Am J Surg Pathol. 1990;4:503-13.
27. Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-22.
28. Lundell L, Miettinen P, Myrvold HE, et al. Nordic GERD Study Group: Lack of effect of acid suppression therapy on gastric atrophy. Gastroenterology. 1999;117:319-26.
29. www.FDA.gov/ohrms/dockets/ac/oo/backgrd/3650b1a_09.pdf
30. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-53.
31. Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-26.
32. Lew EA. Pharmacokinetic concerns in the selection of anti-ulcer therapy. Aliment Pharmacol Ther. 1999;13:11-16.
33. Humphries TJ. Clinical implications of drug interactions with the cytochrome P-450 enzyme system associated with omeprazole. Dig Dis Sci. 1991;36:1665-9.
34. McCarthy DM. Sucralfate. N Engl J Med. 1991;325:1017-25.
35. Wagstaff AJ, Benfield P, Monk JP. Colloidal bismuth subcitrate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in peptic ulcer disease. Drugs. 1988;36:132-57.
36. Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: The second hundred years. Gastroenterology. 1997;112:1000-16.
37. Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981;80:94-8.
38. Chen MC, Amirian DA, Toomey M, et al. Prostanoid inhibition of canine parietal cells: Mediation by the inhibitory guanosine triphosphate-binding protein of adenylate cyclase. Gastroenterology. 1988;94:1121-29.
39. Davis GR, Fordtran JS, Dajani EZ. Dose-response, meal-stimulated gastric antisecretory study of prostaglandin E1 analog, misoprostol, in man. Dig Dis Sci. 1988;33:298-302.
40. Karim A. Antiulcer prostaglandin misoprostol: Single and multiple dose pharmacokinetic profile. Prostaglandins. 1987;33:40-50.
41. Walt RP. Misoprostol for the treatment of peptic ulcer and antiinflammatory-drug-induced gastroduodenal ulceration. N Engl J Med. 1992;327:1575-80.
42. Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: Systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833-55.
43. Sung JJ, Chung SC, Ling TK, et al. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. N Engl J Med. 1995;332:139-42.
44. Malfertheiner P, Kirchner T, Kist M, et al. Helicobacter pylori eradication and gastric ulcer healing—Comparison of three pantoprazole-based triple therapies. BYK Advanced Gastric Ulcer Study Group. Aliment Pharmacol Ther. 2003;17:1125-35.
45. Higuchi K, Fujiwara Y, Tominaga K, et al. Is eradication sufficient to heal gastric ulcers in patients infected with Helicobacter pylori? A randomized, controlled, prospective study. Aliment Pharmacol Ther. 2003;17:111-17.
46. Jaspersen D, Koerner T, Schorr W, et al. Helicobacter pylori eradication reduces the rate of rebleeding in ulcer hemorrhage. Gastrointest Endosc. 1995;41:5-7.
47. Sung JJ, Leung WK, Suen R, et al. One-week antibiotics versus maintenance acid suppression therapy for Helicobacter pylori–associated peptic ulcer bleeding. Dig Dis Sci. 1997;42:2524-8.
48. Gisbert JP, Khorrami S, Carballo F, et al. H. pylori eradication therapy vs. antisecretory non-eradication therapy (with or without long-term maintenance antisecretory therapy) for the prevention of recurrent bleeding from peptic ulcer. Cochrane Database Syst Rev 2004; (2):CD004062.
49. Pang SH, Leung WK, Graham DY. Ulcers and gastritis. Endoscopy. 2008;40:136-9.
50. Lancaster-Smith MJ, Jaderberg ME, Jackson DA. Ranitidine in the treatment of nonsteroidal anti-inflammatory drug associated gastric and duodenal ulcers. Gut. 1991;32:252-5.
51. O’Laughlin JC, Silvoso GK, Ivey KJ. Resistance to medical therapy of gastric ulcers in rheumatic disease patients taking aspirin: A double-blind study with cimetidine and follow-up. Dig Dis Sci. 1982;27:976-80.
52. Goldstein JL, Johanson JF, Hawkey CJ, et al. Clinical trial: Healing of NSAID-associated gastric ulcers in patients continuing NSAID therapy—A randomized study comparing ranitidine with esomeprazole. Aliment Pharmacol Ther. 2007;26:1101-11.
53. Hawkey CJ, Karrasch JA, Szezepanski L, et al. Omeprazole compared with misoprostol for ulcers associated with non-steroidal antiinflammatory drugs: Omeprazole versus Misoprostol for NSAID-Induced Ulcer Management (OMNIUM) study group. N Engl J Med. 1998;338:727-34.
54. Yeomans ND, Tulassay Z, Juhasz L, et al. A comparison of omeprazole with ranitidine for ulcers associated with non-steroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-Associated Ulcer Treatment (ASTRONAUT) study group. N Engl J Med. 1998;338:719-26.
55. Agrawal NM, Campbell DR, Safdi MA, et al. Superiority of lansoprazole versus ranitidine in healing nonsteroidal antiinflammatory drug-associated gastric ulcers: Results of a double-blind, randomized, multicenter study. NSAID-Associated Gastric Ulcer study group. Arch Intern Med. 2000;160:1455-61.
56. Roth S, Agrawal N, Mahowald M, et al. Misoprostol heals gastroduodenal injury in patients with rheumatoid arthritis receiving aspirin. Arch Intern Med. 1989;149:775-9.
57. Bianchi Porro G, Lazzaroni M, Manzionna G, Petrillo M. Omeprazole and sucralfate in the treatment of NSAID-induced gastric and duodenal ulcer. Aliment Pharmacol Ther. 1998;12:355-60.
58. Sawaoka H, Kawano S, Tsuji S, et al. Helicobacter pylori infection induces cyclooxygenase-2 expression in human gastric mucosa. Prostaglandins Leukot Essent Fatty Acids. 1998;59:313-16.
59. Fu S, Ramanujam KS, Wong A, et al. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;16:1319-29.
60. Mizuno H, Sakamoto C, Matsuda K, et al. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387-97.
61. Shigeta J, Takahashi S, Okabe S. Role of cyclooxygenase-2 in the healing of gastric ulcers in rats. J Pharmacol Exp Ther. 1998;86:1383-90.
62. Schmassmann A, Peskar BM, Stettler C, et al. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastrointestinal ulcer models in rats. Br J Pharmacol. 1998;123:795-804.
63. Larkai EN, Smith JL, Lidsky MD, Graham DY. Gastroduodenal mucosa and dyspeptic symptoms in arthritic patients during chronic nonsteroidal anti-inflammatory drug use. Am J Gastroenterol. 1987;82:1153-8.
64. Wong VW, Leong RW, Chan FK. Upper gastrointestinal complications related to non-steroidal anti-inflammatory drugs—What have we achieved so far? Dig Liver Dis. 2004;36:1-3.
65. Singh G, Triadafilopoulus G. Epidemiology of NSAID-induced GI complications. J Rheumatol. 1999;26:18-24.
66. Rostom A, Wells G, Tugwell P, et al. Prevention of NSAID-induced gastroduodenal ulcers (Cochrane Review). Cochrane Database Syst Rev 2000; 4:CD002296.
67. Taha AS, Hudson N, Hawkey CJ, et al. Famotidine for the prevention of gastric and duodenal ulcers caused by non-steroidal antiinflammatory drugs. N Engl J Med. 1996;334:1435-9.
68. Hudson N, Taha AS, Russell RI, et al. Famotidine for healing and maintenance in nonsteroidal anti-inflammatory drug-associated gastroduodenal ulceration. Gastroenterology. 1997;112:1817-22.
69. Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:241-9.
70. Raskin JB, White RH, Jackson JE, et al. Misoprostol dosage in the prevention of nonsteroidal anti-inflammatory drug-induced gastric and duodenal ulcers: A comparison of three regimens. Ann Intern Med. 1995;123:344-50.
71. Chan FK, Sung JJ, Ching JY, et al. Randomized trial of low-dose misoprostol and naproxen vs. nabumetone to prevent recurrent upper gastrointestinal haemorrhage in users of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2001;15:19-24.
72. Raskin JB, White RH, Jaszewski R, et al. Misoprostol and ranitidine in the prevention of NSAID-induced ulcers: A prospective, double-blind, multicenter study. Am J Gastroenterol. 1996;91:223-7.
73. Graham DY, Agrawal NM, Campbell DR, et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: Results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 2002;62:169-75.
74. Regula J, Butruk E, Dekkers CP, et al. Prevention of NSAID-associated gastrointestinal lesions: A comparison study pantoprazole versus omeprazole. Am J Gastroenterol. 2006;101:1747-55.
75. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-10.
76. Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-15.
77. Chan FK, Chung SC, Suen BY, et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med. 2001;344:967-73.
78. Chan FK, Hung LC, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104-10.
79. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2001;284:1247-55.
80. Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR study group. N Engl J Med. 2000;343:1520-8.
81. Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: Randomised controlled trial. Lancet. 2004;364:665-74.
82. Laine L, Curtis SP, Cryer B, et al. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: A randomized comparison. Lancet. 2007;369:465-73.
83. Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: A Cochrane collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818-28.
84. Chan FK, Hung LC, Suen BY, et al. Celecoxib versus diclofenac plus omeprazole in high-risk arthritis patients: Results of a randomized double-blind trial. Gastroenterology. 2004;127:1038-43.
85. Chan FK, Wong VW, Suen BY, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: A double-blind randomized trial. Lancet. 2007;369:1621-6.
86. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092-102.
87. Solomon SD, McMurray JJV, Pfeffer MA, et al. for the Adenoma Prevention with Celecoxib (APC) Study Investigators. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-80.
88. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-91.
89. Cannon CP, Curtis SP, FitzGerald GA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: A randomised comparison. Lancet. 2006;368:1771-81.
90. Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomized trials. BMJ. 2006;332:1302-8.
91. Juni P, Nartey L, Reichenbach S, et al. Risk of cardiovascular events and rofecoxib: Cumulative meta-analysis. Lancet. 2004;364:202.
92. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633-44.
93. www.fda.gov/cder/drug/infopage/cox2/default.htm Accessed July 19, 2005
94. Chan FK. Helicobacter pylori and nonsteroidal anti-inflammatory drugs. Gastroenterol Clin North Am. 2001;30:937-52.
95. Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet. 2002;359:14-22.
96. Vergara M, Catalan M, Gisbert JP, Calvet X. Meta-analysis: Role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21:1411-18.
97. Chan FK, Sung JY, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before starting non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 1997;350:975-9.
98. Chan FK, To KF, Wu JC, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: A randomised trial. Lancet. 2002;359:9-13.
99. Leontiadis GI, Sreedharan A, Dorward S, et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007;11:1-164.
100. Hawkey CJ, Tulassay Z, Szczepanski L, et al. Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs. HELP NSAIDs study. Lancet. 1998;352:1016-21.
101. Chan FK, Sung JJ, Suen R, et al. Does eradication of Helicobacter pylori impair healing of nonsteroidal anti-inflammatory drug associated bleeding peptic ulcers? A prospective randomized study. Aliment Pharmacol Ther. 1998;12:1201-5.
102. Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346:2033-8.
103. Pearson TA, Blair SN, Daniels SR, et al. AHA scientific statement: AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 Update, 71-0226. Circulation. 2002;106:388-91.
104. Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809-17.
105. MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361:573-4.
106. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330:377-81.
107. Jung R, MacLaren R. Proton-pump inhibitors for stress ulcer prophylaxis in critically ill patients. Ann Pharmacother. 2002;36:1929-37.
108. Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients: Resolving discordant meta-analyses. JAMA. 1996;275:308-14.
109. Messori A, Trippoli S, Vaiani M, et al. Bleeding and pneumonia in intensive care patients given ranitidine and sucralfate for prevention of stress ulcer: Meta-analysis of randomised controlled trials. BMJ. 2000;321:1103-6.
110. Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995;311:222-6.
111. Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: A population-based study. Am J Gastroenterol. 1995;90:206-10.
112. Nonvariceal upper gastrointestinal haemorrhage guidelines. Gut. 2002;51(Suppl 4):iv1-6.
113. Barkun A, Bardou M, Marshall JK. Nonvariceal Upper GI Bleeding Consensus Conference Group: Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843-57.
114. Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996 March;38:316-21.
115. Saeed ZA, Winchester CB, Michaletz PA, et al. A scoring system to predict rebleeding after endoscopic therapy of nonvariceal upper gastrointestinal hemorrhage, with a comparison of heat probe and ethanol injection. Am J Gastroenterol. 1993;88:1842-9.
116. Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper gastrointestinal haemorrhage. Lancet. 2000;356:1318-21.
117. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394-7.
118. Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717-27.
119. Therapeutic endoscopy and bleeding ulcers. Natl Inst Health Consens Dev Conf Consens Statement. 1989;7:1-7.
120. Bleau BL, Gostout CJ, Shearman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: A randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc. 2002;56:1-6.
121. Jensen DM, Kovacs TO, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002;123:407-13.
122. Kahi CJ, Jensen DM, Sung JJ, et al. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: A meta-analysis. Gastroenterology. 2005;129:855-62.
123. Johnston JH. The sentinel clot and invisible vessel: Pathologic anatomy of bleeding peptic ulcer. Gastrointest Endosc. 1984;30:313-15.
124. Lin HJ, Wang K, Perng CL, et al. Early or delayed endoscopy for patients with peptic ulcer bleeding: A prospective randomized controlled trial. J Clin Gastroenterol. 1996;22:267-71.
125. Lee JG, Turnipseed S, Romano PS, et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: A randomized controlled trial. Gastrointest Endosc. 1999;50:755-61.
126. Cipolletta L, Bianco MA, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: A randomized controlled trial. Gastrointest Endosc. 2002;55:1-5.
127. Sacks HS, Chalmers TC, Blum AL, et al. Endoscopic hemostasis: An effective therapy for bleeding peptic ulcers. JAMA. 1990;264:494-9.
128. Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology. 1992;102:139-48.
129. Rollhauser C, Fleischer D. Current status of endoscopic therapy for ulcer bleeding. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:391-410.
130. Levy J, Khakoo S, Barton R, Vicary R. Fatal injection sclerotherapy of a bleeding peptic ulcer [letter]. Lancet. 1991;337:504.
131. Rutgeerts P, Rauws E, Wara P, et al. Randomized trial of single and repeated fibrin glue compared with injection of polidocanol in treatment of bleeding peptic ulcer. Lancet. 1997;350:692-6.
132. Johnston JH, Jensen DM, Auth D. Experimental comparison of endoscopic yttrium-aluminum-garnet laser, electrosurgery, and heater probe for canine gut arterial coagulation: Importance of compression and avoidance of erosion. Gastroenterology. 1987;92:1101-8.
133. Sung JJ, Tsoi KK, Lai LH, et al. Endoscopic clipping versus injection and thermocoagulation in the treatment of non-variceal upper gastrointestinal bleeding: A meta-analysis. Gut. 2007;56:1364-73.
134. Calvet X, Vergara M, Brullet E, et al. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004;126:441-50.
135. Vergara M, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev 2007; 2:CD005584.
136. Swain CP, Storey DW, Bown SG, et al. Nature of the bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology. 1986;90:595-608.
137. Labenz J, Peitz U, Leusing C, et al. Efficacy of primed infusions with high dose ranitidine and omeprazole to maintain high intragastric pH in patients with peptic ulcer bleeding: A prospective randomised controlled study. Gut. 1997;40:36-41.
138. Levine JE, Leontiadis GI, Sharma VK, Howden CW. Meta-analysis: The efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther. 2002;16:1137-42.
139. Lau JY, Sung J, Lee K, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310-16.
140. Sung JJY, Barkum A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding. Ann Int Med. 2009;150:455-64.
141. Leontiadis GI, McIntyre L, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev 2004; 3:CD002094.
142. Lau JY, Leung WK, Wu JC, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631-40.
143. Rockall TA. Management and outcome of patients undergoing surgery after acute upper gastrointestinal haemorrhage. Steering Group for the National Audit of Acute Upper Gastrointestinal Haemorrhage. J R Soc Med. 1998;91:518-23.
144. Morris DL, Hawker PC, Brearley S, et al. Optimal timing for bleeding peptic ulcer: A prospective randomized trial. BMJ. 1984;228:1277-80.
145. Poxon VA, Keighley MRB, Dykes PW, et al. Comparison of minimal and conventional surgery in patients with bleeding ulcer: A multicentre trial. Br J Surg. 1991;78:1344-5.
146. Millat B, Hay JM, Valleur P, et al. Emergency surgical treatment for bleeding duodenal ulcer: Oversewing plus vagotomy versus gastric resection, a controlled randomized trial. French Associations for Surgical Research. World J Surg. 1993;17:568-74.
147. Berne CJ, Rosoff L. Peptic ulcer perforation of the gastroduodenal artery complex. Ann Surg. 1969;169:141-4.
148. Lau JY, Sung JJY, Lam YH, et al. Endoscopic re-treatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-6.
149. Ripoll C, Bañares R, Beceiro I, et al. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004;15(5):447-450.
150. Crofts TJ, Park KGM, Steele RJC, et al. A randomized trial of nonoperative treatment for perforated peptic ulcer. N Engl J Med. 1989;320:970-3.
151. Ng EK, Lam YH, Sung JY, et al. Eradication of Helicobacter pylori prevents recurrence of ulcer after simple closure of duodenal ulcer perforation: Randomized controlled trial. Ann Surg. 2000;231:153-8.
152. McGee GS, Sawyers JL. Perforated gastric ulcers: A plea for management by primary gastric resection. Arch Surg. 1987;122:555-61.
153. Lanng C, Hansen CP, Christensen A, et al. Perforated gastric ulcer. Br J Surg. 1988;75:758-9.
154. Lau WY, Leung KL, Kwong KH, et al. A randomized study comparing laparoscopic and open repair of perforated peptic ulcer using suture or sutureless technique. Ann Surg. 1996;224:131-8.
155. Druart ML, Van Hee R, Etienne J, et al. Laparoscopic repair of perforated duodenal ulcer: A prospective multicenter clinical trial. Surg Endosc. 1997;11:1017-20.
156. Siu WT, Leong HT, Law BKB, et al. Laparoscopic repair for perforated peptic ulcer: A randomized controlled trial. Ann Surg. 2002;235:313-19.
157. Siu WT, Chau CH, Law BKB, et al. Routine use of laparoscopic repair for perforated peptic ulcer. Br J Surg. 2004;91:481-4.
158. Boey J, Choi SKY, Alagaratnam TT, Poon A. Risk stratification in perforated duodenal ulcers: A prospective validation of predictive factors. Ann Surg. 1987;205:22.
159. Irvin TT. Mortality and perforated peptic ulcer: A case for risk stratification in elderly patients. Br J Surg. 1989;76:215-18.
160. Lau JY, Chung SC, Sung JY, et al. Through-the-scope balloon dilation for pyloric stenosis: Long term results. Gastrointest Endosc. 1996;43:98-101.
161. Hogan RB, Hamilton JK, Polter DE. Preliminary experience with hydrostatic balloon dilatation of gastric outlet obstruction. Gastrointest Endosc. 1986;32:71-4.
162. Craig PI, Gillespie PE. Through the endoscope balloon dilatation of benign gastric outlet obstruction. BMJ. 1988;297:396.
163. Lam YH, Lau JY, Fung TM, et al. Endoscopic balloon dilation for benign gastric outlet obstruction with or without Helicobacter pylori infection. Gastrointest Endosc. 2004;60:229-33.