Chapter 113 Treatment of Intractable Epilepsy by Electrical Stimulation of the Vagus Nerve
Epilepsy is estimated to affect approximately 50 million people worldwide,1 with a higher incidence in children, estimated at 100 to 200 per 100,000 children per year, compared with 24 to 53 per 100,000 in adults.2 While the overall prognosis of epilepsy is good, approximately 30% of patients do not respond adequately to antiepileptic drugs or ketogenic diet and suffer from medically intractable seizures.2 More than half of these patients are not good candidates or are not controlled by resective surgery, subpial transection, or corpus callosotomy. The effect on health and quality of life, as well as impact on society, is enormous, so the establishment of a new surgical strategy for control of seizures is of significant interest to neurosurgeons. Vagus nerve stimulation (VNS) in the neck, approved since July 1997 by the Food and Drug Administration (FDA) and in much of the European community since 1994, has now been used to treat more than 20,000 patients in at least 26 countries.
Background
The vagus nerve, or tenth cranial nerve, is composed of approximately 80% afferent visceral fibers3 transmitting visceral sensory information from receptors in the heart, aorta, lungs, and gastrointestinal system to the central nervous system (CNS). Accordingly, it is not surprising that VNS can affect CNS activity. A small number of fibers pass directly to the spinal trigeminal nucleus and the reticular formation, but most vagal afferents originate from nodose ganglion neurons with projections primarily to the nucleus of the solitary tract, which has widespread projections to the cerebral cortex, basal forebrain, hypothalamus, dorsal raphe, and cerebellum,4 and important areas for epileptogenesis such as amygdala, hippocampus, and thalamus.
There is also a direct pathway with viscerotopic representation to the insular cortex via the parabrachial nucleus in the pons.5 This is probably the pathway that relays sensation from vagal stimulation to conscious perception.
In addition to the anatomic studies of the vagus nerve showing the vast number of cortical projections, animal studies have demonstrated effects on the EEG with stimulation. Electrical stimulation of the vagus nerve in cats was first shown to cause EEG changes in 1938 by Bailey and Bremer.6 Further studies have shown that, depending on the stimulation intensity and frequency, the cortical EEG can be synchronized or desynchronized.7 Low frequency (1 to 16 Hz) and very high intensity synchronize (increase slow waves), and higher intensity and faster frequencies (greater than 70 Hz) desynchronize (arousal, rapid eye movement), as do high intensity and slower frequency (20 to 50 Hz).8–10 This suggests that different afferent fibers are stimulated under different circumstances, affecting different pathways and connections, with ultimately differing effects on the cortical EEG.
Animal Studies
Based on studies showing that the vagus nerve has a wide “connectivity” to the CNS,11,12 various animal models have been used to investigate the effect of VNS on epilepsy. Some of the early studies in cats, dogs, rats, and monkeys showed reduction or prevention of seizures using a variety of chemically induced seizures. Study of the stimulation parameters required showed maximal anticonvulsant effect with C-fiber stimulation.13–16
In addition, McLachlan17 demonstrated reduction or abolition of interictal spike activity in an acute epilepsy model using rats. Secondarily generalized seizures were also reduced in duration, but only if VNS occurred within 3 seconds of the start of the seizure. After 3 seconds, VNS had no effect on duration. This was also borne out in studies by Woodbury and Woodbury,14,15 who showed that the longer the delay in stimulating the vagal nerve, the longer the duration of a seizure. In the same study, it was also shown that the effect on interictal spiking was seen 1 to 2 seconds after the onset of VNS and could persist for 1 to 3 minutes beyond stimulation; therefore, the anticonvulsant effect appeared to outlast the stimulation. Koo18 showed that during stimulation at the frequency of 20 to 30 Hz, there is synchronization of the epileptiform activity during the time when the stimulus is on, and suggested that there may be desynchronization of EEG results during the time when the stimulator is off. This may contribute to the mechanism of intermittent stimulation of the VNS as opposed to continuous stimulation.
Other parameters for optimal stimulation were assessed and shown in animal studies to be a stimulation of 10 to 20 Hz with a pulse width of 0.5 to 1 millisecond.14,15,19 In practice, human stimulation is usually started with a pulse width of 0.25 millisecond (250 microseconds).
Human Studies and Efficacy
The data show that patients treated with high stimulation rates (≤5 minutes of “off time”) had a mean decrease in seizure frequency of almost 30% versus a mean decrease of 15% in the low-stimulation-rate group (180 minutes off, used as an “active control”). Results also show that the responder rate of at least a 50% reduction in seizure numbers was 30% in the group with the high stimulation rate. The responder rate in the low-stimulation-rate group was 13%, which suggests that VNS works at both rates but the higher stimulation rates are optimal. The use of such “active control” strategies has been critical in minimizing potential placebo effects when interpreting the results of this modality.20,21
There have been a few small studies suggesting that rapid-cycle (7 to 14 seconds on, 30 seconds off) stimulation may be effective in the patient who has not responded to slower cycling rates. This has shown some added efficacy in patients with Lennox-Gastaut syndrome and tuberous sclerosis.22–24
A few studies have included children also.25,26 The numbers are small, but most children had a more than 50% reduction in seizure frequency and a significant number had more than 90% reduction.
Mechanisms of Action
Chronic VNS induces many physiologic changes in the brain. Naritoku and colleagues27 have reported increases in neuronal fos expression with VNS. The increased activity was seen in areas of the medulla, hypothalamus, thalamus, amygdala, and cingulate with connectivity to the vagus nerve. The increased fos activity suggests increased neuronal activity.
Positron emission tomography scanning has also shown activation of CNS structures with VNS. Regional blood flow changes showing increases in the ipsilateral anterior thalamus and cingulate gyrus were reported by Garnett and co-workers,28 and in another study, Ko and associates29 found increased blood flow in the ipsilateral putamen and cerebellum with contralateral increases in the thalamus and temporal lobe.
In addition to reports of increased blood flow, concentrations of inhibitory neurotransmitters and amino acids have been shown to increase. The recent demonstration that magnetoelectroencephalography (MEG) can feasibly be performed on patients with VNS devices in place30 may suggest a direction to collect data to further understanding of the mechanism of action. A coherent understanding of the mechanism of understanding, which has not so far advanced beyond the vague concept of “desynchronization,”31 may help advance appropriate use of the devices.
Patient Selection
Seizure type is not an inclusion criterion. Studies have shown that the efficacy of VNS in generalized seizures and Lennox-Gastaut syndrome is comparable with that in partial seizures.22A clear definition of the best patients for use of this therapy is unknown. Initial studies focused primarily on patients with intractable partial epilepsy. Approximately 20% of the patients studied in this group had a 50% or greater reduction in the number of seizures, with another 50% of the patients having a significant decrease in seizure frequency of at least 20%. Many patients also reported a decrease in seizure intensity, which is more difficult to quantify.
The current stimulator device gives options for telemetrically changing the current intensity in milliamps, pulse width, length of time for stimulus train, and off time or time between stimulus trains. Reprogramming the stimulus output is usually the first parameter to adjust, and it can be increased in increments based on the patient’s tolerance. Other parameters that are adjusted are stimulus on and off times, which can be shortened or lengthened. Changing the on time to as little as 7 seconds and the off time to as little as 30 seconds has been shown to improve seizure control in patients with Lennox-Gastaut syndrome who have shown no improvement with the usual settings.22
Surgical Technique
The patient is positioned supine with the neck in slight extension and with the head turned slightly to the right. If the neck is turned too severely, greater retraction is required on the sternocleidomastoid muscle to allow for the dissection, and a moderate degree of rotation gives the best exposure to a suitable length of the nerve.
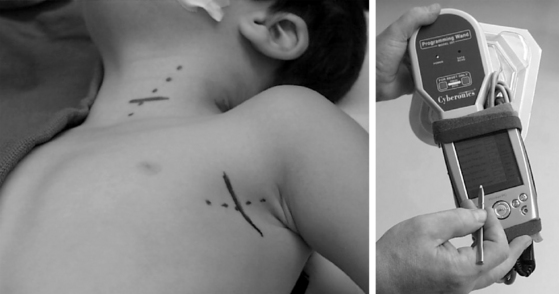
Two skin incisions are made (Fig. 113-1). The cervical incision is made in the direction of a skin crease centered over the anterior edge of the sternocleidomastoid muscle approximately two thirds of the way down from the angle of the jaw to the clavicle. It is helpful to keep the exposure low enough to be well below the carotid bifurcation to ensure the most straightforward dissection of the nerve. Adequate exposure of the nerve can be obtained through an incision 2.0 to 2.5 cm long in a thin patient, but in the presence of a thick neck or prior surgery a longer incision should be made. We prefer to divide the platysma in the direction of its fibers along the anterior border of the sternocleidomastoid muscle, and then follow this anterior border in the avascular plane down to the carotid sheath. A Cloward retractor, usually with the shortest blunt blades, gives excellent exposure of the carotid sheath, and the vagus nerve is identified within the sheath.
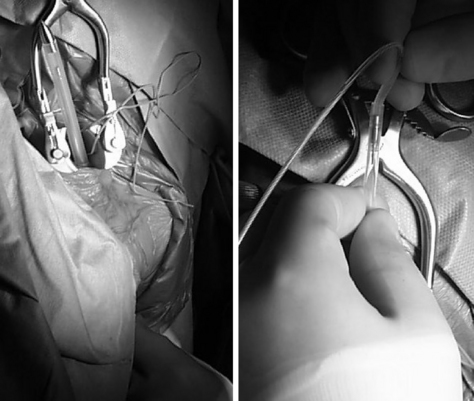
It is important to pay attention to the thickness and nature of the carotid sheath fascia while opening it on either side of the nerve, because this fascia is used for placing anchoring sutures to hold in the electrodes after they are placed on the vagus nerve. The nerve needs to be dissected clear of the sheath for a distance of approximately 3 cm, and this is facilitated by passing a plastic vessel loop around it and using it to elevate the nerve while using sharp dissection to open the sheath of either side of the nerve to free it up (Fig. 113-2). Blunt dissection is then used to open up space within the carotid sheath inferiorly for another 2 cm, because this will be used for a relaxing loop of cable to be fashioned later.
After the nerve is isolated, the subcutaneous pocket or subpectoral pocket is made (Fig. 113-3). Previously, an incision approximately 10 cm below and parallel to the clavicle was recommended, although the stimulator device can be easily implanted into the same pocket by making an incision just at the inferior and lateral border of the pectoralis muscle approximately 2.5 cm in length in the region of the anterior axillary line. We have further modified this technique to make the axillary incision more horizontal and slightly curved, to follow the natural skin creases. This results in improved cosmetic appearance of the scar. Dissection is carried down below all of the subcutaneous tissue and just above the fascia of the pectoralis muscle itself, if a subcutaneous pocket is desired, or deep to the pectoralis for subpectoral placement. The device is relatively elongated in shape, and with practice the surgeon can readily determine when the pocket is large enough to admit the stimulator device in relation to the size of his or her hand through an incision less than 2.5 cm in length. It is useful to have the pocket deep enough that the device is recessed 1 to 2 cm away from the skin incision.
After the pocket is fashioned, the tunneling device can be passed in either direction between the pocket and the surgical incision. We usually do this from the pectoral pocket to the cervical incision above (Fig. 113-4), with care taken to avoid injury to the exposed deep structures and with the ideal level of emergence of the tunneler just below the platysma.
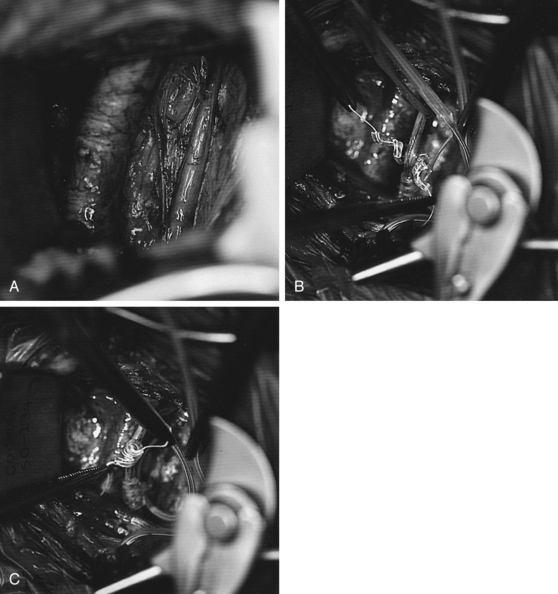
The next step involves putting the coil electrodes onto the vagus nerve (Fig. 113-5). Although this is conceptually simple, it can be frustrating if an attempt is made to put them on in the wrong orientation. We have found the simplest technique is to pull the cable inferiorly somewhat so there is no redundant cable below the coil devices. Starting with the lowest of the three coils (which is really just a retaining coil, and not an electrode), fine threads coming from the two ends of the Silastic coil are grasped and the superior of these is passed from lateral to medial, going deep to the vagus nerve. This is helped by having the assistant retract the nerve gently upward using the plastic loop. If the nerve is allowed to lay in a middle groove of the slightly opened coil, bringing the upper thread laterally and the lower thread medially across that nerve completes one full revolution of the coil, and then releasing the coil causes it to snap back into a good position around the nerve. Usually one to one-and-a-half more revolutions of the helix need to be completed around the nerve, but this is relatively easily done. Each of the two other coils can then be placed around the nerve, and because the superior coils that contain electrodes are shorter and have fewer completed coils than the lower retaining coil, they often go in somewhat more easily. If the nerve is not dissected for an adequate length to begin with, the last coil may be more difficult because of the lack of adequate space.
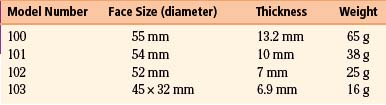
At this point, the connector cable can be placed into the connection socket on the stimulator device. In the current design (Model 103), only one cable is used, but for replacements with the older, two-contact electrode cables it is important to note which cable is positive and which negative. The positive cable is marked with a white band and goes into the stimulator at the level of a port marked with a plus sign. When inserting the cables, a set screw may block the socket if the cable connector does not insert smoothly. Simply backing out the set screws solves this problem. Once these are in place and the set screws are tightened, the electrical integrity and correct impedance of the nerve electrodes can be tested with diagnostics supplied by the computer (Fig. 113-6). This is done by placing the wand of the computer (along with the hand-held programmer, if this is used instead of a laptop computer for programming) in a sterile plastic drape, such as a clear x-ray cassette cover, and passing it onto the field. The device can then be tested and even activated. We turn on the device at the time of placement as noted above, but some clinicians prefer simply to leave it off for a few weeks to be certain that any postoperative symptoms the patient is having can be distinguished from symptoms related to stimulation.
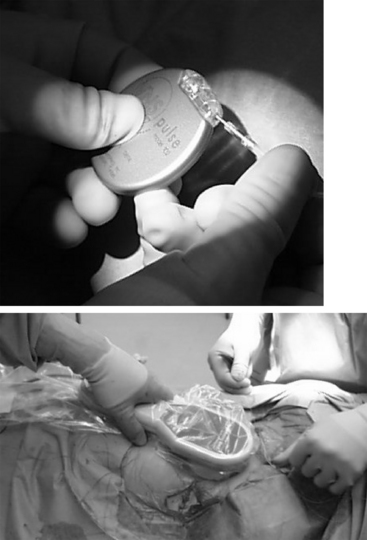
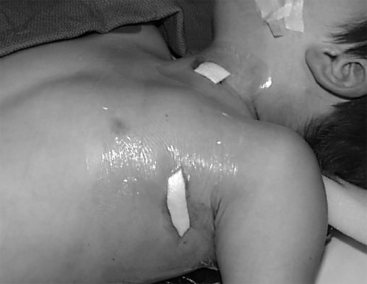
The pulse generator is then inserted into the subcutaneous pocket and secured using a single 2-0 silk stitch to the pectoralis fascia, which keeps it anchored deeply into the pocket. It is useful to have the logo of the device facing toward the outside, which optimally orients the antenna for communication with the wand, especially in patients with thicker subcutaneous tissues. Once this is secured in place, both incisions can be closed with resorbable sutures to the deep fascia in the pocket and the platysma in the neck, followed by subcutaneous closure and subcuticular closure with paper strips to close the edges of the skin. Dry sterile dressings are placed, and we usually instruct patients to leave them on for 7 to 10 days to keep the incision completely dry (Fig. 113-7, Table 113-1).
Al-Jayyousi M., Helmers S.L. Adjunctive treatment in Lennox-Gastaut syndrome using vagal nerve stimulation. Epilepsia. 1998;39(Suppl 6):169.
Amar A.P., Heck C.N., Levy M.L., et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265-1280.
Ben-Menachem E., Hellstrom K., Runmarker B., Augustinsson L- E. A prospective single-center open-label trial of vagal nerve stimulation (VNS) in 59 patients for the treatment of refractory epilepsy. Epilepsia. 1997;38(Suppl 8):208.
Chase M.H., Nakamura Y., Clemente C.D., et al. Afferent vagal stimulation: neurographic correlates of induced EEG synchronization and desynchronization. Brain Res. 1967;5:236-249.
Garnett E.S., Nahmias C., Scheffel A., et al. Regional cerebral blood flow in man manipulated by direct vagal stimulation. Pacing Clin Electrophysiol. 1992;15:1579-1580.
Handforth A. Effect on seizure control of reducing current off period from 5 to 1.8 min in patients receiving cyclic vagus nerve stimulation. Epilepsia. 1997;38(Suppl 8):177.
Handforth A., Degiorgio C.M., Schachter S.C., et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48-55.
Hornig G.W., Murphy J.V., Schallert G. Left vagus nerve stimulation in children with refractory epilepsy: an update. South Med J. 1997;90:484-488.
Jaseja H. EEG-desynchronization as the major mechanism of anti-epileptic action of vagal nerve stimulation in patients with intractable seizures: clinical neurophysiological evidence. Med Hypotheses. 2010;74(5):855-856.
Ko D., Heck C., Grafton S., et al. Vagus nerve stimulation activates central nervous system structures in epileptic patients during PET H215O blood flow imaging. Neurosurgery. 1996;39:426-431.
Koo B. EEG changes with vagus nerve stimulation. J Clin Neurophysiol. 2001;18:434-441.
McLachlan R.S. Suppression of interictal spikes and seizures by stimulation of the vagus nerve. Epilepsia. 1993;34:918-923.
Murphy J.V., Hornig G., Schallert G. Left vagal nerve stimulation in children with refractory epilepsy. Arch Neurol. 1995;52:886-889.
Naritoku D.K., Terry W.J., Helfert R.H. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53-62.
Salinsky M.C., Burchiel K.J. Vagus nerve stimulation has no effect on awake EEG rhythms in humans. Epilepsia. 1993;34:299-304.
Saper C.B. Diffuse cortical projection systems: anatomical organization and role in cortical function. In: Plum F., editor. Handbook of Physiology: the Nervous System V. Bethesda, MD: American Physiological Society; 1987:169-210.
Takaya M., Terry W.J., Naritoku D.K. Vagus nerve stimulation induces a sustained anticonvulsant effect. Epilepsia. 1996;37:1111-1116.
Tanaka N., Thiele E.A., Madsen J.R., et al. Magnetoencephalographic analysis in patients with vagus nerve stimulator. Pediatr Neurol. 2009;41(5):383-387.
Woodbury D.M., Woodbury J.W. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1991;31(Suppl 2):7-19.
Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005-1012.
1. Brodie M.J., Shorvon S.D., Canger R., et al. Commission on European Affairs: appropriate standards of epilepsy care across Europe: ILEA. Epilepsia. 1997;38:1245-1250.
2. Hauser W.A.. Incidence and prevalence, Engel J., Pedley T.A., editors, Epilepsy: A Comprehensive Textbook, Philadelphia, Lippincott-Raven, 1997;vol. 1:47-48.
3. Agostoni E., Chinnock J.E., Daly M.D., Murray J.G. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182-205.
4. Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31(Suppl 2):1-6.
5. Cechetto D.F. Central representation of visceral function. Fed Proc. 1987;46:17-23.
6. Bailey P., Bremer F. A sensory cortical representation of the vagus nerve. J Neurophysiol. 1938;1:4405-4412.
7. Salinsky M.C., Burchiel K.J. Vagus nerve stimulation has no effect on awake EEG rhythms in humans. Epilepsia. 1993;34:299-304.
8. Chase M.H., Sterman M.B., Clemente C.D. Cortical and subcortical patterns of response to afferent vagal stimulation. Exp Neurol. 1966;16:36-49.
9. Chase M.H., Nakamura Y., Clemente C.D., et al. Afferent vagal stimulation: neurographic correlates of induced EEG synchronization and desynchronization. Brain Res. 1967;5:236-249.
10. Magnes J., Moruzzi G., Pompeiano O. Synchronization of the EEG produced by very low frequency electrical stimulation of the region of the solitary tract. Arch Ital Biol. 1961;99:33-67.
11. Ricardo J.A., Koh E.T. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1-26.
12. Saper C.B. Diffuse cortical projection systems: anatomical organization and role in cortical function. In: Plum F., editor. Handbook of Physiology: the Nervous System V. Bethesda, MD: American Physiological Society; 1987:169-210.
13. Lockard J.S., Congdon W.C., DuCharme L.L. Feasibility and safety of vagal stimulation in monkey model. Epilepsia. 1990;31(Suppl 2):20-26.
14. Woodbury D.M., Woodbury J.W. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1991;31(Suppl 2):7-19.
15. Woodbury J.W., Woodbury D.M. Vagal stimulation reduces the severity of maximal electroshock seizures in intact rats: use of a cuff electrode for stimulating and recording. Pacing Clin Electrophysiol. 1991;1:94-107.
16. Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005-1012.
17. McLachlan R.S. Suppression of interictal spikes and seizures by stimulation of the vagus nerve. Epilepsia. 1993;34:918-923.
18. Koo B. EEG changes with vagus nerve stimulation. J Clin Neurophysiol. 2001;18:434-441.
19. Takaya M., Terry W.J., Naritoku D.K. Vagus nerve stimulation induces a sustained anticonvulsant effect. Epilepsia. 1996;37:1111-1116.
20. Amar A.P., Heck C.N., Levy M.L., et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265-1280.
21. Handforth A., Degiorgio C.M., Schachter S.C., et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48-55.
22. Al-Jayyousi M., Helmers S.L. Adjunctive treatment in Lennox-Gastaut syndrome using vagal nerve stimulation. Epilepsia. 1998;39(Suppl 6):169.
23. Ben-Menachem E., Hellstrom K., Runmarker B., Augustinsson L- E. A prospective single-center open-label trial of vagal nerve stimulation (VNS) in 59 patients for the treatment of refractory epilepsy. Epilepsia. 1997;38(Suppl 8):208.
24. Handforth A. Effect on seizure control of reducing current off period from 5 to 1.8 min in patients receiving cyclic vagus nerve stimulation. Epilepsia. 1997;38(Suppl 8):177.
25. Hornig G.W., Murphy J.V., Schallert G. Left vagus nerve stimulation in children with refractory epilepsy: an update. South Med J. 1997;90:484-488.
26. Murphy J.V., Hornig G., Schallert G. Left vagal nerve stimulation in children with refractory epilepsy. Arch Neurol. 1995;52:886-889.
27. Naritoku D.K., Terry W.J., Helfert R.H. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53-62.
28. Garnett E.S., Nahmias C., Scheffel A., et al. Regional cerebral blood flow in man manipulated by direct vagal stimulation. Pacing Clin Electrophysiol. 1992;15:1579-1580.
29. Ko D., Heck C., Grafton S., et al. Vagus nerve stimulation activates central nervous system structures in epileptic patients during PET H215O blood flow imaging. Neurosurgery. 1996;39:426-431.
30. Tanaka N., Thiele E.A., Madsen J.R., et al. Magnetoencephalographic analysis in patients with vagus nerve stimulator. Pediatr Neurol. 2009;41(5):383-387.
31. Jaseja H. EEG-desynchronization as the major mechanism of anti-epileptic action of vagal nerve stimulation in patients with intractable seizures: clinical neurophysiological evidence. Med Hypotheses. 2010;74(5):855-856.