Treatment of ductal carcinoma in situ
Background
The introduction of screening mammography has resulted in a marked increase in the detection rates of ductal carcinoma in situ (DCIS) from 2% of newly diagnosed breast cancers before national screening to 20% of all screen-detected tumours.1 DCIS is a preinvasive breast cancer; the proliferation of malignant ductal epithelial cells remains confined by an intact basement membrane, with no invasion into the surrounding stroma.2 Over 90% of DCIS lesions currently diagnosed are impalpable, asymptomatic and detected by screening. These screening-detected cases are frequently small (< 4 cm) and localised, and breast-conserving surgery is often possible. The remaining 10% present symptomatically, with a palpable breast lump, nipple discharge or Paget’s disease of the nipple. If these symptoms are present, the underlying disease is often extensive and usually requires mastectomy.
Risk factors, natural history, pathology and receptors
Risk factors for the development of DCIS include a family history of breast cancer, older age at first childbirth and nulliparity.3 Although breast epithelial proliferation is increased by the use of the oral contraceptive pill4 and hormone replacement therapy (HRT), particularly combined oestrogen/progestogen HRT for over 5 years,5 there is little evidence to date that either the oral contraceptive pill or HRT increases the risk of DCIS.4 Two studies6,7 have reported a relative risk of 1.4 for the development of DCIS following oestrogen-only HRT preparations and a relative risk of 1.7–2.3 with oestrogen- and progestogen-containing preparations.8 In the Women’s Health Initiative study there were 47 cases of DCIS in the HRT group compared with 37 cases in the control group (hazard ratio 1.18; weighted P = 0.09). Other studies have shown no increased risk following HRT use.9,10
Natural history
Although factors that pertain to an increased risk of developing DCIS have been identified, the natural history of this heterogeneous disease remains poorly understood. It is thought that developmental pathways for low- and intermediate-grade DCIS are distinct from the development of high-grade DCIS and can be explained partly by reference to biological markers. In the sequence of progression from normal breast to DCIS, there is a variable loss of chromosomal heterozygosity dependent on nuclear grade. Low- and intermediate-grade tumours show 16q loss, whereas there is 17q gain in high-grade lesions.11 It is likely that low-grade lesions arise from oestrogen receptor-positive atypical ductal hyperplasia (ADH) or lobular intra-epithelial neoplasia carcinoma and progress to low-grade oestrogen receptor-positive DCIS. High-grade lesions have no obvious precursor, unless they arise from usual ductal hyperplasia or ADH that expresses 17q gain. The progression of well-differentiated/low-grade DCIS to poorly differentiated/high-grade DCIS or high-grade invasive cancer is an uncommon event.12
Retrospective studies of low-grade DCIS misdiagnosed as benign conditions found that, 20 years after local excision, approximately 33% had developed an invasive cancer.14 As not all cases of DCIS progress to invasive disease, detection by mammographic screening may lead to over-treatment of ‘non-progressive DCIS’, i.e. DCIS that would not progress to invasive disease if left untreated. It was hoped that breast screening programmes would, after a lag phase, result in a decreased incidence of invasive breast cancer, secondary to an increase in detection and treatment of DCIS. This has not been demonstrated, as there has been no corresponding decrease in invasive disease in a recent review of American screening data although there appears to be a reduction in grade 3 cancers in women of screening age over time.15 There is therefore now concern that we may be over-treating DCIS and in particular ‘low-risk’ DCIS, and that such lesions may never pose a threat to a patient’s life. Suggestions for alternative management strategies for these patients range from endocrine therapy alone (no surgery)16 to, at the most extreme, ‘watchful waiting’.15 However, what constitutes ‘low-risk’ DCIS remains undefined.
The recent MAP.3 trial showed that exemestane reduces DCIS development in a prevention setting.17 A study looking at ADH (which may be a precursor lesion of low-grade DCIS and has an approximately fivefold increase in risk of subsequent invasive cancer) showed that ADH (and by implication, low-grade DCIS) has become less common since women have stopped using as much HRT.18 The low-grade lesions are the ones that are being potentially over-treated. They are nearly always oestrogen dependent. Removing the oestrogenic drive, either following menopause or with the use of aromatase inhibitors, may allow control of these low-grade cases so they progress to invasive cancer only rarely.
Stem cells
Recent evidence suggests that the breast has stem cells that can reconstitute the various cell types within the breast after trauma. Cancers (including DCIS) arise from accumulations of mutations within stem cells that disrupt their tightly controlled self-renewal and proliferation processes. Stem cells have recently been isolated from human DCIS. In this process, samples of human DCIS tissue have been separated into single cells and a subset of these cells (which are putative stem/progenitor cells) grows, in non-adherent culture conditions, to form three-dimensional branching structures (known as mammospheres). Mammosphere growth is dependent on growth simulation via the epidermal growth factor (EGF) and Notch receptor pathways.19 The DCIS stem cell paradigm could explain the development of both multifocal DCIS and local recurrence. Stem cells could potentially survive after wide local excision with clear margins and regrow, which would also explain the ‘identical’ receptor expression seen in recurrent DCIS as well as early recurrences seen most often in high-grade DCIS, as there are more stem cells found in these high-grade lesions. Potentially, therefore, targeted inhibition of stem cells could reduce the rate of DCIS recurrence.
Pathology
Non-comedo DCIS encompasses all other subtypes and includes the following types:
• Solid – where tumour fills extended duct spaces.
• Micropapillary – where tufts of cells project into the duct lumen perpendicular to the basement membrane.
• Papillary – where the projecting tufts are larger than in the micropapillary type and contain a fibrovascular core.
• Cribriform – where the tumour takes on a fenestrated/sieve-like appearance.
• Clinging (flat) – where there are variable columnar cell alterations along the duct margins. (There remains controversy as to whether clinging DCIS is truly an in situ cancer or whether it should be considered as atypical hyperplasia rather than DCIS.)
Rarer subtypes also exist, including neuroendocrine, encysted papillary, apocrine and signet cell.
The UK- and EU-funded breast screening programmes classify DCIS as of low, intermediate and high nuclear grade. This definition is based on the characteristics of the lesion as seen with a high-power microscope lens (× 40) and uses a comparison of tumour cell size with normal epithelial and red blood cell size:20
• Low nuclear grade DCIS has evenly spaced cells with centrally placed small nuclei and few mitoses and nucleoli that are not easily seen.
• High nuclear grade DCIS has pleomorphic irregularly spaced cells with large irregular nuclei (often three times the size of erythrocytes), prominent nucleoli and frequent mitoses. It is often solid with comedo necrosis and calcification.
• Intermediate-grade DCIS has features between those seen in low- and high-grade DCIS.
Most cases of DCIS are unicentric.21 Following extensive pathological sectioning of DCIS mastectomy specimens, only 1% show multicentric disease.21 A multicentric tumour is defined as separate foci of tumour found in more than one breast quadrant, or more than 5 cm away from the initial primary. A tumour is classified as multifocal if there are separate tumour foci in the same quadrant that are close to each other, although most such lesions have similar morphology and are linked.22 The local spread of DCIS is along branching ducts that form the glandular breast. The ducts, which are ill defined, often extend beyond the borders of a quadrant. Most DCIS is continuous along the branching ductal network. Poorly differentiated high-grade lesions are reported to be more frequently multifocal.23 Most DCIS recurrences are at or near the site of the initial tumour,24 but some recurrences are remote from the initial lesion yet exhibit similar genotypical and phenotypical characteristics to the primary lesion.12
As well as documenting pathological type and grade on the histology report, the pathologist should detail the presence or absence of microinvasion. If microinvasion is detected histologically, a thorough examination of the entire specimen should be undertaken to exclude other previously unnoticed areas of invasive cancer. Lesions that can be mistaken for microinvasion include DCIS involving lobules, branching of ducts, distortion of ducts by acini or fibrosis, crush or cautery artefacts, and DCIS involving a benign sclerosing process (e.g. radial scar).25–29
Lobular intraepithelial neoplasia (LIN)
The current classification combines lobular carcinoma in situ (LCIS) and atypical lobular hyperplasia (ALH) into a single entity known as lobular intraepithelial neoplasia (LIN). Rather than a premalignant lesion, LIN is considered a marker of increased risk. It is often an incidental finding during breast biopsy and accounts for approximately 0.5% of symptomatic and 1% of screen-detected tumours. In situ ductal and lobular tumours show different pathological and clinical features. Compared with DCIS, patients developing LIN tend to be younger and premenopausal, and have bilateral and multicentric disease of lower grade and close to 100% oestrogen receptor expression (Table 11.1). There is an approximate eight- to ninefold increased risk of developing invasive carcinoma after a diagnosis of LCIS compared to the general population.30 Sometimes it is difficult to distinguish histologically between LCIS and DCIS, and the pathology report should state this. The clinical interpretation of the report should take into account the increased risks from both tumour subtypes.
Table 11.1
Comparative clinicopathological features of ductal carcinoma in situ (DCIS) and tabular carcinoma in situ (LCIS)
| Clinicopathological feature | DCIS | LCIS |
| Age at diagnosis (years) | 54–58 | 44–47 |
| Premenopausal | 30% | 70% |
| Absence of clinical signs | 90% | 99% |
| Mammographic findings | Microcalcifications | None |
| Multicentric disease | 30% | 90% |
| Bilateral disease | 12–20% | 90% |
| Histological grade | 65% high grade | 90% low grade |
| Oestrogen receptor status | 65% positive | 95% positive |
| Subsequent invasive disease | 30–40% | 25–30% |
| Ipsilateral–contralateral ratio | 9:1 | 1:1 |
If LIN is detected at core biopsy, the area of suspicion is usually excised to confirm the diagnosis and exclude an adjacent invasive focus. If LIN is diagnosed coincidentally following excision of a coexisting lesion, no further surgical treatment is necessary (even if the area of lobular neoplasia is not fully excised) and the patient should undergo regular review on a ‘watch and wait’ basis or be considered for a preventional strategy. The NSABP P-1 prevention trial showed a 56% reduction in the risk of developing subsequent invasive cancer in women with LCIS who received tamoxifen.31 Further studies are ongoing with aromatase inhibitors in postmenopausal patients with LIN. Chemotherapy and radiotherapy have no place in the treatment of lobular neoplasia. A problem area is pleomorphic LCIS. The current perspective is that this should be treated like DCIS rather than lobular neoplasia but the scientific basis for this is minimal. Further studies and clarification of the behaviour and most appropriate treatment of pleomorphic LCIS is needed urgently.
Receptors and markers
To advance our understanding of the development and behaviour of DCIS, there has been interest in cell receptor expression and signalling pathways controlling growth. These studies have been mainly based on immunohistochemical assessment and show that poorly differentiated high-grade comedo DCIS has low oestrogen receptor expression, high rates of cell proliferation32 (as expressed by Ki67, a nuclear antigen expressed in late G1 S, G2 and M phases of the cell cycle but not in the quiescent G0),33 high rates of apoptosis,34 and over-expression of HER-2 and epidermal growth factor receptor (EGFR (HER-1)).32 Low-grade lesions in contrast have high oestrogen receptor expression, with lower rates of cell proliferation32 and apoptosis than high-grade lesions,34 and they rarely overexpress HER-2.32 Progesterone receptor expression correlates with oestrogen receptor expression in both low- and high-grade tumours.32 In comparison, normal breast epithelium has low levels of expression of oestrogen receptor and progesterone receptor,35 and a very low rate of apoptosis and HER-2 expression.
The increased rate of apoptosis seen in DCIS is lost on progression to invasive cancer, but the high proliferative rate is maintained.36 Cyclin D1, an oncogene responsible for G1 cell cycle proliferation/progression and induction of apoptosis, is overexpressed in approximately 90% of in situ and invasive ductal cancers.37 It also appears to be associated with a loss of differentiation (measured by p27Kip1).38 In oestrogen receptor-positive tumours, the driving force behind this increase in cell proliferation is the nuclear action of the activated oestrogen receptor, which increases growth-promoting gene transcription. In oestrogen receptor-negative DCIS, the driving pathway is thought to be predominantly via EGFR/HER-2/RAS/MAP kinase activation (Fig. 11.1). This leads to a subsequent increase in transcription of both proliferative and, via Akt, anti-apoptotic genes. Activation of this pathway also induces the expression of cyclo-oxygenase-2 (COX-2), which is an inducible enzyme that converts arachidonic acid to prostaglandins. It has been found to be overexpressed in up to 80% of DCIS.39 COX-2-positive DCIS shows increased cell proliferation, and is related to increased tumour recurrence and decreased survival in invasive cancer.40
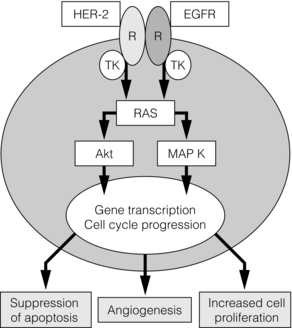
Figure 11.1 The basic growth pathway in oestrogen receptor-negative breast tumour cells. The oestrogen receptor-positive signalling pathway is mediated via oestrogen attaching to its receptor, which then moves down its concentration gradient to the cell nucleus. The presence of oestrogen receptor in the cell nucleus subsequently increases gene transcription and expression of growth-promoting factors, leading to increased cell proliferation and tumour growth. In cells that do not express oestrogen receptors, the main signalling pathway for growth is via the epidermal growth factor (EGF)/c-erbB-2 receptor; this activates the RAS intracellular messenger, which increases cell proliferation and tumour growth via MAP kinase. RAS stimulation also leads to the suppression of the apoptosis cascade via Akt and BAD phosphorylation (an apoptotic protein). MAP K, MAP kinase; R, receptor; TK, tyrosine kinase.
In addition to alterations in cell proliferation and apoptosis, the development of neovascularisation is necessary for the growth of solid tumours. It is driven in part by angiogenic factors expressed in hypoxic areas of the tumour. Hypoxic areas of DCIS show a less well differentiated, more malignant phenotype, with increased HIF-1α (a hypoxia-induced transcription factor), decreased oestrogen receptor expression and increased expression of cytokeratin-19 (a breast stem cell marker).27 It is felt that hypoxia-induced dedifferentiation could be a factor promoting tumour progression.41
Presentation, investigation and diagnosis
Over 90% of DCIS is detected by mammographic screening. Approximately 70% of these mammographically detected cases present as microcalcifications with no associated mass lesion. Calcifications may be heterogeneous, fine, linear, branching, malignant or of indeterminate appearance. Microcalcifications with an associated mass lesion are seen in approximately 30% of DCIS diagnosed by screening.42 Circumscribed nodules, ill-defined masses, duct asymmetry and architectural distortion are sometimes seen in association with DCIS.43 When diagnosed clinically, DCIS is often extensive or associated with a concurrent invasive tumour. It may present as a palpable mass, Paget’s disease of the nipple or nipple discharge.44
Investigation and diagnosis
Stereotactic core biopsy and vacuum-assisted biopsy
In the NHS Breast Screening Programme, the primary method of diagnosis was formerly by stereotactic core biopsy with a 14 G needle. If image-guided core biopsy was inconclusive then vacuum-assisted biopsy (VAB) using a device such as a Mammotome, which takes several contiguous biopsies of a wider calibre (11 G) during a single pass, can be used. A metal clip can be inserted during the procedure to aid future localisation. VAB has a higher sensitivity and specificity than core biopsy and is now the biopsy technique of choice for microcalcification,45 but still underdiagnoses a coexisting invasive tumour in 10–20% of cases due to sampling error.46 Other factors that have been shown to underestimate the presence of associated invasive disease include high-grade lesions, imaging size > 2 cm, Breast Imaging and Reporting Data System (BI-RADS) score of 4 or 5, a visable mass at mammography (versus only calcification) and a palpable abnormality.45 If the area of DCIS is extensive (> 4 cm in size), two or more areas should be biopsied preoperatively to increase the chance of detecting any associated invasive component.
Localisation-guided biopsy
If a definitive histological diagnosis cannot be made with core biopsy or VAB, this due to failure to sample the calcification adequately, or doubt exists as to whether DCIS is present on histology, then open biopsy is necessary (see Chapter 1). The excised specimen should be sent for immediate radiography, after careful orientation with Liga-clips or metal markers, to confirm that all microcalcification of concern has been excised and is clear of margins. The guidelines of the Association of Breast Surgeons at BASO47 recommends that 90% of diagnostic guided biopsies for screen-detected abnormalities should weigh less than 20 g. Due to improved preoperative diagnosis, wire-guided localisation procedures are usually therapeutic rather than diagnostic. However, DCIS is often pathologically larger than mammographically suggested; this is especially true if magnification views are not used, and up to 30% of cases need re-excision to clear margins adequately.48 Accurate orientation of the specimen is essential to direct re-excision of the relevant margins and to minimise the volume of any re-excision.
Other diagnostic procedures
Ductoscopy: Ductoscopy (see Chapter 3) is an appealing option for DCIS. There has been interest in its use both in diagnosis and potential for treatment with direct instillation of chemotherapy into the ducts49 (see Chapter 3).
Magnetic resonance imaging (MRI): MRI can be used to image DCIS and is currently being investigated in a number of trials. DCIS may identify occult multifocal or contralateral disease, but there are concerns about the potential of MRI to overestimate the extent of disease, leading to wider than necessary excisions, and unnecessary mastectomy or identifying high numbers of contralateral lesions that turn out to be benign. There are also concerns that MRI might detect biologically insignificant disease that could increase ‘over-treatment’. It therefore remains under investigation in trials and has no routine role in assessing DCIS.
Treatment: mastectomy versus breast-conserving surgery
Mastectomy
The long-term recurrence rate following mastectomy for DCIS is less than 1%.50 As current evidence (see p. 181) points to DCIS being predominantly unicentric in origin, it is now recognised that mastectomy is over-treatment for the majority of patients.50 In 1983 mastectomy was performed for 71% of cases of DCIS in the USA but this had dropped to 44% by 1992.51 Mastectomy is now reserved for patients with larger areas of DCIS (arbitrarily considered as > 4 cm), for multicentric disease and for patients where radiotherapy is contraindicated. Women should also be offered mastectomy if the excision margins are involved following breast-conserving surgery and the patient is not deemed suitable for re-excision. Rates of re-excision versus mastectomy vary widely in different units. Women with DCIS requiring mastectomy are excellent candidates for skin-sparing mastectomy and immediate breast reconstruction.
Breast-conserving surgery
The recommended treatment protocol for DCIS is shown in Fig. 11.2.
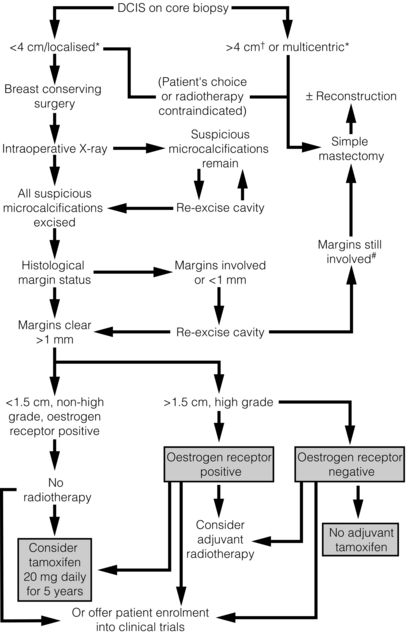
Figure 11.2 Recommended treatment algorithm for ductal carcinoma in situ (DCIS). *Determined mammographically. Shaded boxes indicate those treatments suggested by the results of recent trials.55,66 †Areas of DCIS > 4 cm can be treated by breast conservation if unifocal and patient has large breasts, or is suitable for an oncoplastic procedure. #A further re-excision can be attempted providing the final cosmetic result is acceptable to the patient.
Axillary staging
The incidence of macroscopic lymph node metastasis in DCIS is less than 1% and should prompt thorough pathological examination for occult invasion. Formal axillary staging in women with DCIS should not be performed alongside breast-conserving surgery.52 However, National Institute for Health and Clinical Excellence (NICE) guidelines recommend that a sentinel lymph node biopsy should be performed at the same time as mastectomy for DCIS52 and women should be counselled as to the indications for this. The rationale for performing sentinel lymph node biopsy with mastectomy is the potential of occult invasive disease that may be identified histologically in a large area of DCIS. This would subsequently require axillary staging. A sentinel lymph node biopsy cannot easily be performed after a mastectomy. Patients found to have positive lymph nodes have occult invasive disease and should be managed accordingly. A study by Veronesi et al. of 508 patients with pure DCIS found that nine patients (1.8%) had epithelial cells found in the sentinel node (five of these nine cases were micrometastases alone). None of the cases showed further lymph node involvement at formal axillary dissection.53 A further study that looked retrospectively at the NSABP B-17 and B-24 data, from patients who had undergone local excision of DCIS with clear margins (no axillary surgery at initial treatment), showed that the ipsilateral nodal recurrence rate was 0.83 per 1000 patient-years in the B-17 trial and 0.36 per 1000 patient-years in the B-24 trial. Meta-analysis of the published literature showed approximately 1.8% of DCIS (almost entirely G3 or high-grade disease) had involved sentinel nodes.54
Recurrence: rates and predictors
No trial has specifically evaluated breast-conserving surgery versus mastectomy in DCIS. The long-term recurrence rate following mastectomy is known to be very low at less than 1%.50 The majority of these recurrences are invasive disease. This reflects the fact that after mastectomy no imaging is performed routinely of the ipsilateral side and further disease is only detected when it becomes clinically apparent – at which stage it is most likely to be invasive. The recurrence rate for breast-conserving surgery alone has been reported to be up to 25% at 8 years follow-up, with up to 50% of recurrences (i.e. 12.5% of all cases) being invasive disease.13,48,55 The remaining 50% of recurrences are in situ tumours.56 Reviews of clinical and pathological variables have demonstrated certain unfavourable tumour characteristics and these are outlined below. Recurrence rates have fallen significantly over time.
Assessment of excision margins
A fundamental risk factor for recurrence is inadequate excision following breast-conserving surgery. This is judged as close (< 1 mm) or involved margins48 and/or failure to remove all suspicious microcalcifications.57 Excision margin width has three times the power of tumour grade in predicting local recurrence.58 The NSABP-B17, NSABP-B24 and EORTC clinical trials all revealed that the presence of clear margins after local excision significantly decreased tumour recurrence.13,59–61 On multivariate analysis of the EORTC trial, non-specified, close or involved margins conferred a hazard ratio of 2.07 (95% confidence interval (CI) 1.35–3.16, P = 0.0008) compared with clear margins.61 The NSABP-B24 trial found a covariate relative risk of 1.68 (95% CI 1.20–2.34) if the margins were involved.59 No prospective trials have looked at the optimum excision width required for in situ or invasive cancer. When considering the extent of surgical excision there has to be balance between minimising recurrence and producing an acceptable cosmetic outcome. A retrospective study by Chan et al.48 reported that women with clear margins (judged as greater than 1 mm) had an 8.1% recurrence at a median follow-up of 47 months compared with 37.9% recurrence where excision margins were close (1 mm). There was no improvement in recurrence rates in more widely excised lesions. The recent meta-analysis performed by Wang et al.62 suggested a 10-mm margin was superior to lesser margins for local recurrence but this conflicts with an earlier meta-analysis, which suggested 2 mm.63 Part of the problem of defining an optimum margin is that these analyses are affected by confounding factors. The 10- and 2-mm distances also rely on a small proportion of patients treated in very few centres. At present, as Morrow and Katz conclude, there is no compelling evidence that bigger is better for margins in DCIS.64
High-grade/comedo tumours
High-grade tumours and tumours showing comedo necrosis are independent risk factors for recurrence. In a review of the EORTC 10853 trial,61 high nuclear grade had a hazard ratio of 2.23 (95% CI 1.41–3.51, P = 0.0011) for local recurrence, with 22% of high-grade tumours and 11% of intermediate-grade tumours developing either recurrent DCIS or invasive tumour. Comedo necrosis was also shown to be related to local recurrence, 18% of patients with DCIS having comedo necrosis developing recurrence (hazard ratio 1.80, 95% CI 1.08–3.00, P = 0.0183).
Histological type and tumour architecture
The degree of tumour differentiation is predictive of both local recurrence and metastatic disease. In the EORTC trial,13,61 poorly differentiated tumours were at significantly higher risk of developing DCIS recurrence (hazard ratio 3.58, 95% CI 1.68–7.62, P = 0.0001) and metastasis (hazard ratio 6.65, 95% CI 1.46–30.22, P = 0.00083) compared with well-differentiated tumours. In this same trial, histological type was also strongly related to DCIS recurrence, though not to invasive recurrence. Both solid/comedo DCIS (hazard ratio 4.40, 95% CI 2.28–8.48, P = 0.0001) and cribriform DCIS (hazard ratio 3.74, 95% CI 1.91–7.30, P = 0.0001) were found to be much more likely to recur than clinging or micropapillary tumours. Within the well-differentiated group, no tumours with clinging DCIS recurred.61 It has been suggested that this well-differentiated clinging DCIS should be reclassified separately as ‘columnar alteration with prominent apical snouts and secretion’,65 with debate as to whether this subtype should be managed as atypical ductal hyperplasia or LCIS.
Age at diagnosis
A further risk factor for recurrence irrespective of tumour grade or type is young age (< 40 years) at diagnosis. The EORTC 10853 trial13,61 found that women less than 40 years at diagnosis were more likely to recur (hazard ratio 2.54, 95% CI 1.53–4.23, P = 0.010) than older women. The NSABP B-24 trial59 found that the rate of ipsilateral breast tumours (in the placebo population) in women aged 49 years or less at diagnosis was 33.3 per 1000, compared with 13.0 for those aged 50 and above. In the UK/ANZ DCIS trial,66,67 only a small proportion (9.5%) of women was less than 50 years old at diagnosis. The power of this study is thus limited, but of these younger women, 26% recurred after excision and tamoxifen compared with only 17% of women older than 50 years. Rodrigues et al.68 studied women aged 42 years or less (mean age 38.5) or women aged 60 years or more (mean age 67.8) at diagnosis. They found that although there was no difference in tumour grade, comedo necrosis or overall histology (also found in the EORTC trial) between the groups, compared with older patients HER-2 was more frequently overexpressed in the younger patient population. Approximately 65% of the younger age group were HER-2 positive compared with 38% of the older age group (P = 0.06). No significant difference was found between oestrogen receptor, progesterone receptor, p53, Ki67, cyclin D1 or bcl-2 expression.
Tumour size and palpability
None of the major trials have found any statistical significance between recurrence and size. The NSABP-B17 trial55 found that the size of mammographically detected tumours was not significant in predicting ipsilateral recurrence. However, when researchers examined clustering of microcalcifications in women whose mammograms did not show a tumour mass, they found that clustered microcalcifications greater than 10 mm (relative risk 2.06, 95% CI 1.36–3.10) or scattered calcifications (relative risk 2.41, 95% CI 1.40–4.16) had a significantly higher ipsilateral recurrence than clustered calcifications of 10 mm or less. The problem is that there may be differences in histology between the groups, so counfounding the analysis of size. The EORTC 10853 trial61 found no difference in recurrence rates between tumours less than 10 mm in size and those 10–20 mm or greater than 20 mm in size (P = 0.2127). However, tumours that were clinically apparent rather than mammographically detected were more likely to recur (covariate relative risk 2.17, 95% CI 1.53–3.08).61
Scoring systems
In order to bring together the most clinically relevant risk factors, Silverstein and Lagois69 developed the Van Nuys Prognostic Index, with the aim of predicting which women would be at risk of recurrence following breast-conserving surgery. This numerical algorithm was derived from regression analysis of retrospective data pooled from patients with DCIS treated at two centres in the USA. Recurrence is clearly multifactorial but the problem with the Van Nuys Index is that the data derived were not randomised and used historical controls. The formula encompassed tumour size, margin width and pathological classification. The index has since been modified as the University of Southern California/Van Nuys Prognostic Index (USC/VNPI) and now includes patient age.69 Each criterion is weighted and scored 1, 2 or 3 and the individual scores combined to give an overall score from 4 to 12. Scores of 4–6, 7–9 and 10–12 are said to be at low, moderate and high risk of 5-year recurrence, respectively. It was designed to achieve a less than 20% recurrence rate at 12 years. The data are skewed by the fact that 80% of large tumours (> 4 cm) recurred, whereas in the UK these women would have undergone mastectomy. These large tumours were also more likely high grade and incompletely excised. The value of the scoring system for a UK population, where the majority of cases of DCIS are small (< 2 cm) and screen detected (patients usually over 50 years old), may be limited. For instance, Boland et al.58 were unable to demonstrate that size was a marker of recurrence in screen-detected DCIS in the UK.
Markers of recurrence
There has also been interest in another member of the type 1 tyrosine kinase receptor family, HER-4. DCIS and invasive tumours that show co-expression of HER-2 and HER-4 have a better prognosis (reduced recurrence) than HER-2-positive, HER-4-negative tumours.71–72 A summary of the risk factors for DCIS recurrence is shown in Table 11.2 (see Lari SA and Kuerer HM73 review of DCIS biological prognostic markers).
Table 11.2
Risk factors for recurrence of ductal carcinoma in situ
| Excision margins | Margins < 1 mm after breast-conserving surgery |
| Tumour grade | High grade (III) |
| Comedo necrosis | Present |
| Histological type | Poorly differentiated |
| Patient age | Younger age at diagnosis (< 40 years) |
| Biological markers | |
| Negativity | Oestrogen receptor |
| Progesterone receptor | |
| bcl-2 | |
| HER-4 | |
| Positivity | HER-2 |
| p21 | |
| p53 | |
| Ki67 (high-percentage expression) | |
| Patient presentation | Symptomatic |
| Tumour size | Not significant |
Adjuvant therapy
Four main trials and a subsequent Cochrane review74 have examined the value of radiotherapy following breast-conserving surgery for DCIS. The NSABP-B17,55 EORTC 10853,13 UK/ANZ DCIS66,67 and SWEDCIS75 trials each studied a radiation dose of 50 Gy in 25 fractions. All found a significant reduction in ipsilateral recurrence following radiotherapy. However, none of the trials have shown any impact on mortality (Table 11.3, Fig. 11.3).
Table 11.3
Summary of major radiotherapy/tamoxifen clinical trials following breast-conserving therapy for ductal carcinoma in situ
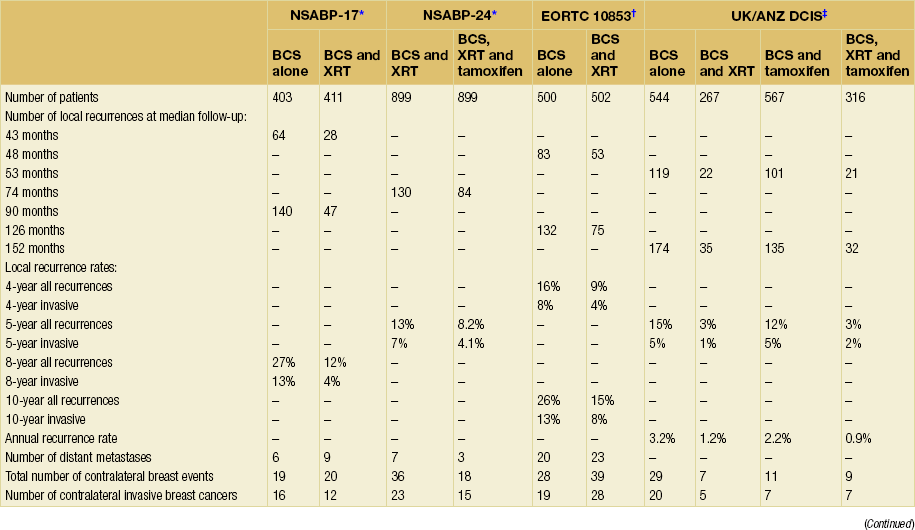

BCS, breast-conserving surgery; XRT, radiotherapy.
*Fisher ER, Dignam J, Tan-Chiu E et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 1999; 86:429–38.
†Julien J, Bijker N, Fentimen I et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomized phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy group. Lancet 2000; 355:528–33. Bijker N, Meijnen P. Peterse JL et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853 – a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 2006; 24(21):3381–7.
‡UK Coordinating Committee on Cancer Research (UKCCCR). Ductal carcinoma in situ (DCIS) Working Party on behalf of DCIS trialists in the UK, Australia and New Zealand, Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia and New Zealand: randomised controlled trial. Lancet 2003; 362:95–103. Cuzick J, Sestaka I, Pinder S, et al Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2010; 12(1): 21–9.
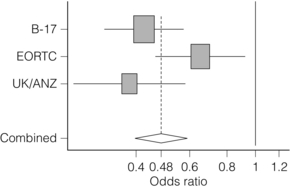
Figure 11.3 Radiotherapy trials overview: ipsilateral ductal carcinoma in situ (DCIS) and invasive recurrences. This Forrest plot of the major randomised controlled trials of radiotherapy in DCIS (B17,55 EORTC13 and UK/ANZ66) shows a significant reduction in ipsilateral recurrence risk following radiotherapy for all trials, with a combined odds ratio for the reduction in recurrence of DCIS and invasive disease of 0.48 for all trials. Reproduced from Cuzick J. Treatment of DCIS – results from clinical trials. Surg Oncol 2003; 12:213–9. With permission from Elsevier.
The reduction in recurrence was similar for both in situ and invasive disease. In the EORTC trial, radiotherapy reduced the risk of DCIS recurrence by 48% (P = 0.0011) and invasive local recurrence by 42% (P = 0.0065) at a median of 10.7 years’ follow-up.76 The UK DCIS trial found that after a median follow-up of 12.5 years there was a reduced incidence of ipsilateral invasive disease (0.32, 0.19–0.56; P < 0.0001) and ipsilateral DCIS (0.38, 0.22–0.63; P < 0.0001).67 Both groups had similar low risks of metastases and death. No survival advantage following radiotherapy was found in any trial.
The Cochrane review concluded that ‘nine women require treatment with radiotherapy to prevent one ipsilateral recurrence’.74
Two studies have looked at avoiding radiotherapy in ‘low-risk’ cases of pure DCIS; one by Wong et al.77 was stopped in line with the trial protocol because of high recurrence rates, although most recurrences were mammographically detected DCIS and the remainder node-negative invasive cancers and thus did not impact overall survival. They concluded that it remained unclear how to identify patients who had a low recurrence risk with excision alone, and that despite margins > 1 cm (or having had re-excision) the local recurrence rate was still high. The second, the Eastern Cooperative Oncology Group study 5194 (the study in which the Oncotype DX DCIS score was validated (see previously)),78 included 558 cases of low- or intermediate-grade DCIS, which measured 2.5 cm or less, and 103 cases of high-grade DCIS of 1 cm or smaller which had been excised completely with 3-mm margins, none of which had radiation therapy but they may have had tamoxifen. The 5-year ipsilateral overall breast event rate for low/intermediate-grade DCIS was 6.1% (3% for invasive disease alone). For the high-grade lesions this was increased to 15.3% (7.5% for invasive disease). They concluded that a 6% 5-year ipsilateral breast event rate for low/intermediate-grade tumours may be acceptable to patients and physicians, but that the 15% high-grade event rate would not be. They suggested that specimens need to be rigorously evaluated to ensure they are actually ‘low risk’. This study included patients with DCIS with a median size of 1 cm. Seventy-five per cent of the patients were classified by the Oncotype DX assay as low risk, 14% intermediate risk and 11% high risk. Low-risk patients had a 10-year event rate of 12% and a 5% rate of developing an invasive cancer with no radiotherapy. This event rate is higher than one currently sees in invasive cancer after breast conservation surgery and radiotherapy but many of these events will be further DCIS which can be treated effectively by further surgery +/- radiotherapy.
Endocrine therapy
Although radiotherapy reduces tumour recurrence following breast-conserving surgery, there is still an overall recurrence rate of between 3% and 13%13,55,66 at 5 years, and research into the use of additional adjuvant therapies for women with ‘high-risk’ DCIS remains important.
In this trial, 30% of women were younger than 50 years at diagnosis and the effect of tamoxifen was largely due to a 40% reduction in this younger age group, with only a 20% reduction in the age group greater than 50 years. On this basis adjuvant tamoxifen after wide local excision for DCIS could be discussed in this younger (under 50) age group; however, current NICE guidelines indicate that tamoxifen should not be used in DCIS.52 A retrospective review of the NSABP-B24 results showed that tamoxifen was only beneficial in oestrogen receptor-positive cases, as one would expect. The relative risk of recurrence of any breast cancer in the oestrogen receptor-positive cohort was 0.41 (95% CI 0.25–0.65, P = 0.0002), whereas there was little benefit in the oestrogen receptor-negative cases (relative risk 0.80, P = 0.51).80 The UK/ANZ DCIS trial found that adjuvant tamoxifen reduced overall DCIS recurrence (0.70, 0.51–0.86; P = 0.03) and contralateral tumours (0.44, 0.25–0.77; P = 0.005) but had no effect on ipsilateral invasive disease67 (see Table 11.3). The UK/ANZ DCIS trial has not published a breakdown of tamoxifen response in relation to oestrogen receptor status, but tamoxifen was found to be more effective in low- and intermediate-grade compared with high-grade DCIS and this is likely a surrogate (though not completely accurate) reflection of oestrogen receptor status; low-grade DCIS tends to be nearly 100% oestrogen receptor positive, compared with only 60% of high-grade cases expressing oestrogen receptor.81 The UK/ANZ DCIS trial authors suggested that the variation in findings, as to the benefit of tamoxifen in preventing ipsilateral invasive recurrence between the two trials, may have been a product of the American B24 trial having approximately 34% of women aged under 50, whereas in the UK trial > 90% of participants were older than 50.67 No significant effects were seen on mortality with the use of tamoxifen in either trial.
In the randomised controlled trials, the rate of contralateral breast cancer after DCIS is 0.5% per year for 10 years. As tamoxifen can halve the risk of breast cancer in the contralateral breast, its effects in part are as a chemopreventive agent. This may possibly justify its use in some women with oestrogen receptor-positive disease. Approximately 60% of DCIS cases express HER-2. Oestrogen receptor-positive tumours that also express HER-2 are considered to be more often resistant to tamoxifen but do respond to aromatase inhibitors. The ERISAC trial showed that exemestane, at a dose of 25 mg/day, inhibits epithelial proliferation in DCIS by 39% (hazard ratio 0.61, 95% CI 0.41–0.91, P = 0.016) compared with placebo.82 This suggests that aromatase inhibition of oestrogen receptor-positive DCIS could potentially be used to prevent local recurrence.
The use of aromatase inhibitors is currently being investigated in the IBIS II DCIS trial, which is a comparison of tamoxifen with anastrozole or placebo, after complete excision of oestrogen receptor-positive DCIS. The NSABPB35 trial is comparing anastrozole with tamoxifen for patients with DCIS after lumpectomy and radiation therapy. The NSABP P-1 chemoprevention trial83 compared tamoxifen to placebo in patients at high risk of breast cancer. The study reported a 49% reduction in incidence of invasive cancer and a 50% reduction of DCIS in the tamoxifen-treated group. The reduction in contralateral breast cancer was only seen in oestrogen receptor-positive cases, with no benefit being seen for oestrogen receptor-negative patients.
The recent MAP.3 trial looked at exemestane in a prevention setting in postmenopausal women and showed a reduction in both new cases of DCIS and further breast events in women with a prior diagnosis of DCIS, though numbers were small.17
Management of recurrence
Invasive recurrence
The management of invasive recurrence is dependent on the initial therapy for DCIS. If the patient did not receive radiotherapy after initial DCIS excision, then wide local excision and radiotherapy may be an option depending on the size and location of the invasive tumour. If wide local excision is not an option, then mastectomy and axillary staging is the treatment of choice, with adjuvant therapy dictated by standard protocols for primary invasive cancers. Studies following salvage treatment for both in situ and invasive recurrences of DCIS have shown overall cause-specific survival rates in excess of 90% at 8 years after recurrence.57
DCIS of the male breast
DCIS accounts for approximately 5% of breast cancers in men.84 It usually presents clinically with symptoms of a retro-areola cystic-type mass or bloody nipple discharge. Clinical, rather than mammographic, detection possibly accounts for the different incidence of DCIS between men and women. The predominant histological subtypes of DCIS in men are papillary and cribriform. Standard treatment is total mastectomy with excision of the nipple–areola complex but wide excision and radiotherapy is being used more frequently.85 Pure DCIS in men is usually of low or intermediate grade; less than 3% of cases are high grade. In a series of 114 patients, 84 with pure DCIS and 30 with DCIS and invasive cancer, there were no cases of high-grade comedo DCIS in men without an invasive tumour.86 The percentage of men with DCIS that eventually develop an invasive cancer is not known.
The future
DCIS stem cell therapy
Breast cancers have been shown to consist of a mixture of stem cells and proliferating cells.19 The stem cells appear to be more resistant to both chemotherapy and endocrine therapy than proliferating cells. Stem cells evade death and subsequently may re-grow and may be a source of breast recurrence. Farnie et al.19 showed that primary cultures of DCIS using a mammosphere technique identified Notch and the epidermal growth factor receptor/HER-1 as key receptors that stem cells use to avoid death. Thus strategies aimed at inhibiting both stem cell self-renewal and proliferating progeny may increase the DCIS cure rate. HER-2-amplified DCIS has an increased stem cell population and this population is targeted by lapatinib, trastuzumab and other anti-HER-2 therapies but not by chemotherapy. In vitro lapatinib (an HER-1/2 inhibitor) has been shown to reduce stem cell renewal by 70% in HER-2-amplified DCIS.87
References
1. NHS Cancer Screening ProgrammesAll breast cancer report. An analysis of all symptomatic and screen detected breast cancers diagnosed in 2006. NHS Breast Screening Programme, October 2009.
2. Lagios, M.D., Heterogeneity of ductal carcinoma in situ of the breast. J Cell Biochem Suppl 1993; 17G:49–52. 8007709
3. Rakovitch, E., Part 1 Epidemiology of ductal carcinoma in situ. Curr Probl Cancer 2000; 24:100–111. 10919313
4. Williams, G., Anderson, E., Howell, A., et al, Oral contraceptive (OCP) use increases proliferation and decreases oestrogen receptor content of epithelial cells in the normal human breast. Int J Cancer 1991; 48:206–210. 2019467
5. Hofseth, L.J., Raafat, A.M., Osuch, J.R., et al, Hormone replacement therapy with oestrogen or oestrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal post-menopausal breast. J Clin Endocrinol Metab 1999; 84:4559–4565. 10599719
6. Schairer, C., Byrne, C., Keyl, P.M., et al, Menopausal estrogen and estrogen–progestin replacement therapy and the risk of breast cancer (United States). Cancer Causes Control 1994; 5:491–500. 7827235
7. Longnecker, M.P., Bernstein, L., Paganini-Hill, A., et al, Risk factors for in situ breast cancer. Cancer Epidemiol Biomarkers Prev 1996; 5:961–965. 8959317
8. Chlebowski, R.T., Hendrix, S.L., Langer, R.D., WHI Investigators, Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. 12824205
9. Henrick, J.B., Kornguth, P.J., Viscoli, C.M., et al, Postmenopausal estrogen use and invasive versus in situ breast cancer risk. J Clin Epidemiol 1998; 51:1277–1283. 10086820
10. Gapstur, S.M., Morrow, M., Sellars, T.A., Hormone replacement therapy and risk of breast cancer with a favourable histology: results of the Iowa Women’s Health Study. JAMA 1999; 281:2091–2097. 10367819
11. Hwang, E.S., DeVries, S., Chew, K.L., et al, Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res. 2004;10(15):5160–5167. 15297420
12. Bijker, N., Peterse, J.L., Duchateau, L., et al, Histological type and marker expression of the primary tumour compared with its local recurrence after breast-conserving therapy for ductal carcinoma in situ. Br J Cancer 2001; 84:539–544. 11207051
13. Julien, J., Bijker, N., Fentimen, I., et al, Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomized phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet 2000; 355:528–533. 10683002 The results of a multicentre, randomised, controlled trial of 1010 patients with DCIS treated with breast-conserving surgery, randomised to receive no further treatment or radiotherapy. The study found that radiotherapy reduced overall invasive (40% reduction, P = 0.04) and non-invasive (35% reduction, P = 0.06) ipsilateral recurrences (median follow-up 4.25 years).
14. Page, D.L., Dupont, W.D., Rogers, L.W., et al, Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 1995; 76:1197–1200. 8630897
15. Ozanne, E.M., Shieh, Y., Barnes, J., et al, Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat. 2011;129(1):165–173. 21390494
16. Chen, Y.Y., DeVries, S., Anderson, J., et al, Pathologic and biologic response to preoperative endocrine therapy in patients with ER-positive ductal carcinoma in situ. BMC Cancer 2009; 9:285. 19689789
17. Goss, P.E., Ingle, J.N., Alés-Martínez, J.E., et alNCIC CTG MAP.3 Study Investigators, Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. 21639806
18. Menes, T.S., Kerlikowske, K., Jaffer, S., et al, Rates of atypical ductal hyperplasia have declined with less use of postmenopausal hormone treatment: findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2822–2828. 19900937
19. Farnie, G., Clarke, R.B., Spence, K., et al, Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst 2007; 99:616–627. 17440163
20. NHS Breast Screening ProgrammePathology reporting of breast disease. Sheffield: National Pathology Co-ordinating Group, January 2005. [Publication no. 58].
21. Holland, R., Hendriks, J.H., Vebeek, A.L., et al, Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet 1990; 335:519–522. 1968538
22. Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer, The management of ductal carcinoma in situ (DCIS). Can Med Assoc J. 1998;158(Suppl):S27–S34. 9484276
23. Faverley, D.R.G., Burgers, L., Bult, P., et al, Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol 1994; 11:193–198. 7831530
24. Holland, P.A., Ghandi, A., Knox, W.F., et al, The importance of complete excision in the prevention of local recurrence of ductal carcinoma in situ. Br J Cancer 1998; 77:110–114. 9459154
25. Kerner, H., Lichtig, C., Lobular cancerisation: incidence and differential diagnosis with lobular carcinoma in situ of the breast. Histopathology 1986; 10:621. 3733006
26. Fisher, E.R., Pathobiological considerations relating to the treatment of intraductal carcinoma (ductal carcinoma in situ) of the breast. CA Cancer J Clin. 1997;47(1):52–64. 8996078
27. Eusebi, V., Collina, G., Bussolati, G., Carcinoma in situ in sclerosing adenosis of the breast. An immunocytochemical study. Semin Diagn Pathol 1989; 6:146. 2762670
28. Youngston, B.J., Cranor, M., Powell, C., et al, Epithelial displacement in surgical breast specimens following needling procedures. Am J Surg Pathol 1994; 18:896. 8067510
29. Akashi-Tanaka, S., Fukotomi, T., Nanasawa, T., et al, Treatment of non-invasive carcinoma: fifteen-year results at the National Cancer Centre Hospital in Tokyo. Breast Cancer 2000; 7:341–344. 11114862
30. O’Malley, F.P., Lobular neoplasia: morphology, biological potential and management in core biopsies. Mod Pathol. 2010;23(Suppl. 2):S14–S25. 20436498
31. Dunn, B.K., Ford, L.G., Breast cancer prevention: results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) breast cancer prevention trial (NSABP P-1: BCPT). Eur J Cancer. 2000;36(Suppl. 4):S49–S50. 11056316
32. Millis, R.R., Bobrow, L.G., Barnes, D.M. Immunohistochemical evaluation of biological markers in mammary carcinoma in situ: correlation with morphological features and recently proposed schemes for histological classification. Breast. 1996; 5:113–122.
33. Sullivan, R.P., Mortimer, G., Muircheartaigh, I.O., Cell proliferation in breast tumours: analysis of histological parameters Ki67 and PCNA expression. Ir J Med Sci 1993; 162:343–347. 7903289
34. Boland, G.P., Knox, W.F., Bundred, N.J., Molecular markers and therapeutic targets in ductal carcinoma in situ. Microsc Res Tech 2002; 59:3–11. 12242692
35. Shoker, B.S., Jarvis, C., Clarke, R.B., et al, Estrogen receptor-positive proliferating cells in the normal and pre-cancerous breast. Am J Pathol 1999; 155:1811–1815. 10595909
36. Parton, M., Dowsett, M., Smith, I., Studies of apoptosis in breast cancer. Br Med J 2001; 322:1528–1532. 11420276
37. Weinstat-Saslow, D., Merino, M.J., Manrow, R.E., et al, Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med 1995; 1:1257–1260. 7489405
38. Zhou, Q., Hopp, T., Fuqua, S.A., et al, Cyclin D1 in breast pre-malignancy and early breast cancer: implications for prevention and treatment. Cancer Lett 2001; 162:3–17. 11121857
39. Soslow, R.A., Dannenberg, A.J., Rush, D., et al, COX-2 is expressed in human pulmonary, colonic and mammary tumours. Cancer 2000; 89:2637–2645. 11135226
40. Ristimaki, A., Sivula, A., Lundin, J., et al, Prognostic significance of elevated COX-2 expression in breast cancer. Cancer Res 2002; 62:632–635. 11830510
41. Helcynska, K., Kronblad, Å., Jögi, A., et al, Hypoxia induces a dedifferentiated phenotype in ductal carcinoma in situ. Cancer Res 2003; 63:1441–1444. 12670886
42. Dershaw, D.D., Abramson, M.D., Kinne, D.W., Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology 1989; 170:411–415. 2536185
43. Ikeda, D.M., Andersson, I., Ductal carcinoma in situ: atypical mammographic appearances. Radiology 1989; 172:661–666. 2549563
44. Schuh, M.E., Nemoto, T., Penetrante, R.B., et al, Intraductal carcinoma. Analysis of presentation, pathologic findings, and outcome of disease. Arch Surg 1986; 121:1303–1307. 3022676
45. Brennan, M.E., Turner, R.M., Ciatto, S., et al, Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128. 21493791
46. Lee, C.H., Carter, D., Philpotts, L.E., et al, Ductal carcinoma in situ diagnosed with stereotactic core needle biopsy: can invasion be predicted? Radiology 2000; 217:466–470. 11058647
47. Association of Breast Surgery at BASO, Surgical guidelines for the management of breast cancer. Eur J Surg Oncol. 2009;35(Suppl. 1):1–22. 19299100
48. Chan, K.C., Knox, W.F., Sinha, G., et al, Extent of excision margin width required in breast conserving surgery for ductal carcinoma in situ. Cancer 2001; 91:9–16. 11148554
49. Tang, S., Twelves, D., Isacke, C., et al, Mammary ductoscopy in the current management of breast disease. Surg Endosc. 2010;25(6):1712–1722. 21170661
50. Silverstein, M.J., Barth, A., Poller, D.N., et al, Ten year results comparing mastectomy to excision and radiation therapy for ductal carcinoma in situ of the breast. Eur J Cancer 1995; 31A:1425–1427. 7577065
51. Fonseca, R., Hartmenn, L., Petersen, I., et al, Ductal carcinoma in situ of the breast. Ann Intern Med 1997; 127:1013–1022. 9412283
52. , NICE clinical guideline 80. Early and locally advanced breast cancer: diagnosis and treatment. Developed by the National Collaborating Centre for Cancer. February, 2009.
53. Veronesi, P., Intra, M., Vento, A.R., et al, Sentinel lymph node biopsy for localised ductal carcinoma in situ? Breast. 2005;14(6):520–522. 16185871
54. Julian, T.B., Land, S.R., Fourchotte, V., et al, Is sentinel node biopsy necessary in conservatively treated DCIS? Ann Surg Oncol. 2007;14(8):2202–2208. 17534687
55. Fisher, E.R., Dignam, J., Tan-Chiu, E., et al, Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 1999; 86:429–438. 10430251 The 8-year update of 623 women in a randomised controlled trial of 814 women with DCIS treated with local excision who were randomised to receive radiotherapy or no additional treatment. The study found that women who received additional radiotherapy following breast-conserving surgery had a significant reduction in ipsilateral breast tumours (31% vs. 13% at 8 years, P = 0.0001). The authors also analysed a range of clinicopathological characteristics of the patients and the tumours to assess predictors of recurrence; findings suggested that the presence of comedo necrosis was an independent risk factor for recurrence.
56. Solin, L.J., Fourquet, A., Vincini, F.A., et al, Salvage treatment for local recurrence after breast-conserving surgery and radiation as initial treatment for mammographically detected carcinoma in situ of the breast. Cancer 2001; 91:1090–1097. 11267953
57. Sneige, N., McNeese, M.D., Atkinson, E.N., et al, Ductal carcinoma in situ treated with lumpectomy and irradiation: histopathological analysis of 49 specimens with emphasis on risk factors and long term results. Hum Pathol 1995; 26:642–649. 7774895
58. Boland, G.P., Chan, K.C., Knox, W.F., et al, Value of the Van Nuys Prognostic Index in prediction of recurrence of ductal carcinoma in situ after breast-conserving surgery. Br J Surg 2003; 90:426–432. 12673743
59. Fisher, B., Dignam, J., Wolmark, N., et al, Tamoxifen in the treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 1999; 353:1993–2000. 10376613 Double-blind, randomised, controlled trial of 1804 women with completely or incompletely excised DCIS at breast-conserving surgery who were randomised to receive radiotherapy plus or minus tamoxifen. The women receiving tamoxifen had fewer breast cancer events at 5 years compared with placebo (8.2 vs. 13.4, P = 0.0009), mainly due to a decrease in invasive cancer in the ipsilateral breast. A retrospective review of the results (Ref. 80) showed that this benefit was confined to oestrogen receptor-positive cases.
60. Fisher, B., Constantino, J., Redmond, C., et al, Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med 1993; 328:1581–1586. 8292119
61. Bijker, N., Peterse, J.L., Duchateau, L., et al, Risk factors for recurrence and metastasis after breast conserving therapy for ductal carcinoma in situ: analysis of EORTC trial. J Clin Oncol 2001; 19:2263–2271. 11304780 A review of 843 women of the 1010 randomised cases from the EORTC 10853 trial (local excision of DCIS plus or minus radiotherapy) that examined the clinicopathological characteristics of the women. The authors found that clear margins were the most important factor in reducing local recurrence (hazard ratio 2.07, P = 0.0008). Patients with poorly differentiated DCIS were at higher risk of metastatic disease (hazard ratio 6.57, P = 0.01) and other poor prognostic factors included young age (< 40 years) at diagnosis (hazard ratio 2.14, P = 0.02) and symptomatic detection (hazard ratio 1.8, P = 0.008).
62. Wang, S.Y., Chu, H., Shamliyan, T., et al, Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst 2012; 104:507–516. 22440677
63. Dunne, C., Burke, J.P., Morrow, M., et al, Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27(10):1615–1620. 19255332
64. Morrow, M., Katz, S.J., Margins in ductal carcinoma in situ: is bigger really better? J Natl Cancer Inst. 2012;104(7):494–495. 22440679
65. Fraser, J., Raza, S., Chorny, K., et al, Columnar alteration with prominent apical snouts and secretions: a spectrum of changes frequently present in breast biopsies with microcalcifications. Am J Surg Pathol 1998; 22:1521–1527. 9850178
66. UK Coordinating Committee on Cancer Research (UKCCCR), Ductal Carcinoma In Situ (DCIS) Working Party on behalf of DCIS trialists in the UK, Australia and New Zealand. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia and New Zealand: randomised controlled trial. Lancet 2003; 362:95–103. 12867108 A 2 × 2 factorial design, randomised controlled trial of 1701 screen-detected patients with completely excised DCIS, randomised to receive tamoxifen, radiotherapy, both treatments or none. The authors found that radiotherapy reduced the incidence of both ipsilateral invasive recurrence (hazard ratio 0.45, P = 0.01) and DCIS recurrence (hazard ratio 0.36, P = 0.0004). Tamoxifen reduced overall DCIS recurrence (hazard ratio 0.68, P = 0.03) but not invasive disease. The trial has not yet published results with regard to oestrogen receptor status.
67. Cuzick, J., Sestaka, I., Pinder, S., et al, Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2010;12(1):21–29. 21145284
68. Rodrigues, N., Dillon, D., Parisot, N., et al, Differences in the pathologic and molecular features of intraductal breast carcinoma between younger and older women. Cancer 2003; 97:1393–1403. 12627502
69. Silverstein, M.J., Lagios, M.D., Choosing treatment for patients with DCIS: fine tuning the University of Southern California/Van-Nuys Prognostic Index. J Natl Cancer Inst Monogr 2010; 41:193–196. 20956828
70. Provenzano, E., Hopper, J.L., Giles, G.G., et al, Biological markers that predict clinical recurrence in ductal carcinoma in situ of the breast. Eur J Cancer 2003; 39:622–630. 12628841
71. Witton, C.J., Reeves, J.R., Going, J.J., et al, Expression of the HER 1–4 family of receptor tyrosine kinases in breast cancer. J Pathol 2003; 200:290–297. 12845624
72. Barnes, N.L.P., Khavari, S., Boland, G.P., et al, Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res 2005; 11:2163–2168. 15788662
73. Lari, S.A., Kuerer, H.M., Biological markers in DCIS and risk of Breast recurrence: A systematic review. J Cancer 2011; 2:232–261 May 1. 21552384
74. Goodwin, A., Parker, S., Ghersi, D., et al, Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 2009;(1) CD000563. 19160183
75. Emdin, S.O., Granstrand, B., Ringberg, A., et al, SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45(5):536–543. 16864166
76. Bijker, N., Meijnen, P., Peterse, J.L., et al, Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853 – a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. 16801628
77. Wong, J., Kaelin, C., Troyan, S., et al, Prospective study of wide local excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24(7):1031–1036. 16461781
78. Hughes, L., Wang, M., Page, D., et al, Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(32):5319–5324. 19826126
79. , 2009 National Institutes for Health State-of-the-Science meeting on Ductal Carcinoma in Situ: Management and Diagnosis. J Natl Cancer Inst Monogr. 2010;2010(41):111–222. 21312388
80. Allred, D.C., Anderson, S.J., Paik, S., et al, Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP Protocol B-24. J Clin Oncol. 2012;30(12):1268–1273. 22393101
81. Boland, G.P., McKeowan, A., Chan, K.C., et al, Biological response to hormonal manipulation in oestrogen receptor positive ductal carcinoma in situ of the breast. Br J Cancer 2003; 89:277–283. 12865917
82. Bundred, N.J., Cramer, A., Cheung, K.L., et al. ERISAC trial: evidence exemestane effects oestrogen receptor (ER) positive ductal carcinoma in situ (DCIS) proliferation. Breast Cancer Res Treat. 2007; 106(Suppl. 1):S5043.
83. Fisher, B., Constantino, J.P., Wickerman, D.L., et al, Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. 9747868
84. Pappo, I., Wasserman, I., Halevy, A., Ductal carcinoma in situ of the breast in men: a review. Clin Breast Cancer. 2005;6(4):310–314. 16277880
85. Simmons, R.M., Male ductal carcinoma in situ presenting as bloody nipple discharge: a case report and literature review. Breast J 2002; 8:112–114. 11896758
86. Hittmair, A.P., Liniger, R.A., Tavassoli, F.A., Ductal carcinoma in situ (DCIS) in the male breast. A morphological study of 84 cases of pure DCIS and 30 cases of DCIS associated with invasive carcinoma: a preliminary report. Cancer 1998; 83:2139–2149. 9827718
87. Li, X., Lewis, M.T., Huang, J., et al, Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. 18445819