Treatment of Dry Eye Disease
Introduction
Dry eye develops when disease or dysfunction of one or more components of the integrated lacrimal functional unit is no longer able to maintain a stable tear film.1 Dry eye is a common condition with a reported prevalence ranging from 2% to 14.4%.2–6 Patients with dry eye typically complain of eye irritation, such as foreign body sensation, burning, and dryness, as well as visual symptoms, including photophobia and fluctuating vision. Dry eye causes pathological changes to the ocular surface epithelium with disruption of corneal epithelial barrier function and loss of mucus-secreting goblet cells. Dry eye may decrease quality of life, and in the most severe cases, dry eye disease can cause functional and occupational disability. The impact of dry eye on quality of life was rated to be equivalent to unstable angina using utility assessments.7
Therapy of dry eye is aimed at improving irritation symptoms, visual quality and sight-threatening corneal epithelial disease. This chapter reviews current evidence and consensus-based treatment recommendations for dry eye, that are based on previously published recommendations of the International Dry Eye Workshop (DEWS) and Meibomian Gland Workshop.8,9
Diagnostic Classification of Dry Eye
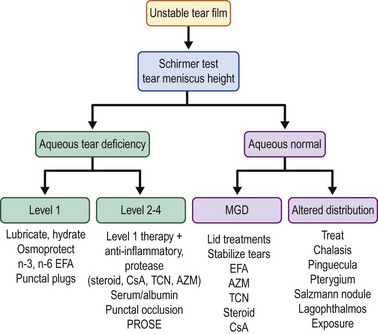
Optimal management of dry eye or tear dysfunction requires performance of a minimal number of diagnostic tests to: identify character, frequency and severity of patient complaints; confirm presence of an unstable tear film; identify aqueous tear deficiency; detect meibomian gland disease; identify conditions altering tear clearance and/or distribution (conjunctivochalasis, lid or punctal ectropion, punctal stenosis, pterygium, pingueculum, Salzmann’s nodular degeneration) and detect location and extent of ocular surface epithelial disease. Results of these tests, combined with clinical impressions, can be used to grade disease severity. The Dry Eye Workshop proposed four levels of severity (Table 12.1);8 however, disease severity may be better characterized on a continuous spectrum.
Table 12.1
Severity Grading Scheme for Dry Eye
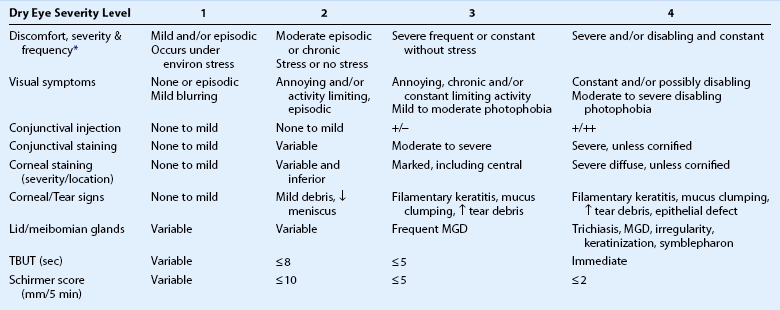
eviron: environmental; MGD: meibomian gland disease.
*Discomfort symptoms may be minimal or absent in levels 3 and 4 due to nerve degeneration.
Modified from scheme proposed by Dry Eye Workshop (Pflugfelder S (committee chairman) Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:163–78.)
Aqueous deficiency requires therapies to minimize or eliminate environmental factors, lubricate and hydrate the ocular surface, protect against osmotic stress and stimulate tear production. Replacement of tear constituents with autologous serum or plasma and protection of the ocular surface with therapeutic contact lens, such as PROSE are necessary in more severe cases (levels 3 and 4). Ocular surface inflammation is a pathological feature shared by aqueous deficient and aqueous sufficient dry eye conditions and many currently utilized therapies, inhibit production of inflammatory cytokines and proteases. A severity-based treatment algorithm for treatment of dry eye is presented in Figure 12.1. Therapies can be added or removed based on objective and subjective response.
Environmental Modification
Systemic and environmental factors contributing to dry eye should be modified or eliminated. Systemic medications with anticholinergic side effects (e.g. antihistamines, antidepressants, and antispasmodics) should be eliminated if possible. Exposure to desiccating environmental stresses (e.g. low humidity and air conditioning drafts) that cause irritation symptoms or increase tear evaporation, should be minimized or eliminated. The height of video display terminals should be adjusted to sit at slightly below eye level to decrease the interpalpebral aperture and exposure. Room humidifiers may be beneficial in dry climates and high altitudes. Moisture chamber eyeglasses can reduce tear evaporation and maintain high humidity around the eye. Nocturnal lagophthalmos can be treated with swim goggles, taping the eyelids closed, or performing a lateral tarsorrhaphy. Patients should be encouraged to eat a balanced diet, rich in n-3 polyunsaturated fatty acids from fish and low in saturated n-6 fatty acids.10 Smoking has been identified to destabilize the tear film and should be stopped.11
Artificial Tears
Artificial tears are considered a first-line therapy for dry eye. They increase tear volume, minimize desiccation and lubricate the ocular surface. They often provide temporary relief of irritation symptoms in many dry eye conditions. There are a variety of commercially available artificial tears, that contain various agents (Table 12.2) that increase their viscosity, lubricity, retention time and adhesion to the ocular surface. Certain artificial tear formulations contain electrolytes or ions, such as potassium and bicarbonate ions, that are found in normal tears. Tears with a lipid component, such as castor oil (Refresh Optive™ ADVANCED, Systane® Balance, and Soothe® XP) may retard tear evaporation and prevent intrusion of irritating skin lipids.
Table 12.2
Composition of Artificial Tears
| Viscosity Enhancing Agent | Concentration | Found in Commercial Brands |
| Cellulose derivatives [carboxymethylcellulose (CMC), hydroxypropylmethylcellose (HPMC)] | 0.2–1% | Refresh Tears, TheraTears® (CMC); Bion® Tears, Tears Naturale®, Visine®, GenTeal®, Artelac® (HPMC) |
| CMC + glycerin | 0.5-0–9 | Refresh Optive™ |
| Polyols [polyethylene glycol (PEG), propylene glycol, glycerin] | 0.25–1% | Systane®, Systane Ultra®, Soothe® Lubricant preservative free, Advanced Eye Relief®, Oasis® |
| Polyvinyl alcohol (PVA) | 1–1.4% | HypoTears®, Akwa Tears®, Tears Again®, Tears Naturale® PM, Freshkote |
| Hyaluronic Acid | 0.1–0.18% | Blink Tears®, Hyalistil™ Oasis®, Vismed® |
| Oil-based emulsions (mineral and castor oil) | Solutions: Refresh Optive™ ADVANCED, Systane® Balance, Soothe® XP Emollient, Ointments: AKWA Tears, LACRI-LUBE®, Systane Nighttime Ointment®,Tears Naturale® PM, REFRESH PM® |
Artificial tears lubricate the ocular surface, reduce tear osmolarity and protect the ocular surface from desiccation. They often provide temporary improvement in eye irritation and blurred vision symptoms, visual contrast sensitivity, tear break-up time, corneal surface regularity and ocular surface dye staining. They have not been found to reverse conjunctival squamous metaplasia.12–19
Benzalkonium chloride is known to disrupt tight junctions and accelerate desquamation of the apical epithelial layer of the cornea in a dose-dependent fashion.20 BAC has detrimental effects on cell membrane integrity and viability. BAC was found to promote apoptosis at lower concentrations and necrosis at higher concentrations following treatment of a conjunctival epithelial cell line.21 It has also been found to stimulate production of inflammatory cytokines (IL-1 and TNF-α) by cultured ocular surface epithelial cells.22
Punctal Occlusion
Punctal occlusion should be considered for patients with aqueous tear deficiency and low tear volume. Punctal occlusion is a simple and practical method for conserving endogenously produced tears or instilled artificial tears, increasing tear volume and decreasing tear osmolarity. Punctal occlusion has been reported to decrease ocular irritation symptoms, to improve ocular surface dye staining, and to decrease dependence on artificial tears.23–27 It should be used with caution in patients with normal aqueous tear production (Schirmer 1 scores > 10 mm) and poor tear clearance or in patients with overt lid or ocular surface inflammation, such as meibomian gland disease or ocular rosacea. In these cases, ocular surface inflammation should be controlled prior to punctal occlusion.
Dissolvable intracanalicular plugs (lasting 7–10 days for collagen and up to 3 months for synthetic polymers) can be placed into the canaliculis on a trial basis, to determine the efficacy and tolerability of punctal occlusion prior to placement of semipermanent plugs or permanent occlusion. Reversible punctal occlusion can be accomplished with a variety of ‘semipermanent’ dumbbell-shaped silicone plugs that are inserted into the punctal orifice. These plugs are available in a variety of sizes ranging in diameter from 0.4 to 0.8 mm and a gauge can be used to determine the size of the punctum prior to insertion. Silicone plugs are typically retained for weeks to years and have a reported extrusion rate of approximately 7% at 1 month, increasing to 37% at 6 months.28 They can be easily removed with forceps. They are generally well tolerated, but occasionally patients will experience discomfort from the head of the plug rubbing against the medial bulbar conjunctiva. There is also a small risk of pyogranuloma formation at the punctal opening and migration of the plug into the lacrimal canaliculus, that may require surgical removal of the displaced plug.
Anti-inflammatory Therapy
Cyclosporine A
Cyclosporine emulsion (Restasis™, Allergan) is the only FDA approved anti-inflammatory therapy of dry eye. Administered twice a day, topical cyclosporine stimulates aqueous tear production by suppressing ocular surface and glandular inflammation and inhibiting apoptosis (programmed cell death) of the tear-producing epithelial cells in the lacrimal glands and ocular surface. In FDA clinical trials, cyclosporine emulsion was found to be effective in decreasing corneal fluorescein staining, improving blurred vision symptoms and decreasing artificial tear use in patients with moderate to severe keratoconjunctivitis sicca. Clinical improvement may be observed within several weeks and continues up to 6 months. Preliminary clinical experience suggests that cyclosporine may be effective in patients with less severe disease. Cyclosporine emulsion is a non-toxic medication that can be continued indefinitely, although once daily dosing may be sufficient after 1 year29 and discontinuing routine use may lead to worsening symptoms.30 Topical cyclosporine has also been found to increase density of mucus-filled goblet cells in the conjunctiva.31,32 Some dry eye patients benefit from more frequent dosing than the standard twice daily regimen.33
Tacrolimus
Tacrolimus is a calcineurin inhibitor with a similar mechanism of action to cyclosporine, reduction in T-cell activation. Although tacrolimus is not approved for the treatment of dry eye in the United States, a recent small study suggests that it may benefit patients with dry eye.34 Further studies are needed to address safety and efficacy.
Corticosteroids
Corticosteroids are potent immunosuppressors, and when administered topically they inhibit multiple pathways of the inflammatory response. Topical corticosteroids also decrease ocular surface inflammation and have been reported to improve corneal epithelial disease in dry eye. They are most appropriately used for pulse therapy, such as 2–4 times per day for up to 4 weeks, to minimize their well-known side effects.35,36 Clinical improvement is usually observed within a week and the therapeutic effect may be sustained for weeks or months after a pulse.35 To minimize the potential to raise intraocular pressure, agents such as fluorometholone and loteprednol etabonate or low-dose dexamethasone (0.01%) should be considered.36,37 Topical steroids can be combined with other anti-inflammatory therapies, such as cyclosporine, to provide more rapid relief of symptoms and greater improvement of ocular surface disease.
Tetracyclines
In addition to their antibiotic activity, tetracyclines are recognized to have numerous anti-inflammatory properties. They inhibit the production of inflammatory cytokines, decrease nitric oxide production and inhibit matrix metalloproteinase production.38 The semisynthetic tetracycline doxycycline has been recognized to improve irritation symptoms, increase tear film stability and decrease the severity of ocular surface disease in meibomian gland dysfunction and ocular rosacea.8 Doxycycline has also been reported to be effective for treating recurrent corneal epithelial erosions.39
Oral tetracycline therapy should be considered in patients with ocular rosacea, severe corneal epithelial disease, corneal vascularization, marginal infiltrates, non-healing corneal epithelial defects and corneal ulceration associated with tear film deficiency. In a randomized clinical trial of doxycycline for treatment of meibomian gland dysfunction, a dose of 20 mg orally twice a day, for 1 month was found to be as effective as 200 mg orally twice a day.40 The trend has been to treat patients with lower doses (20–50 mg orally twice a day) to minimize gastrointestinal upset and candidiasis, for 4 weeks and stop or taper to once a day if improvement in signs and symptoms is observed.
Azithromycin
Azithromycin is an azalide, macrolide antibiotic with broad-spectrum antibacterial activity and an antimicrobial mechanism of action that involves binding to the 50S subunit of the bacterial ribosome. Like the tetracycline, antibiotics, azithromycin may also have anti-inflammatory properties41 beneficial to treating the ocular surface.42 Azithromycin is available as a topical ophthalmic preparation (Azithromycin 1% solution) approved for the treatment of bacterial conjunctivitis. Azithromycin applied to the lids may have an antibacterial effect on common agents of anterior blepharitis, including Staphylococcus. A number of studies have shown improvement in signs and symptoms of meibomian gland dysfunction-related dry eye in patients using topical azithromycin solution.43–46 Topical azithromycin ophthalmic solution on the lid margin may be a practical alternative in patients unwilling or unable to take oral doxycycline.
Essential Fatty Acids
Diet has been implicated as a cause for dry eye. In the Woman’s Health Study, the group with the highest dietary consumption of omega-3 (n-3) polyunsaturated fatty acids (PUFA) carried a significantly lower risk of dry eye than the group with the lowest consumption. Furthermore, a high dietary omega-6 (n-6) to n-3 ratio was associated with a twofold higher risk of developing dry eye.10 This benefit was attributed to the anti-inflammatory properties of n-3 PUFAs that are found in certain cold water fish (salmon, cod, mackerel). The n-3 PUFA eicosapentaenoic acid (EPA) found in fish oil has been found to decrease production of arachidonic acid-derived eicosanoids, to decrease the production of proinflammatory cytokines and reactive oxygen species and inhibit lymphocyte proliferation.47,48 The n-6 PUFA γ-linolenic acid (GLA) also has anti-inflammatory activity.48 Supplementation with EPA has been found to potentiate the anti-inflammatory activity of GLA by decreasing synthesis of arachidonic acid. Formulations containing one or both of these PUFAs have been observed to be effective in treating chronic inflammatory diseases, such as rheumatoid arthritis.49 Clinical trials of nutritional supplements containing linoleic acid and/or GLA alone or combined with fish oil50–53 for treatment of dry eye have reported improvement in symptoms and/or signs.
Secretogogues
The oral secretogogues pilocarpine and cevilemine stimulate aqueous tear production. They should be considered in conditions, such as Sjögren’s syndrome and neurotrophic disease, where the ability to reflex tear is lost. Oral pilocarpine (Salagen, MGI Pharma, Minneapolis) is a muscarinic cholinergic parasympathomimetic agonist that stimulates exocrine glands. Subjective improvement in dry eye symptoms has been reported54–56 and one study also noted improvement in conjunctival staining.55 Pilocarpine is typically prescribed at a dose of 5 mg q.i.d. The most common side effects from this medication are excessive sweating and gastrointestinal cramping, occurring in approximately 40% of patients.56
Cevimeline (Evoxac; Daiichi, Montvale, NJ) is another oral cholinergic agonist that has been found to improve ocular irritation symptoms57 and aqueous tear production.57–59 This agent appears to have fewer adverse systemic side effects than oral pilocarpine, although it may also cause mild to moderate gastrointestinal symptoms and sweating.59 It is typically prescribed at a dose of 30 mg p.o. t.i.d.
Serum/Plasma
Autologous serum, which is known to contain albumin and various growth and anti-inflammatory factors, can be used to treat Sjögren’s syndrome-associated keratoconjunctivitis sicca60,61 and to promote healing of persistent corneal epithelial defects.62–65 Autologous plasma has also been reported to heal neurotrophic keratopathy in dry eye.66 Because of the difficulty and expense in preparing serum drops and the potential for microbial contamination, they should be reserved for patients with severe disease who are unresponsive to other therapies. Furthermore, studies have reported varying degrees of efficacy with serum ophthalmic preparations, possibly due to different concentrations and preparation regimens. Close cooperation with a blood bank or formulation pharmacy with an established protocol for serum/plasma may improve outcomes and minimize complications.
Contact Lenses
Patients with severe corneal epithelial disease (e.g. filamentary keratitis and non-healing corneal epithelial defects) and those with lid margin keratinization, irregularity or trichiasis (as typically seen with Stevens Johnson syndrome) may benefit from therapeutic contact lenses. Hydrogel and silicone hydrogel soft lenses provide some protection from microtrauma, from irregular lids and trichiasis, but they are often poorly tolerated in severe aqueous tear deficiency. A better option for this subset of patients is the prosthetic replacement of the ocular surface ecosystem (PROSE TM), a specially designed scleral-bearing contact lens with a fluid-filled reservoir over the cornea. PROSE has proven to be an excellent option for improving irritation symptoms and visual acuity.67–69 The fluid-filled reservoir hydrates the cornea and shields it from blink trauma, noxious environmental stimuli and inflammatory mediators in the tears. The body-temperature saline reservoir also prevents corneal cooling and nerve firing that occurs during the inter-blink intervals. Patients may experience almost immediate relief in photophobia and irritation symptoms after placing the device on the cornea. The PROSE is an excellent substitute for tarsorrhaphy; however, this surgical procedure should be considered in patients where PROSE is not an option. Other options for treatment of filamentary keratitis include topical corticosteroids, topical acetylcysteine and onabotulinumtoxin A injection into the orbicularis muscle to decrease lid frictional force on the corneal surface.70
References
1. Stern, ME, Beuerman, RW, Fox, RI, et al. The pathology of dry eye: The interaction between ocular surface and lacrimal glands. Cornea. 1998;17:584–589.
2. Moss, SE, Klein, R, Klein, BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268.
3. Moss, SE, Klein, R, Klein, BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–373.
4. Schaumberg, DA, Sullivan, DA, Buring, JE, et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326.
5. Schaumberg, DA, Dana, R, Buring, JE, et al. Prevalence of dry eye syndrome among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127:763.
6. Shimmura, S, Shimazaki, J, Tsubota, K. Results of a population-based questionnaire on the symptoms and lifestyles associated with dry eye. Cornea. 1999;18:408–411.
7. Schiffman, RM, Walt, JG, Jacobsen, G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419.
8. Pflugfelder, S. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). (committee chairman). Ocul Surf. 2007;5:163–178.
9. Geerling, G, Tauber, J, Baudouin, C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–2064.
10. Miljanovic′, B, Trivedi, KA, Dana, MR, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893.
11. Altinors, DD, Akça, S, Akova, YA, et al. Smoking associated with damage to the lipid layer of the ocular surface. Am J Ophthalmol. 2006;141(6):1016–1021.
12. Nelson, JD, Farris, RL. Sodium hyaluronate and polyvinyl alcohol artificial tear preparations: a comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 1988;106:484–487.
13. Lemp, MA. Artificial tear solutions. Int Ophthalmol Clin. 1973;13:221–229.
14. Toda, I, Shinozaki, N, Tsubota, K. Hydroxypropyl methylcellulose for the treatment of severe dry eye associated with Sjögren’s syndrome. Cornea. 1996;15:120–128.
15. Huang, FC, Tseng, SH, Shih, MH, et al. Effect of artificial tears on corneal surface regularity, contrast sensitivity and glare disability in dry eyes. Ophthalmology. 2002;109:1934–1940.
16. Iskeleli, G, Kizilkaya, M, Arslan, OS, et al. The effect of artificial tears on corneal surface regularity in patients with Sjögren syndrome. Ophthalmologica. 2002;216:118–122.
17. Liu, Z, Pflugfelder, SC. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology. 1999;106:939–943.
18. Donshik, PC, Nelson, JD, Abelson, M, et al. Effectiveness of BION tears, Cellufresh, Aquasite, and Refresh Plus for moderate to severe dry eye. Adv Exp Med Biol. 1998;438:753–760.
19. Calonge, M. The treatment of dry eye. Surv Ophthalmol. 2001;45(suppl. 2):S227–S239.
20. Pauly, A, Meloni, M, Brignole-Baudouin, F, et al. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest Ophthalmol Vis Sci. 2009;50:1644–1652.
21. De Saint Jean, M, Brignole, F, Bringuier, AF, et al. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:619–630.
22. Epstein, SP, Chen, D, Asbell, PA. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25:415–424.
23. Freeman, JM. The punctum plug: evaluation of a new treatment for the dry eye. Trans Am Acad Ophthalmol Otolaryngol. 1975;79:874–879.
24. Tuberville, AW, Frederick, WR, Wood, TO. Punctal occlusion in tear deficiency syndromes. Ophthalmology. 1982;89:1170–1172.
25. Willis, RM, Folberg, R, Krachmer, JH, et al. The treatment of aqueous deficient dry eye with removable punctual plugs. A clinical and impression-cytological study. Ophthalmology. 1987;94:514–518.
26. Gilbard, JP, Rossi, SR, Azar, DT, et al. Effect of punctal occlusion by Freeman silicone plug insertion on tear osmolarity in dry eye disorders. CLAO J. 1989;15:216–218.
27. Baxter, SA, Laibson, PR. Punctal plugs in the management of dry eyes. Ocul Surf. 2004;2:255–265.
28. Balaram, M, Schaumberg, DA, Dana, MR. Efficacy and tolerability outcomes after punctal occlusion with silicone plugs in dry eye syndrome. Am J Ophthalmol. 2001;131:30–36.
29. Su, MY, Perry, HD, Barsam, A, et al. The effect of decreasing the dosage of cyclosporine A 0.05% on dry eye disease after 1 year of twice-daily therapy. Cornea. 2011;30:1098–1104.
30. Rao, SN. Reversibility of dry eye deceleration after topical cyclosporine 0.05% withdrawal. J Ocul Pharmacol Ther. 2011;27:603–609.
31. Pflugfelder, SC, De Paiva, CS, Villarreal, AL, et al. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta 2 production. Cornea. 2008;27:64–69.
32. Kunert, KS, Tisdale, AS, Gipson, IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–337.
33. Dastjerdi, MH, Hamrah, P, Dana, R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28:1091–1096.
34. Moscovici, BK, Holzchuh, R, Chiacchio, BB, et al. Clinical treatment of dry eye using 0.03% Tacrolimus eye drops. Cornea. 2012;31:945–949.
35. Marsh, P, Pflugfelder, SC. Topical non-preserved methylprednisolone therapy of keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999;106:811–816.
36. Pflugfelder, SC, Maskin, SL, Anderson, B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–457.
37. Jonisch, J, Steiner, A, Udell, IJ. Preservative-free low-dose dexamethasone for the treatment of chronic ocular surface disease refractory to standard therapy. Cornea. 2010;29:723–726.
38. Pflugfelder, SC. Anti-inflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–342.
39. Dursun, D, Kim, MC, Solomon, A, et al. Treatment of recalcitrant recurrent corneal epithelial erosions with inhibitors of matrix metalloproteinases-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13.
40. Yoo, SE, Lee, DC, Chang, MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005;19:258–263.
41. Ianaro, A, Ialenti, A, Maffia, P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292:156–163.
42. Igami, TZ, Holzchuh, R, Osaki, TH, et al. Oral azithromycin for treatment of posterior blepharitis. Cornea. 2011;30:1145–1149.
43. Foulks, GN, Borchman, D, Yappert, M, et al. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29:781–788.
44. Luchs, J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008;25:858–887.
45. Haque, RM, Torkildsen, GL, Brubaker, K, et al. Multicenter, open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea. 2010;29:871–877.
46. Opitz, DL, Tyler, KF. Efficacy of azithromycin 1% ophthalmic solution for treatment of ocular surface disease from posterior blepharitis. Clin Exp Optom. 2011;94:200–206.
47. Zurier, RB, Rossetti, RG, Seiler, CM, et al. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: effects of unsaturated fatty acids. Prost Leuk Essent Fatty Acids. 1999;60:371–375.
48. DeMarco, DM, Santoli, D, Zurier, RB. Effects of fatty acids on proliferation and activation of human synovial compartment lymphocytes. J Leuk Biol. 1994;56:612–615.
49. Calder, PC, Zurier, RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4:115–121.
50. Aragona, P, Bucolo, C, Spinella, R, et al. Systemic omega-6 essential fatty acid treatment and PGE1 tear content in Sjogren’s syndrome patients. Invest Ophthalmol Vis Sci. 2005;46:4474–4479.
51. Barabino, S, Rolando, M, Camicione, P, et al. Efficacy of systemic linoleic and gamma-linolenic acid therapy in dry-eye syndrome with inflammatory component. Cornea. 2003;22:97–101.
52. Kokke, KH, Morris, JA, Lawrenson, JG. Oral omega-6 essential fatty acid treatment in contract lens association dry eye. Cont Lens Anterior Eye. 2008;31:141–146.
53. Creuzot, C, Passemard, M, Viau, S, et al. Improvement of dry eye symptoms with polyunsaturated fatty acids. J Fr Ophtalmol. 2006;29:868–873.
54. Tsifetaki, N, Kitsos, G, Paschides, CA, et al. Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren’s syndrome: a randomised 12 week controlled study. Ann Rheum Dis. 2003;62:1204–1207.
55. Papas, AS, Sherrer, YS, Charney, M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169–177.
56. Vivino, FB, Al-Hashimi, I, Khan, Z, et al. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren syndrome: a randomized, placebo-controlled, fixed-dose, multicenter trial. P92-01 Study Group. Arch Intern Med. 1999;159:174–181.
57. Fife, RS, Chase, WF, Dore, RK, et al. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome: a randomized trial. Arch Intern Med. 2002;162:1293–1300.
58. Petrone, D, Condemi, JJ, Fife, R, et al. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002;46:748–754.
59. Ono, M, Takamura, E, Shinozaki, K, et al. Therapeutic effect of cevimeline on dry eye in patients with Sjögren’s syndrome: a randomized, double-blind clinical study. Am J Ophthalmol. 2004;138:6–17.
60. Fox, RI, Chan, R, Michelson, JB, et al. Beneficial effects of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27:459–461.
61. Yoon, KC, Heo, H, Im, SK, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144:86–92.
62. Schrader, S, Wedel, T, Moll, R, et al. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006;244:1345–1349.
63. Jeng, BH, Dupps, WJ, Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–1108.
64. Del Castillo, JM, de la Casa, JM, Sardina, RC. Treatment of recurrent corneal erosions using autologous serum. Cornea. 2002;21:781–783.
65. Young, AL, Cheng, AC, Ng, HK, et al. The use of autologous serum tears in persistent epithelial defect. Eye. 2004;18:609–614.
66. Rao, KV, Leveque, C, Pflugfelder, SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;51:844–849.
67. Romero-Rangel, T, Stavrou, P, Cotter, J, et al. Gas-permeable scleral contact lens therapy in ocular surface disease. Am J Ophthalmol. 2000;130:25–32.
68. Rosenthal, P, Croteau, A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31:130–134.
69. Gumus, K, Gire, A, Pflugfelder, SC. The impact of the Boston ocular surface prosthesis on wavefront higher-order aberrations. Am J Ophthalmol. 2011;151:682–690.
70. Gumus, K, Lee, S, Yen, MT, et al. Botulinum toxin injection for the management of refractory filamentary keratitis. Arch Ophthalmol. 2012;130:446–450.