Travel-Acquired Illnesses
Sources of Information
The Centers for Disease Control and Prevention (CDC) publishes several authoritative sources of information on travel medicine. Health Information for International Travel (the “yellow book”) is updated annually. Two other periodicals, the weekly Morbidity and Mortality Weekly Report (MMWR) and Summary of Health Information for International Travel (the “blue sheet,” published biweekly), provide updated information on the status of immunization recommendations, worldwide disease outbreaks, and changes in health conditions. A reliable way to obtain current travel health information, including vaccine requirements, malaria chemoprophylaxis, and disease outbreaks for various regions of the world, is to consult the CDC Travelers’ Health website at http://wwwnc.cdc.gov/travel/. See Chapter 49 for more information on immunizations for travel. For nonmedical information of interest to the traveler, the U.S. State Department can be accessed at http://travel.state.gov/. Additional resources for travel medicine information are listed at the end of the chapter.
Aside from traveler’s diarrhea (see Chapter 44), traffic-related accidents, and purified protein derivative conversion, the major travel-acquired illnesses in descending order from most to least common are as follows:
Dengue Fever
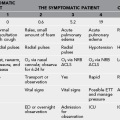
Signs and Symptoms (Compare Dengue With Malaria in Table 48-1)
Table 48-1
Clinical Illness in Malaria and Dengue Fever
| SIGNS AND SYMPTOMS | MALARIA | DENGUE FEVER |
| Fever | +++ | +++ |
| Chills | +++ | ++ |
| Headache | +++ | +++ |
| Malaise | ++ | |
| Anorexia | ++ | |
| Nausea, vomiting | ++ | ++ |
| Abdominal pain | ++ | |
| Myalgia | ++ | ++ |
| Arthralgia | ++ | |
| Backache | + | |
| Dark urine | + |
+++, >90% of patients; ++, >50% of patients; +, <10% of patients.
1. Clinically, may range from undifferentiated viral symptoms with fever and mild respiratory/gastrointestinal symptoms to dengue hemorrhagic fever
2. Incubation period: 4 to 6 days, although may be as long as 14 days
3. Early prodromal symptoms of fever (temperature usually greater than 39° C [102.2° F]), myalgias, headache, arthralgias, and rash. May also have gastrointestinal symptoms of nausea and vomiting
4. After several days, often maculopapular or morbilliform rash spreading outward from chest
5. Dengue can progress to severe forms, referred to as dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS). Severe DHF/DSS may progress to circulatory failure with shock and spontaneous bleeding from almost any site, most commonly the skin, nose, and gastrointestinal tract
6. After infection, patients often develop extreme fatigue persisting for weeks or months
7. DHF/DSS is unlikely in travelers not previously infected with dengue
8. Awareness of the local epidemiology of DHF/DSS is important in establishing the diagnosis. Definitive diagnosis of all forms requires serologic examination (antibody identification via enzyme-linked immunosorbent assay [ELISA] or polymerase chain reaction [PCR]) or viral isolation from serum
9. Predictors of more severe disease manifestations, including DHF/DSS, include serotype (DEN-2 is the most severe), prior exposure, age, malnutrition, and genetic factors such as human leukocyte antigen (HLA) type
Treatment
2. Administer acetaminophen for fever and myalgias (do not use salicylates).
3. Provide aggressive fluid and electrolyte resuscitation.
4. Be aware that severe DHF or any DSS is a medical emergency and requires immediate evacuation and hospitalization.
5. No specific therapy exists for any form of dengue.
6. Vaccine initiatives are ongoing; however, no licensed vaccine against dengue viruses currently exists.
Yellow Fever
Signs and Symptoms
1. May appear as an undifferentiated viral syndrome
2. Specific diagnosis in the wilderness is extremely difficult; clinical suspicion is based on immune status, geographic distribution of the disease, travel history, and characteristic triphasic fever, as follows:
a. Infection phase: After 3 to 6 days of incubation period, onset of headache, photophobia, fever, malaise, back pain, epigastric pain, anorexia, and vomiting. May also have “Faget sign” (bradycardia occurring at the height of the fever), conjunctival injection, and a coated tongue with pink edges
b. Remission phase: The 3 to 4 days of infection phase is followed by up to 48 hours of brief remission
c. Intoxication phase (up to 15% of individuals infected with yellow fever virus): Onset of jaundice, fever, encephalopathy, and in severe cases, hypotension, shock, oliguria, coma, and multiorgan failure. Hemorrhage usually manifested as hematemesis, but bleeding from multiple sites possible
d. Signs of a poor prognosis include early onset of the intoxication phase, hypotension, severe hemorrhage with disseminated intravascular coagulation, renal failure, shock, and coma
Treatment
1. Perform a careful physical examination. Be aware that the laboratory evaluation includes thick and thin blood smears to rule out malaria and blood cultures for bacterial pathogens; both of these necessitate evacuation to a qualified medical facility. If the patient’s condition progresses to the intoxication phase, arrange for immediate evacuation to an intensive care unit (ICU).
2. Note that no effective antiviral treatment is available for yellow fever.
Prevention
1. Give yellow fever vaccine (>95% of those vaccinated achieve significant antibody levels). Be aware that booster doses of vaccine are recommended every 10 years.
2. Current recommendations for yellow fever vaccine can be obtained online at http://wwwnc.cdc.gov/travel/ (see Chapter 49).
3. Avoid the causative organism through mosquito protection measures in endemic areas, including repellent and proper netting.
Rabies Exposure
Rabies exists almost everywhere in the world with the exception of Antarctica and a few island nations. Most cases of human disease in the developing world result from multiple, deep bites to the face, scalp, or upper extremities from an unimmunized canine. In more developed countries, however, the majority of rabies transmission occurs from wild animal carriers (see Chapter 42). Travelers who plan on spending more than 1 month in regions at high risk for rabies should consider preexposure rabies immunization. CDC recommendations for rabies prophylaxis can be found at http://www.cdc.gov/diseases/rabies.html. Rabies is almost universally fatal within a few weeks of symptom onset. If a potential rabies exposure occurs, immunized patients will require only two subsequent rabies immunization boosters on days 0 and 3 post exposure with close follow up. Patients without preexposure immunizations should receive rabies immune globulin and rabies immunization as outlined in Chapter 43.
Hepatitis Viruses
Hepatitis A
Signs and Symptoms
1. Incubation period ranging from 2 to 7 weeks
2. Infection is often asymptomatic or mild, especially in children
3. Classic syndrome: early onset of anorexia, followed by nausea, vomiting, fever, and abdominal pain
4. Symptoms possibly accompanied by hepatosplenomegaly
5. Jaundice after gastrointestinal syndrome by several days to a few weeks; resolution of jaundice lasting another 3 to 4 weeks
6. Although rare, HAV sometimes follows a fulminant course (0.5% to 1% of patients), resulting in hepatic necrosis, hepatic encephalopathy, and death. The incidence of fulminant hepatitis increases with age
7. Clinical presentation is often milder than with other types of viral hepatitis, but not distinctive enough to allow clinical differentiation
8. A number of laboratory tests are available to confirm the diagnosis, but diagnosis in the field is clinical
Treatment
1. No specific therapy exists for HAV infection.
2. Instruct affected persons to adhere to enteric precautions to avoid transmission to others (compulsive hand washing).
3. Although infectivity drops sharply soon after the onset of jaundice, to be safe, continue enteric and blood-drawing precautions for 2 weeks.
Hepatitis B
Signs and Symptoms
1. Range of incubation period is 7 to 22 weeks
2. Manifestations are similar to those of HAV: fever, anorexia, nausea, vomiting, abdominal pain.
3. Additional prodrome of rash, arthralgias, or arthritis and fever occurs in up to 20% of hepatitis B patients (rare in HAV).
4. Jaundice developing a short time after gastrointestinal symptoms
5. With self-limited disease, recovery is complete by 6 months.
6. With fulminant course (1% to 3% of patients), hepatic necrosis, hepatic encephalopathy, and death can occur.
8. Definitive diagnosis requires antigen and antibody tests.
Treatment
1. No specific field therapy exists for HBV.
2. Note that prolonged and sometimes persistent viremia makes blood and body fluid precautions necessary until antigen and antibody testing show noninfectivity.
3. Note: For patients with chronic infection, therapy with interferon-α and lamivudine is recommended.
Prevention
Universal immunization of U.S. infants beginning at birth and catch-up immunization of children and adolescents are the current recommendations. Vaccination for at-risk adults is also advised. Travelers to highly endemic areas who stay for 6 or more months or have close contact with inhabitants should be vaccinated (see Chapter 49).
Hepatitis C
Signs and Symptoms
1. Acute disease is indistinguishable from hepatitis A or hepatitis B virus infections.
2. Most infections are asymptomatic with jaundice occurring in fewer than 20% of infected individuals.
3. Transition to chronic hepatitis after an insidious asymptomatic infection is the usual pattern.
4. Chronic hepatitis may be asymptomatic or associated with nonspecific symptoms, such as lethargy, nausea, and abdominal discomfort.
5. Extrahepatic syndromes associated with hepatitis C infection include porphyria cutanea tarda, membranous glomerulonephritis, and mixed cryoglobulinemia.
6. Definitive diagnosis requires antigen and antibody tests.
Prevention
1. Prevention of hepatitis C largely depends on risk reduction, especially with respect to IV drug use.
2. Pooled immunoglobulin has been used after exposure, but this should be procured from donors screened for hepatitis C. It is, however, not generally recommended.
3. Unlike hepatitis B, protective antibody responses have not been demonstrated.
Hepatitis D
1. Hepatitis D infection is found only in patients concomitantly or previously infected with hepatitis B.
2. Transmission follows a pattern similar to hepatitis B.
3. In the United States, affected populations are IV drug abusers and multiply transfused hemophiliacs.
4. Serologic evidence of hepatitis D disease has been documented in the Mediterranean basin, West Africa, and parts of South America.
5. The diagnosis should be suspected in previously well patients infected with hepatitis B who are now having recurrent symptoms.
6. The diagnosis is made based on serologic examination.
7. Management is supportive, and only interferon-α has proved antiviral activity against hepatitis D virus. There are no specific vaccines for hepatitis D virus.
Hepatitis E, F, and G
1. Hepatitis E is an RNA virus and is the second most common cause of viral hepatitis transmitted via the enteric route.
2. The epidemiologic characteristics are similar to hepatitis A. However, hepatitis E has animals (pigs and deer) as its reservoir.
3. This group of infections is especially important in the Indian subcontinent, the Middle East, and Africa.
4. The incubation period is 2 to 6 weeks.
5. The disease is usually self-limited but may be associated with severe illness in pregnant women.
6. Diagnosis in travelers from endemic areas can be made on the basis of IgM antibody to hepatitis E in serum or testing of stool for viral antigen.
7. Vaccines are not available. Prophylaxis is appropriate advice for travelers and involves counseling with respect to precautions regarding ingestion of food and water in endemic areas.
8. Hepatitis F is a putative hepatitis virus of uncertain significance, first described in France.
9. Hepatitis G is a member of the flavivirus family with limited homology to hepatitis C. Its significance as a cause of hepatitis is unclear.
Typhoid and Paratyphoid Fever
Signs and Symptoms
1. Onset of illness is usually 10 to 14 days after exposure to the pathogen.
2. Gastroenteritis is possible early in the course of the disease with associated abdominal pain and constipation. Diarrhea is more common in younger children.
a. Usually the first sign of disease
b. May be accompanied by bradycardia
c. Increases slowly over several days and remains constant for 2 to 3 weeks, after which defervescence begins
4. Headaches, malaise, anorexia
5. “Rose spots” (2- to 4-mm maculopapular blanching lesions) classically described on the trunk, although not seen in most patients
6. Hepatomegaly, splenomegaly in many patients
7. Uncomplicated, untreated typhoid fever usually resolves spontaneously in 3 to 4 weeks.
8. Life-threatening complications:
9. Definitive diagnosis possible by bacterial culture
10. Possible for patients to remain asymptomatic carriers and continue to shed organisms for years
Treatment
a. Ciprofloxacin 500 mg q12h for 2 weeks (or other quinolone)
b. In cases of quinolone resistance (either laboratory or clinical unresponsiveness), ceftriaxone or other third-generation cephalosporins are indicated.
c. In the absence of the previously mentioned antibiotics, use one of the following, but remember that resistance has been noted:
2. Make certain that the patient has adequate nutrition and food support.
3. High-dose steroids are not recommended for those with mild to moderate disease but may be used cautiously in those with severe disease. The current recommendation in severe disease is dexamethasone 3 mg/kg for the first dose, followed by 1 mg/kg every 6 hours for eight more doses.
4. Be aware that relapse can occur after 2 weeks of therapy and necessitates retreatment with the same regimen.
Cholera
Signs and Symptoms
1. Initial abdominal pain, cramping, nausea, and vomiting
2. Subsequent “rice water” diarrhea with “fishy” smell
3. Severe dehydration, dry mucous membranes, fast pulse rate, hypotension, sunken eyes, decreased skin turgor, decreased urine output
4. Decreased level of consciousness, lethargy, stupor
5. Muscle and abdominal cramping with electrolyte deficiency
Treatment
1. Aggressive oral, IV, or intraosseous (IO) fluid replacement in two phases: (1) rapid rehydration of existing fluid deficits over 2 to 4 hours, and (2) maintenance hydration for duration of illness.
2. Severe dehydration is typically 10% to 15% of patient’s body weight; 30% of the estimated fluid losses should be replaced in the first 30 minutes in adults, and first hour in children, following fluid initiation.
3. After rehydration has been achieved, measure fluid losses over a 4-hour period and replace on top of maintenance fluids over the next 4 hours. Oral hydration should be attempted first, with IV or IO hydration reserved for significant ongoing fluid losses and inadequate oral hydration alone.
4. Oral hydration can be achieved with homemade or commercially available oral rehydration solution (ORS) (see Chapter 44) or parenterally with lactated Ringer’s solution with 20 mEq of KCl added to every 1 L of solution. Alternatively, normal saline with 20 mEq of KCl added to every 1 L of solution can be used if lactated Ringer’s solution is not available.
5. If IV or IO access is unobtainable and the patient is not taking adequate volume of ORS, a nasogastric tube can be placed with administration of ORS at the same rate. Be sure to elevate the head of the bed 30 degrees to prevent aspiration.
6. Initiate antibiotic therapy with tetracycline 500 mg PO q6h in adults and 50 mg/kg/day PO divided q6h in children older than 8 years for 3 to 5 days. Alternatively, use doxycycline 300 mg PO once daily in adults and 4 to 6 mg/kg PO once daily in children older than 8 years for 3 to 5 days. In pregnant women, children younger than 8 years, or in tetracycline/doxycycline-resistant areas, use azithromycin 1 g as a single dose in adults or 20 mg/kg as a single dose in children, or ciprofloxacin 500 mg PO once daily for 1 to 3 days in adults or 20 mg/kg PO once as a single dose in children.
7. Transport or evacuate the patient as soon as possible to definitive medical care while continuing rehydration and antibiotic therapy.
Japanese B Encephalitis
1. Japanese encephalitis is a viral infection transmitted by Culex mosquitoes. Transmission takes place year-round in tropical and subtropical areas and during the late spring, summer, and early fall in temperate climates. This disease occurs primarily in rural areas, often associated with pig farming.
2. Encephalitis is caused by a neurotropic flavivirus.
3. After initial replication near the mosquito bite, viremia occurs and may seed infection to the brain.
4. The virus causes central nervous system nerve cell destruction and necrosis.
5. Most infections in endemic areas involve children, but this disease may occur in any age-group.
6. Japanese encephalitis virus is the leading cause of viral encephalitis in Asia, with recent expansion into northern Australia.
7. There have been rare reported outbreaks in U.S. territories and in the western Pacific.
Signs and Symptoms
1. No clinical illness in most infections
2. Encephalitis (about 1 in 300 infections)
3. Mild, undifferentiated febrile illness (encephalitis patients often with a similar prodrome)
4. Headache, lethargy, fever, confusion, abdominal pain, nausea, and vomiting; possible tremors or seizures
5. Approximately 30% reported mortality rate with clinical encephalitis; 50% of survivors have severe neurologic sequelae
6. Encephalitis syndrome not easily distinguished from other arboviral encephalitis
Treatment
1. No specific therapy exists for this disease.
2. Provide supportive care and hydration.
3. Transport severe cases to a medical facility with an ICU.
4. Practice blood and body fluid precautions.
5. Therapies such as corticosteroids, interferon-α, and ribavirin have all been studied in randomized, double-blind, placebo-controlled trials and do not seem to benefit patients with JE.
Prevention
1. The main interventions are prophylactic: vaccination and reduced arthropod exposure.
2. Vaccination is recommended for persons older than 9 months of age traveling to endemic areas during the transmission season who will be staying for longer than 1 month, and if travel includes rural areas.
3. See discussion of Japanese encephalitis vaccine in Chapter 49.
Meningococcal Disease
Signs and Symptoms
1. Variety of forms, including but not limited to the following:
2. Sustained meningococcemia may lead to severe toxemia with hypotension, fever, and disseminated intravascular coagulation.
3. Meningitis caused by N. meningitidis is marked by the classic triad of fever, headache, and stiff neck and possibly accompanied by bacteremia and any of several skin manifestations including petechiae, pustules, or maculopapular rash.
4. Severe meningitis may progress to mental status deterioration, hypotension, congestive heart failure, disseminated intravascular coagulation, and death.
5. During an epidemic a presumptive diagnosis can be made on the basis of clinical presentation.
6. Definitive diagnosis requires culture of the organism from cerebrospinal fluid or blood.
7. Several commercial kits for measuring meningococcal antigen are now available for use on cerebral spinal fluid or blood samples.
Treatment
1. Be aware that meningococcal meningitis, or sepsis, is a medical emergency, with suspected patients requiring immediate evacuation to an appropriate medical facility.
2. Note that, fortunately, the organism remains sensitive to many antibiotics, such as the following:
a. Penicillin G, 300,000 units/kg/day (up to 24 million units/day) IV in divided doses q2h for 7 to 10 days for serious disease
c. Chloramphenicol, 100 mg/kg/day divided q6h IV (note that there has been emergence of chloramphenicol-resistant strains of N. meningitidis)
3. Give supportive care, including close monitoring for hypotension and cardiac failure (will necessitate ICU-level care) and IV fluid support.
4. Dexamethasone may be of value for patients in a coma or with evidence of increased intracranial pressure.
5. Make sure that close contacts receive prophylaxis to eradicate the organism (ciprofloxacin 500 mg PO as a single dose, or rifampin 600 mg PO q12h for four doses).
Travel Medicine Information Resources
Official References
• Centers for Disease Control and Prevention: Health Information for International Travel, 2012, Washington, DC, USA: http://wwwnc.cdc.gov/travel/page/yellowbook-2012-home.htm
• Centers for Disease Control and Prevention travel immunization information: http://wwwnc.cdc.gov/travel/page/vaccinations.htm
• Centers for Disease Control and Prevention destination-specific health information: http://wwwnc.cdc.gov/travel/destinations/list.htm