Traumatic spinal cord injury
MYRTICE B. ATRICE, PT, BS, SARAH A. MORRISON, PT, BS, SHARI L. McDOWELL, PT, BS, PAULA M. ACKERMAN, MS, OTR/L, TERESA A. FOY, OT, BS and CANDY TEFERTILLER, DPT, ATP, NCS
After reading this chapter the student or therapist will be able to:
1. Describe the demographics, etiology, and mechanism of injury of spinal cord injury.
2. Discuss the acute medical management of person with spinal cord injury.
3. Describe the secondary complications of spinal cord injury, the appropriate interventions, and the impact of complications on the rehabilitation process.
4. Identify the basic components of the examination process.
5. Identify patient problems based on the examination, establish appropriate goals, and plan individualized treatment programs for patients with a spinal cord injury.
6. Describe adaptive equipment available to increase function.
7. Discuss progression of each individual and the process of discharge planning throughout the rehabilitation process.
8. Describe functional expectations for individuals with complete spinal cord injuries.
9. Identify equipment needs for a given spinal cord injury lesion.
10. Describe various aspects of activity-based therapies to promote recovery after spinal cord injury.
Spinal cord injury (SCI) is a catastrophic condition that, depending on its severity, may cause dramatic changes in a person’s life. SCI usually happens to active, independent people who at one moment are in control of their lives and in the next moment are paralyzed, with loss of sensation and loss of bodily functions, which can lead to dependence on others for even the most basic needs. To reduce negative impact, individuals with SCI need a well-coordinated, specialized rehabilitation program to assist them in maximizing the development of skills necessary to live a satisfying and productive postinjury life.1,2
A successful rehabilitation program requires a team of health care professionals who work in unison to address alterations in body function, increase the individual’s independence in all daily activities, and return the individual to the highest level of community participation specific to that individual’s life situations. Minimally, the team should include a physician, case manager, occupational therapist, physical therapist, therapeutic recreation specialist, prosthetist or orthotist, nurse, speech-language pathologist, dietician, assistive technologist, respiratory care practitioner, psychologist, social worker, vocational counselor, rehabilitation engineer, and chaplain.3–5 The most important element determining success in any rehabilitation program is the patient’s and family’s active participation throughout the rehabilitation process.
Spinal cord lesions
Tetraplegia
Tetraplegia (preferred to quadriplegia) refers to impairment or loss of motor and/or sensory function as a result of damage to the cervical segments of the spinal cord. Function in the upper extremities, lower extremities, and trunk is affected. It does not include brachial plexus lesions or injury to peripheral nerves outside the neural canal.6
Paraplegia
Paraplegia refers to impairment or loss of motor or sensory function as a result of damage to the thoracic, lumbar, or sacral segments of the spinal cord. Depending on the level of the damage, function may be impaired in the trunk and/or lower extremities. This term is used to refer to cauda equina and conus medullaris injuries but not to lumbosacral plexus lesions or injury to peripheral nerves, which are considered outside of the central nervous system.6
Complete, discomplete, and incomplete lesions
In a complete lesion, sensory and motor function in the lowest sacral segments (S4-S5) is absent postinjury.6 The American Spinal Injury Association (ASIA) classification for this type of injury is ASIA Impairment Scale (AIS) A. Complete injuries to the spinal cord are usually the result of extensive trauma or disease and are often segmentally associated with damage to the nerve roots in the intervertebral foramina.7 Function of the roots originating from the more cranial portion of the intact cord can be expected to return within 6 months.7
Discomplete injury is a relatively new term in SCI research and practice. It is defined as a lesion that is “clinically complete but which is accompanied by neurophysiological evidence of residual brain influence on spinal cord function below the level of the lesion.”8 Studies of persons whose spinal cord injuries were considered complete under ASIA standards have shown that in a large percentage (84%) there was residual brain influence on the spinal cord below the level of the lesion.8,9 The current gold standard for testing, the AIS, is unable to detect this residual function, which suggests that AIS testing may be providing an inaccurate picture of the patients’ neurological plasticity and recovery potential. The Brain Motor Control Assessment (BMCA) is emerging as a desirable adjunct to the standard ASIA testing.9 In the BMCA, surface electromyography (EMG) is used to quantify the motor unit activity of the lower extremities in response to a standard testing protocol including active and passive movement of the lower extremities, reinforcement maneuvers (e.g., Jendrassik or Valsalva) performed above the level of injury, tendon taps and vibration, and elicitation and suppression of reflex activity. The motor unit responses are quantified and compared with normative data to establish a voluntary response index and a similarity index. In other words, the results of the BMCA help to determine how different the subjects’ motor responses are from those of persons with intact neurological systems.8–11 This testing requires specialty equipment but can easily be administered by physical therapists once they have received the appropriate training.
With incomplete lesions there is detectable residual sensory or motor function below the neurological level and specifically in the lowest sacral segment. According to ASIA standards, any sensation in the anal mucocutaneous junction, or deep anal sensation, indicates that the lesion is incomplete. If only sensation is preserved, the injury is classified as AIS B. If motor function in key muscles is maintained to some degree, patients may achieve level C, D, or E classification. This testing will be reviewed further in this chapter.6,12
Demographics
The incidence of traumatic SCI in the United States is approximately 12,000 new cases per year.13 Approximately 3000 new cases of spinal cord impairment resulting from disease and congenital anomalies occur each year. The number of people living in the United States today with SCI is between 231,000 and 311,000.13 Fifty-three percent of traumatic SCIs occur in persons aged 16 to 30 years. However, the median age of the general population of the United States has increased by 8 years since the mid 1970s, and the average age of the SCI population has steadily increased. Since 2005, the mean age at the time of injury is 40.2 years.13,14 Persons older than 60 years of age at injury have increased from 4.7% before 1980 to 11.5% for injuries occurring since 2000. This trend explains the increase in the median age during this same time period from 27.9 years to 35.3 years. Table 16-1 lists additional demographics.
TABLE 16-1 
SPINAL CORD INJURY DEMOGRAPHICS
| Mean age at injury | 40.2 years |
| Most common age at injury | 19.0 years |
| SEX | |
| Male | 80.9% |
| Female | 19.1% |
| CAUSES OF INJURY | |
| Motor vehicle accident | 41.3% |
| Falls | 27.3% |
| Violent acts | 15.0% |
| Sports injuries | 7.9% |
| Other | 8.5% |
| NEUROLOGICAL CATEGORIES AT DISCHARGE | |
| Incomplete tetraplegia | 38.3% |
| Complete paraplegia | 22.9% |
| Incomplete paraplegia | 21.5% |
| Complete tetraplegia | 16.9% |
| No deficits | 0.7% |
| COMMON INJURY SITES64 | |
| C5 | 14.9% |
| C4 | 13.6% |
| C6 | 10.8% |
| T12 | 6.7% |
| C7 | 5.3% |
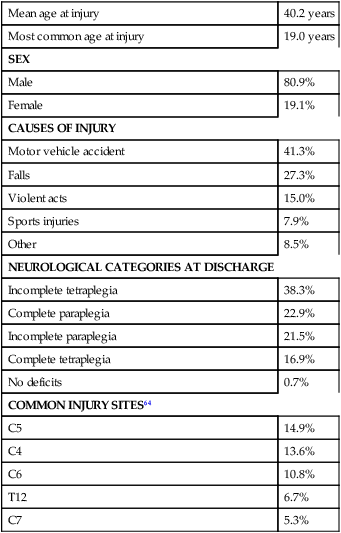
Data from National Spinal Cord Injury Statistical Center: Spinal cord injury: facts and figures at a glance, February 2010, Birmingham, AL, 2010, University of Alabama, National Spinal Cord Injury Statistical Center. Available at www.uab.edu/NSCICSC.
In 2005 the average length of inpatient stay was 50 days (12 days in an acute-care facility and 38 days in rehabilitation). The average yearly health care and living expenses vary according to severity of injury. In the first year, individuals with high tetraplegia spend $829,843, whereas individuals with paraplegia spend an average of $303,220.13 Today 87.7% of persons with SCI are discharged to a noninstitutional residence. Life expectancies for patients with SCI continue to increase but are still below the national average of persons without SCI. Mortality rates are significantly higher during the first year after injury, especially for severely injured persons. According to the National SCI Database, the leading causes of death after an SCI are pneumonia, pulmonary emboli, and septicemia.13
Statistics suggest a high incidence of multiple trauma associated with a traumatic SCI (55.2%).15 The most common injuries are fractures (29.3%) and loss of consciousness (28.2%).15 Traumatic pneumothorax or hemothorax are reported in 17.8% of persons with SCI. Traumatic head injuries of sufficient severity to affect cognitive or emotional functioning are reported in 11.5% of all cases.15 Skull and facial fractures, along with traumatic head injuries and vertebral artery and esophageal disruptions, are common in cervical injuries.16 Limb fractures and intrathoracic injuries (rib fractures and hemopneumothorax) are frequent in thoracic injuries, whereas intraabdominal injuries to the liver, spleen, and kidneys are associated with lumbar and cauda equina injuries.16
Sequelae of traumatic spinal cord injury
As stated previously, most spinal cord injuries occur as a result of trauma, be it motor vehicle accidents, falls, violence, or sports-related injury. The degree and type of forces that are exerted on the spine at the time of the trauma determine the location and severity of damage to the spinal cord.17 Injuries to the vertebral column can be classified biomechanically as flexion or flexion-rotation injuries, hyperextension injuries, and compression injuries.18 Penetrating injuries to the cord are usually the result of gunshot or knife wounds.18
Spinal cord damage can also be caused by nontraumatic mechanisms. Circulatory compromise to the spinal cord resulting in ischemia causes neurological damage at and below the involved cord level. This can be caused by a thrombus, swelling, compression, or vascular malformations and dysfunction. Degenerative bone diseases can cause compression of the spinal cord by creating a stenosis of the spinal canal and intervertebral foramina. Stenosis can also result from the prolapse of the intervertebral disc into the neural canal. The encroachment of tumors or abscesses within the spinal cord, the spinal canal, or the surrounding tissues can also lead to SCI. Congenital malformation of the spinal structures, as in spina bifida, can also compromise the spinal cord and its protective layers of connective tissue. Some of the more common diseases and conditions that result in compromise of the spinal cord include Guillain-Barré syndrome, transverse myelitis, amyotrophic lateral sclerosis, and multiple sclerosis.12
After the spinal cord has sustained damage, cellular events occur in response to the injury and are classified in three phases of progression: acute, secondary, and chronic responses. The acute process begins on occurrence of an injury and continues for 3 to 5 days.19 Abrupt necrosis or cell death can result from both mechanical and ischemic events. The impact of an SCI often causes direct mechanical damage to neural and other soft tissues as well as severe hemorrhaging in the surrounding gray and white matter, resulting in immediate cell death.20,21 In the next few minutes after the insult, injured nerve cells respond with trauma-induced action potentials, which lead to increased levels of intracellular sodium. The result of this influx is an increase in osmotic pressure movement of water into the area. Edema generally develops in up to three levels above and below the original insult and leads to further tissue deconstruction.19,21,22 Increased levels of extracellular potassium and intracellular concentrations of calcium also result in an electrolyte imbalance that contributes to a toxic environment.23–25 Abnormal concentrations of calcium within the damaged cells disrupt their functioning and cause breakdown of protein and phospholipids, leading to demyelination and destruction of the cell membrane.25 The cascade of these events consequentially contributes to a dysfunctional nervous system.
During this acute phase, evidence of spinal shock may be present. Spinal shock occurs 30 to 60 minutes after spinal trauma and is characterized by flaccid paralysis and absence of all spinal cord reflex activity below the level of the spinal cord lesion.26,27 This condition lasts for about 24 hours after injury, represents a generalized failure of circuitry in the spinal neural network, and is thought to be directly related to a conduction block resulting from leakage of potassium into the extracellular matrix.28 The completeness of the lesion cannot be determined until spinal shock is resolved. The signs of spinal shock resolution are controversial; however, the return of reflexes may be a good indication.
The secondary phase of the injury occurs within the course of minutes to weeks after the acute process and is characterized by the continuation of ischemic cellular death, electrolytic shifts, and edema. Extracellular concentrations of glutamate and other excitatory amino acids reach concentrations that are six to eight times greater than normal within the first 15 minutes after an injury.24 In addition, lipid peroxidation and free radical production also occur.29 Apoptosis (a secondary programmable cell death) occurs and involves reactive gliosis. There is also an important immune response that adds to the secondary damage that may be a result of a damaged blood-brain barrier, microglial activation, and increased local concentrations of cytokines and chemokines.30 The lesion enlarges from the initial core of cell death, expanding from the perilesional region to a larger region of cell loss.
In the chronic phase, which occurs over a period of days to years, apoptosis continues both rostrally and caudally. Receptors and ion channels are altered, and with penetrating injuries scarring and tethering of the cord occurs. Conduction deficits persist owing to demyelination, and permanent hyperexcitability develops with consequential chronic pain syndromes and spasticity in many SCI patients.26 Changes in neural circuits result from alterations in excitatory and inhibitory inputs, and axons may exhibit regenerative and sprouting responses but go no farther than 1 mm.24
Medical interventions are evolving to limit the impact of the acute SCI and the subsequent progression that follows. Growing interest in protection and repair of the injured nervous system has led to an improved understanding of the pathophysiology associated with SCI and has resulted in the implementation of several therapeutic strategies that are currently being investigated in phase 1 and 2 clinical trials. The effects of methylprednisolone sodium succinate, tirilizad mesylate, monosialotetrahexosylganglioside, thyrotropin-releasing hormone, gacyclidine, naloxone, and nimodipine have all been examined in randomized controlled trials over the last few years. Although the primary outcomes in these studies did not demonstrate statistically significant effects, a secondary analysis demonstrated that methylprednisolone sodium succinate given within 8 hours of injury was associated with modest clinical benefits.17 Phase 2 trials with monosialotetrahexosylganglioside and thyrotropin-releasing hormone also yielded some therapeutic benefits, but further studies need to be completed to determine efficacy. Several current or planned studies exist to evaluate the potential benefits of early surgical decompression and electrical field stimulation, neuroprotective strategies such as riluzole and minocycline, the inactivation of myelin inhibition by blocking Nogo and Rho, and the transplantation of various substrates into the injured spinal cord.17 Promising clinical trials are also underway to minimize the secondary phase of injury and to promote healing and neuronal regeneration (Table 16-2). If medical interventions can bridge the central lesion or limit the secondary progression, the functional loss that follows SCI will be minimized and chances of recovery improved.
TABLE 16-2 
| PHASE | DESCRIPTION |
| Acute | Systemic hypotension and spinal shockHemorrhage Cell death from direct insult or ischemia Edema Vasospasm Shifts in electrolytes Accumulation of neurotransmitters Induced hypothermic treatment |
| Secondary | Continued cell deathContinued edema Continued shifts in electrolytes Free-radical production Lipid peroxidation Neutrophil and lymphocyte invasion and release of cytokines Apoptosis Calcium entry into cells |
| Chronic | Continued apoptosis radiating from site of injuryAlteration of ion channels and receptors Formation of fluid-filled cavity Scarring of spinal cord by glial cells Demyelination Regenerative processes, including sprouting by neurons Altered neurocircuits Syringomyelia |
Data from Hulsebosch CE: Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ 26:238–255, 2002; and Sekhon LH, Fehlings MG: Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 26(24 suppl):S2–S12, 2001.
Clinical syndromes
Some incomplete lesions have a distinct clinical picture with specific signs and symptoms. An understanding of the various syndromes can be helpful to the patient’s team in planning the rehabilitation program. Figure 16-1 depicts the anatomy of the spinal cord.30,31 This basic anatomy of the spinal cord can be referred to as the various syndromes are described.
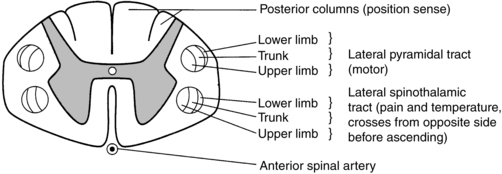
 Cross-sectional anatomy of the spinal cord.
Cross-sectional anatomy of the spinal cord.Central cord syndrome
Hyperextension injuries usually result in a central cord syndrome.31 This injury causes bleeding into the central gray matter of the spinal cord, resulting in more impairment of function in the upper extremities than in the lower extremities.31 Most incomplete lesions result in this syndrome, especially in elderly individuals when cervical stenosis is present.30 Although the prognosis for functional recovery is good for individuals with central cord syndrome, the pattern of recovery is such that intrinsic hand function is the last thing to return. Approximately 77% of clients with central cord syndrome will attain some level of ambulatory function, 53% bowel and bladder control, and 42% hand function.12,32,33
Anterior spinal artery syndrome
Anterior spinal artery syndrome is usually caused by flexion injuries in which bone or cartilage spicules compromise the anterior spinal artery.31 Motor function and pain and temperature sensation are lost bilaterally below the injured segment.31 The prognosis is extremely poor for return of bowel and bladder function, hand function, and ambulation.12,33
Brown-séquard syndrome
Occasionally, as a result of penetrating injuries (gunshot or stab wounds), only one half of the spinal cord is damaged. The Brown-Séquard syndrome is characterized by ipsilateral loss of motor function and position sense and contralateral loss of pain sensation several levels below the lesion.31 The prognosis for recovery is good. Nearly all clients attain some level of ambulatory function, 80% regain hand function, 100% have bladder control, and 80% have bowel control.12,33
Medical management
Surgical stabilization
One of the first interventions after acute traumatic SCI is to stabilize the spine to prevent further cord or nerve root damage. In the emergency department, diagnostic studies reveal the severity of the spinal injury and the type and degree of the instability. On the basis of these findings, the physician, client, and family decide on treatment. Many options must be considered regarding the optimal operative strategy. Indications for surgical intervention include, but are not limited to, signs of progressive neurological involvement, type and extent of bony lesions, and degree of spinal cord damage.34 The following discussion describes nonsurgical and surgical interventions.
Cervical spine
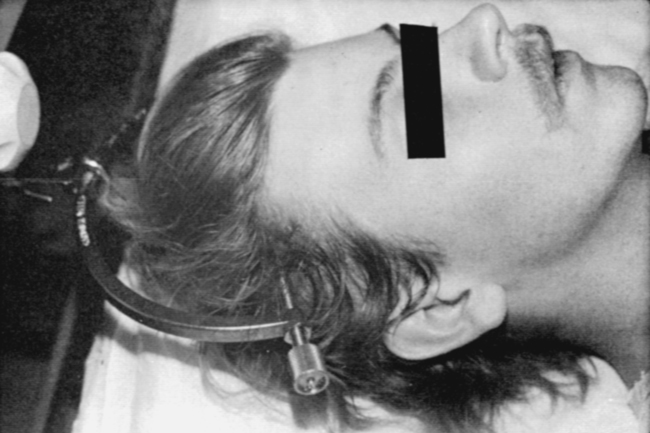
At the scene of the accident, emergency medical professionals exercise extreme caution to immobilize the injured patient and prevent excessive movement. If there is compression of neurological tissue, vertebral fracture, or dislocation, reduction must occur to minimize ischemia and edema formation.35 In the emergency department, reduction is accomplished by cervical traction with the goal of immediate and proper alignment of bone fragments and decompression of the spinal cord until further stabilization.34,36,37 The most widely used traction method is the Gardner-Wells tongs (Figure 16-2), which are inserted into the skull. Weights are added at approximately 5 pounds of traction per level of injury to achieve reduction of the dislocation and to maintain alignment.36
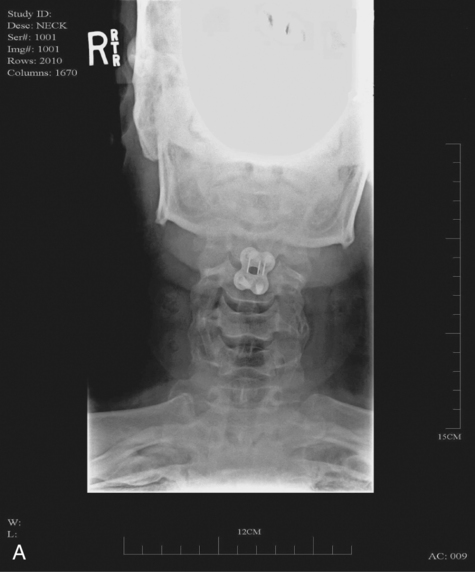
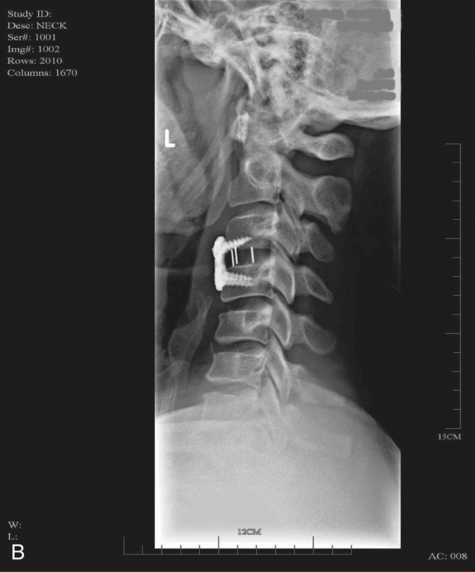
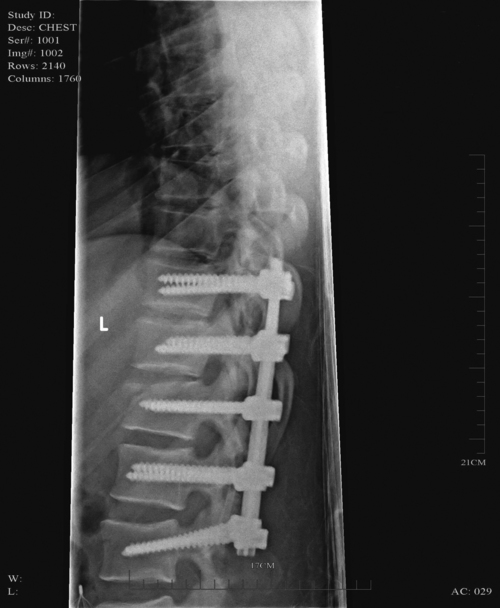
When surgical stabilization is indicated, common surgical protocols include posterior and anterior approaches. Figure 16-3 shows radiographs of a person who had an anterior and lateral cervical fusion at C3-C4. Unstable compression injuries are usually managed by a posterior procedure except when there is a deficient anterior column. Anterior approaches are indicated for patients with evidence of residual anterior spinal cord or nerve root compression and persistent neurological deficits.34
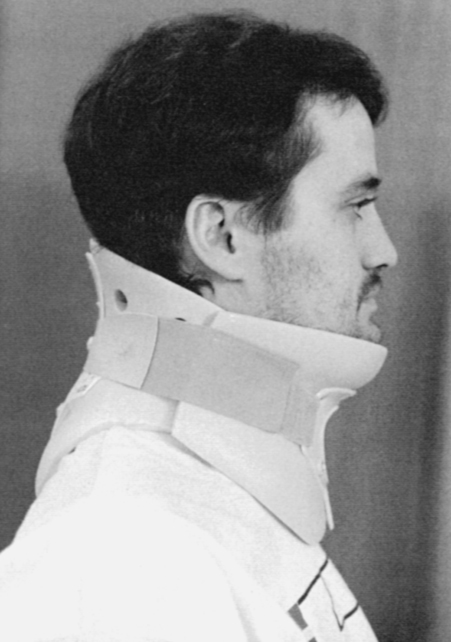
After cervical surgical stabilization, a hard collar such as a Philadelphia collar (Figure 16-4) or sternal-occipital-mandibular immobilizer (SOMI) brace is used until solid bony fusion has developed. The Aspen collar also provides this stability (Figure 16-5). The solid bony fusion usually takes 6 to 8 weeks. Postoperatively, care must be taken to protect the bony fusion.
When surgery is not indicated, or when more postoperative stabilization is required, halo traction may be indicated. The halo device restricts more movement in the upper cervical spine compared with the lower cervical spine.38 The halo traction device consists of three parts: the ring, the uprights, and the jacket (Figure 16-6). The ring fits around the skull, just above the ears. It is held in place by four pins that are inserted into the skull. The uprights are attached to the ring and jacket by bolts. The jacket is usually made of polypropylene and lined with sheepskin. This equipment is left in place for 6 to 12 weeks until bony healing is satisfactory.5 The advantage of using the halo device is the ability to mobilize the client as soon as the device has been applied without compromising spinal alignment. This allows the rehabilitation program to commence more rapidly. It also allows for delayed decision making regarding the need for surgery.
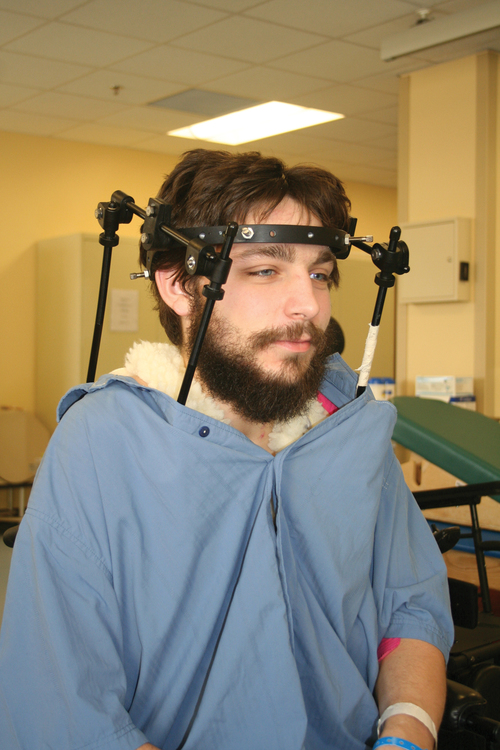
 Halo vest. Basic components are the halo ring, distraction rods, and jacket (jacket not pictured).
Halo vest. Basic components are the halo ring, distraction rods, and jacket (jacket not pictured).The disadvantage of the halo device is that pressure and friction from the vest or jacket may lead to altered skin integrity.7 Special attention must be given to ensure the skin remains intact. During more active phases of the rehabilitation process, the halo device may slow functional progress because of added weight and interference with the middle to end range of upper-extremity movement. In a small percentage of patients, there are complications of dysphagia and temporomandibular joint dysfunctions associated with wearing the halo device.7
Thoracolumbar spine
Internal fixation of the thoracolumbar region is necessary when stability and distraction cannot be maintained by other means.39 Common thoracic stabilization procedures include transpedicular screws (Figure 16-7) and a hybrid type of instrumentation.
Postoperatively, an external trunk support may be necessary to limit excessive vertebral motion and to maintain proper thoracic and lumbar alignment.39 This may be achieved by a custom thoracolumbosacral orthosis (Figure 16-8) or a Jewett brace (Figure 16-9). Initially the client’s activity may be limited to allow for a complete fusion to take place and to minimize the possibility of rod displacement. All spinal limitations should be discussed with the surgeon postoperatively.
The goals of the operative procedures at any spinal level discussed are to reverse the deforming forces, to restore proper spinal alignment, and to stabilize the spine.40 All these procedures have advantages and disadvantages. The surgeon, client, and family must be involved in the decision-making process to select the most appropriate method of treatment. This will allow the therapeutic rehabilitation process to begin.
Pharmacological management immediately after traumatic spinal cord injury
The National Acute Spinal Cord Injury Study41 (NASCIS-2) used high doses of methylprednisolone and showed significant improvements in sensory and motor function 6 months after injury.42 Young and Flamm43 showed that methylprednisolone enhanced the flow of blood to the injured spinal cord, preventing the typical decline in white matter, extracellular calcium levels, and evoked potentials, thus preventing progressive posttraumatic ischemia.44–47 The dosage recommended by the NASCIS-2 study is 30 mg/kg of methylprednisolone followed by an infusion of 5.4 mg/kg/hr for 23 hours.41 The therapist must be aware of side effects that may occur with such high doses of steroids, including gastric ulcers, decreased wound-healing time, hypertension, cardiac arrhythmias, and alteration in mental status.42
Therapeutic rehabilitation continuum of care
Therapeutic rehabilitation can be effectively delivered beginning in an acute-care setting at the time of injury and continuing on through a lifetime of care. Rehabilitation teams may use one of three models: multidisciplinary, interdisciplinary, and transdisciplinary.3 The standards set forth by the Commission on Accreditation of Rehabilitation Facilities (CARF) suggest that the interdisciplinary model of team structure is optimal in the rehabilitation setting.31
Inpatient rehabilitation
As the acute phase progresses, out-of-bed activities are tolerated for longer periods of time and the patient begins to work toward specific long-term goals. In accordance with Medicare guidelines for inpatient rehabilitation, the client is able to participate in therapeutic programs a minimum of 3 hours a day.48,49 The intensity of therapy may continue to be limited according to unresolved medical issues.
Outpatient rehabilitation and community reentry
Discharge from an inpatient rehabilitation program marks only the beginning of the lifelong process of adjustment to changes in physical abilities, community reintegration, and participation in life activities. Inpatient rehabilitation provides an environment best suited for learning self-care skills, yet “the implications of living in the community with SCI can scarcely be anticipated accurately by the newly injured individual or the able-bodied staff.”50 Because of the shortened lengths of hospitalization, services provided after discharge are becoming increasingly important. A direct consequence of this shift results in outpatient treatment of patients who have more acuity, greater care needs, and fewer skills attained in the inpatient rehabilitation program before entry into the outpatient arena. Common outpatient therapy treatment programs have included advanced transfer training, advanced wheelchair mobility training, locomotor training, upgraded ADL training, and upgraded home exercise program instruction. This is a shift in the typical program structure because these skills were traditionally a part of the inpatient rehabilitation.
Examination and evaluation of body function and structure
Regardless of where the patient begins the rehabilitation process, an examination is completed on admission. The examination and evaluation will assist in establishing the diagnosis and the prognosis of each patient as well as determining the appropriate therapeutic interventions. The client and caregivers participate by reporting activity performance and functional ability.51 Any pertinent additions to the history stated by the client should be described. The client’s statement of goals, problems, and concerns should be included. The main areas of the examination are outlined here.
History
A review of the medical record is the first step toward the examination because it provides the background information and identifies medical precautions. The history should include general demographics, social history, occupation or employment, pertinent growth and development, living environment, history of current condition, functional status and activity level, completed tests and measures, medications, history of current condition if applicable, medical and surgical history, family history, reported patient and family health status, and social habits.52 If the history suggests a loss of consciousness or brain injury, the clinician should consider the possibility of compromised cognition and should include tests and measures during the examination and assessment appropriate to that impairment.
Tests and measures
Depending on the data generated during the history and systems review, the clinician performs tests and measures to help identify impairments, activity limitations, and participation restrictions and to establish the diagnosis and prognosis of each client. Tests and measures that are often used for persons with SCI are included in Box 16-1. For more detail related to specific tools, refer to the Guide to Physical Therapist Practice.52
Neurological examination
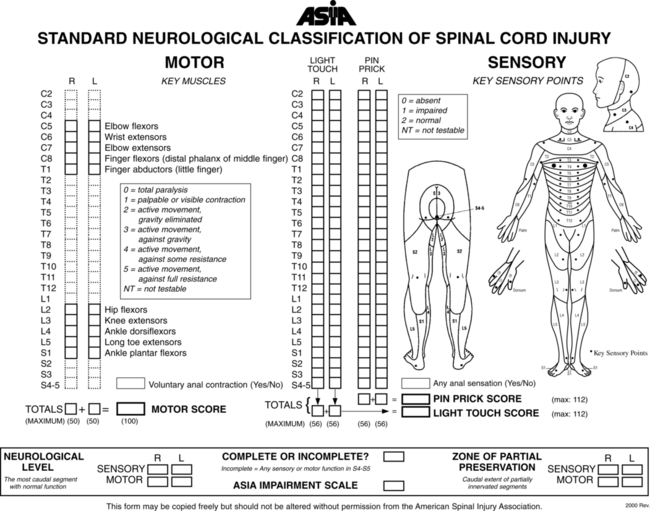
American spinal cord injury association examination
It is recommended that the international standards of ASIA be used for the specific neurological examination after an SCI.53 See Figure 16-10 for the ASIA motor and sensory examination form. Assessment of muscle performance allows for specific diagnosis of the level and completeness of injury. The examination of muscle performance includes each specific muscle and identifies substitutions from other muscles.
Along with the strength of each muscle, the presence, absence, and location of muscle tone should be described. The Modified Ashworth Scale is a common tool used to describe hypertonicity.54 The client’s sensation is described by dermatome. The recommended tests include (1) sharp-dull discrimination or temperature sensitivity to test the lateral spinothalamic tract, (2) light touch to test the anterior spinothalamic tract, and (3) proprioception or vibration to test the posterior columns of the spinal cord. Sensation is indicated as intact, impaired, or absent per dermatome. A dermatomal map is helpful and recommended for ease of documentation.
Functional examination
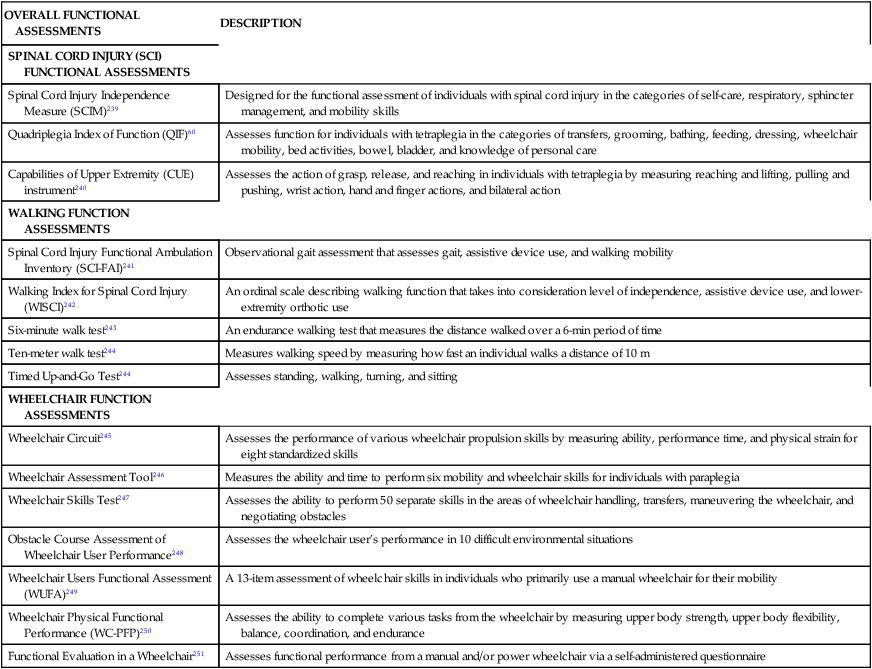
It is recommended that a complete functional assessment be performed on initial examination and thereafter as appropriate. Myriad tools exist to assess functional skills. Many institutions develop functional assessments that address home, community, and institutional mobility and ADL functional skills. The Functional Independence Measure (FIM) is one of the more commonly used tools that is currently applied for many impairment diagnostic groups, including SCI.55 Another tool that is recognized as a primary outcome measure to assess functional recovery for the client with SCI is the Spinal Cord Injury Independence Measure III (SCIM III).56 This tool was specifically designed for the functional assessment of individuals with SCI. The SCIM III has been shown to be valid, reliable, and easily administered.57–59 Other tools, such as the Quadriplegia Index of Function (QIF)60 and the Craig Handicap Assessment and Reporting Technique (CHART),61 are options. Additional assessments for patients with SCI are described in Table 16-3.
TABLE 16-3 
ASSESSMENT OF FUNCTION SUMMARY TABLE
| OVERALL FUNCTIONAL ASSESSMENTS | DESCRIPTION |
| SPINAL CORD INJURY (SCI) FUNCTIONAL ASSESSMENTS | |
| Spinal Cord Injury Independence Measure (SCIM)239 | Designed for the functional assessment of individuals with spinal cord injury in the categories of self-care, respiratory, sphincter management, and mobility skills |
| Quadriplegia Index of Function (QIF)60 | Assesses function for individuals with tetraplegia in the categories of transfers, grooming, bathing, feeding, dressing, wheelchair mobility, bed activities, bowel, bladder, and knowledge of personal care |
| Capabilities of Upper Extremity (CUE) instrument240 | Assesses the action of grasp, release, and reaching in individuals with tetraplegia by measuring reaching and lifting, pulling and pushing, wrist action, hand and finger actions, and bilateral action |
| WALKING FUNCTION ASSESSMENTS | |
| Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI)241 | Observational gait assessment that assesses gait, assistive device use, and walking mobility |
| Walking Index for Spinal Cord Injury (WISCI)242 | An ordinal scale describing walking function that takes into consideration level of independence, assistive device use, and lower-extremity orthotic use |
| Six-minute walk test243 | An endurance walking test that measures the distance walked over a 6-min period of time |
| Ten-meter walk test244 | Measures walking speed by measuring how fast an individual walks a distance of 10 m |
| Timed Up-and-Go Test244 | Assesses standing, walking, turning, and sitting |
| WHEELCHAIR FUNCTION ASSESSMENTS | |
| Wheelchair Circuit245 | Assesses the performance of various wheelchair propulsion skills by measuring ability, performance time, and physical strain for eight standardized skills |
| Wheelchair Assessment Tool246 | Measures the ability and time to perform six mobility and wheelchair skills for individuals with paraplegia |
| Wheelchair Skills Test247 | Assesses the ability to perform 50 separate skills in the areas of wheelchair handling, transfers, maneuvering the wheelchair, and negotiating obstacles |
| Obstacle Course Assessment of Wheelchair User Performance248 | Assesses the wheelchair user’s performance in 10 difficult environmental situations |
| Wheelchair Users Functional Assessment (WUFA)249 | A 13-item assessment of wheelchair skills in individuals who primarily use a manual wheelchair for their mobility |
| Wheelchair Physical Functional Performance (WC-PFP)250 | Assesses the ability to complete various tasks from the wheelchair by measuring upper body strength, upper body flexibility, balance, coordination, and endurance |
| Functional Evaluation in a Wheelchair251 | Assesses functional performance from a manual and/or power wheelchair via a self-administered questionnaire |
Goal setting for activity and participation skills
Goal setting is a dynamic process that directly follows the examination. Each activity limitation identified should be addressed with specific short- and long-term goals. The clinician must interpret new information continuously, which leads to continuing reevaluation and revision of goals.62 Goals are always individualized and should be established in collaboration with the treatment team, the client, and the caregiver, and with realistic consideration of anticipated needs on return to the home environment. Factors to consider in the goal-setting process include age, body type, associated injuries, premorbid medical conditions, additional orthopedic injury, cognitive ability, psychosocial issues, spasticity, endurance, strength, ROM, funding sources, and motivation.
Long-term goals for the rehabilitation of patients with SCI reflect functional outcomes and are based on the strength of the remaining innervated or partially innervated musculature. Short-term goals identify components that interfere with functional ability and are designed to “address these limiting factors while building component skills”7 of the desired long-term goals.63
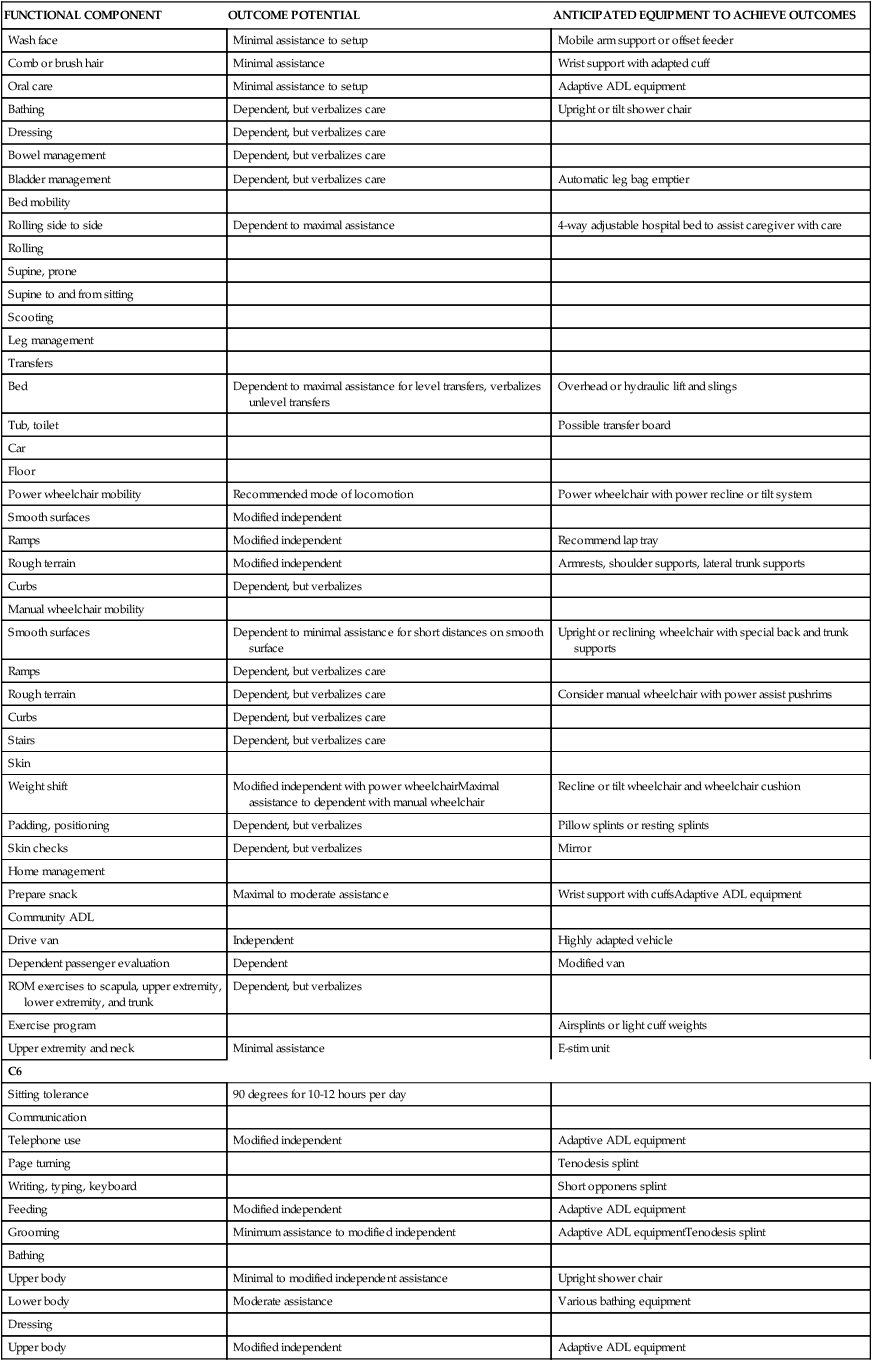
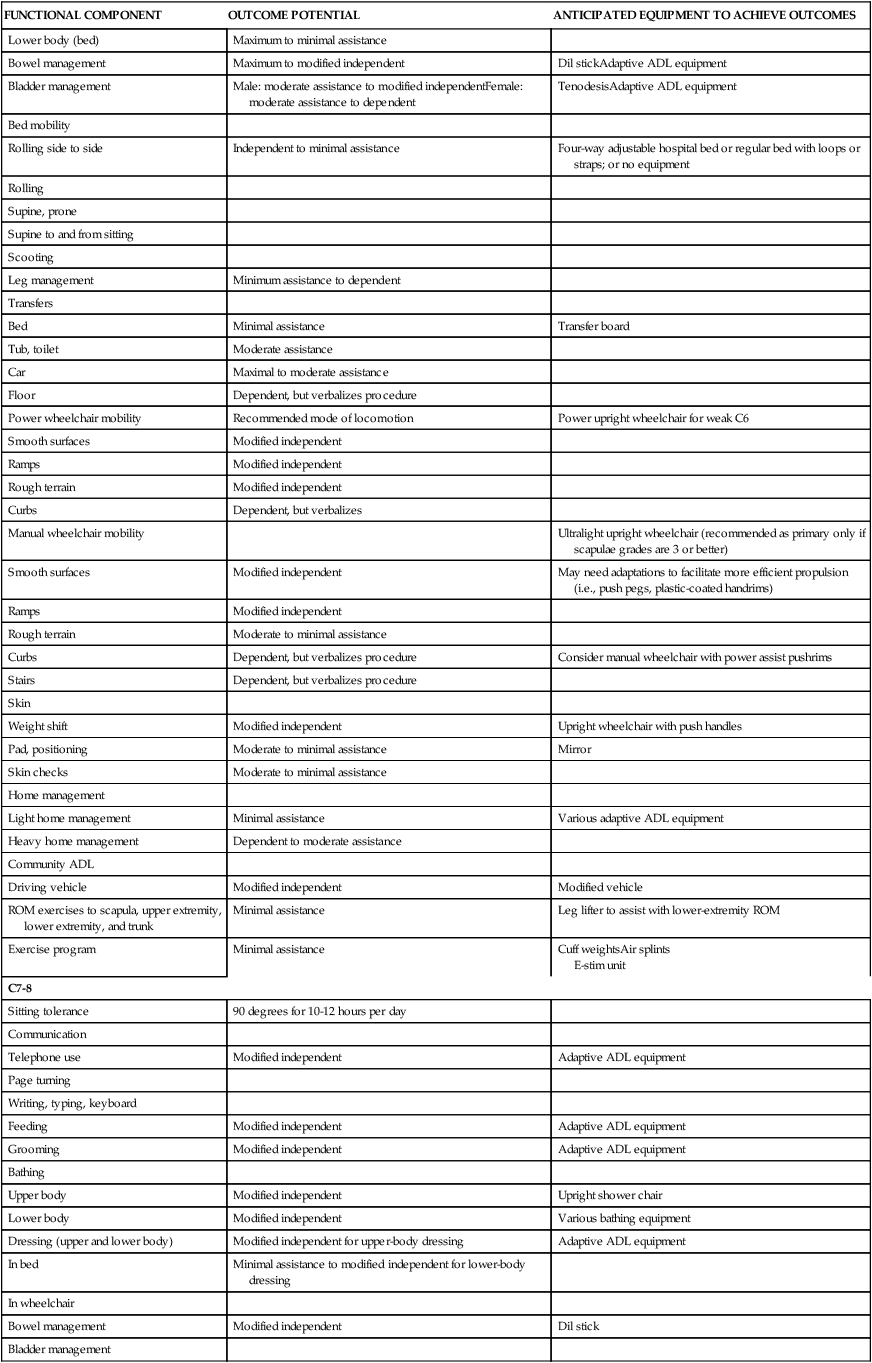
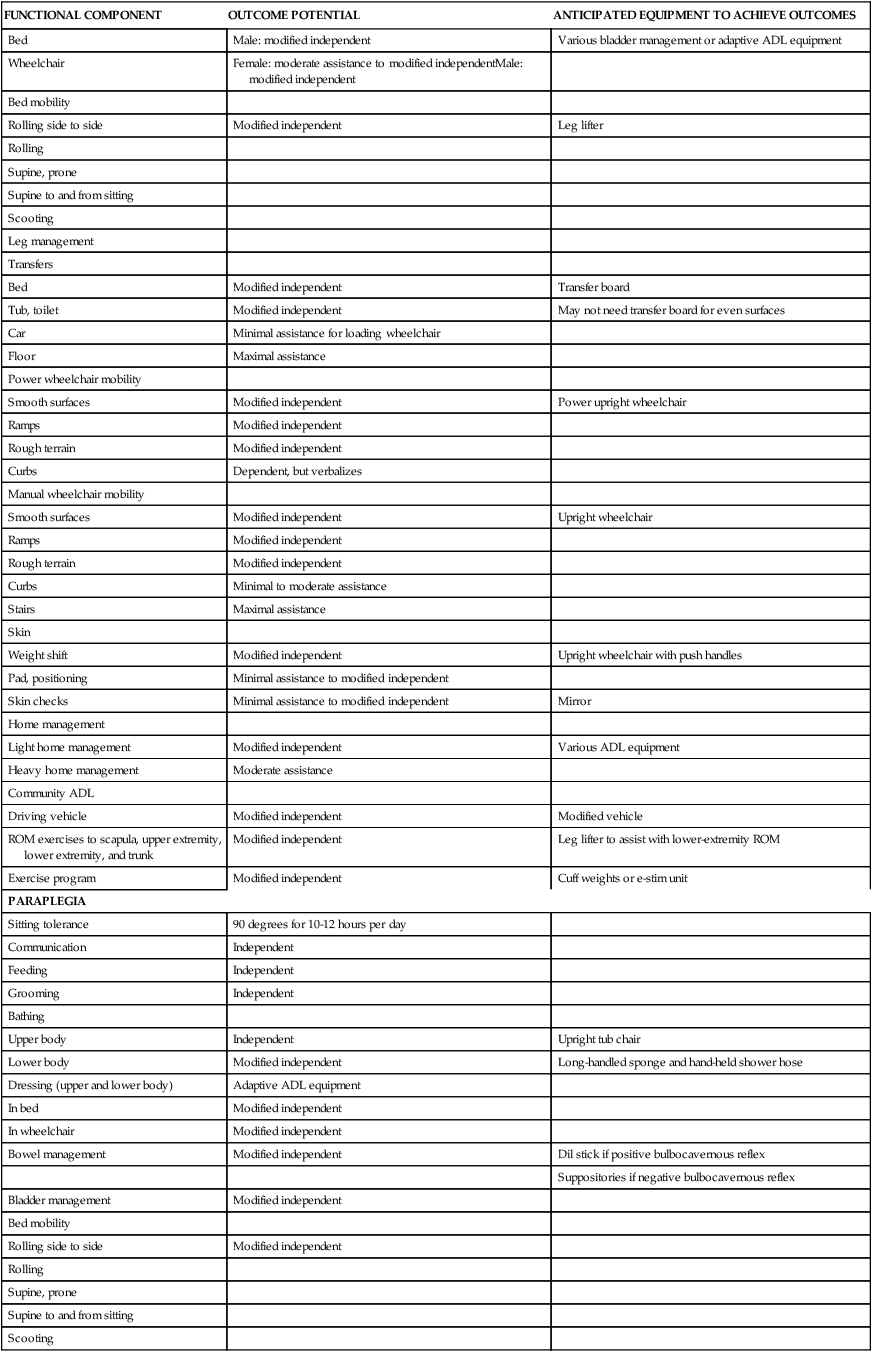
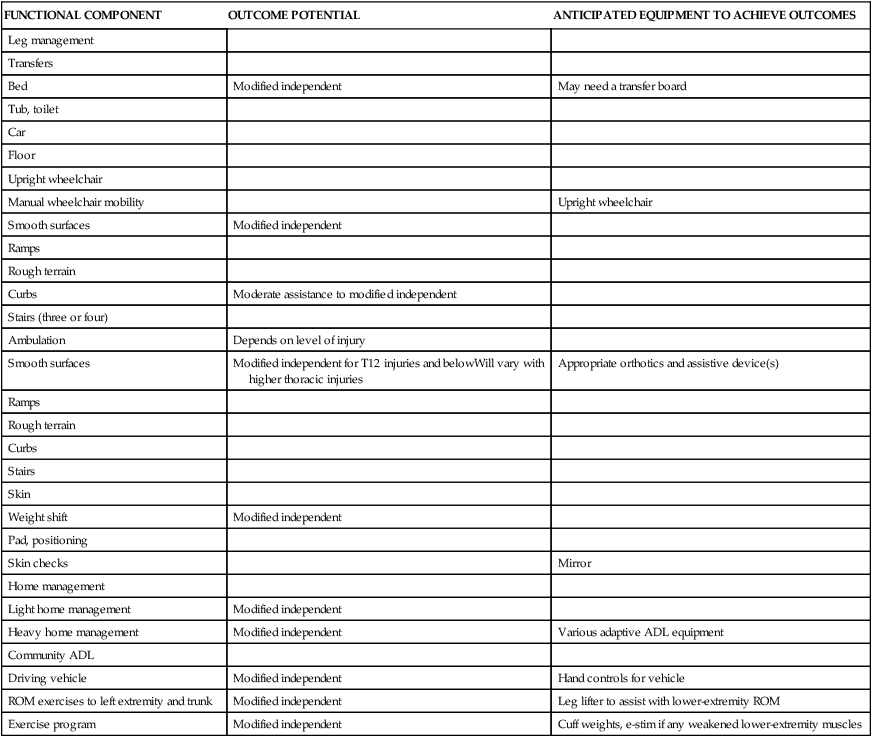
Functionally based goals are established in the following areas: bathing, bed mobility, bladder and bowel control, communication, environmental control and access, feeding, dressing, gait, grooming, home management, ROM and positioning, skin care management, transfers, transportation and driving, wheelchair management, and wheelchair mobility. Refer to Table 16-4 for anticipated goals for each level of injury. Information presented in this table should be recognized as general guidelines because variability exists. These guidelines are most usefully applied to patients with complete SCI. Goal setting for individuals with incomplete SCI is often more challenging, given the greater variability of client presentations and the uncertainty of neurological recovery. As with any patient, continual reevaluations provide additional insight into functional limitations or progression and potential and thereby direct the goal-setting process. In addition to specific functional goals and expectations, family training; home, work, or school modifications; and community reentry should be considered.
TABLE 16-4 
FUNCTIONAL EXPECTATIONS FOR COMPLETE SPINAL CORD INJURY LESIONS
| FUNCTIONAL COMPONENT | OUTCOME POTENTIAL | ANTICIPATED EQUIPMENT TO ACHIEVE OUTCOMES |
| C1-4 | ||
| Sitting tolerance | 80-90 degrees for 10-12 hours per day | Power wheelchair with power tilt, reclineWheelchair cushion |
| Communication | ||
| Mouth stick writing | Minimal assistance | Mouth sticks and docking station |
| ECU | Setup | ECU |
| Page turning | Minimal assistance to setup | Book holder |
| Computer operation | Minimal assistance to setup | Computer |
| Call-system use | Setup | Call system or speaker phone |
| Cuff-leak speech (ventilator dependent) | Up to 6 hours | |
| Feeding | Dependent, but verbalizes care | |
| Grooming | Dependent, but verbalizes care | |
| Bathing | Dependent, but verbalizes care | Reclining shower chair |
| Dressing | Dependent, but verbalizes care | |
| Bowel management | Dependent, but verbalizes care | |
| Bladder management | Dependent, but verbalizes care | |
| Bed mobility | ||
| Rolling side to side | Dependent, but verbalizes care | Four-way adjustable hospital bed to assist caregiver with task |
| Rolling | ||
| Supine, prone | ||
| Supine to and from sitting | ||
| Scooting | ||
| Leg management | ||
| Transfers | ||
| Bed | Dependent, but verbalizes care | Overhead lift system |
| Tub, toilet | Hydraulic lift | |
| Car | Slings | |
| Floor | ||
| Power wheelchair mobility | ||
| Smooth surfaces | Modified independent | Power wheelchair with power recline or tilt system |
| Ramps | Modified independent | |
| Rough terrain | Modified independent | Lap tray |
| Curbs | Dependent, but verbalizes | Armrests, shoulder supports, and lateral trunk supports |
| Manual wheelchair mobility | ||
| Smooth surfaces | Dependent, but verbalizes | Manual reclining or tilt wheelchair with same options as power wheelchair |
| Ramps | ||
| Rough terrain | ||
| Curbs | ||
| Stairs | ||
| Skin | ||
| Weight shift | Modified independent with power wheelchair | Recline or tilt wheelchair |
| Padding, positioning | Dependent, but verbalizes | Wheelchair cushion |
| Skin checks | Dependent, but verbalizes | Pillow splints, resting splintsMirror |
| Community:ADL- | ||
| dependent passenger evaluation | Dependent, but verbalizes | Modified van |
| ROM exercises to scapula, upper extremity, lower extremity, and trunk | Dependent, but verbalizes | |
| Exercise program | Independent for respiratory and neck exercises | Portable or bedside ventilator (C1-3 only) |
| C5 | ||
| Sitting tolerance | 90 degrees for 10-12 hours per day | Power recline or tilt wheelchairWheelchair cushion |
| Communication | ||
| Telephone use | Modified independent | Telephone adaptations |
| ECU | Setup | ECU |
| Page turning | Setup | Book holder, wrist support with cuff |
| Computer operation | Supervision | Computer |
| Writing, typing | Setup | Long Wanchik brace |
| Feeding | Minimal assist to setup | Mobile arm support or offset feederAdaptive ADL equipment |
| Grooming | ||
| Wash face | Minimal assistance to setup | Mobile arm support or offset feeder |
| Comb or brush hair | Minimal assistance | Wrist support with adapted cuff |
| Oral care | Minimal assistance to setup | Adaptive ADL equipment |
| Bathing | Dependent, but verbalizes care | Upright or tilt shower chair |
| Dressing | Dependent, but verbalizes care | |
| Bowel management | Dependent, but verbalizes care | |
| Bladder management | Dependent, but verbalizes care | Automatic leg bag emptier |
| Bed mobility | ||
| Rolling side to side | Dependent to maximal assistance | 4-way adjustable hospital bed to assist caregiver with care |
| Rolling | ||
| Supine, prone | ||
| Supine to and from sitting | ||
| Scooting | ||
| Leg management | ||
| Transfers | ||
| Bed | Dependent to maximal assistance for level transfers, verbalizes unlevel transfers | Overhead or hydraulic lift and slings |
| Tub, toilet | Possible transfer board | |
| Car | ||
| Floor | ||
| Power wheelchair mobility | Recommended mode of locomotion | Power wheelchair with power recline or tilt system |
| Smooth surfaces | Modified independent | |
| Ramps | Modified independent | Recommend lap tray |
| Rough terrain | Modified independent | Armrests, shoulder supports, lateral trunk supports |
| Curbs | Dependent, but verbalizes | |
| Manual wheelchair mobility | ||
| Smooth surfaces | Dependent to minimal assistance for short distances on smooth surface | Upright or reclining wheelchair with special back and trunk supports |
| Ramps | Dependent, but verbalizes care | |
| Rough terrain | Dependent, but verbalizes care | Consider manual wheelchair with power assist pushrims |
| Curbs | Dependent, but verbalizes care | |
| Stairs | Dependent, but verbalizes care | |
| Skin | ||
| Weight shift | Modified independent with power wheelchairMaximal assistance to dependent with manual wheelchair | Recline or tilt wheelchair and wheelchair cushion |
| Padding, positioning | Dependent, but verbalizes | Pillow splints or resting splints |
| Skin checks | Dependent, but verbalizes | Mirror |
| Home management | ||
| Prepare snack | Maximal to moderate assistance | Wrist support with cuffsAdaptive ADL equipment |
| Community ADL | ||
| Drive van | Independent | Highly adapted vehicle |
| Dependent passenger evaluation | Dependent | Modified van |
| ROM exercises to scapula, upper extremity, lower extremity, and trunk | Dependent, but verbalizes | |
| Exercise program | Airsplints or light cuff weights | |
| Upper extremity and neck | Minimal assistance | E-stim unit |
| C6 | ||
| Sitting tolerance | 90 degrees for 10-12 hours per day | |
| Communication | ||
| Telephone use | Modified independent | Adaptive ADL equipment |
| Page turning | Tenodesis splint | |
| Writing, typing, keyboard | Short opponens splint | |
| Feeding | Modified independent | Adaptive ADL equipment |
| Grooming | Minimum assistance to modified independent | Adaptive ADL equipmentTenodesis splint |
| Bathing | ||
| Upper body | Minimal to modified independent assistance | Upright shower chair |
| Lower body | Moderate assistance | Various bathing equipment |
| Dressing | ||
| Upper body | Modified independent | Adaptive ADL equipment |
| Lower body (bed) | Maximum to minimal assistance | |
| Bowel management | Maximum to modified independent | Dil stickAdaptive ADL equipment |
| Bladder management | Male: moderate assistance to modified independentFemale: moderate assistance to dependent | TenodesisAdaptive ADL equipment |
| Bed mobility | ||
| Rolling side to side | Independent to minimal assistance | Four-way adjustable hospital bed or regular bed with loops or straps; or no equipment |
| Rolling | ||
| Supine, prone | ||
| Supine to and from sitting | ||
| Scooting | ||
| Leg management | Minimum assistance to dependent | |
| Transfers | ||
| Bed | Minimal assistance | Transfer board |
| Tub, toilet | Moderate assistance | |
| Car | Maximal to moderate assistance | |
| Floor | Dependent, but verbalizes procedure | |
| Power wheelchair mobility | Recommended mode of locomotion | Power upright wheelchair for weak C6 |
| Smooth surfaces | Modified independent | |
| Ramps | Modified independent | |
| Rough terrain | Modified independent | |
| Curbs | Dependent, but verbalizes | |
| Manual wheelchair mobility | Ultralight upright wheelchair (recommended as primary only if scapulae grades are 3 or better) | |
| Smooth surfaces | Modified independent | May need adaptations to facilitate more efficient propulsion (i.e., push pegs, plastic-coated handrims) |
| Ramps | Modified independent | |
| Rough terrain | Moderate to minimal assistance | |
| Curbs | Dependent, but verbalizes procedure | Consider manual wheelchair with power assist pushrims |
| Stairs | Dependent, but verbalizes procedure | |
| Skin | ||
| Weight shift | Modified independent | Upright wheelchair with push handles |
| Pad, positioning | Moderate to minimal assistance | Mirror |
| Skin checks | Moderate to minimal assistance | |
| Home management | ||
| Light home management | Minimal assistance | Various adaptive ADL equipment |
| Heavy home management | Dependent to moderate assistance | |
| Community ADL | ||
| Driving vehicle | Modified independent | Modified vehicle |
| ROM exercises to scapula, upper extremity, lower extremity, and trunk | Minimal assistance | Leg lifter to assist with lower-extremity ROM |
| Exercise program | Minimal assistance | Cuff weightsAir splints E-stim unit |
| C7-8 | ||
| Sitting tolerance | 90 degrees for 10-12 hours per day | |
| Communication | ||
| Telephone use | Modified independent | Adaptive ADL equipment |
| Page turning | ||
| Writing, typing, keyboard | ||
| Feeding | Modified independent | Adaptive ADL equipment |
| Grooming | Modified independent | Adaptive ADL equipment |
| Bathing | ||
| Upper body | Modified independent | Upright shower chair |
| Lower body | Modified independent | Various bathing equipment |
| Dressing (upper and lower body) | Modified independent for upper-body dressing | Adaptive ADL equipment |
| In bed | Minimal assistance to modified independent for lower-body dressing | |
| In wheelchair | ||
| Bowel management | Modified independent | Dil stick |
| Bladder management | ||
| Bed | Male: modified independent | Various bladder management or adaptive ADL equipment |
| Wheelchair | Female: moderate assistance to modified independentMale: modified independent | |
| Bed mobility | ||
| Rolling side to side | Modified independent | Leg lifter |
| Rolling | ||
| Supine, prone | ||
| Supine to and from sitting | ||
| Scooting | ||
| Leg management | ||
| Transfers | ||
| Bed | Modified independent | Transfer board |
| Tub, toilet | Modified independent | May not need transfer board for even surfaces |
| Car | Minimal assistance for loading wheelchair | |
| Floor | Maximal assistance | |
| Power wheelchair mobility | ||
| Smooth surfaces | Modified independent | Power upright wheelchair |
| Ramps | Modified independent | |
| Rough terrain | Modified independent | |
| Curbs | Dependent, but verbalizes | |
| Manual wheelchair mobility | ||
| Smooth surfaces | Modified independent | Upright wheelchair |
| Ramps | Modified independent | |
| Rough terrain | Modified independent | |
| Curbs | Minimal to moderate assistance | |
| Stairs | Maximal assistance | |
| Skin | ||
| Weight shift | Modified independent | Upright wheelchair with push handles |
| Pad, positioning | Minimal assistance to modified independent | |
| Skin checks | Minimal assistance to modified independent | Mirror |
| Home management | ||
| Light home management | Modified independent | Various ADL equipment |
| Heavy home management | Moderate assistance | |
| Community ADL | ||
| Driving vehicle | Modified independent | Modified vehicle |
| ROM exercises to scapula, upper extremity, lower extremity, and trunk | Modified independent | Leg lifter to assist with lower-extremity ROM |
| Exercise program | Modified independent | Cuff weights or e-stim unit |
| PARAPLEGIA | ||
| Sitting tolerance | 90 degrees for 10-12 hours per day | |
| Communication | Independent | |
| Feeding | Independent | |
| Grooming | Independent | |
| Bathing | ||
| Upper body | Independent | Upright tub chair |
| Lower body | Modified independent | Long-handled sponge and hand-held shower hose |
| Dressing (upper and lower body) | Adaptive ADL equipment | |
| In bed | Modified independent | |
| In wheelchair | Modified independent | |
| Bowel management | Modified independent | Dil stick if positive bulbocavernous reflex |
| Suppositories if negative bulbocavernous reflex | ||
| Bladder management | Modified independent | |
| Bed mobility | ||
| Rolling side to side | Modified independent | |
| Rolling | ||
| Supine, prone | ||
| Supine to and from sitting | ||
| Scooting | ||
| Leg management | ||
| Transfers | ||
| Bed | Modified independent | May need a transfer board |
| Tub, toilet | ||
| Car | ||
| Floor | ||
| Upright wheelchair | ||
| Manual wheelchair mobility | Upright wheelchair | |
| Smooth surfaces | Modified independent | |
| Ramps | ||
| Rough terrain | ||
| Curbs | Moderate assistance to modified independent | |
| Stairs (three or four) | ||
| Ambulation | Depends on level of injury | |
| Smooth surfaces | Modified independent for T12 injuries and belowWill vary with higher thoracic injuries | Appropriate orthotics and assistive device(s) |
| Ramps | ||
| Rough terrain | ||
| Curbs | ||
| Stairs | ||
| Skin | ||
| Weight shift | Modified independent | |
| Pad, positioning | ||
| Skin checks | Mirror | |
| Home management | ||
| Light home management | Modified independent | |
| Heavy home management | Modified independent | Various adaptive ADL equipment |
| Community ADL | ||
| Driving vehicle | Modified independent | Hand controls for vehicle |
| ROM exercises to left extremity and trunk | Modified independent | Leg lifter to assist with lower-extremity ROM |
| Exercise program | Modified independent | Cuff weights, e-stim if any weakened lower-extremity muscles |
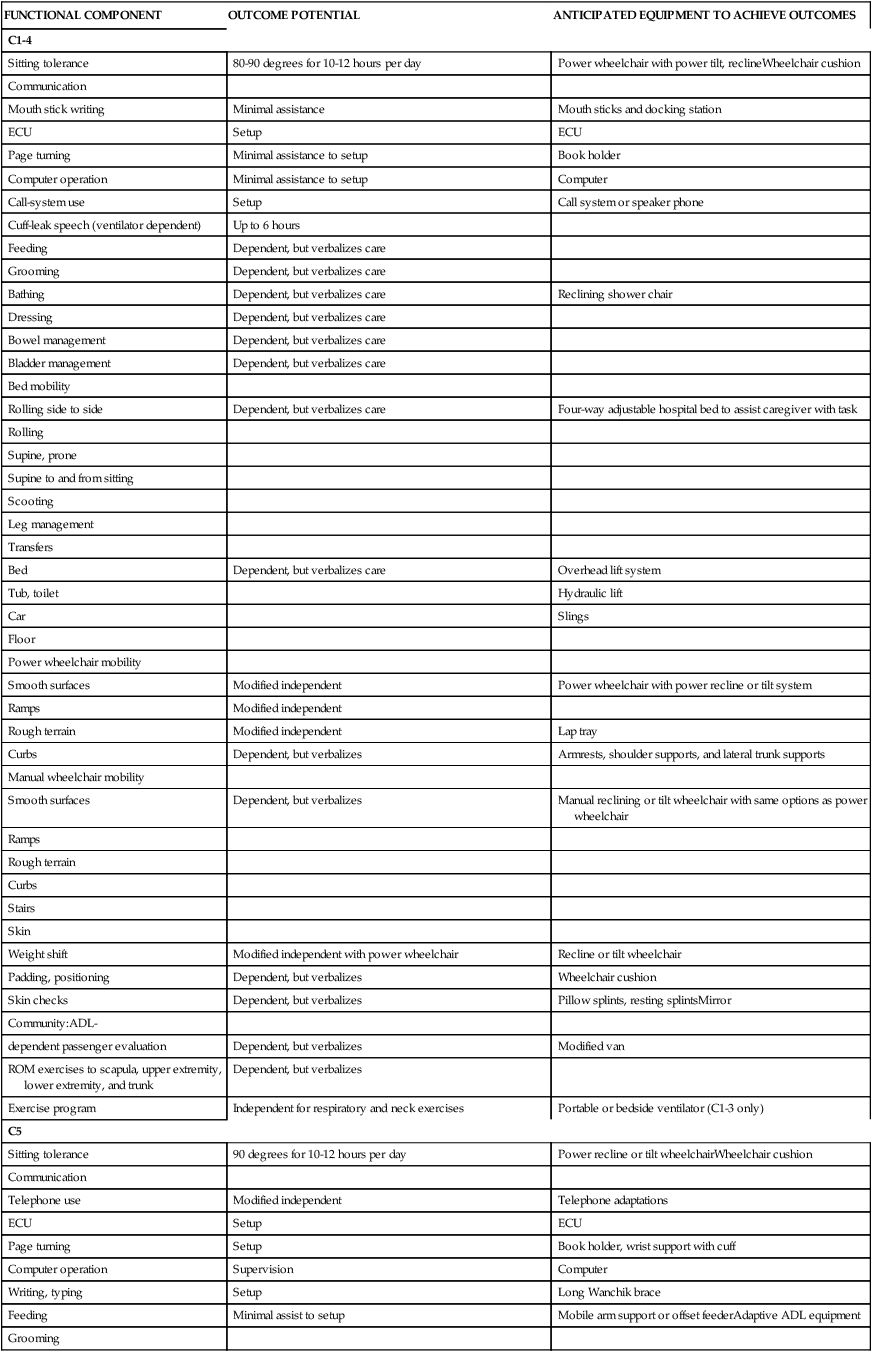
ADL, Activity of daily living; ECU, enviornmental control unit; ROM, range of motion.
Early rehabilitation and complication prevention
Table 16-5 describes an overview of the primary complications that can arise after an SCI. In this table, known causes and common management activities are reviewed. Tests and measures commonly used to determine the complication and the recommended medical and/or therapeutic interventions are listed in the table. Although various reports of incidences are published, the largest database is the Model Spinal Cord Injury Care Systems report.41,64 Because of their high incidence and potential effect on long-term outcomes, the following complications require further discussion: skin compromise, loss of ROM or joint contractures, and respiratory compromise after SCI.
TABLE 16-5 
COMPLICATIONS AFTER SPINAL CORD INJURY
| COMPLICATION | CAUSE | DIAGNOSTIC TESTS AND MEASURES | MEDICAL TREATMENT OR INTERVENTION | THERAPEUTIC INTERVENTION |
| CARDIOPULMONARY | ||||
| Pneumonia Atelectasis | Bacterial or viral infection, prolonged immobilization, prolonged artificial ventilation, general anesthesia | Radiographic studies, diagnostic bronchoscopy | Antibiotics, bronchodilator therapy, therapeutic bronchoscopy, suctioning | Chest physical therapy: percussion, vibration, postural drainage, mobilization, inspiratory breathing exercises |
| Ventilatory failure | Weakness or paralysis of the inspiratory muscles, unchecked bronchospasm | Pulmonary function tests (PFTs), arterial blood gases (ABGs), end-tidal CO2 monitoring, pulse oximetry | Artificial ventilation and supportive therapy, management of underlying cause (e.g., pneumonia), oxygen therapy | Airway and secretion management treatment as above, early mobilization once stabilized, biofeedback to assist with ventilator weaning as appropriate |
| Deep vein thrombosis (DVT)* | Venous status, activation of blood coagulation, pressure on immobilized lower extremity, and endothelial damage65–67 | Doppler studies, leg measurements, extremity visual observation and palpation, low-grade fever of unknown origin | Subcutaneous heparin3,252Prophylactic anticoagulation can decrease incidence to 1.3%5 Vena cava filter for failed anticoagulant prophylaxis |
Early mobilization and range of motion (ROM) for prevention, centripetal massage for prevention, compression garments, education about smoking cessation, weight loss, and exercise; avoid constricting garments and monitor overly tight leg bag straps and pressure garments (Paralyzed Veterans of America DVT guidelines) |
| Pulmonary embolus | Dislodging of DVT | Ventilation-perfusion lung scan, signs and symptoms including chest pain, breathlessness, apprehension, fever, and cough | Vena cava filterAnticoagulation therapy | None |
| Orthostatic hypotension | Vasodilation and decreased venous return, loss of muscle pump action in dependent lower extremities and trunk253 | Monitor blood pressure with activity and changes in position, observation for signs and symptoms | Medications to increase blood pressure, fluids in the presence of hypovolemia | Gradient compression garments: Ace wraps, abdominal binders, appropriate wheelchair selection to prevent rapid changes in position early in rehabilitation |
| Apneic bradycardia | True origin unknown; believed to be caused by sympathetic disruption resulting in vagal dominance in response to a noxious stimulus or hypoxia254 | ElectrocardiogramHeart rate Respiratory rate |
Hyperventilation | Remove noxious stimulus |
| INTEGUMENTARY SYSTEM | ||||
| Pressure ulcers | Prolonged external skin pressure exceeding the average arterial or capillary pressure255 | Wound measurements, staging classification, nutritional assessment71 | Nutritional support as needed, surgical or enzymatic debridement, surgical closure, muscle flap, skin flap or graft, antibiotics as appropriate | Irrigation and hydrotherapy, dressing management, electrotherapy71 |
| Shearing | Stretching and tearing of the blood vessels that pass between the layers of the skin7 | See pressure ulcers | See pressure ulcers | Add protective padding during functional activities, skill perfection, correct handling techniques |
| Moisture | Excessive sweating below the level of injury, urinary and bowel incontinence, poor hygiene | See pressure ulcers | See pressure ulcers, treat possible urinary tract infection, medications for bladder incontinence | Protective barrier ointments and powders, establish effective bowel and bladder programs, educate for improved hygiene, and refine activity of daily living (ADL) skills |
| NEUROMUSCULAR | ||||
| Spasticity | Upper motor neuron lesion73Deep tendon reflex spasticity scale evaluation | Ashworth or Modified Ashworth ScaleBaclofen pump insertion75 | Antispastic pharmacological agents: baclofen, diazepam (Valium), dantroleneSurgical intervention: myelotomy, rhizotomy, peripheral neurotomy73Botox injection | Prolonged stretching; inhibitive positioning or castingCryotherapy, weight-bearing exercise, and aquatic therapy |
| Flaccidity | Lower motor neuron lesion7,256Most often in injuries at L1 level and below | Deep tendon reflexes (would be absent) | None | None for treating flaccidity; however, secondary treatments that need to be considered include positioning to improve postural support, education for skin protection, and bracing and splinting to maintain joint integrity |
| Neurogenic bowel† | Refer to bowel management | Positive bulbocavernosus reflex: indicates reflexic bowel | Oral laxative, suppositories, and enemas | Establish comprehensive bowel program |
| Autonomic dysreflexia | Triggering of an uncontrolled hyperactive response from the sympathetic nervous system by a noxious stimulus7; noxious stimuli may include bowel or bladder distention, urinary tract infection, ingrown toenail, tight clothing, and pressure sore | Sudden rise in systolic blood pressure of 20-40 mm Hg above baseline254Observation of signs and symptoms: Sweating above level of injuryGoose bumpsSevere headacheFlushing of skin from vasodilation above level of injury254 |
Catheterization of the bladder, irrigation of indwelling catheter, pharmacological management if systolic blood pressure is greater than 150 mm HgRemove ingrown toenail if present | Immediately position the client in upright position, identify and remove noxious stimuli, check clothing and catheter tubing for constriction, and perform bowel program if fecal impaction is suspected |
| Ulcers, gastrointestinal | Venous status, activation of blood | Radiographic studies, diagnostic | Medications to increase blood pressure | Gradient compression garments; Ace wrap |
| OTHER | ||||
| Thermoregulation problems | Interruption between communication with autonomic nervous system and hypothalamusLack of vasoconstriction and inability to shiver or perspire254 | Body temperature | Cooling or warming blanket if extreme | Education about risk and proper protection from elements; behavior modification, education for proper hydration and appropriate clothing |
| Pain | Radicular pain originating from the injury,7,257,258 kinematic or mechanical pain, direct trauma, referred pain258,259 | Pain scales, functional assessment,260 taxonomy | Immobilization and rest, pain medications, injections for pain or antiinflammatory measures | Restore ideal alignment and posture; thermal modalities and electromodalities; manual therapy, improve movement patterns |
| Urinary tract infections | Presence of excessive bacteria in urine | Urinalysis, urine culture and sensitivity, temperature | Antibiotics | Monitor fluid intake and educate for proper technique during bladder care |
| Contractures | Muscle imbalance around joint; prolonged immobilization, unchecked spasticity, pain | Goniometric measurements | Tendon release; Botox injection for isolated spasticity | ROM functional use of extremity, casting or splinting, achieving and maintaining optimal postural alignment |
| Heterotopic ossification (HO) | Unknown | Alkaline phosphatase levels (increase after 6 weeks)261,262; observation for sudden loss of ROM, local edema, heat, erythema, nonseptic fever | Etidronate disodium (Didronel): use prophylactically or during inflammatory stageSurgical resection | Maintain available ROM; avoid vigorous stretching during inflammatory stage; achieve and maintain optimal wheelchair positioning |
| Osteoporosis and joint changes degenerative | Bone demineralization263 | Bone scan | None; calcium supplement for prevention | Weight-bearing techniques: amount and type unknown, specific to spinal cord injury |
| Spinal deformities | Muscle imbalance or weakness around spinal column; poor postural support, asymmetrical functional activities | Posture evaluation, seating evaluation | If severe: surgical fixation, thoracic orthosis | Restore postural alignment, avoid repetitive asymmetrical activities, control spasticity |
| Gastroduodenal ulcers, gastrointestinal bleeding | Acute: disruption of central nervous system, abdominal trauma or stress response to neuroendocrine system264Chronic: impairment of autonomic nervous system7 | Hematocrit and hemoglobin; observation of gastrointestinal fluids | Surgical intervention; restore normal gastrointestinal function | Establish effective bowel program, establish high-fiber diet, provide education and stress management |
| Metabolic, endocrine | Impairment of autonomic nervous system | Observe for fatigue, malaise; undesirable weight gain62 | None known | Education, exercise, and weight control |
*Consortium for Spinal Cord Medicine Clinical Practice Guidelines: Prevention of thromboembolism in spinal cord injury, Washington, D.C., February 1997, Paralyzed Veterans of America.
†Consortium for Spinal Cord Medicine Clinical Practice Guidelines: Neurogenic bowel management in adults with spinal cord injury, Washington, D.C., March 1998, Paralyzed Veterans of America.
Preventing and managing pressure ulcers and skin compromise
After SCI and during the period of spinal shock, patients are at greater risk for development of pressure ulcers.65,66 The use of backboards at the emergency scene and during radiographic procedures contributes to potential skin compromise; therefore, immediate concern for tissue death, especially at the sacrum, should be taken into account. Recently, padded spine boards have become available and are recommended to reduce the risk of skin complications.
Preventive skin care begins with careful inspection. Soft tissue areas over a bony prominence are at greatest risk for acquiring a pressure sore.67 Key areas to evaluate include the sacrum, ischia, greater trochanters, heels, malleoli, knees, occiput, scapulae, elbows, and prominent spinous processes. A turning schedule should be initiated immediately. Even if the patient has unstable fractures or is in traction, he or she can be turned and positioned with flat pillows using the logroll technique. Even small changes off the sacrum and coccyx are helpful. The patient’s position in bed should be initially established for turns to occur every 2 to 3 hours.66 This interval can be gradually increased to 6 hours with careful monitoring for evidence of skin compromise. A reddened round area over the bone that does not disappear after 15 to 30 minutes is the hallmark start of a pressure sore, and action to avoid or minimize pressure in the area must be taken immediately to avoid progression. Turning positions include prone, supine, right and left side-lying, semiprone, and semisupine positions.68,69 Secondary injuries such as fractures and the presence of vital equipment, such as ventilator tubing, chest tubes, and arterial lines, should be considered when choosing turning positions. The prone position is the safest position for maintaining skin integrity but may not always be feasible.
Keeping the head of the bed as low as tolerated minimizes the risk for shearing and excessive sacral pressure. For patients who are not appropriate for rigorous turning schedules (e.g., patients with unstabilized fractures), specialty alternating pressure mattresses are available. Low air loss, alternating pressure, or even air-fluidized mattresses are available for those who require the head of the bed to be elevated more than 30 degrees for prolonged periods and also have other extenuating conditions such as respiratory distress, diabetes, and/or low prealbumin.70
Should skin compromise occur, the first intervention is to identify and remove the source of the compromise. Modifications to the seating system or changing to a more pressure-reducing mattress system or cushion may be necessary. Examination and treatment will then need to focus on healing the wound and preventing other secondary complications that may occur as a result of potential immobility and delayed physical rehabilitation. The reader is encouraged to refer to Pressure Ulcer Treatment: Clinical Practice Guideline, developed by the Agency for Health Care Research and Quality, for examination tools, including the classification of pressure ulcers.71,72
Treatment interventions may include hydrotherapy, specialty wound dressings, electro-modalities, and thermal modalities to increase circulation.71 Mechanical, autolytic, enzymatic, or surgical debridement may be necessary to obtain and maintain a viable wound bed. If the wound does not heal, surgical interventions with myocutaneous or muscle flaps may be necessary for closure. Coordinated return-to-sit programs or protocols after such medical interventions are necessary to prevent opening of the surgical site. Such surgical procedures are costly and significantly delay functional rehabilitation.
Prevention and management of joint contractures
The development of a contracture may result in postural misalignment or impede potential function. Daily ROM exercises, proper positioning, and adequate spasticity control may help prevent contractures.66 Contracture prevention includes the use of splints for proper joint alignment, techniques such as weight bearing, ADLs, and functional exercises. Patients exhibiting spasticity may require more frequent ROM intervention.66,73
Adaptive shortening or adaptive lengthening of muscles
Although isolated joint ROM should be normal for all patients, allowing adaptive shortening or adaptive lengthening of particular muscles is recommended to enhance the achievement of certain functional skills.69,74 Likewise, unwanted shortening or lengthening of muscles should be prevented. The following section reviews a few examples of these concepts as they relate to SCI.
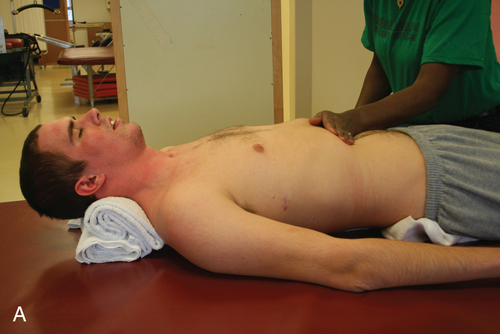
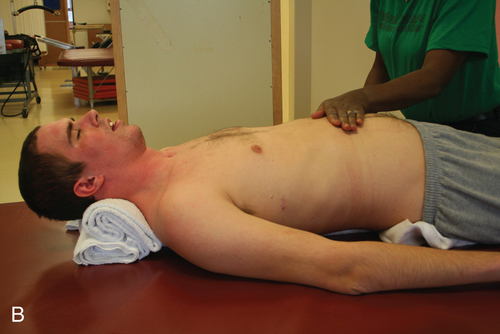
Tenodesis is described as the passive shortening of the two-joint finger flexors as the wrist is extended. This action creates a grasp, which assists performance of ADLs (Figure 16-11).69,75 A patient with mid to low tetraplegia may rely on adaptive shortening of these long finger flexors to replace active grip.69 If the finger flexors are stretched across all joints during ROM exercises, the achievement of some functional goals may be limited. ROM to the finger flexors should be applied only while the wrist is in a neutral position. There is controversy over shortening of the flexor tendons. Some clinicians argue that the client can develop a fixed flexion contracture of the proximal interphalangeal joints, interfering with future surgical attempts to restore finger function.38 It is recommended to promote tenodesis functioning via adaptive shortening while maintaining joint suppleness.

 Tenodesis grasp.
Tenodesis grasp.In the presence of weakened or paralyzed elbow extensors, shortening of the elbow flexors should be prevented because it will impair ADL function and transfer skills.7,69 Contracted elbow flexors or pronator muscles in a client with an SCI level of C6 can cost this client his or her independence. Likewise, the rotator cuff and the other scapular muscles should be assessed for their length-tension relationships and their ability to generate force. Normal length of these muscles should be maintained. For example, achieving external rotation of the shoulder (active and passive) is critical for clients with low-level tetraplegia. Shortening of the subscapularis and other structures can quickly result in a decrease in motion, limiting bed mobility, transfers, feeding, and grooming skills. Patients with complete paraplegia who are candidates for ambulation require normal ROM in the lower extremities. If the hip flexors or knee flexors are allowed to shorten, achieving standing and ambulation goals will be more difficult.
Splinting to prevent joint deformity
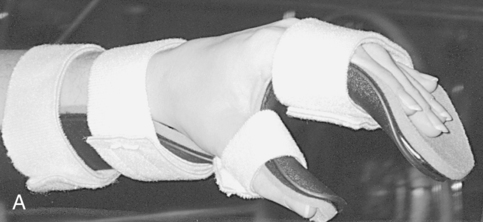
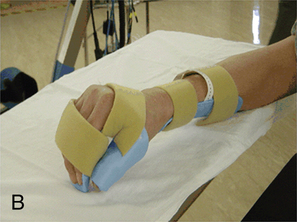
Deformity prevention is the first goal of splinting.76 Patients with cervical spinal cord injuries may have lost normal neural input to musculature in their wrists and hands. Other clients may have partial motor control, which may lead to muscle imbalances and loss of ROM. In the absence or weakness of elbow extensors, a bivalve cast or an elbow extension splint at night may be beneficial to prevent joint contractures. At the wrists, a volar wrist support is commonly used initially and may be progressed to a longer-term option of a definitive wrist orthosis. Other splints often used for deformity prevention of the hands include resting hand splints with proper positioning to maintain the support of the wrist and web space (Figure 16-12, A).77 Another hand-based option is the intrinsic plus splint (Figure 16-12, B), which places the metacarpophalangeal joints closer to 90 degrees of flexion and decreases intrinsic hand muscle tightness.
Patients who are not strong enough to use their wrists for tenodesis may require splinting to support their wrists until they can perform wrist extension against gravity. Long opponens splints can be used to position the thumb for function but support the weak wrist (Figure 16-13). Once the wrist muscles strengthen, the long opponens splint can be cut down to a hand-based short opponens to maintain proper web space and thumb positioning while maximizing tenodesis.
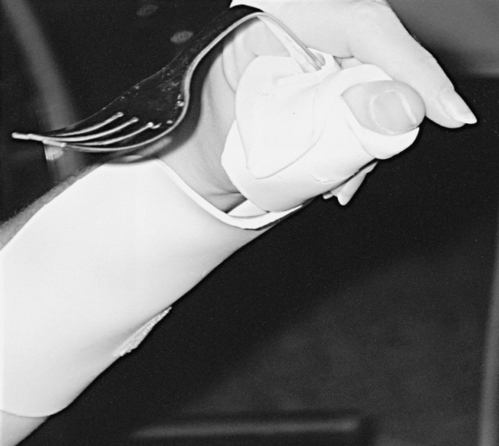
 Long opponens splint with fabricated utensil holder.
Long opponens splint with fabricated utensil holder.As mentioned previously, clients with injuries at the C6 level can use their wrists for a tenodesis grasp.75,78,79 Critical components of the splint assessment for these clients are the positioning of the thumb, web space, and index finger observed during the grasp. It is recommended that the client’s hand be positioned with the thumb in a lateral pinch position because this is the most commonly used prehension pattern to pick up objects. Clients who are not splinted may not have the proper positioning to pick up objects because their tenodesis is “too tight” or “too loose.”
Clients with C8 to T1 injuries or clients who have incomplete injuries may have “clawing” or hyperextension of the metacarpophalangeal joints. This is caused by finger extensor musculature that is stronger than finger flexor musculature.75,80 To prevent this, a splint can be made to block the metacarpophalangeal joints and promote weak intrinsic muscle function. Depending on the extent of the imbalance, these splints can be used during function or worn only at night. Cost, time, material, and clinician experience are important considerations when deciding between custom and prefabricated splints. A well fitting, prefabricated splint can be as effective as a custom-fabricated splint in certain situations. Custom splints require additional resources and clinician expertise. One way to minimize time spent in fabrication of splints is to use a good pattern and premade straps. Finally, educating the client on the splint-wearing schedule, skin checks, and splint care is important for preventing skin breakdown.
Treatment for joint deformity
If a joint contracture occurs despite preventive measures, more aggressive treatments are necessary. This may include more aggressive use of splinting, plaster or fiberglass casting techniques, or botulinum toxin type A (Botox) injections.81–83 When splinting is not effective, fabrication of serial or bivalve casts may be indicated. The client with minimal ROM limitations may require only one cast. Most commonly, the client has a significant limitation and requires serial casts, in which several casts are applied and then removed over a period of weeks to increase extensibility in the soft tissues surrounding the casted joint.84 The involved joint is placed at submaximal ROM.85 Once the cast is removed, the joint should have an increase of approximately 7 degrees of ROM.85 This process continues until the deformity is minimized or resolved. The final cast is a bivalve so that the cast can act as a positioning device that can be easily removed. Casting contraindications are skin compromise over the area to be casted, heterotopic ossification, edema, decreased circulation, severe fluctuating tone, and inconsistent monitoring systems. The elbow, wrist and hand, and finger joints are the most common joints casted for clients with SCI. Casting for most of these clients may be the last resort to regain increased ROM before a client can begin using feeding, grooming, or communication skills. Long-arm casts are used when elbow and wrist contractures must be managed simultaneously. If evaluation of the upper extremity reveals a pronation or supination contracture, a long-arm cast would also be the cast of choice. Dropout casts are used with severe elbow flexor or extensor contractures, but the patient should be in a position in which gravity can assist. Wrist-hand and finger casts are indicated for contractures that prevent distal upper-extremity function. Most commonly, a client will have a wrist flexion-extension contracture or have finger flexor-extensor tone and will require a cast to use the tenodesis or individual fingers for fine motor skills. Sometimes wrist casts with finger shells or resting hand extensions on casts are needed to ensure that the hand, fingers, and web space are maintained in a position of optimal function. Casting is an expensive and labor-intensive treatment modality, but if indicated and used appropriately it can assist a client in regaining lost joint ROM needed for increased independence and function.
Botox may be used in conjunction with casting. In a study conducted by Corry and colleagues83 tone reduction was evident when botulinum toxin type A was used; however, ROM and functional improvement varied among subjects. Pierson and co-workers82 found that, with careful selection, subjects who received botulinum toxin type A had significant improvements in active and passive ROM. Research indicates that patients who have flexor spasticity without fixed contracture will benefit the most.
Surgical intervention may be recommended by an orthopedic physician in severe cases of joint contracture.86 Some of the more commonly used surgical options include joint manipulation under anesthesia, arthroscopic surgical releases, open surgical releases, and rotational osteotomy.
Prevention and management of respiratory complications
Early management must focus heavily on preventing pulmonary complications and maximizing pulmonary function so the patient may perform physical activities. The clinician should first determine which ventilatory muscles are impaired. The primary ventilatory muscles of inspiration are the diaphragm and the intercostals. The diaphragm is innervated by the phrenic nerve at C3 through C5. The intercostals are innervated by the intercostal nerves positioned between the ribs. If the diaphragm is weak or paralyzed, its descent will be lessened, reducing the patient’s ability to ventilate.87–90
Accessory muscles of ventilation are primarily located in the cervical region.91 The accessory muscles are used to augment ventilation when the demand for oxygen increases, as during exercise. Accessory muscles may also be recruited to generate an improved cough effort.66 The most commonly cited accessory muscles are the sternocleidomastoids, the scalenes, the levatores scapulae, and the trapezius muscles.88,89 The erector spinae group may also assist by extending the spine, thus improving the potential depth of inspiration.89
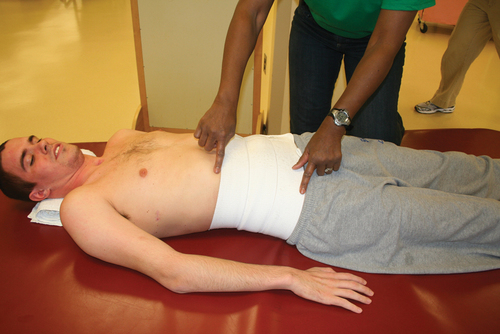
The abdominals are the primary muscles used for forced expiration in such maneuvers as coughing or sneezing. The latissimus dorsi, the teres major, and the clavicular portion of the pectoralis major are also active during forced expiration and cough in the client with tetraplegia.92 Alterations in the function of these muscles will have an impact on the patient’s ability to clear secretions and produce loud vocalization. Gravity plays a crucial role in the function of all ventilatory muscles.89 Neural input to the diaphragm increases in the upright position in persons with intact nervous systems. As one moves into an upright position, the resting position of the diaphragm drops as the abdominal contents fall.89 The diaphragm is effectively shortened, which makes generating a strong contraction more difficult. With intact abdominal musculature, however, a counter pressure is produced and adequate intraabdominal pressure is maintained, allowing the diaphragm to perform work. If weakness or paralysis of the abdominal wall is present, the client may need a binder or corset to maintain the normal pressure relationship.69,87,93–95 Unless the SCI has affected only the lowest sacral and lumbar areas, some degree of ventilatory impairment is present and should be addressed in therapeutic sessions.
Many treatment techniques are available to address the myriad causes of ventilatory impairment. Decreased chest wall mobility and the inability to clear secretions should always be addressed. Interventions may include inspiratory muscle training, chest wall mobility exercises, and chest physical therapy.69,74,90,96,97
Inspiratory muscle training
Inspiratory muscle training may be used to train the diaphragm and the accessory muscles that are weakened by partial paralysis, disuse from prolonged artificial ventilation, or prolonged bed rest. In the presence of significant impairments, it is generally recommended that training be initiated in the supine or side-lying position and progressed to the sitting position when tolerated. When training a moderately weak diaphragm, gentle pressure during inspiration may be used to facilitate the muscle (Figure 16-14). Accessory muscle training may be facilitated with the client in the supine position while a slight stretch is placed on these muscles.74 The stretch is accomplished by shoulder abduction and external rotation, elbow extension, forearm supination, and neutral alignment of the head and neck. A more challenging position incorporates upper thoracic extension. The clinician’s hands are placed directly over the muscle to be facilitated. The patient is instructed to breathe into the upper chest (Figure 16-15). As the treatment progresses, the diaphragm may be inhibited for short training periods by applying pressure over the abdomen in an upward direction. Care must be taken to avoid excessive pressure to prevent occlusion of vital arteries.
As the inspiratory muscles strengthen, resistive inspiratory devices may be used. Inspiratory devices are relatively inexpensive and most function similarly. Most devices have a one-way valve that closes when the patient inspires, forcing him or her to breathe either through a small aperture or against a spring-loaded resistance. Although evidence fully supporting this intervention remains inconclusive,98 some researchers have shown improvements in total lung capacity99 and improved endurance measures.100 The diaphragm may also be trained by using weights on the abdominal wall with the client positioned supine. Derrickson and colleagues101 concluded that both inspiratory muscle training devices and abdominal weights are effective in improving ventilatory mechanics. Muscle trainers, however, appear to promote more of an endurance effect than the use of abdominal weights.
Diaphragm and phrenic nerve pacing
When the primary inspiratory muscles are no longer volitionally active as a result of SCI, diaphragm or phrenic nerve pacing may be used to cause the diaphragm to contract. These interventions are most commonly indicated when the lesion is at or above the C3 level.101–106 Electrical stimulation may be applied directly or indirectly through a vein wall or the skin or directly to the phrenic nerve via thoracotomy. Transdiaphragmatic pacing, in which electrodes are placed laparoscopically on the diaphragm, is also an option.107 Transdiaphragmatic pacing is less invasive than direct phrenic nerve pacing, may be implanted and initiated on an outpatient basis, and may result in improved outcomes. Both of these procedures require a reconditioning program that involves extensive caregiver and client training. Many clients require some residual use of mechanical ventilation even after maximal tolerance has been achieved so as not to overfatigue the phrenic nerve. Other researchers are considering also pacing intercostal muscles108 or using a combination of diaphragmatic and intercostal pacing. There is limited evidence comparing the outcomes associated with these devices in isolation or in combination therapies.
Glossopharyngeal breathing
Glossopharyngeal breathing is another way of increasing vital capacity in the presence of weak inspiratory muscles.90,94,97 Moving the jaw forward and upward in a circular opening and closing manner traps air in the buccal cavity. A series of swallowing-like maneuvers forces air into the lungs, increasing the vital capacity. This technique has been reported to increase vital capacity by as much as 1 L.74 Although this technique is rarely used to sustain ventilation for long periods of time,109 it may be used in emergency situations and to enhance cough function. The client with high tetraplegia should attempt to master this skill.
Secretion clearance
Ventilatory impairment occurs when the client is unable to clear secretions.87,110 Factors such as artificial ventilation and general anesthesia hamper secretion mobilization. With artificial ventilation, clients may require an artificial airway.110,111 The presence of this airway in the trachea is an irritant, and the client subsequently produces more secretions.87 A description of various types and parameters of ventilation is beyond the scope of this chapter. Clinicians working with clients requiring artificial ventilation are referred to other publications.110,112
Secretions are most commonly removed by tracheal suctioning, unassisted coughing, or assisted coughing. Recently there has been a resurgence of previously used technologies that provide rapidly alternating pressures through a mouthpiece or an endotracheal tube to remove secretions. This is commonly referred to as insufflation-exsufflation.113 To date, conclusive research determining which single technique or combination of techniques achieves the best outcome is not available. Insufflation-exsufflation may result in fewer complications and is reported to be more comfortable to the client. Barriers to implementation of these techniques may include expense of the equipment and competency barriers in that training is required. Postural drainage, percussion or clapping, and shaking or vibration are used to assist with moving secretions toward larger airways for expectoration.17,69,79
Assisted coughing is typically used with people who are unable to generate sufficient effort.97 The assistant places both hands firmly on the abdominal wall. After a maximal inspiratory effort, the patient coughs and the assistant simply supports the weakened wall. A gentle upward and inward force may be used to increase the intraabdominal pressure, yielding a more forceful cough (Figure 16-16).84,97 Excessive pressure over the xiphoid process should be avoided to prevent severe injury.
Patients may learn independent coughing techniques. In preparation for a cough, the patient positions an arm around the push handle of the wheelchair, opening the chest wall to enhance inspiratory effort. The other arm is raised over the head and chest during inspiration. This procedure is followed by a breath hold, strong trunk flexion, and then a cough (Figure 16-17).97 Another technique for independent coughing is accomplished by placing the forearms over the abdomen and delivering a manual thrust during cough. This technique is more difficult and may not provide an inspiratory advantage.
Early mobilization
Getting the patient upright as soon as possible promotes self-mobility and should be planned carefully. An appropriate seating system for pressure relief and support should be chosen. Most patients require a reclining wheelchair with elevating footrests or tilt-in-space wheelchairs when they are first acclimating to the upright position.69,74,97
Because of paralysis, the abdominal wall may not support the internal organs and viscera. In these cases an abdominal binder or corset should be applied to all clients with lesions above T12 to assist in venous return74,95,97; as discussed, this will enhance ventilatory function. If the client has a history of vascular insufficiency or prolonged bed rest, wrapping the lower extremities with elastic bandages while applying the greatest pressure distally may be beneficial.
Abdominal binders and corsets are fitted so that the top of the corset lies just over the lower two ribs.95 The bottom portion is placed over the anterior iliac spine and iliac crest (Figure 16-18). The corset or binder should be adjusted slightly more tightly at the bottom to assist in elevating the abdominal contents.69,74,97 Properly fitting the abdominal binder is essential. If it is placed too high or allowed to ride up, ventilation may be impaired by restriction of chest wall excursion. If placed too low, it will not provide the necessary abdominal support.
Once the client is out of bed, a weight shift or pressure relief schedule is immediately established. Initially, weight shifts are performed at 30-minute intervals and modified according to skin tolerance.67 A timer may be issued to ensure reminders for weight shifts. This is particularly important if the client has cognitive deficits. The skin is inspected thoroughly before and immediately after out-of-bed activities. Total sitting time is progressed according to tolerance.