Chapter 29 Traumatic Facial Paralysis
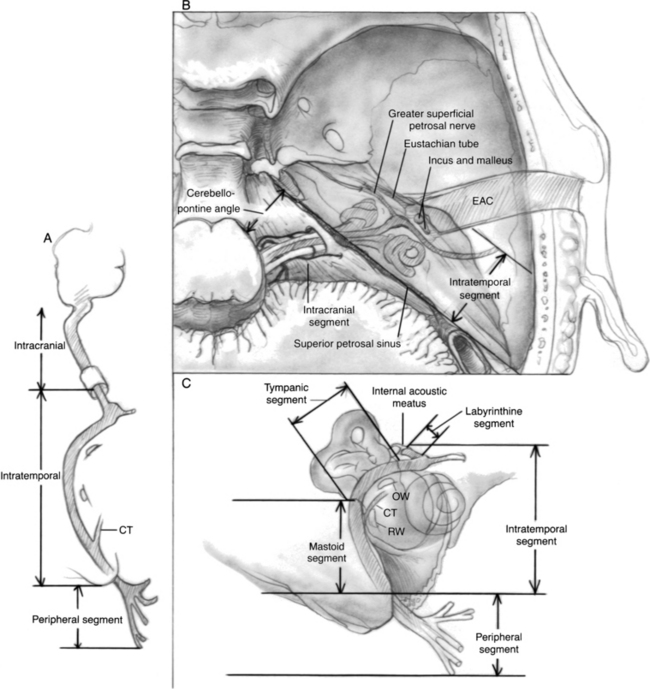
The course of the nerve from the brainstem to the facial musculature can be divided into three segments: intracranial, intratemporal, and extratemporal or peripheral (Fig. 29-1). The pathophysiology of facial nerve disorders varies according to the segment of the nerve involved. Each segment is discussed individually.
INTRATEMPORAL INJURY TO THE FACIAL NERVE
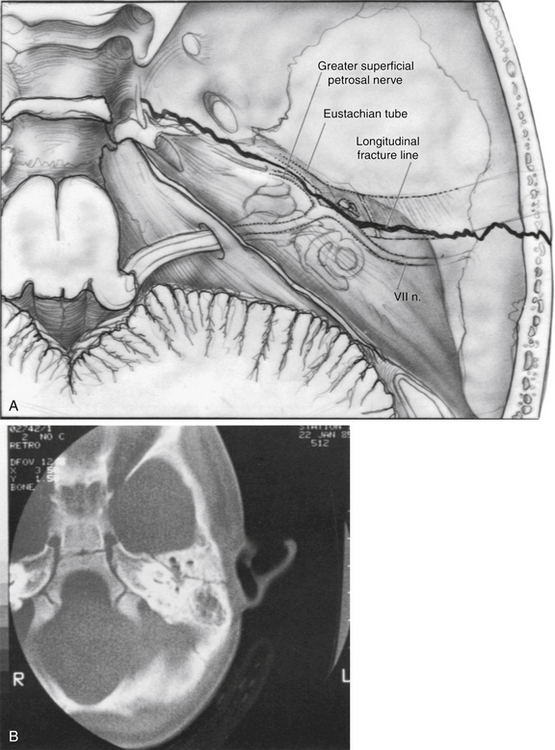
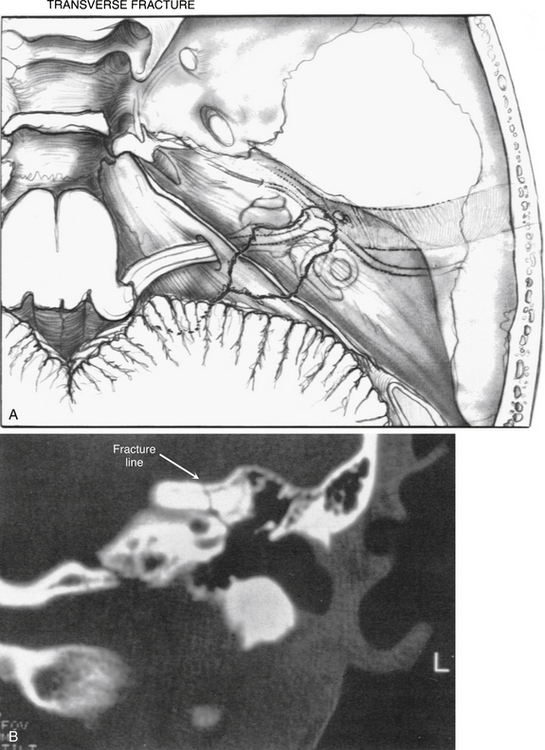
The intratemporal facial nerve, extending from the internal auditory canal fundus to the stylomastoid foramen, is frequently damaged from blunt trauma to the skull that leads to temporal bone fracture. Fractures produced by blunt trauma have been traditionally grouped into longitudinal and transverse varieties (Table 29-1), although almost any type of fracture can be encountered. Fractures with the main component parallel to the long axis of the petrous pyramid are classified as longitudinal (Fig. 29-2), whereas fractures perpendicular to the long axis (Fig. 29-5) are classified as transverse. Longitudinal fractures are produced by trauma to the lateral aspects of the skull in the temporoparietal region, and compose 80% of fractures in most series.1 Transverse fractures are produced by trauma to the occipital or frontal regions of the skull, and compose about 20% of fractures. Many fractures are oblique or combine elements of longitudinal and transverse fractures.2 Severely comminuted and complex fractures of the temporal bone are commonly produced by penetrating gunshot wounds of the temporal bone.3
| Longitudinal | Transverse | |
|---|---|---|
| Mechanism | Temporal or parietal blow | Frontal or occipital blow |
| Incidence | 80% | 20% |
| Incidence of facial paralysis | 20% | 50% |
| Type of hearing loss | Conductive | Sensorineural |
| External auditory canal | Torn, bloody | Intact |
| Tympanic membrane | Perforated | Intact, hemotympanum |
| Ossicular damage | Common | Uncommon |
| Vertigo | Uncommon | Common |
| Skull base foramen | Ovale | Spinosum, lacerum |
A longitudinal fracture (Figs. 29-2 and 29-3) is suspected when a step-off is present in the external auditory canal and is frequently accompanied by blood in the external auditory canal. A perforation or tear of the tympanic membrane may be present, and cerebrospinal fluid (CSF) otorrhea is occasionally seen. Sterile instruments should be used during the examination of the external auditory canal to avoid introducing contamination into the area and producing retrograde meningitis. A conductive hearing loss usually is present and can have numerous causes. Perforation of the eardrum, hematoma in the middle ear cleft, disruption of ligaments supporting the ossicles in the attic region, and disruption of the ossicular joints all can lead to varying degrees of conductive hearing loss (see section on ossicular damage).
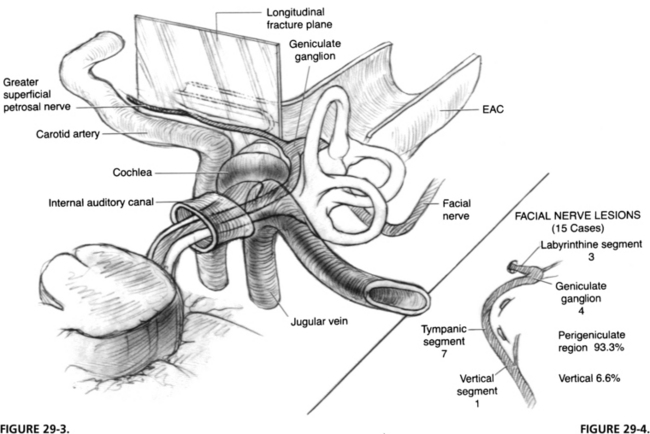
FIGURE 29-3 Longitudinal fracture of temporal bone. EAC, external auditory canal.
FIGURE 29-4. Location of lesion to facial nerve in 15 cases of longitudinal fractures.
(From Coker NJ, Kendall KA, Jenkins HA, Alford BR: Traumatic intratemporal facial nerve injury: Management rationale for preservation of function. Otolaryngol Head Neck Surg 97:262-269, 1987.)
A longitudinal fracture often extends to the foramen ovale. Facial paralysis is seen in only 20% of longitudinal fractures, but is the most common cause of facial paralysis in blunt trauma of the temporal bone because of the relative infrequency of transverse fractures. The facial nerve is involved in the perigeniculate region in 90% of cases4,5 and less commonly in the mastoid segment by fractures of the posterior external auditory canal (Fig. 29-4). The pathology of the facial nerve injury in blunt temporal bone trauma, in decreasing order of occurrence, consists of intraneural hemorrhage, bony fragment impingement, and nerve transection.6
A transverse temporal bone fracture is suspected when a patient presents with sensorineural hearing loss and vertigo accompanied by facial paralysis. The external canal is frequently intact, and no evidence of canal wall discontinuity and hemotympanum may be present (Figs. 29-5 and 29-6). Transverse fractures can extend into the foramen spinosum or lacerum. These patients have a 50% incidence of facial paralysis, which occurs from damage to the geniculate ganglion region (Fig. 29-7).7 The causes of injury are the same as for longitudinal fractures, and intraneural hemorrhage is the most common.
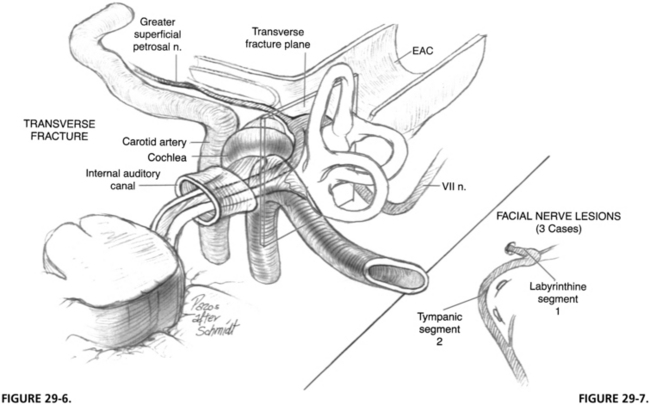
FIGURE 29-6 Transverse fracture of temporal bone. EAC, external auditory canal.
FIGURE 29-7. Location of lesion in three cases of transverse temporal bone fracture.
(From Coker NJ, Kendall KA, Jenkins HA, Alford BR: Traumatic intratemporal facial nerve injury: Management rationale for preservation of function. Otolaryngol Head Neck Surg 97:262-269, 1987.)
The traditional classification scheme, based on anatomic cadaveric studies performed more than 50 years ago,8 has been criticized because of its inability to predict complications of temporal bone fractures. More recently, several authors have proposed new classification schemes with predictive ability.9–12 These schemes are similar and are based on whether the otic capsule or petrous apex or both are violated. Fractures involving the otic capsule were significantly more likely to result in the complications of facial nerve injury, sensorineural hearing loss, and CSF leak than fractures that did not. The new classification systems seem to have utility for predicting these serious sequelae of temporal bone fractures.
Gunshot wounds to the temporal bone region typically produce extensive damage, the degree of which is determined by the velocity of the projectile. Low-velocity civilian projectiles have low energy and produce mainly locally destructive manifestations. In contrast, high-velocity, large-caliber weapons, which are increasingly being seen on city streets, are capable of widespread destruction, with extensive local and regional manifestations produced by the concomitant shock wave. Severe life-threatening injuries including vascular and intracranial damage are seen in one third to one half of patients and must be stabilized first. Angiography and computed tomography (CT) scan of the head are needed as part of the initial evaluation.13,14
In gunshot wounds to the temporal bone, the incidence of facial nerve injury is about 50%, with the vertical segment being the most frequently damaged.3,13 Less frequent sites of injury include the tympanic segment, the main trunk just distal to the stylomastoid foramen, and the labyrinthine segment. At the time of surgery, two thirds to three fourths of patients were found to have complete transection of the facial nerve13; interposition grafts and transmastoid decompression have been the primary modalities of treatment. Because residual bullet fragments can remain lodged in the temporal bone and can become a nidus for infection, a canal wall down or radical mastoidectomy has been advocated as the approach of choice.13,15 Gunshot wounds of the temporal bone frequently result in loss of a segment of the nerve, usually in the vertical portion, requiring interposition grafting for repair. There is also a high incidence of concomitant vascular and central nervous system injuries.
PATIENT EVALUATION
The presentation of facial nerve injuries greatly affects their management. The presence of a tightly enclosing fallopian canal around the intratemporal facial nerve makes the nerve much more susceptible to all types of trauma. Lack of any space to accommodate edema, which inevitably accompanies soft tissue trauma, leads to further neural injury. An injured nerve may not manifest significant clinical dysfunction initially, but later, after sufficient edema has occurred to prevent axoplasmic flow, the injury manifests. Fisch6 and others16–18 have shown that the area of the fallopian canal with the least expansion room for neural swelling is in the region of the meatal foramen. Because most injuries to the facial nerve occur in the perigeniculate area just distal to the meatal foramen, the edema produced in facial nerve injury is quite critical in the pathophysiology of this disorder.
The findings elicited from a careful history and physical examination on the patient’s presentation to the emergency department provide prognostic data and determine appropriate management. Eyewitness accounts of facial nerve function immediately after the injury and of any progression during transport to the emergency department are often unreliable and likely to be fraught with inaccuracy, but still can provide important information, especially if an initial examination is impossible because of other life-threatening injuries. An accurate analysis of facial nerve function might be impossible if the patient has been intubated and sedated as part of the primary survey, but every effort should be made to elicit some sort of facial movement, even a grimace, in a comatose patient. Patients with any facial movement after the injury and before the onset of paralysis rarely need surgical intervention.19 A nerve with diffuse weakness in all branches can be observed clinically, and if some function persists, expectant management can be employed. If this situation deteriorates to total paralysis, electric testing should be used to follow the nerve to ensure that total degeneration does not occur.
Audiometric evaluation should also be performed as soon as the patient’s condition permits. The type of hearing loss can corroborate CT scan findings. If surgical exploration is warranted, the severity of the hearing loss in the affected ear guides the surgeon in determining the best approach,19–21 and serves as a baseline with which to compare postoperative results.22
Prognosis Based on Electric Studies
Fisch23 and Esslen16 have postulated that surgery can facilitate return of facial nerve function if performed before complete degeneration. A level of 90% degeneration or less, as determined by electroneuronography (ENoG), has been correlated with a uniformly good prognosis for return of function. If the nerve is nonfunctional at the initial examination, the chance of a complete transection is high and will likely require surgery.
Patients with complete facial paralysis at the initial examination are screened daily with nerve excitability testing. This test uses direct transcutaneous stimulation of the nerve on each side of the face and determines a stimulation threshold that produces perceptible movement. The normal side is used as a control. If the threshold difference between the normal and dysfunctional sides exceeds 2.5 mA, ENoG is performed regularly thereafter. ENoG uses transcutaneous supramaximal stimulation of the facial nerve while recording the evoked potential from anterograde stimulation in the periphery of the face.24 The maximal evoked response of the nerve is measured on each side by use of a nonfixed recording electrode technique. A side-to-side comparison is made, with the normal side serving as the control. The percentage of degeneration is calculated as the difference between the two sides. More recent data have shown that a correlation exists between ENoG and nerve excitability testing: a 90% degeneration score on ENoG correlates to about a 3.5 mA difference on nerve excitability testing.25
Chang and Cass,26 in a comprehensive and critical assessment of the available literature on facial nerve injury secondary to temporal bone trauma, proposed that serial ENoG be performed in any patient with acute-onset complete facial nerve paralysis or acute-onset incomplete paralysis that subsequently progresses to complete paralysis. Only patients progressing to greater than 95% degeneration within 14 days are at risk for poor outcomes and should be offered facial nerve exploration. Patients with incomplete paralysis at presentation who do not progress to complete paralysis and patients who have a normal initial examination with subsequent delayed-onset facial nerve paralysis, whether complete or not, have an excellent prognosis for recovery and can be observed.
In a nonacute injury, ENoG can be relied on for up to 3 weeks, but after this period, a desynchronization (deblocking) of electrically evoked facial nerve discharge can occur, preventing a single unified discharge of all neurons in the trunk. This effect occurs because of the differing time courses over which recovering neurons re-establish electric conductivity and the capability to conduct an action potential. At this stage, it is no longer possible to compare the diseased, asynchronously discharging side with the unaffected, synchronously discharging side, making accurate determination of the severity of degeneration by this technique alone impossible. If by 3 weeks after injury the patient has not progressed to 90% degeneration, however, it is unlikely surgery would be needed.26
After 3 weeks, electromyography (EMG) may be used to establish whether recovering axons are present. This information is useful if delayed intervention is being considered. Voluntary motor units and polyphasic potentials indicate that regeneration is in progress. Lack of the foregoing and fibrillation potentials indicate a fully degenerated nerve without evidence of ongoing recovery. EMG can detect signs of wallerian degeneration, such as fibrillation potentials, only after the 10th day following nerve injury.6,7,21 A limitation of EMG is that it cannot be used initially following injury; however, it does not seem to have the reliability and reproducibility issues that several authors have found with ENoG.27,28
Timing of Surgery
Timing and even the necessity of surgery in some cases of facial nerve injury remain controversial. McCabe29 suggested that exploration and repair be done at 21 days after injury based on studies of motor neuron proteosynthetic activity levels and maximal repair activity at a neural anastomosis. More recent evidence does not support this theory, but does show a trend toward lower regeneration rates with increasing time after onset of injury.30
Facial nerve paresis arising from blunt trauma to the peripheral portion of the facial nerve should be managed expectantly. If no recovery is evident at the end of 6 months, reconstitution of the dysfunctional portion of the nerve may be required. Facial paralysis ensuing after laceration or iatrogenic injury to the parotid region is likely a transection, and is best repaired primarily and as soon as the patient’s condition permits. If a divided nerve cannot be repaired as soon as possible after the injury, at least limited exploration of the wound should be performed to identify the severed ends of the nerve for subsequent repair. The use of an electric nerve stimulator can be helpful for 48 hours after injury for stimulation of the distal ends of the severed nerve. The regional twitching of facial musculature can be used as an aid to nerve identification.4
Patients with immediate onset of complete facial nerve paralysis after a penetrating injury should also be offered surgery as soon as their condition permits because of the high likelihood of facial nerve transection and the need for nerve repair.20,25 This approach allows facial nerve recovery to proceed without delay.
ASSOCIATED TRAUMA
Ossicular Damage
Trauma to the ossicular chain frequently occurs in concert with damage to the intratemporal facial nerve. The ossicles are most often damaged in longitudinal temporal bone fractures as the fracture line passes through the vicinity of the attic and posterosuperior external auditory canal wall. Ossicles may be damaged by dislocation brought about by relative movement of supporting structures or by inertial factors associated with sudden movements of the supporting structures.31 Many different types of injury to the ossicles can occur, but the most frequent are dislocation of the incudostapedial joint, fractures of the stapes crura, and subluxation of the stapes footplate.32 The malleus is rarely injured, but occasional fractures of the long process of the malleus are seen.
Traumatic Otorrhea
The presence of CSF drainage from the ear or nose of a patient with head trauma is not unusual and represents a defect in the dural covering of the brain.33 The incidence of meningitis in patients with CSF leaks lasting longer than 7 days is 23% to 88%,19,34–36 and mortality may be 10% in traumatic cases.35 Only otologic sources are considered in this chapter, but an anterior or middle cranial fossa defect in the sinus region should always be considered in the differential diagnosis of CSF rhinorrhea, especially in traumatic injuries. Fluid originating from a posterior or middle fossa fracture defect may enter the mastoid and middle ear and drain into the nose or the oropharynx. CSF otorrhea is frequently associated with longitudinal temporal bone fractures because the fracture defect may result in a dural tear, whereas a step-off in the external ear canal provides a direct channel for egress of the fluid from the middle ear.
Clear fluid in the ear should alert the examiner to the presence of CSF, and the diagnosis should be straightforward. The diagnosis of CSF rhinorrhea is typically confounded, however, by the appearance of clear nasal secretions frequently accompanying nasal trauma. An informal test to make this differentiation is the halo test, whereby the fluid is placed on filter paper and allowed to diffuse. Blood in the sample is left behind, and a ring of clear fluid surrounds the red ring of blood products. The glucose levels in nasal secretions have also been used to identify CSF; high levels (>50 mg/100 mL) are considered to be indicative of CSF. The most sensitive and specific method of differentiation seems to be protein electrophoresis of the sample: the β2 fraction of transferrin is specific to CSF.37
Pneumocephalus is a dreaded, potentially treacherous complication of a defect in the dural protective barrier of the brain.38 It occurs when air is introduced into the cranial cavity via a fistula in the dura. Frequently, pneumocephalus is accompanied by CSF leakage, but it may occur in the absence of clinical evidence of fluid leakage. The major difficulty in this entity is the potential formation of a tension pneumocephalus from a ball-valve defect in the dura. Continued accumulation of air may induce intracranial hypertension, with resultant brain herniation. Aggressive management is required in CSF fistulas accompanied by persistent pneumocephalus.
The treatment of CSF otorrhea must take into account the natural history of the entity and, in particular, the incidence of meningitis. Conservative management is possible because of the high probability that the condition will heal spontaneously, and because of the efficacy of present generation antibiotics in treating meningitis. A more recent meta-analysis has shown that the use of prophylactic antibiotics has led to a significant reduction in the incidence of meningitis.39 In a series of anterior cranial fossa CSF fistulas, 35% resolved by 24 hours, and 85% healed by 1 week.34 The most common organisms isolated from post-traumatic meningitis cases are Pneumococcus spp., and antibiotics are effective against them.
The indications for operative management of CSF fistula include persistent leakage despite adequate conservative measures, recurrent meningitis, and persistent pneumocephalus.40 These cases may be approached via the middle ear, mastoid, or middle fossa, depending on the extent and localization of the defect. Generally, the defect should be identified and repaired with some form of soft tissue reinforced by bone, where possible. Discrete defects in the dura of less than 1 cm should be closed, and the area should be reinforced with fascia. If a mastoid tegmen defect is identified, the dural defect is repaired, reinforced with soft tissue, and finally a bone graft is used to stabilize the repair in the face of continuous CSF pulsation pressure and gravity.
SURGICAL TREATMENT
Technique
Intratemporal Nerve Segment
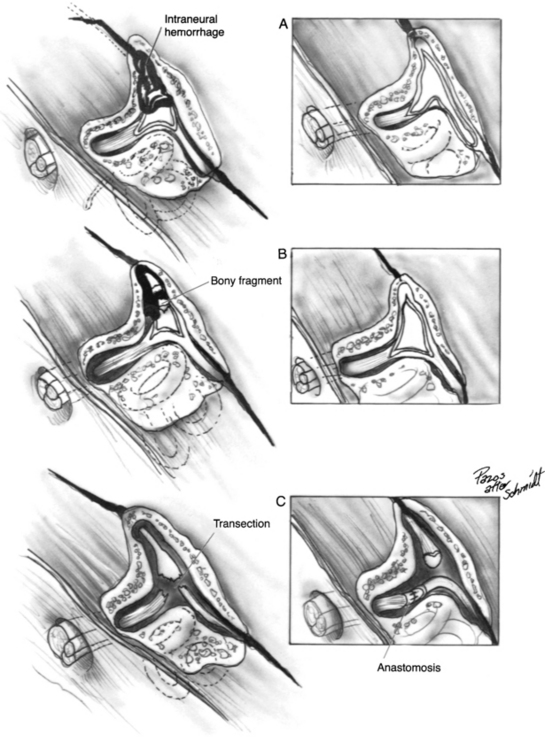
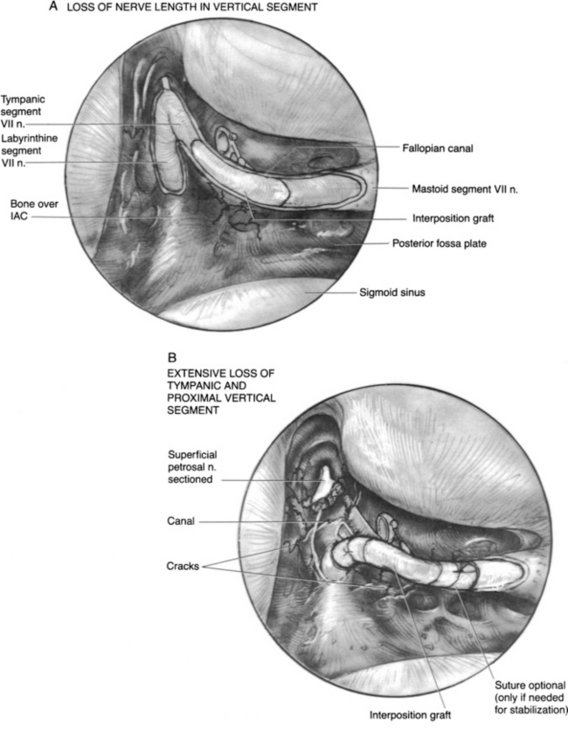
The surgical technique for mastoid facial nerve exploration is discussed in Chapter 16. Details specific to post-traumatic treatment for paralysis are presented here. Surgery for intratemporal traumatic facial paralysis centers on exposure of the damaged segment of the fallopian canal, facilitating surgical repair and providing the nerve room to expand. In treating damage to the nerve in the intratemporal segment, several factors must be taken into account, including status of individual nerve fibers, percentage of nerve loss, and length of nerve trunk loss. The techniques used for the repair of common lesions are shown in Figure 29-8. In injuries to the fallopian canal, the bone fragments may be in good reduction, betraying the true extent of injury to the nerve. In these cases, an intraneural hematoma can be produced by free blood within the intact nerve epineurium. Blood staining is frequently observed, but in some cases, the nerve appears diffusely enlarged without areas of visible sheath staining. The nerve sheath should be incised using sharp, atraumatic technique and taking special care to preserve all underlying nerve fascicles (see Fig. 29-8A). The Ziegler ophthalmic knife (Storz) is useful for incising the nerve sheath.
After the nerve sheath has been opened, the degree of nerve loss is assessed. If a significant portion of the nerve fibers seems to be divided, clean division of the remaining trunk followed by an interposition graft should be considered. Bony fragments impinging on the nerve sheath should be atraumatically removed, and the nerve should be assessed for intraneural hematoma and treated as discussed earlier (Fig. 29-8B). Complete transection situations can be repaired using nerve rerouting, when the anatomy permits, to avoid the need for an interposition graft (Fig. 29-8C and 29-9B). Gunshot wounds to the temporal bone with a tympanic or labyrinthine segment injury and a dead ear are the typical situation (Fig. 29-9).6 The interposition graft should be performed with a donor nerve graft of the appropriate diameter. In many situations, creation of a bony channel can enhance nerve and graft alignment without the need for multiple stabilization sutures and attendant postoperative inflammation (Fig. 29-9A).5 In all cases, the nerve should be widely decompressed until nonedematous nerve is encountered. In situations in which the loss of mastoid segment nerve tissue is minimal, the nerve can be dissected from the stylomastoid foramen region, and the parotid facial nerve can be mobilized proximally to obviate an interposition graft.
The most common area of damage to the intratemporal segment is in the perigeniculate and labyrinthine regions, but location of the precise area of damage should be ascertained on preoperative CT scans. The middle fossa approach to decompression is used when labyrinthine function must be preserved. If clinical suspicion is confirmed by preoperative CT scans, and intraoperative findings warrant, decompression of the mastoid segment of the facial nerve can be performed via a mastoid approach (see Chapter 16). During middle fossa decompression of the proximal facial nerve, the condition of the distal nerve can be determined by examination of the tympanic portion as it is exposed. If the nerve appears to be in good condition with no hemorrhage or edema evident, and no other indications exist for exploration of the mastoid portion of the nerve, exploration need not be done.4
Repair should be performed at all anastomoses by use of neuroscopic technique, atraumatic handling of tissues, exact end-to-end anastomosis, tension-free closure, and the use of monofilament 9-0 or 10-0 suture.5 Interposition grafts are employed when nerve tissue loss would produce tension in a direct anastomosis, or when diseased nerve segments must be excised. The greater auricular nerve is adequate for defects less than 7 cm, and the sural nerve can bridge defects 30 cm long. The superiority of perineurial over epineurial repair has not been shown, but meticulous techniques are useful in reconstitution of the facial nerve.41
Extratemporal Nerve Segment
Establishing a functionally intact nerve in the frontal and marginal mandibular distribution of the face is important because of the limited anastomotic interconnection from adjacent branches of the facial nerve in these regions. Conversely, small branches in the midface region do not require extensive procedures to re-establish continuity because of the rich anastomotic network that already exists. This quality leads to a much higher probability of a favorable outcome. Details of neural anastomosis techniques can be found in Chapter 30.
The technique for exposure of the peripheral nerve is based on techniques commonly used in parotid surgery. Penetrating trauma to the nerve at the stylomastoid foramen region may prevent identification of the nerve proximally; the nerve would need to be found in the periphery and traced back proximally. Finding a branch of the marginal mandibular or frontal distribution is frequently useful.42 If the exploration is performed within 2 days of the injury, the distal branches of the nerve can be stimulated by electric current, and observation of facial musculature twitching can help find the distal stump.
RESULTS
The postoperative result depends largely on the severity of injury to the nerve and to a lesser extent the timing of intervention. Recovery is excellent for Sunderland’s neurapraxia (class I) or axonotmesis (class II) lesions, which involve intact endoneurial tubules. Primary anastomosis or interposition is required for neurotmesis (class III-V) injuries because complete separation of the nerve has occurred.43 The best surgical result in this condition is a House-Brackmann grade III or grade IV (American Academy of Otolaryngology–Head and Neck Surgery), with the latter being more typical. Crush and compression injuries are Sunderland class III or IV lesions, with varying degrees of endoneurial and perineurial disruption and no gross disruption of the nerve. These injuries have varying degrees of recovery, but grades III to V are usually attainable. The decision to resect and perform an anastomosis or simply to decompress is left to the surgeon; few physiologic data on which to base such a decision exist.43 The best prognosis is obtained with early intervention in the acute phase of degeneration and before the onset of continuing endoneurial degeneration and fibrosis, which impede the eventual re-establishment of competent axons.
COMPLICATIONS
Infection of a middle fossa wound has never occurred in the senior author’s (H.A.J.) series.44 Because of the low incidence of postoperative infection, it has not been necessary to modify our surgical approach to produce a staggered incision through the skin and temporalis muscles,44 but rather we use an incision straight through the skin down to bone.
1. Cannon C.R., Jahrsdoerfer R.A. Temporal bone fractures: Review of 90 cases. Arch Otolaryngol. 1983;109:285-288.
2. Ghorayeb B.Y., Yeakley J.W. Temporal bone fractures: Longitudinal or oblique? The case for oblique temporal bone fractures. Laryngoscope. 1992;102:129-134.
3. Duncan N.O.3rd, Coker N.J., Jenkins H.A., Canalis R.F. Gunshot injuries of the temporal bone. Otolaryngol Head Neck Surg. 1986;94:47-55.
4. Coker N.J., Kendall K.A., Jenkins H.A., Alford B.R. Traumatic intratemporal facial nerve injury: Management rationale for preservation of function. Otolaryngol Head Neck Surg. 1987;97:262-269.
5. Coker N.J. Management of traumatic injuries to the facial nerve. Otolaryngol Clin North Am. 1991;24:215-227.
6. Fisch U. Facial paralysis in fractures of the petrous bone. Laryngoscope. 1974;84:2141-2154.
7. Lambert P.R., Brackmann D.E. Facial paralysis in longitudinal temporal bone fractures: A review of 26 cases. Laryngoscope. 1984;94:1022-1026.
8. Gurdjian E.S., Lissner H.R. Deformation of the skull in head injury studied by “stresscoat” technique: Quantitative determinations. Surg Gynecol Obstet. 1946;83:219-233.
9. Yanagihara N., Murakami S., Nishihara S. Temporal bone fractures inducing facial nerve paralysis: A new classification and its clinical significance. Ear Nose Throat J. 1997;76:79-80. 83-86
10. Dahiya R., Keller J.D., Litofsky N.S., et al. Temporal bone fractures: Otic capsule sparing versus otic capsule violating clinical and radiographic considerations. J Trauma. 1999;47:1079-1083.
11. Ishman S.L., Friedland D.R. Temporal bone fractures: Traditional classification and clinical relevance. Laryngoscope. 2004;114:1734-1741.
12. Little S.C., Kesser B.W. Radiographic classification of temporal bone fractures: Clinical predictability using a new system. Arch Otolaryngol Head Neck Surg. 2006;132:1300-1304.
13. Shindo M.L., Fetterman B.L., Shih L., et al. Gunshot wounds of the temporal bone: A rational approach to evaluation and management. Otolaryngol Head Neck Surg. 1995;112:533-539.
14. Moore P.L., Selby G., Irving R.M. Gunshot injuries to the temporal bone. J Laryngol Otol. 2003;117:71-74.
15. Bento R.F., de Brito R.V. Gunshot wounds to the facial nerve. Otol Neurotol. 2004;25:1009-1013.
16. Esslen E. The acute facial palsies: Investigations on the localization and pathogenesis of meato-labyrinthine facial palsies. Schriftenr Neurol. 1977;18:1-164.
17. Lang J. Anatomy of the brainstem and the lower cranial nerves, vessels, and surrounding structures. Am J Otol. 1985;6(Suppl):1-19.
18. Ge X.X., Spector G.J. Labyrinthine segment and geniculate ganglion of facial nerve in fetal and adult human temporal bones. Ann Otol Rhinol Laryngol. 1981;90(Suppl):1-12.
19. Brodie H.A., Thompson T.C. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18:188-197.
20. McKennan K.X., Chole R.A. Facial paralysis in temporal bone trauma. Am J Otol. 1992;13:167-172.
21. Darrouzet V., Duclos J.Y., Liguoro D., et al. Management of facial paralysis resulting from temporal bone fractures: Our experience in 115 cases. Otolaryngol Head Neck Surg. 2001;125:77-84.
22. Nosan D.K., Benecke J.E.Jr., Murr A.H. Current perspective on temporal bone trauma. Otolaryngol Head Neck Surg. 1997;117:67-71.
23. Fisch U. Prognostic value of electrical tests in acute facial paralysis. Am J Otol. 1984;5:494-498.
24. Gantz B.J., Gmuer A.A., Holliday M., Fisch U. Electroneurographic evaluation of the facial nerve: Method and technical problems. Ann Otol Rhinol Laryngol. 1984;93:394-398.
25. Coker N.J., Fordice J.O., Moore S. Correlation of the nerve excitability test and electroneurography in acute facial paralysis. Am J Otol. 1992;13:127-133.
26. Chang C.Y., Cass S.P. Management of facial nerve injury due to temporal bone trauma. Am J Otol. 1999;20:96-114.
27. Adour K.K., Sheldon M.I., Kahn Z.M. Maximal nerve excitability testing versus neuromyography: Prognostic value in patients with facial paralysis. Laryngoscope. 1980;90:1540-1547.
28. Sittel C., Guntinas-Lichius O., Streppel M., Stennert E. Variability of repeated facial nerve electroneurography in healthy subjects. Laryngoscope. 1998;108:1177-1180.
29. McCabe B.F. Injuries to the facial nerve. Laryngoscope. 1972;82:1891-1896.
30. Barrs D.M. Facial nerve trauma: Optimal timing for repair. Laryngoscope. 1991;101:835-848.
31. Hough J.V., Stuart W.D. Middle ear injuries in skull trauma. Laryngoscope. 1968;78:899-937.
32. Bellucci R.J. Traumatic injuries of the middle ear. Otolaryngol Clin North Am. 1983;16:633-650.
33. Canniff J.P. Otorrhoea in head injuries. Br J Oral Surg. 1971;8:203-210.
34. Mincy J.E. Posttraumatic cerebrospinal fluid fistula of the frontal fossa. J Trauma. 1966;6:618-622.
35. Leech P.J., Paterson A. Conservative and operative management for cerebrospinal-fluid leakage after closed head injury. Lancet. 1973;1:1013-1016.
36. Westmore G.A., Whittam D.E. Cerebrospinal fluid rhinorrhoea and its management. Br J Surg. 1982;69:489-492.
37. Oberascher G. Cerebrospinal fluid otorrhea—new trends in diagnosis. Am J Otol. 1988;9:102-108.
38. Andrews J.C., Canalis R.F. Otogenic pneumocephalus. Laryngoscope. 1986;96:521-528.
39. Brodie H.A. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123:749-752.
40. Marentette L.J., Valentino J. Traumatic anterior fossa cerebrospinal fluid fistulae and craniofacial considerations. Otolaryngol Clin North Am. 1991;24:151-163.
41. Orgel M.G. Epineurial versus perineurial repair of peripheral nerves. Clin Plast Surg. 1984;11:101-104.
42. Lore J.M.Jr. The parotid salivary gland. In: Lore J.M.Jr., editor. An Atlas of Head and Neck Surgery. Philadelphia: Saunders; 1988:708-713.
43. Coker N.J., Jenkins H.A., Psifidis A. Electrophysiological prognostication of acute facial nerve trauma. In: Fisch U., Valavanis A., Yasargil M.G., editors. Neurological Surgery of the Ear and the Skull Base. Amsterdam: Kugler & Ghedini; 1989:355-362.
44. Holsinger F.C., Coker N.J., Jenkins H.A. Hearing preservation in conservation surgery for vestibular schwannoma. Am J Otol. 2000;21:695-700.