23 Trauma Management
After reading this chapter, you should be able to:
• identify the benefits and limitations of an organised trauma system
• describe the rationale for a systematic approach to the patient who has sustained injuries
• discuss the benefits of appropriate nursing care of the patient with serious injury and/or multitrauma
• describe the acute nursing management of the patient with multiple serious fractures
• describe the acute nursing management of patients with burn injuries, abdominal injuries and chest trauma
• describe the nurse’s role in managing the trauma patient undergoing interim damage-control surgery.
Introduction
Trauma refers to physical injury that is caused by mechanical injury, also known as kinetic injury. Injury remains the leading cause of death in adults under 45 years of age, and is a leading cause of preventable mortality and morbidity in Australia and New Zealand, as well as the rest of the world.1–4 Furthermore, injury represents a major cost to injured individuals, the healthcare system and society.5,6 More than 5.2 million people throughout the world die due to injury, with 90% occurring in low- to middle-income countries. According to the World Health Organization, injury accounts for 16% of the world’s disease burden.7
Trauma Systems and Processes
A trauma system can be defined as:
an assembly of health care processes intended to improve survival among injured patients by reducing the time interval between injury and definitive treatment, and by assuring that appropriate resources and personnel are immediately available when a patient presents to a hospital’.8, p. 643
Without trauma systems in place, a range of organisational and clinical errors in the management of trauma patients have been identified. These errors occur at all stages of care, including prehospital, emergency, operating theatre, intensive care unit, wards, and during transfers between hospitals.9 The majority of errors identified were errors in management of patients, although approximately 20% of errors occurred as a result of system inadequacies. A smaller number of technique or diagnostic errors occurred.
Over the past 20 years there has been increasing emphasis on the development of trauma systems that cover geographical areas, such as a nominated state or region. The introduction of trauma systems has resulted in a 15–30% reduction in the risk of death, primarily in the area of preventable deaths.10 Although this reduction appears widespread, it has not been replicated in remote areas,11 and is limited by the lack of examination of deaths that occur before a patient reaches hospital or after discharge. Additionally, the lack of examination of functional outcomes limits interpretation of the trauma system, as it is not clear whether the patients who survive have altered functional capacity. Despite these limitations, there is widespread agreement on the benefits of trauma system implementation, although the contribution of nursing care in such trauma systems is rarely considered or measured. Furthermore, the precise components of a trauma system that prove beneficial have not been identified.12
Prehospital Care
The debate regarding the relative benefits of stabilising a patient at the scene versus proceeding to the hospital as quickly as possible, is not new.13 Benefits are somewhat dependent on the proximity of effective trauma facilities, the level of knowledge and skills of the prehospital personnel available and the specific injuries and condition of the patient. The principle of the ‘golden hour’ remains in place today and suggests that, in order to improve outcomes, definitive care should be provided to patients as soon as possible, and preferably within 1 hour of the injury being sustained.13,14 In countries with large distances and sparse populations this aim presents particular challenges and cannot be met in many regions. Despite these distances and transport challenges, recognition of life-threatening conditions, application of appropriate emergency interventions and prompt transport to the nearest appropriate hospital remain the principles of prehospital care.13–15
In a number of regions, processes are in place to facilitate prehospital admission: personnel can notify the receiving hospital in advance, for those patients who meet predefined criteria. Identified patients generally have severe physiological compromise, or injuries from high-velocity causes that result in significant injury and associated poor outcomes. Early notification allows the assembly of a multidisciplinary group of health professionals who can provide immediate, expert assessment, resuscitation and treatment of critically injured patients.16,17 Such trauma teams have been shown to provide benefit in the early management of multiply-injured trauma patients, and are reviewed later in this chapter.10,16
Transport of the Critically Ill Trauma Patient
Transport of critically injured patients occurs at two stages in the patient’s care. Primary transport occurs from the place of injury to the first healthcare facility to provide care to the patient; this is sometimes referred to as prehospital transport. Secondary transport occurs between healthcare facilities; this is sometimes referred to as interhospital transport. This chapter concentrates on secondary transport, although many of the principles are similar for both stages of transport. Intrahospital transport principles are also relevant for critically injured patients being transferred within departments in a healthcare facility (see Chapter 6). Transport of a patient between healthcare facilities may occur for clinical reasons, such as specialist or higher levels of care being required, or for non-clinical reasons, such as bed availability. It is preferable for patient transfer to be for clinical reasons only; however non-clinical transfer is sometimes unavoidable.
• the condition of the patient
• the potential impact of the transport medium on the patient
• the urgency of the transport
Amenities such as landing sites, particularly for helicopters, being in close proximity to healthcare facilities must also be considered. Different jurisdictions activate air retrieval using helicopters when the distance for the transport is beyond a certain point, with the minimum distance ranging from 16–80 km.14,15,19,20
It is essential that the standard of care is not compromised during transport of critically injured patients. Minimum standards exist that outline the requirements for transport of critically injured patients, and these should be referred to for full details.13,18,19 The following principles apply during such phases of care:
• There must be adequate preparation of the patient and equipment.
• Transport must occur by personnel with appropriate levels of expertise.
• Necessary equipment, including batteries and pumps, should be secured.
• Patients should be stabilised prior to transport (whilst balancing the need for timely transport).
• Monitoring of relevant aspects of the patient’s care is essential.
• Adequate vascular access and airway control must be secured prior to commencing transport.
• Effective communication is mandatory between referring, transporting and receiving personnel.
• Documentation, including X-rays and scans, should accompany the patient and should cover the patient’s status, assessment and treatment before, during, and on completion of, the transport.
• Relatives should be informed of the transfer, including destination, and provided with assistance for their own travel arrangements.18 Checklists itemising many of these principles, sometimes attached to an envelope containing all transfer documentation, are often used to ensure that all necessary actions are undertaken.20
Trauma Reception
The formal process of triage provides a means of categorising patients based on threat to life. Although there are many different triage systems in use, within Australia and New Zealand, the five-level triage categorisation of the Australasian Triage Scale (ATS) is widely used.23 See Chapter 22 for further description of the ATS.
Primary Survey
Priorities of care are similar to those in all health settings, with airway, breathing and circulation taking precedence, and disability and exposure/environment being part of the primary survey (see Chapter 22). These components of care will often occur simultaneously rather than sequentially. Compromise to airway and breathing may result from direct injury, for example to the trachea, or indirectly through decreased level of consciousness. Compromise to circulation is usually as a result of significant blood loss, although it may occur as a result of injuries, such as cardiac contusions in chest trauma, or the patient’s preexisting disease. The priorities of care during this time reflect the principles of care in any setting, and include:
Secondary Survey
Following stabilisation of the life-threatening problems identified during the primary survey, patients should undergo a secondary survey (see Chapter 22). This is a systematic examination of the body regions to identify injuries that have not yet been recognised. It is essential that both the front and the back of the patient, as well as areas covered by clothing, are examined during this process.
Radiological and Other Investigations
If the patient is sufficiently stable after the secondary survey, more extensive investigation in the radiology department should be undertaken. This will include CT scans. It is essential that clinicians consider investigations carefully, to ensure that all necessary imaging is undertaken; for example, where a CT scan of the brain is required it is often prudent to also undertake a CT scan of the cervical spine. However care should be taken to avoid investigations that will not change the planned treatment but may delay urgent interventions such as surgery. Current controversies in radiation exposure and lifetime-associated cancer risks need to be considered.21 Furthermore, the implications of moving the patient on and off imaging tables for repeated imaging is problematic. The patient should be accompanied and monitored by an appropriately competent nurse during all transfers for investigation. Where the patient is requiring ongoing advanced life support such as fluid resuscitation or airway monitoring, it may also be appropriate for a medical officer to accompany the patient.
Focused assessment with sonography for trauma
Where abdominal trauma is suspected, a focused assessment with sonography for trauma (FAST) examination22,23 is likely to be used as part of the secondary survey to determine whether free fluid is present in the abdominal cavity. The abdomen is scanned in four zones – pericardial, Morison’s pouch (right upper quandrant), splenorenal (left upper quadrant), and pelvis (Douglas’ pouch). This generally takes 1–2 minutes when performed by an experienced, credentialled clinician. Findings are regarded as positive (fluid [blood] observed), negative or equivocal. Technical difficulties can be experienced with obese patients. While a positive FAST is useful in identifying if a patient should receive urgent surgical intervention, a negative FAST does not rule out significant abdominal trauma, and the low sensitivity of FAST remains a concern for trauma clinicians.22 Where a patient is undergoing a prolonged trauma resuscitation phase, there may be an indication to repeat the FAST after 20 minutes. The use of FAST examination outside the trauma resuscitation and reception phase is occurring more often and can be undertaken in any clinician setting where there is a suspicion of internal haemorrhage or pneumothorax.24
Trauma Teams
There are a number of different ways to organise the early care of trauma patients. The most common method used is through the establishment of multidisciplinary trauma teams that can provide immediate, expert assessment, resuscitation and treatment of traumatised patients, especially those with multiple injuries. Many hospitals that receive trauma cases operate trauma teams that are either activated or placed on standby, via pagers or telephone, based on communications from paramedic personnel in the prehospital setting.25 This activation is based on a combination of physiological and injury criteria (see Table 23.1). Age is sometimes added to the patient criteria, with those under 5 years or over 65 years receiving particular attention. A number of hospitals have two levels of trauma team activation, with more severe injuries activating the full trauma team and less severe activating a partial team. The use of two-tiered trauma team activation has not been shown to affect patient outcomes.17
| Physiological criteria | Injury criteria |
|---|---|
| Heart rate <50 or >120 beats/min Respiratory rate <10 or >29 breaths/min Systolic blood pressure <90 mmHg Glasgow Coma Scale Score <10 Skin pale, cool or moist Paralysis Trauma arrest |
Penetrating injury to head, neck or torso Burn to ≥20% body surface area Fall ≥5 metres Multiple trauma Crush or degloving injury to extremity Amputation proximal to the wrist or ankle Motor vehicle crash with ejection |
Common Clinical Presentations
Patients with multitrauma will also be cared for according to the principles of care for each specific injury, although consideration of priorities is essential. Care should follow the common principles of airway, breathing and circulation, therefore concentrating on respiratory and circulatory compromise first, before moving on to the treatment of other injuries. The relative importance of other injuries, for example neurological trauma or skeletal trauma, will vary for each individual patient and will be dependent on the physiological impact of the injuries. Neurological and spinal cord injury are reviewed in Chapter 17.
Mechanism of Injury
The most common causes of traumatic injury include road traffic crashes, falls and collisions. While falls account for the greatest number of injuries requiring hospitalisation,28 injuries sustained in road traffic crashes tend to be more severe given the high velocity of the trauma, and account for the greatest number of major injuries, including those injuries requiring a critical care admission.28–30
The mechanism of injury is recognised as affecting both survival and requirement for admission to the intensive care unit. Patients who are injured in a road traffic crash experience a similar mortality to those injured through falls (approx 3% in all patients and 10–17% in major injury patients), with both groups having a higher mortality than patients injured in assaults and collisions with objects (<1% in all patients and 12% in major injury patients).28,29 The older age group, with associated comorbidities, is likely to account for many of the deaths in the group injured through falls. In addition, patients injured in road traffic crashes tend to spend longer in the intensive care unit than patients injured through falls or assaults and collisions, and experience a greater number of injuries.28
Generic Nursing Practice
Positioning and Mobilisation of the Trauma Patient
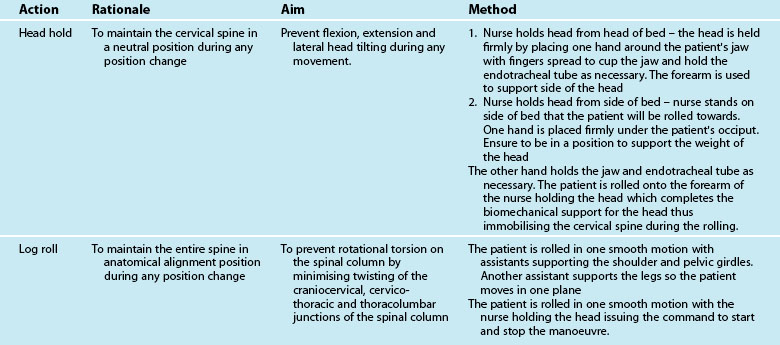
Difficulty in positioning and mobilisation is often experienced when there is concern for the stability of the patient’s cervical spine, particularly in unconscious patients. Specific protocols for confirming the absence of injury to the cervical spine in unconscious patients, or those complaining of cervical soreness or abnormal neurology, vary between institutions and regions, but generally incorporate the following principles:31
• Obtain a detailed history of the injury wherever possible, including specific investigation of mechanisms of injury that might exert force on the cervical spine. A high index of suspicion should remain, particularly in the setting of injuries often associated with cervical spine injury, including craniofacial trauma rib fractures, pneumothoraces and damage to the great vessels and/or trachea.
• Undertake plain X-rays of the full length of the spine, interpreted by a radiologist.
• Where any abnormality exists in clinical or radiological assessment, or the patient remains unconscious, a CT or MRI may be undertaken, and this must be reported on by a radiologist.
• A correctly fitted hard collar should remain in place only until the patient is appropriately reviewed and the chance of a cervical spine injury is eliminated. If a collar is required for more than 4 hours, a long-term collar (e.g. Philadelphia, Aspen or Miami J) should be used.
• Maintain appropriate pressure area care to areas under the hard collar as well as usual pressure points until cervical clearance is gained.32
The two methods available for moving the trauma patient are staff manual handling and lifting hoists. Generally, trauma patients can be log-rolled (see Figure 23.1 for initial care and p. 635 for later care) as frequently as required for nursing care. Any restrictions to patient positioning and weight bearing due to injuries or physiological status must be considered through this process; it is essential that care be taken to prevent any worsening of injuries due to handling of the patient. Knowledge of the position restrictions for each limb, including all weight-bearing joints and the vertebrae, is imperative to avoid secondary iatrogenic injury. Certain injuries will impose position and mobility restrictions (see Table 23.2).
TABLE 23.2 Position and mobility restrictions in trauma patients
| Type of injury | Restrictions |
|---|---|
| Traumatic brain injury |
• Position restrictions are dependent on severity of fracture(s), use of external fixateurs and degree of stabilisation.
• Some patients may sit out of bed and ambulate with external pelvis fixateur in situ.
• Position restrictions require regular review, as changed or loss of fixation may affect recovery.
ICP = intracranial pressure.
Practice tip
The NEXUS low-risk criteria have been widely accepted as identifying patients in whom further examination is unnecessary and cervical spine injury can be excluded on the basis of clinical examination.82 These criteria include absence of midline cervical spine tenderness, no focal neurological deficit, no intoxication, no painful distracting injury and normal alertness.
The ‘Trauma Triad’
The critically injured patient can experience the ‘trauma triad’ of hypothermia, acidosis and coagulopathy. While it is possible to experience these pathophysiological conditions individually, they often occur simultaneously. Additionally, hypothermia is a common contributor to the exacerbation of both acidosis and coagulopathy.33–38 Acidosis has been discussed in earlier chapters so is reviewed here only as it interacts with hypothermia and coagulopathy in the trauma setting. Low cardiac output, hypotension, hypoxia, hypothermia and rhabdomyolysis are common causes of acidosis in the trauma setting. The increased recognition of the importance of this triad in the trauma setting has led to the development of damage control surgery. The principle of this surgery is reviewed below.
Hypothermia
Hypothermia is defined as a core temperature <35°C and is associated with high morbidity and mortality. Even in sub-tropical environments, hypothermia is identified in approximately 10% of major trauma cases during the prehospital or in-hospital phase of care.36,39
Uncontrolled causes of hypothermia can be endogenous or accidental.33,34,37,39 Endogenous causes include metabolic dysfunction with decreased heat production, or central nervous system dysfunction with insufficient thermoregulation such as in neurological trauma. Dermal dysfunction, such as a burn, is another endogenous cause of hypothermia.
Accidental hypothermia can occur without thermoregulatory dysfunction, and generally occurs in the trauma patient as a result of environmental exposure either at the injury site or during transport to, or between, healthcare facilities, as a result of large-volume fluid resuscitation or during prolonged surgical procedures. The pathophysiological changes associated with hypothermia vary depending on the severity, and are outlined in Chapter 22. Of particular relevance, shivering leads to increased oxygen consumption and acidosis, and platelet dysfunction leads to impaired clotting.33,36,39
Coagulopathy
Coagulation is widespread in the trauma setting, and ranges from a mild defect in coagulation function to life-threatening coagulopathy. Defects in coagulation may be caused by dilution, hypothermia, acidosis, tissue damage or the effects of underlying disease.34,35
Dilution results from the transfusion of either crystalloid or colloid fluids, and occurs as the concentration of coagulation factors in the patient’s blood is diluted with the transfused fluid. It should be remembered that transfusion of red blood cells has the same effect, as whole blood or packed cells have undergone some dilution and have reduced viability of platelets.38 Hypothermia causes coagulopathy because many of the enzymatic reactions in coagulation are temperature-dependent. Platelet and thromboplastin function both decline with even moderate (34°C) hypothermia, while hypothermia stimulates fibrinolysis.34,40
Acidosis reduces the activity of both the extrinsic and the intrinsic coagulation pathways, as well as platelet function. This is particularly pronounced with a pH below 6.8.34 Tissue damage causes endothelial disruption and defibrination, which promote the systemic activation of coagulation; this is particularly profound in patients with brain injury due to the high level of thromboplastin in brain tissue.34,37,38 The final cause of coagulopathy in trauma is the underlying disease present in many patients. Patients may have a coagulation defect such as haemophilia or von Willebrand’s disease, or liver disease with resultant compromise to coagulation on an ongoing basis. Alternatively, patients may be taking anticoagulants, such as aspirin or warfarin, as treatment for other health conditions.37,41
Treatment of coagulopathy should focus first on prevention of coagulopathy and then on the treatment as required. Prevention strategies include:40
• maintaining normothermia in critically injured patients through the use of blankets, warming devices, and minimisation of exposure and theatre time
• administering as little resuscitation fluid as is necessary to maintain adequate circulation
• achieving control of haemorrhage as soon as possible, through techniques such as low-pressure resuscitation and damage-control surgery.
Treatment includes transfusion of platelets, fresh frozen plasma (FFP) and cryoprecipitate, as well as the plasma derivatives showing promise in this area of treatment.35 While transfusion of platelets is specifically directed towards increasing the circulating concentration of platelets, administration of FFP is directed at increasing the levels of fibrinogen and other coagulation factors. Cryoprecipitate is made by freezing and thawing individual units of FFP and collecting the precipitate, a process that concentrates fibrinogen, von Willebrand factor, factor VIII and factor XIII.
Damage-control Surgery
Damage-control surgery can be defined as a four-stage procedure, involving early recognition of relevant patients and ‘rapid termination of an operation after control of life-threatening bleeding and contamination followed by correction of physiological abnormalities and definitive management’.42,43 This approach to surgical correction of traumatic injuries gained favour through the latter part of the 1990s and is intended to reduce the development of the triad of complications of hypothermia, acidosis and coagulopathy. The intention is that surgery is initiated rapidly, only the most rapid and simplest interventions that are required to stop bleeding and contamination are undertaken, then surgery is completed and the patient moved to definitive care, usually in the ICU.42 Care can then be undertaken to ensure that hypothermia, acidosis and coagulopathy do not develop or, if present, are rapidly reversed, thereby ensuring correction of physiological abnormalities as quickly as possible. Definitive surgical correction of injuries is undertaken during the ensuing days when the patient is physiologically stable. Damage-control surgery can apply to a range of patients, including those with abdominal, skeletal and thoracic trauma.
Skeletal Trauma
Skeletal trauma involves injury to the bony structure of the body. While skeletal injuries alone rarely result in the patient being admitted to critical care, damage to surrounding blood vessels and nerves, as well as potential complications such as fat embolism syndrome (FES) and rhabdomyolysis, may cause the patient to become seriously ill. Patients with skeletal trauma who require admission to ICU include those with multiple injuries, severe pelvic fractures (often associated with significant blood loss), long bone fractures (often associated with FES) and thoracic injuries such as flail segment. A small number of people with crush injuries that cause significant damage to muscles, often resulting in rhabdomyolysis, also require admission to the ICU.44,45
Skeletal trauma is the form of trauma that causes the highest number of patients to be admitted to hospital for 24 hours or more, with approximately 50% of patients experiencing a fracture as their main injury.28 Of those patients admitted to an ICU, fractures are the second most common type of injury (after head injury), with approximately 20% of patients experiencing this type of injury.
Pathophysiology
Bone is composed of an organic matrix as well as bone salts. The majority of the organic matrix is collagen fibres and the remainder is ground substance, a homogeneous gelatinous medium composed of extracellular fluid plus proteoglycans.46 Calcium and phosphate are the primary bone salts, although there are smaller amounts of magnesium, sodium, potassium and carbonate ions. These ions combine to form a crystal known as hydroxyapatite.
A fracture is simply defined as a break in the continuity of a bone. Fractures generally occur when there is force applied that exceeds the tensile or compressive strength of the bone. In patients sustaining a major injury (injury severity score [ISS] ≥16) fractures are the primary injury in more than 15% of cases, although many patients experience a fracture in addition to other serious injury resulting in ICU admission.28
A fracture causes disruption to the periosteum, blood vessels, marrow and surrounding soft tissue, resulting in a loss of mechanical integrity of the bone. Bone is one of only two sites (the other being the liver) that will reform itself, not forming scar tissue when it heals.47 When a fracture occurs, there is initial bleeding and soft tissue damage around the site, with haematoma formation within the medullary canal. The healing sequence that follows a fracture depends on the type of fracture fixation that is used. When a fracture is fixed in a method that eliminates the interfragmentary gap and provides stability to the site, such as in screwing or wiring, primary healing takes place. When a fracture is fixed in a manner that reduces but does not eliminate movement around the fracture site, secondary healing takes place.48
In primary healing, also referred to as direct union, the haematoma that initially formed is eliminated by the apposition of fracture ends during reduction. Once the bone ends are intact, osteoclasts form cutting cones that in turn form new haversian canals across the fracture gap. These contain blood vessels that are essential to primary bone healing. By 5–6 weeks after the fracture, osteoblasts will fill the canals with osteons, which are the basic structure of the new bone.47 Although the bone is now formed, the strength and shape continues to develop over coming weeks.
In contrast to primary healing, secondary healing is characterised by an intermediate phase, where a callus of connective tissue is first formed and then replaced by bone.47,49 The secondary healing phase begins with an inflammatory phase in which the haematoma clots and provides initial support, then inflammatory cells invade the haematoma to remove necrosed bone and debris. The reparative phase begins 1–2 weeks after the fracture and consists of immature woven bone being laid down and strengthened through a process known as mineralisation. The final remodelling stage consists of replacement of the woven bone by lamellar bone, through osteoblasts secreting osteoid that is mineralised and forms interstitial lamellae. The remodelling of these structures occurs in response to appropriate levels of mechanical loading during this phase.47,48
Fat embolism
Fat embolism syndrome (FES) may occur in patients who have experienced a fracture of a long bone, particularly if multiple fractures or fractures to the middle or proximal parts of the femur are experienced. Fractures to the pelvis can also lead to a fat embolism. Incidence of FES is low (<1%). FES consists of fat in the blood circulation associated with an identifiable pattern of clinical signs and symptoms that include hypoxaemia, neurological symptoms and a petechial rash.49 Patients generally present 12–48 hours after they have experienced a relevant fracture and often require admission to a critical care unit for assessment and treatment, including mechanical ventilation.
Internationally, there continues to be disagreement regarding the pathophysiological changes associated with FES, although there is general consensus on the following principles. It has been accepted that there is a mechanical component to the changes that take place in FES, where fat is physically forced into the venous system and causes physical obstruction of the vasculature. Although marrow pressure is normally 30–50 mmHg, it can be increased up to 600 mmHg during intramedullary reaming (the process where the medullary cavity of the bone is surgically enlarged to fit a surgical implant such as a tibial nail), consequently reaching a pressure significantly above pressures throughout the vasculature.49 A second theory, associated with the biochemical changes that occur during trauma, proposes that trauma is associated with a higher level of circulating free fatty acids, which cause destabilisation of circulating fats and/or direct toxicity to specific tissues, including pulmonary and vascular endothelium.49
Rhabdomyolysis
Rhabdomyolysis is the breakdown of muscle fibres resulting in the distribution of the cellular contents of the affected muscle throughout the circulation, and occurs during the reperfusion of injured muscle. The cellular contents that are circulated include potassium, phosphate, organic acids, myoglobin, creatine kinase and thromboplastin.44 Two phases of injury are essential for the development of rhabdomyolysis: the first is when muscle ischaemia occurs, and the second is with reperfusion of the injured muscle. The length of time that muscle is ischaemic affects the development of rhabdomyolysis, with periods of less than 2 hours generally not producing permanent damage, but periods above this time resulting in irreversible anatomical and functional changes.44 The clinical sequelae of rhabdomyolysis include electrolyte abnormalities such as hypocalcaemia, hyperkalaemia and acidosis, hypovolaemia, acute renal failure and multiorgan failure.
Clinical Manifestations
Common forms of skeletal trauma include the following:
• Long bone fractures. The long bones are the humerus, radius, ulna, femur, tibia and fibula. Fractures of these bones are serious and can carry a high level of morbidity, especially if they involve a joint such as a trimalleolar fracture of the ankle (distal tibia and fibula). In many cases definitive surgical management is required, with internal fixation.
• Dislocations. All joints are at risk of traumatic dislocation, depending on the mechanism of injury. Dislocations can be limb-threatening if they cause neurovascular compromise. Reduction of traumatic dislocation is a medical emergency.
• Open fractures (compound). Any break in the skin that communicates directly with the fracture is classified as an open fracture. Open fractures carry a higher infection risk and require surgical treatment within 8 hours.50,51
• Traumatic amputation. Amputation refers to an avulsion in which the affected limb or body appendage is completely separated from the body. This can occur when a digit or extremity is sheared off by either mechanical or severing forces, for example amputation of a thumb by a bandsaw. Traumatic amputations vary in severity and ongoing compromise, with a cleancut amputation more likely to be successfully reattached than a crushed extremity. Criteria that inform the surgical decision-making process include the amount of tissue loss, location on the body at the connection site, damage to underlying and surrounding tissues, bones, nerves, tendons/muscles and vessels, and condition of the amputated part.
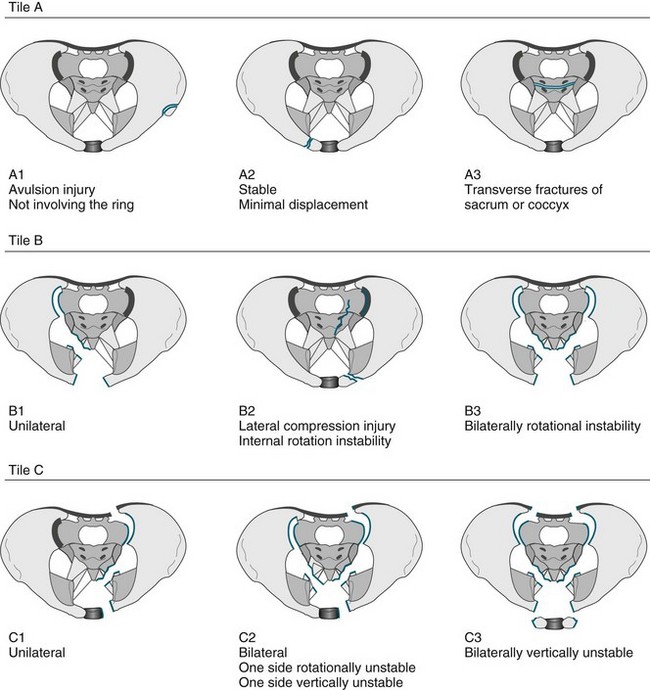
• Fractures of the pelvis. The pelvis is the largest combined bony structure in the body and serves to provide an essential supporting framework for ambulation and protection of pelvic organs. Major blood vessels and nerves traverse the pelvic bones, supplying the lower limbs and pelvic organs. Therefore, injury to any part of the pelvis is serious. The three bones that comprise the pelvic ring are the two innominate bones (ilium and pubic rami) and the sacrum. Due to its reinforced structure, the amount of force required to fracture the pelvis is substantial. Fractures of the pelvis can affect one or both sides of the pelvis, and be stable or unstable. A variety of classification systems exist to describe the severity of pelvic fractures, the most common being the Tile classification (see Figure 23.2).
• Fractures of the spinal column. (see also Chapter 17). The spinal column includes all of the bony components in the cervical, thoracic and lumbar vertebral regions. Fractures of the vertebra are common in trauma patients, but the actual incidence of fracture without spinal cord injury in multitrauma patients is not well described. Not all fractures cause vertebral column instability with the subsequent risk of spinal cord damage. A spine column fracture will be diagnosed as mechanically stable or unstable and this will affect the positioning and possible activity of the patient.
• Discoligamentous injuries of the spinal columns (see also Chapter 17). The soft tissue components of the spinal column include the spinal cord, the inter-vertebral discs and the spinal ligaments. An injury to the spinal column can disrupt one or more of these structures with or without fracture. These injuries can be highly unstable and the nurse must be vigilant with spinal precautions and the fitting and management of the patient requiring a spine orthoses (refer to Figure 23.1).
Nursing Practice
Independent practice
Bones are very vascular structures and can be the cause of substantial blood loss in the trauma patient. The critical care nurse should therefore be cognisant of the potential for extensive blood loss in common fractures (see Table 23.3).
| Fracture | Blood loss (mL) |
|---|---|
| Humerus | 500–1500 |
| Elbow | 250–750 |
| Radius/ulna | 250–500 |
| Pelvis | 500–3000 |
| Femur | 500–3000 |
| Tibia/fibula | 250–2000 |
| Ankle | 250–1000 |
Given the potential for extensive blood loss, as well as the frequent close proximity of nerves and blood vessels to bones, neurovascular assessment of the patient with skeletal trauma is essential (see Table 23.4).
TABLE 23.4 Neurovascular observations of the skeletal trauma patient
Should be undertaken on all injured limbs both pre- and postoperatively as required
| Observation | Process | Comments |
|---|---|---|
| Skin colour | State the skin colour of the area inspected as it compares with the unaffected part. NB: Distal limb pulses may be difficult to palpate in the injured limb; a warm pink limb is a perfused limb. |
Pink: normal perfusion |
| Pale: reduced perfusion | ||
| Dusky, purple or cyanotic discolouration: usually indicating significantly reduced perfusion | ||
| Demarcated: a distinct line where the skin colour changes to dusky (usually follows the vessel path) | ||
| Skin temperature to touch | State the ambient temperature of the skin to touch as it compares with normally perfused skin at room temperature. | Normal: not discernibly cold to touch. Reduced skin temperature indicates reduced perfusion. |
| Voluntary movement | The patient should be able to move the non-immobilised distal part of any injured limb (i.e. fingers and toes of a plastered limb). | It is important to assess range of motion where that is possible, provided this will not aggravate the injury. Reduced movement may indicate compromise to either the nerve or blood supply to the limb. |
| Sensation | The patient should be able to report normal sensation to touch. | Sensation should be assessed in nerve distributions (i.e. all fingers and toes). Reduced sensation may indicate compromise to either the nerve or blood supply to the limb. |
Collaborative practice: splinting
• Positioning of injured limbs. All patients who have any form of splint in situ should not have the affected limb below the level of the patient’s body, and may need to have it elevated to promote venous return and minimise tissue oedema. In the ICU the trauma patient will often be nursed flat, with the bed on tilt for a head-elevation position. In these circumstances, the injured dependent limb must be elevated on pillows so that it is no longer dependent. Care must be taken to ensure that elevation does not place pressure on any part of the limb: for example, a hand sack made from a pillowcase tied to an IV pole should not be used, as it places direct pressure on the path of the median nerve and can cause an iatrogenic neurapraxia.
• Wooden/air splints. These are padded appliances that are strapped to the injured limb. Ideally, no patient should remain in wooden splints for longer than 4 hours, as pressure may build up on pressure points.
• Plaster backslab. Limbs with fractures will often swell as a physiological response to injury; a plaster backslab composed of layered Plaster of Paris is the preferred treatment, as it accommodates swelling and can easily be loosened by nursing staff at any time of day. It is imperative that this be adequately padded within the limitations of providing structural support to the limb. Poorly made or ill-fitting backslabs can cause major complications, such as pressure sores or displacement of fractures.
• Traction. Traction may be required as part of fracture management, and involves the application of a pulling force to fractured or dislocated bones. There are three types of traction:
1. The grip or hold on the body must be adequate and secure.
2. Provision for countertraction must be made.
3. There must be minimal friction.
4. The line and magnitude of the pull, once correctly established, must be maintained.
5. There must be frequent checks of the apparatus and of the patient to ensure that: (a) the traction set-up is functioning as planned; and (b) the patient is not suffering any injury as a result of the traction treatment.
Collaborative practice: traumatic amputations
• appropriate positioning of the affected limb, usually based on surgical orders
• frequent neurovascular observations, particularly observing for reperfusion injury, which manifests as an acute compartment syndrome or vascular trashing of distal vessels from a clot
• implementing changes in treatment initiated in response to altered perfusion in a timely manner
• psychological support to assist the patient in dealing with the injury.
Collaborative practice: pelvic stabilisation
The initial management of the patient with a fractured pelvis involves assessment and splinting. Assessment should encompass the following two aspects:45,51
1. haemodynamic status: to identify signs of ongoing blood loss and determine fluid resuscitation requirements
2. stability of pelvic ring: assessed with the aid of clinical examination and diagnostic imaging. Palpation and inspection of the anterior and posterior pelvis for signs of trauma, including tenderness in the conscious patient, is generally adequate.45,51
Non-invasive pelvic binding, in the form of either a bedsheet or a proprietary pelvic binder, may make a significant impact on patient morbidity and mortality.45,51 Such a manoeuvre will stabilise the pelvis and assist in approximating bleeding vessels, thereby assisting in haemostasis (see Figure 23.3).
Pelvic binders are temporary devices,45,51 and ideally will not be left in situ for longer than 4 hours. If a patient is to remain in the binder longer than 4 hours, nursing staff must take care to minimise pressure. Conscious patients should be advised to report signs of increasing pressure, such as positional paraesthesia. Increasing abdominal swelling may indicate a need to reposition the binder. Position restrictions should be clarified by all members of the healthcare team, especially if the patient will be in the binder for a lengthy period. The patient may be able to be log-rolled and side-lain with a pelvic binder in situ. Release of a pelvic binder should by undertaken only with caution and as part of definitive care (e.g. within the operating theatre), with all relevant members (particularly the orthopaedic or trauma surgeon) of the healthcare team present.
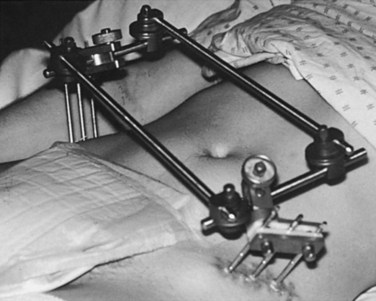
Invasive pelvic fixation uses an external fixateur (see Figure 23.4) to achieve pelvic stabilisation.45,51 The application of an external bridging frame (either anterior or posterior) to stabilise the pelvis may be an interim or definitive treatment measure that may be in situ for days or weeks. Patients in external fixation may be permitted to mobilise, although the extent of mobilisation will depend on the stability of the fracture. While the external fixateur is in place, the following nursing care is required:
• pin site care: usually cleaned with isotonic saline and covered with dry absorbent dressing; care should be taken to identify gaping or stretched skin around the site, as this may require surgical intervention
• analgesia: based on patient reports of pain and taking into account planned activities, such as mobilisation and physiotherapy
• mobilisation: based on stability of pelvis, and in consultation with the surgeon
• patient education: particularly regarding the safety of the procedure and mobilisation and rehabilitation plans.
Pelvic embolisation involves interventional radiology to control haemorrhage in patients with pelvic fractures. Because of the large arteries that traverse the pelvis, arterial bleeding can be the cause of substantial blood loss in 10–20% of cases.45,51 The timing of embolisation, particularly in relation to stabilisation, remains controversial.45,51,55
Collaborative practice: spine orthoses
The cervical collar or orthosis is the most commonly used splint to immobilise the cervical spine. It commonly remains in situ for >24 hours in an ICU setting. This particular type of splinting is associated with an increased risk of pressure ulceration in immobile patients.32 Collar care is an essential component of critical care practice. Any dirt, grit, glass and road grime must be removed as soon as possible from under the collar, particularly in the occipital regions. The patient should side-lie as much as possible and the collar should be removed while maintaining spinal precautions (see Table 23.5) and the underlying skin integrity assessed at least every 8 hours.52 Other examples of spine orthoses include a halothoracic brace and thoracolumbar/truncal anti-flexion bracing.
Chest Trauma
Chest trauma represents approximately 10% of injuries that require admission to hospital for more than 24 hours,28 although this proportion grows to over 15% when only patients with major injury (injury severity score >15) are considered.28,53 Chest trauma also represents approximately 15% of the injured patients requiring admission to the ICU.28 The incidence of chest trauma varies, depending on the external cause of the injury, with approximately 20% of road traffic crash injuries occurring to the chest, 30% of stabbing injuries occurring to the chest and only 10–15% of assault and fall injuries occurring to the chest.54 Associated mortality ranges from 4% to 9%.28,55
Description
Chest trauma covers a broad array of injuries and severity, and ranges from relatively minor injuries (e.g. abrasions and fracture of a single rib) to major, immediately life-threatening injuries (e.g. cardiac rupture or tension pneumothorax). Chest trauma is often associated with injuries to other regions of the body, including the head, neck, spine, abdomen and limbs.57
• rib fractures: a very common form of chest trauma, often a source of severe pain and often associated with other injuries such as haemothorax, pneumothorax and pulmonary contusion.57
• flail chest: fractures to two or more ribs, in two or more places, resulting in a freely-moving section of the rib cage. Usually such fractures occur in the anterior or lateral sections of the rib cage, where there is less muscle protection. The significant impact of this injury is paradoxical movement of the flail segment during spontaneous ventilation, so that when a patient inspires, the flail segment moves inwards with the negative intrapleural pressure instead of expanding with the rib cage. Compromised respiratory function is caused by the increased work of breathing that this ineffective flail segment creates, as well as the contused lung that normally occurs underneath the flail segment.57
• diaphragmatic injuries: generally consist of diaphragmatic rupture when there has been a significant rise in intra-abdominal pressure, usually with compression injuries. When the rupture is sufficiently large, protrusion of the abdominal contents into the thoracic space, resulting in respiratory compromise, is likely.58
• pulmonary contusion: consists of bruising to the lung tissue, usually as a result of mechanical force. This bruising is followed by diffuse haemorrhage and interstitial and alveolar oedema, resulting in impaired gas exchange.57,59
• pneumothorax: the accumulation of air in the pleural space. A pneumothorax may be closed (no contact with the external atmosphere) or open (a communicating channel with the atmosphere).57 Closed pneumothoraces are generally caused by blunt chest trauma and result from a fractured rib puncturing the lung parenchyma. Open pneumothoraces generally occur in the setting of penetrating trauma, where air is able to move from the external atmosphere to the pleural space during inspiration. If not all of the inspired air is able to escape during expiration, due to a tissue flap or similar obstruction covering the opening, the volume of the pneumothorax will gradually expand and cause collapse of the adjacent lung, with resultant hypoxaemia. Where air is not able to escape at all from the pleural space, this is referred to as a tension pneumothorax, and rapidly becomes a life-threatening event due to the increasing pressure on the lungs, heart and trachea.
• haemothorax: the accumulation of blood in the pleural space. Blood may collect from the chest wall, the lung parenchyma or major thoracic vessels.57 Breath sounds are usually reduced on the side of the haemothorax. Small haemothoraces (<200 mL blood) may not be apparent on clinical or radiological investigation, although respiratory compromise is likely to be present.
• cardiac trauma: encompasses a number of different injuries, ranging from relatively mild bruising of the heart muscle to rupture of the heart wall, septum or valves or damage to the coronary arteries.57 The right side of the heart is most commonly injured, probably as a result of the anterior placement of this side of the heart in the thorax.
• aortic injuries: generally, injuries to the brachiocephalic, left subclavian or right subclavian branches of the aorta and associated with high mortality at the scene.57
• tracheobronchial injuries: tend to occur as a result of direct blunt trauma and in close proximity to the carina, but are relatively rare.60
Clinical Manifestations
Injuries to the thoracic cavity can manifest according to the structures and systems involved (see Table 23.6). When multiple organs and systems are involved, the combined injuries pose an increased threat to life.
| System | Manifestation | Clinical signs and symptoms |
|---|---|---|
| Respiratory |
Nursing Practice
Given the underlying structures of heart, lungs and great vessels, chest trauma can cause rapid deterioration in the patient. Ongoing and thorough assessment, particularly in relation to the signs and symptoms outlined in Table 23.6, is essential. Other essential aspects of care include patient positioning and management of pain relief.
Independent practice: assessment
• Cardiac tamponade: as blood collects in the pericardium, the venous return to the heart is impeded, resulting in reduced cardiac output. Signs of cardiac tamponade include:
• Tension pneumothorax: the lung or lungs collapse as the pleural space fills with air that cannot escape (see Figure 23.5). As the volume of air grows with each breath, the thoracic cavity contents are compressed or pushed against the opposite side of the chest. Signs of tension pneumothorax include:
Independent practice: pain relief
The principles of managing pain in chest trauma patients are similar to those for other patients, although the potential severity of pain, particularly as a result of fractured ribs, should not be underestimated. Effective pain management in the chest trauma patient is a major determinant of maintaining adequate spontaneous breathing. Avoiding mechanical ventilation is a major goal in the less-severe group of chest trauma patients, so effective deep-breathing and coughing must be promoted. Pain relief will normally include IV opioids, but may also include intercostal or epidural analgesia and non-steroidal anti-inflammatory agents in selected patients (see Chapter 7). Non-pharmacological means such as the use of supplemental oxygen, use of cold packs early and heat packs late in the treatment course, massage, relaxation and diversion techniques should also be considered. Providing and maintaining a comfortable posture for the patient that includes the elevation and support of injured limbs has remarkable analgesic properties. A confident, competent and efficient nurse that engenders trust from both the patient and family is very comforting.
Collaborative practice: surgical management of injury
The emergency thoracotomy has proven beneficial in a select group of patients with penetrating trauma and less than 15 minutes of cardiopulmonary resuscitation; however, it is generally recognised as not providing benefit in patients with blunt chest trauma.61 While different techniques are used in different settings, the main access to the thoracic cavity is via a left thoracotomy, a midline sternotomy or a ‘clam shell’ incision. Initial assessment of the patient is used to determine the need for a thoracotomy in either the emergency department or the operating room. Nurses working in a trauma reception facility that has the capacity for emergency thoracotomy should be familiar with the equipment and process for this procedure. Postoperative nursing care of these patients should follow the same principles as those for patients who have undergone routine cardiothoracic surgery.
Collaborative practice: chest drainage
• The lungs are encased in a potential space. The visceral pleura attaches to the parietal pleura via surface tension, creating a negative intrapleural pressure and attaching the lung to the chest wall. During inspiration the rib cage moves out and the diaphragm contracts and moves down, increasing the size of the intrathoracic space. Air moves from an area of higher pressure in the environment to an area of lower pressure within the lungs along a pressure gradient.
• An intercostal catheter is inserted into the pleural space, passing between the ribs. The ICC is designed to drain both air and fluid as required.
• The drainage system and seal provides an ongoing means of removing air and/or fluid from the pleural space, while preventing air from the atmosphere entering via the ICC. The seal is provided by placing the distal end of the ICC under water (usually 2 cm). The catheter should not be placed under excessive levels of water, as this creates resistance and will limit air and fluid escaping from the pleural space.
• Suction is often added to the drainage system to promote drainage of fluid.
Care of the chest trauma patient with intercostal drainage is directed towards ensuring sterility and patency of the system, assessing the amount and type of drainage, as well as the impact on the patient (see Table 23.7). Additional considerations include the following:
• ICC may be positional, or alternatively haemo/pneumothoraces may be loculated. Repositioning of either the patient or the catheter may be necessary.
• Side-lying or lifting the patient, especially with a frame, may kink or disconnect the ICC.
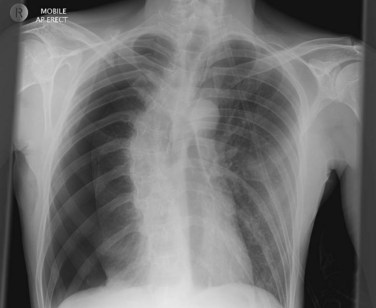
• Surgical emphysema around the site of the ICC may dislodge the tip of the catheter out of the pleural cavity as the emphysema swells. Ongoing assessment, including a chest X-ray, will be required to confirm the position of the ICC.
• Movement of the patient, including sitting upright, will assist with fluid drainage; the volume of drainage should be assessed after moving the patient.
• Monitoring of respiratory function should continue after removal of the ICC to detect recollection of air or fluid.
| Characteristic | Description |
|---|---|
| Water seal | Ensure there is sufficient water in the water seal chamber. |
| Bubbling | Continued bubbling indicates an air leak. |
| Drainage | Observe the nature and volume of fluid exudate (NB: >1500 mL stat or 200/mL/hour for 2–4 hours; surgical exploration may be required. |
| Patency | Ensure the intercostal catheter is not blocked, remove any blood clots. |
| Swinging | Oscillation of fluid in the ICC confirms patency, as this reflects the changes in intrapleural pressure with respiration; such oscillation should continue even when the lung has re-expanded. |
| Suction | If suction is ordered, check the appropriate level is being delivered. |
Collaborative practice: ventilatory support
Ventilatory support is often required for patients with chest trauma (see Chapter 15 for general principles). The following specific considerations apply:
• Non-invasive ventilation: care should be taken based on associated injuries, with contraindications including fractured base of skull or facial fractures.
• Intubation: haemoptosis is relatively common in patients with lung injury, and care must be taken to ensure removal of blood clots from the ETT. Heated, humidified air and regular suctioning will assist with maintaining ETT patency.
• Airway injury: initiation of positive pressure ventilation in the chest trauma patient may identify damage to a small airway that previously went unnoticed (damage to a large airway will usually have been detected early in the assessment phase). Treatment will depend on the severity and location of the rupture, but usually requires decompression of the pleura with an ICC, possibly surgical intervention and advanced respiratory support such as independent lung ventilation.
• Use of tracheostomy: this may be required for patients with injury to the trachea and is managed using the same principles as with any patient with a tracheostomy.
Abdominal Trauma
Recent advances in diagnostic and treatment techniques for abdominal trauma have seen an increased emphasis on non-operative management for solid organ injury, with more recent increases in the use of angioembolisation. These two clinical treatment innovations place an emphasis on excellent patient monitoring and, in some instances, higher ICU utilisation for selected cases.62,63
Patients who experience abdominal trauma as their main injury comprise only 3–5% of injured patients requiring admission to ICU, although up to a quarter of trauma patients experience some form of abdominal injury.28 Of all patients who present to the emergency department with serious injury, approximately 15–20% have abdominal injury.26
Description
The abdomen is susceptible to injury from a variety of external causes, both blunt and penetrating (see discussion of penetrating injuries below). A key aspect to remember with any abdominal injury is that the superficial injury does not always reflect what lies below. For example, it is not possible to be certain of the trajectory that a bullet took after it passed through the skin.
Perforation
Full-thickness injury, or perforation, to a hollow viscus organ is life-threatening. Perforation of the intestine can result in peritoneal soiling and ischaemic bowel. Small bowel injuries are particularly difficult to diagnose; if diagnosis is delayed, morbidity can be severe. The abdominal seatbelt sign – in other words, bruising across the anterior abdominal wall that follows the path of the lap and sash of the seatbelt – is a sentinel sign for hollow viscus perforation.64 Importantly, patients with this type of abdominal trauma can present late (by days). If presenting late, the usual clinical manifestations are pain, peritonitis and sepsis.64
Secondary injury: abdominal compartment syndrome (ACS)
The abdominal viscera are highly vascular and subject to vascular engorgement during massive fluid resuscitation. Where this occurs, there is an acute rise in intra-abdominal pressure (IAP). In severe cases, the IAP will rise to the point where cardiorespiratory function is compromised. This is a surgical emergency and the abdominal cavity requires decompression immediately. The incidence of ACS is difficult to determine because of the different assessment and measurement techniques that exist, but has been reported to be between 1% and 33%.65
High IAP can have effects on multiple systems throughout the body, as follows:65,66
• gut and hepatic effects: reduced blood flow to abdominal organs
• renal effects: reduced renal blood flow and glomerular filtration rate
• cardiovascular effects: decreased venous return through pressure on the inferior vena cava and raised intrathoracic pressure, leading to reduced cardiac output
• respiratory effects: where pressure on the abdominal side of the diaphragm increases abdominal resistance to inspiration. In ventilated patients this is usually demonstrated by elevated peak inspiratory pressures, resulting in reducing tidal volume and minute volume as the ventilator cycles off when either the preset pressure is reached or pressure alarms are triggered
• central nervous system effects: reduced cerebral blood flow due to the raised intracranial pressure from impaired venous drainage. When this is coupled with a lower cerebral perfusion pressure that results from the reduced cardiac output, it is deleterious to the injured brain
• cytokine response: activation of the stress response, seen through raised interleukins IL-6 and IL-1 alpha, as well as tumour necrosis factor.
A high level of suspicion for ACS should be retained for all patients with abdominal trauma as well as those who have had abdominal surgery for other reasons. Clinical examination, looking for a distended and firm abdomen, is insensitive in the early stages of ACS; however, these signs should be identified if ACS progresses to a late state. Proactive detection of ACS is more effectively carried out through the use of routine IAP measurements in all patients who have the potential to develop ACS. While agreement as to the precise levels of IAP that indicate ACS is yet to be achieved, there is widespread agreement that values above approximately 20 mmHg require investigation; and pressures above 25 mmHg, in association with other clinically relevant findings such as firm or distended abdomen and the systemic effects outlined above, often indicate a need for urgent surgery.65,66
IAP can be measured directly by laparoscopy, but is more effectively measured on an ongoing basis, either intermittently or continuously, via an indirect technique of measuring bladder pressures. IAP measurements are achieved using an indwelling urinary catheter with a pressure transducer or manometer levelled to the midaxillary line and attached via a T piece to allow continuous sterile access.67 According to the World Society of the Abdominal Compartment Syndrome Guidelines, intermittent measurements are obtained as follows:67
1. Lay the patient flat, or head-up if undergoing head injury management. If the IAP is measured with the patient head-up, the level of elevation should be documented to ensure that future measurements are done with the patient in the same position.
2. The catheter is clamped and 25 mL (use consistent amount for all measurements) of room-temperature 0.9% saline is infused into an empty bladder via the indwelling urinary catheter. This will create the static column of fluid for pressure measurement. Higher infused volumes may create a falsely elevated intraabdominal pressure.
3. After 30–60 seconds of dwell time, the pressure is measured via the transducer or manometer.
4. The catheter is unclamped to allow fluid to drain out. It must be remembered to deduct the fluid installation amount from any future urine output measurements.
There is some evidence that accurate IAP measurements can be obtained on a continuous basis using a three-way catheter.67 The benefits of this method include the provision of a continuous measurement as well as the absence of instillation of additional fluid into the bladder. The primary disadvantage is the potential for inaccuracy, depending on the volume of urine in the bladder.
Nursing Practice
Independent practice
With the high use of nonoperative management techniques for solid organ injury, the role of monitoring of patients with abdominal trauma is pivotal. Nurses must be cognisant of the clinical signs of abdominal injury, especially haemorrhage, and act on these immediately (see Table 23.8). Specific aspects of nursing care for patients after abdominal trauma include pain management, monitoring and postoperative care. Abdominal trauma patients will often experience severe pain, as a result of both the primary trauma and any surgical intervention for repair (see Chapter 7).
| Sign | Description | Suspected injury |
|---|---|---|
| Grey Turner’s sign | Blueish discolouration of the lower abdomen and flanks 6–24 hours after onset of bleeding | Retroperitoneal haemorrhage |
| Kehr’s sign | Left shoulder tip pain caused by diaphragmatic irritation | Splenic injury, although can be associated with any intra-abdominal bleeding |
| Cullen’s sign | Bluish discolouration around the umbilicus | Pancreatic injury, although can be associated with any peritoneal bleeding |
| Coopernail’s sign | Ecchymosis of scrotum or labia | Pelvic fracture or pelvic organ injury |
Where the patient has undergone a trauma laparotomy, postoperative care is standard as for any patient who has undergone an abdominal surgical procedure. The specific nursing care elements will depend on what organ has been injured and the surgical procedure that has been undertaken to repair the injury. Careful attention must be paid to those general nursing care elements that all patients require (see Chapter 6).
Postoperative feeding and bowel care should be discussed with the healthcare team and plans made early to avoid delays and adverse events such as constipation (see Chapter 19 for principles of feeding). A paralytic ileus is a common manifestation of the critically-ill abdominal trauma patient. Ensuring that the gut is decompressed, with a functional enterogastric tube that is correctly positioned, is essential. Because constipation is a common problem, early intervention and implementation of a bowel-care protocol for trauma should be considered (see Chapter 6).
Collaborative practice: diagnostic peritoneal lavage
The diagnostic peritoneal lavage (DPL) is a diagnostic procedure that can be undertaken rapidly to assess for intraabdominal bleeding. It can identify the presence of haemorrhage but gives no indication of its source. DPL may be performed on a patient with unexplained persistent signs of shock (hypotension ± tachycardia); where the abdominal clinical examination and FAST is inconclusive; where there is a high index of suspicion of intraabdominal injury; or alternative diagnostic evaluation such as CT is unavailable. Disadvantages of the DPL include the high level of invasiveness and associated complications, its inability to detect retroperitoneal injuries, the high rate of non-therapeutic laparotomies and its low specificity or high number of false-positive results.22
Prior to DPL, time permitting, the bladder should be decompressed with an indwelling urinary catheter and the stomach decompressed with an enterogastric tube. The DPL procedure involves an incision below the umbilicus, then a catheter passed into the peritoneal cavity and aspirated to determine peritoneal contents. Differing results in terms of colour and volume indicate different potential injuries. When blood, red blood cells, white blood cells, bacteria, faecal matter, bile or food particles are aspirated, the peritoneal lavage is considered to be positive.22
Collaborative practice: abdominal computed tomography
Abdominal computed tomography (CT) is recognised as having high sensitivity and specificity in the setting of abdominal trauma and is therefore accepted as a diagnostic mainstay in this group of patients, particularly for blunt trauma. The main exception to this is where the results of a FAST examination are positive and the patient is taken to surgery urgently. Abdominal CT is used less often in patients with penetrating trauma, primarily due to its lower sensitivity in diagnosing the hollow visceral injuries common in penetrating trauma.22 An important pitfall for CT imaging in abdominal trauma occurs when the patient has arrived at the scanner so quickly after the injury that major blood loss is not apparent and the extent of the injury is missed or underevaluated. A high index of suspicion in the setting of a negative CT and extensive abdominal trauma should remain, particularly if signs of shock develop.
Collaborative practice: laparotomy/laparoscopy
The role of diagnostic operations such as laparotomy/laparoscopy is well described in the literature,22 and is essential to aid diagnosis (laparoscopy) and provide appropriate treatment to control haemorrhage and repair organ injury (laparotomy). When this procedure is considered appropriate, rapid transit to the operating room should be undertaken. As the consequences of missed or delayed diagnosis of abdominal injury can be catastrophic for the patient, opening the peritoneal cavity to exclude injury in selected cases is a necessity.
Collaborative practice: embolisation
Interventional radiology is a treatment option in the management of abdominal trauma. Via an arterial approach, the interventional radiologist can insert cannulae to identify arterial blushes (bleeders). Once identified, the vessel can be ligated via mechanical coiling or blocked chemically. Embolisation has been shown to be effective and safe for a wide range of patients in the setting of splenic trauma, renal trauma and pelvic trauma.62 The patient undergoing embolisation as a treatment option for the control of haemorrhage requires meticulous monitoring and an ability to respond immediately to hypovolaemic shock should the bleeding worsen.
Collaborative practice: management of the patient with an open abdomen
In cases of severe abdominal trauma, the abdominal trauma patient may be returned to the ICU with an open abdomen, or laparostomy, covered with a temporary wound-closure system. There are various types of open abdominal dressings, but the principal aim of the dressing is to provide a coverage for the contents of the peritoneum if these are too swollen to fit beneath the closed skin or where there is a need for repeated opening of the abdomen.40 Ultimately, the aim is to close the skin as soon as possible, when the patient’s physiological status normalises. It is possible for these abdominal dressings to cause a secondary ACS if they are too restrictive.
Specific Abdominal Injuries: Spleen
The spleen is the solid organ most commonly injured in blunt trauma.62 Its location (under the ribs) also makes it vulnerable to secondary injury from fractured ribs. Splenic injury should always be suspected in those patients who have sustained a direct blow to the abdomen, as it is a large organ. Signs of splenic injury are generally pain over the left upper quadrant. There may be no changes to vital sign parameters until the patient has incurred significant circulating blood loss. Splenic injury is categorised in a scale consisting of five levels; this scale is designed to aid classification for management and research purposes62 (see Table 23.9).
| Grade* | Injury description | |
|---|---|---|
| I | Haematoma | Subcapsular, <10% surface area |
| Laceration | Capsular tear, <1 cm parenchymal depth | |
| II | Haematoma | Subcapsular, 10–50% surface area |
| Intraparenchymal, <5 cm in diameter | ||
| Laceration | Parenchymal depth 1–3 cm not involving a trabecular vessel | |
| III | Haematoma | Subcapsular, >50% surface area or expanding; |
| Ruptured subcapsular or parenchymal haematoma | ||
| Intraparenchymal haematoma >5 cm or expanding | ||
| Laceration | Parenchymal depth >3 cm or involving trabecular vessels | |
| IV | Laceration | Laceration involving segmental or hilar vessels producing major devascularisation (>25% of spleen) |
| V | Laceration | Completely shattered spleen |
| Vascular | Hilar vascular injury that devascularises spleen |
* Advance one grade for multiple injuries, up to grade III.
The spleen has an immunological function that is not well understood. After splenectomy, patients are at increased risk of infection and therefore require careful education regarding lifelong risks. The role of immunisation after splenectomy is very important, and the patient must be counselled regarding the necessity for follow-up on immunisations.68 Prior to discharge from the hospital, the patient should be administered the first round of immunisations. The current recommendation for predischarge immunisations include:
The patient will also be commenced on antibiotic prophylaxis and should be advised to wear a medi-alert disk or card and consult specialist travel advice when travelling.69
Specific Abdominal Injuries: Liver
The liver is a vital organ, with liver failure being a fatal condition unless reversible. After the spleen, the liver is the next most common solid organ injured. Any injury to this highly vascular organ is serious and requires surgical review. As the largest abdominal solid organ traversing the midline, the liver is susceptible to injury from any external forces applied to the abdomen, for example seatbelt injuries and abdominal blows from an assault. The liver is also at risk of secondary injury from fractured ribs.62 Liver injuries are graded using the six-level liver injury scale (see Table 23.10). The treatment of liver injuries is largely dependent on the nature of the injury or injuries to the liver itself, presence of concomitant injuries, premorbid status and overall injury severity. The treatment options may also be guided by the services and expertise that your health agency can offer the patient.
| Grade* | Injury description | |
|---|---|---|
| I | Haematoma | Subcapsular, <10% surface area |
| Laceration | Capsular tear, <1 cm parenchymal depth | |
| II | Haematoma | Subcapsular, 10%–50% surface area |
| Intraparenchymal, <10 cm in diameter | ||
| Laceration | Parenchymal depth 1–3 cm, <10 cm in length | |
| III | Haematoma | Subcapsular, >50% surface area or expanding |
| Ruptured subcapsular or parenchymal haematoma | ||
| Laceration | Parenchymal depth >3 cm | |
| IV | Laceration | Parenchymal disruption involving 25–75% of hepatic lobe or 1–3 Couinaud’s segments within a single lobe |
| V | Laceration | Parenchymal disruption involving >75% of hepatic lobe or >3 Couinaud’s segments within a single lobe |
| Vascular | Juxtahepatic venous injuries; i.e. retrohepatic vena cava/central major hepatic veins | |
| VI | Vascular | Hepatic avulsion |
* Advance one grade for multiple injuries, up to grade III.
The overwhelming aim of the management of liver injuries is to preserve liver function. This is achieved by controlling haemorrhage, resting the patient and close monitoring. Most liver injuries can be managed nonoperatively. In these cases it is imperative that the patient be closely monitored for signs of haemorrhage and that the capacity for laparotomy is available at short notice if required. In some cases, embolisation may be considered for arterial haemorrhage.62 Late complications of liver injury include infection, haematoma, bile leak and late haemorrhage.
Penetrating Injuries
Clinical Manifestations
1. conspicuous: where the penetrating article is grossly visible (e.g. a shard of glass, a branch or a knife). Care must be taken not to focus solely on the visible cause of injury but to continue to undertake a systematic trauma assessment
2. inconspicuous: where the penetrating article is not immediately visible and may become apparent only during the systematic trauma assessment of the patient (e.g. with gunshot wounds and projectiles). In these injuries the visual signs on the external skin may not reflect the catastrophic injury underlying it (e.g. ventricle lacerations or serious vascular injury).
Nursing Practice
• Stabilise the foreign object. This may require padding and/or taping an object, for example a knife, to ensure minimal movement and prevent further damage until definitive care to remove the object.
• Care for the patient in a non-standard position. This will be dependent on how and where any foreign object is protruding from the body. For example, it may be necessary to care for a patient in the side-lying or prone position until the object is removed.
• Minimal volume resuscitation. This describes the practice of only resuscitating a patient sufficiently to maintain adequate perfusion to essential organs until definitive repair of the wound can be undertaken.43
• Psychosocial care of the patient and family. It is possible that patients with penetrating injury will need specific psychosocial care, particularly when the injury has occurred as a result of assault.
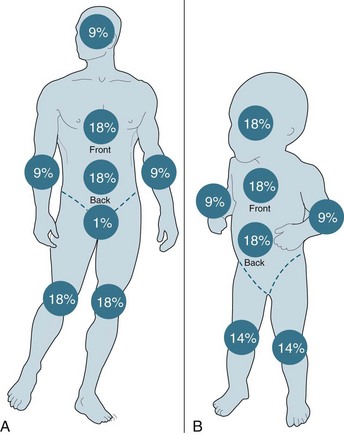
Burns†
Burn injuries are highly variable and individual injuries affect all ages and social groups. In general terms, assessment is based on the size, depth and anatomical site of the injury, mechanism of injury and the presence of coexisting conditions. The World Health Organization estimates that more than 300,000 deaths are fire-related every year, the majority occurring in developing countries.70
Burn injuries occur as a result of thermal, electrical or chemical injury and cause both local and systemic changes to a patient. An understanding of these changes will assist with planning appropriate care for this group of patients. All patients with a serious burn injury should be referred to a specialised burns unit that is staffed and equipped appropriately to manage burns. The Australian and New Zealand Burns Association (ANZBA) criteria outline which burns patients require treatment in a specialised burns unit (see Box 23.1).
Box 23.1
Criteria for treatment in a specialised burn centre77
• Burns greater than 10% of total body surface area (TBSA)
• Burns to special areas: face, hands, feet, genitalia, perineum, major joints
• Full-thickness burns greater than 5% of TBSA
• Burns with an associated inhalation injury
• Circumferential burns of the limbs or chest
• Burns in the very young or very old
• Burns in people with preexisting medical disorders that could complicate management, prolong recovery or increase mortality
Pathophysiology
The skin consists of three layers: the epidermis, the dermis and subcutaneous tissue.68 The epidermis is the outer layer, and is composed of stratified epithelial cells that protect against infection and conserve moisture. This layer is characterised by having regenerative ability. The dermis, as the middle layer, is between 1 and 4 mm thick, although thinner in the elderly and the very young. It is composed of an outer papillary dermis and an inner reticular dermis, and supplies nutrients to the epidermis. The dermis contains all the accessory structures including blood vessels, nerve endings, the sweat and sebaceous glands and the hair follicles. The dermis itself does not have regenerative ability, but because the glands, vessels and follicles are lined with epidermis, burns that involve this layer may still regenerate. The innermost layer, the subcutaneous tissue, consists of adipose and connective tissue. This layer has no regenerative ability.
Local changes
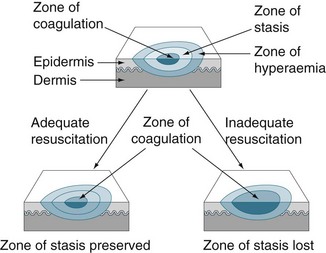
Local changes include the zones of coagulation, stasis and hyperaemia (see Figure 23.6) and the specific changes are outlined below.71
• Zone of coagulation: occurs at the point of maximum damage. Irreversible tissue loss occurs in this zone due to coagulation of the constituent proteins.
• Zone of stasis: surrounds the zone of coagulation and is an area of decreased tissue perfusion. Changes that contribute to this stasis include microthrombus formation, neutrophil adherence, fibrin deposition and endothelial swelling. Tissue in this zone is potentially salvageable if sufficient resuscitation is achieved to increase tissue perfusion. If insufficient resuscitation occurs, or if there are additional insults of hypotension, infection or oedema, tissue within this zone may convert to the zone of coagulation.
• Zone of hyperaemia: the outermost zone. It experiences increased tissue perfusion as a result of local inflammatory response, which results in local vasodilation and an increase in vascular permeability. Tissue in this zone will usually recover, unless there are prolonged or severe periods of hypotension, infection or oedema.
Systemic changes
With a burn injury of >30% total burn surface area (TBSA) microcirculation vessel wall integrity is altered resulting in fluid and protein loss into the interstitium. The protein loss results in a reduction in osmotic pressure which further insults circulating volume. Table 23.11 contains a description of the changes to the cardiovascular, respiratory, metabolic and immunological systems that occur as a result of the release of cytokines and other inflammatory mediators in response to the injury.
TABLE 23.11 Systemic changes that occur with burn injuries71
| System affected | Pathophysiological change |
|---|---|
| Cardiovascular system |
Inhalation injury
The presence of an inhalation injury will increase mortality and morbidity in people with a dermal burn injury.71,72 Inhalation injury consists of three components that may occur independently but often occur simultaneously, and include heat injury to the upper airways, effects of smoke on the respiratory system and inhalation of toxic gases.71 Diagnosis of an inhalation burn injury remains problematic, but it should be suspected if the injury was sustained in a closed spaced as well as if there are facial burns, singed nasal hairs or carbonaceous debris in the mouth or pharynx or in the sputum.71 The specific changes are dependent on the type of substances inhaled at the time of injury. In addition, the size of the smoke particles that are inhaled will affect the location of any injury. If coarse smoke particles are inhaled, these will primarily be deposited in the upper tracheobronchial tree, whilst fine smoke particles will usually be lodged in the alveoli. Patients with inhalation burn injury will usually experience upper airway oedema and bronchospasm in the early stages, with the airway disease progressing to the small airways in subsequent days.71,72,75
Clinical Manifestations
The most prominent clinical manifestations of burn injury are the dermal signs of injury. ANZBA categorise burns as follows:74
1. Epidermal burns are limited to injury to the epidermis and tend to be very painful, with a common example being sunburn. The skin is pink to red in colour and remains intact. The surrounding tissues may be oedematous and there is no blistering. This burn injury will usually heal within 7 days.
2. Superficial partial-thickness burn injury involve the epidermal and superficial dermal layers and are generally red or mottled in appearance and the underlying skin will blanch with pressure, demonstrating that perfusion is intact; blisters are a hallmark symptom. This degree of burn injury is very painful and healing may take up to 14 days. There is usually a lot of wound exudate in the first 72 hours where the skin is broken.
3. Mid-dermal partial-thickness injuries extend a part way into the dermis. They have a large zone of damaged non-viable tissue extending into the dermis, with damaged but viable tissue at the base. Preservation of the damaged but viable tissue (particularly in the initial period following injury) is pivotal to preventing burn wound progression. As some of the nerve endings remain viable, pain is present but is less severe when compared to superficial burns. Similarly, as some of the capillaries remain viable, capillary return is present, albeit delayed. Blisters may be present and the underlying dermis is a variable colour (pale to dark pink).
4. Deep partial-thickness burns extend into the deep dermal layer. The tissue is a characteristic pink to pale ivory in appearance. It can also have a blotchy red base due to extravasation of red blood cells. The underlying tissue does not blanch and the hair is easily removed; sensation is reduced. These burns usually take in excess of 3 weeks to heal and are managed with surgical excision and closure.
5. Full-thickness burns destroy both layers of skin (epidermis and dermis) and may penetrate more deeply into underlying structures. These burns have a dense white, waxy or even charred appearance. The sensory nerves in the dermis are destroyed in a full thickness burn, and so sensation to pinprick is lost. The coagulated dead skin of a full thickness burn, which has a leathery appearance, is called eschar.
Assessment of the total body surface area (TBSA) of burns
• Rule of Nines: for the adult population, the most widely known and easily applied method of estimating TBSA is the ‘rule of nines’ (see Figure 23.7). The principle of this assessment method is that most areas of the body constitute 9% (or multiples of 9%) of the TBSA.
• Palmar surface: the surface area of a patient’s palm (including fingers) is about 1% total body surface area. This method of estimating TBSA is commonly taught in emergency medicine courses but is yet to be validated. The Palmar surface method can be used to estimate relatively small burns (<15% of total surface area) or very large burns (>85%, when unburnt skin is counted). For medium sized burns, it is inaccurate.
Nursing Practice
Care can be considered in two categories; the first is the immediate priorities of care (outlined below) and including emergency principles, assessment and management of airway, breathing and circulation, and minimisation of hypothermia and hyperkalaemia. The second category of care is that provided throughout the first 24 hours (see Table 23.12). Care of the burn patient beyond that time will follow the general principles for patients with compromise to one or more of the systems, with additional considerations relating to wound care.
Airway
All patients with burn injury require supplemental oxygen. Facial burns or carbonaceous sputum (sputum with signs of smoke or charcoal) may indicate a burn injury to the airway. A carboxyhaemoglobin of >10% within the first hour post-injury is strongly indicative of inhalation injury. Classic signs of obstruction including stridor, dyspnoea and hoarse voice warrant immediate intubation and should be considered early as worsening oedema can make intubation difficult. Airway stability is mandatory for safe transfer.71,72
The Multitrauma Burns Patient
• Spinal injury: if the patient has potential spinal injuries in addition to the burn, spinal precautions must be maintained; however, cervical collars should not be used over a burnt neck or upper chest due to the potential for swelling and subsequent restriction. If a collar is used, changing to an appropriate size as the swelling worsens or goes down is essential.
• Skeletal injury: skin traction cannot be used in a patient with burn injury over a limb that also has a skeletal fracture; this will necessitate early internal fixation or the use of an external fixateur.
• Electrocution injuries: electrocution burns are largely internal burns that potentially cause devastating multiple internal injuries. The electrical current causes a burn at both the entry and exit sites. Where electrocution is confirmed or suspected the body must be inspected to identify all injuries. These may be in obscure places such as the hands and feet or even the back and scalp. Close monitoring for cardiac damage and rhabdomyolysis is essential.
Burn Dressings
Mitigating infection is the primary aim of good burns nursing.68 The greatest challenge is minimising the risk for cross-contamination, and patients should be nursed in a single room where possible. Burn dressings present a physical challenge, particularly when large areas of the body are affected.
• Debridement: this refers to the excision of dead skin. Gentle scrubbing is generally used to remove loose tissue and burst blisters. Forceps and scissors may be required to lift and remove smaller areas of tissue. Extensive areas of debridement will usually be undertaken in the operating room.
• Blisters: small blisters should be left intact, large blisters may be aspirated or deroofed during debridement, although it should be noted that evidence regarding blister management is poor. Blisters over joints that are restricting movement should also be debrided.
• Escharotomy: an escharotomy is undertaken to a limb or side of the trunk for circumferential burns that are contracting and creating vascular compromise to the underlying and distal tissues. The escharotomy is an incision through the eschar, and does not involve opening muscle fascia. The escharotomy immediately relieves the compression and is a limb/lifesaving surgical manoeuvre. The escharotomy is dressed as a burn to prevent infection.
• Skin grafts: these are required to cover the skin defect. They may be full-thickness or partial-thickness grafts, and may be harvested from the patient or, in some cases, obtained from a cadaver donor. Regardless of the type of skin graft, nursing care remains the same, with the aim being to maximise adherence. Specific nursing care of the graft site includes leaving the site intact and immobilising the graft site, applying the appropriate wound care regimen, preventing shearing injury to the graft site, and minimising the risk for infection. With autografts, wound care will also be required for the donor site.71
• Skin substitutes: some products are available to cover partial-thickness wounds that provide a moist environment that stimulates epithelialisation. These are best reserved for ‘clean’ wounds. Some products are able to act as full-thickness substitutes that provide wound closure, protection from mechanical trauma and bacteria and a vapour barrier. Once the new dermis is created the substitute is removed.71
Summary
Case study‡
• HR 110, RR not recorded, sBP 155 mmHg on palpation, GCS 15, SpO2 89%
• There was palpable surgical emphysema of the chest wall; a tension pneumothorax was diagnosed. A pneumocath was inserted and an audible hiss was heard, with subsequent improvement in the patient’s vital signs.
• IV cannula inserted with 1000 mL of Hartmann’s solution administered during extrication. Extrication was slow due to the door of the car having to be removed and the patient’s body habitatus, being 120 kg and 168 cm tall.
• Chris met the major trauma triage criteria and was transferred via helicopter, with a trauma team call activated prior to arrival. When Chris arrived at the emergency department, a full team was present to assess and treat him. This included a trauma surgeon, emergency physician, trauma nurse leader, specialist nurses and support staff.
Treatment in the emergency department consisted of the following:
• primary survey and associated resuscitation
• intubation and mechanical ventilation
• no cervical collar would fit him, so he was nursed with bilateral sandbag and head strapping
Teams involved in Chris’ care, and their reports, included:
• Cardiothoracic: flail chest, however no surgical intervention required.
• Orthopaedics: # humerus, surgically fixed and placed in sling with no internal rotation, and minimal external rotation for dressings only.
• Plastics: degloving injuries, with a radial reverse forearm flap performed.
• Neurosurgery and orthotics: C2 # with Halothoracic brace applied, posterior spinal fixation and reapplication of halo thoracic brace for 3 weeks to provide further stabilisation. Intermittently pre- and post-surgical fixation, Chris was noted to have absent movement in his lower limbs. Somato-Sensory Evoked Potentials were undertaken which demonstrated bilateral nerve conduction present. Chris’ girth precluded MRI investigation.
• Trauma Team: grade 3 liver laceration with positive FAST. As initial vital signs were stable, interventional radiology consulted for hepatic angiogram. A vessel of the hepatic artery was embolised. Follow-up hematocrits were low but stable. Follow-up CT showed no further increase in size of haematoma.
Learning activities
Learning activities 1–5 relate to the clinical case study.
1. Describe the implications of the type of accident experienced by Chris for his likely injuries and treatment.
2. Explain the rationale for pleural decompression and management of a pneumothorax in the early care of Chris.
3. Discuss the components of the ‘trauma triad’ and outline practices that should be undertaken to prevent or ameliorate the triad.
4. Identify the likely causes of Chris’ respiratory failure and agitation and discuss the various preventive and treatment approaches that are available.
5. Describe the practices that could be incorporated in Chris’ care to reduce his psychological distress.
6. Briefly describe the tenets on which all trauma systems are based.
7. Undertake a trauma tertiary survey assessment, discussing the process and findings with a senior clinical colleague.
8. Briefly describe why the mechanism of injury is important information in diagnosing injuries.
9. Describe why the patient’s positioning in the bed is an important consideration for trauma nursing care.
10. What is ‘damage-control surgery’ and why is this so important to survival in trauma patients?
11. Describe the nursing observations of a patient with an intercostal underwater seal drainage system in situ.
American College of Surgeons. www.facs.org.
Australasian College of Emergency Medicine. www.acem.org.au.
Australasian Trauma Society. www.atsoc.com.au.
Australian and New Zealand Burn Association. http://www.anzba.org.au/.
Eastern Association for the Surgery of Trauma. www.east.org.
An independent, non-profit organisation providing global education, information and communication resources for professionals in trauma and critical care. www.trauma.org.
NSW Trauma System. http://www.itim.nsw.gov.au/index.cfm.
NSW Trauma Management Guidelines. http://www.itim.nsw.gov.au/go/itim-trauma-guidelines.
Royal Australasian College of Surgeons. www.surgeons.org.
Society of Trauma Nurses. www.traumanursesoc.org.
Victorian State Trauma System. http://www.health.vic.gov.au/trauma/review99/index.htm.
World Health Organization. http://www.who.int/topics/injuries/en/.
Moloney-Harmon PA, Czerwinski SJ. Nursing care of the paediatric trauma patient. Cambridge: Elsevier; 2003.
Langstaff D, Christie J. Trauma care: a team approach. Oxford: Butterworth-Heinemann; 2000.
McQuillan K, Whalen E, Flynn-Makick M. Trauma nursing: from resuscitation through rehabilitation, 4th edn. Philadelphia: Saunders; 2008.
1 New Zealand Health Information Service. Selected morbitity data for publicly funded hospitals 2000/01. Wellington: Ministry of Health; 2004.
2 Peden M, McGee K, Krug E. Injury: a leading cause of the global burden of disease. Geneva: World Health Organization; 2002.
3 Bonnie RJ, Fulco CE, Liverman CT. Reducing the burden of injury: advancing prevention and treatment. Washington: National Academy Press; 1999.
4 Australian Institute of Health and Welfare (AIHW). Australia’s Health, 2010. Canberra: AIHW; 2010.
5 Tobias M. The burden of disease and injury in New Zealand. Wellington: Ministry of Health; 2001.
6 Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia. Canberra: Australian Institute of Health and Welfare; 1999.
7 Mock C, et al. Guidelines for trauma quality improvement programmes. Geneva: World Health Organization; 2009.
8 Mann NC, Mullins RJ, Hedges JR, Rowland D, Arthur M, et al. Mortality among seriously injured patients treated in remote rural trauma centers before and after implementation of a statewide trauma system. Medical Care. 2001;39(7):643–653.
9 Hoyt DB, Coimbra R. Trauma systems. Surgical Clinics of N America. 2007;87:21–35.
10 Cameron PA, Gabbe BJ, Cooper DJ, Walker T, Judson R, McNeil J. A statewide system of trauma care in Victoria: effect on patient survival. Med J Aust. 2008;189(10):546–550.
11 Liberman M, Mulder DS, Jurkovich GJ, Sampalis JS. The association between trauma systems and trama center components and outcom in a mature regionalized trauma system. Surgery. 2005;137(6):647–658.
12 Moore L, Hanley JA, Turgeon AF, Lavoie A. Evaluation of the long-term trend in mortality from injury in a mature inclusive trauma system. World J Surg. 2010;34:2069–2075.
13 Nirula R, Maier R, Moore E, Sperry J, Gentiello L. Scoop and run to the trauma center or stay and play at the local hospital: hospital transfer’s effect on mortality. J Trauma. 2010;69:595–601.
14 Brown JB, Stassen NA, Bankey PE, Sangosanya AT, Cheng JD, Gestring ML. Helicopters and the civilian trauma system: national utilization patterns demonstrate improved outcomes after traumatic injury. Trauma. 2010;69(5):1030–1036.
15 Shepherd MV, Trethewy CE, Kennedy J, Davis L. Helicopter use in rural trauma. Emerg Med Australas. 2008;20(6):494–499.
16 Cherry R, King TS, Carney DE, Bryant P, Cooney RN. Trauma team activation and the impact on mortality. JTrauma. 2007;63(2):326–330.
17 Kouzminova N, Shatney C, Palm E, McCullough M, Sherck J. The efficacy of a two-tiered trauma activation system at a level I trauma center. J Trauma. 2009;67(4):829–833.
18 Zalstein S, Danne P, Taylor D, Cameron P, McLellan S, Fitzgerald M, et al. The Victorian major trauma transfer study. Injury. 2010;41(1):102–109.
19 Garwe T, Cowan LD, Neas BR, Sacra JC, Albrecht RM. Directness of transport of major trauma patients to a level I trauma center: a propensity-adjusted survival analysis of the impact on short-term mortality. J Trauma. 2010. DOI: 10.1097/TA.0b013e3181e243
20 Nocera N, Schoettker P. NEWS Checklist (Abstract). Aust Emerg Nurs. 2001;4(2):31.
21 Inaba K, Branco BC, Lim G, Russell K, Teixeira PGR, Lee K, et al. The increasing burden of radiation exposure in the management of trauma patients. J Trauma. 2010. DOI: 10.1097/TA.0b013e3181ebb4d4.
22 Rozycki GS, Root HD. The diagnosis of intrabdominal visceral injury. J Trauma. 2010;68(5):1019–1023.
23 Gaarder C, Kroepelien CF, Loekke R, Hestnes M, Dormage J, Naess PA. Ultrasound performed by radiologist – confirming the truth about FAST in trauma. J Trauma. 2009;67(2):323–329.
24 Kanz KG, Paul AO, Lefering R, Kay MV, Kreimeier U, et al. Trauma management incorporating focused assessment with computed tomography in trauma (FACTT) – potential effect on survival. J Trauma Manag Outcomes. 2010;4:4.
25 Delprado A. Trauma systems in Australia. Trauma Nursing. 2007;14:93–97.
26 Kohn MA, Hammel JM, Bretz SW, Stangby A. Trauma team activation criteria as predictors of patient disposition from the emergency department. Acad Emerg Med. 2004;11(1):1–9.
27 Richards CF, Mayberry JC. Initial management of the trauma patient. Crit Care Clin. 2004;20(1):1–11.
28 Aitken LM, Lang JH, Bellamy N. Queensland Trauma Registry: description of serious injury throughout Queensland, 2003. Herston: Centre of National Research on Disability and Rehabilitation Medicine; 2004.
29 National Trauma Registry Consortium (Australia and New Zealand). National Trauma Registry report, 2002. Herston: NTRC; 2004.
30 Department of Human Services.Victorian State Trauma Outcome Registry and Monitoring Group. Victorian State Trauma Registry: 1 July 200230 June 2003, summary report. Melbourne: Department of Human Services; 2004.
31 Hadley MN, Walters BC, Grabb PA, Oyesiku NM, Przybylski GJ, et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407–498.
32 Jacobson T, Tescher A, Miers A, Downer L. Improving practice: efforts to reduce occipital pressure ulcers. J Nurs Care Qual. 2008;23(3):283–288.
33 Kheirbek T, Kochanek A, Alam H. Hypothermia in bleeding trauma: a friend or foe? Scand J Trauma, Resusc Emerg Med. 2009;17:65–80.
34 Hess J, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–754.
35 D’Angelo MR, Dutton RP. Management of trauma-induced coagulopathy: trends and practices. AANA. 2010;78:35–40.
36 Langhelle A, Lockey D, Harris T, Davies G. Body temperature of trauma patients on admission to hospital: a comparison of anaesthetised and non-anaesthetised patients. Emerg Med J. 2010. DOI: 10.1136/emj.2009.086967
37 Frith D, Brohi K. The acute coagulopathy of trauma shock: clinical relevance. Surgeon. 2010;8(3):159–163.
38 Clement N, Tennant C, Muwanga C. Polytrauma in the elderly: predictors of the cause and time of death. Scand J Trauma Resusc Emerg Med. 2010;18:26.
39 Ireland S, Endacott R, Cameron P, Fitzgerald M, Paul E. The incidence and significance of accidental hypothermia in major trauma – A prospective observation study. Resuscitation. 2011;82(3):300–306.
40 Larson CR, White CE, Spinella P, Jones JA, Holcomb JB, et al. Association of shock, coagulopathy and initial vital signs with massive transfusion in combat casualties. J Trauma. 2010;69(Suppl1):S26–S32.
41 Ganter M, Pittet J. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol. 2010;24:15–25.
42 Fabian T. Damage control in trauma: laparaotomy wound management acute to chronic. Surg Clin N Am. 2007;87(1):73–93.
43 Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, et al. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. 2010;69(1):46–52.
44 Bosch X, Poch E, Grau J. Rhabdomyolysis and acute kidney injury. New Eng J Med. 2009;361(1):62–72.
45 Williams-Johnson J, Williams E, Watson H. Management and treatment of pelvic and hip injuries. Emerg Med Clin North Am. 2010;28(4):841–859.
46 Guyton AC, Hall JE. Textbook of medical physiology. Philadelphia: WB Saunders; 2011.
47 Pape H, Marcucio R, Humphrey C, Colnot C, Knobe M, Harvey EJ. Trauma-induced inflammation and fracture healing. J Orthop Trauma. 2010;24(9):522–525.
48 Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing–an update. Injury. 2007;38(S1):S3–10.
49 Akhtar S. Fat embolism. Anesthesiology Clin. 2009;27(3):533–550.
50 Lenarz CJ, Watson JT, Moed BR, Israel H, Mullen JD, Macdonald JB. Timing of wound closure in open fractures based on cultures obtained after debridement. J Bone Joint Surg Am. 2010;92(10):1921–1926.
51 Fulkerson E, Egol K. Timing issues in fracture management: a review of current concepts. Bulletin of the NYU Hospital for Joint Diseases. 2009;67(1):58–67.
52 Consortium for Spinal Cord Medicine. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care providers. Washington DC: Paralyzed Veterans of America; 2008.
53 Department of Human Services (Victoria). Victorian State Trauma Registry, 1 July 2002-30 June 2003. Melbourne: Department of Human Services; 2004.
54 NSW Department of Health. NSW Trauma Minimum Data Set, 2003. Sydney: NSW Department of Health; 2004.
55 Kulshrestha P, Munushi I, Wait R. Profile of chest trauma in a level I trauma center. Trauma. 2004;57(3):576–581.
56 The Alfred Hospital. Spinal Clearance Management Protocol, Updated 1.6.06. Victoria: The Alfred Hospital; 2006.
57 Keel M, Meier C. Chest injuries – what is new? Curr Opin Crit Care. 2007;13(6):674–679.
58 Lopez P, Arango J, Gallup TM, Cohn SM, Myers J, et al. Diaphragmatic injuries; what has changed over 20-year period? Am Surg. 2010;76(5):512–516.
59 Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32(2):122–130.
60 Schneider T, Volz K, Dienemann H, Hoffmann H. Incidence and treatment modalities of tracheobronchial injuries in Germany. Interact Cardiovasc thoracid Surg. 2009;8(5):571–576.
61 Soreide K, Petrone P, Asensio J. Emergency throacotomy in trauma: rationale, risks and realities. Scand J Surg. 2007;96(1):4–10.
62 Holden A. Abdomen–Interventions for solid organ injury. Injury. 2008;39(11):1275–1289.
63 Peitzman AB, Richardson JD. Surgical treament of injuries to the solid abdominal organs: a 50-year perspective from the Journal of Trauma. J Trauma. 2010;69(5):1011–1021.
64 Alsayali M, Atkin C, Winnett J, Rahim R, Niggemeyer LE, Kossmann T. Management of blunt bowel and mesenteric injuries: experience at the Alfred Hospital. Eur J Trauma Emerg Surg. 2009;35(5):482–488.
65 Rizoli S Mamtani A, Scarpelini S, Kirkpatrick AW. Abdominal compartment syndrome in trauma resuscitation. Curr Opin Anaesthesiol. 2010;23(2):251–257.
66 Cheatham M, Safcsak K. Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med. 2010;38(2):402–407.
67 Malbrain M, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intens Care Med. 2006;32(11):1722–1732.
68 Langley JM, Dodds L, Fell D, Langley GR. Pneumococcal and influenza immunization in asplenic persons: a retrospective population based cohort study 1990–2002. BMC Infectious Diseases. 2010;10:219.
69 Victorian Spleen Registry. Recommendations for the prevention of infection in asplenic or hyposplenic patients 2010. Victoria: The Alfred Hospital; 2010.
70 World Health Organization. The WHO plan for burn prevention and care. Geneva: WHO; 2008.
71 Grunwald T, Garner W. Acute burns. Plast Reconstr Surg. 2008;121(5):311.
72 Latenser BA. Critical care of the burn patient: the first 48 hours. Crit Care Med. 2009;37(10):2819–2826.
73 Sheridan RL, Tompkins RG. What’s new in burns and metabolism. J Am Coll Surg. 2004;198(2):243–263.
74 Edgar D, Katsu A. Burn survivor rehabilitation: principles & guidelines for the allied health professional. Albany Creek: Australian & New Zealand Burns Association, 2007.
75 Cancio LC. Airway management and smoke inhalation injury in the burn patient. Clin Plast Surg. 2009;36(4):555–567.
76 McQuillan K, Whalen E, Flynn-Makic M. Trauma nursing: from resuscitation through rehabilitation. Philadelphia: Saunders, 2008.
77 Australian and New Zealand Burn Association. Referral criteria to a burn unit. [Cited April 2011]. Available from http://www.anzba.org.au/index.php?option=com_content&view=article&id=51&Itemid=58
78 Newberry L, ed. Sheehy’s emergency nursing: principles and practice, 5th edn, St Louis: Mosby, 2003.
79 Kozin S, Bertlet A. Pelvis and acetabulum. Kozin S, Bertlet A. Handbook of common orthopaedic fractures, 2nd edn, Chester: Medical Surveillance, 1992.
80 Maher AB, Salmond SW, Pellino TA. Orthopedic nursing, 2nd edn. Philadelphia: WB Saunders; 1998.
81 Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328(7453):1427–1429.
82 Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. New Eng J Med. 2000;343(2):94–99.
83 Eckert KL. Penetrating and blunt abdominal trauma. Crit Care Nurse Q. 2005;28(1):41–59.