38 Trauma
ANESTHESIOLOGISTS COMMONLY PROVIDE CARE TO CHILDREN who have suffered traumatic injuries. These injuries vary in complexity, and patients range from the healthy older child with an isolated elbow fracture to the infant with a life-threatening epidural hematoma. The anesthesiologist should view the management of children with traumatic injuries as a continuum of care that may originate in the prehospital setting with emergency medical services (EMS), progress to the emergency department (ED), and continue through the operating room, the postanesthesia care unit, and the intensive care unit. Anesthesiologists may become involved in all phases of care of the traumatically injured child, with the possible exception of the prehospital setting. The care required is often complex but can be effectively accomplished in a collaborative environment incorporating a standardized process for initial evaluation and management.1 Anesthesiologists should be familiar with these processes to effectively continue this care in the perioperative setting.
Anesthesiologists are essential participants in many capacities during the care of injured children. Operative interventions demand full involvement of the anesthesiologist. In many institutions, anesthesiologists also provide emergency airway and critical care management. From the perspectives of airway control, ventilation, hemodynamic resuscitation, metabolic management, and effective control of pain, anesthesiologists can play a central role in the care of the injured child. Common diseases of childhood are now better understood and more effectively treated, which has resulted in more cures, better outcomes, and an improved quality of life. Despite major reductions in the overall mortality rate for pediatric trauma in recent years, unintentional injuries remain the leading cause of death and disability in the pediatric population of the United States.2 Injury has also emerged as the most common public health threat to children around the world.3
This chapter reviews the key principles of anesthesia management of traumatic injuries to children. This discussion is intended to augment the principles established in the widely accepted Advanced Trauma Life Support (ATLS) program, which is produced by the American College of Surgeons Committee on Trauma.4 Additional resources for the management of the pediatric trauma patient include the Advanced Pediatric Life Support (APLS) course administered by the American Academy of Pediatrics and the American College of Emergency Physicians.5 Many of the topics discussed in this chapter are being investigated to determine the most effective strategy, including volume resuscitation, evaluation of the cervical spine, and prehospital tracheal intubation.
Epidemiology of Pediatric Trauma
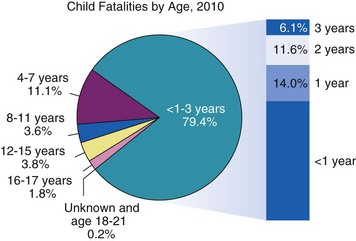
Traumatic injuries are the most common cause of death within the United States for children older than 1 year of age.6 Approximately 20,000 deaths of children occur annually as a result of trauma. Most traumatic injuries in children are a result of motor vehicle accidents (the leading cause of death), falls, nonaccidental trauma (Fig. 38-1), drowning, or extremes of temperature.
The epidemiology of trauma reflects its continued growth as a significant health risk for children of the world. Table 38-1 illustrates the recent incidence of injury types per 100,000 U.S. children.2 Motor vehicle trauma is a major threat to the health of children in the United States, which has spurred research to identify methods to improve their protection.7–9
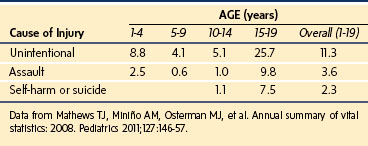
Pediatric and adult injury patterns and treatment protocols are different.10 Head injuries are the leading cause of death in children. One explanation for this is the proportionately large head of children compared with adults. Thoracic injuries are the second leading cause of death for pediatric trauma patients. Due to increased rib cage pliability from a lack of bony calcification and the flexible cartilaginous component, severe internal injury can occur in children without obvious external signs such as rib fractures. Blunt abdominal trauma frequently can be treated with close observation, avoiding operative intervention. Penetrating abdominal trauma usually requires surgical exploration. However, diagnostic laparoscopy is being used in lieu of exploratory laparotomy in hemodynamically stable children to evaluate and repair many types of abdominal injuries.11
Nonaccidental Trauma
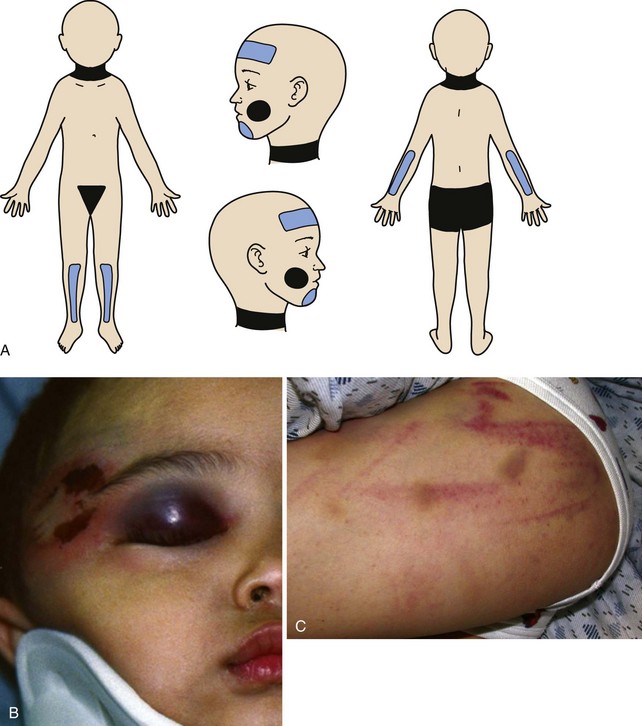
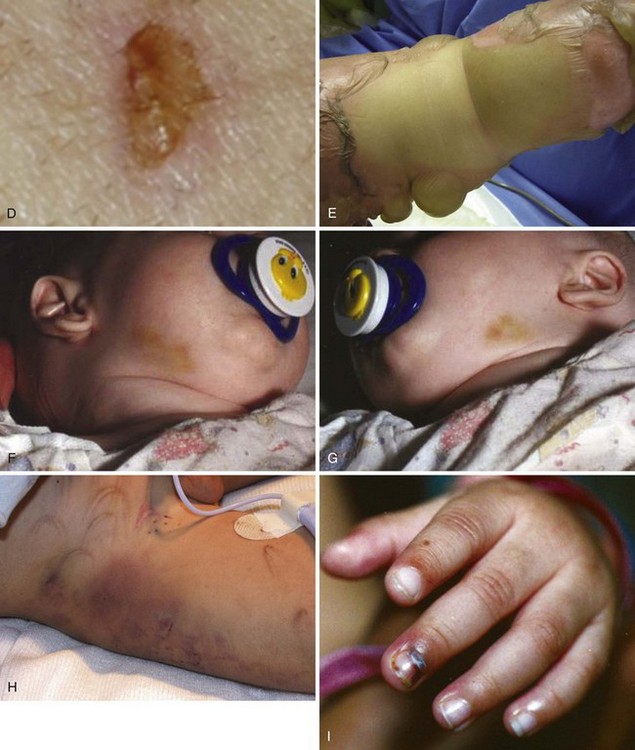
Nonaccidental trauma, also referred to as shaken baby syndrome or child abuse, is an epidemic that continues to grow in virtually all parts of the world. Although 3 million reports of nonaccidental trauma are filed every year in the United States, most experts believe that this represents less than one third of the actual cases.12,13 Every state has stringent laws for reporting nonaccidental trauma. These laws are designed to assist the health care provider who suspects mistreatment and to punish health care providers who do not appropriately report potential neglect. It is the responsibility of every physician involved, including the anesthesiologist, to be aware of the potential for nonaccidental trauma in all infants and children and to report all suspicious observations accurately to the appropriate authorities.
Characteristics of nonaccidental trauma include a history inconsistent with the character and extent of the injuries and a delay in seeking medical assistance.14,15 Nonaccidental trauma should be considered when the history appears improbable. Funduscopic examination may disclose retinal hemorrhages or papilledema, which may suggest forceful shaking of the head or increased intracranial pressure (ICP), respectively. Examination of the skin may show bruises, burns, or other injuries in several stages of healing (Fig. 38-2). A skeletal survey may reveal multiple fractures of various ages occurring typically at the metaphyses of long bones. Occasionally, a child who has previously been silent in the company of the parents or caregiver tells operating room or recovery room personnel about the events surrounding his or her injuries. These reports should be carefully documented and relayed to the appropriate personnel. Maltreated children are often terrified of painful procedures, and the need for sensitivity and reassurance in these settings cannot be overemphasized.
The anesthesiologist encountering a potential victim of nonaccidental trauma may provide the first impartial assessment of the child’s status.16 Physicians, nurses, and other professionals who provide initial resuscitation may be preoccupied with the small child’s acute and potentially life-threatening injuries. Historical data associated with the event may be inaccurate or fictitious at the time of initial presentation. An objective evaluation by the anesthesiologist in preparation for operative intervention may reveal the first objective evidence of nonaccidental trauma. Indications of mistreatment may be subtle, ranging from lack of parental availability for perioperative counseling and consent to irrational refusal of permission for a necessary operative intervention.17,18
Prehospital Care of the Pediatric Trauma Patient
Trauma Systems
The evolution of pediatric trauma systems has significantly improved outcomes and quality of life for trauma victims.19,20 Countries such as the United States have a trauma system philosophy more in line with the military approach. in which prehospital personnel such as paramedics are the initial team to make rapid assessments, initiate efforts at stabilization, obtain radio contact with the medical facility, and transport the child to the trauma center as rapidly as possible.21 This philosophy of minimizing the time on the scene and emphasizing prompt transport to the closest trauma center is called scoop and run.
In parts of Canada and several European countries, initial resuscitation of injured children is commonly performed by physicians who are charged with evaluating the child at the scene, securing the airway, initiating resuscitative measures to maintain hemodynamic stability, and transporting the child to an appropriate trauma center (Fig. 38-3). Instituting these management procedures may result in additional time on the scene. This approach has been called stay and play. Many aspects of this system are being integrated into existing American trauma systems as part of mass casualty disaster management plans.22,23
Developing a systematic approach to the care of the pediatric trauma patient is crucial. This includes acquiring age- and size-appropriate equipment. Luten and Broselow developed a system that provides immediate guidance for the identification of appropriate equipment and drug doses based on the child’s length.24–27 This system assigns a specific color based on the child’s body length. A corresponding color-coded, length-based wristband can then be placed onto the child, which designates drug doses and devices that match the assigned color. The system is designed to minimize delays, medication miscalculations, and equipment errors.
As trauma systems have evolved and EDs have become overwhelmed by larger patient volumes, effective triage for the pediatric trauma patient has become increasingly important. Care of traumatically injured children requires the use of significant personnel and resources from many services, including the operating room and ancillary services such as diagnostic imaging and transfusion services.28 Inappropriate triage may waste precious resources and potentially limit access to patients most in need if every patient with traumatic injuries, regardless of their severity, is sent to a trauma center. Conversely, not recognizing that a child must be brought to a trauma center may increase morbidity and result in preventable deaths.29,30
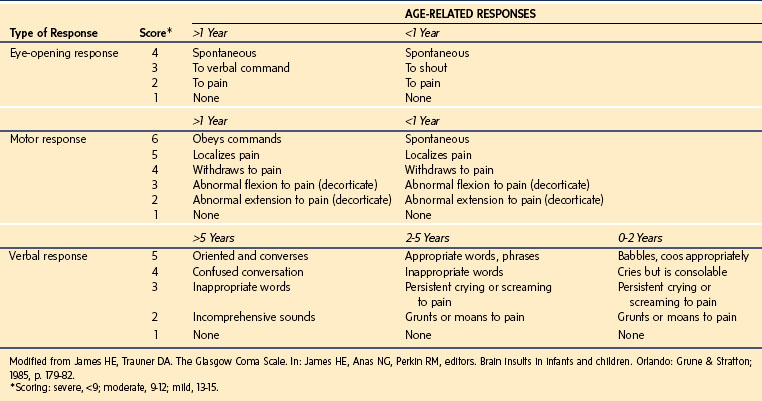
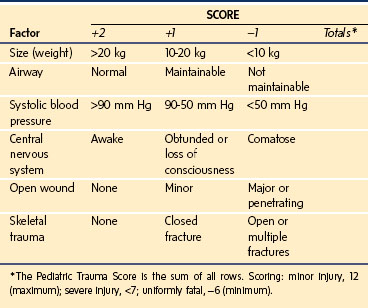
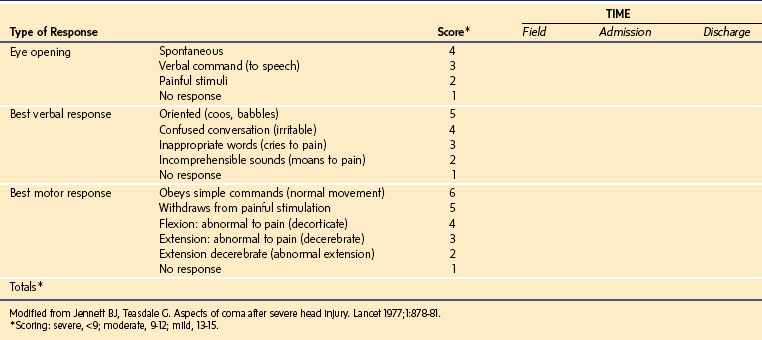
The Glasgow Coma Scale (GCS) (E-Table 38-1) and the modified GCS for children (Table 38-2) are the most common scales used to estimate the severity of neurologic injury. The GCS assigns a total score with a range of 3 to 15 that quantifies eye opening, verbal, and motor functions. The Pediatric Trauma Score (PTS) was developed to facilitate the initial assessment and triage of injured children (Table 38-3). As trauma systems have matured and prehospital providers have become more experienced with assessment and field management, the GCS and PTS have emerged as effective tools for determination of appropriate direct transfer to a trauma center.31,32
Prehospital Airway Management
Effective airway management in the prehospital environment has many challenges, including poor access to the child, lack of use of pharmacologic agents, inclement weather conditions, trauma to the face, and providing care in challenging environments such as an ambulance. Several studies of adult patients undergoing tracheal intubation in the prehospital setting have reported an increased incidence of difficult tracheal intubation,33 need for multiple attempts,34 and undiagnosed esophageal intubation. These events have been reported by all levels of providers, including anesthesiologists.35 However, anesthesiologists have increased success rates and lower complication rates during tracheal intubation compared with other providers.36–38
Unsuccessful prehospital airway management in children may be due to a combination of training, experience, equipment, and environmental issues. A significant proportion of the unsuccessful prehospital tracheal intubations result from ineffective operator training and lack of professional experience. Most prehospital providers, such as paramedics, receive minimal dedicated training in pediatric airway management, including tracheal intubation.39 These providers may not have an opportunity to use their skills on a routine basis. Over time, the psychomotor skills required for pediatric tracheal intubation decay, which likely contributes to the increased complication and failure rates associated with tracheal intubation in children.
Guidelines have proposed that avoidance of airway instrumentation in the prehospital setting may be just as effective as tracheal intubation for pediatric trauma patients. For example, the American Heart Association Pediatric Advanced Life Support (PALS) guidelines state, “Bag-mask ventilation can be as effective as ventilation through an endotracheal tube for short periods and may be safer.”40 The 2010 PALS guidelines state, “In the prehospital setting, ventilate and oxygenate infants and children with a bag-mask device, especially if transport time is short.”40 As a result, many EMS personnel have developed policies containing a scoop and run philosophy for pediatric trauma patients that avoid definitive airway management if the transport time is brief and bag-mask ventilation is effective. Several investigators have also questioned whether prehospital tracheal intubation is the best approach in children who require positive-pressure ventilation. The infrequent need for tracheal intubation in pediatric trauma patients has created obstacles for members of the emergency medical teams to maintain their skills. Multiple studies have demonstrated an increased mortality rate or worsening neurologic outcomes for adult patients who received prehospital tracheal intubation compared with those who received standard bag-mask ventilation.41,42 Several studies that focused on children also reported increased complication rates associated with prehospital tracheal intubation.43–46
There is a trend for using alternative airway devices in the field in adult and pediatric trauma patients. Among the devices that have found favor, supraglottic airway devices have proved easy to use and reliable in the field. Although these devices do not protect the airway from regurgitation and aspiration, they may prevent airway obstruction during transport. One meta-analysis of prehospital alternative airway devices in adults and children indicated that LMAs were very successful in the hands of anesthesiologists and nonphysician flight crews (success rate of 96%) and slightly less successful in the hands of nonphysician clinicians (83%).47 However, there is a dearth of evidence to support a role for the LMA in pediatric trauma.
Emergency Department Evaluation and Management of the Pediatric Trauma Patient
Anesthesiologists should familiarize themselves with the initial management of pediatric trauma patients in the ED because they may be asked to assist in emergency airway management and intraoperative care. Rapid establishment of provisional diagnoses and priorities of care is essential in the ED management of trauma victims.48 Most trauma centers use a multilayered assessment system consisting of a primary survey with resuscitation, a secondary survey, and definitive management.1
Based on the ATLS course, evaluation of the trauma patient occurs in three progressive steps: primary survey, secondary survey, and definitive care.4 The initial evaluation of all trauma patients is the primary survey. The sequence of the primary survey can be remembered as “ABCDE”: airway (A), breathing (B), circulation (C), disability (D), and exposure or environment (E). Many trauma centers have the personnel and resources to simultaneously perform several activities and have prepared trauma rooms, usually in the ED. A trauma cart should be maintained daily in the trauma room and be prepared with all necessary equipment to resuscitate a child (E-Fig. 38-1 and E-Table 38-2). In this circumstance, it would be acceptable for the primary and secondary surveys to occur simultaneously—that is, the primary survey (e.g., volume resuscitation) can occur as a part of the secondary survey (e.g., drawing blood samples).
The airway (A) should be evaluated for patency and opened using a jaw-thrust technique if obstruction is encountered. Immobilization of the cervical spine should be maintained. The child’s breathing (B) and ventilation should be evaluated, and immediate intervention should take place if they are inadequate. Circulation (C) is evaluated by palpation of peripheral pulses, blood pressure, sensorium, and skin turgor. Control of external hemorrhage by the application of direct pressure is also part of the circulation phase. Disability (D) is evaluated by examining the child for neurologic injuries. A GCS score of 8 or less implies severe neurologic injury (see Table 38-2), and immediate intubation (with in-line neck stabilization if indicated) is strongly recommended. Exposure (E) of the whole child is essential for a complete examination. The environment (E) should consist of a heated treatment area that is ideally prepared in advance of the child’s arrival.
E-TABLE 38-2 Recommended Equipment for a Trauma Resuscitation Room
Appropriate sizes (neonate to adult) of facemasks, endotracheal tubes, oral airways, nasal airways, and laryngeal mask airways
Appropriate size stylettes for the endotracheal tubes
Self-inflating ventilating devices capable of administering at least 90% oxygen
Difficult airway and cricothyrotomy kits of appropriate sizes
The importance of an adequate and secure airway cannot be overemphasized. All trauma patients should initially receive 100% supplemental oxygen. Tracheal intubation of most children with major trauma occurs by using a rapid-sequence induction (RSI) with appropriate medications or, in some situations, while the child remains awake. When increased ICP is suspected, measures to prevent further increases during tracheal intubation should be undertaken. Regardless of site or mode of tracheal intubation, proper position and patency of the tracheal tube must be confirmed as soon as the child arrives in the ED.49 Techniques to verify correct tracheal tube position may initially include auscultation, direct laryngoscopy, and determination of the tracheal tube length from the lips and the detection of end-tidal carbon dioxide (E-Fig. 38-2). All children should be monitored with frequent blood pressure measurements and continuous electrocardiography and pulse oximetry.
For traumatic injuries that are primarily located in the abdomen, attempts should be made to place intravenous access in the upper extremities. Traumatic injuries that are located in the chest should have vascular access placed in the upper and lower extremities to account for disruption of a major vessel above or below the right atrium. If vascular access cannot be obtained promptly, femoral venous or intraosseous access should be strongly considered (see Fig. 48-6, A-D, and E-Fig. 48-1, A-E).50
Diagnostic testing is completed during the secondary survey.51,52 Imaging studies, including computed tomography (CT) scans of the brain, neck, chest, abdomen, and pelvis, may be obtained, along with a bedside focused abdominal sonography for trauma (FAST) examination, which is used to detect intraperitoneal free fluid. Standard radiographs of the chest and extremities may also be obtained during the secondary survey. Laboratory studies, including blood count, electrolytes, and blood product crossmatching, are completed during the secondary survey. If the child remains unstable despite aggressive resuscitation, the patient should be considered for emergent transfer to the operating room for surgical intervention. After the child has been stabilized and all injuries have been identified, plans for definitive care can be made. This may include admission to the intensive care unit, an acute care bed, or an observation unit; discharge home with outpatient follow-up and consultation with specialists; or transfer to the operating room.
Anesthesia Management of the Pediatric Trauma Patient
Anesthesiologists, surgeons, and other personnel should work as a coordinated team when managing children with trauma. This collaborative approach can optimize the prompt and reliable identification of suspected injuries so that the anesthesiologist can more effectively anticipate the magnitude of bleeding, physiologic effects, and nature of the surgical procedures. By understanding the appropriate evaluation and initial management of the pediatric trauma patient, the anesthesiologist can recognize injuries that might have been undiagnosed and anticipate the resultant effects intraoperatively.53
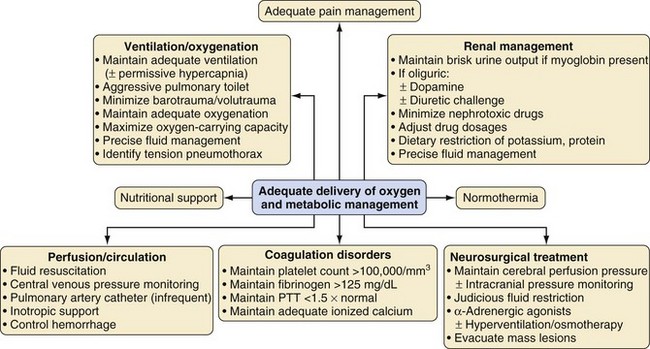
The approach to diagnosis and treatment for the pediatric trauma patient is dictated by the degree of urgency (see Fig. 38-3). For critically ill and hypotensive children who need immediate surgical intervention, resuscitation and the administration of anesthesia may need to be provided simultaneously. Basic principles are applied in this circumstance, including establishment and protection of the airway, maintenance of adequate ventilation, and support of hemodynamics with fluids, blood products, and vasoactive medications. Establishment of generous vascular access is recommended in the critically injured child; this may necessitate a vascular cutdown or placement of an intraosseous needle for volume resuscitation. Anesthetic medications should be cautiously administered in this situation by carefully titrating the dose of the medication to the child’s hemodynamic status.
For the child who requires emergent transfer to the operating room, does not have a secure airway in place, and is not anticipated to have a difficult airway, induction of anesthesia should begin with preoxygenation and be followed by intravenous RSI. If the child is thought to be hypovolemic, a preoperative fluid bolus of balanced salt solution (20 mL/kg) should be administered before induction of anesthesia and drugs selected that maintain circulatory homeostasis. If concern exists regarding a possible cervical spine injury, which applies to most trauma patients, the practitioner should also incorporate in-line immobilization of the cervical spine during airway management and all transfers (Fig. 38-4).54 Arterial and central venous line placements should be selected on a case-by-case basis. The anesthesiologist should be vigilant for the development of undiagnosed traumatic injuries that may manifest in the operating room. For a child who is critically ill, surgery should proceed without delay, and monitoring may initially include only a blood pressure cuff, pulse oximeter, and electrocardiogram.
After oxygenation, ventilation, and circulation have been stabilized, the anesthesiologist may need to address other concerns. If it has not been possible to administer acceptable doses of anesthetic medications, they are administered in stepwise increments after hemodynamic stability has been achieved. Evidence from adult victims of major trauma indicates that recall is more common in these cases. It is reasonable to assume that a similar risk holds true in children.55–57 An intravenous dose of midazolam is strongly recommended,58 although a single agent may be insufficient to guarantee amnesia. If inhalational anesthetics cannot be tolerated, the anesthesiologist may include a ketamine infusion (1 to 5 mg/kg/hr, titrated to the child’s hemodynamic responses; infants may require up to 15 mg/kg/hr).
An important principle is maintenance of body temperature. Hypothermia occurs commonly in victims of major trauma. Hypothermia may potentiate neuromuscular blockade, exacerbate coagulopathy, and contribute to delayed emergence, although moderate hypothermia (34.5° C) may be protective in children with traumatic brain injury.59 Measures to warm the child should be instituted as early as possible. They include warming the blood products and intravenous fluids, using forced-air warming devices and heat lamps, wrapping the head and extremities in plastic bags, and increasing the temperature of the operating room. Ideally, the operating room should be warmed in advance of the child’s arrival to minimize radiant heat loss.
The features and mechanisms of trauma are different in children and adults. In children, abdominal trauma is more common than thoracic trauma, and blunt traumatic injuries are more common than penetrating traumatic injuries. In thoracic trauma, hemothorax and pneumothorax are common; needle decompression followed by chest tube placement may be lifesaving for a tension pneumothorax. Strong consideration should be given to needle decompression or the placement of a chest tube before instituting positive-pressure ventilation in a child with a tension pneumothorax. The greater mobility of the mediastinum in children makes it more likely for tension pneumothorax to occur and to produce hemodynamic instability. Chest injuries in children may be overlooked because rib fractures are less common than in adults.60
Head injury is the most common cause of traumatic death in children.61 Although the principles of managing head injuries in polytrauma patients are mostly similar in adults and children, there are some differences. In neonates and infants, unlike adults, intracranial bleeding can lead to hypovolemic shock because the head is a significantly larger fraction of the body. The open fontanelles in children may provide greater compliance and a potential space for a proportionately larger amount of blood to accumulate. However, the open fontanelles may also provide additional protection against initial increases in ICP. A key goal for children with neurosurgical emergencies is maintenance of hemodynamic stability to preserve cerebral perfusion pressure. A child with suspected intracranial hemorrhage is at risk for increased ICP. Acute treatment for increased ICP due to intracranial hemorrhage includes hyperventilation, intravenous administration of hyperosmolar medications such as hypertonic saline, administration of medications such as opioids, cerebrospinal fluid drainage, and surgical removal of an intracranial hematoma.
Preoperative Evaluation
Emergency surgery is a great source of fear and anxiety for children and their parents.62 The unexpectedness of the event provides little time for the child and family to adjust to the crisis and often limits the time the anesthesiologist has to develop rapport with the child and parents. The anesthesiologist who appears calm and reassuring is of great benefit to all parties.
Evidence suggests that the gastric residual volume in children undergoing emergency surgery is greater than in those undergoing elective surgery.63,64 There is evidence to suggest that in emergency cases, the size of the gastric residual volume (measured in milliliters per kilograms) depends in part on the time interval between the last food ingestion and the injury.64 There is some reassurance in these numbers, but the anesthesiologist should consider these children to have a full stomach and take appropriate measures to reduce the risk of aspiration.65–68 The possible value of H2-blocking agents, metoclopramide, and clear antacids may be considered but their use in these circumstances is not evidence based.
Cervical Spine Evaluation
Cervical spine injuries occur less often in children than in adults and typically occur in different locations.69 Cervical spine injuries in children tend to be located at a more cephalad spinal level than in adults, usually at or above C3. In contrast to actual injuries, pseudosubluxation of the cervical spine is a common and benign finding in children. Pseudosubluxation of the cervical spine usually occurs as the anterior displacement of C2 on C3. In the pediatric trauma patient, the examiner may need to differentiate benign pseudosubluxation from a true cervical spine injury.70 After consultation with the surgeon, pseudosubluxation can be excluded by placing the child’s head in the sniffing position and repeating the radiograph; pseudosubluxation is reduced with this maneuver. In older children, an odontoid or open mouth view can also be considered to evaluate the superior cervical vertebrae. The physician must assume a cervical spine injury exists if the child complains of tenderness in response to palpating the spinous processes of the cervical spine, if the sensorium is decreased, or if neurologic deficits are found. Any of these findings demand a neurosurgical consultation and cervical spine immobilization.
Injury to the cervical spine is less common in children than in adults because the child’s spine is more elastic and mobile, and their incompletely calcified vertebrae are less likely to fracture with minor trauma. Nevertheless, the risk of spine injury is increased when the child is subjected to a substantial force from a fall or the substantial forces associated with motor vehicle crashes.71 Any child with a suspected neck injury should have cervical spine precautions implemented (e.g., placement of a cervical collar, backboard to provide immobilization). Cervical spine immobilization (see Fig. 38-4) should always be maintained when airway management is attempted (Fig. 38-5).
Challenges in obtaining plain radiographs of the cervical spine include difficulty in obtaining a complete view of the spine below C6 and the odontoid process. As CT technology has advanced to more rapid and precise imaging, many centers have replaced standard radiographic examinations with CT scans for evaluation of the cervical spine in the trauma patient. The American College of Surgeons updated the ATLS guidelines, stating that a CT scan of the neck can be substituted for a cervical spine radiograph.4 Replacing plain films with CT scans resulted in a doubling of the rate of cervical spine fractures identified in one study.72
It is challenging to rule out spinal cord injuries in children by standard radiography alone because up to 50% of spinal cord injuries may exist without positive radiographic findings. However, spinal cord injury without radiographic abnormality (SCIWORA) has been estimated in as many as 25% to 50% of children with spinal cord injuries.73,74 These occult cases may reflect ligamentous damage that cannot be detected with standard radiographic examinations or CT scans but can be visualized with magnetic resonance imaging (MRI).
Imaging must be used in conjunction with the physical examination to evaluate the cervical spine, but the cervical spine cannot be considered cleared of pathology only on the basis of diagnostic imaging studies.75,76 Appropriate spine immobilization should continue during the intraoperative and postoperative periods if the cervical spine of the child cannot be confirmed to be uninjured by a negative imaging study and by unremarkable results of the physical examination. The cervical spine cannot be considered undamaged if the child is not alert, is nonconversant, has positive neurologic deficits, has midline cervical tenderness, or has a painful, distracting injury. If the cervical spine cannot be clinically cleared in the postoperative period, MRI should be considered after the child is stable.
Airway Management
Children undergoing emergency surgery are assumed to have a full stomach. They often have considerable gastric contents because of a recent meal,65 increased acid secretion, and delayed gastric emptying caused by pain, trauma, and the administration of opioids. Many experts recommend using an RSI to secure the airway to protect against pulmonary aspiration of gastric contents in the pediatric trauma patient. However, evidence-based data supporting this approach are lacking.77 The anesthesiologist must balance the risks of an RSI with the risks of other airway management techniques. RSI is associated with an inability to intubate the trachea and rapid desaturation during periods of apnea. The risks of using this approach must be weighed against the benefit of reducing the risk for aspiration. The decision to use an RSI assumes that the anesthesiologist has completed an airway evaluation and predicts that the intubation will be uncomplicated and that mask ventilation while maintaining cricoid pressure will be possible as a backup temporizing measure.
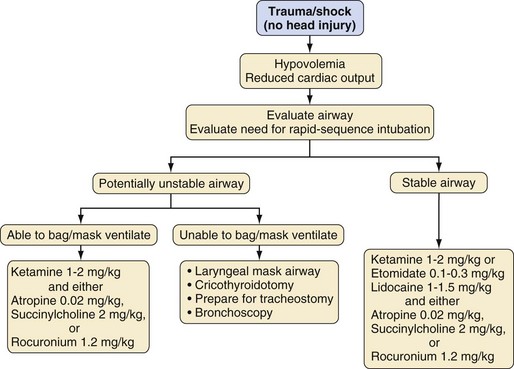
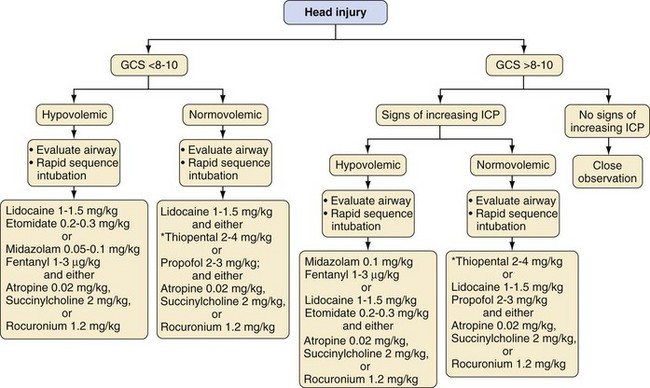
In the operating room, induction of anesthesia depends on the child’s injuries, condition at the time of presentation, whether the airway has been secured, and anticipated airway difficulty. The most common approach to the initial airway management is to follow a protocol for RSI.78,79 Awake intubation is another approach for airway management and usually is reserved for those with severely depressed consciousness, cardiac arrest, or suspected difficult airway. The selection of medications depends on individual circumstances (e.g., head injury, hypovolemia, contraindication to succinylcholine) and preferences of the anesthesiologist. Table 38-4 lists dosages, advantages, and disadvantages of selected anesthetic medications for RSI. Figure 38-6 presents an algorithm for management of children with multiple traumatic injuries but without a suspected traumatic brain injury. Specific considerations for children with multiple trauma that include a traumatic brain injury are outlined in Figure 38-7.
Before an RSI is performed, the anesthesiologist must ensure that the proper equipment is present and functioning, including laryngoscope blades and handles, suction, a leak-free anesthesia circuit, an anesthesia machine, monitors, tracheal tubes of appropriate sizes, and a tracheal tube stylet. All monitors should be properly functioning, and at a minimum, the pulse oximeter and blood pressure cuff should be applied before induction, even though these monitors may not function properly in a crying, uncooperative child until after induction of anesthesia. After intravenous access is confirmed, the child’s lungs are denitrogenated with 100% oxygen for several breaths (as tolerated). Studies of adult patients demonstrate that oxygen saturation remains greater than 95% for 6 minutes after only four vital capacity breaths of 100% oxygen by mask.80 Similar studies have not been performed in children, although the rates at which the Pao2 decreases81 and the Sao2 decreases to 95%82 in infants and younger children after breathing 100% oxygen through a tracheal tube and then performing an apnea maneuver was more rapid than in older children and adults. Even with an uncooperative child, it is possible to increase the Pao2 by enriching the immediate environment with high flows of oxygen. Preoxygenation should not involve forcefully holding the facemask on the awake child; this will likely result in increased anxiety, increased oxygen consumption, and decreased effectiveness of denitrogenation. Premedication (e.g., 0.05 to 0.1 mg/kg of intravenous midazolam) in divided doses may be considered to alleviate fear and anxiety before induction. All drugs intended for use during the RSI should be prepared and labeled along with the doses predetermined for the child’s weight and hemodynamic status.
RSI consists of preoxygenation with 100% oxygen and application of cricoid pressure, followed by the bolus administration of an induction agent such as ketamine (2 mg/kg), etomidate (0.2 to 0.3 mg/kg), or propofol (2 to 3 mg/kg), as well as atropine (20 µg/kg) if succinylcholine is selected and along with a neuromuscular blocking agent such as succinylcholine (2 mg/kg) or high-dose rocuronium (1.2 mg/kg), depending on the age of the child. This is followed immediately by laryngoscopy and tracheal intubation (see Chapters 4 and 12). We recommend using a modified Bier block technique with 1 mg/kg of lidocaine applied using an occlusive tourniquet for 30 to 60 seconds to prevent pain from injection before the administration of propofol or etomidate through a small peripheral vein. Positive-pressure ventilation before tracheal intubation is avoided during an RSI. Cervical spine immobilization is typically indicated for most trauma patients during RSI (see Fig. 38-4) and requires an assistant to perform manual in-line stabilization during laryngoscopy (see Fig. 38-5).
Succinylcholine is the neuromuscular blocking agent of choice because of its rapid onset and short duration of action. A Cochrane Review concluded that succinylcholine created superior intubating conditions compared with rocuronium using doses of less than 1.2 mg/kg.83 However, high-dose rocuronium may also be effectively used as an alternative to succinylcholine for an RSI in children.84 If large doses of rocuronium (e.g., 1.2 mg/kg) are used in infants and children, the time to recovery of the train-of-four to 25% averages about 45 minutes, and it may take as long as 75 minutes.84 It is possible that the duration of action may be longer than the planned procedure. Moreover, if the child’s lungs are impossible to ventilate and the trachea cannot be intubated, the situation may become life-threatening. The use of high-dose sugammadex if available (see Chapter 6) can abbreviate the duration of the neuromuscular block in these situations. If rocuronium (or any nondepolarizing muscle relaxant) is combined with thiopental, the thiopental must be cleared from the intravenous tubing before administering the relaxant to prevent the former from precipitating.85 The routine use of succinylcholine for elective tracheal intubation in children has lost popularity among pediatric anesthesiologists due to reports of hyperkalemia-induced cardiac dysrhythmias and cardiac arrest, which led the U.S. Food and Drug Administration to issue a black box warning about the use of succinylcholine in children undergoing elective surgery.86 Many of the male children who developed hyperkalemic cardiac arrest were subsequently diagnosed with various forms of muscular dystrophy (mostly Duchenne’s muscular dystrophy, which may manifest with few clinical signs in boys 8 years of age or younger).
Establishing a definitive airway may be required for children with a suspected difficult airway due to facial, laryngeal, or thoracic trauma. Alternative approaches for airway management should be considered when difficult intubation is anticipated.87,88 In these situations, the urgency of securing the airway takes priority over the increased risk of tracheal aspiration of gastric contents. These cases can be managed by using an awake intubation approach, which commonly includes topical anesthesia and sedation combined with one of the following airway techniques:
• Awake intubation under direct laryngoscopy or rigid bronchoscopy
• Indirect laryngoscopy using a fiberoptic bronchoscope (FOB) or video laryngoscope
• Supraglottic airway device–assisted tracheal intubation with or without an FOB
In a nonemergent situation, the FOB is the gold standard approach to manage a difficult airway. An FOB can be very effective, especially in children with limited mouth opening, restricted neck movement (common in the trauma patient with a cervical collar in place), or associated congenital syndromes that make direct laryngoscopy difficult or impossible.89,90 The alternative approaches are advantageous because their main goals are to maintain spontaneous ventilation, to maintain oropharyngeal reflexes to prevent aspiration if regurgitation occurs, and to allow the option of aborting the procedure if the airway cannot be secured. However, these airway techniques can be challenging in small children, in those whose airway anatomy is severely distorted, and those with copious secretions or blood in the pharynx. Large amounts of sedation to achieve effective patient cooperation during airway interventions may approach levels of general anesthesia and produce significant respiratory depression.
In emergency situations, blind placement of a supraglottic airway device such as an LMA is recommended as part of the American Society of Anesthesiologists’ difficult airway algorithm.91 The LMA does not protect the airway from tracheal aspiration of gastric contents, but it may be used as a conduit to facilitate blind or fiberoptic intubation of the trachea.92 The development of an LMA (LMA North America, San Diego, Calif.) with a modified cuff and drainage tube (ProSeal) and that is available in sizes 1 to 5 may make this process even safer by allowing evacuation of gastric contents and providing a better airway seal than earlier LMA devices.93,94 The Fastrach LMA (LMA North America) provides an additional supraglottic device that is useful when direct visualization of the laryngeal inlet is not possible. This device has been designed to blindly place a tracheal tube into the trachea from within the supraglottic airway.95 However, this is only of benefit in children larger than 30 kg because pediatric sizes (1 to 2) are not available.
Cricoid Pressure
Cricoid pressure was popularized for RSI by Sellick in his seminal report in 1961,96 which carefully described the position of the head and neck during the maneuver. The patient assumes the supine position with the neck fully extended in a slight head-down tilt, and the nasogastric tube, if present, is removed. The concavity of the cervical neck is maintained during cricoid pressure using a firm support placed under the cervical spine. Cricoid pressure is applied by an assistant using the thumb and second finger; the first finger stabilizes the thumb and finger on the cricoid ring. Pressure is applied firmly as consciousness is lost and released only after the tracheal tube cuff has been inflated.
In current practice, the head and neck are often not positioned as described by Sellick. Although Sellick’s preliminary evidence suggested that his technique was very effective for occluding the esophagus, there have been no randomized, controlled trials that document its effectiveness or its superiority over other techniques in preventing aspiration during RSI.97,98 Radiologic evidence suggests that cricoid pressure may not completely occlude the lumen of the esophagus because the contours of the vertebral bodies are asymmetric and the esophagus is not aligned directly over the vertebral bodies.99 The application of cricoid pressure in children may result in even further lateral displacement of the esophagus than it does in adults.100 Sellick never described the amount of force required to occlude the lumen of the esophagus, although in adults, a force of 30 to 40 N (equivalent to a 3- to 4-kg mass) is considered sufficient to occlude the esophagus. In a study of the force required to occlude the esophagus in infants, investigators determined that a force of only 5 N distorts the airway in infants weighing less than 5 kg and that a force of only 12 N produces the same distortion in 15-year-old adolescents.101 This begs the question of how much force is required to reliably occlude the esophagus without distorting the airway in infants and children. The answer is unknown.
Many assistants who perform cricoid pressure apply insufficient force,102 apply force at an incorrect location103 or use excessive force that may distort the larynx and make tracheal intubation more difficult. One study found that correctly applied cricoid pressure in children 2 weeks to 8 years of age with or without paralysis resulted in no leak of air into the stomach with up to 40 cm H2O of peak inflation pressure.104
In children, the application of cricoid pressure has been viewed with some skepticism because the force required has been considered excessive in younger children. However, in the current medicolegal climate, many have perceived an obligation on their part to apply it to prevent regurgitation. In support of this perception, the results of a survey documented that even though 71% do not believe cricoid pressure occludes the esophagus, 90% use it when there is an increased risk for aspiration.105
Volume Administration
Better understanding of the mechanisms of the stress response and particularly of the factors that contribute to sepsis and ongoing proliferation of proinflammatory cytokines has helped redefine the immediate and long-term goals of acute resuscitation and definitive management of the unstable trauma patient.106 The original concept of resuscitation was restoration of adequate circulating volume with appropriate red cell mass and adequate oxygenation, but this has evolved to become a continuum of critical care focused on restoration of total body homeostasis. The initial priority is focused on hemodynamic stabilization and should progress seamlessly to the aggressive management of metabolic derangements. The anesthesiologist who is involved in immediate resuscitation of the severely injured child should focus on these initial hemodynamic goals.
The preservation of intravascular volume is a primary priority during resuscitation of the severely injured child. When immediate surgical intervention in the operating room is not necessary, it is advisable to replace fluid deficits before induction of anesthesia. Mild to moderate deficits can be replaced with isotonic crystalloid solutions. In severe hypovolemia with ongoing blood loss, colloids such as 5% albumin can be considered for use as an intravascular volume expander along with packed red blood cells. If time permits and if clinically indicated, crossmatched blood products should be administered, starting with an initial dose of 10 mL/kg. Type-specific uncrossmatched blood has a very low incidence of transfusion reactions and is typically available before crossmatched blood.107 In an emergency situation requiring the immediate transfusion of blood products, type O negative packed red blood cells should be administered if the ABO blood type of the child has not been determined.
Life-threatening exsanguination is most commonly caused by a massive disruption of the solid viscera or major vascular structures within the abdomen and chest. The most important part of this resuscitation process is restoration of adequate red cell mass and circulating volume to promote effective tissue perfusion and oxygenation. Evaluation of 103,434 cases in phases II and III of the National Pediatric Trauma Registry (NPTR) study suggests that massive exsanguination is relatively uncommon.108 Children who sustain massive exsanguinating injuries typically die at the scene.
If volume resuscitation is vigorous and large volumes of crystalloid solutions are administered, hemorrhage-induced red blood cell loss may be further exacerbated by dilution with crystalloids. Overzealous fluid resuscitation may worsen bleeding by abrupt elevation of the systolic blood pressure from circulatory expansion that disrupts clots from injured vessels. Clinical evidence of this has been limited to assessment of penetrating injury in adults and elective cardiac surgery, emphasizing the necessity of a proper balance between restoration of adequate peripheral perfusion and avoidance of gross fluid overload.109–110 Effective circulatory resuscitation of the injured child requires a combination of clinical data, anticipation of pending physiologic challenges, and coordination of planned operative interventions by all specialists involved in the child’s acute care. Large-volume transfusions without consideration of coagulation status, electrolyte shifts, or critical serum protein depletion may produce coagulopathy. Injudicious or inadequate crystalloid infusions may worsen capillary leak locally in injured organs and remotely in the interstitium of lung and brain. It is postulated that the concentrations of systemic inflammatory mediators increase after traumatic injuries. Increased serum secretory phospholipase A2 levels have been associated with increased injury magnitudes in patients after traumatic injuries,111 and increased plasma high-mobility group box 1 (HMGB1) levels have been identified after traumatic injuries, although clear correlations with patient outcomes were not established.112
Injured children tend to be reasonably free of the acquired cardiovascular diseases that complicate the care of injured adults. However, transient myocardial ischemia can quickly undermine contractility of even the healthiest heart. In the presence of cardiovascular collapse from massive or ongoing hemorrhage, this can rapidly lead to cardiac failure and peripheral hypoperfusion. Children who are being resuscitated from major hemorrhage and who have been exposed to prolonged low flow states may require inotropic support to maintain adequate peripheral perfusion. Because an accurate definition for what was previously referred to as myocardial contusion is not easily quantifiable, a high index of suspicion based on an understanding of the child’s mechanism of injury is the best guide to preemptive management, especially if rhythm disturbances are observed.113,114
Studies are being conducted to determine the ideal products and dosing schedules for fluid resuscitation of injured children. Several studies have evaluated hypertonic saline for use as a resuscitation solution and for the management of traumatic brain injury.115–117 Investigators from several studies suggest that no definitive recommendations can be made because no fluid product is superior.118–120 However, children with evidence of significant hypovolemia who are bleeding significantly and receiving isotonic crystalloids probably should be initially resuscitated with lactated Ringer’s solution due to its pH of 6.6, compared with a pH of 5.5 for normal saline, although Plasmalyte has a more normal physiologic pH. The critical objectives are immediate control of the sources of hemorrhage, restoration of red cell mass to ensure adequate oxygen delivery, and appropriate rheology to enhance tissue microperfusion.
Vascular Access
Vascular access can be overlooked as one of the more important priorities in the initial care of injured children. In some circumstances, placement of two large-bore intravenous lines may be very challenging in the hypovolemic child. In some cases, the child arrives in the ED with no vascular access. In the setting of the ED or operating room, adequate vascular access can usually be readily obtained by a standard percutaneous approach through a peripheral vein or by direct percutaneous cannulation of the femoral vein. These two methods are consistently successful in achieving immediate vascular access without resorting to invasive cutdown techniques. The success rate for percutaneous femoral catheterizations using a Seldinger technique is so high that using a cutdown approach is rarely required (see also Fig. 48-2). If the child is severely exsanguinated and percutaneous femoral vascular access is unsuccessful, surgical exposure through an incision 1 cm below the inguinal ligament can expose the saphenous vein at the saphenofemoral junction. This provides immediate access to a large-caliber vessel and facilitates the placement of a femoral arterial line for ongoing management. Ultrasound-guided vascular access techniques may also be used to place peripheral and central lines in the venous and arterial systems.121,122 Many trauma centers do not routinely use subclavian or internal jugular veins for vascular access during acute resuscitation. Personnel responsible for airway management usually obstruct access to the neck, as does the cervical collar that should be in place as part of standard prehospital management protocols. In an orchestrated approach, one individual or group of individuals should concentrate fully on management and support of the airway while a second group addresses access to the vascular system.
Intraosseous infusion devices are being increasingly used in adults and children for whom percutaneous vascular access is unavailable or unsuccessful.123 In the pediatric population, intraosseous needles are usually placed in the tibial plateau medial to the tibial tuberosity approximately 3 to 5 cm below the knee. These devices can be lifesaving and usually are inserted after several attempts at venous access have failed. If appropriately placed, they are useful conduits for infusion of all medications, crystalloids, and dilute blood products.124 Intraosseous needles should be replaced by effective and reliable venous access as soon as possible (see also Fig 48-6, A-D). Pneumatic-driven devices for placement of intraosseous needles have also been described. Some of these devices have been extraordinarily difficult to remove and require specific training from the product manufacturer; their overall usefulness remains to be evaluated. One type of intraosseous infusion device, the EZ-IO (Vidacare Corporation; San Antonio, Tex.) operates like a battery-powered drill and is relatively simple to train providers in its use (see E-Fig. 48-1, A-E).125
Damage Control Surgery
Children with massive anatomic derangements associated with uncontrollable hemorrhage usually require emergent operative intervention. In many trauma centers, these hemodynamically unstable children are treated by damage control surgery,126 which is based on the premise that rapid control of abdominal bleeding by placing abdominal packing and immediate coverage of the open abdomen enables more effective resuscitation before exposing the child to a more prolonged definitive repair.
Over the past decade, the use of damage control surgery for the initial stabilization rather than definitive repair has moved the focus of resuscitation away from simple circulatory volume management to a need for immediate correction of whole-body metabolic derangements associated with the stress response.127 Acute abdominal packing and coverage avoids development of abdominal compartment syndrome, allows reevaluation of the injured tissue, and provides access for repeated lavage of contaminated spaces. Although there have been concerns that this approach may be used more often than necessary, the risks of leaving an abdomen open are minimal compared with the ventilatory and circulatory consequences that may develop from an abdominal compartment syndrome. Damage control surgery has proved to be a lifesaving technique in isolated circumstances by allowing an unstable child to receive comprehensive resuscitation in a more controlled environment.
Pain Management
Acute pain occurring as a result of traumatic injuries is one of the most common adverse stimuli experienced by children.128 There has been fear that treating pain due to trauma may mask the symptoms of progressive injury. Historically, adults have received significantly more analgesia after injuries than children.129 As a result, many physicians withhold significant pain relief, especially opioids, because they fear serious side effects such as respiratory depression or hypotension may develop. All medications used for analgesia and sedation in children can have significant side effects; however, careful titration to the desired effect along with appropriate monitoring should ensure safe administration.
The management of acute pain in the injured child can be accomplished by using nonpharmacologic interventions and pharmacologic therapies. Nonpharmacologic interventions include positive reinforcement, distraction, hypnotherapy, acupuncture, massage and touch relaxation, and guided imagery.130 Pharmacologic therapies include oral and intravenous acetaminophen and nonsteroidal antiinflammatory drugs (NSAIDs) for mild to moderate pain and oral and intravenous opioids for moderate to severe pain.131 Regional anesthesia can be very useful in the acutely injured child, especially for orthopedic injuries.
In certain cases, regional nerve blocks can be used to provide analgesia in the ED; as primary anesthesia in a cooperative or older child to avoid some of the risks associated with general anesthesia, including aspiration; and as a supplement to general anesthesia for postoperative analgesia.132,133 For example, analgesia for children with midshaft fractures of the femur can be provided by a traditional femoral nerve block or fascia iliaca compartment block, diminishing pain from the femur and quadriceps muscle spasm. Similarly, an axillary or supraclavicular nerve block may be used in children with forearm fractures. Central neuraxial techniques, such as epidural analgesia, can be useful for children with major thoracoabdominal trauma, including rib fractures. Close communication with orthopedic and general surgical colleagues is advised so that there are no misunderstandings about the ability to perform sensory and motor examinations and about coordination of additional analgesic medications.
Future Directions in Pediatric Trauma
As traumatic injuries have become recognized as a significant public health threat, numerous initiatives have emerged to improve prevention of this hazard, management of its victims, and measurement of system performance and outcomes. Exciting areas of investigation continue to emerge in aspects of clinical management, systems development, patient safety, and injury prevention.134–136
Prevention and public education are important areas of ongoing research in pediatric trauma. There has been great progress in educating the public that many injuries are avoidable, but there are still many factors that undermine the best intentions to produce a safe and nurturing environment for children. A significant advance in effective prevention and public education has occurred through the Injury Free Coalition for Children sponsored by the Robert Wood Johnson Foundation.137–139 This coalition has evolved into a network of 40 institutions throughout the country, and each focuses on specific areas of enhanced public education to define opportunities for improvement within the environment of children. Although there is no such thing as a completely risk-free environment, ongoing and effective public awareness campaigns can enhance the likelihood that risks to children throughout the world will diminish.
As the transition from fluid resuscitation to metabolic management has evolved, there has been increasing interest in identifying tissue-specific biomarkers that reflect the severity and prognosis of injury.140,141 The efficacy of resuscitation could be enhanced by routine assay of biomarkers that accurately reflect levels of physiologic derangements or effective restoration of homeostasis. Numerous studies are focusing on identification of various components of neuronal tissue markers that reflect the presence and severity of brain injury.
The combination of hypovolemia, coagulopathy, hypothermia, and acidosis is associated with increased morbidity and mortality. There have been many efforts to control these issues, and most significant has been the evolution of damage control surgery. There is growing laboratory animal evidence that hypothermia, if induced in a rapid and controlled fashion, is cytoprotective.59,142–144 In the near future, the use of hypothermia in the minutes after traumatic injury may widen the margin of safety for injured children, especially those with significant cerebral injury. This is an area that must make the transition from animal observations in the basic science laboratory to well-designed, randomized, controlled trials involving prehospital providers and established trauma programs. Emerging evidence suggests that premature death in bleeding patients with severe trauma may be attenuated by administering antifibrinolytics, such as tranexamic acid.145–147 Institution of tranexamic acid within 3 hours of the insult, particularly in countries with low to middle incomes, is likely to have the greatest impact on outcome. However, there is insufficient evidence to recommend antifibrinolytics in patients with traumatic brain injury.148 Randomized, controlled trials enrolling traumatized children are warranted to identify a possible role for antifibrinolytics to prevent premature death.
Acknowledgment
We thank J. Tepas, MD, and H. DeSoto, MD, for their prior contributions to this chapter.
Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest registry. Anesth Analg. 2007;105:344–350.
De Ross AL, Vane DW. Early evaluation and resuscitation of the pediatric trauma patient. Semin Pediatr Surg. 2004;13:74–79.
This review article focuses on the initial treatment of the pediatric trauma patient.
Hubble MW, Wilfong DA, Brown LH, et al. A meta-analysis of prehospital airway control techniques. Part II: alternative airway devices and cricothyrotomy success rates. Prehosp Emerg Care. 2010;14:515–530.
Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71.
Ross AK. Pediatric trauma. Anesthesia management. Anesthesiol Clin North Am. 2001;19:309–337.
This article reviews the implications of anesthesia in managing pediatric trauma.
1 Kortbeek JB, Al Turki SA, Ali J, et al. Advanced trauma life support, 8th edition: the evidence for change. J Trauma. 2008;64:1638–1650.
2 Mathews TJ, Miniño AM, Osterman MJ, et al. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–157.
3 Rivara FP. The global problem of injuries to children and adolescents. Pediatrics. 2009;123:168–169.
4 American College of Surgeons, Committee on Trauma. Advanced trauma life support (ATLS), 8th ed. Chicago: American College of Surgeons; 2008.
5 American Academy of Pediatrics and the American College of Emergency Physicians. Advanced pediatric life support: the pediatric emergency medicine resource, 4th ed. Elk Grove Village, IL: American Academy of Pediatrics and the American College of Emergency Physicians; 2006.
6 American Academy of Pediatrics Section on Orthopaedics, American Academy of Pediatrics Committee on Pediatric Emergency Medicine, American Academy of Pediatrics Section on Critical Care. Management of pediatric trauma. et al. Pediatrics. 2008;121:849–854.
7 Rasouli MR, Rahimi-Movaghar V, Maheronnaghsh R, et al. Preventing motor vehicle crashes related spine injuries in children. World J Pediatr. 2011;7:311–317.
8 Judy K. Unintentional injuries in pediatrics. Pediatr Rev. 2011;32:431–438.
9 Brown JK, Jing Y, Wang S, et al. Patterns of severe injury in pediatric car crash victims: Crash Injury Research Engineering Network database. J Pediatr Surg. 2006;41:362–367.
10 Koepsell TD, Rivara FP, Vavilala MS, et al. Incidence and descriptive epidemiologic features of traumatic brain injury in King County, Washington. Pediatrics. 2011;128:946–954.
11 Marwan A, Harmon CM, Georgeson KE, et al. Use of laparoscopy in the management of pediatric abdominal trauma. J Trauma. 2010;69:761–764.
12 U.S. Department of Health and Human Services, Administration for Children and Families, Administration on Children, Youth, and Families, Children’s Bureau; Child Maltreatment, 2010. Available at http://www.acf.hhs.gov/programs/cb/pubs/cm10/cm10.pdf#page=70 (accessed September 2012)
13 Knight LD, Collins KA. A 25-year retrospective review of deaths due to pediatric neglect. Am J Forensic Med Pathol. 2005;26:221–228.
14 Mulpuri K, Slobogean BL, Tredwell SJ. The epidemiology of nonaccidental trauma in children. Clin Orthop Relat Res. 2011;469:759–767.
15 Hobbs CJ, Bilo RA. Nonaccidental trauma: clinical aspects and epidemiology of child abuse. Pediatr Radiol. 2009;39:457–460.
16 Sullivan CM. Child abuse and the legal system: the orthopaedic surgeon’s role in diagnosis. Clin Orthop Relat Res. 2011;469:768–775.
17 Chang DC, Knight V, Ziegfeld S, et al. The tip of the iceberg for child abuse: the critical roles of the pediatric trauma service and its registry. J Trauma. 2004;57:1189–1198.
18 Coffey C, Haley K, Hayes J, et al. The risk of child abuse in infants and toddlers with lower extremity injuries. J Pediatr Surg. 2005;40:120–123.
19 Celso B, Tepas JJ, Pracht E, et al. A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma. 2006;60:371–378.
20 Durham R, Pracht E, Orban B, et al. Evaluation of a mature trauma system. Ann Surg. 2006;243:775–783.
21 Nirula R, Maier R, Moore E, et al. Scoop and run to the trauma center or stay and play at the local hospital: hospital transfer’s effect on mortality. J Trauma. 2010;69:595–599.
22 Chung S, Shannon M. Hospital planning for acts of terrorism and other public health emergencies involving children. Arch Dis Child. 2005;90:1300–1307.
23 Michael WS, Julia AM. Chemical-biological terrorism and its impact on children. Pediatrics. 2006;118:1267–1278.
24 Luten R, Wears RL, Broselow J, et al. Managing the unique size-related issues of pediatric resuscitation: reducing cognitive load with resuscitation aids. Acad Emerg Med. 2002;9:840–847.
25 Luten R. Error and time delay in pediatric trauma resuscitation: addressing the problem with color-coded resuscitation aids. Surg Clin North Am. 2002;82:303–314.
26 Agarwal S, Swanson S, Murphy A, et al. Comparing the utility of a standard pediatric resuscitation cart with a pediatric resuscitation cart based on the Broselow tape: a randomized, controlled, crossover trial involving simulated resuscitation scenarios. Pediatrics. 2005;116:e326–e333.
27 Rosenberg M, Greenberger S, Rawal A, et al. Comparison of Broselow tape measurements versus physician estimations of pediatric weights. Am J Emerg Med. 2011;29:482–488.
28 Faul M, Wald MM, Sullivent EE, et al. Large cost savings realized from the 2006 Field Triage Guideline: reduction in overtriage in U.S. trauma centers. Prehosp Emerg Care. 2011;16:222–229.
29 Acosta CD, Kit Delgado M, Gisondi MA, et al. Characteristics of pediatric trauma transfers to a level 1 trauma center: implications for developing a regionalized pediatric trauma system in California. Acad Emerg Med. 2010;17:1364–1373.
30 Williams D, Foglia R, Megison S, et al. Trauma activation: are we making the right call? A 3-year experience at a level I pediatric trauma center. J Pediatr Surg. 2011;46:1985–1991.
31 Potoka DA, Schall LC, Ford HR. Development of a novel age-specific pediatric trauma score. J Pediatr Surg. 2001;36:106–112.
32 Tude Melo JR, Di Rocco F, Blanot S. Mortality in children with severe head trauma: predictive factors and proposal for a new predictive scale. Neurosurgery. 2010;67:1542–1547.
33 Timmermann A, Eich C, Russo SG, et al. Prehospital airway management: a prospective evaluation of anaesthesia trained emergency physicians. Resuscitation. 2006;70:179–185.
34 Krisanda TJ, Eitel DR, Hess D, et al. An analysis of invasive airway management in a suburban emergency medical services system. Prehosp Disaster Med. 1992;7:121–126.
35 Timmermann A, Russo SG, Eich C, et al. The out-of-hospital esophageal and endobronchial intubations performed by emergency physicians. Anesth Analg. 2007;104:619–623.
36 Timmermann A, Braun U, Panzer W, et al. Out-of-hospital airway management in northern Germany. Physician-specific knowledge, procedures and equipment. Anaesthesist. 2007;56:328–334.
37 Helm M, Hossfeld B, Schäfer S, et al. Factors influencing emergency intubation in the prehospital setting—a multicentre study in the German Helicopter Emergency Medical Service. Br J Anaesth. 2006;96:67–71.
38 Timmermann A, Russo SG, Hollmann MW. Paramedic versus emergency physician emergency medical service: role of the anaesthesiologist and the European versus the Anglo-American concept. Curr Opin Anaesthesiol. 2008;21:222–227.
39 Warner KJ, Carlbom D, Cooke CR. Paramedic training for proficient prehospital endotracheal intubation. Prehosp Emerg Care. 2010;14:103–108.
40 Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(Suppl 3):S876–S908.
41 Wang HE, Mann NC, Mears G, et al. Out-of-hospital airway management in the United States. Resuscitation. 2011;82:378–385.
42 Dunford JV, Davis DP, Ochs M, et al. Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid sequence intubation. Ann Emerg Med. 2003;42:721–728.
43 Cooper A, DiScala C, Foltin G, et al. Prehospital endotracheal intubation for severe head injury in children: a reappraisal. Semin Pediatr Surg. 2001;10:3–6.
44 Ehrlich PF, Seidman PS, Atallah O, et al. Endotracheal intubations in rural pediatric trauma patients. J Pediatr Surg. 2004;39:1376–1380.
45 Gausche M, Lewis RJ, Stratton SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783–790.
46 DeBoer S, Seaver M, McNeil M, et al. Prehospital airway management. It’s time to reconsider how we maintain pediatric airways. EMS Mag. 2009;38:42–48.
47 Hubble MW, Wilfong DA, Brown LH, et al. A meta-analysis of prehospital airway control techniques. Part II: alternative airway devices and cricothyrotomy success rates. Prehosp Emerg Care. 2010;14:515–530.
48 Eppich WJ, Zonfrillo MR. Emergency department evaluation and management of blunt abdominal trauma in children. Curr Opin Pediatr. 2007;19:265–269.
49 Grmec S, Mally S. Prehospital determination of tracheal tube placement in severe head injury. Emerg Med J. 2004;21:518–520.
50 Hansen M, Meckler G, Spiro D, et al. Intraosseous line use, complications, and outcomes among a population-based cohort of children presenting to California hospitals. Pediatr Emerg Care. 2011;27:928–932.
51 Moore MA, Wallace EC, Westra SJ. Chest trauma in children: current imaging guidelines and techniques. Radiol Clin North Am. 2011;49:949–968.
52 Fox JC, Boysen M, Gharahbaghian L, et al. Test characteristics of focused assessment of sonography for trauma for clinically significant abdominal free fluid in pediatric blunt abdominal trauma. Acad Emerg Med. 2011;18:477–482.
53 Krug SE, Tuggle DW. Management of pediatric trauma. Pediatrics. 2008;121:849–854.
54 Vanderhave KL, Chiravuri S, Caird MS, et al. Cervical spine trauma in children and adults: perioperative considerations. J Am Acad Orthop Surg. 2011;19:319–327.
55 Engelhardt T, Petroz GC, McCheyne A, et al. Awareness during pediatric anesthesia: what is the position of European pediatric anesthesiologists? Paediatr Anaesth. 2007;17:1066–1070.
56 Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–1108.
57 Phelan L, Stargatt R, Davidson AJ. Long-term posttraumatic effects of intraoperative awareness in children. Paediatr Anaesth. 2009;19:1152–1156.
58 Buffett-Jarrett SE, Stewart SH, Finley GA, Loughlan HL. Effects of benzodiazepines on explicit memory in a paediatric surgery setting. Psychopharmacology. 2003;168:377–386.
59 Li H, Lu G, Shi W, Zheng S. Protective effect of moderate hypothermia ion severe traumatic brain injury in children. J Neurotrauma. 2009;26:1905–1909.
60 Tovar JA. The lung and pediatric trauma. Semin Pediatr Surg. 2008;17:53–59.
61 Durkin MS, Olsen S, Barlow B, et al. The epidemiology of urban pediatric neurological trauma: evaluation of, and implications for, injury prevention programs. Neurosurgery. 1998;42:300–310.
62 Helfricht S, Latal B, Fischer JE, et al. Surgery-related posttraumatic stress disorder in parents of children undergoing cardiopulmonary bypass surgery: a prospective cohort study. Pediatr Crit Care Med. 2008;9:217–223.
63 Schurizek BA, Rybro L, Boggild-Madsen NB, Julh B. Gastric volume and pH in children for emergency surgery. Acta Anaesthesiol Scand. 1986;30:404–408.
64 Bricker SRW, McLuckie A, Nightingale DA. Gastric aspirates after trauma in children. Anaesthesia. 1989;44:721–724.
65 Sagarin MJ, Chiang V, Sakles JC, et al. Rapid sequence intubation for pediatric emergency airway management. Pediatr Emerg Care. 2002;18:417–423.
66 Ehrenfeld JM, Cassedy EA, Forbes VE, Mercaldo ND, Sandberg WS. Modified rapid sequence induction and intubation: a survey of United States current practice. Anesth Analg. 2012;115:95–101.
67 El-Orbany M, Connolly LA. Rapid sequence induction and intubation: current controversy. Anesth Analg. 2010;110:1318–1325.
68 Gencorelli FJ, Fields RG, Litman RS. Complications during rapid sequence induction of general anesthesia in children: a benchmark study. Paediatr Anaesth. 2010;20:421–424.
69 Parent S, Mac-Thiong JM, Roy-Beaudry M, et al. Spinal cord injury in the pediatric population: a systematic review of the literature. J Neurotrauma. 2011;28:1515–1524.
70 Easter JS, Barkin R, Rosen CL, et al. Cervical spine injuries in children, part II: management and special considerations. J Emerg Med. 2011;41:252–256.
71 Rasouli MR, Rahimi-Movaghar V, Maheronnaghsh R, et al. Preventing motor vehicle crashes related spine injuries in children. World J Pediatr. 2011;7:311–317.
72 Griffen MM, Frykberg ER, Kerwin AJ, et al. Radiographic clearance of blunt cervical spine injury: plain radiography or computed tomography scan? J Trauma. 2003;55:222–226.
73 Pang D. Spinal cord injury without radiographic abnormality in children, 2 decades later. Neurosurgery. 2004;55:1325–1342.
74 Yucesoy K, Yuksel KZ. SCIWORA in MRI era. Clin Neurol Neurosurg. 2008;110:429–433.
75 Chung S, Mikrogianakis A, Wales PW, et al. Trauma association of Canada Pediatric Subcommittee National Pediatric Cervical Spine Evaluation Pathway: consensus guidelines. J Trauma. 2011;70:873–884.
76 Hutchings L, Willett K. Cervical spine clearance in pediatric trauma: a review of current literature. J Trauma. 2009;67:687–691.
77 Neilipovitz DT, Crosby ET. No evidence for decreased incidence of aspiration after rapid sequence induction. Can J Anaesth. 2007;54:748–764.
78 Brownstein D, Shugerman R, Cummings P, et al. Prehospital endotracheal intubation of children by paramedics. Ann Emerg Med. 1996;28:34–39.
79 Zuckerbraun NS, Pitetti RD, Herr SM, et al. Use of etomidate as an induction agent for rapid sequence intubation in a pediatric emergency department. Acad Emerg Med. 2006;13:602–609.
80 Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anaesth. 2009;56:449–466.
81 Cook TM, Wolf AR, Henderson AJW. Changes in blood-gas tensions during apneic oxygenation in paediatric patients. Br J Anaesth. 1998;81:338–342.
82 Kinouchi K, Fukumitsu K, Tahsiro C, et al. Duration of apnoea in anaesthetized children required for desaturation of haemoglobin to 95%: comparison of three different breathing gases. Paediatr Anesth. 1995;5:115–119.
83 Perry JJ, Lee JS, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2, 2008. CD002788
84 Mazurek AJ, Rae B, Hann S, et al. Rocuronium versus succinylcholine: are they equally effective during rapid-sequence induction of anesthesia? Anesth Analg. 1998;87:1259–1262.
85 Morton WD, Lerman J. The effect of pancuronium on the solubility of aqueous thiopentone. Can J Anaesth. 1987;34:87–89.
86 Lerman J, Berdock SE, Bissonnette B, et al. Succinylcholine warning. Can J Anaesth. 1994;41:165.
87 Auden SM. Additional techniques for managing the difficult pediatric airway. Anesth Analg. 2000;90:878–880.
88 Weiss M, Engelhardt T. Proposal for the management of the unexpected difficult pediatric airway. Paediatr Anaesth. 2010;20:454–464.
89 Xue FS, Luo MP, Xu YC, et al. Airway anesthesia for awake fiberoptic intubation in management of pediatric difficult airways. Paediatr Anaesth. 2008;18:1264–1265.
90 Walker RW, Ellwood J. The management of difficult intubation in children. Paediatr Anaesth. 2009;19(Suppl 1):77–87.
91 American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
92 Selim M, Mowafi H, Al-Ghamdi A, et al. Intubation via LMA in pediatric patients with difficult airways. Can J Anaesth. 1999;46:891–893.
93 Wheeler M. ProSeal laryngeal mask airway in 120 pediatric surgical patients: a prospective evaluation of characteristics and performance. Paediatr Anaesth. 2006;16:297–301.
94 Sinha A, Sharma B, Sood J. ProSeal as an alternative to endotracheal intubation in pediatric laparoscopy. Paediatr Anaesth. 2007;17:327–332.
95 Gerstein NS, Braude DA, Hung O, et al. The Fastrach Intubating Laryngeal Mask Airway: an overview and update. Can J Anaesth. 2010;57:588–601.
96 Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anesthesia. Lancet. 1961;2:404–406.
97 Kron SS. Questionable effectiveness of cricoid pressure in preventing aspiration. Anesthesiology. 1995;83:431–432.
98 Lerman J. Cricoid pressure: “May the force be with you.”. Anesth Analg. 2009;109:1363–1366.
99 Dotson K, Kiger J, Carpenter C, et al. Alignment of cricoid cartilage and esophagus and its potential influence on the effectiveness of Sellick maneuver in children. Pediatr Emerg Care. 2010;26:722–725.
100 Smith KJ, Dobranowski J, Yip G, et al. Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imagining. Anesthesiology. 2003;99:60–64.
101 Walker RW, Ravi R, Haylett K. Effect of cricoid force on airway calibre in children: a bronchoscopic assessment. Br J Anaesth. 2010;104:71–74.
102 Herman NL, Carter B, Van Decar TK. Cricoid pressure: teaching the recommended level. Anesth Analg. 1996;83:859–863.
103 Nafiu OO, Bradin S, Tremper KK. Knowledge, attitude, and practice regarding cricoid pressure of ED personnel at a large U.S. teaching hospital. J Emerg Nurs. 2009;35:11–15.
104 Moynihan RJ, Brock-Utne JG, Archer JH, et al. The effect of cricoid pressure on preventing gastric insufflation in infants and children. Anesthesiology. 1993;78:652–656.
105 Ahmed Z, Zestos M, Chidiac E, et al. A survey of cricoid pressure use among pediatric anesthesiologists. Pediatr Anesth. 2009;19:183–187.
106 Blackburn GL. Metabolic considerations in management of surgical patients. Surg Clin North Am. 2011;91:467–480.
107 Dutton RP, Shih D, Edelman BB, et al. Safety of uncrossmatched type-O red cells for resuscitation from hemorrhagic shock. Trauma. 2005;59:1445–1449.
108 Tepas JJ. The National Pediatric Trauma Registry: a legacy of commitment to control of childhood injury. Semin Pediatr Surg. 2004;13:126–132.
109 de Guzman E, Shankar MN, Mattox KL. Limited volume resuscitation in penetrating thoracoabdominal trauma. AACN Clin Issues. 1999;10:61–68.
110 Vretzakis G, Kleitsaki A, Stamoulis K, et al. Intra-operative intravenous fluid restriction reduces perioperative red blood cell transfusion in elective cardiac surgery, especially in transfusion-prone patients: a prospective, randomized controlled trial. J Cardiothorac Surg. 2010;5:7–15.
111 Neidlinger NA, Hirvela ER, Skinner RA, et al. Postinjury serum secretory phospholipase A2 correlates with hypoxemia and clinical status at 72 hours. J Am Coll Surg. 2005;200:173–178.
112 Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22.
113 Kaptein YE, Talving P, Konstantinidis A, et al. Epidemiology of pediatric cardiac injuries: a National Trauma Data Bank analysis. J Pediatr Surg. 2011;46:1564–1571.
114 Bromberg BI, Mazziotti MV, Canter CE, et al. Recognition and management of nonpenetrating cardiac trauma in children. J Pediatr. 1996;128:536–541.
115 Knapp JM. Hyperosmolar therapy in the treatment of severe head injury in children: mannitol and hypertonic saline. AACN Clin Issues. 2005;16:199–211.
116 Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350–1357.
117 Tyagi R, Donaldson K, Loftus CM, et al. Hypertonic saline: a clinical review. Neurosurg Rev. 2007;30:277–289.
118 Akech S, Ledermann H, Maitland K. Choice of fluids for resuscitation in children with severe infection and shock: systematic review. BMJ. 2010;341:c4416.
119 Roberts I, Alderson P, Bunn F, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 4, 2004. CD000567
120 Wills BA, Nguyen MD, Ha TL, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877–889.
121 Samoya SW. Real-time ultrasound-guided peripheral vascular access in pediatric patients. Anesth Analg. 2010;111:823–825.
122 Skippen P, Kissoon N. Ultrasound guidance for central vascular access in the pediatric emergency department. Pediatr Emerg Care. 2007;23:203–207.
123 Mahajan R, Nazir R, Mehta S. An overview of intraosseous access. Anesth Analg. 2010;111:825–826.
124 Tobias JD, Ross AK. Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use. Anesth Analg. 2010;110:391–401.
125 Ong ME, Chan YH, Oh JJ, et al. An observational, prospective study comparing tibial and humeral intraosseous access using the EZ-IO. Am J Emerg Med. 2009;27:8–15.
126 Fabian TC. Damage control in trauma: laparotomy wound management acute to chronic. Surg Clin North Am. 2007;87:73–93.
127 Wetzel RC, Burns RC. Multiple trauma in children: critical care overview. Crit Care Med. 2002;30(Suppl):S468–S477.
128 Bauman BH, McManus JG, Jr. Pediatric pain management in the emergency department. Emerg Med Clin North Am. 2005;23:393–414.
129 Friedland LR, Kulick RM. Emergency department analgesic use in pediatric trauma victims with fractures. Ann Emerg Med. 1994;23:203–207.
130 Rusy LM, Weisman SJ. Complementary therapies for acute pediatric pain management. Pediatr Clin North Am. 2000;47:589–599.
131 Nelson KL, Yaster M, Kost-Byerly S, et al. A national survey of American Pediatric Anesthesiologists: patient-controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesth Analg. 2010;110:754–760.
132 Tsui B, Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology. 2010;112:719–728.
133 Tsui B, Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of extremity and trunk blocks. Anesthesiology. 2010;112:473–492.
134 Stelfox HT, Bobranska-Artiuch B, Nathens A, et al. A systematic review of quality indicators for evaluating pediatric trauma care. Crit Care Med. 2010;38:1187–1196.
135 Mello MJ, Getz MA, Lapidus G, et al. Innovations in injury prevention education. J Trauma. 2007;63(Suppl):S7–S9.
136 Kendrick D, Barlow J, Hampshire A, et al. Parenting interventions for the prevention of unintentional injuries in childhood. Cochrane Database Syst Rev. 4, 2007. CD006020
137 Gittelman MA, Pomerantz WJ, McNealy T. Reducing injury rates using a community-based approach. J Trauma. 2007;63(Suppl):S44–S49.
138 Pressley JC, Barlow B. Child and adolescent injury as a result of falls from buildings and structures. Inj Prev. 2005;11:267–273.
139 Pressley JC, Barlow B, Durkin M, et al. A national program for injury prevention in children and adolescents: the injury free coalition for kids. J Urban Health. 2005;82:389–402.
140 Li J, Li XY, Feng DF, et al. Biomarkers associated with diffuse traumatic axonal injury: exploring pathogenesis, early diagnosis, and prognosis. J Trauma. 2010;69:1610–1618.
141 Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg Clin North Am. 2010;90:665–677.
142 Varon J. Therapeutic hypothermia: implications for acute care practitioners. Postgrad Med. 2010;122:19–27.
143 Kochanek PM, Drabek T, Tisherman SA. Therapeutic hypothermia: the Safar vision. J Neurotrauma. 2009;26:417–420.
144 Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71.
145 Roberts I, Shakur H, Afolabi A, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101.
146 Shakur RI, Ker K, Coats T, Antifibrinolytic drugs for acute traumatic injury. on behalf of the CRASH-2 Trial collaborators. Cochrane Database Syst Rev. 2011;1. CD004896
147 Ker K, Kiriya J, Perel P, et al. Avoidable mortality from giving tranexamic acid to bleeding trauma patients: an estimation based on WHO mortality data, a systematic literature review and data from the CRASH-2 trial. BMC Emerg Med. 2012;12:3.
148 Perel P, Roberts I, Shakur H, et al. Haemostatic drugs for traumatic brain injury. Cochrane Database Syst Rev. 1, 2010. CD007877