Transposition Flaps
Introduction
Transposition flaps can be designed in a number of different configurations; however, their linear dimension is always longer than their width. Common configurations of transposition flaps are listed in Table 8-1. The majority of facial cutaneous defects are repaired with transposition flaps designed as a rectangle, parabola, or rhombus. A rhombic flap and its modifications are transferred to the recipient site by advancement as well as by pivoting. However, I discuss them here because their major method of movement is pivotal, and like pure pivotal flaps, they develop a single standing cutaneous deformity at their base. Z-plasty and bilobe flaps represent the use of two transposition flaps concomitantly. In the case of Z-plasty, diagonally opposing triangle-shaped flaps trade locations after transposition. A bilobe flap represents two transposition flaps designed on a common base. However, each lobe has an independent pivotal point, and each lobe develops an independent standing cutaneous deformity.
Because transposition flaps are pivotal flaps, the greater the arc of pivotal movement, the greater will be the size of the standing cutaneous deformity and the less will be the effective length of the flap. Pivoting a transposition flap 45° from its in situ position reduces the effective length 5%. A 90° and 180° pivot reduces length by 15% and 40%, respectively (see discussion in Chapter 6).1 The reduction in effective length must be accounted for when transposition flaps are designed, so that greater pivoting requires a longer design of the flap. The most common error in using transposition flaps of any kind is to design the flap too short because of a poor understanding of this concept. As transposition flaps turn in an arc around their relatively fixed pivotal point, a standing cutaneous deformity forms. Similar to the relationship between the arc of flap movement and effective length, there is a direct and positive association between the degree of pivoting and the size of the standing cutaneous deformity. The greater the pivot of the flap, the larger is the deformity that develops from the pivotal movement. Thus, increasing the flap’s pivot will shorten the effective length, increase wound closure tension (if it is not designed sufficiently long), and change the flap’s shape as a result of forming a standing cutaneous deformity. To limit these restricting factors, whenever possible, transposition flaps should be designed not to pivot more than 90°.
In the majority of cases, standing cutaneous deformities resulting from transposing flaps can be resected at the time of the initial flap transfer to the recipient site. Excision should always be in a vector that diverges from the base of the flap so vascularity is not unduly impaired (Fig. 8-1). For scalp and large transposition flaps of the face, it may be prudent to delay excision of the deformity for 4 to 6 weeks to allow revascularization of the distal flap.
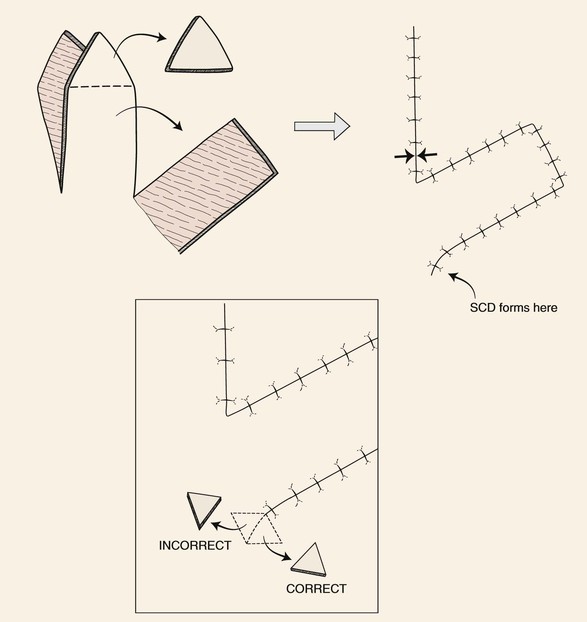
FIGURE 8-1 Transposition flaps are pivotal flaps that form single standing cutaneous deformities (SCD) at base of flap. Only base of flap must be contiguous with defect. Excision of SCD should always be in vector that diverges from base of flap to minimize impairment of blood supply. Opposing arrows indicate area of greatest wound closure tension.
A disadvantage of transposition flaps is the potential for development of a trap-door deformity.2,3 The deformity is present when the flap appears bulky, protruding above the surface of the surrounding skin and giving the appearance of a pincushion. This complication tends to occur a few weeks after transfer. Trap-door deformity occurs most often when curvilinear incisions are made at the distal border of flaps. Concentric contraction of the resulting scar presumably causes the curvilinear arc of the scar to contract toward its center, which in turn forces the skin to protrude outward above the surface of the surrounding skin. Creating straight rather than curved incisions and designing the distal border of the flap with an angle instead of an arc prevents curvilinear scars. Trap-door deformity may also develop because adjacent tissue surrounding the recipient site is not sufficiently undermined at the time of transposition. The sheet of scar that forms between the undersurface of the flap and the depth of the defect contracts in a concentric fashion. Contraction of the scar beneath the flap causes the flap to bulge outward. Fortunately, trap-door deformity usually resolves with time. Trap-door deformities can be minimized or prevented by wide subdermal undermining of the margins of the primary defect and using a flap with the same thickness as the depth of the recipient site. For defects located in skin with sebaceous gland hypertrophy, it is helpful to maintain the bevel of the defect resulting from micrographic surgery or to create one and counterbevel the edge of the flap. This creates a diagonally sloping scar beneath the epidermis that may reduce concentric scar contraction. As noted, concentric scars are a common cause of trap-door deformity.
Applications
Classic Design
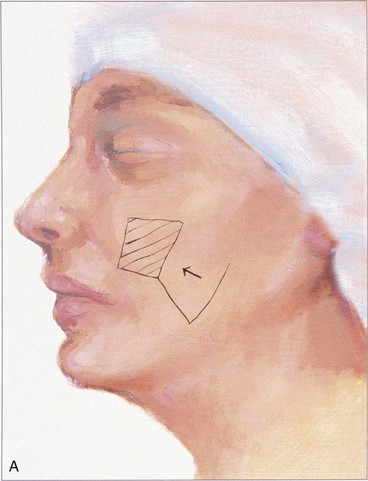
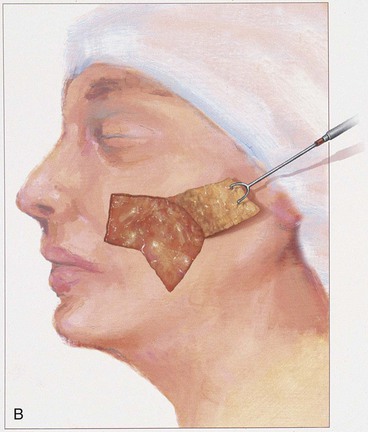
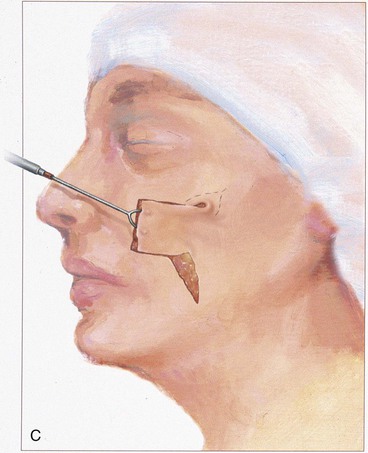
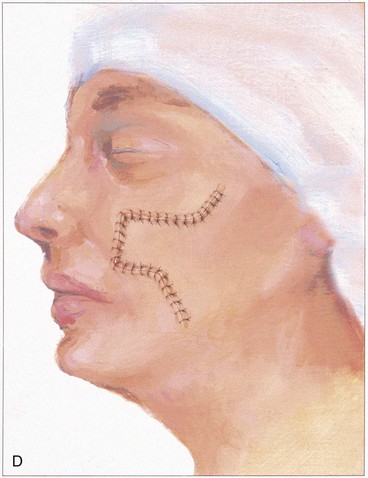
The rectangle- or parabola-shaped transposition flap is commonly used for repair of cutaneous defects of the medial and lateral cheek, temple, and glabellar area (Fig. 8-2). A linear closure of cutaneous defects of the temple often results in elevation of the lateral brow or webbing of the lateral canthus. The use of transposition flaps frequently can avoid these problems. Small flaps of this design can also be used to repair defects of the dorsum and sidewall of the nose, chin, and upper and lower lips. Transfer of skin or of skin and muscle from the upper to the lower eyelid to repair anterior lamellar defects may readily be accomplished by using either medially or laterally based cutaneous or musculocutaneous (containing orbicularis muscle) transposition flaps.
Island Transposition Flaps
Most transposition flaps used on the face have a cutaneous pedicle and a random blood supply. However, island transposition flaps based on an axial blood supply can be harvested from the region of the forehead and medial cheek. In the forehead, the flap is transferred with the supratrochlear artery and vein. Such flaps have limited use for facial reconstruction but can be used to repair defects located on the upper nasal dorsum or medial canthus. Park and colleagues have shown that the superior labial artery gives rise to an artery to the nasal septum that divides into a superficial and deep branch and a second independent artery to the nasal ala.4 The deep division of the septal artery can be used to nourish a sizeable mucosal island axial transposition flap harvested from the inner aspect of the upper lip. This flap can be used to reconstruct large defects of the vermilion. Similarly, a sizeable island transposition flap harvested with the skin and subcutaneous tissue of the upper melolabial fold can be transferred as an axial flap based on the alar artery, which arises from the facial artery just before the vessel gives rise to the superior labial artery. Common examples of island transposition flaps used in the head and scalp are flaps from the forehead transferred to the upper nose, temporal hair-bearing scalp flaps transferred to the anterior scalp to treat male pattern baldness, and palatal mucoperiosteal flaps transferred to the nasal surface of the velum.
Note Flap
The note flap described by Walike and Larrabee is an angular transposition flap that, when designed, looks like a musical eighth note.5 This flap allows one to close a circular defect with a triangular transposition flap that maximizes the use of surrounding tissue (Fig. 8-3).6 The flap is designed by first drawing a tangent on either side of the circle parallel to relaxed skin tension lines (RSTLs). The tangent should have the length of 1.5 times the diameter of the circular defect. At the end of the tangent line, a 50° to 60° angled flap is designed, with the second side of the flap having a length approximately equal to the diameter of the circle.6 The flap is transposed into the defect, and the distal tip of the flap is trimmed so that there is no wound closure tension. As with rectangular and parabolic transposition flaps, the greatest wound closure tension is at the closure of the donor site. In the case of the note flap, the greatest wound closure tension is approximately perpendicular to the tangent line created when the flap is designed. The surface area of the note flap is designed so that it is 25% less than the area of the defect; thus clinical judgment is required when this flap is used. The flap is recommended primarily for small (2 cm or less) skin defects. Because the pivotal arc of the note flap is approximately 45°, a minimal standing cutaneous deformity develops that may not require excision. Standing cutaneous deformities of larger flaps will require subsequent surgical revision unless the deformities are removed at the time of flap transfer. I use the note flap primarily for defects on the cheek, temple, and lateral nasal sidewall. However, it may be used everywhere on the head and neck, including the lip.
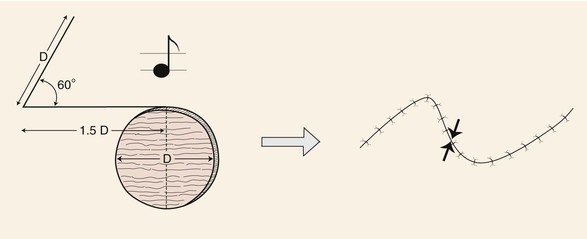
FIGURE 8-3 Note flap has configuration of musical eighth note. The note flap is used for repair of circular skin defects 2 cm or less in size. One side of flap drawn parallel to relaxed skin tension lines and tangent to defect has length of 1.5 times diameter (D) of defect. Second side of triangle-shaped flap forms 50° to 60° angle with first side and is length of diameter of defect. Point of greatest wound closure tension is indicated by opposing arrows.
Rhombic Flap
The rhombic flap is a common flap used by plastic surgeons. The flap is discussed in great detail in Chapter 11. Transfer of the flap involves advancement and pivotal tissue movement. A rhombus can be considered a square usually tilted toward one side. The greater the tilt of the rhombus, the greater is the discrepancy in length between the short and long diagonals of the rhombus. A rhombus may have right angles, in which case it is also a square; however, in addressing rhombic flaps, the rhombus-shaped defect is usually not square in configuration, so opposing interior angles are obtuse or acute. The classic rhombic flap described by Limberg is used to repair a defect that has a configuration of a rhombus with two opposing 60° and two opposing 120° interior angles (Fig. 8-4).7 The 60° to 120° rhombus can be thought of as two equilateral triangles placed base to base. This means the short diagonal of the rhombus is equal in length to the sides of the rhombus. The Limberg flap is designed to repair a defect of this configuration. The flap is designed by extending the line of the short diagonal a length equal to the diagonal, which is also the length of the side of the defect. This creates the first side of the flap. A second line is then drawn of equal length parallel to either adjacent side of the defect. Because there are two sides adjacent to the extended diagonal, two flaps can be designed. In addition, because the short diagonal can be extended in two directions from the rhombic defect, a total of four flaps can be designed around a rhombus-shaped defect. The majority of wound closure tension is at the donor site and has been calculated to be 20° to the short diagonal of the rhombus defect (see Fig. 8-4).8 Skin mobility and extensibility are important to consider in designing a rhombic flap, and an understanding of the resultant wound closure tension vector is critical to avoid distortion of surrounding facial structures.
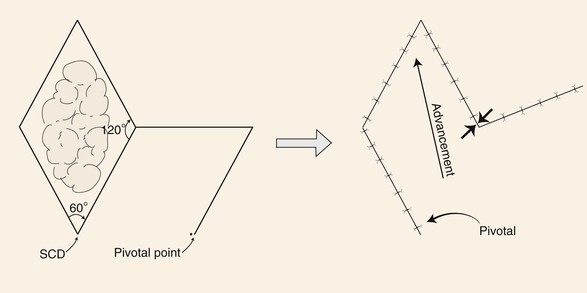
FIGURE 8-4 Limberg flap used for repair of skin defects that have configurations of a rhombus with two opposing 60° and two opposing 120° interior angles. Flap designed by extending line of short diagonal equal to diagonal length. Second line drawn of equal length parallel to either adjacent side of defect. Point of greatest wound closure tension is indicated by opposing arrows. Standing cutaneous deformity (SCD) forms at base of flap.
There are a number of variations in the design of rhombic flaps. A useful variation is a 30° rhombic flap. An M-plasty may be combined with transposition of the flap.9 The 30° rhombic flap has the advantage of minimizing the standing cutaneous deformity because there is less pivotal movement than with the classic Limberg flap. When it is used, the M-plasty reduces the length of excision necessary to remove the standing cutaneous deformity. The 30° rhombic flap can be designed by approximately halving an equilateral triangle.6 The length of the side of the triangle should be the length of a side of the defect (Fig. 8-5). The angle of the apex of the transposition flap is 30°. The width of the base of the flap is half the greatest width of the defect (see Fig. 8-5).6
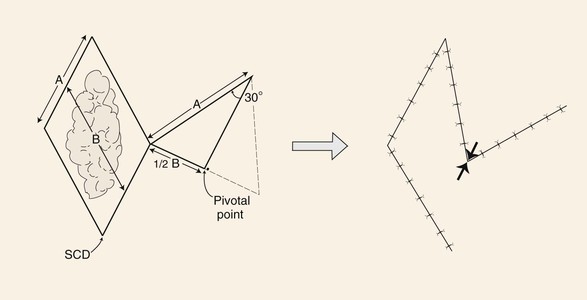
FIGURE 8-5 A 30° rhombic transposition flap designed by approximately halving an equilateral triangle. Length of triangle side is length of side of defect (A). Angle formed at apex of triangle-shaped flap is 30°. Width of flap is half widest width of defect (B). Point of greatest wound closure tension is indicated by opposing arrows. Standing cutaneous deformity (SCD) forms at base of flap.
The Dufourmentel modification of the rhombic flap can be used for rhombus-shaped defects that have any combination of interior angles, not just those of 60° to 120°. It is particularly useful for repair of rhombic defects with acute angles of 60° to 90° when the surgeon does not wish to excise additional tissue to create a 60° to 120° rhombus.6 The flap is designed first by bisecting the angle between the extension of the short diagonal and one of the sides of the defect (Fig. 8-6). The bisecting line is extended a length equal to the length of a side of the defect. This creates the first side of the flap. A second line of the same length is drawn parallel to the long diagonal of the rhombus defect. This creates the second side of the flap that is not parallel to the side of the defect, as with the Limberg flap. Like in other transposition flaps designed to close rhombus-shaped defects, the greatest wound closure tension is at the closure site of the donor defect. Therefore, whenever possible, the donor site closure should be planned so that it is parallel to the lines of maximum extensibility (LME) and perpendicular to RSTLs.
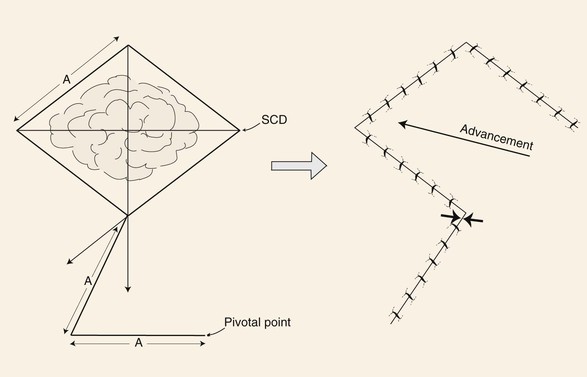
FIGURE 8-6 Dufourmentel flap designed by bisecting angle between extension of short diagonal and one side of defect. Length of bisecting line extended length of side of defect (A). Line of same length creating second side of flap drawn parallel to long diagonal of rhombic defect. Point of greatest wound closure tension is indicated by opposing arrows. Standing cutaneous deformity (SCD) forms at base of flap.
Rhombus-shaped transposition flaps are useful for repair of defects anywhere on the face and neck but are particularly helpful for defects on the cheek and temple. When designing a rhombic flap, the surgeon must select the optimum flap for a given defect. The surgeon first draws two lines parallel to the LME and creates rhomboids. The four possible rhombic flaps are then conceptualized. Of the four flaps, two will have a short diagonal parallel to the LME, and one of these should be selected.6 If one flap is near a mobile facial structure, the other should be used. Design of a rhombic flap is more complex than that of most other facial skin flaps because of the exact geometry and option of placing the flap in four separate locations about the defect. I do not frequently use the rhombic flap in my practice because most micrographic defects are not rhombus shaped, and to convert the defect to a rhombus requires discarding normal tissue unnecessarily. In addition, the scar is usually more visible than when other flaps are used because approximately half of the entire length of the resulting scar does not parallel or does not fall within the RSTLs of the face. This disadvantage is more important in the area of the forehead and lips, where skin creases are more prominent, and less important in the cheek and temple, where creases are not as prominent, the skin is thinner, and the resulting scar tends to blend better with the adjacent skin.
Z-Plasty
Z-plasty is discussed in detail in Chapter 14. Z-plasty can be considered a double transposition of triangular flaps, each with independent points of pivot. When the flaps are rounded at their ends, the process is sometimes referred to as S-plasty. In Z-plasty, one flap is transposed about its pivotal point in a clockwise direction, the other in a counterclockwise direction. In addition to pivoting, the flaps are advanced into their triangular recipient sites. Thus, to achieve proper flap movement, it is necessary to undermine widely at the base of each flap. When Z-plasty is used for scar revision, it is designed so the scar is positioned in and oriented along the central limb of the Z. With this design, Z-plasty changes the direction of the old scar and relieves scar contracture at the expense of increasing the final length of the scar that results from revision. Z-plasty can be used to “lengthen” or to release a segment of skin distorted by scar contraction. Advancement, not the pivotal movement of the flaps, accounts for the lengthening in the axis of scar contracture. This is because, in essence, skin adjacent to the scar is recruited through the mechanism of advancement to augment the contracted area. The larger the triangular flaps used in Z-plasty, in terms of both the angles of the tips of the flaps and the length of the sides of the flaps, the greater the amount of skin advanced toward the contracted site and the greater the lengthening of the contracted segment. However, the greater the angles of the triangular flaps, the greater are the standing cutaneous deformities that result from the pivotal movement of the flaps. Likewise, the longer the limbs of the Z-plasty, the greater will be the length of the final revised scar. Used appropriately, Z-plasty is a highly effective method of improving the appearance of scars, relieving tension during wound closure, and correcting scar contraction causing distortion of facial structures.
The standard Z-plasty design is a Z-shaped figure with a central limb and two peripheral limbs, all of equal length. The peripheral limbs are extensions from each end of the central limb and are on opposite sides and parallel to each other so that the two angles thus formed are equal. The central limb represents the area of desired lengthening or alterations in orientation. The principle of Z-plasty can be better understood if the figure is completed by drawing lines across the bases of the two triangular flaps outlined by the Z-plasty, thus forming a parallelogram (Fig. 8-7).10 The central limb of the Z becomes the short diagonal of the parallelogram, and an imaginary line joining the outer ends of the two peripheral limbs becomes the long diagonal. Transposing the two triangles formed by the flaps will result in replacement of the short diagonal of the parallelogram (central limb of the Z) with the long diagonal.10 Thus a Z-plasty has the effect of interchanging the existing short diagonal for the long diagonal of the parallelogram. The difference between the lengths of the two diagonals is the amount of lengthening in the direction of the central limb of the Z that may be obtained after the flaps are transposed.10 This difference in length of the two diagonals becomes greater by lengthening the limbs of the Z. Similarly, the wider the angle of the triangular flaps, the greater is the difference between the short and long diagonals. The wider the angle, the greater the gain but also the greater the pivotal arc of the two flaps, resulting in larger standing cutaneous deformities. Larger deformities make it more difficult to achieve transposition without the need for excision of the deformities. Likewise, flaps with smaller angles are easy to transpose with no significant standing cutaneous deformities but afford minimal change in length and risk vascular compromise of their tips. The approximate percentages of elongation in the direction of the central limb that can be expected from Z-plasties of 30°, 45°, and 60° are 25%, 50%, and 75%, respectively. Most often, Z-plasty flaps with 60° angles provide an adequate margin of safety from the standpoint of flap vascularity and also provide for significant lengthening of scar contracture. The angle of the Z-plasty is also influenced by the anatomic location of the Z-plasty and the clinical situation.
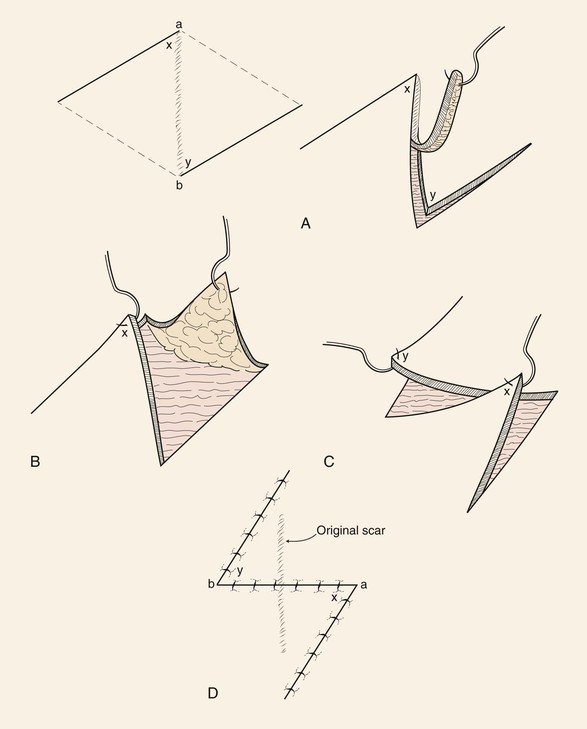
FIGURE 8-7 Principle of Z-plasty may be understood if parallelogram is formed containing two opposing triangular flaps of Z-plasty. Central limb of Z-plasty is short diagonal of parallelogram. Transposing triangular flaps result in replacement of short diagonal with long diagonal of parallelogram. Difference in length between diagonals is amount of lengthening in direction of central limb of Z that may be obtained after transposing flaps. Difference in length of two diagonals becomes greater by increasing angle of flaps.
Because creating a Z-plasty with longer limbs will provide greater release of scar contracture, it is tempting to design Z-plasties with long limbs. However, the longer the limbs of the Z, the greater will be the length and visibility of the overall scar. This is because the length of a Z-plasty scar consists of the central limb plus the two peripheral limbs, which are new scars created by the flaps; thus overall length of the scar compared with the original one is significantly increased. This is calculated to be approximately 200% of the length of the central limb of the Z.10 Although relatively large Z-plasties may be effectively used in the neck, Z-plasties on the face are best designed so that the limbs are no greater than 0.5 cm. If greater release of scar contracture is required than what a 0.5-cm Z-plasty can provide or the scar being revised with Z-plasty is longer than 0.5 cm, multiple Z-plasties should be used. In such circumstances, the number of flaps designed depends on the length of the scar being revised. The angles of the flaps of a Z-plasty do not always have to be equal; however, the three limbs of the Z must be of similar length owing to the requirement that the flaps outlined by the limbs are interchanged and their common borders sutured together.
Z-plasty may be an effective method of effacing a skin web. By transposing the short diagonal, which is positioned on the crest of the web, with the long diagonal position at an angle to the web, the transposition of the Z-plasty flaps creates an elongation of tissue in the place of the previous web. Some underlying hypertrophic scar must also usually be removed for this procedure to be successful. Z-plasty is particularly useful in correcting scars that cause webs in the area of the medial canthus. Multiple Z-plasties are often required to correct the web (Fig. 8-8).
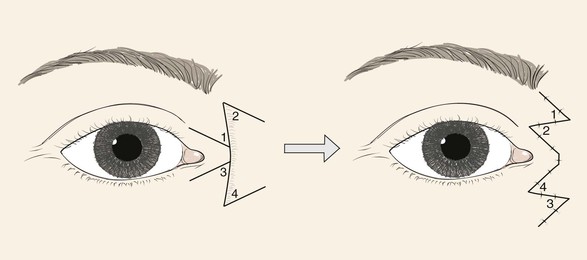
FIGURE 8-8 Z-plasties may be an effective method of correcting skin webs in region of medial canthus. Central limb of Z placed along crest of web.
Standing cutaneous deformities, rather than being excised, may sometimes be treated by Z-plasty. This method is most useful with unipedicle advancement flaps (Fig. 8-9). Z-plasties on either side of the base of the advancement flap are designed so that their limbs extend outward away from the base of the flap. This design prevents the Z-plasties from compromising the vascularity of the flap. The Z-plasties in effect redistribute the tissue of the standing cutaneous deformities by moving the deformities in the same vector as the flap movement.
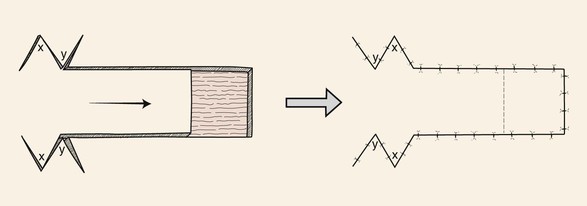
FIGURE 8-9 Z-plasties on either side of base of advancement flap redistribute tissue of standing cutaneous deformities by moving deformities in same vector of advancement as flap movement.
A Z-plasty may be helpful in repair of an oval skin defect whose long axis is not parallel to RSTLs. Triangular flaps are designed adjacent to the long axis of the oval so that when the flaps are transposed, the central limb of the Z (long axis of the oval) is realigned with the RSTLs (Fig. 8-10). Similarly, a circular skin defect may also be repaired by modified Z-plasties, which cause the suture line of the wound closure to assume a zigzag shape. This is accomplished by designing two modified Z-plasties, one on either side of the circle and with opposite orientation.11 With use of a compass, two circles of the same size as the skin defect are drawn opposite each other on each side of the defect. The centers of the circles are shifted the length of one radius toward the defect so that two new circles can be constructed in which points on their circumferences touch in the center of the defect. The four circles provide a template for designing two modified Z-plasties with flaps that have curved borders (Fig. 8-11). These borders can be designed more angulated if desired. The flaps are transposed without the need to excise standing cutaneous deformities, and the resulting scar has a zigzag configuration.
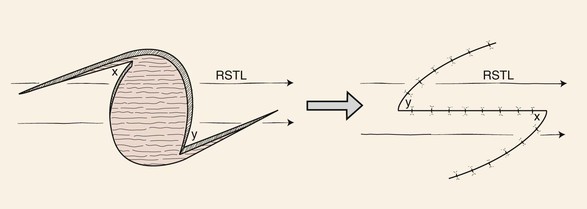
FIGURE 8-10 Z-plasty may be helpful in repairing oval skin defect when long axis is not parallel to relaxed skin tension lines (RSTLs). Triangular flaps are designed adjacent to long axis of oval so that when flaps are transposed, central limb of Z (long axis of oval) is realigned with RSTLs.
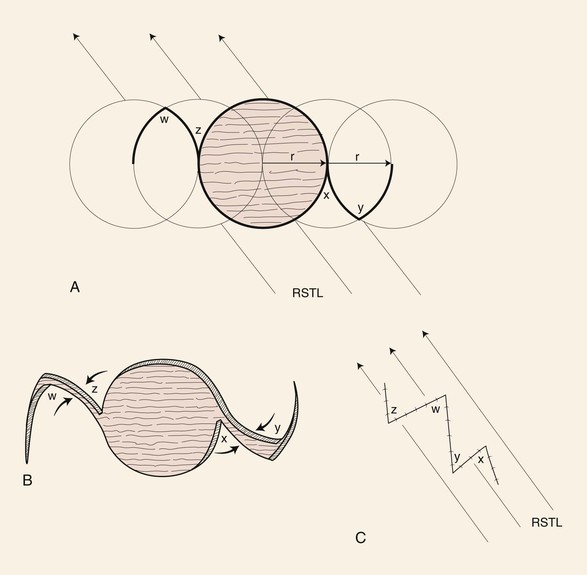
FIGURE 8-11 Circular skin defects may be repaired by modified Z-plasties, which cause suture line of wound closure to assume zigzag configuration. Two circles of size of defect are drawn opposite each other on each side of defect. Centers of circles are shifted the length of one radius toward defect, and two new circles are drawn. Four circles provide template for designing two modified Z-plasties with flaps that have curved borders. RSTL, relaxed skin tension line.
Bilobe Flaps
Bilobe flaps are discussed in detail in Chapter 12. Bilobe flaps are double transposition flaps that share a single base. Similar to single transposition flaps, bilobe flaps move around pivotal points located at their base and develop standing cutaneous deformities as they pivot. Because each flap or lobe moves around an independent pivotal point, each lobe develops an individual standing cutaneous deformity. The greater the arcs of rotation about their pivotal points, the larger are the standing cutaneous deformities. To minimize these deformities, it is wise to limit the degree of movement through the arc by orienting the linear axis of each lobe approximately 45° from the other, with the axis of the first lobe oriented 45° from the axis of the defect for a total arc of movement of 90° to 110°. This is more important on the nose than on the cheek. When bilobe flaps are used on the cheek, the primary factors in determining the position of the first and second lobes of the flap are the location of the defect and the availability of donor skin for construction of the two lobes. In such instances, the first and second lobes are designed in areas of greatest skin laxity or in areas where scar camouflage will be maximized. This may on occasion necessitate design of the second lobe along an axis that is 180° to the axis of the defect. The major advantage of bilobe flaps is the ability to recruit redundant skin for construction of the flap from areas that are not adjacent to the defect. This skin redundancy may be located at some distance from the defect and may be difficult to transfer to the recipient site by other surgical approaches. In general, bilobe flaps are designed in such a way that the first lobe is immediately adjacent to the defect and has a surface area that is less than the surface area of the defect. Thus, part of the closure of the defect is achieved by secondary movement of surrounding skin through direct advancement. The relationship of the surface area of the first lobe compared with the surface area of the defect depends on the location of the defect on the face and the elasticity of skin surrounding the defect. On the lower nose, for instance, the first lobe must nearly approximate the size of the defect because of the inelasticity of nasal tip skin. However, on the cheek, the first lobe may be designed considerably smaller than the size of the defect (up to 25% less in surface area) because of the elasticity of the skin of the cheek. The second lobe of a bilobe flap is usually designed so that its surface area is less than the surface area of the defect left by harvesting of the first lobe. Again, advancement of adjacent skin assists the second lobe in repair of the donor site of the first lobe. The defect left by the second lobe is closed primarily by direct advancement of surrounding skin. Thus, for the bilobe flap to work, there must be considerable skin laxity in the vicinity of the first and second lobes to achieve wound repair without excessive wound closure tension.
The primary disadvantage of the bilobe flap is that most of the incisions necessary to create the flap produce scars that do not parallel RSTLs. The resulting scar is also lengthy due to the need to elevate two lobes. Because bilobe flaps have curvilinear incisions, they are prone to develop a trap-door deformity. This is especially true when they are used on the nose in patients with thick skin or with sebaceous gland hyperplasia. Trap-door deformity may be minimized by extensive peripheral undermining of the nasal skin as far laterally as the cheek. Adjusting the thickness of the first lobe so that it matches the depth of the recipient site is accomplished by thinning the flap if necessary, and it may also be helpful in preventing the deformity.
Case Reports
Case 1
A 43-year-old woman developed a basal cell carcinoma of the right temple. It was removed with micrographic surgery, leaving a 2.2 × 2-cm skin defect (Fig. 8-12). Reconstruction of the defect with a skin flap could be readily accomplished by tissue transposition, rotation, or advancement. An appealing option would have been to use bilateral unipedicle advancement flaps in the form of an H-plasty (see discussion in Chapter 9). A transposition flap was selected and was designed in the form of a note flap.5 This flap enables the closure of circular skin defects with a triangular transposition flap that maximizes the use of skin surrounding the defect (see discussion in earlier section of chapter). A tangent to the circular defect was drawn. The length of the tangent was 1.5 times the diameter of the defect. The second side of the flap equal to the diameter of the defect was drawn in RSTLs. The angle of the tip of the flap was 45°. The flap was dissected in the subcutaneous tissue plane and transposed without trimming of the distal tip of the flap.
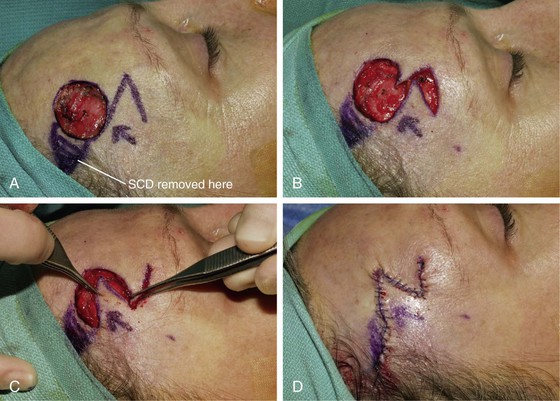
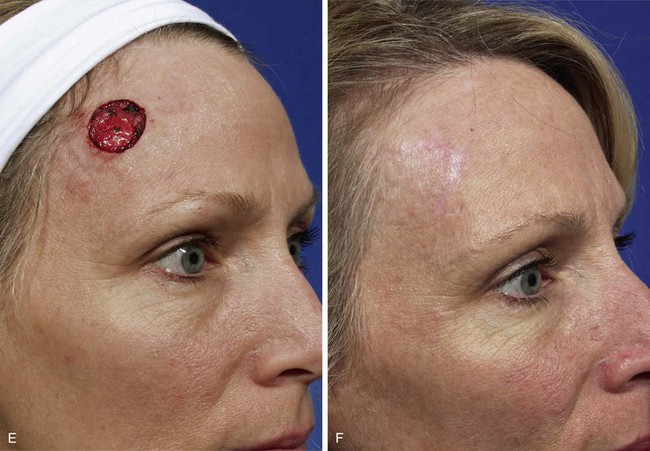
FIGURE 8-12 A, A 2.2 × 2-cm skin defect of right temple. Transposition note flap designed for repair of wound. Anticipated standing cutaneous deformity (SCD) marked by diagonal lines. B-D, Flap transposed. SCD excised. E, F, Preoperative and 5-month postoperative views. No revision surgery performed.
The note flap is recommended primarily for circular skin defects 2.0 cm or less in diameter. The pivotal arc of the flap is approximately 45°, so the standing cutaneous deformity is small and may not require excision. In the case shown in Figure 8-12, a small standing cutaneous deformity was excised at the superolateral aspect of the skin defect.
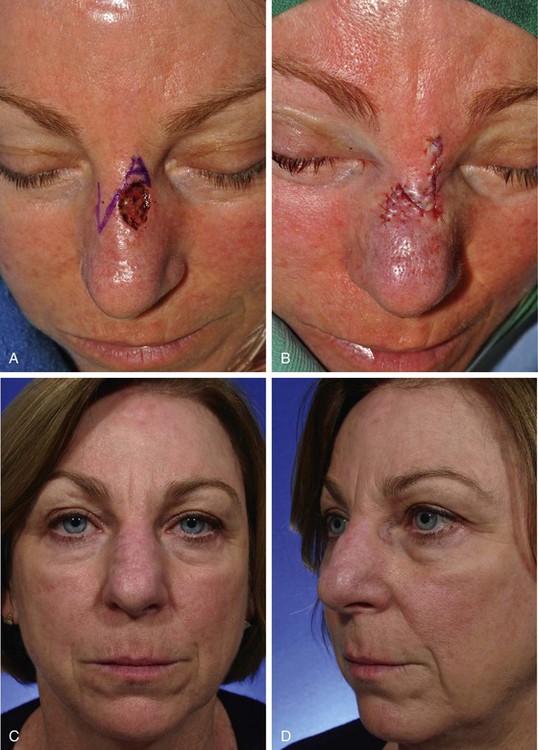
In Figure 8-13, a melanoma in situ is outlined in blue and measures 3.5 × 1.5-cm. It is located immediately superior to the medial eyebrow. Although the skin lesion has a rectangular configuration, a triangular flap was designed to repair the defect after surgical resection of the melanoma. Similar to circular defects, rectangular defects may be repaired with triangular transposition flaps. In the case presented, selection of such a flap design enabled a greater length of the final scar to lie parallel to RSTLs compared with the use of a rectangular transposition flap.
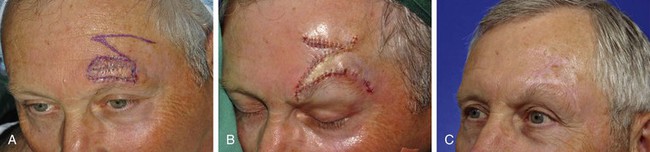
FIGURE 8-13 A, A 3.5 × 1.5-cm melanoma in situ outlined in blue. Adjacent transposition flap designed for repair of defect after excision of melanoma. B, Melanoma excised and flap transposed. Standing cutaneous deformity excised along superior aspect of lateral eyebrow. C, Postoperative view at 10 months. No revision surgery performed.
Case 2
Figure 8-14 shows a 58-year-old woman with a 1.4 × 1.1-cm skin defect of the nasal dorsum after micrographic surgery for a basal cell carcinoma. The wound could have been reconstructed with primary wound closure or with a local flap using advancement. Primary wound closure would have necessitated excision of two standing cutaneous deformities. A triangular transposition flap was selected for repair of the wound. The vertical axis of the defect was longer (1.4 cm) than the horizontal axis. For this reason, the axis of the transposition flap was designed parallel to the long axis of the defect. The advantage of this design is that as the flap pivots toward the recipient site, a portion of the standing cutaneous deformity will cover the superior aspect of the defect. Use of the standing cutaneous deformity to assist with closure of the wound means that less of the deformity will require excision, preserving more of the nasal skin.
Case 3
A 76-year-old woman was treated for two independent melanoma in situ skin lesions of the upper and lower cheek. The margins of the skin lesions were cleared by the square technique, which consists of the following (Fig. 8-15).12,13 A Wood’s lamp (UVA black light) is used to visualize the skin lesion. This may help distinguish any subclinical extension. With use of the lamp, a margin is outlined at 0.5 to 1 cm beyond the clinically apparent borders of the lesion with the intent to establish margins that are free of atypical junctional melanocytic hyperplasia (AJMH), which is thought to represent a precursor of melanoma. The margins around the lesion are marked such that the borders of the margins are straight with geometric sharp-angled corners. Thus, a shape with a square, rectangle, or diamond configuration is created around the lesion. The geometric configuration with angled corners facilitates tissue processing and precise orientation for total peripheral margin histologic examination. After the area is injected with local anesthetic, a double bladed scalpel is used to remove a 2-mm-wide strip of skin along each of the marked linear margins. A “square” of tissue is removed in block from the patient. The tissue square, which contains 100% of the peripheral skin margin of the lesion, is tagged with a suture for orientation. The resulting geometric-shaped wound strip is sutured closed with a running nonabsorbable suture. The central island of skin that contains the melanoma or AJMH is left intact while the skin margins are assessed. The tissue specimen is embedded in paraffin, and routine vertical histologic sections that contain 100% of the peripheral margins are processed. The specimen is inked, and a map is drawn and included with the specimen for precise anatomic orientation of the peripheral margins. The dermatopathologist examines the permanent vertical histologic sections and notes any areas that are positive for melanoma or AJMH. By use of the tissue map, any remaining positive areas are outlined in a fashion similar to the creation of the first geometric shape, and margins around this second shape are sent for permanent vertical histologic sectioning as described before. This process is repeated until all of the margins are free of disease. When the entire peripheral margin is interpreted as being tumor free, the central island of skin along with any other islands necessitated by additional margin control is excised within the defined clear peripheral margins. These islands of skin are sent to the laboratory for serial sectioning and histopathologic examination for any invasive component. The reconstruction is performed with the confidence that 100% of the peripheral margins of a skin lesion have been evaluated and interpreted as being free of melanoma.
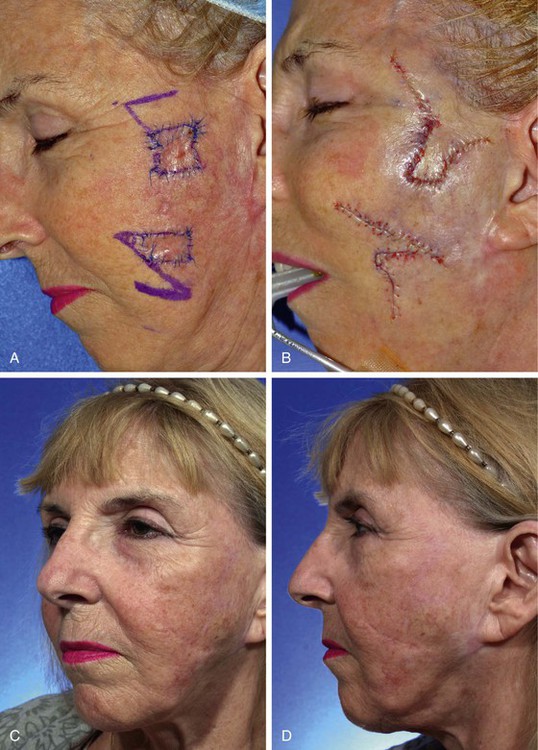
FIGURE 8-15 A, A 1.7 × 1.7-cm and a 2 × 1.3-cm melanoma in situ skin lesion outlined in blue. Adjacent transposition flaps designed for repair of defects after excision of melanomas. B, Melanomas excised and flaps transposed. C, D, Postoperative views at 1 year. No revision surgery performed.
In the case shown in Figure 8-15, the square technique used for margin control created two areas on the left cheek for planned excision. Both lesions are outlined by blue sutures. The superior lesion measured 1.7 × 1.7-cm, and the inferior lesion measured 2 × 1.3-cm. The planned superior excision had nearly a square configuration. A rhombus-shaped flap was used to repair the defect resulting from excision. The flap was designed by extending superomedially a diagonal of the rhombus. The length of the extension was the length of a side of the rhombus. This created the first side of the flap. A second line was drawn of equal length parallel to the superior border of the planned excision to create the second side of the flap. The flap design used the advantage of the relative skin laxity of the temple for construction of the flap and to minimize wound closure tension. Rhombus-shaped flaps may be used for repair of skin defects that have a rhomboid configuration with two sets of equal or nearly equal interior angles. Rhombic flaps and their many modifications are discussed in detail in Chapter 11.
Similar to Case 2, the linear axis of a triangular flap was oriented parallel to the long axis of the anticipated defect after excision of the melanoma in situ. This design maximizes the use of the standing cutaneous deformity for resurfacing of the adjacent primary defect and minimizes the necessary excision of skin at the base of the flap. The orientation of the triangular flap in this particular patient allowed the recruitment of skin from the jowl for construction of the flap. The jowl is the most redundant skin of the face. Local flaps harvested from the area of the jowl minimize donor site wound closure tension.
Case 4
Transposition flaps are almost always a reasonable reconstructive option for repair of skin defects of the superior medial cheek measuring 1 to 4 cm. An example of this is shown in Figure 8-16. This 68-year-old man presented with a 3 × 2.5-cm skin defect of the malar eminence after micrographic surgery for a basal cell carcinoma. This defect could have easily been repaired with primary wound closure but would have necessitated excision of two large standing cutaneous deformities, one of which would have extended into the lower eyelid skin. The use of an advancement flap would also have been a reliable option. In this case, an inferiorly based transposition flap was designed with plans to excise the standing cutaneous deformity at the inferior aspect of the defect. Designing the base of the flap inferiorly allowed recruitment of skin for construction of the flap from the lower temple, where skin laxity is relatively greater than in the medial superior cheek region. This design also ensured that the greatest wound closure tension, which is at the flap donor site, was oriented horizontally. This prevented the exertion of excessive wound closure tension on the lower eyelid.
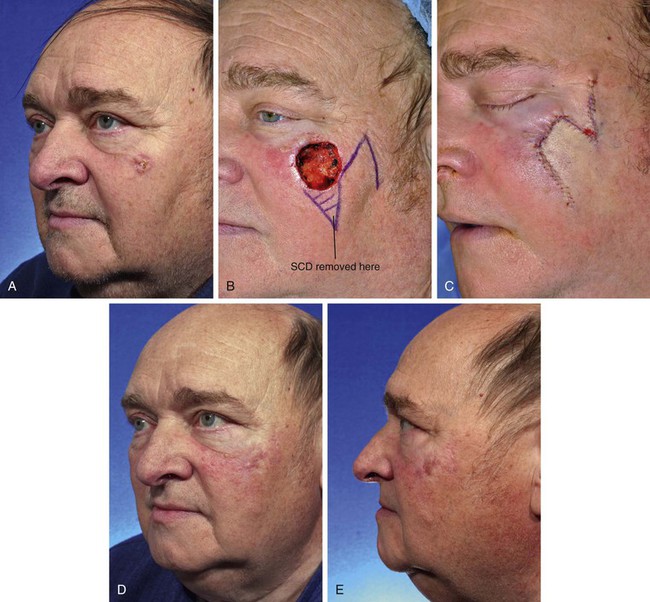
FIGURE 8-16 A, Basal cell carcinoma of malar eminence. B, A 3 × 2.5-cm cutaneous defect after micrographic surgical resection. Transposition flap designed for repair. Standing cutaneous deformity (SCD) marked for excision. C, Flap transposed. SCD removed. D, E, Postoperative views at 6 months. No revision surgery performed.
Figure 8-17 also shows a skin defect of the superior medial cheek that measured 3 × 4-cm. This defect could have been reconstructed with an advancement or rotation flap based inferiorly. A rotation flap would have required a lengthy curvilinear incision extending laterally perhaps as far as the auricle. If a rotation flap had been selected, it would have been necessary to design the flap with a surface area considerably larger than the transposition flap used for this case. The transposition flap was based superolaterally, and the standing cutaneous deformity was excised at the extreme superolateral aspect of the defect. Designing the base of the flap superolaterally enabled the construction of the flap from the lax skin of the jowl and minimized the wound closure tension of the flap donor site repair.
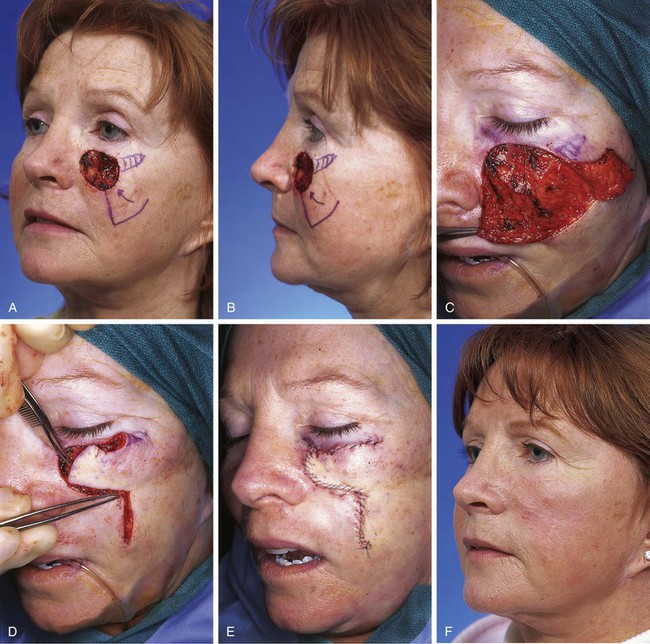
FIGURE 8-17 A, B, A 3 × 4-cm skin defect of superior medial cheek. Superolaterally based transposition flap designed for repair of wound. Anticipated standing cutaneous deformity marked with vertical lines lateral to defect. C, Flap dissected in subcutaneous tissue plane. D, Flap transposed. Standing cutaneous deformity developed superiorly at base of flap. E, Deformity excised and wound repaired. F, Postoperative view at 10 months. No revision surgery performed.
The patient shown in Figure 8-18 developed a melanoma in situ of the superior medial cheek. Tumor-free margins were obtained by the square technique as described for Case 3. The area requiring resection measured 3 × 5-cm and had a rhomboid configuration. After resection, the resulting skin defect was repaired with a rhombus-shaped transposition flap, similar to the previously discussed case. The long linear axis of the skin defect enabled primary wound repair of the most superior aspect of the defect. This approach ensured less wound closure tension between the superior aspect of the flap and the lower eyelid skin. Similar to the previously discussed case, the transposition flap was based superolaterally to facilitate recruitment of skin from the inferior cheek for construction of the flap.
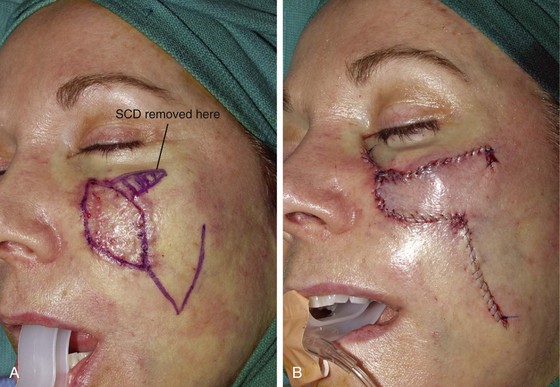
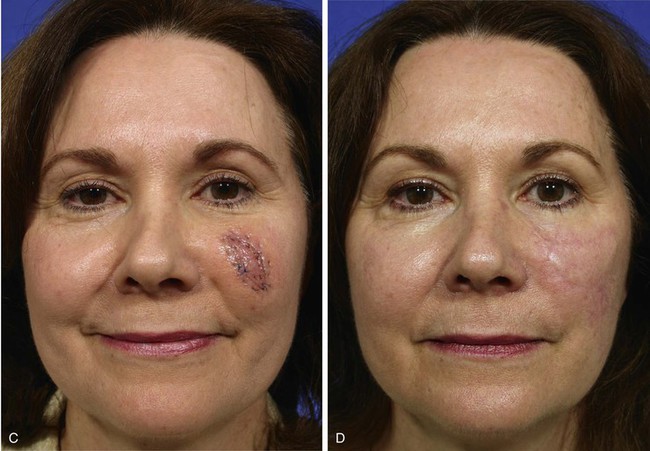
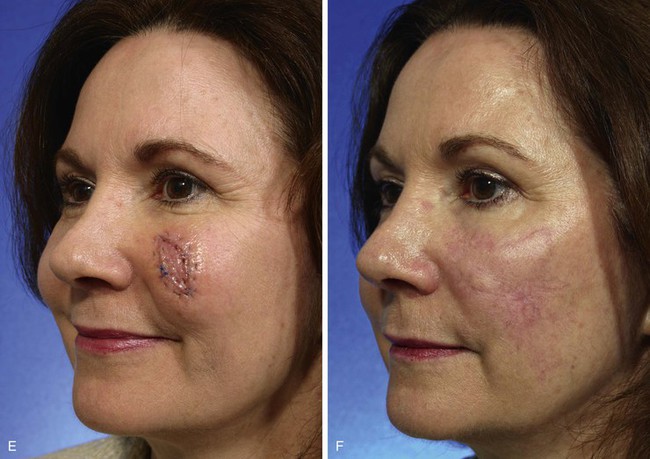
FIGURE 8-18 A, A 3 × 5-cm melanoma in situ of medial cheek outlined in blue. Superiorly based transposition flap designed for repair of defect after excision of melanoma. Anticipated standing cutaneous deformity (SCD) marked for excision. B, Melanoma excised. Flap transposed. Superior portion of primary defect closed by advancement of wound margins. C-F, Preoperative and 4-year postoperative views. No revision surgery performed.
Case 5
Skin defects of up to 5 cm of the inferior medial cheek are readily reconstructed with transposition flaps. An example of this is shown in Figure 8-19. This 72-year-old man presented with a 3.5 × 3-cm skin defect of the medial cheek after micrographic excision of a basal cell carcinoma. A portion of the defect extended into the base of the ala. Although this defect could have been reconstructed with a large rotation or V-Y subcutaneous tissue pedicled advancement flap, a transposition flap was selected for repair of the wound. The flap was based superiorly and designed to recruit the redundant skin in the region of the jowl. The flap was dissected in the subcutaneous tissue plane, and the standing cutaneous deformity was excised at the superolateral aspect of the defect. Keeping with the concept of not transposing a local flap across a facial aesthetic boundary, the flap was not used to resurface the defect of the alar base. This small component of the facial defect was covered with a full-thickness skin graft. This approach enabled the leading border of the flap to be positioned in the alar-facial sulcus for scar camouflage while at the same time maintaining the concave contour of the sulcus. The skin graft used to cover the alar defect was obtained from the excised standing cutaneous deformity of the cheek flap.
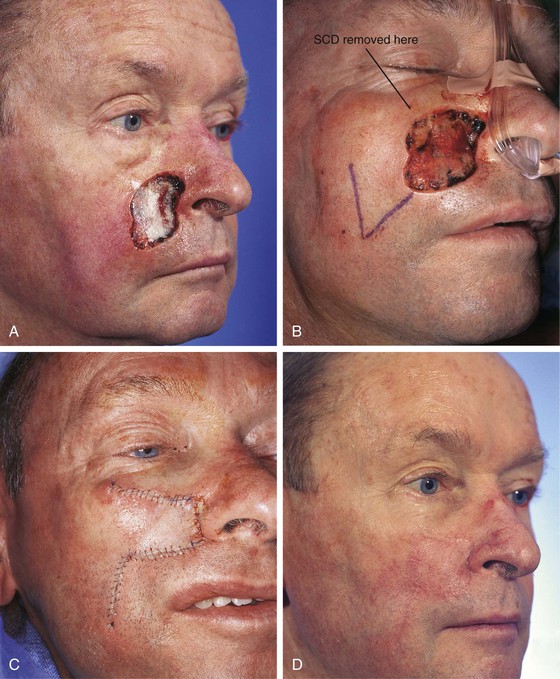
FIGURE 8-19 A, A 3.5 × 3-cm skin defect of medial cheek. B, Superiorly based transposition flap designed for repair of wound. Standing cutaneous deformity (SCD) excised superolaterally. C, Flap transposed. Small defect involving alar base repaired with full-thickness skin graft harvested from SCD excised superolaterally to cheek defect. D, Postoperative view at 2 months. No revision surgery performed. (From Baker SR: Reconstruction of facial defects. In Cummings CW, Fredrickson JM, Harker LA, et al, editors: Otolaryngology—head and neck surgery, ed 3, Philadelphia, Mosby, 1998.)
The patient shown in Figure 8-20 has a skin defect similar in size and location to that of the patient shown in Figure 8-19. The defect is irregular in shape and has a limited extension into the upper lip. Although the patient was of similar age (74 years) to the patient in Figure 8-19, she did not display facial skin laxity because she previously had a rhytidectomy. Because of this, the patient did not demonstrate a discernible melolabial crease or fold. For this reason, the lip component of the defect was not repaired with a separate flap but was reconstructed together with the cheek portion of the defect with a single large transposition flap. The flap was based superolaterally to take advantage of the greater skin laxity of the face located inferomedially. The flap was designed with an irregular shape to accommodate the limited extension of the defect into the upper lip. The flap was dissected in the subcutaneous tissue plane. After transposition of the flap, it was not possible to completely close the donor site deformity because of the tautness of the patient’s facial skin. Therefore, a small full-thickness skin graft was used to assist with the repair of the donor site. The graft was obtained from the standing cutaneous deformity excised from the superolateral aspect of the defect. The graft is seen as a triangle of skin beneath the inferior border of the flap in Figure 8-20B. The concept of employing full-thickness skin grafts together with local cutaneous flaps is discussed in detail in Chapter 16.
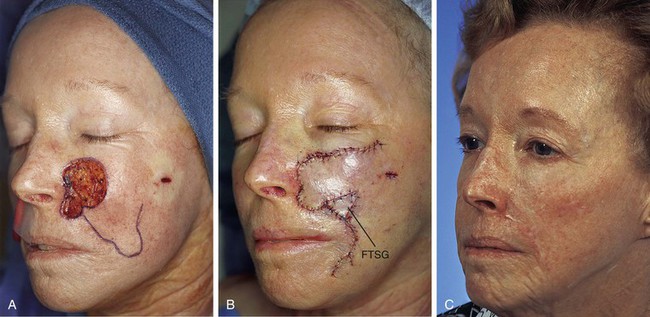
FIGURE 8-20 A, A 3.5 × 3-cm skin defect of medial cheek. Superiorly based transposition flap designed for repair of wound. B, Flap transposed. Small portion of donor site closed with full-thickness skin graft (FTSG) to reduce wound closure tension. Graft obtained from standing cutaneous deformity excised superolaterally to cheek defect. C, Postoperative view at 8 months. No revision surgery performed.
Case 6
A 91-year-old woman was treated for a melanoma in situ of the right cheek and nasal sidewall by the square technique to obtain clear surgical margins (Fig. 8-21). Once tumor-free margins of the skin lesion were confirmed, the area marked by sutures was removed, resulting in a 4 × 3-cm skin defect. The primary defect was reconstructed with a superolaterally based transposition cheek flap. A single flap was used to cover the defect, which occupied two aesthetic regions, the nasal sidewall and the cheek. Because of her advanced age, her facial skin was sufficiently thin to allow the flap to cross over the nasofacial sulcus without blunting or distortion of the sulcus.
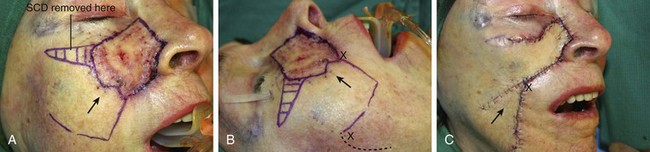
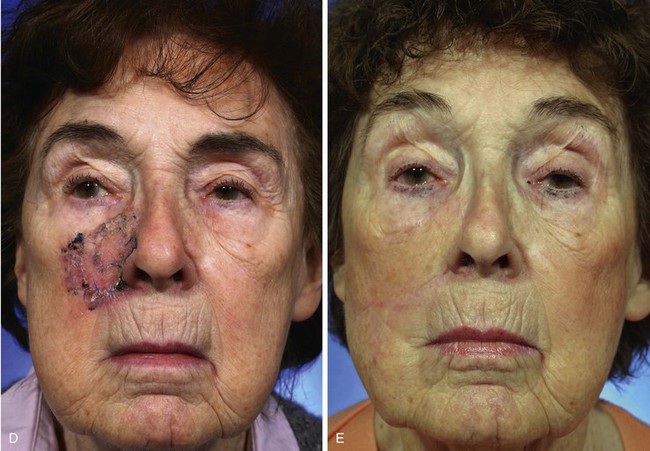
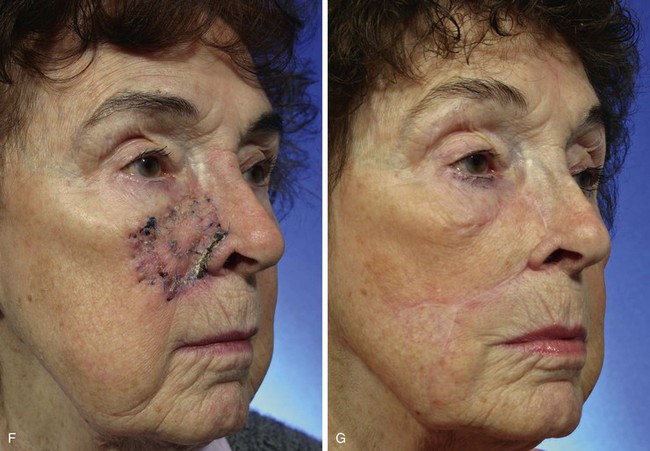
FIGURE 8-21 A, A 4 × 3-cm melanoma in situ marked in blue. Superolaterally based transposition flap designed for repair of defect after excision of melanoma. Anticipated standing cutaneous deformity (SCD) marked for excision. B, Incision for second transposition flap marked by interrupted line. Flap designed to assist with closure of donor defect. Distal end of flap marked with X. After transposition and advancement, distal end must meet upper lip at point marked with second X. C, Melanoma excised. Flaps transposed. X marks junction of distal end of second flap with upper lip. D-G, Preoperative and 7-month postoperative views. No revision surgery performed.
To repair the flap donor site on the inferior cheek, it was necessary to design and transfer a second flap, which was constructed from the jowl skin. The second flap is shown in Figure 8-21B. It was designed as a transposition flap, but considerable advancement of the flap was also necessary. The distal tip of the flap marked with an X was transposed and advanced to the upper lip at the junction of the lip with the melolabial crease, also marked with an X. Figure 8-21C shows the second flap in position. The X marks the junction of the distal tip of the second flap with the upper lip. As demonstrated in this case, it is sometimes necessary to use a skin flap to assist with closure of a secondary defect when an especially large cutaneous flap has been used to repair the primary facial defect. In this case, redundant skin in the area of the jowl and superior neck was used to repair the secondary defect.
Case 7
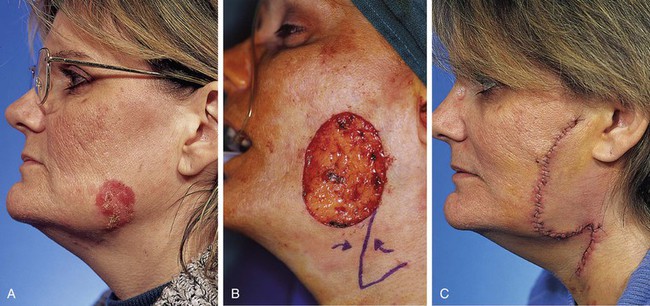
A 47-year-old woman developed a squamous cell carcinoma of the skin of the inferior cheek. The tumor was resected by frozen-section margin control. This resulted in a skin defect measuring 4 × 4.5-cm (Fig. 8-22). Reconstruction of the defect with a local flap could have been accomplished by a large cervicofacial rotation advancement flap, recruiting skin from the posterosuperior neck, or by a sizeable rotation flap based medially, recruiting skin from the submental area of the neck. Another effective alternative was to use a transposition flap. Such a flap was selected in this case. The flap was based posterosuperiorly and recruited redundant skin from the superior cervical region. The surface area of the flap was designed smaller than the surface area of the defect because of moderate skin laxity in the facial skin adjacent to the defect. Thus, part of the wound closure was achieved by advancing the borders of the defect. The transposition flap was dissected in the subcutaneous tissue plane and transposed, removing the standing cutaneous deformity at the superior aspect of the defect. Although the use of a transposition flap to repair this defect required shorter incisions in the neck compared with a cervicofacial rotation advancement flap, the incisions on the cheek were longer because of the necessity of removing a large standing cutaneous deformity resulting from pivoting of the transposition flap upward into the cheek.
Case 8
A 46-year-old woman had micrographic surgical excision of a microcystic carcinoma of the chin and lower lip. Her defect measured 5 × 4-cm and occupied nearly half of the surface area of the chin and lip (Fig. 8-23). The defect was large for reconstruction with a local flap. The location and size of the defect prevented repair of the wound with a single unipedicle advancement flap because of the inelasticity of the chin skin. The defect could probably have been reconstructed with a transposition or rotation flap harvested from the submental area of the neck. Another alternative was to transfer a flap from the cheek in the form of a transposition or advancement flap. An inferiorly based melolabial transposition flap was selected (Fig. 8-23A). The flap was designed to recruit skin from the melolabial fold. It was slightly curved in its linear axis to parallel the melolabial crease. This facilitated placement of the flap donor site scar directly within the melolabial crease. Because the flap was long relative to the width of the base, the standing cutaneous deformity that formed on transposition of the flap was not excised for fear of compromising the vascularity of the flap. The standing cutaneous deformity was resected in a second stage 4 months later. As seen in Figure 8-23B, the flap was not constructed with sufficient width to prevent downward displacement of the vermilion. The mild retraction of the vermilion persisted after healing and was corrected during the second stage when the standing cutaneous deformity was excised. The correction of the lip displacement was accomplished by performing four Z-plasties along the scar delineating the superior border of the flap from the adjacent remaining skin of the lip between the vermilion and the flap. The patient’s 4-year postoperative result is shown in Figure 8-23D, F. The inferior retraction of the vermilion was completely corrected, and there was no significant ablation of the melolabial fold, providing nearly perfect symmetry of the cheeks. The flap’s donor site scar was camouflaged well by its location within the melolabial crease.
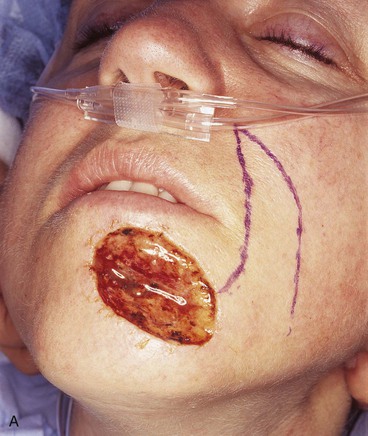
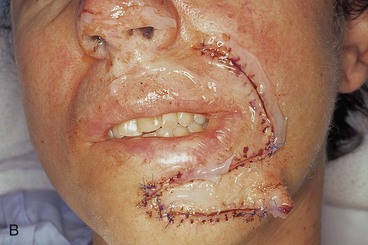
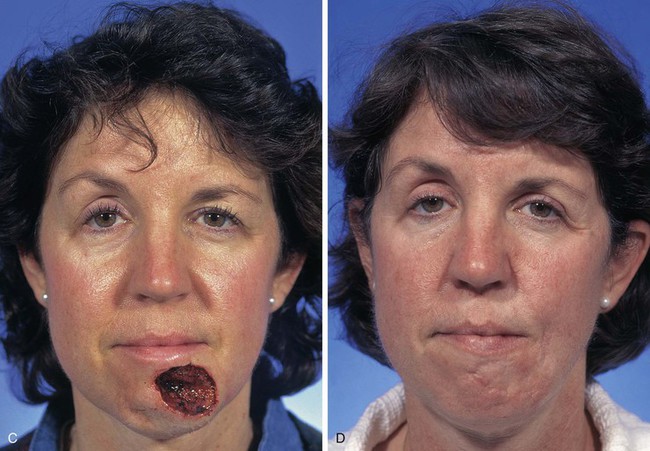
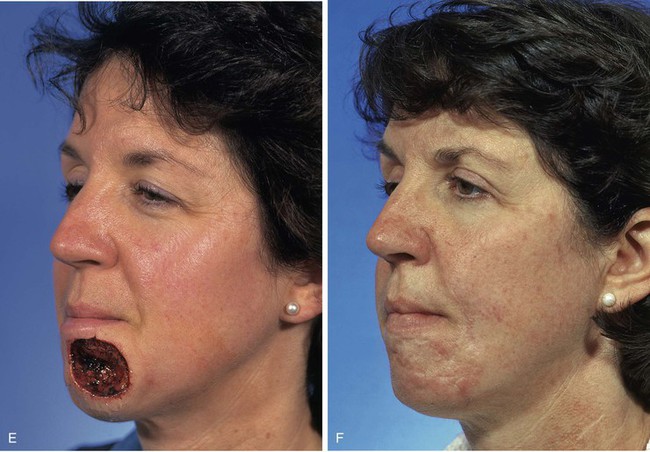
FIGURE 8-23 A, A 5 × 4-cm skin defect of lower lip and chin. Long inferiorly based melolabial transposition flap designed with base adjacent to defect. B, Flap transposed. Standing cutaneous deformity formed from transposition left in situ. Deformity subsequently excised and scar revision, consisting of several Z-plasties, performed along superior border of flap. C, E, Preoperative views. D, F, Postoperative views at 3 years, 7 months.
References
1. Gorney, M. Tissue dynamics and surgical geometry. In: Kernakan DA, Vistnes LM, eds. Biological aspects of reconstructive surgery. Boston: Little, Brown, 1977.
2. Koranda, FC, Webster, RC. Trap-door effect in nasolabial flaps. Arch Otolaryngol. 1985; 111:421.
3. Webster, RC, Benjamin, RJ, Smith, RC. Treatment of “trap-door deformity.”. Laryngoscope. 1978; 88:707.
4. Park, C, Lineaweaver, WC, Buncke, HJ. New perioral arterial flaps: anatomic study and clinical applications. Plast Reconstr Surg. 1994; 94:268.
5. Walike, JW, Larrabee, WF, Jr. The note flap. Arch Otolaryngol. 1985; 111:430.
6. Larrabee, WF, Jr. Design of local skin flaps. Otolaryngol Clin North Am. 1990; 23:899.
7. Limberg, AA, Wolf, SA, The planning of local plastic operations on the body surface: theory and practice trans. Collamore Press, Lexington, Mass, 1984.
8. Bray, DA. Rhombic flaps. In: Baker SR, Swanson NA, eds. Local flaps in facial reconstruction. St. Louis: Mosby, 1995.
9. Webster, RC, Davidson, TM, Smith, RC. The thirty degree transposition flap. Laryngoscope. 1978; 88:85.
10. Bernstein, L. Transposed and interposed flaps in head and neck surgery. Otolaryngol Clin North Am. 1972; 5:531.
11. Yabe, T, Takemura, H, Muraoka, M. Circle-to-W flap. Plast Reconstr Surg. 1999; 104:1430.
12. Anderson, KW, Baker, SR, Lowe, L. Treatment of head and neck melanoma, lentigo maligna subtype. Arch Facial Plast Surg. 2001; 3:202.
13. Anderson, KW, Baker, SR. Management of early lentigo and lentigo maligna melanoma of the head and neck. Facial Plast Surg Clin North Am. 2003; 11:93.