Chapter 60 Transplantation of Organs
Rehabilitation to Maximize Outcomes
Historical Success and Rehabilitation Options
As the volume of organ transplants performed each year surges and the types of transplants become diversified, there is an increasing need for proper rehabilitation care for transplant recipients (Figure 60-1).155 Functional restoration of persons who have undergone organ transplant surgery is an important priority of the physical medicine and rehabilitation team and has emerged as a global rehabilitation priority.113 Just as transplantation surgery can add years to life, transplant rehabilitation can add life to years.
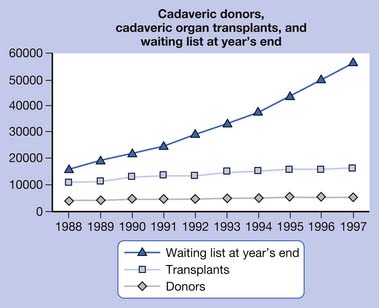
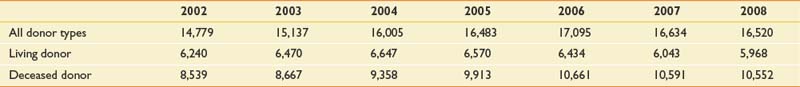
FIGURE 60-1 Cadaveric donors, cadaveric organ transplants, and waiting list at year’s end
(From http://optn.transplant.hrsa.gov/latestData/rptData.asp; and http://www.ustransplant.org/annual_reports/current/103_dh.htm.)
The rapidly evolving, innovative methods of preventing or minimizing organ transplant-related infection and rejection have resulted in improved survivorship among organ transplant recipients.1 The availability of more potent immunosuppressive drugs with reduced side effects has significantly improved health outcomes and enabled patients to be transferred earlier to the acute rehabilitation unit.125 The preemptive application of acute immunosuppressive therapies, such as monoclonal and polyclonal antibodies, has facilitated successful organ transplantation surgery and subsequent rehabilitation functional restoration.44 Ongoing developments in the science of human leukocyte antigen (HLA) matching and the perfection of the HLA Registry process have considerably improved outcomes for bone marrow transplant recipients.59 Methods of transplanting end-stage renal disease (ESRD) patients by combining renal transplantation with perioperative bone marrow transplant (without the benefit of HLA matching and without maintenance immunosuppression) have recently been reported.68 Optimization of surgical technique has led to both an increase in the number of transplant surgeries performed annually, as well as an expansion in the diversity and complexity of these procedures. Prominent examples include the emergence of groundbreaking new transplantation procedures, including face transplant,37 multiple limb transplantation,38 and “domino organ transplants.”2 Many of these breakthroughs have sparked a surging demand for rehabilitation services. A renewal of academic interest in organ transplantation rehabilitation has resulted in a growing body of medical literature, including journal articles and book chapters dedicated to transplantation rehabilitation.114,155
Federal agencies in cooperation with the National Institute of Allergy and Infectious Diseases and the National Library of Medicine have established a unique public database containing results of blood and marrow stem cell transplants involving unrelated donors.48 This database is particularly useful to patients undergoing blood and marrow transplants and is accessible to physicians, researchers, and patients online at http://www.ncbi.nih.gov/mhc. This database includes fundamental demographic information such as gender, ethnicity, age, and genetic data on more than 1400 transplant donors and recipients worldwide, transplant recipient survival rates, as well as data on the relationship between transplantation and major histocompatibility complex genes. This latter information can assist doctors to better predict whether a recipient will “accept” or “reject” transplantation from a particular donor source. This novel resource promises to assist physicians in the evaluation of risks and benefits of transplantation for various clinical conditions. For the physiatrist providing preoperative/pretransplant consultative input, the data gleaned from this source can help predict outcomes and guide posttransplant rehabilitation.
Organ transplantation, despite its recognition as one of the “modern miracles” of contemporary medicine, is also fraught with many ethical and moral dilemmas. Choosing appropriate selection criteria is just one of the myriad philosophical issues present in organ transplantation.139 Despite the advances in organ transplantation medicine, an international crisis continues to loom in organ transplantation because the demand for organs radically outnumbers the available organ donors. (UNOS/OPTN unpublished data, 2010). This unmet need creates many ethical questions.21 National organizations within the United States such as the United Network for Organ Sharing (UNOS) and the Organ Procurement and Transplant Network (OPTN) exist to alleviate this exigency. OPTN is a private, not-for-profit, federally contracted transplant network established by the U.S. Congress under the National Organ Transplant Act of 1984 that united all the professionals involved in the transplantation and donation system. OPTN works to increase the effectiveness and efficiency of organ sharing as well as equalize the national system of organ allocation. A secondary goal is to increase the supply of donated organs available for transplantation. UNOS is responsible for administering the OPTN under contract with the Health Resources and Services Administration of the U.S. Department of Health and Human Services. Together UNOS and OPTN work collaboratively to establish organ transplantation policies and procedures. Assurance of positive functional outcomes posttransplantation remains a major goal. As a result of these efforts, physiatrists are likely to see a future surge in the number of transplantation patients seeking rehabilitation.
Chapter Focus
This chapter provides a general overview of the subject of organ transplantation and rehabilitation. Although solid organ transplantation rehabilitation is the primary focus, reference is made to recent developments in bone marrow and hematologic transplantation that have an impact on physiatry. A general background discussion of the relevance of physical medicine and rehabilitation to the transplantation process is offered. This is followed by a systematic consideration of rehabilitation principles and techniques that can improve patient outcome and quality of life. Of the more than 21 transplantable organ systems, this chapter will address the four most common ones seen in physiatric practice, that is, heart, lung, liver, and kidney. Special attention is also given to new frontiers in organ transplantation rehabilitation. Because transplant rehabilitation is such a nascent field, there is a scarcity of literature outlining the fundamental principles of the transplantation rehabilitation process.153
Physiatric Interventions in Enhancing Outcomes
Enhancement of quality of life is a primary goal of transplantation rehabilitation.155 Maintaining the physiatric spirit of “adding life to years as well as adding years to life” holds particular significance to rehabilitation of transplant survivors because these patients have often endured years of chronic end organ deterioration followed by prolonged organ waiting. The goal of transplant rehabilitation is to improve functional outcome and facilitate the return to a fulfilling life and lifestyle. Optimal physiatric management of a person who has survived transplantation requires a comprehensive, multidisciplinary, structured, and integrated approach.137
It is essential that a functional and medical baseline before surgery is established. The physiatric evaluation should include a comprehensive medical history and elaboration of functional deficits associated with end-stage organ damage. The physiatrist should conduct a thorough musculoskeletal, neurologic, and functional assessment of the patient (Box 60-1). Emphasis should be placed on the maintenance of bodily systems that are likely to be adversely affected by immobilization. This includes contracture prevention, deep vein thrombosis and pulmonary embolism preventive measures, skin maintenance, and preservation of bowel and bladder function. The prevention of disuse atrophy of major muscle groups can be addressed through bedside isometric exercise protocols. A therapeutic plan emphasizing exercise and remobilization should be developed and implemented that takes into account the patient’s presurgical functional status. As always, physiatrists must consider the psychosocial status of each patient and the impact the impairment will have on handicap and disability.130 Communication with other members of the transplant team is essential during each step of the process.
Close ongoing daily observation is essential after transplantation to ensure adequate immune system suppression, prevent opportunistic infection, and maintain rehabilitation goals.22 Emphasis should be placed on the underlying functional, medical, socioeconomic, and psychologic needs of the patient.98 The rehabilitation team should possess knowledge of the clinical aspects of acute and chronic rejection in all types of transplantation surgeries. Because transplant recipients typically present with a multitude of medical comorbidities, it is important to include any appropriate precautions in the rehabilitation plan. Careful consideration of exercise prescription should be made.26
The physiatrist must be aware of the side effects of the numerous medications used posttransplant. A summary of the side effects of some of the most frequently used immunosuppressive agents is provided in Table 60-1. See Table 60-2 for the monitoring parameters for immunosuppressive medication. Neuromusculoskeletal complications after transplant are extremely common. The physiatrist needs to sort through the extensive differential diagnoses of these impairments (Table 60-3).
Table 60–1 Systemic and Metabolic Effects of Transplant Rejection Drugs
| Drug | Adverse Effects | Clinical Manifestations |
|---|---|---|
| Azathioprine |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; GI, gastrointestinal; TTP, thrombotic thrombocytopenic purpura.
Table 60-2 Immunosuppressive Medication Monitoring for Physiatrists
| Drug | Side Effects | Monitor |
|---|---|---|
| Prednisone | ||
| Cyclosporine | ||
| Azathioprine |
BUN, Blood urea nitrogen; CBC, complete blood count; GI, gastrointestinal; Hct, hematocrit; LFT, Liver function test.
Table 60-3 Neuromusculoskeletal Complications After Transplantation and Their Possible Etiologies
| Complication | Etiologies |
|---|---|
| Delirium | Intensive care unit psychosis, hyponatremia, hypernatremia, hypoglycemia, sepsis, hypotension, hypoxemia, medications, encephalitis (bacterial, fungal, or viral) |
| Stroke | Embolic, watershed infarct from hypotension, mycotic aneurysm, vasculitis (sirolimus-induced) |
| Paraparesis | Spinal cord infarction, myelopathy |
| Peripheral neuropathy | Critical illness, metabolic, diabetic, transplant medications (cyclosporine, FK506, sirolimus), nerve impingements (brachial plexus, accessory, axillary, median, ulnar, femoral, sciatic, peroneal) |
| Tremor | Cyclosporine, tacrolimus, sirolimus |
| Myopathy | Critical illness, steroid, statin, cyclosporin |
| Contracture | Positioning, graft vs. host disease, nephrogenic |
| Osteoporotic fracture | Steroids, immobility |
Rehabilitation Through Renal Transplantation
As the number of people with end-stage renal failure continues to increase, kidney transplantation is being performed with increased frequency and improved outcome. From 1998 to 2004, there has been a steady increase in the number of kidneys transplanted (Table 60-4). In 2003 to 2004, there were 16,004 kidney transplants, representing an increase of 3552 since 1998. Despite the increase in kidney transplants, there has been a decrease in the number of kidney–pancreas transplants (Table 60-5). Compared with the 972 simultaneous kidney–pancreas transplants (SKPT) performed in the United States in 1998, there has been a significant decrease to approximately 881 of these procedures in 2003 to 2004. Recent literature has demonstrated that the long-term survival of SKPT recipients is superior to that of cadaver kidney transplant recipients with type 1 diabetes. There is no difference, however, in survival of SKPT recipients and living donor kidney recipients with type 1 diabetes at up to 8 years follow-up.109
Fatigue and problems with the performance of the activities of daily living (ADL) pose limitations to patients with ESRD.52 Many renal failure patients are unable to perform even the most basic tasks of everyday life. A majority of patients are unable to work because they are physically unable to sustain their energy levels. However, the greatly enhanced survival rates of patients who have received a transplant, coupled with the net increase in procedures performed annually, have resulted in a growing number of patients who now require rehabilitation and restorative services.
Complications that can surface during the postoperative period include infection, bleeding, and rejection. The rehabilitation team must be aware of the signs of kidney rejection. Acute rejection is frequently heralded by anorexia, malaise, fever, hypertension, leukocytosis, blood urea nitrogen (BUN) elevation, and kidney enlargement with localized tenderness. Immunosuppressant medications, including prednisone, azathioprine, and cyclosporine, carry a host of side effects and reactions. Evidence of kidney rejection, which includes graft site tenderness, reduced urinary output, elevated temperature, and edema, can arise during postacute rehabilitation. (See Box 60-2 for a list of the clinical signs associated with kidney transplant rejection.)
Options to Enhance Exercise After Kidney Transplantation
A number of important physiologic factors affect the renal transplant recipient’s ability to exercise. Chronic renal failure is well known to induce stress on the cardiovascular system.101 Metabolic abnormalities, such as imbalance of electrolytes, including sodium and water retention, can augment right and left ventricular preload. As circulatory volume expands, hypertension is exacerbated and increased afterload is maintained. This situation frequently leads to the development of cardiac hypertrophy, greater ventricular stiffness, and diminished compliance.
The ability to exercise can be maximized in several ways, including exercise training and treatment with recombinant human erythropoietin (epoetin). However, recent data have uncovered physiologic limitations to exercise capacity that are not simply overcome by exercise training or normalization of hematocrit.99 Exercise training after renal transplant results in higher levels of measured and self-reported physical functioning, although exercise alone does not affect body composition.100 A successful kidney transplant can greatly increase exercise capacity to near-normal values for sedentary healthy individuals.41
Exercise training after transplant further increases exercise capacity and counteracts some of the negative side effects of glucocorticoid therapy, such as muscle wasting and excessive weight gain. Studies have shown that before epoetin administration, exercise training alone in patients on dialysis can increase exercise tolerance by 25%. Similar but smaller increases are observed after correction of anemia with epoetin. Physiatrists frequently need to encourage renal patients to exercise and improve their physical functioning by overcoming their fatigue.100 Renal rehabilitation is an essential therapeutic method for improving physical fitness, social functioning, and well-being, and is reflected by an increase in health-adjusted quality of life among ESRD and posttransplant patients.
Just as the ability to exercise is significantly impaired in chronic renal patients, patients who have received transplanted kidneys also demonstrate exercise intolerance.42 Several factors contribute to this challenge, including skeletal muscle atrophy, anemia, and cardiovascular deconditioning.
Kidney transplants are often performed in conjunction with pancreas transplants for patients with severe end-organ damage resulting from diabetes mellitus. An increasing number of these patients are surviving and moving on to rehabilitation and vocational rehabilitation services (Figure 60-2).93
Rehabilitation of the Person Through Cardiac Transplantation
Heart Transplant Epidemiology
Since the inauguration of the procedure in 1967, the survival rate of patients undergoing heart transplant continues to improve.126 With constant refinements in surgical technique, more than 2000 heart transplants had been performed throughout the world by 1990. As a result of the increase in organ availability and surgical capacity in the United States, the waiting list of candidates decreased 45%, from 2414 in 1997 to 1327 in 2006. Patients on the waiting list are assigned a level of urgency based on medical condition and requirements for continuous circulatory support. The highest priority candidates, designated as status 1A, require hospitalization with continuous mechanical circulatory support, such as an implanted cardiac pump or aortic balloon pump. Status 1B patients can remain at home but require continuous intravenous inotropes and an occasional ventilator. Most wait-list candidates are listed as status 2. Outcomes are improving as well, and heart transplant recipient survival has exceeded 92% at 3 months, 88% at 1 year,92 and 85% at 5 years.32 The Stanford Group has recently reported on their 20-year survivors, who require ongoing treatment for a variety of problems including hypertension (87%), malignancy (44%), vasculopathy (43%), and diabetes (14%).32
It is estimated that survival rates after heart transplantation will continue to improve with careful systematic patient selection, better surgical techniques, lower rejection rates, and continuous rehabilitation.66,122,152
Rehabilitation Before the Heart Transplant
Congestive heart failure (CHF) that is unresponsive to medical therapy is not only a primary impairment but also a major indicator for heart transplantation. The disease processes leading to CHF include idiopathic cardiomyopathy, viral myocarditis, ischemic heart disease, and valve dysfunction.62,79 It is essential that the assessments of both the cardiologist and the physiatrist be integrated to better understand the disease process and its probable impact on the patient’s quality of life. This assessment includes quantification of circulatory impairments, exercise performance, associated diagnoses, and the health of other organ systems.19
The success of heart transplantation depends on careful selection of suitable patients (Box 60-3). The most successful outcomes depend on identifying candidates who have the physical capacity that would enable them to maximally benefit from the new heart and rehabilitation.126 During the period of heart procurement, implantation of devices, such as the Ventes left ventricular assist devices, the HeartMate, and total artificial heart, can improve tolerance for activity and allow preliminary rehabilitation.43,108 Careful surveillance for declining cardiac function and irreversible heart disease helps manage a patient before transplantation. Several criteria used for cardiac transplant candidate selection are outlined in Box 60-4.133
Complications After Cardiac Transplantation
The cardiac rehabilitation team should be familiar with the complications of transplantation to recognize and treat problems that might affect patients’ functional performance. Coordination of care and communication between the cardiac surgeon, cardiologist, and the physiatrist is essential for success. The main complication posttransplant is allograft failure from rejection. Secondary complications include problems related to immunosuppression, such as infection, neurotoxicity, renal toxicity, hypertension, and various metabolic abnormalities. One study of cardiac transplant recipients on an inpatient rehabilitation unit uncovered multiple secondary complications, including hypertension, nutritional limitations, neuromuscular deficits, and compression fractures.62 Stress fractures of the weight-bearing limbs have also been described83 and are most likely due to steroid-induced osteoporosis. Monitoring vitamin D levels and supplementation with 1000 to 2000 units daily reduce risk. Physiatrists should be clinically familiar with these problems and be prepared to evaluate and treat them.
Many patients develop hypertension as a result of cyclosporine-induced renal vasoconstriction superimposed on chronic renal hypoperfusion, third spacing of fluids, and an abnormal distribution of blood flow.24,45,86,95 Cyclosporine is believed to cause afferent glomerular arteriolar vasoconstriction through an increase in transmembrane calcium flux in mesangial and vascular smooth muscle cells.83,95 Blood pressures should be closely monitored, with morning blood pressure values used as a guide for antihypertensive therapy.24,99 In most cases this can be achieved without interruption of the exercise therapy regimen. Alternative cyclosporine dosing regimens, calcium channel antagonists, and angiotensin-converting enzyme inhibitors are preferred therapies to promote arteriolar dilation.18,82,142 Proper management of the hypertension facilitates full participation in rehabilitation.
Cardiac transplantation itself can cause neurologic complications, including metabolic encephalopathy, stroke, central nervous system infection, seizures, and psychosis. These potential complications are most likely to present during the acute posttransplant period, although they can also surface during the rehabilitative/restorative phase.124 The mechanisms for strokes include particulate embolism, air embolism, and inadequacy of perfusion during the transplantation procedure. Careful review of mental status, perceptual sensation, and motor function is an essential part of the physiatric consultation in the postoperative phase.
Acute rejection in cardiac transplantation is a major complication that can be heralded by fulminant CHF, accumulation of peripheral edema, premature atrial contractions, a diastolic gallop, and sudden marked reduction in exercise capacity. Chronic rejection can also progress with accelerated graft atherosclerosis.30,146 At 1 year posttransplantation, 10% to 15% of patients develop accelerated graft atherosclerosis, which increases to 35% to 50% by the fifth postoperative year.35,122 Cardiac denervation produces an up-regulation of muscarinic receptors, which facilitates increased calcium influx in the coronary arteries of the transplanted heart. This causes diffuse circumferential narrowing of the arterial luminal diameters. This type of coronary artery disease ultimately is a key barrier to the long-term survival of cardiac transplant patients. Recent studies, however, suggest that it can be prevented and improved with calcium channel blockers.119,122 This condition should be placed on the problem list, and a plan should be designed for its prevention, surveillance, and acute management.
Beyond the postoperative complications outlined above, the leading cause of death in post–cardiac transplant patients is infection.88,143 The types of infections include mediastinitis, pneumonia, urinary tract infections, and intravenous catheter-induced sepsis.55,88,143 Such problems tend to develop during the first 2 years after the cardiac transplant.14,89 Bacterial and viral infections account for 47% and 41% of infections, respectively. Infections caused by fungus and protozoa account for 12% of posttransplant morbidity. This makes it imperative that infection control techniques be used, especially adequate washing of the hands for a full 30 seconds or use of an equivalent topical antiseptic or both before and after direct contact with the transplant patient.
Physiology of the Transplanted Heart
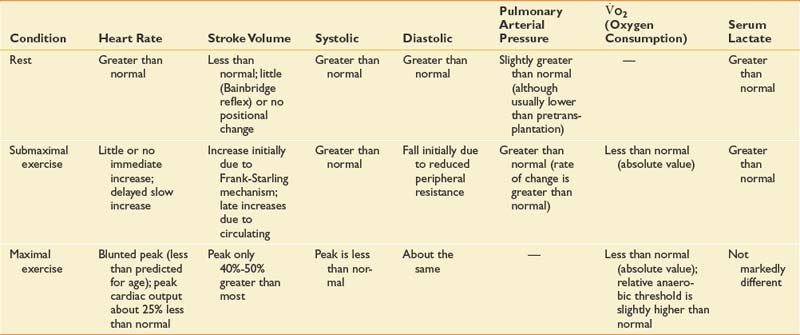
The customization of exercise programs for each transplant patient entering rehabilitation requires a dynamic understanding of the physiology of the transplanted heart. The normal heart is innervated and is influenced by the sympathetic nervous system (which has chronotropic and inotropic effects).3,6 The sympathetic nervous system enhances venous return, stroke volume, and cardiac output. A transplanted heart is denervated and consequently achieves a maximal heart rate more slowly than a normal heart. It does this primarily through a response to circulating catecholamines and to a limited extent via partial and inconsistent gradual sympathetic reinnervation.149 After an exercise session or ambulation activity, the heart transplant patient experiences a more gradual return to baseline. Despite the denervation, cardiac output in the transplanted heart increases in response to dynamic total body activity. This promotes venous return and increases stroke volume through increased preload volume of blood filling the left ventricle.
When orthotropic heart transplant is performed, the complete denervation of the heart leads to a loss of the autonomic nervous system control mechanism. The denervated heart has a higher than normal resting heart rate that can typically be modulated by carotid massage, Valsalva maneuver, and body inclination.81,148 The most widely accepted explanation for this higher than normal heart rate is the loss of vagal tone associated with denervation.6,117,118,148
As the patient gradually begins exercise, a slight increase in heart rate is immediately observable. This is attributed to the Bainbridge reflex,120 which is an increase in heart rate in response to increased pressure in the veins entering the right heart or the increased rate of ventricular work. In either situation, this acceleration will continue for 3 to 5 minutes. The gradual ongoing heart rate increase continues into the recovery period and can contribute to a slower than normal return to preexercise heart rate.85 This requires teaching the patient to pace with a slow warm-up period and a gradual ramping up of activity intensity. The peak heart rate achieved during maximal exercise is considerably lower in cardiac transplant recipients than in age-matched control subjects.23,81,85
The transplanted heart compensates for output demand primarily by increasing stroke volume. The resting stroke volume of patients with transplanted hearts is less than that of individuals without transplantation.67 Despite this, cardiac output is virtually normal.65,67,87 Most heart recipients experience a rapid increase in stroke volume of about 20% when they begin their exercise regimen.81,87 Subsequent increases in stroke volume or cardiac output during prolonged submaximal exercise are mediated by inotropic responses to circulating catecholamines.63,65,69,81 After gradual conditioning, higher intensity training can be achieved in a period of more than 15 months to achieve athletic capabilities in some younger transplant recipients.107
See Table 60-4 for a summary of the effects of cardiac transplant on various cardiovascular parameters.
Because heart transplant patients display this unusual catecholamine-driven cardioacceleratory response to exercise, empiric exercise prescriptions based on target heart rates are not recommended. More beneficial measures of exercise intensity that have been suggested include blood pressure reserve (the difference between systolic and diastolic pressure), the Borg scale of perceived exertion (a categorical scale from 1 to 10 with 10 representing the maximal exertion), the Dyspnea Index (a scale from 0 to 4 with 4 being the maximum shortness of breath, which prevents counting or speaking), or defined exercise tasks and pace (see also Chapter 34).12,476,67
The effect of transplantation on both systolic and diastolic blood pressure is higher than expected but minimal on resting pulse pressure.46,67 Diastolic blood pressure can decrease early in submaximal exercise because of reduced peripheral resistance.46,47,62,63 The peak systolic blood pressure is less than that of individuals without cardiac transplants, but diastolic blood pressure remains essentially unchanged.
After heart transplant, patients consume less oxygen during submaximal exercise than normal control subjects.65,69,104,127 Oxygen consumption at the anaerobic threshold is also considerably lower than that of age-matched normal individuals.65,104,127 According to Braith and Edwards,15 the decrement in peak oxygen consumption seen in transplant recipients is partly due to changes in skeletal muscle. Skeletal muscle myopathy associated with the heart failure syndrome produces atrophy, decreased mitochondrial counts, and decreased oxidative enzymes. Corticosteroids also promote muscle atrophy affecting primarily type II fibers, and cyclosporine further decreases oxidative enzymes.15
Aerobic cardiovascular conditioning programs and exercise regimens emphasizing endurance tasks have been shown to improve the ability of heart transplant patients to achieve sustained participation in higher levels of ADLs and in the community.51 Aerobic capabilities such as O2max can increase from 12% to 49% with 7 to 11 months of three times per week training.15 Excessive exertion can be prevented by training with a cycle ergometer and limiting intensity to 15 on the Borg scale. Alternatively, exercise can be dosed by time and rate on the cycle.121 It is generally held that cardiac transplant survivors can perform exercise and physical training routines and achieve improvements comparable with those achieved by nontransplant individuals of similar age.72 Studies in the cardiac rehabilitation literature have focused on the hemodynamic responses to upright exercise after cardiac transplantation, as well as the cardiovascular response to gait training and ambulation in hemiparetic heart recipients.123,124 A regular scheduled practical group exercise regimen is recommended for heart recipients. The fellowship and support from exercising together is valuable as is the safety from supervision by others who could administer cardiac pulmonary resuscitation or call for help in an emergency.
Therapeutic Exercise After Cardiac Transplantation
Early in the history of cardiac transplantation, it was considered inadvisable to start an exercise protocol immediately after the surgery. New research suggests, however, that it is vitally important to initiate exercise therapy as soon as 1 month after transplant surgery.13,142 Benefits that accrue from this plan include improved strength, enhancement of aerobic capacity,76,145 and improved physical work capability. As a result, the number of heart transplantation recipients enrolled in rehabilitation and maintenance exercise programs continues to increase.12
Evidence suggests that exercise programs that are supervised should be a standard of care for heart transplant patients.74,80,129 A revealing study74 evaluated 27 patients discharged within 2 weeks after receiving a heart transplant who were randomly divided into two groups. One group of 14 patients was assigned to participate in a 6-month structured aerobics exercise program involving sitting-to-standing exercises. Each cardiac transplant patient in the structured exercise group worked with a physical therapist and had a customized program of muscular strength and aerobics training. The second group of 13 patients received only written instructions about exercises to do at home, with no supervised sessions. All 27 patients were tested for muscle strength, aerobic capacity, and flexibility within 1 month of receiving a heart transplant, and tested again 6 months later. Although all the patients showed an improvement in all areas, those in the structured exercise group showed significantly better results. Muscle strength, measured by the number of times a patient could stand from a sitting position repetitively for 1 minute, improved 125% for the exercise group (from a mean of 10.6 times/min to a mean of 23.9 times). The control group of patients who had received only written instructions showed an 18% gain, increasing from 10.4 times/min to 12.3. Aerobic capacity, tested by peak oxygen consumption, increased 49% in the group receiving formal exercise training compared with just 18% in the control group.
Patients tolerate exercise well after cardiac transplantation,73,74 and progressive resistance training is also beneficial.96,106,121,135,150 Resistance training should not begin until 6 to 8 weeks after transplantation, permitting time for sternal healing and corticosteroid tapering.134 A controlled study designed to determine the effect of resistance exercise training on bone metabolism in heart transplant recipients also produced positive results. As early as 2 months after heart transplantation, about 3% of whole-body bone mineral density (BMD) has been lost as a result of decreases in trabecular bone.132 Six months of resistance exercise, consisting of low back exercises that isolate the lumbar spine and a regimen of variable resistance exercises, restored BMD toward pretransplantation levels. Research suggests that resistance exercise is osteogenic and should be initiated early after heart transplantation (see also Chapter 41).15 Progressive resistance exercise with lumbar extension and upper and lower limb resistance machines has been demonstrated to limit muscle mass loss after corticosteroid use.16 The initial training resistance is set at 50% of the one repetition maximum, and repetitions are limited to 15 per session.
Although almost every cardiac transplant patient faces episodes of graft rejection, it is only rarely necessary to curtail the exercise workout during episodes of moderate rejection. When the patient shows signs of new arrhythmias, hypotension, or fever, however, the physiatrist can adjust the exercise regimen to balance medical management with restorative rehabilitative services.14,70 The patient’s long-term prognosis generally becomes less favorable as rejection episodes increase in frequency and severity. Clinical and physiologic monitoring of the patient and regular review of personal life and family goals are essential to maximize the patient’s prognosis, life plans, and family functioning. Patient and family education plays a critical role in transplantation rehabilitation (Box 60-5).58
BOX 60-5 Transplantation Rehabilitation: Patient-Family Education
Rehabilitation Through Lung Transplantation
Lung Transplants and Patient Outcomes
The lung is a bellows that promotes carbon dioxide escape and oxygen acquisition from the atmosphere. Organ failure becomes a life-limiting impairment treatable only with replacement. Hardy brought the lung transplant to surgical care at the University of Mississippi in 1963, as an extension of his laboratory investigation.49 The most common indications for single-lung transplants are chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis (Box 60-6). Bilateral lung transplants are often done for cystic fibrosis and pulmonary hypertension. Lung transplantation referral should be considered in patients with 1-second forced expiratory ventilation (FEV1) less than 30% of predicted, hypoxia (PO2 <60 mm Hg), or hypercarbia (PCO2 >50 mm Hg).102 The median wait-list time has decreased 87% over the past decade to 132 days in 2006.92 The number of bilateral lung transplants has more than doubled over the decade to 887, which was 64% of all the lung transplants done in 2008.92 The International Society for Heart and Lung Transplantation and the St. Louis International Lung Transplantation Registry report 1-year survival rates of 71% and 5-year survival rates of 45% after lung transplantation.
BOX 60-6 Most Common Primary Diagnosis in Pulmonary Transplant Rehabilitation Patients
Contemporary physiatric practice includes providing pulmonary rehabilitation to patients with varied ventilation and oxygen exchange impairments. As rehabilitation is delivered, patients not fully responsive can be identified and might be transplantation candidates. This requires close cooperation with pulmonary medicine, as well as sufficient institutional program development, to provide effective pulmonary rehabilitation treatment to meet the needs at each setting. Compared with education alone, pulmonary rehabilitation (education and exercise) has been shown to increase exercise performance and to decrease muscle fatigue and shortness of breath.112
Pretransplant Rehabilitation: Assessment, Education, and Conditioning
Once identified, workup for lung transplant candidates includes chest radiographs, computed tomography scans, and ventilation perfusion scans. To assess for ischemia, cardiac assessment with catheterization or a chemical stress test (persantine/thallium) is used. A pulmonary rehabilitation program is designed to document baseline functions and maximize performance before surgery. Oxygen saturation at rest and exercise requirements are determined with variable flow tanks and ambulatory O2 saturation meters. A manual muscle testing identifies problem areas and guides a program of resistance exercises. Nutritional rehabilitation of these patients can improve postoperative outcomes.50 Careful review of patient body mass index, diet, prealbumin, and albumins provides a useful baseline.
To assess pulmonary functional capacity, the rehabilitation team should quantify ventilatory effort, vital capacity, and FEV1. Auscultation of the chest for wheezing and bronchial breath sounds is essential for assessment of the effectiveness of bronchodilators, mucociliary clearance, and expectoration efficiency.34 The 6-minute walk test is a standard assessment of exercise tolerance used in patients with various pulmonary diseases. During this test the transplant patient is instructed to ambulate as fast as possible over a flat measured course for exactly 6 minutes. Performance is maximized by tuning O2 saturations to stay above 90% and use of the most efficient ambulation pattern. Higher-level testing can also use a cycle ergometer or treadmill. After an individualized plan is designed, patients are initially encouraged to exercise repeatedly for short periods to avoid prolonged breathlessness. Lung transplant candidates with end-stage pulmonary disease often do better with interval exercise training rather than continuous training because less ventilatory demand is required. The goals of therapy are to gradually decrease the number of required rest periods, extend exercise duration, and reduce the amount of limiting symptoms. Each program should include instructions on efficient ventilation, expectoration, stretching, strengthening, and low-level aerobic endurance. An exercise program that gradually approaches and maintains 60% of peak heart rate can effectively condition patients.34 Upper limb exercise has also been safely used in rehabilitation programs,7 although it can contribute to dyspnea. Inspiratory muscle exercise training can also optimize function.110 Energy conservation exercises can help the patient adjust to the low functional capacity caused by advanced pulmonary disease. Occupational therapy instruction can aid in the formulation of appropriate work simplification strategies and energy conservation measures.
Identifying the optimal level of exercise intensity suitable for each patient is an important goal of the rehabilitation team. Target heart rates can be applied to patients with lung disease, just as they are used in patients with cardiac disease. The high resting heart rates of this population, however, must be considered. Exercise regimens using 60% of peak heart rate as a target have been demonstrated to increase exercise tolerance.7,19 Patients with severe lung disease typically do not attain predicted maximal heart rates because exercise is limited by pulmonary rather than cardiac function. Traditional cardiac rehabilitation target exercise formulas do not universally apply to the pulmonary rehabilitation patient.20
The Dyspnea Index is a helpful and simple clinical tool for monitoring and prescribing exercise intensity in patients with dyspnea.64 Dyspnea can be assessed using the 5-level index developed at Stanford, based on the number of breaths the patient must take to count to 15. The index runs from level 0, in which the patient can count to 15 in one breath, to level 4, in which the patient is too short of breath to count. An alternative measure is the dyspnea scale in which the patient rates the degree of dyspnea during exercise.7 A third alternative is the Borg rating scale of perceived exertion,12 which requires the patient to evaluate self-perceived effort during exercise.
Because of deteriorating disease, alterations in the patient’s health status can often occur during the acute pretransplant period. As pulmonary reserves worsen, the pre–lung transplant patient might require abrupt cessation of exercise until clinically stable.7 Hospitalization might be required if lung function continues to decline. This deterioration should move the patient’s name closer to the top of the transplant waiting list. Some lung transplant programs require the patient move closer to the operating center to enable close monitoring.39,40
Acute Postoperative Rehabilitation
Single-lung transplants are preferentially performed on the left because of surgical ease through a standard posterolateral thoracotomy. Bilateral lung transplants have been recently performed through bilateral thoracotomies with sequential single-lung transplants, each with individual bronchial anastomoses. The transplanted lung is denervated, which leads to impairments of the cough reflex, gas exchange, circulatory autoregulation, mucociliary clearance, and fluid balance. This can result in ineffective clearance of airway secretions, necessitating chest physical therapy. Lymphatic disruption contributes to fluid retention and congestion that hinders gas exchange, and reduces lung compliance. Diaphragmatic dysfunction can also be present in lung transplant recipients. These can be evaluated with electrodiagnostic studies.7,19,25
Prolonged bed rest in these patients can cause orthostatic intolerance, reduced ventilation, increased resting heart rate, and decreased oxygen uptake.116 The goals of immediate postoperative rehabilitation are to minimize atelectasis, clear airway secretions, and normalize gas exchange. Altering the patient’s position from supine to side-lying or upright can increase drainage from chest tubes, as well as promote drainage of pulmonary secretions. The decreased mucociliary clearance associated with denervated lungs33 can contribute to increased susceptibility to infection in the early postoperative period. The patient should be assisted with airway clearance, beginning on the first postoperative day if the patient is stable. Patients who are mechanically ventilated can benefit from a combination of shaking (such as with the Pneumovest) and hyperinflation with a manual ventilation bag.147
After extubation, the patient can use the active cycle of breathing technique or a flutter valve device. Positive expiratory pressure therapy has been used in the posttransplant period. Secretion expectoration requires an effective cough, but efforts are hampered by incisional pain and bronchial sensory defects from denervation.39,111 Coughing technique can be improved using adequate pain control and optimal positioning.77 The patient should be encouraged to sit upright during coughing because this produces the greatest expiratory flow rates.77,147
Huff coughing is performed without closing the glottis and has been shown to produce a larger volume of expired air at a higher flow rate of secretions than conventional coughing.53 Huffing produces lower sound, vibrates the pharynx less, and can be more comfortable after surgery. For patients unable to generate substantial airflow, the techniques of stacking breaths and positive pressure breathing, used before the expulsion phase, can increase the effectiveness of a cough. Splinted coughing, with a pillow against the incision, can help reduce postoperative pain. Incisional pain can limit activity progression, deep breathing exercises, and coughing.147 Patients might complain of pain originating from the chest tube sites. Epidural analgesia can help in pain management and allows the patient to participate more enthusiastically in rehabilitation (see also Chapter 34).
Medical Complications
To prevent acute and chronic rejection, patients are now commonly placed on triple-drug immunosuppressant induction regimens (e.g., basiliximab, daclizumab, and antithymocyte globulin). Patients are later discharged on baseline therapy that typically includes corticosteroids, tacrolimus, and an antimetabolite such as azathioprine. Recently a new generation of immunosuppressant medications has emerged, including FK506 (tacrolimus), sirolimus, and leflunomide. Although these drugs are very helpful in averting acute and chronic rejection, they have significant side effects.94 A majority of acute rejection episodes occur during the initial 3 months after transplantation. Chronic rejection can also occur and can manifest as a sudden decrease in FEV1.31 This is known histologically as bronchiolitis obliterans and can be exacerbated by gastroesophageal reflux.28
Infection is the most common complication in lung transplantation and can lead to premature death if not properly recognized and treated.158 Clinicians should be aware of common pathogens associated with infection. Cytomegalovirus is a common viral pathogen and typically appears 14 to 100 days postoperatively. The diagnosis of its infection can be made with bronchoscopic lavage and biopsy. Typical fungal pathogens include Candida, Aspergillus, and Pneumocystis.
Postoperative Exercise Considerations
Progressive activity should be initiated on the first postoperative day, beginning with range of motion exercises.102 These can be advanced to transfers out of bed to a chair and then to ambulation. After the patient leaves the intensive care unit, rehabilitation should continue to focus on alveolar ventilation, mucociliary transport, and ventilation perfusion matching to optimize the efficiency of oxygen transport. Thoracic mobility might be improved by instructing the patient in chest and upper limb mobilization exercises.20,34 Breathing exercises should be included in the thoracic mobility and cardiovascular exercise programs, as well as coughing and airway clearance, and general activities.
As the patient progresses, a treadmill and cycle ergometer can be introduced in the isolation exercise room, allowing the patient to improve cardiovascular endurance and strength and reduce infection risk. Pulmonary transplant recipients can often reach exercise intensities comparable with recovering fully able-bodied patients of similar ages.102 Denervation of the lungs does not impair the ability to increase ventilation during physical exertion. In fact, most studies show that physical training results in improved endurance and strength.72 Before discharge from the hospital, the patient should progress to stair climbing, which is one of the major hallmarks of recovery. This is because advanced pulmonary disease typically makes it impossible for most patients to climb stairs for weeks to years.
Cardiopulmonary exercise testing has demonstrated areas of limitation in exercise capacity after lung transplantation. Aerobic capacity, measured by maximal oxygen uptake, typically remains reduced to 32% to 60% of the predicted value.151 This reduction in aerobic capacity is thought to underlie the exercise limitations in lung transplant patients. Abnormalities of gas exchange and ventilation–perfusion are not thought to play a major role in the reduced exercise capacity of single-lung transplant patients. Many other factors can contribute to reduced exercise reserve, including chronic deconditioning and muscle atrophy. Peripheral muscle work capacity is reduced after lung transplantation and is predominantly responsible for exercise performance limitations.
After lung transplantation, patients usually achieve considerable restoration of functional ability.36 Improvement in exercise tolerance has been demonstrated by an increase in 6-minute walk distances after transplantation.7,40,84,151 One center reported that none of the lung transplant recipients failed to complete a maximal symptom-limited exercise test because of dyspnea. The main complaint was lower limb discomfort or pain.56
Vocational Rehabilitation After Lung Transplant
Once surgery has been performed and the recuperative process has been successful, the lung transplant survivor faces a unique set of challenges, especially because the majority of patients need to resume work or homemaking duties. More than 90% of lung transplant patients report satisfaction with their health while performing these tasks.29 One leading study compared return to work rates among lung transplant survivors with other forms of transplant surgery.103 This study concluded that there was a 37% employment rate among posttransplant survivors. Employment was not determined by the type of lung transplantation procedure (single or bilateral).57
Rehabilitation Through Liver Transplantation
The art and science of liver transplantation has improved considerably within the past decade. This is in part due to the widespread availability of new surgical techniques and the emergence of new pharmacologic options aimed at preventing rejection and infection of transplanted organs.71,136
Liver transplantation offers the only life-saving cure for people with end-stage liver disease (ESLD). ESLD is frequently associated with a variety of common liver diseases, including141:
The National Institutes of Health, in collaboration with several leading academic transplant centers, is now in the process of exploring the risks, benefits, and outcomes of the adult to adult living donor liver transplantation procedure (also known as A2ALL).138
Liver Transplant in Children
For children requiring liver transplantation, adult living donor transplant has proven to be an accepted medical option.11 Unlike adult liver transplant candidates who require a larger liver segment (as much as half of the donor liver) and far more complex surgery, pediatric transplantation calls for a relatively small donor segment. More than half of the living donor transplants performed to date have occurred since 2000.
The first liver transplant was performed at the University of Colorado in 1963. Clinical outcomes of this complex surgical procedure improved dramatically in the 1980s, when antirejection medications such as cyclosporine became widely available. Improved survival as a result of better surgical techniques and optimization of immunosuppression has led to improved survival rates and a larger number of referrals to rehabilitation centers. The current 1-year survival rate after liver transplantation is approximately 80% to 90%, and the 5-year patient survival rate is about 70%. The commitment of the transplant team (Box 60-7) to rehabilitative care is a key factor in enhancing longevity and quality of life for the patient.
Many patients are awaiting liver transplantation, representing an extreme shortage of human livers. This shortage was, in fact, central to the debate on revamping the way human organs are distributed.97 According to UNOS, there are currently about 16,000 patients on the waiting list for a liver transplant. In 2008 there were 6319 liver transplants performed in the United States.
Quality of life issues after liver transplantation are becoming increasingly important as survival rates continue to improve. A survey conducted by Robinson et al.115 tracked the progress of 31 patients after liver transplantation. Forty-seven percent of the patients surveyed reported abnormal function in at least one limb, and 13% reported developing gout. Sixty-one percent reported severe impairment in endurance before the transplant, with 48% unable to ambulate outside the house. After the transplant, only 6% reported severely impaired endurance. Three years after the transplant, 39% of patients reported that they were employed full-time, and 26% were homemakers.
Indications for Liver Transplant and Presurgical Considerations
Other features associated with the disease include neurologic dysfunction, fatigue, and forgetfulness. Bronster et al.17 report that neurologic complications are common after liver transplantation, causing considerable morbidity and mortality. Neurologic complications are frequently the result of the therapeutic interventions required to maintain function of the transplanted liver. Recognizing the early signs of drug-related neurotoxicity is the first step in preventing the development of more severe problems. (Box 60-8). Careful perioperative management of fluids, particularly sodium and glucose levels, can help reduce the risk of postoperative changes.157
The enzymes typically used to track graft function after liver transplantation are specified in Box 60-9.
Rehabilitation Concerns in the Liver Transplant Patient
A number of critical priorities exist in the promotion of functional well-being in the transplant patient.61 A customized rehabilitation plan should begin with a comprehensive presurgical evaluation. Physical therapy should be initiated immediately after the transplant recipient has achieved surgical stabilization. A special emphasis must be placed on sustaining the patient’s nutritional status. Priorities should include restoration of muscle mass and strength through graded isometric exercises, as well as enhancement of aerobic endurance. Early mobilization postoperatively should be achieved. The liver transplant patient receiving rehabilitative services should be monitored carefully for signs of rejection and liver failure.
Emerging Developments in Transplantation
New Indications for Bone Marrow Transplantation
Bone marrow transplantation is no longer limited to indications related to hematologic malignancy. It has become a widely accepted treatment for metastatic breast cancer. Progression-free survival at 1 year ranges from 8% to 23% depending on the technique used.140 It is also being used in a range of autoimmune disorders, for example, systemic lupus erythematosus, systemic sclerosis, Crohn’s disease, and multiple sclerosis.128 Clinical trials have been promising for multiple sclerosis, even in cases of severe progressive disease.128 In 19 patients with a mean follow-up period of 36 months, only one patient deteriorated in functional status compared with baseline.
Airway Transplants
Progress in transplants of the larynx and trachea has been somewhat hampered compared with other vital organs. The high vascularity of these structures makes them particularly prone to tissue rejection. Tissue rejection in these vital structures unfortunately can lead to catastrophic airway compromise. The complex motor function of the larynx can also be slow to recover after transplantation, leading to potential problems with aspiration. Only one true larynx transplant has been performed since 1998.8,9
Facial Transplantation
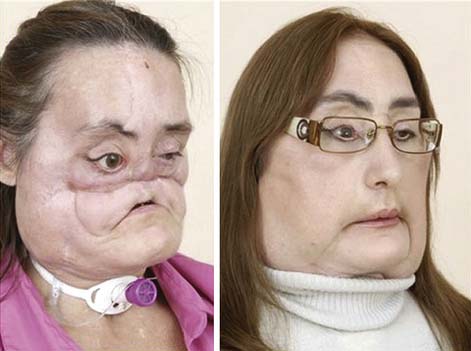
Facial deformity from congenital defect or trauma can have a devastating impact on the psychosocial aspects and quality of life for affected individuals (Figure 60-3). It can lead to dramatic restrictions in social participation. Because these deformities are not life threatening, however, there has been much ethical debate about facial transplantation.60 The need for lifelong antirejection medications, with potentially fatal side effects, has tempered the enthusiasm of some surgeons for these procedures. Only a few of these procedures have been done to date.91 The use of extracorporeal photopheresis might obviate the need for lifelong antirejection medications.54 Screening criteria for institutional review boards have been proposed.105 Realistic patient expectations are necessary to obtain truly informed consent. Disfigured patients’ expectations differed significantly from plastic surgeons’ expectation in one study,5 raising concern over this potential ethical dilemma.
Hand and Forearm Transplantation
Similar ethical concerns are raised about hand transplantation.91 Despite advances in prosthetic technology, prosthetic upper limbs have not provided great functional solutions for many amputees. The advent of neural-controlled prostheses, however, might surpass hand transplantation as a viable option for these patients.75 Good reason for optimism, however, exists regarding hand and forearm transplantation. A registry has recorded 24 hand and forearm transplants, with no episodes of permanent rejection in 2 years and 100% patient survival.78 Sensation improved in 17 patients, and 90% returned to work. Fifteen of the 24 patients reported improved quality of life overall (Figure 60-4).
Nerve Transplantation
Nerve transplantations have offered exciting opportunities as alternatives to autografts, which are not always readily available in cases of major peripheral nerve trauma.90 One patient successfully underwent brachial plexus transplantation in 2008, with good functional outcome at 1 year (Belzberg A, Personal communication, 2009).
Intestinal and Multivisceral Transplantation
Between 1990 and 2003, 122 children received intestinal transplants, with a 5-year survival of 61%.10 Many of these patients also need liver and pancreatic transplantation. This is due in part to late referral, with secondary liver failure occurring before intestinal transplantation.144 Earlier referral for intestinal transplantation might alleviate the need for multivisceral transplantation.
Xenotransplantation
Xenotransplantation refers to the transplantation of animal tissues into humans. This has become a routine practice in the case of some tissues, such as porcine heart valves, which seem to have a low risk of rejection because of their acellular composition. There has been successful xenotransplantation in a number of tendon ruptures, including rotator cuff injuries4 and anterior cruciate ligaments.131 These do not require antirejection medication. Precautions for rehabilitation are similar to those of standard tendon repairs.
Xenotransplantation of major organs remains problematic, with a high risk for rejection. Transplantation into humans has been largely unsuccessful, and even porcine organ transplantation into nonhuman primates under experimental conditions has had less than 60% graft survival rates for hearts and lungs.27
Summary
Our society has been dramatically affected by the development of organ transplantation science. Recent technologic discoveries and breakthroughs have created a new type of physiatric practice, that of transplant rehabilitation. Life-saving treatment of disease by organ transplantation is becoming standard practice. Transplant patients are enjoying more active and meaningful lives as a result of early rehabilitation intervention.156 A cautious and progressive rehabilitation program typically results in organ recipients returning to a more active lifestyle—one that includes exercise, work, recreation, and travel. Another role of the rehabilitation team has been the education of patients and families regarding the transplant process and coping strategies (see Box 60-7). As transplant science progresses, it is predicted that the need for transplant rehabilitation services will continue to grow.
1. American Transplant Congress 2003: The Fourth Joint American Transplant Meeting. May 30-June 4, 2003; Washington, DC.
2. Anyanwu A.C., Banner N.R., Radley-Smith R., et al. Long-term results of cardiac transplantation from live donors: the domino heart transplant. J Heart Lung Transplant. 2002;21(9):971-975.
3. Auerbach I., Tenenbaum A., Motro M., et al. Attenuated responses of Doppler-derived hemodynamic parameters during supine bicycle exercise in heart transplant recipients. Cardiology (Switzerland). 1999;92(3):204-209.
4. Badhe S.P., Lawrence T.M., Smith F.D., et al. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17(suppl 1):35S-39S.
5. Barker J.H., Furr L.A., McGuire S., et al. Patient expectations in facial transplantation. Ann Plast Surg. 2008;61(1):68-72.
6. Beck W., Barnard C.N., Schrire V. Heart rate after cardiac transplantation. Circulation. 1969;40:437-445.
7. Biggar D.G., Malen J.F., Trulock E.P., et al. Pulmonary rehabilitation before and after lung transplantation. In: Kasaburi R., Petty T.L., editors. Principles and practice of pulmonary rehabilitation. Philadelphia: WB Saunders, 1993.
8. Birchall M., Macchiarini P. Airway transplantation: a debate worth having? Transplantation. 2008;85(8):1075-1080.
9. Birchall M.A., Lorenz R.R., Berke G.S., et al. Laryngeal transplantation in 2005: a review. Am J Transplant. 2006;6(1):20-26.
10. Bond G.J., Mazariegos G.V., Sindhi R., et al. Evolutionary experience with immunosuppression in pediatric intestinal transplantation. J Pediatr Surg. 2005 Jan;40(1):274. 9; discussion 279–280
11. Borenstein S., Diamond I.R., Grant D.R., et al. Outcome of pediatric live-donor liver transplantation: the Toronto experience. J Pediatr Surg. 2003;38(5):668-671.
12. Borg G. Psychophysical basis of perceived exertion. Med Sci Sports Exer. 1982;14:377-381.
13. Braith R.W. Exercise training in patients with CHF and heart transplant recipients. Med Sci Sports Exerc. 1998;30(10 suppl):S367-S378.
14. Braith R.W., Clapp L., Brown T., et al. Rate-responsive pacing improves exercise tolerance in heart transplant recipients: a pilot study. J Cardiopulm Rehabil. 2000;20(6):377-382.
15. Braith R.W., Edwards D.G. Exercise following heart transplantation. Sports Med. 2000;30(3):171-192.
16. Braith R.W., Welsch M.A., Mills R.M.Jr., et al. Resistance exercise prevents glucocorticoid-induced myopathy in heart transplant recipients. Med Sci Sports Exer. 1998;30(4):483-489.
17. Bronster D.J., Emre S., Mor E., et al. Neurologic complications of orthotopic liver transplantation. Mount Sinai J Med. 1994;61(1):63-69.
18. Bunke M., Ganzel B. Effects of calcium antagonists on renal function in hypertensive heart transplant recipients. J Heart Lung Transplant. 1992;2:1194-1199.
19. Bunzel B., Laederach-Hofmann K. Long-term effects of heart transplantation: the gap between physical performance and emotional well-being. Scand J Rehabil Med. 1999;31(4):214-222.
20. Butler B.B. Physical therapy in heart and lung transplantation. In Hillegas E., Sadowski S., editors: Cardiopulmonary physical therapy, ed 3, St Louis: Mosby–Year Book, 1995.
21. Caplan A., Coelho D. The ethics of organ transplants: the current debate. New York: Prometheus Books; 1998.
22. Carlin B.W., Lega M., Veynovich B. Management of the patient undergoing lung transplantation: an intensive care perspective. Crit Care Nurs Q. 2009;32(1):49-57.
23. Carter R., Al-Rawas O.A., Stevenson A., et al. Exercise responses following heart transplantation: 5 year follow-up. Scott Med J. 2006;51(3):6-14.
24. Cavero P.G., Sudhir K., Galli F., et al. Effect of orthotopic cardiac transplantation on peripheral vascular function in congestive heart failure: Influence of cyclosporine therapy. Am Heart J. 1994;127:1581-1587.
25. Chlan L., Snyder M., Finkelstein S., et al. Promoting adherence to an electronic home spirometry research program after lung transplantation. Appl Nurs Res. 1998;11(1):36-40.
26. Coon S.K., Coleman E.A. Exercise decisions within the context of multiple myeloma, transplant, and fatigue. Cancer Nurs. 2004;27(2):108-118.
27. Cooper D.K., Keogh A.M., Brink J., et al. Report of the xenotransplantation advisory committee of the international society for heart and lung transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant. 2000;19(12):1125-1165.
28. Corris P.A., Christie J.D. Update in transplantation 2007. Am J Respir Crit Care Med. 2008;177:1062-1067.
29. Craven J.L., Bright J., Dear C.L. Psyciatric, psychosocial and rehabilitative aspects of lung transplantation. Clin Chest Med. 1990;11:247-257.
30. Dandel M., Wellnhofer E., Hummel M., et al. Early detection of left ventricular dysfunction related to transplant coronary artery disease. J Heart Lung Transplant. 2003;22(12):1353-1364.
31. De Vito Dabbs A., Hoffman L.A., Swigart V., et al. Using conceptual triangulation to develop an integrated model of the symptom experience of acute rejection after lung transplantation. ANS Adv Nurs Sci. 2004;27(2):138-149.
32. Deuse T., Haddad F., Pham M., et al. Twenty-year survivors of heart transplantation at Stanford University. Am J Transplant. 2008;8:1769-1774.
33. Dolovich M., Rossman C., Chambers C., et al. Mucociliary function in patients following single lung or lung/heart transplantation. Am Rev Respir Dis. 1987;135:363. [abstract]
34. Downs A.M. Physical therapy in lung transplantation. Phys Ther. 1996;76(6):626-642.
35. Drexler H., Schroeder J.S. Unusual forms of ischemic heart disease. Curr Opin Cardiol. 1994;9:457-464.
36. Duarte A.G., Terminella L., Smith J.T., et al. Restoration of cough reflex in lung transplant recipients. Chest. 2008;134(2):310-316.
37. Dubernard J.M., Lengele B., Morelon E., et al. Outcomes 18 months after the first human partial face transplantation. Ann Chir. 2002 Jan;127(1):19-25.
38. Dubernard J.M., Petruzzo P., Lanzetta M., et al. Functional results of the first human double-hand transplantation. Ann Surg. 2003;238(1):128-136.
39. Egan T.M., Kaiser L.R., Cooper J.D. Lung transplantation. Curr Prob Surg. 1989;26:673-752.
40. Egan T.M., Westerman J.H., Lambert C.J.Jr., et al. Isolated lung transplantation for end-stage lung disease: a viable therapy. Ann Thorac Surg. 1992;53:590-596.
41. Fuhrmann I., Krause R. Principles of exercising in patients with chronic kidney disease, on dialysis and for kidney transplant recipients. Clin Nephrol. 2004;61(suppl. 1):S14-S25.
42. Gallagher-Lepak S. Functional capacity and activity level before and after renal transplantation. ANNA J. 1991;18(4):378-382. 406
43. Gammie J.S., Edwards L.B., Griffith B.P., et al. Optimal timing of cardiac transplantation after ventricular assist device implantation. J Thorac Cardiovasc Surg. 2004;127(6):1789-1799.
44. Gelling L. Quality of life following liver transplantation: physical and functional recovery. J Adv Nurs. 1998 Oct;28(4):779-785.
45. Greenberg A., Egel J.W., Thompson M.E., et al. Early and late forms of cyclosporine nephrotoxicity: studies in cardiac transplant recipients. Am J Kidney Dis. 1987;9:12-22.
46. Greenberg M.L., Uretsky B.F., et al. Long-term hemodynamic follow-up of cardiac transplant patients treated with cyclosporine and prednisone. Circulation. 1985;71:487-494.
47. Griepp R.B., Stinson E.D., Dong E.Jr., et al. Hemodynamic performance of the transplanted human heart. Surgery. 1971;70:88-96.
48. Hampton T. Transplant outcomes database. JAMA. 2004;291:1434.
49. Hardy J.D., Webb W.R., Dalton M.L., et al. Lung homotransplantation in man. JAMA. 1963;186:1065.
50. Hasse J.M. Diet therapy for organ transplantation: a problem-based approach. Nurs Clin North Am. 1997;32(4):863-880.
51. Haykowsky M., Taylor D., Kim D., et al. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9(4):734-739.
52. Heiwe S., Clyne N., Dahlgren M.A. Living with chronic renal failure: patients’ experiences of their physical and functional capacity. Physiother Res Int. 2003;8(4):167-177.
53. Hietpas B., Roth R., Jensen W. Huff coughing and airway patency. Respir Care. 1979;24:710-714.
54. Hivelin M., Siemionow M., Grimbert P., et al. Extracorporeal photopheresis: from solid organs to face transplantation. Transpl Immunol. 2009;21(3):117-128.
55. Hosenpud J.D., Novick R.J., Breen T.J., et al. Registry of the International Society for Heart and Lung Transplantation: Eleventh official report—1994. J Heart Lung Transplant. 1994;13:561-570.
56. Howard D.K., Iademarco E.J., Trulock E.P. The role of cardiopulmonary exercise testing in lung and heart-lung transplantation. Clin Chest Med. 1994;15:405-420.
57. Huddleston C.B., Bloch J.B., Sweet S.C., et al. Lung transplant in children. Ann Surg. 2002;236(3):270-276.
58. Hummel M., Michauk I., Hetzer R., et al. Quality of life after heart and heart-lung transplantation. Transplant Proc. 2001;33(7-8):3546-3548.
59. Hurley C.K., Fernandez V.M., Setterholm M. Maximizing optimal hematopoietic stem cell donor selection from registries. Tissue Antigens. 2003;61(6):415-424.
60. Johnson S.E., Corsten M.J. Facial transplantation in a new era: What are the ethical implications? Curr Opin Otolaryngol Head Neck Surg. 2009.
61. Jones J.B. Liver transplant recipients’ first year of posttransplant recovery: a longitudinal study. Prog Transplant. 2005;15(4):345-352.
62. Joshi A., Kevorkian C.G. Rehabilitation after cardiac transplantation: case series and literature review. Am J Phys Med Rehabil. 1997 May-Jun;76(3):249-254.
63. Kao A.C., Van Trigt P.R., Shaeffer-McCall G.S., et al. Allograft diastolic dysfunction and chronotropic incompetence limit cardiac output response to exercise two to six years after heart transplantation. J Heart Lung Transplant. 1995;14:11-22.
64. Karam V., Castaing D., Danet C., et al. Longitudinal prospective evaluation of quality of life in adult patients before and one year after liver transplantation. Liver Transplant. 2003;9(7):703-711.
65. Kavanagh T., Yacoub M.H. Exercise training in patients after heart transplantation. Ann Acad Med Singapore. 1992;21:372-378.
66. Kavanagh T., Yacoub M.H., Kennedy J., et al. Return to work after heart transplantation: 12-year follow-up. J Heart Lung Transplant. 1999;18(9):846-851.
67. Kavanagh T., Yacoub M.H., Mertens D.J., et al. Cardiorespiratory responses to exercise training after orthotopic cardiac transplantation. Circulation. 1988;77:162-171.
68. Kawai T., Cosimi A.B., Spitzer T.R., et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353-361.
69. Keteyian S., Marks C.R., Levine A.B., et al. Cardiovascular responses of cardiac transplant patients to arm and leg exercise. Eur J Appl Physiol. 1994;68:441-444.
70. Kevorkian C.G. Stroke rehabilitation and the cardiac transplantation patient. N Engl J Med. 1999;340(12):976.
71. Kim W.R., Poterucha J.J., Kremers W.K., et al: Outcome of liver transplantation for hepatitis B in the United States, Liver Transpl 10(8):968-974
72. Kjaer M., Beyer N., Secher N.H. Exercise and organ transplantation. Scand J Med Sci Sports. 1999;9(1):1-14.
73. Kobashigawa J.A., Laks H., Marelli D., et al. The University of California at Los Angeles experience in heart transplantation. Clin Transpl. 1998:303-310.
74. Kobashigawa J.A., Leaf D.A., Lee N., et al. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med. 1999;340(4):272-277.
75. Kuiken T.A., Li G., Lock B.A., et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301(6):619-628.
76. Lampert E., Mettauer B., Hoppeler H., et al. Skeletal muscle response to short endurance training in heart transplant recipients. J Am Coll Cardiol. 1998;32(2):420-426.
77. Lannefors L., Wollmer P. Mucus clearance with three chest physiotherapy regimens in cystic fibrosis: a comparison between postural drainage, PEP, and physical exercise. Eur Respir J. 1992;5:748-753.
78. Lanzetta M., Petruzzo P., Dubernard J.M., et al. Second report (1998-2006) of the international registry of hand and composite tissue transplantation. Transpl Immunol. 2007;18(1):1-6.
79. Latlief G., Young M.A.: Cardiac transplantation in a post-partum female, presented at the Annual Assembly of the American Academy of physical Medicine and Rehabilitation, Anaheim, CA, October, 1994.
80. Le Jemtel T.H. Review of a controlled trial of exercise rehabilitation after heart transplantation. Transplant Proc. 2003;35(4):1513-1515.
81. Leenen F.H., Davies R.A., Fourney A. Role of cardiac beta 2-receptors in cardiac responses to exercise in cardiac transplant patients. Circulation. 1995;91:685-690.
82. Legault L., Olgilvie R.I., Cardella C.J., et al. Calcium antagonists in heart transplant recipients: effects on cardiac and renal function and cyclosporine pharmacokinetics. Can J Cardiol. 1993;9:398-404.
83. Lucas T.S., Einhorn T.A. Stress fracture of the femoral neck during rehabilitation after heart transplantation. Arch Phys Med Rehabil. 1993;74(9):1004-1006.
84. Mal H., Sleiman C., Jebrak G., et al. Functional results of single-lung transplantation for chronic obstructive lung disease. Am J Respir Crit Care Med. 1994;149:1476-1481.
85. Martin T.W., Gaucher J., Pupa L.E., et al. Response to upright exercise after cardiac transplantation. Clin Cardiol. 1994;17:292-300.
86. McGiffin D., Kirklin J.K., Nafiel D.C. Acute renal failure after heart transplantation and cyclosporine therapy. J Heart Transplant. 1985;4:396-399.
87. Meyer M., Rahmel A., Marconi C., et al. Adjustment of cardiac output to step exercise in heart transplant recipients. Z Kardiol. 1994;83(suppl 3):103-109.
88. Miller L.W., Naftel D.C., Bourge R.C., et al. Infection after heart transplantation: a multi-institutional study—Cardiac Transplant Research Database Group. J Heart Lung Transplant. 1994;13:381-393.
89. Mills R.M. Jr: Transplantation and the problems afterward including coronary vasculopathy. Clin Cardiol. 1994;17:287-290.
90. Moore A.M., Ray W.Z., Chenard K.E., et al. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand (N Y). 2009.
91. Morris P., Bradley A., Doyal L., et al. Face transplantation: a review of the technical, immunological, psychological and clinical issues with recommendations for good practice. Transplantation. 2007;83(2):109-128.
92. Mulligan M.S., Shearon T.H., Weill D., et al. Heart and lung transplantation in the United States 1997-2006. Am J Transplant. 2008;(8):977-987.
93. Neipp M., Karavul B., Jackobs S., et al. Quality of life in adult transplant recipients more than 15 years after kidney transplantation. Transplantation. 2006;81(12):1640-1644.
94. Ng C.Y., Madsen J.C., Rosengard B.R., et al. Immunosuppression for lung transplantation: Front Biosci. 2009;14:1627-1641.
95. O’Connell J.B., Bourge R.C., Costanzo-Nordin M.R., et al. Cardiac transplantation: recipient selection, donor procurement, and medical follow-up—a statement for health professionals from the Committee on Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1992;86:1061-1079.
96. Oliver D., Pflugfelder P.W., McCartney N., et al. Acute cardiovascular responses to leg-press resistance exercise in heart transplant recipients. Int J Cardiol. 2001;81(1):61-74.
97. Organ Procurement and Transplantation. Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Institute of Medicine: Committee on Organ Procurement and Transplantation Policy, Division of Health Sciences Policy. Washington, DC: National Academy Press; 1999.
98. Ozcurumez G., Tanriverdi N., Colak T., et al. The psychosocial impact of renal transplantation on living related donors and recipients: preliminary report. Transplant Proc. 2004;36(1):114-116.
99. Painter P., Moore G., Carlson L., et al. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39(2):257-265.
100. Painter P.L., Hector L., Ray K., et al. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74(1):42-48.
101. Painter P.L., Hector L., Ray K., et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2003;42(2):362-369.
102. Palmer S.M., Tapson V.F. Pulmonary rehabilitation in the surgical patient: lung transplantation and lung volume reduction surgery. Respir Care Clin North Am. 1998;4(1):71-83.
103. Paris W., Diercks M., Bright J., et al. Return to work after lung transplantation. J Heart Lung Transplant. 1998;17(4):430-436.
104. Paterson D.H., Cunningham D.A., Pickering J.G., et al. Oxygen uptake kinetics in cardiac transplant recipients. J Appl Physiol. 1994;77:1935-1940.
105. Pomahac B., Aflaki P., Chandraker A., et al. Facial transplantation and immunosuppressed patients: a new frontier in reconstructive surgery. Transplantation. 2008;85(12):1693-1697.
106. Quittan M., Wiesinger G.F., Sturm B., et al. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2001 Mar;80(3):206-214. quiz 215-216, 224
107. Rajendran A.J., Pandurangi U.M., Mullasari A.S., et al. High intensity exercise training programme following cardiac transplant. Indian J Chest Dis Allied Sci. 2006;48(4):271-273.
108. Rao V., Oz M.C., Flannery M.A., et al. Revised screening scale to predict survival after insertion of a left ventricular assist device. J Thorac Cardiovasc Surg. 2003;125(4):855-862.
109. Reddy K.S., Stablein D., Taranto S., et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis. 2003;41(2):464-470.
110. Reid W.D., Dechman G. Considerations when testing and training the respiratory muscles. Phys Ther. 1995;75:971-982.
111. Richard C., Girard F., Ferraro P., et al. Acute postoperative pain in lung transplant recipients. Ann Thorac Surg. 2004;77(6):1951-1955.
112. Ries A.L., Kaplan R.M., Limberg T.M., et al. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1995;122:823-832.
113. Ring H. International rehabilitation medicine: closing the gaps and globalization of the profession. Am J Phys Med Rehabil. 2004;83(9):667-669.
114. Ring H.: Strategic Plan for rehabilitation services: education, Turkish J Phys Med Rehap 53(suppl 2):1-5,2007
115. Robinson L.R., Switala J., Tarter R.E., et al. Functional outcome after liver transplantation: A preliminary report. Arch Phys Med Rehabil. 1990;71(6):426-427.
116. Saltin B., Blomquist G., Mitchell J.H. Response to exercise after bed rest and after turning: A longitudinal study of adaptive changes in oxygen transport and body composition. Circulation. 1968;38(suppl 7):1-78.
117. Savin W.M., Haskell W.L., Schroeder J.S., et al. Cardiorespiratory responses of cardiac transplant patients to graded symptom limited exercise. Circulation. 1980;62:55-60.
118. Schrire V., Barnard C.N., Beck W. Some electrocardiographic changes in human heart transplants. Isr J Med Sci. 1969;5:931-937.
119. Schroeder J.S., Gao S.Z., Alderman E.L., et al. A preliminary study of diltiazem in the prevention of coronary artery disease in heart transplant recipients. N Engl J Med. 1993;328:164-170.
120. Shaver J.A., Leon D.F., Gray S.D., et al. Hemodynamic observations after cardiac transplantation. N Engl J Med. 1969;281:822-827.
121. Shephard R.J., Kavanagh T., Mertens D.J., et al. The place of perceived exertion ratings in exercise prescription for cardiac transplant patients before and after training. Br J Sports Med. 1996;30(2):116-121.
122. Shiba N., Chan M.C., Kwok B.W., et al. Analysis of survivors more than 10 years after heart transplantation in the cyclosporine era: Stanford experience. J Heart Lung Transplant. 2004;23(2):155-164.
123. Sliwa J.A., Andersen S., Griffin J. Cardiovascular responses to gait training and ambulation in a hemiparetic heart recipient. Arch Phys Med Rehabil. 1990;71(6):424-425.
124. Sliwa J.A., Blendonohy P.M. Stroke rehabilitation in a patient with a history of heart transplantation. Arch Phys Med Rehabil. 1988;69(11):973-975.
125. Smith S. Immunosuppressive therapies in organ transplantation. WebMD Medscape Health Network. Jun 25, 2002. Available at http://www.medscape.com/viewarticle/437182 Accessed April 4, 2009
126. Solomon N.A., McGiven J.R., Alison P.M., et al. Changing donor and recipient demographics in a heart transplantation program: influence on early outcome. Ann Thorac Surg. 2004;77(6):2096-2102.
127. Squires R.W. Exercise training after cardiac transplantation. Med Sci Sports Exerc. 1991;23:686-694.
128. Statkute L., Verda L., Oyama Y., et al. Mobilization, harvesting and selection of peripheral blood stem cells in patients with autoimmune diseases undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39(6):317-329.
129. Stewart K.J., Badenhop D., Brubaker P.H., et al. Cardiac rehabilitation following percutaneous revascularization, heart transplant, heart valve surgery, and for chronic heart failure. Chest. 2003;123(6):2104-2111.
130. Stiens S., O’Young B., Young M.A. Person-centered rehabilitation: interdisciplinary intervention to enhance patient enablement. Physical medicine and rehabilitation secrets, ed 3. Philadelphia: Hanley & Belfus, 2002.
131. Stone K.R., Abdel-Motal U.M., Walgenbach A.W., et al. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83(2):211-219.
132. Streiff N., Feurer I., Speroff T., et al. The effects of rejection episodes, obesity, and osteopenia on functional performance and health-related quality of life after heart transplantation. Transplant Proc. 2001;33(7-8):3533-3535.
133. Tayler A., Bergin J. Cardiac transplantation for the cardiologist not trained in transplantation. Am Heart J. 1995;129:578-592.
134. Tegtbur U., Busse M.W., Jung K., et al. Time course of physical reconditioning during exercise rehabilitation late after heart transplantation. J Heart Lung Transplant. 2005;24(3):270-274.
135. Tegtbur U., Pethig K., Machold H., et al. Functional endurance capacity and exercise training in long-term treatment after heart transplantation. Cardiology. 2003;99(4):171-176.
136. Tome S., Wells J.T., Said A., et al. Quality of life after liver transplantation: a systematic review. J Hepatol. 2008;48:567-577.
137. Toronto Lung Transplant Group. Experience with single-lung transplantation for pulmonary fibrosis. JAMA. 1988;259:2258-2262.
138. Trotter J.F., Wachs M., Everson G.T., et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346(14):1074-1082.
139. Truong R.D. Consent for organ donation: Balancing conflicting ethical obligations. N Engl J Med. 2008;358(12):1209-1211.
140. Ueno N.T., Rizzo J.D., Demirer T., et al. Allogeneic hematopoietic cell transplantation for metastatic breast cancer. Bone Marrow Transplant. 2008;41(6):537-545.
141. United Network for Organ Sharing. Transplant living: organ donation and transplantation information for patients. http://www.transplantliving.org/BeforeTheTransplant/OrganFacts/liver.aspx. Available atAccessed Mar 4, 2009
142. Valentine H., Keogh A., McIntosh N., et al. Cost containment: co-administration of diltiazem with cyclosporine, after heart transplantation. J Heart Lung Transplant. 1992;2(part 1):1-7.
143. Vaska P.L. Common infections in heart transplant patients. Am J Crit Care. 1993;2:145-156.
144. Vianna R.M., Mangus R.S., Tector A.J. Current status of small bowel and multivisceral transplantation. Adv Surg. 2008;42:129-150.
145. Ville N.S., Varray A., Mercier B., et al. Effects of an enhanced heart rate reserve on aerobic performance in patients with a heart transplant. Am J Phys Med Rehabil. 2002;81(8):584-589.
146. Von Scheidt W., Kembes B.M., Reichart B., et al. Percutaneous transluminal coronary angioplasty of focal coronary lesions after cardiac transplantation. Clin Invest. 1993;71:524-530.
147. Webber B.A., Pryor J.A. Physiotherapy skills: techniques and adjuncts. In: Webber B.A., Pryor J.A., editors. Physiotherapy for respiratory and cardiac problems. Edinburgh: Churchill Livingstone, 1993.
148. Wechsler M.E., Giardina E.G., Sciacca R.R., et al. Increased early mortality in women undergoing cardiac transplantation. Circulation. 1995;91:1029-1035.
149. Wenting G.J., vd Meiracker A.H., Simoons M.I., et al. Circadian variation of heart rate but not of blood pressure after heart transplantation. Transplant Proc. 1987;19:2554-2555.
150. Wiesinger G.F., Crevenna R., Nuhr M.J., et al. Neuromuscular electric stimulation in heart transplantation candidates with cardiac pacemakers. Arch Phys Med Rehabil. 2001;82(10):1476-1477.
151. Williams T.J., Grossman R.F., Maurer J.R. Long-term functional follow-up of lung transplant recipients. Clin Chest Med. 1990;11:347-358.
152. Young L., Little M. Women and heart transplantation: an issue of gender equity? Health Care Women Int. 2004;25(5):436-453.
153. Young M.: (Book review) Physical medicine & rehabilitation, JAMA 278: 1037, 1994
154. Young M. Physical medicine and rehabilitation. JAMA. 2002;287:1050-1051.
155. Young M.A., Steins S.A. Rehabilitation of the transplant patient. In O’Young B.J., Young M.A., Stiens S.A., editors: PM&R secrets, ed 2, Philadelphia: Elsevier Health Sciences, 2002.
156. Young M.A., Steins S.A., McGill D., et al. Rehabilitation of the patient requiring transplantation. In: Grabois M., editor. Physical medicine & rehabilitation: the complete approach. Cambridge, MA: Blackwell Science, 1999.
157. Young M.A., Young M.M. Organ transplant rehabilitation perspectives. Sweden: Abstract Proceedings of the 11th European Congress of PM&R, Gothenburg; May 27, 1999.
158. Yun J.J., Mason D.P. Lung transplantation: past, present, and future. Minerva Chir. 2009;64(1):37-44.