Chapter 78 Transplantation
Hematopoietic Stem Cell
PATHOPHYSIOLOGY
Hematopoietic stem cell transplantation (HSCT), formerly known as bone marrow transplantation, is performed for the treatment of malignancies (leukemia, lymphoma, and solid tumors), bone marrow dysfunction and failure, immunodeficiencies, and congenital metabolic disorders (Box 78-1). The bone marrow, where all blood cells originate, is involved in a number of functions: (1) transportation of oxygen throughout the body by the erythrocytes; (2) infection protection by granulocytes, lymphocytes, and monocytes; and (3) control and prevention of bleeding by platelets. The goal of HSCT is to restore bone marrow and its hematologic, and immune functions, and to reverse immune dysfunction or failure. HSCT also is used to treat and prevent further progression of genetic diseases by replacing the enzyme-deficient cells with genetically normal bone marrow and subsequently normal blood cells.
Box 78-1 Conditions Treated with Hematopoietic Stem Cell Transplantation
Malignancies: Leukemias, Lymphomas, and Solid Tumors
Bone Marrow Dysfunction or Failure
In HSCT, donor stem cells are removed, which is termed harvesting, and then transplanted into the recipient. Once the stem cells are infused into the recipient on the day of transplant, they migrate to the marrow’s spaces and eventually begin to produce new cells. Additional information on the process of transplantation is provided in the Medical Management section of this chapter.
Stem cells harvested from and transplanted back into the recipient are termed autologous; those collected from someone other than the recipient are termed allogeneic and may be matched related (from a sibling), syngeneic (from a twin sibling), matched unrelated (from a donor pool), or mismatched. Stem cells can be collected from bone marrow, peripheral blood, or the placenta and umbilical cord blood obtained after birth. Cord blood stem cells show neonatal naiveté and have less risk of viral transmission and graft versus host disease (GVHD) when compared to other sources of stem cells. Rate of engraftment following transplantation for all types of stem cells is dependent on a number of factors such as cell dose, the conditioning regimen, the recipient’s disease, and posttransplant infection. Peripheral blood stem cells engraft faster and are associated with a decreased risk of tumor contamination when compared to autologous bone marrow.
INCIDENCE
1. Survival rates following transplantation are 60% to 90%.
2. Of children under the age of 10 years, 10% develop GVHD between the second and tenth week after HSCT.
3. Among older children, 30% to 60% develop GVHD.
4. More than 70% of children lack a human lymphocyte antigen (HLA)–matched sibling.
5. Children transplanted early in their disease for chronic myelogenous leukemia have disease-free survival rates between 70% and 86%.
6. Survival rates for individuals with acute leukemia following transplantation are 30% to 60%.
7. HSCT remains the most successful treatment for children with acute myelogenous leukemia (AML) in second remission; disease-free survival is 40% in allogeneic HSCT, and 35% for autologous.
8. Patients with AML or acute lymphocytic leukemia (ALL) who receive HSCT while in remission have relapse rates of 5% to 30%. Those who receive an HSCT during a more advanced stage of disease have relapse rates of 40% to 80%.
CLINICAL MANIFESTATIONS
Clinical manifestations experienced by children during the transplant process are commonly related to the toxicities of the preparative regimen (chemotherapy with or without radiation), infection, and the occurrence of GVHD in the posttransplant period:
2. Respiratory infections, pulmonary edema
3. Hepatomegaly, venoocclusive disease, ascites
5. Renal insufficiency (increased or decreased fluid volume)
COMPLICATIONS
1. Bone marrow suppression: Approximately 7 to 10 days following the preparative regimen, all cell lines will be eradicated, causing profound neutopenia, anemia, and thrombocytopenia. During this very vulnerable period, children are at risk for (a) infection (due to immunosuppression and low or absent bacteria fighting ability); (b) adverse effects of anemia such as lethargy, dizziness, headaches, and pallor; and (c) bleeding due to low platelet count and reduced clotting ability. Supportive care to minimize the risks of these complications until engraftment includes (a) the administration of colony-stimulating factors (G-CSF, GM-CSF) starting immediately after transplant to stimulate recovery of neutrophil count and engraftment; (b) administration of packed red blood cells and platelets to correct anemia; and (c) thrombocytopenia and/or bleeding.
2. Infection: Infection is a leading cause of morbidity and mortality among children undergoing HSCT. They are at increased risk for bacterial, viral, and fungal infections owing to their immunocompromised state. In the first 30 days posttransplant, the most common infections are gram-negative bacteremia and herpes simplex infections; from 30 to 100 days, children are at most risk for fungal infections such as Aspergillus and Candida, cytomegalovirus, and Pneumocystis jiroveci; following the 100-day phase and up to 1 year after HSCT, the most prevalent infections are viral infections including varicella zoster (shingles) and herpes infections. Prophylactic antiviral therapy to prevent reactivation of viruses is an essential part of the supportive care plan. Aggressive and appropriate antibiotic therapy is instituted with the onset of fevers, and early addition of antifungal therapy for unresolved fever in a posttransplant patient receiving antibiotic therapy is recommended to decrease the mortality rate due to sepsis. Common indicators of infection include fever, change in vital signs from baseline, diaphoresis, irritability, pain and/or erythema at affected site, behavioral changes, pain on urination, cough, and new onset of drainage from any site.
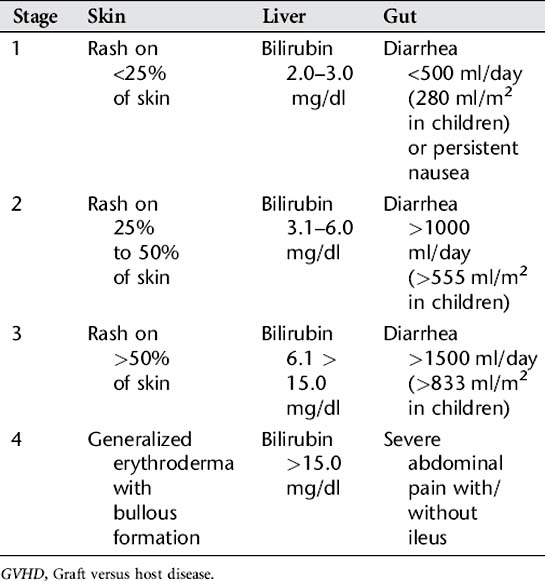
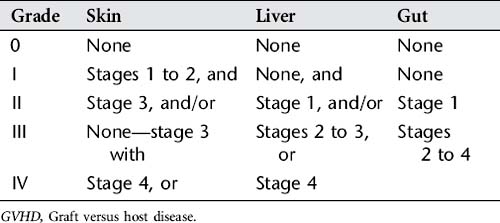
3. GVHD: This is one of the most serious complications of allogeneic HSCT. GVHD occurs when transplanted T lymphocytes from the donor’s bone marrow react against the immunocompromised host tissues. GVHD is classified as acute if it develops in the first 100 days following a stem cell transplant. Chronic GVHD may occur following the acute phase and up to 1 year after the transplantation. Severity of manifestations vary once vital organs are involved, and death can occur. Signs and symptoms of acute GVHD appear in the following order from the mildest (stage 1) to the most severe (stage 4) (Table 78-1):
Acute GVHD is staged by the degree of organ involvement (skin, gut, and liver), and these stages are summed into an overall grade (Table 78-2). This system is used by health care providers as a universal measurement of GVHD for diagnosis, management, and evaluation of treatment for this disease.
4. Gastrointestinal: Stomatitis and esophagitis secondary to chemotherapy and radiation appears with oral lesions and mucosal breakdown, dysphagia, and sloughing of the mucosal lining of the gut. This occurs immediately after transplant following chemotherapy and usually resolves with engraftment, with a recovering white blood cell count at 2 to 3 weeks after transplant. Changes in taste or sensation and dry mouth (hyposalivation) are all side effects of the preparative regimen. Antibacterial mouthwashes, topical analgesia, and systemic pain medications are essential to support the patient with these symptoms. Anorexia, nausea, and vomiting are common during the high-dose emetogenic chemotherapy. Antinausea medications are often given continuously or at scheduled intervals. Patients require enteral feeding or total parenteral feeding to provide nutrition during this period of mucositis to promote healing and maintain caloric intake. Diarrhea can occur also as a result of chemotherapy but also may be due to GVHD or infection.
5. Dental: Dental caries, enamel changes, and poor root development are common in patients after HSCT, especially if they received total body irradiation (TBI) or radiation to the mandible or head. Dental follow-up and care is an essential part of posttransplant care. Also, all patients must have a complete dental evaluation before HSCT to rule out any caries or infection, since this can be a source of abscess or infection, in the transplant phase.
6. Pulmonary: Pulmonary edema due to capillary leak syndrome may be experienced in the first 2 weeks after transplant. Cytomegalovirus (CMV) pneumonitis occurs because of reactivation of CMV approximately 2 to 4 months after HSCT. These patients experience low-grade fever, tachypnea, cough, and increased oxygen requirements owing to interstitial lung disease.
7. Renal insufficiency: Acute renal failure may develop as a result of damage to the epithelial cells of the lining of the renal tubules. This can be due to chemotherapy used in preparative regimen, antimicrobial use, and immunosuppressive medications. Blood chemistries including blood urea nitrogen (BUN) and creatinine are monitored carefully, and medications are renal-dosed to reduce the risk of further renal damage.
8. Genitourinary: Hemorrhagic cystitis sometimes develops in the posttransplant period. This may occur if cyclophosphamide or ifosfamide are used as preparative chemotherapy because, when excreted, these drugs irritate the bladder wall and cause bleeding. Viruses such as BK virus and adenovirus also can cause a similar picture. These patients require vigorous hydration to flush the bladder wall. Mesna may be given in cases of chemotherapy-related cystitis. Analgesia and antispasmodic medications are given to relieve the symptoms. If hematuria is severe, bladder irrigation may be needed. These symptoms can occur within 24 hours after transplant and continue for up to 2 months. Platelet transfusions are administered more frequently when there is evidence of bleeding.
9. Relapse: Relapse of the primary malignancy after HSCT is associated with poor prognosis, and limited treatment options remain available. Relapse is more common in patients with aggressive disease.
10. Endocrine dysfunction: Thyroid dysfunction can occur in patients who have received TBI. Patients show an increase in thyroid-stimulating hormone (TSH) and a decrease in thyroid hormone (T4), requiring thyroid replacement therapy. Growth and development delays are common in the growing child who receives TBI or steroid therapy, or who suffers from GVHD. Careful monitoring of growth patterns with each visit is essential, and early intervention with growth hormone replacement is essential to promote optimal development and prevent early closure of growth plates. Impaired fertility is a risk related to specific chemotherapy agents received in HSCT. Prepubertal patients are affected less than those treated during or after puberty, and girls are usually less affected than boys.
11. Secondary malignancies: Risk factors for the development of secondary tumors include preoperative combination chemotherapy, TBI, viral infections, chronic GVHD, and genetic cancer predisposition. The most common secondary malignancies are leukemia and lymphoma.
12. Posttransplant lymphoproliferative disease (PTLD) occurs in approximately 1% of posttransplant patients owing to Epstein-Barr virus (EBV) causing a lymphoma in an immunocompromised host. Children or adolescents with normal immune systems may have EBV but have immunity to keep it under control. Prognosis to date for PTLD has been very poor; however, emerging therapies such as alpha interferon and rituximab (monoclonal antibodies) and modifications to immunosuppression are making an impact on this disease.
LABORATORY AND DIAGNOSTIC TESTS
Refer to Appendix D for normal values and ranges of laboratory and diagnostic tests.
1. HLA typing—to identify compatibility of potential donors
2. Mixed lymphocyte culture analyses—to determine histoincompatibility between HLAs of potential donor and recipient
3. Microlymphocytotoxicity test—to identify HLAs
4. Type and cross-match—to determine if the donor is ABO-compatible with the recipient
5. Complete blood count, differential, and platelet count—to assess for adequate blood cell counts before harvesting
6. Chemistry panel, levels of electrolytes, creatinine, magnesium—to monitor renal function and to assess for any electrolyte imbalances
7. Liver function tests, hepatitis screen (A, B, C and E )—to assess liver function
8. Coagulation tests—to determine clotting ability and risk for bleeding
9. Immunoglobulins: IgG, IgA, IgM, and IgE—to assess immune functioning
10. Viral testing: cytomegalovirus, herpes simplex virus, varicella-zoster virus, Epstein-Barr virus, human immunodeficiency virus, toxoplasmosis—to determine presence of viral infection
11. Urinalysis and culture—to determine presence of infection or bleeding
12. Endocrine function testing—to assess for post-HSCT endocrine dysfunction
13. Pregnancy test for all adolescent females before preconditioning therapy
14. CT chest, abdomen, pelvis, and sinuses, chest x-ray—to rule out infection
15. Cardiac evaluation: electrocardiogram, echocardiogram, multiple-gated acquisition—to assess cardiac function before HSCT and to provide baseline to monitor for post-HSCT complications
16. Pulmonary function tests—to assess for post-HSCT pulmonary complications
17. Glomerular filtration rate, renogram—to determine the presence of renal insufficiency before and after HSCT
18. Disease staging: X-ray, computed tomography (CT), magnetic resonance imaging (MRI), bone scan, surgical biopsy, lumbar puncture, or bone marrow evaluation dependent on disease—to determine the extent of the disease
19. Neuropsychologic or developmental evaluation—to determine HSCT effects upon neuropsychologic and developmental functioning
20. Interdisciplinary evaluations—to assess and determine the need for ancillary services such as audiology, dentistry, nutrition, ophthalmology, occupational therapy, physical therapy, child life services, social work, and pastoral care
21. Counseling regarding sperm banking and egg donation should be included in all pretransplant evaluations.
MEDICAL MANAGEMENT
There are three types of hematopoietic stem cell transplants: autologous, allogeneic, and syngeneic. Autologous transplantation (auto-HSCT) uses the child’s own bone marrow and is performed for treatment of malignant diseases. Auto-HSCT is used as a form of consolidation therapy when an initial remission is achieved, when there is progressive disease, or when a patient’s relapse is showing poor response to conventional therapy. In these patients, transplantation is used as “rescue therapy” so that higher bone marrow–ablative doses of chemotherapy may be administered in an effort to cure the disease. Allogeneic transplantation (allo-HSCT), the most frequent type of HSCT, uses bone marrow from someone other than the child. The donor of choice is first a sibling, followed by a matched unrelated donor (obtained through a national registry). Alternative donor matching options include mismatched related donors (including haplotype parent donors), and matched or mismatched unrelated donors. Syngeneic HSCT is used when stem cells are used from an identical twin. The chances of a sibling match are about 35%; parents and relatives have only a remote chance of being matches, so increasing numbers of children with no matched siblings are transplanted from a closely matched unrelated donor located through the national bone marrow and cord blood stem cell transplantation registries.
Critical to ensuring appropriate matching between donor and recipient is HLA typing. HLA matching is important to minimize the complication of GVHD. Rejection risk increases as the incompatibility between the donor and recipient increases. A majority of transplants are allogeneic, but methods to decrease graft rejection and GVHD have improved. HLAs are protein antigens on the surface of cells that are used for immune recognition. The HLA system is responsible for recognizing foreign tissues and activating an immune response. There are approximately 100 HLA antigens. The most important HLAs for matching tissue for HSCT are HLA-A, HLA-B, HLA-DR, and HLA-DQ antigens. The three most important genes to match for compatibility as a predictor of GVHD are HLA-A, HLA-B, and HLA-DR. HLAs may be typed by a blood test or buccal swabs.
Before the transplantation, a conditioning (ablative) regimen is followed. Conditioning destroys cancer cells to prevent relapse, suppresses the immune system to prevent rejection of the marrow, and enables engraftment of the infused stem cells to occur in the child’s marrow. In autologous transplants, the stem cells may be treated with tumor antibodies to eradicate any residual disease before the stem cells are infused. The conditioning regimen involves high-dose cytotoxic drugs with or without TBI. Common chemotherapeutic agents used in conditioning regimens include cyclophosphamide, busulfan, cytosine arabinoside, melphalan, thiotepa, carmustine, carboplatin, cisplatin, and etoposide. Once the conditioning is completed, the harvested marrow is infused into the child via a central venous access catheter. With auto-HSCT, stem cells are either harvested from the bone marrow of the donor’s iliac crest under general anesthesia or collected peripherally via a pheresis process, and frozen several weeks before the HSCT. With allo-HSCT, stem cells are harvested, processed, sometimes manipulated to remove red cells and/or T cells, and infused directly into the recipient within a 48- to 72-hour period. Between 12 and 56 days after the transplantation, engraftment occurs. This is dependent on stem cell dose and source, and the patient’s diagnosis, condition, and prior history of therapy. Engraftment is demonstrated by an absolute neutrophil count (ANC) greater than 500 for 3 consecutive days following discontinuation of colony-stimulating factors and a platelet count greater than 20,000/mm3 for 3 consecutive days without the transfusion of platelets.
The most serious complication of allogeneic HSCT is GVHD (see the Complications section of this chapter). Acute GVHD usually occurs within 30 days of the transplantation (the most critical posttransplantation period). Immunosuppressive agents such as cyclosporine, methotrexate, antithymocyte globulin, steroids, tacrolimus, sirolimus, daclizumab, mycophenolate mofetil (MMF), or azathioprine (Imuran) are used to prevent or treat GVHD and prevent graft rejection. Those who undergo HSCT for leukemia and experience the complications of GVHD (acute or chronic form) have less risk of experiencing relapse of their leukemia. This immune effect is called the graft versus leukemia effect, providing immunity from donor cells against their disease.
NURSING ASSESSMENT
1. Assess psychologic status before and after HSCT.
2. Assess for signs and symptoms of sepsis.
3. Assess for gastrointestinal complications of GVHD.
4. Assess for hepatic complications of GVHD (venoocclusive disease).
5. Assess for renal complications of GVHD.
6. Assess skin and mucous membranes for breakdown, infection, or GVHD.
7. Focus of assessments identified in items 2 through 5 is on monitoring for life-threatening effects.
NURSING DIAGNOSES
NURSING INTERVENTIONS
1. Answer parent’s questions and reinforce information about research on HSCT, alternative treatments, and transplant centers.
2. Answer child’s questions and reinforce information using age-appropriate terminology and explanations (see Appendixes B and F).
3. Provide orientation and reinforce information about HSCT routines, restrictions of personal items, isolation procedures and policies, and average length of hospital stay (see Appendix F).
4. Provide meticulous oral and dental care.
5. Provide meticulous skin care.
6. Monitor for signs and symptoms of infection (refer to Complications section).
7. Monitor hydration status, intake, and output.
8. Monitor side effects and complications of conditioning drug therapies (refer to Complications section).
9. Monitor for signs and symptoms of GVHD (refer to Complications section).
10. Monitor bone marrow infusion and assess for reactions (nausea, vomiting, fever, rigors, hypertension, tachycardia, tachypnea).
11. Assess pain status, and administer pharmacologic and nonpharmacologic therapies (see Appendix I).
12. Ensure adequate nutritional support.
13. Serve as service coordinator and communication liaison regarding treatment and care concerns.
14. Provide psychosocial support to child and family as indicated by psychologic assessment and ongoing needs (see Appendix F).
15. Refer to child and/or family mental health professional as needed for counseling to deal with the challenges of living in social isolation during HSCT process (see Appendix F).
16. Encourage child or youth’s expression of feelings using age-appropriate methods (see Appendix F).
17. Ensure continuity with child or youth’s lifestyle activities as condition permits (academics, peers, hobbies).
18. Use behavioral techniques (i.e., hypnosis, muscle relaxation, visual imagery) for chemotherapy-related symptoms of anxiety, nausea, and vomiting.
Discharge Planning and Home Care
At selected HSCT centers, early discharge with intensive clinic follow-up and home care is being implemented. Discharge criteria under these circumstances are the following: child must be afebrile and have an ANC of 500 polymorphonuclear cells/mm3 for up to 72 hours before discharge.
1. Instruct family and child about disease, long-term treatment, medication administration, symptom recognition, and complications.
2. Coordinate with school personnel. Children with autologous transplants return to school in 3 to 6 months; those with allogeneic transplants, in 9 to 12 months (see Appendix G).
3. Instruct about long-term effects.
4. Refer child and family to community resources based on individualized needs (mental health services, community support groups) (refer to Appendix G).
CLIENT OUTCOMES
Alcoser P, Rodgers C. Treatment strategies in childhood cancer. J Pediatr Nurs. 2003;18(2):103.
Benjamin DK, et al. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J. 2002;21(3):227.
Gonzales L, et al. Hematopoietic stem cell transplantation. In Baggot, et al, eds.: Nursing care of children and adolescents with cancer, ed 3, Philadelphia: WB Saunders, 2002.
Guinan EC, Krance RA, Lehman LE. Stem cell transplantation in pediatric oncology. Pizzo PA, Popock DG. Principles and practice of pediatric oncology, ed 5, Philadelphia: Lippincott, Williams & Wilkins, 2005.
Lennard AL, Jackson GH. Stem cell transplantation. West J Med. 2001;175(1):42.
Norville R, Monroe R, Forte K. Hematopoietic stem cell transplantation. Kline NE, ed. Essentials of pediatric oncology nursing: A core curriculum, ed 2, Glenview, IL: Association of Pediatric Oncology Nurses, 2004.
Ringden O, et al. Allogeneic hematapoietic stem cell transplantation for inherited disorders: Experience in a single center. Transplantation. 2006;81(5):718.
Senior K. Umbilical cord blood transplants as good as bone marrow? Lancet. 2001;357(9273):2031.