Transplantation
INTRODUCTION
1. Solid whole-organ transplantation has been one of the main events in the evolution of 20th and 21st-century medicine. A kidney transplant offers a quality of life unattainable by long-term dialysis, and the lack of long-term artificial support for end-stage disease of the liver, heart and lungs makes it likely that there will be a demand for organ transplantation into the foreseeable future.
2. Immunosuppressive agents that reduce or abolish graft rejection are vital to the success of organ transplantation. Ciclosporin, acting by calcineurin inhibition within T-cell lymphocytes, blocks progression of the cell cycle from G0 to G1 and the production of interleukin 2. In the early 1980s, when used alone or with steroids it improved the 2-year graft survival following kidney transplantation from 50% to 75–80%. Newer drugs include tacrolimus, rapamycin and mycophenolate mofetil. Other interesting molecules differing in their mode of action are leflunomide, brequinar, mizoribine, deoxyspergualin.
Brain-stem dead, heart-beating ‘cadavers’ – over 80% of solid-organ donors, usually providing multiple organs.
Non-heart-beating cadavers, providing suitable organs for kidney and increasingly for liver transplantation, occasionally lung transplants but not for hearts.
Living related donors, such as identical twins, siblings, parents, children, first-order cousins, providing excellent donor organs for kidney transplants; and, with appropriate techniques, segments of livers, pancreas and lung can be grafted.
Living unrelated donors: spouses, partners, friends, altruists and paid donors (the latter illegal in the UK).
4. The success of organ transplantation has resulted in a shortage of solid organs for transplantation. There are almost 8000 patients waiting in the UK and 70 000 in the USA. The shortfall results in part from cultural, religious, financial, legal and political conditions and varies in different countries. In addition, permission to remove organs after death is usually determined by prior consent of the donor or of the surviving relatives. In some countries, although not in the UK, potential donors are required to ‘opt out’ – that is, state while alive that they refuse to have organs removed – otherwise it is assumed that they permit it. In the UK ‘opt out’ legislation is currently being debated. Other discussions taking place include the practicalities and ethics of xenotransplantation (Greek: xenos = strange, foreign) – the use of animal organs – and cloning (using nuclear replacement technology) as a means of providing auto-transplantable tissues.
5. Brain-stem death must be established before organs are removed from a mechanically ventilated patient with irreversible cerebral destruction. If the plasma electrolytes or blood gases are abnormal, or if there is suspicion of drug intoxication, organ donation is not pursued. The diagnosis must be made by qualified medical practitioners independent of the transplant team, carrying out the examination individually on two separate occasions. The following signs must be absent:
 Spontaneous breathing when disconnected from the ventilator under hypercarbic conditions monitored by blood gases.
Spontaneous breathing when disconnected from the ventilator under hypercarbic conditions monitored by blood gases.
6. Tissue transplantation has been successfully practised for many years, including the cornea, which as a ‘privileged site’ evokes no rejection and requires no immunosuppression. Bone and skin are also transplanted, but essentially serve to provide a non-cellular matrix into which recipient cells can grow as the donor cells are destroyed. Immunosuppressive agents are again unnecessary.
THE MULTIPLE ORGAN DONOR
Appraise
Donor screening and exclusions
1. Exclude potential donors if there is any possibility of transmitting to potential recipients the following:
2. Scrutinize the social history of the donor as far as possible. Exclude prospective donors with a clear history of intravenous drug abuse, prostitution or homosexuality.
Prepare
1. The ventilated heart-beating brain-dead donor may be physiologically unstable, requiring inotropes to maintain systemic vascular resistance and other pharmacological agents such as pitressin or desmopressin (des-amino-des-aspartate-arginine vasopressin, DDAVP) to control diabetes insipidus. Carefully maintain fluid balance, so be willing to institute invasive monitoring such as Swan-Ganz catheterization and direct arterial pressure measurements. Pulse oximetry and cardiac monitoring are essential. Have available body-warming equipment to compensate for hypothermia.
2. Have an anaesthetist in attendance. A general anaesthetic is unnecessary, but give a neuromuscular blocking agents such as curare before making the first incision, to prevent muscular spasms and spinal reflexes, which may be induced by the surgical procedure.
3. Blood loss during the surgical procedure may profoundly destabilize the donor’s cardiovascular status and you must therefore take great care to minimize blood loss at all times. Have available 4–6 units of cross-matched bank blood for use during the surgical procedure.
4. The donor surgical team must be self-sufficient and provide surgical instruments, cannulae, sterile bags for the excised organs, ice, cooling fluids, preservation fluids and cardioplegic solution.
Access
1. Make a midline incision from the jugular notch to the symphysis pubis.
2. Split the sternum longitudinally with a Gigli saw. Leave the pericardium and pleural cavities intact if possible.
3. Retract the sternal edges with a self-retaining retractor after securing haemostasis by liberal application of bone wax.
Assess
1. Perform a detailed inspection of all abdominal contents to exclude unsuspected pathology, the incidence of which increases in proportion to age. Absolute contraindications to proceeding with organ retrieval are peritoneal contamination due to ruptured bowel and the presence of disseminated intra-peritoneal cancer. Cirrhosis of the donor liver precludes transplantation of that organ.
2. Focal abnormalities found in one or more sites in the abdomen or chest need not prohibit organ retrieval, but remove biopsy specimens of all suspicious lesions for histological examination. You must not re-implant retrieved organs before excluding malignancy histologically.
Action
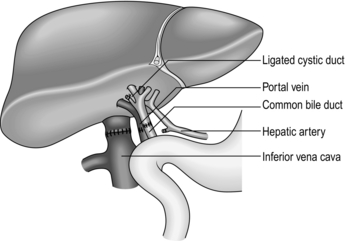
1. Commence careful dissection of the structures in the free edge of the lesser omentum leading to the porta hepatis. Ligate and divide the common bile duct just above the duodenum. Dissect and control the portal vein with a rubber sling. Carefully search for abnormalities of the hepatic arterial supply. Seventeen per cent of donors have an accessory or aberrant right hepatic artery passing to the porta hepatis posterior to the portal vein and common bile duct. Twenty-three per cent have an accessory left hepatic artery arising from the left gastric artery. Identify these variations early and preserve the vessels.1
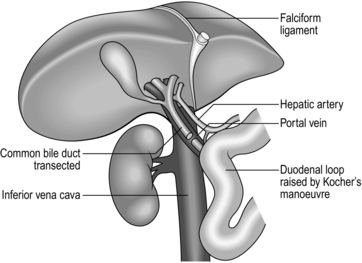
2. Mobilize the duodenum by Kocher’s manoeuvre. Isolate the inferior vena cava above the renal veins and below the liver, and control it with a nylon tape.
3. Divide the peritoneal attachments of the liver, starting with the left triangular ligament and proceeding to the falciform ligament, thus exposing the anterior surface of the suprahepatic vena cava (Fig. 26.1).
4. Divide the right triangular ligament and continue by dividing the upper and lower layers of the coronary ligament while progressively dislocating the liver upwards and to the left, thus separating the liver from the bare area of the diaphragm. Ligate and divide the right adrenal vein, which has now been exposed, and continue dissecting to free the retrohepatic vena cava from the posterior abdominal wall. Achieve complete mobilization of the vena cava by dividing the peritoneum of the right side of the lesser sac. Divide the remnant of the lesser omentum, leaving the liver attached by its blood vessels only.
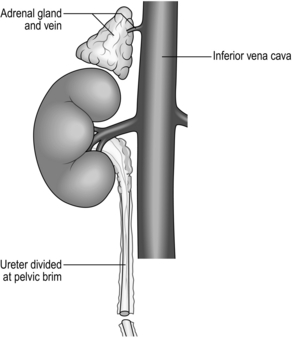
5. Begin mobilization of the right kidney by dissecting the peritoneum from its anterior surface and controlling the right renal vein and vena cava below the renal vein with rubber slings.
6. Gently lift the kidney from the renal fossa and sweep away surrounding fatty tissue to expose the renal artery or arteries and vein, and control them with further rubber slings.
7. Dissect the ureter with plenty of surrounding connecting tissue down as far as the pelvic rim and then divide it (Fig. 26.2).
8. Repeat the procedure for the left kidney. In addition, ligate and divide the left adrenal and gonadal veins. Also identify, ligate and divide the large lumbar vein opening into the posterior aspect of the left renal vein.
9. Expose and control the lower aorta just above the bifurcation, for subsequent cannulation and flush-cooling of the liver and kidneys.
10. Dissect and control the superior mesenteric vein below the transverse mesocolon for later cannulation of the portal vein and flush-cooling of the liver.
11. Mobilize the pancreas at this stage if it is required. Completely divide the gastrocolic and gastrosplenic omentum. Retract the stomach to expose the pancreas. Divide the lienorenal ligament and, using the spleen as a ‘handle’, carefully mobilize the tail and the body of the pancreas, working towards the midline. Dissect and control with a rubber sling the origin of the splenic artery at the coeliac axis.
12. The cardiac surgical team should now prepare the required thoracic organs for removal. When all the dissections have been completed give 30 000 IU of heparin intravenously. Place a cannula (size 16–20 F) in the portal vein via the superior mesenteric vein and place another in the infrarenal abdominal aorta. Place a third cannula in the infrarenal vena cava.
13. Stop mechanical ventilation. Stop circulation with cardioplegic solution infused through the aortic root. Start flush-cooling of abdominal organs via the portal and aortic cannulas using 3–6 L of University of Wisconsin solution (Viaspan) at 4 °C. Simultaneously exsanguinate the donor via the inferior vena caval cannula to ensure venous decompression and thus effective flush-cooling of the abdominal organs.
14. Following removal of the thoracic organs by the cardiac surgical team wait until the abdominal organs are visibly pale and palpably cold before removing them by dividing the remaining vascular connections. Follow the coeliac axis and the renal arteries back to the aorta. In each case excise a rim of aorta – a ‘Carrel patch’.
15. Remove all cannulas. Suck out all free fluid and make a careful watertight wound closure using continuous 0 or no. 1 nylon.
ORGAN PRESERVATION
1. Cool the organs in situ by infusing large volumes of isotonic cold crystalloid solution such as Viaspan, thus reducing the temperature of the perfused organs to 8–15 °C.
2. Excise the organs when they are visibly pale and palpably cold. Flush them through once more with approximately 1 L of a preservation solution (University of Wisconsin solution for the liver and Marshall’s hypertonic citrate solution for the kidneys) which will equilibrate throughout the extracellular space of the organ during the preservation period.
3. Double-wrap the organs in sterile bags and place them in boxes of ice where they will remain until re-implantation. Cooling continues to approximately 0 °C in ice over the next few hours.
4. Currently used preservation solutions are hypertonic, contain non-diffusible large anions and usually have a high potassium content corresponding with that of intracellular fluid. These solutions are more effective than physiological saline for cold preservation of organs because they prevent cell swelling and intracellular electrolyte loss during the hypothermic inactivation of the sodium pump.
5. Immediate life-supporting function can be expected from a kidney that has been preserved in one of these solutions for up to 24 hours. Between 24 and 72 hours viability will be preserved but, because of acute tubular necrosis, delayed function is likely.
6. Immediate life-supporting function is an absolute prerequisite for the transplanted liver or heart, thus reducing the safe preservation time dramatically. For the liver, University of Wisconsin solution is clearly the best preservation solution, allowing preservation time for up to 20 hours. For the heart, 4–6 hours is currently the safe limit.
7. Record on the forms provided the donor’s demographic details, blood group, anatomical abnormalities and time of circulatory arrest. Record the type and volume of preservation solution used. Arrange for samples of donor spleen and lymph node to accompany each organ, together with the copy of the data form, to its final destination.
TISSUE TYPING
1. Despite 30 years of clinical organ transplantation, the role of human leucocyte antigen (HLA) matching remains controversial and enigmatic. At least four different gene loci on chromosome 6 code for the human major histocompatibility complex (MHC) and are known as HLA A, B, C and D. Currently, antigens that are cell-surface gene products, usually glycoproteins, of the A B and D loci are routinely determined and matched for donor and recipients in renal transplantation.
2. For renal transplantation from closely related donors such as a parent or sibling, graft survival appears to be directly related to the degree of HLA matching, whereas in the unrelated cadaver donor situation, kidneys fully matched at the A, B and D loci (six antigens) enjoy outstandingly good survival, but any lesser degree of matching significantly prejudices survival.
3. In liver transplantation and heart transplantation no convincing relationship has yet been demonstrated between HLA matching and graft survival. Donors and recipients are usually paired on the basis of ABO blood group compatibility only.
KIDNEY TRANSPLANTATION
Appraise
1. Donor kidneys for transplantation may be obtained from:
 Living related donors – usually confined to those with close genetic links such as mothers and fathers, sisters and brothers and, occasionally, first-order cousins. The results of living related kidney transplantation have always been superior to those of cadaver kidney transplantation. In the UK, the Human Organ Transplant Act 1989 stipulates that for any proposed living related organ donation genetic relationship between the donor and the recipient must be established by DNA fingerprinting before the transplant can legally proceed.
Living related donors – usually confined to those with close genetic links such as mothers and fathers, sisters and brothers and, occasionally, first-order cousins. The results of living related kidney transplantation have always been superior to those of cadaver kidney transplantation. In the UK, the Human Organ Transplant Act 1989 stipulates that for any proposed living related organ donation genetic relationship between the donor and the recipient must be established by DNA fingerprinting before the transplant can legally proceed.
 Living unrelated donors – a small but increasing number of transplants have been performed between related, but not genetically linked, individuals such as husbands and wives and vice versa. Close friends may also donate. Before a living unrelated donation can legally take place in the UK, a dispensation from the Human Tissue Authority must be obtained, and is a statutory requirement.
Living unrelated donors – a small but increasing number of transplants have been performed between related, but not genetically linked, individuals such as husbands and wives and vice versa. Close friends may also donate. Before a living unrelated donation can legally take place in the UK, a dispensation from the Human Tissue Authority must be obtained, and is a statutory requirement.
 Unrelated brain-stem-dead heart-beating cadaver donors – organs are removed immediately following arrest of the circulation. Eighty per cent of all kidney transplants occurring in the UK are based on this type of donor.
Unrelated brain-stem-dead heart-beating cadaver donors – organs are removed immediately following arrest of the circulation. Eighty per cent of all kidney transplants occurring in the UK are based on this type of donor.
 Unrelated non-heart-beating cadaver donors – kidneys may be used from a donor following a cardiac standstill as long as the kidneys can be safely removed and cooled within 60 minutes of circulatory arrest. This type of donation is often associated with delayed initial function of the transplant kidney due to acute tubular necrosis.
Unrelated non-heart-beating cadaver donors – kidneys may be used from a donor following a cardiac standstill as long as the kidneys can be safely removed and cooled within 60 minutes of circulatory arrest. This type of donation is often associated with delayed initial function of the transplant kidney due to acute tubular necrosis.
2. Kidney transplantation currently offers the best chance of long-term survival combined with near-normal quality of life for those suffering from end-stage chronic renal disease.
3. The cost of a well-functioning kidney graft, or any other organ graft, is lifelong treatment with non-specific immunosuppressive agents. Ultimately, the main threat to long-term survival of the kidney graft recipient is likely to be the complications of long-term immunosuppressive therapy in the form of infections and an increased incidence of neoplasia. In addition, hypertension and accelerated cardiovascular disease are commonly seen. After 5 years, recipient death with a functioning graft is the commonest cause of graft loss.
4. End-stage renal disease of all types comprises the indications for renal transplantation. Chronic glomerulonephritis accounts for 60% of all cases of chronic renal failure. Diabetic nephropathy, refluxing pyelonephritis, polycystic disease and previously failed kidney grafts are the other main indications for transplantation.
THE OPEN APPROACH
Prepare
1. Perform HLA tissue typing and ABO blood grouping. Ensure compatibility with the recipient. Screen for hepatitis B and C and HIV. Demonstrate proof of genetic relationship between donor and recipient by DNA fingerprinting (restriction fragment length polymorphism).
2. Perform diethylenetriamine pentaacetic acid (DTPA) scan to ensure that each of the potential donor’s kidneys contributes roughly 50% to total renal function. Gross functional asymmetry between the two kidneys will prohibit donation.
3. Perform an aortogram with selective views of both renal arteries, or magnetic resonance angiography. A kidney with a single renal artery is desirable. Multiple renal arteries supplying both kidneys prohibit kidney donation.
4. Ensure that kidney preservation solutions, ice, organ bags, and cannulae are available for the operative procedure.
Access
1. Place the anaesthetized donor on the operating table in the lateral position.
2. Make a 20-cm muscle-cutting loin incision over the 12th rib. Gain access to the retroperitoneal space through the bed of the 12th rib. Take care to avoid the pleura, which is attached to the medial half of the upper border of the 12th rib. Sweep the peritoneum forward, keeping it intact.
Action
1. Carefully dissect the kidney free of its perirenal fat and the adrenal gland and gently draw it up into the wound to facilitate dissection of the renal pedicles.
2. Dissect both renal vein and renal artery and control each with a soft rubber sling. On the left side divide and ligate the gonadal vein and the adrenal vein. Look for a large lumbar vein entering the posterior aspect of the renal vein. Ligate and divide it. Mobilize the renal vein fully to its confluence with the inferior vena cava.
3. Dissect the renal artery to the aorta. On the right side this requires elevation and retraction of the inferior vena cava and necessitates ligation and division of one or more lumbar veins.
4. Dissect the ureter to the pelvic brim. Ligate it distally at this point and divide it.
5. When attached by its blood vessels only, confirm that the kidney is undergoing diuresis. Give 30 g of mannitol intravenously. Apply vascular clamps to the renal vessels and rapidly excise the kidney. Take it to a side trolley and rapidly flush-cool via the renal artery using a kidney preservation solution at 4 °C. Continue perfusion of the kidney until the effluent from the renal vein is clear and the kidney is palpably cold.
6. Double-wrap the kidney in sterile polythene bags and place it in a bag of ice until the time for reimplantation.
7. Carefully ligate the cut donor renal vessels and close the loin incision in layers, inserting a silicone tube drain.
TRANSPLANTATION OF THE DONOR KIDNEY
Appraise
1. Eighty per cent of renal transplants performed in the UK use unrelated ‘cadaver’ donor kidneys. Less than 20% of kidney transplants are donated by a close living relative.
2. ‘Cadaver’ kidneys are exchanged between transplant centres on the basis of clinical need and tissue matching.
3. Organ exchange and transport are arranged by a central distribution agency, the UK Blood and Transplant Authority.
4. Organs are transported in boxes of crushed ice by special courier.
Prepare
1. Before commencing the recipient operation carefully check the information sheet accompanying the donor kidney, paying particular attention to ABO blood group compatibility and any anatomical abnormality recorded.
2. Ensure that an immunological cross-match is negative between recipient serum and donor lymphocytes from the spleen and lymph node samples accompanying the kidney. A positive cross-match absolutely prohibits transplantation as it demonstrates preformed antibodies in the recipient’s serum that are capable of killing donor lymphocytes. If the transplant were to proceed in the face of a positive cross-match, the kidney would be hyper-acutely rejected.
3. Remove the kidney from its box of ice and check its anatomical details. Some dissection of excess donor tissues may be necessary. Perform this on a sterile trolley. Ensure that you have an adequate source of light and an assistant. When the dissection is completed, replace the kidney in sterile bags and pack it away once again in the box of ice.
KIDNEY TRANSPLANT OPERATION
Appraise
1. The donor kidney is re-implanted into the right or left iliac fossa and vascularized from the iliac vessels. This is an example of heterotopic (Greek: heteros = other + topos = a place) transplantation, as the kidney is not re-implanted into its normal anatomical—orthotopic (Greek: orthos = straight, right)—position.
2. The reasons why this heterotopic position is most appropriate are:
Access
1. Place the patient on the operating table in the supine position.
2. Pass a urinary catheter and attach it to a 1-L bag of saline via an infusion line.
3. Make a ‘hockey-stick’ incision starting 1 cm above the symphysis pubis and curving laterally to the pararectal line. Proceed vertically up the pararectal line to just above the level of the umbilicus. Incise the abdominal wall along the pararectal line and make an extraperitoneal approach to the external iliac vessels. Ligate and divide the inferior epigastric vessels. Control the spermatic cord with a nylon tape.
4. Insert a self-retaining ring retractor, such as Denis Browne’s, and adjust it to provide full access to the operative field.
Action
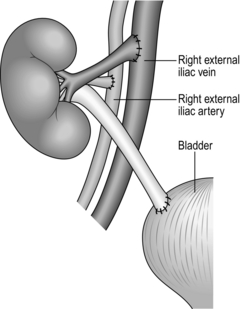
1. Mobilize both the external iliac artery and vein and control them with nylon tapes. Carefully ligate and seal the perivascular lymphatic vessels with diathermy.
2. Remove the prepared donor kidney from ice.
3. Perform an end-to-side anastomosis between the renal vein and the external iliac vein using continuous 5/0 polypropylene or PDS (polydioxane sulphate).
4. Perform an end-to-side anastomosis between the donor renal artery and the external iliac artery using similar suture materials.
5. Remove the clamps from the iliac vessels, thus perfusing the graft and secure haemostasis.
6. Fill the bladder with physiological saline from the previously attached infusion line to distend it and help identify the bladder in the pelvis.
7. Spatulate the end of the ureter, pass below the spermatic cord and perform an anastomosis to the dome of the bladder using continuous 4/0 synthetic absorbable suture such as PDS. Fashion a submucosal tunnel by incising the bladder muscle down to the mucosa over a 2-cm distance in line with the ureter. Lay the distal ureter in the groove created and close the bladder muscle loosely over the top of the ureter using interrupted absorbable sutures. Test the anastomosis by refilling the bladder with saline (Fig. 26.3).
8. Take a renal biopsy before closing the wound in layers over a large silicone tube drain.
Postoperative
1. Leave the urethral catheter in situ for a minimum of 5 days.
2. Ensure meticulous fluid replacement to maintain a urine output of more than 100 ml/hour.
3. Perform daily full blood count, blood urea, electrolyte and creatinine estimations.
4. Record the patient’s weight and fluid balance accurately.
Complications
1. Delayed function of cadaver grafts occurs in up to 40%, due to acute tubular necrosis. Acute tubular necrosis results from ischaemic injury to the kidney graft, which may occur in the donor before removal, during the preservation period, if it is excessively long, or during the implantation period, if this is excessively long. Acute tubular necrosis is confirmed on DTPA scan and renal biopsy and usually resolves within 6 weeks.
2. Vascular thrombosis may occur rarely (approximately 5%), leading to infarction and loss of the graft, which must then be removed. A transplant nephrectomy requires removal of all donor tissue, reconstitution of iliac vessels by vein patch if necessary and oversewing of the bladder.
3. Urinary leakage may occur as a result of terminal ischaemic necrosis of the donor ureter. Operative intervention is almost always necessary. Reimplant the ureter into the dome of the bladder or perform uretero-ureterostomy to the recipient’s own ureter.
4. Acute cellular rejection is seen in up to 80% of cases within 1 week of transplantation. Clinically there is oliguria, pyrexia and a rising creatinine and blood urea. You should obtain histological confirmation by percutaneous biopsy. Give antirejection therapy, usually high-dose steroids, to suppress the immune response. In a small minority of cases rejection is uncontrollable and results in infarction of the graft leading to the need to remove it.
LIVER TRANSPLANTATION
Appraise
1. More than 200 000 liver transplants have been performed in over 250 liver transplant centres worldwide since Starzl reported the first liver graft in 1963. The introduction of ciclosporin in 1979 was associated with a significant improvement in both short-term and long-term survival following liver grafting. The longest surviving recipient of a liver graft is now more than 43 years post-transplant.
2. Auxiliary heterotopic transplantation of the liver for non-malignant cases of liver disease has had its proponents from time to time. The problems of creating sufficient space for an extra liver, or part of a liver, kinking of the vascular anastomoses and the development of malignancy in the recipient’s own liver have precluded its widespread adoption. Auxiliary partial orthotopic liver transplantation, described by Gubernatis,2 is an interesting technical variant where a donor right or left lobar graft is implanted orthotopically following resection of the same lobe of the recipient’s own acutely failing liver. The expectation here is that full recovery of the recipient’s native liver will occur so that the grafted lobe may subsequently be removed or abandoned and immunosuppression withdrawn. This expectation is not always achieved.
3. Orthotopic liver transplantation as originally described and practised required the removal of the recipient’s own liver together with the retrohepatic inferior vena cava between the renal veins and the diaphragm. For very small recipients, usually children, transplantation of part of a larger liver, obtained either by splitting a cadaver graft or by left hemihepatectomy of a living related donor, requires the recipient’s own retrohepatic vena cava to be left in situ. For children, the partial liver graft usually consists of segments 2 and 3 and possibly 4 of the donor organ. For adults, a partial graft consisting of segments of 5, 6, 7 and 8, whether obtained from a ‘split’ cadaver graft or from a living related donor, usually provides sufficient functional liver cell mass. A graft weight to recipient body weight ratio of 0.8–1% is essential to immediately sustain life. Alternatively, using volumetry based on three-dimensional computed tomography (CT) or magnetic resonance imaging (MRI), the living donor graft should be 40% of the recipient’s calculated standard liver volume. Leaving the recipient’s own vena cava in situ also allows the option of a single cavocaval side-to-side anastomosis (the so-called piggy-back technique). Several large series of living related donor liver transplants have recently been reported demonstrating excellent outcomes for both adult and paediatric recipients but also showing significant early morbidity for both donor and recipient. Biliary tract complications currently occur in up to 36% of recipients and 20% of donors. Donor mortality appears to be just under 1% at present, an unacceptably high figure in the view of many surgeons. I shall describe the original orthotopic liver transplant procedure.
4. In 70% of cases transplantation is for end-stage chronic liver disease due to cirrhosis arising from a variety of different causes such as primary biliary cirrhosis, primary sclerosing cholangitis, post-hepatitic cirrhosis, autoimmune chronic active hepatitis and alcohol-related liver disease. In approximately 12% it is for primary hepatic malignancy and in another 10% for acute liver failure resulting from fulminant hepatitis, acute (drug) poisoning or idiosyncratic drug reactions. Metabolic diseases account for 6% of cases, including:
 Where the liver is itself a target organ of the metabolic abnormality such as Wilson’s disease, α1-antitrypsin deficiency and tyrosinosis
Where the liver is itself a target organ of the metabolic abnormality such as Wilson’s disease, α1-antitrypsin deficiency and tyrosinosis
 Where a hepatic enzyme defect leads to damage and failure of other organs such as primary hyperoxaluria, familial hypercholesterolaemia and some forms of familial amyloidosis. Here, liver transplantation is used as a highly effective form of gene therapy.
Where a hepatic enzyme defect leads to damage and failure of other organs such as primary hyperoxaluria, familial hypercholesterolaemia and some forms of familial amyloidosis. Here, liver transplantation is used as a highly effective form of gene therapy.
Prepare
1. Carry out a full biochemical, haematological, bacteriological and virological screen including hepatitis viruses A-G. Replicating HBV must be suppressed by antiviral treatment with lamuvidine or adefovir to prevent post-transplant recurrence in the graft, which may cause its rapid destruction. Similarly, HCV may be treated to suppress or eliminate it preoperatively with interferon and ribavirin, if time and liver function permit.
2. Magnetic resonance angiography and cholangiography are required to assess the vasculature and biliary tract of the recipient liver. An occluded portal vein may be a relative contraindication to transplantation, although transplantation may still be possible if the splenic vein or the superior mesenteric vein is patent and accessible.
3. Perform lung function tests and carefully evaluate cardiac function. Measure pulmonary artery pressure and perform cardiac output studies followed by a detailed anaesthetic assessment.
4. Assess the neuropsychiatric state, including CT scans of the brain.
5. Control ascites, oesophageal varices and encephalopathy by maximizing medical treatment.
6. Immediately before operation have 20 units of blood, fresh-frozen plasma and platelets cross-matched. Give prophylactic broad-spectrum antibiotics at the induction of anaesthesia.
7. Inspect the donor liver graft before starting the recipient operation.
Resect
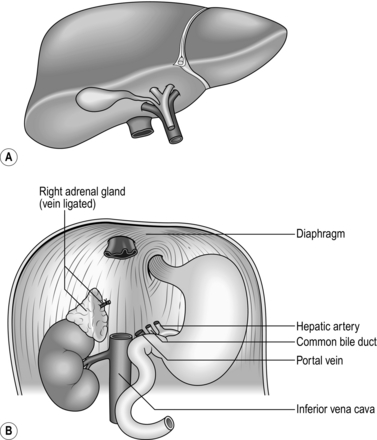
1. Dissect the structures in the free edge of the lesser omentum leading to the porta hepatis.
2. Ligate and divide the common bile duct as close as possible to the porta hepatis.
3. Dissect and control the common hepatic artery and portal vein with rubber slings.
4. Dissect the infrahepatic vena cava above the renal veins and control it with a nylon tape.
5. Divide the peritoneal attachments of the liver, starting with the left triangular ligament and proceeding to the falciform ligament, thus exposing the anterior surface of the suprahepatic vena cava.
6. Progressively dislocate the liver from the hepatic fossa while dissecting the liver from the bare area of the diaphragm.
7. Completely mobilize the retrohepatic vena cava from the posterior abdominal wall, ligating and dividing the right adrenal vein.
8. Apply vascular clamps to the vascular connections of the liver and excise it, maximizing the length of vessels for the subsequent reimplantation of the new graft (Fig. 26.4).
9. In recipients with borderline renal or cardiac function institute venovenous bypass during the anhepatic period, inserting percutaneously placed cannulas in the left femoral vein and left internal jugular vein. Flows of up to 3 L/minute are observed, which support cardiac output and effective renal perfusion during the anhepatic period.
Replace
1. Remove the new liver from ice.
2. Begin the reimplantation with the suprahepatic vena caval anastomosis using a continuous 2/0 polypropylene (Prolene), polydioxanone (PDS) or polyester (Merseline) suture. Follow this with the infrahepatic vena caval anastomosis, using a continuous 3/0 polypropylene suture. Before completing the infrahepatic anastomosis insert a 16 F flexible cannula into the retrohepatic vena cava for subsequent flushing of the liver.
3. Anastomose the donor and recipient portal veins end-to-end using 5/0 polypropylene. Before completing this anastomosis flush out the graft via the portal vein with 500 ml of 5% human albumin solution at room temperature. This removes air and preservation fluid from the liver, which escapes through the retrohepatic caval vent.
4. Complete the anastomoses of the portal vein and infrahepatic vena cava and remove clamps from the infrahepatic vena cava, suprahepatic vena cava and portal vein in that order, thus perfusing the new graft with portal-venous blood (Fig. 26.5).
5. Reconstruct the hepatic arterial supply as an end-to-end anastomosis between the donor and recipient common hepatic arteries using interrupted 6/0 polypropylene.
6. Reconstruct the biliary tract as an end-to-end, duct-to-duct anastomosis using interrupted 5/0 PDS. Splinting is not necessary. Use a 50-cm Roux loop of upper jejunum if there is no recipient common bile duct, as in biliary atresia or primary sclerosing cholangitis.
7. Perform a donor cholecystectomy and a liver biopsy.
8. Check thoroughly for haemostasis, inspecting all anastomoses and placing extra sutures when necessary, and then close the abdomen in layers, inserting two large silicone tube drains.
Postoperative
1. Transfer the patient to the intensive care unit.
2. Continue ventilation until the patient’s cardiovascular condition is stable.
3. Carefully monitor temperature, blood pressure, pulse, central venous pressure (CVP), ECG, pulmonary artery wedge pressure, cardiac output and systemic vascular resistance.
4. Monitor haemoglobin, haematocrit (maintaining at 30%), potassium, calcium and magnesium, blood gases, blood lactate and pH.
5. Measure and replace fluid losses, including urine output, abdominal drainage and nasogastric losses.
6. Begin immunosuppressive therapy immediately.
7. Cover with broad-spectrum antibiotics for at least 48 hours.
8. Check liver function tests and coagulation profiles twice daily.
Complications
1. Bleeding may occur within the first 24 hours resulting from vasodilatation as the patient warms up. Bleeding may also occur as a result of poor synthesis of clotting factors by the new graft. Correct any coagulation abnormalities with fresh-frozen plasma, platelets and cryoprecipitate as necessary. If bleeding continues, intra-abdominal haematoma may accumulate sufficiently to cause tamponade, resulting in hypotension and anuria. Re-explore the abdomen to evacuate haematoma and improve haemostasis as much as possible. The retrohepatic area, particularly in the region of the right adrenal, is a common site for postoperative bleeding.
2. A right pleural effusion develops in nearly all patients during the first postoperative week. Treat it expectantly unless it rapidly increases in size or causes compression or collapse of the underlying lung. In this case aspirate it or insert an underwater-seal drain.
3. Vascular occlusion is a rare (5–10%) but serious complication, particularly in the first few postoperative weeks. Hepatic arterial thrombosis may lead to extensive patchy infarction of the graft or acute liver failure, requiring urgent re-transplantation. Portal vein thrombosis may be clinically silent. Hepatic arterial thrombectomy or thrombolysis is rarely of benefit, but portal vein thrombectomy is frequently effective.
4. Diagnose acute rejection on biopsy in up to 80% of grafts within the first postoperative week. Treat it by increasing immunosuppression, for example giving 1 g of methylprednisolone (Solu-Medrone) intravenously once daily for 3 days. Repeat the biopsy to assess the response.
5. Send samples of urine, saliva, faeces, blood and appropriate skin swabs every 48 hours for bacteriological and virological surveillance.
6. Perform a HIDA (hepatobiliary imidodiacetic acid) scan 10–14 days postoperatively to assess the integrity of the biliary tract. Proceed to ERCP (endoscopic retrograde cholangiopancreatography) or transhepatic percutaneous cholangiography if the HIDA scan is equivocal. If there is a small leak at the anastomosis it may heal following careful stenting or nasobiliary drainage inserted endoscopically. Large biliary leaks are usually associated with a substantial infrahepatic bile collection and may signify donor duct necrosis. Reconstructive surgery using a Roux loop is usually the best approach. For an obstruction of the anastomosis, once again temporary stenting may suffice if it is possible to pass a stent across the obstruction; otherwise proceed to reconstructive surgery, performing a choledochojejunostomy using a 50-cm Roux loop.
Results
1. An 85% 1-year patient survival and 65% 5-year patient survival are currently being achieved by most major liver transplant centres. A recurrence rate of about 65% at 5 years for those transplanted for malignant disease of the liver underscores the poor long-term prognosis of transplantation for malignancy, but still compares well with resectional surgery for primary liver cancer.
2. Chronic rejection of the graft occurs in up to 10% of liver transplant recipients, appearing histologically as progressive dropout of liver parenchymal cells and loss of portal tract bile ducts. These changes are due to a progressive obliterative arteriopathy, where the lumina of the major arteries of the graft become progressively obstructed by the accumulation of foamy macrophages of recipient origin. Re-transplantation is the only solution. The falling incidence of chronic rejection seen in recent years has been attributed to more effective immunosuppressive drugs and a more proactive approach to liver graft dysfunction through biopsy.
REFERENCES
1. Michels NA. The hepatic, cystic and retroduodenal arteries and their relations to the biliary ducts. Ann Surg 1951;133:503–23.
2. Gubernatis G, Pichlmayr R, Kemnitz J, et al. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg 1991;15:660–6.
PANCREAS TRANSPLANTATION
VASCULARIZED GRAFTS
Appraise
1. No single technique has been universally adopted in the 40 years since Kelly performed the first vascularized graft in 1966.1 The question of whether it is better to graft the whole pancreas or a segment comprising the body and the tail of the organ is still unresolved, but the balance of opinion now favours the whole-organ graft. Those who advocate the whole-organ preparation claim that it is beneficial to engraft as much of the donor endocrine mass as possible, thus minimizing islet exhaustion due to too small a graft, as in the segmental technique. Those advocating the simpler segmental graft claim that it is a lower-morbidity, lower-mortality procedure for the recipient. Other controversial areas include:
2. Despite these technical difficulties the results of pancreas transplantation have improved substantially over the last decade. In recent years, the most favoured technique is that of transplanting the whole pancreas of the donor into the left or right iliac fossa of the recipient, draining the pancreatic exocrine secretion directly into the bladder, as described by Sollinger et al.2 These authors also found that a fall in the urinary amylase output from the graft was an early marker of impending graft rejection, indicating the need for antirejection therapy. More recently, because of sometimes insurmountable bladder problems with frequency, dysuria and cystitis, many surgeons have reverted to pancreatic duct drainage into a Roux-en-Y loop of jejunum.
Achievements
1. A well-functioning pancreas graft exerts good control of blood glucose excursions without dietary restrictions and 24-hour blood glucose profiles are normal or near normal.
2. Glucose-tolerance curves show an abnormal pattern of insulin release leading to a slower return of blood glucose to basal levels.
3. Glucose-tolerance curves are similar whether the pancreatic graft venous effluent is drained into the systemic circulation or into the portal circulation, but plasma insulin levels are at least twice as high in those with a systemic venous drainage. Glycosylated haemoglobin values (HbA1C) are maintained within the normal range.
4. Evidence for pancreas transplantation altering the natural history of the secondary complications of diabetes is slowly emerging. Diabetic nephropathy in transplanted kidneys appears to be prevented by a well-functioning pancreas graft. There are also some reports of improved peripheral nerve conduction time following pancreas transplantation. In general, retinopathy is not objectively improved but many patients with diabetic eye problems have found an improvement in acuity.
Techniques
1. The artery and vein of the pancreas graft are anastomosed end-to-side to the common iliac artery and vein of the recipient.
2. For the whole-organ graft some ‘bench’ surgery is required at a separate sterile work table.
3. An interposition graft comprising the donor common iliac artery bifurcation is anastomosed to the splenic artery and the superior mesenteric artery of the graft so that only a single anastomosis to the recipient artery is required. In addition, a 6-cm length of donor duodenum is retained and anastomosed to either the dome of the bladder or a loop of jejunum to provide exocrine drainage. The graft is placed intra-peritoneally. In contrast, the segmental graft requires no bench procedure and is usually placed extraperitoneally. Exocrine drainage is into a Roux loop or obturated by duct injection with Neoprene.
Results
1. More than 20 000 pancreas grafts have now been reported worldwide; 94% of these have been associated with a kidney graft. In 80%, the pancreas is transplanted simultaneously with a kidney from the same donor. In 14%, a successful pancreas transplant is followed some months later by a kidney transplant, usually from a third-party donor. Only 6% of pancreas transplants are performed alone.
2. 1-year patient survival is currently 95%, with a graft survival of over 80%. The use of the new immunosuppressants, such as tacrolimus and mycophenolate mofetil, appears to have significantly improved outcome in recent years.
REFERENCES
1. Kelly WD, Lillehei RC, Merkel FK, et al. Allotransplantation of pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967;61:827–33.
2. Sollinger HW, Kalayoglu M, Hoffman RM, et al. Results of segmental and pancreaticosplenic transplantation with pancreaticocystostomy. Transplant Proc 1985;17:360–2.
SMALL-BOWEL TRANSPLANTATION
Appraise
1. Transplantation of the small bowel may be indicated for small numbers of adults and children who have suffered massive small-bowel loss and are unable to sustain body weight by either enteric feeding or total parental nutrition. In most countries the demand for small-bowel transplantation to date has been low. In small children, transplantation of the liver together with the small bowel has produced better results than transplantation of the small bowel alone.
2. Lillehei was the first to report the technical feasibility of small-bowel transplantation in the dog.1 Several attempts at human small-bowel transplantation followed, with extremely poor results. The introduction of ciclosporin A in 1978 revived interest in clinical small-bowel transplantation and this has been reinforced by the development of new agents such as tacrolimus and mycophenolate mofetil. Many major transplant centres now have modest series of small-bowel grafts, but graft survival longer than 3 years remains a rare event.
 Immunological rejection of the graft
Immunological rejection of the graft
 Septicaemia due to bacteriological translocation across the graft during rejection episodes
Septicaemia due to bacteriological translocation across the graft during rejection episodes
 Fluid and electrolyte losses from the graft
Fluid and electrolyte losses from the graft
 Graft-versus-host disease, which may be fatal
Graft-versus-host disease, which may be fatal
 Technical problems, including vascular thrombosis and torsion of the graft pedicle.
Technical problems, including vascular thrombosis and torsion of the graft pedicle.
3. Small-bowel grafts can be obtained from cadaveric donors or from living related donors. The potential of careful and close HLA matching in grafts taken from living related donors may prove critical for the long-term survival of the small-bowel graft.
4. Access to the recipient’s major abdominal blood vessels may be very difficult as candidates for small-bowel transplantation have invariably undergone numerous previous surgical procedures.
5. Implantation of the graft is straightforward, with the superior mesenteric artery of the graft anastomosed end-to-side to the recipient abdominal aorta and the vein anastomosed to the inferior vena cava, or the portal vein if available.
HEART TRANSPLANTATION
Appraise
1. Eight years after the first successful series of orthotopic heart transplants in dogs, carried out by Lower and Shumway,1 Barnard,2 using the same technique, performed the first human heart transplant in 1967. The wave of worldwide enthusiasm for human heart grafting that followed Barnard’s contribution was almost totally dissipated by 1970, when it had become clear that the development of a successful surgical technique had preceded the development of suitable immunological means and immunosuppressive agents to monitor and prevent graft rejection.
2. Heart transplantation continued in a very few centres over the following decade. The introduction in 1980 of ciclosporin A, a potent new non-steroidal immunosuppressant, sparked a second wave of worldwide enthusiasm, which has been sustained and has established heart transplantation as an extremely effective therapy for various types of end-stage heart disease. More than 50 000 transplants have now been performed.
Technique
1. Perform a median sternotomy.
2. Establish full cardiopulmonary bypass, placing cannulae in the superior and inferior vena cava transatrially and a return cannula in the ascending aorta.
3. Apply aortic and pulmonary artery clamps and excise the recipient’s heart across a transatrial plane just dorsal to the atrial appendages. Leave the posterior atrial wall with the orifices of the systemic and pulmonary veins intact. Divide the aorta and pulmonary trunk just distal to their respective valves.
4. Insert a cannula (12–14 F) in the left ventricle, through the apex of the myocardium, to act as a vent.
5. Implant the new heart, which consists of more of the donor atria in order to preserve the sinoatrial node.
6. Perform left and right atrial anastomoses, followed by aortic and pulmonary artery anastomoses, using continuous polypropylene sutures.
7. On completion of the anastomoses, release the aortic clamp and allow the heart to fill via the coronary circulation, displacing air through the left ventricular vent.
8. When all air is displaced, clamp and remove the vent, release all other clamps and snuggers and reduce bypass flow rate. If the heart does not restart spontaneously, apply a direct current (DC) shock if necessary.
9. Place temporary pacing wires in the donor right atrial wall and stop bypass. Remove cannulae.
10. Repair the pericardium and close the chest with pericardial drainage.
HEART–LUNG TRANSPLANTATION
The first reported human heart–lung transplant was performed by Cooley in 1968.1 The recipient survived only 4 hours after surgery. Further attempts were reported by Lillehei in 1969 and Barnard in 1971, with similar short-term survival.
Reitz, working at Stanford, California, performed the first heart–lung transplant under ciclosporin immunosuppression in 1981.2 This patient survived 5 years. Heart–lung transplantation is now a well-established therapy for certain cardiopulmonary disorders.
Indications
Donor and recipient matching
1. This is similar to that for heart transplantation. Size matching of the donor lungs to the recipient thoracic cage is very important, as over-inflation occurs if the lungs are relatively too small and inadequate ventilation occurs if the lungs are too large.
2. Donor organs are much more difficult to obtain than hearts alone. Many potential donors are precluded on account of pulmonary infection due to mechanical ventilation. Thus, in view of size matching and scarcity of suitable uninfected donors, a potential heart–lung recipient may need to wait a long time before transplantation.
Technique
2. Establish full cardiopulmonary bypass as described for heart transplantation.
3. Excise both lungs and heart of the recipient, separately if necessary.
4. Take care not to damage the phrenic nerves or the vagal trunks.
5. Leave the posterior wall of the right atrium intact and in continuity with the superior and inferior vena cavae.
6. Carefully insert the donor heart–lung block.
7. Anastomose right atrium, trachea and aorta in that order, using polypropylene sutures and venting the right and left ventricles as described for the heart graft.
8. On filling the heart by releasing the aortic clamp, the cardiac cycle usually starts spontaneously.
9. Remove vents and withdraw bypass.
10. Close the chest with appropriate pericardial and pleural drainage.
REFERENCES
1. Cooley DA, Bloodwell RD, Hallman GL, et al. Organ transplantation for advanced cardiopulmonary disease. Ann Thorac Surg 1969;8:30–46.
2. Reitz BA, Burton NA, Jamieson SW, et al. Heart and lung transplantation. Autotransplantation and allotransplantation in primates with extended survival. J Thorac Cardiovasc Surg 1980;80:360–71.
Advisory Group on the Ethics of Xenotransplantation. Animal tissues into humans: recommendations and report. London: Department of Health; 1996.
Bismuth, H., Houssin, D. Reduced size orthotopic liver transplantation in children. Surgery. 1984; 95:367–370.
Campbell, K.W.S., McWeir, J., Ritchie, W.A., et al. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996; 280:54–66.
Couinaud, C. Controlled hepatectomies and exposure of the intrahepatic bile ducts. Paris: Couinaud; 1981.
Lancet. Diagnosis of brain death (editorial). Lancet. 1976; ii:1069–1070.
Medawar, P.B. The behaviour and fate of skin autografts and skin homografts in rabbits. J Anat. 1944; 78:176–199.
National Institutes of Health Consensus Development Conference. Statement: liver transplantation 1983. Hepatology. 1984; 4(Suppl. 1):1075–1105.
Starzl, T.E. Experience in Hepatic Transplantation. Philadelphia: Saunders; 1969.
Strong, R.W., Lynch, S.V., Ong, T.H., et al. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990; 322:1505–1507.
Warnock, G.L., Kneteman, N.M., Ryan, E.A., et al. Long term follow-up after transplantation of insulin producing pancreatic islets into patients with type I (insulin dependent) diabetes mellitus. Diabetologia. 1992; 35:89–95.