17 Transcatheter Valve Therapies
Recent advances in percutaneous-based catheter technologies have allowed for development of novel therapies for valvular heart disease, including transcatheter valve implantation and transcatheter mitral valve repair (Table 17-1). Although these devices are still investigational in the United States, many have incorporated transcatheter valve procedures into their daily practice. This chapter briefly reviews two transcatheter valve procedures: transcatheter aortic valve replacement and percutaneous edge-to-edge mitral valve repair.
Table 17-1 Transcatheter Valve Therapies
| Transcatheter Valve Implantation |
| Percutaneous Mitral Valve Repair |
Transcatheter Aortic Valve Replacement (TAVR)
Indications
Aortic valve replacement (AVR) is indicated in all symptomatic patients with severe AS (2006 ACC/AHA Valvular Heart Disease Guidelines, Class I recommendation, Level of Evidence: B). Despite this recommendation, a significant number of elderly patients (up to one third) do not undergo AVR because of comorbidities, including advanced age and the associated increased morbidity and mortality with surgery. Additionally, many elderly patients that are suitable surgical candidates will refuse surgery because they perceive themselves as “too old” to survive open AVR. Transcatheter aortic valve replacement (TAVR) is a promising alternative to surgical AVR in those patients deemed high risk for surgery (Table 17-2).
STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement.
Transcatheter Valves
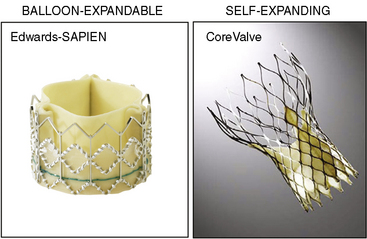
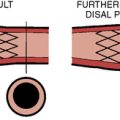
There are two catheter-implantable valves commercially available overseas. They are the balloon-expandable Edwards SAPIEN valve and the self-expanding Medtronic CoreValve (Fig. 17-1). The Edwards SAPIEN valve was studied in the PARTNER trial, which completed randomized enrollment in fall 2009. The Medtronic CoreValve randomized trial started enrollment in December 2010. The initial international experience and the results of PARTNER cohort A and B suggest that outcomes compare favorably with conventional valve surgery in selected patients. In November 2011, the Edwards SAPIEN valve was FDA approved and became commercially available in the United States for PARTNER cohort B (inoperable) patients.
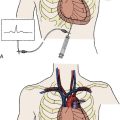
Transfemoral TAVR Procedure With Edwards SAPIEN valve
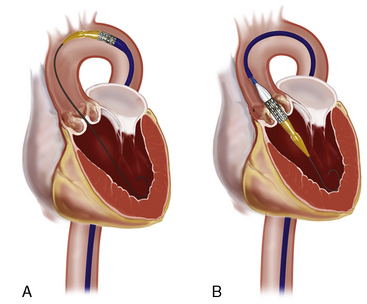
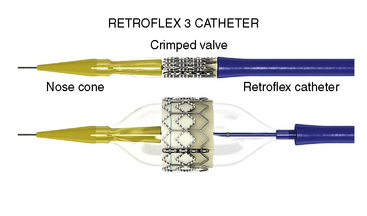
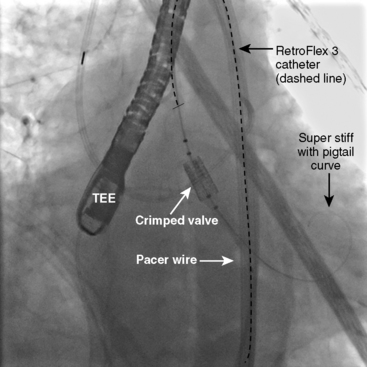
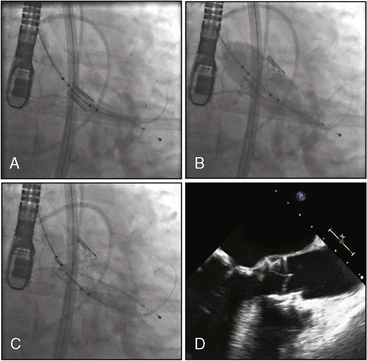
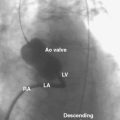
Next, the RetroFlex 3 delivery prosthetic valve system with the crimped SAPIEN valve is advanced through the delivery sheath over the stiff wire. The delivery system has a flexing mechanism that assists in steering through the aortic arch and minimizes trauma on the outer curvature of the arch (Fig. 17-2). When crossing the native aortic valve, the delivery system is fully retroflexed, which gives a central and coaxial orientation of the prosthesis to the native valve. The delivery catheter also has a nose cone, which facilitates crossing of the native valve (Fig. 17-3). After the valve has been crossed, the retroflex catheter is retracted to fully expose the delivery balloon. The valve is positioned using fluoroscopy, aortography, and TEE (Fig. 17-4). Approximately 60% of the valve assembly should be on the ventricular side of the aligned sinuses. Once positioning is confirmed, rapid pacing is performed and the delivery balloon is inflated when the blood pressure falls below 50 mm Hg (Fig. 17-5). The balloon is inflated for 5 seconds, then deflated. After stent valve deployment, aortography is repeated to assess for paravalvular leak, and if significant, repeat balloon inflations may be necessary. The double-lumen pigtail catheter is again placed and final LV-aortic gradients are measured.
TAVR, transcatheter aortic valve replacement
Percutaneous Mitral Valve Repair
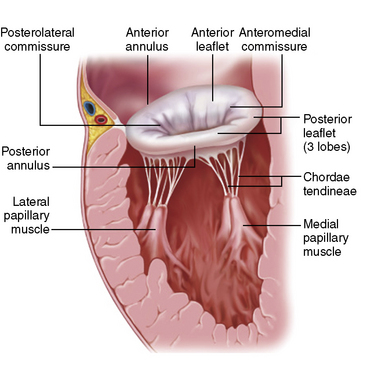
The mitral valve is composed of two leaflets (anterior and posterior), the mitral annulus, the chordae, and the papillary muscles (Fig. 17-6). Dysfunction of any of these structures can cause incompetence of the valve and result in regurgitation. Mitral regurgitation (MR) is the most common form of valvular heart disease, and about half of the cases are due to myxomatous degeneration of the valve, which leads to stretching of the valve leaflets and chordae tendineae. Elongation of these structures causes prolapse of the leaflets into the left atrium and failure of the leaflets to coapt properly. If left untreated, severe MR leads to LV failure due to chronic volume overload.
There are numerous transcatheter mitral valve repair strategies that have been developed over the recent years. These include leaflet repair, direct and indirect annuloplasty, and ventricular and annular remodeling devices (see Table 17-1).
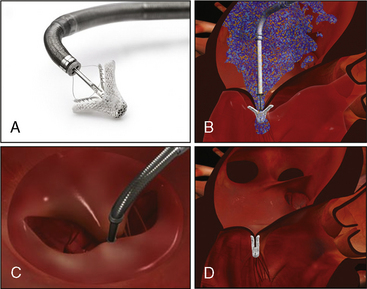
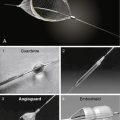
The MitraClip (Fig. 17-7) currently represents the most widely used percutaneous repair technique. In 1991, Dr. Alfieri performed a mitral valve repair without annuloplasty using a stitch “edge-to-edge” technique to create a double-orifice valve. The durability of this procedure (>1500 patients) led to the development of a percutaneous technique using a clip to approximate the valve leaflets instead of a suture. The MitraClip system has been evaluated in the EVEREST I and EVEREST II studies.
Indications
Patients with a class I indication for mitral valve surgery according to the AHA/ACC 2006 guidelines for valvular heart disease were eligible for the EVEREST studies (Table 17-4). Mitral valve surgery is indicated in all symptomatic patients with severe MR in the absence of severe LV dysfunction and/or LV end-systolic dimension >55 mm (Class I recommendation, Level of Evidence: B). In asymptomatic patients with severe MR, mitral valve surgery is indicated if LV dysfunction is present (Class I recommendation, Level of Evidence: B). Additionally, patients with severe MR and new onset of atrial fibrillation or pulmonary hypertension were also candidates.
EF, ejection fraction; LV, left ventricular; MI, myocardial infarction; NYHA, New York Heart Association.
Mitral Valve Anatomy
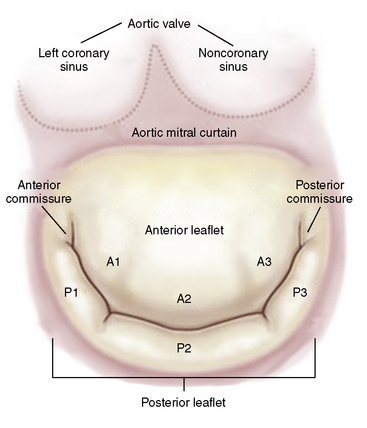
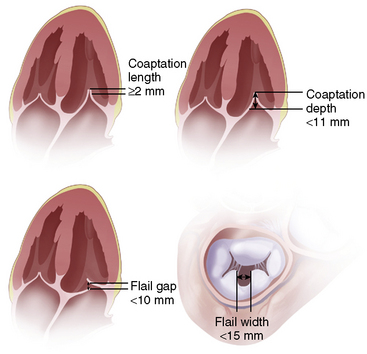
The mitral valve anatomy must have sufficient leaflet tissue amenable for mechanical coaptation with the MitraClip. A key anatomical inclusion criterion includes a regurgitant jet origin associated with the A2 to P2 segments of the mitral valve (Fig. 17-8). For patients with functional MR, a coaptation length of at least 2 mm and a coaptation depth of no more than 11 mm are required. For patients with leaflet flail, a flail gap less than 10 mm and a flail width less than 15 mm are required (Fig. 17-9).
Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system. J Am Coll Cardiol. 2009;54:686–694.
Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243.
Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607.
Ross JJr, Braunwald E. Aortic stenosis. Circulation. 1968;38(1 suppl):61–67.