20 Transcatheter Closure of Atrial Septal Defects and Patent Foramen Ovale
Secundum ASD
Clinical Presentation
Symptoms may include the following:
• Reduced exercise tolerance or fatigue
• Palpitations (due to supraventricular arrhythmias, frequent atrial fibrillation/atrial flutter in older age), or syncope for sick sinus syndrome
• Atypical chest pain (right ventricular ischemia)
• Frequent respiratory tract infections
• Signs of right-heart failure
• Paradoxical embolism from peripheral venous or pelvic vein thrombosis, atrial arrhythmias, unfiltered intravenous infusion, or indwelling venous catheters
Echocardiography
Table 20-1 summarizes the key points regarding TEE evaluation of intracardiac anatomy.
Table 20-1 Transesophageal Echocardiogram Features to Consider for Atrial Septal Defect Closure
Contraindications for Percutaneous Closure of Secundum ASD
An absolute contraindication for percutaneous ASD closure is the presence of severe and irreversible PAH, with no evidence of a left-to-right shunt. See Table 20-2 for a summary of the indications and contraindications to ASD closure.
Table 20-2 Indications and Contraindications to Atrial Septal Defect Closure
| Indications |
|---|
1. Small ASD (diameter <5 mm) and no evidence of right ventricular volume overload
2. Irreversible pulmonary hypertension
3. Other contraindications include the following:
ASD Closure Devices
Amplatzer Septal Occluder (ASO)
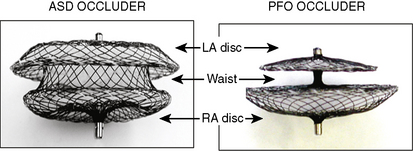
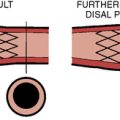
The Amplatzer septal occluder (ASO) is a self-expandable double disc device made of nitinol (55% nickel, 45% titanium) wire mesh. The ASO is tightly woven into two flat discs (Fig. 20-1). There is a 3- to 4-mm connecting waist between the two discs, corresponding to the thickness of the atrial septum. Nitinol has super elastic properties, with shape memory. This allows the device to be stretched into an almost linear configuration and placed inside a small sheath for delivery and then return to its original configuration within the heart when not constrained by the sheath. The device size is determined by the diameter of its waist and is constructed in various sizes ranging from 4 to 40 mm (1-mm increments up to 20 mm; 2-mm increments up to the largest device currently available, 40 mm; the 40-mm size is not available in the United States). The two flat discs extend radially beyond the central waist to provide secure anchorage.
Amplatzer Delivery System
For device deployment, we recommend using appropriately sized sheaths for the device as summarized in Table 20-3. The delivery system is supplied sterilized and separate from the device. It contains all the equipment needed to facilitate device deployment. It consists of the following:
1. Delivery sheath of specified French size and length, and appropriate dilator
2. Loading device, used to collapse the device and introduce it into the delivery sheath
3. Delivery cable (internal diameter [ID] 0.081 inch): the device is screwed onto its distal end allowing for loading for loading, placement, and retrieval of the device
4. Plastic Pin-vice: this facilitates unscrewing of the delivery cable from the device during device deployment
5. Tuohy Borst valve adapter with a side arm for the sheath, to act as a one-way stop-bleed valve
Table 20-3 Sheath Delivery System Sizing for Atrial Septal Defect Devices
Optional but Recommended Equipment
Amplatzer Super Stiff Guidewire
The 0.035-inch Amplatzer super stiff exchange guidewire is used to advance the delivery sheath and dilator into the left upper pulmonary vein. Table 20-4 summarizes all the necessary materials for ASD closure.
Table 20-4 Materials/Equipment Required for Transcatheter ASD Closure Procedures
| Item | Size | Cost each (US$) |
|---|---|---|
| Amplatzer Septal Occluder | 4–40 mm | 5500 |
| Amplatzer PFO Occluder | 18, 25, 35 mm | 5000 |
| Amplatzer Delivery System | 7–12F | 580 |
| Amplatzer Super Stiff exchange | 0.035-inch, 100 cm length guidewire | 45 |
| Multipurpose catheter | 6–7F | 10 |
| Amplatzer Sizing Balloon | 24, 34 mm | 265 |
| Amplatzer Rescue System | 9 F, 12F | 580 |
PFO, patent foremen ovale.
Step-by-Step Technique: Transcatheter Device Closure of Secundum ASD
Materials and Equipment
1. Single- or bi-plane cardiac catheterization laboratory
3. Full range of device sizes, delivery and exchange (rescue) systems, sizing balloons
4. A multipurpose catheter to engage the defect and the left upper pulmonary vein
5. Extra-stiff exchange length wire, for example, a 0.035-inch Amplatzer super stiff exchange length guidewire with a 1-cm floppy tip, but any extra-stiff J-tipped wire may be used.
Personnel
Method
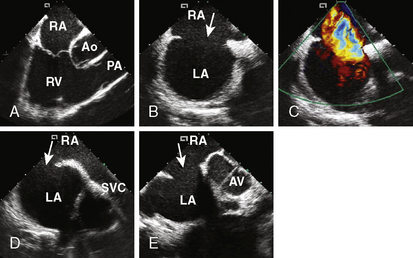
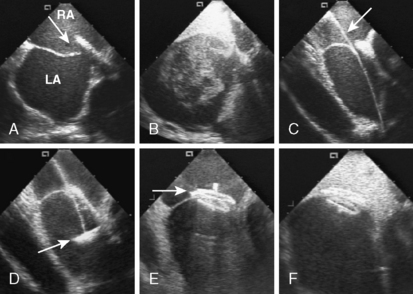
4. Echocardiographic assessment of the secundum ASD is performed simultaneously using either TEE or ICE. Figure 20-2 demonstrates full assessment of the defect by ICE.
The important ASD rims to look for are:
• Superior/SVC rim—best achieved using the bicaval view
• Superior posterior/right upper pulmonary vein rim
• Anterior superior/aortic rim—the least important rim; often, patients lack it
• Inferior/IVC and coronary sinus rim—an important rim to have
• Posterior rim—seen best in the short-axis view at the aortic valve level
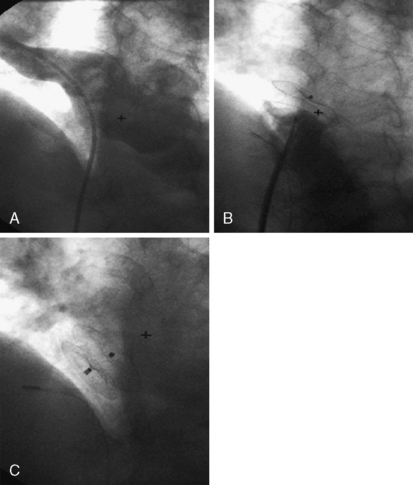
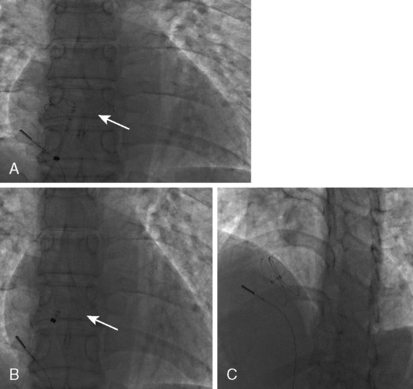
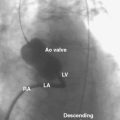
6. Perform a right upper pulmonary vein angiogram (Fig. 20-3A) in the hepatoclavicular projection (35-degree left anterior oblique/35-degree cranial). This delineates the anatomy, shape, and length of the septum. This may come in handy when the device is deployed but not released—the operator can position the imaging tube in the same view of the angiogram and compare the position of the device with that obtained during the deployment (Fig. 20-3B, C).
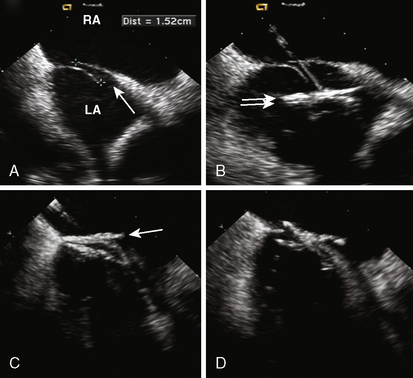
7. Defect Sizing. Position the multipurpose catheter in the left upper pulmonary vein. Prepare the appropriate size of balloon according to the manufacturer’s guidelines. We prefer to use the 34-mm balloon because it is longer and during inflation it sits nicely across the defect. Pass an extra-stiff, floppy/J-tipped 0.035-inch exchange length guidewire (Fig. 20-4A). This gives the best support within the atrium for the balloon, especially in large defects. Remove the multipurpose catheter and the femoral sheath. We advance the sizing balloon catheter directly over the wire without a venous sheath. Most sizing balloons require an 8F or 9F sheath. The balloon catheter is advanced over the wire and placed across the defect under both fluoroscopic and echocardiographic guidance. The “stop-flow” balloon sizing is performed by inflating the balloon (previously prepared with 1:4 diluted contrast) until the left-to-right shunt ceases, as observed by color flow Doppler TEE/ICE. Once the shunt ceases, deflate the balloon slightly until shunt reappears. This “stop-flow” balloon sizing technique is used to select an ASO device size. The best echo view for measurement is to observe the balloon in its long axis (Fig. 20-4B). In this view the indentation made by the margins of the ASD can be visualized and precise measurement can be made.
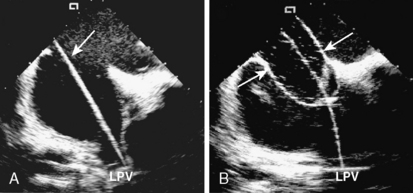
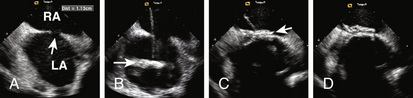
Figure 20-4 Intracardiac echocardiographic images of the patient in Figure 20-2, showing defect sizing. A, The exchange wire (arrow) across the defect into the left upper pulmonary vein (LPV). B, Sizing balloon occluding the defect. This is the stretched diameter (arrows) of the defect.
8. Fluoroscopic Measurement. Angulate the x-ray tube so the beam is perpendicular to the balloon. Various calibration markers can be helpful. Ensure that the markers are separated and discrete. Measure the balloon diameter at the site of the indentation (or at the middle of the balloon) as per the diagnostic function of the laboratory (Fig. 20-5). We have found that when a discrepancy exists between the echocardiographic and the fluoroscopic measurements, the echocardiographic measurement is usually more accurate.
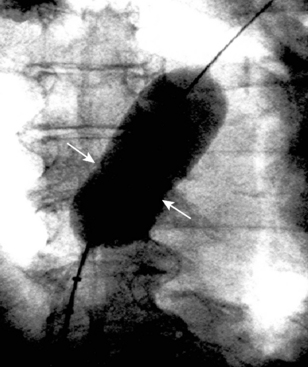
Figure 20-5 Cineangiographic image of the patient in Figure 20.3 during balloon sizing of the defect, demonstrating the stretched diameter (arrows) of the defect.
Recheck the ACT and give the first dose of antibiotics.
10. Device Delivery. Open the appropriate-sized delivery system. Flush the sheath and dilator. The delivery sheath is advanced over the guidewire to the left upper pulmonary vein (Fig. 20-6A). Both dilator and wire are removed, keeping the tip of the sheath inside the left upper pulmonary vein. Use extra care and do not allow air inside the delivery sheath. An alternative technique to minimize air embolism is passage of the sheath with the dilator over the wire until the IVC, at which point the dilator is removed and the sheath is advanced over the wire into the left atrium while continuously flushing the side arm of the sheath.
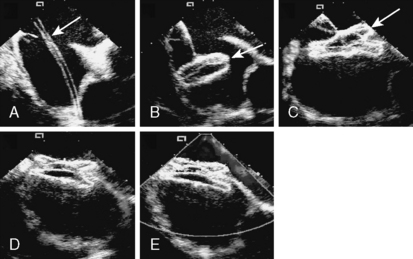
Figure 20-6 Intracardiac echocardiographic images of the patient in Figure 20-2, showing device delivery and deployment. A, Delivery sheath (arrow) across the defect into the left upper pulmonary vein. B, The left atrial disc (arrow) deployed in the left atrium. C, The right atrial disc (arrow) deployed in the right atrium. D, The device released, demonstrating good position. E, Color Doppler demonstrating no residual shunt and patent superior vena cava.
11. Device Deployment. The LA disc is deployed first under fluoroscopic and/or echocardiographic guidance by pulling back the sheath, while leaving the disc fixed in the LA away from the LA appendage (Fig. 20-6B). Part of the connecting waist should be deployed in the left atrium, very close (a few millimeters) to the atrial septum (the mechanism of ASD closure using the ASO is stenting of the defect). While applying constant tension on the entire assembly and withdrawing the delivery sheath off the cable, the connecting waist and the RA disc are deployed in the ASD itself and in the right atrium respectively (Fig. 20-6C).
12. Device Positioning. Proper device position can be verified using different techniques:
TEE/ICE. The echocardiographer should make sure that one disc is in each chamber. The long-axis view should be sufficient to evaluate the superior and inferior part of the septum and the short-axis view for the anterior and posterior part of the disc (Fig. 20-6D,E ).
Troubleshooting
Complications
1. Device embolization, the majority of which were encountered during the early learning curve of the investigators.
2. Heart block: rarely reported. Most likely related to the use of an oversized device.
3. Atrial arrhythmia: significant increase in atrial arrhythmias following device placement, generally resolving by 6 months.
4. Headaches: reported in about 5% of patients following device placement, resolving within 6 months. The use of clopidogrel for 2 to 3 months after device closure has minimized this complication significantly.
HELEX Septal Occluder Device
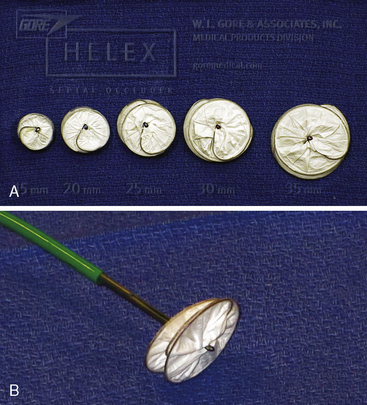
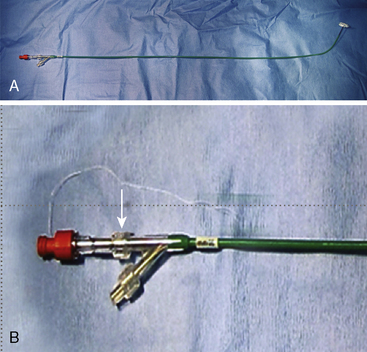
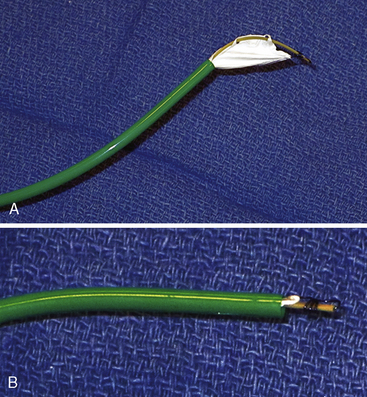
The Gore HELEX septal occluder device (WL Gore & Associates, Flagstaff, AZ) is a non-self-centering double-disc device made of nitinol and expanded polytetrafluoroethylene (ePTFE) (Fig. 20-7). The device is designed such that, following introduction across the septum, one disc is constituted on the LA side and the other on the RA side of the septum. The construction of the device consists of a curtain of ePTFE (gore-tex, WL Gore & Associates Flagstaff, Arizona) bonded to a single-piece wire frame of nitinol (0.012 inch). The nitinol is manufactured into a helical pattern of opposing rotations which, on full configuration, assumes two parallel discs. The device is delivered through its own composite triaxial 10 F delivery catheter with a workable length of 75 cm. This obviates the need for a long transseptal sheath. For patent foramen ovale (PFO) closure, the delivery system can be “monorailed” through a hole close to its distal end using a wire placed through a diagnostic catheter positioned in one of the left pulmonary veins. The device is available in 15-, 20-, 25-, 30- and 35-mm diameters. The devices are delivered through a short 10 F femoral vein sheath; however, if using the monorail technique, a short 11F or 12F femoral sheath is required.
Technique of Closure
Loading the Device
The HELEX device is supplied with its own delivery catheter (Fig. 20-8A) such that a long Mullins-type sheath is not necessary. The delivery system consists of three distal coaxial components transitioning to a parallel component arrangement at the proximal Y-arm hub: a 10 F green delivery catheter, a gray control catheter, and a tan mandrel (Fig. 20-8B). The proximal end of the control catheter exits the Y-arm hub and is terminated by a red retrieval cord cap. The proximal end of the mandrel exits the side port of the Y-arm hub and is terminated by a clear Luer-Lok. The control catheter is equipped with a retrieval cord if occluder repositioning or retrieval is deemed necessary (Fig. 20-8B). A 0.035-inch guidewire channel is incorporated into the distal end of the delivery catheter.
Loading the Occluder Into the Green Delivery Catheter
Remove the device from the sterile tray. Discard the sterile tray and follow the following steps:
1. To reduce the chance of air entrapment in the delivery system, loading of the occluder should be conducted with the occluder and catheter tip submerged in a heparinized saline bath.
2. Fill a large volume (20–30 mL) syringe with heparinized saline.
3. Attach the syringe to the red retrieval cord cap.
4. Tighten the mandrel Luer-Lok.
5. Loosen the control catheter Luer-Lok.
6. Flush the control catheter into the bowl or sterile tray.
7. When the initial flushing is completed, draw back on the gray control catheter with the attached syringe until only about 3 cm of the occluder remains outside the delivery catheter and the tan mandrel appears slightly curved (Fig. 20-9A).
8. Loosen the mandrel Luer-Lok.
9. Complete loading by continuing to draw back on the gray control catheter hub until the entire occluder has been withdrawn into the green delivery catheter (Fig. 20-9B).
10. Flush the control catheter into the bowl or sterile tray.
11. Keep the flushing syringe attached to the red cap to prevent the entrance of air into the delivery system until the catheter tip is placed inside the introducer sheath.
Device Delivery
Load the delivery catheter onto a guidewire through the guidewire port from the luminal surface out; ensure that the occluder is sufficiently withdrawn into the green delivery catheter to avoid interference with the guidewire (monorail system) (Fig. 20-10A). Load the delivery system into the appropriately sized introducer sheath. At this stage, remove the flushing syringe. Verify that the red retrieval cord cap affixing the retrieval cord is securely attached to the gray control catheter.
Deployment
LA Disc Deployment
1. Under direct fluoroscopic visualization, advance the catheter tip across the ASD until the radiopaque marker at the tip of the green delivery catheter is positioned within the middle left atrium. Verify that the tip of the green delivery catheter is across the defect and away from the LA appendage using TEE or ICE.
2. At this stage, the guidewire should be removed before attempting to deploy the occluder.
3. Use the following “push-pinch-pull” method to deploy the LA occluder disc:
4. Once the LA disc is deployed, hold the entire system as one unit and pull it back until the LA disc is in contact with the atrial septum under echocardiographic and fluoroscopic guidance.
RA Disc Deployment
1. To prepare for RA disc deployment, hold the gray control catheter in a fixed position and gently expose a portion of the RA side by withdrawing the green delivery catheter until the mandrel Luer-Lok stops on the Y-arm hub. Tighten the mandrel Luer-Lok.
2. Deploy the RA disc by holding the green delivery catheter in a fixed position with left hand and pushing the gray control catheter with the right hand until the control catheter Luer-Lok contacts the Y-arm hub. Then tighten the control catheter Luer-Lok.
3. Confirm that both left and right discs appear planar and apposed to the septum with septal tissue trapped between the discs (Figs. 20-10C, 20-11B).
4. Confirm proper position using TEE/ICE and angiography in the LAO-cranial (35-35) position (Figs. 20-10D, 20-11C). If the position is not correct, refer to the repositioning steps later in this chapter.
5. Completely remove the red retrieval cord cap and set it aside.
Occluder Lock and Release
1. It is important to note that the occluder can only be repositioned prior to lock release.
2. If position and occlusion are acceptable, loosen the mandrel Luer-Lok. Hold the green delivery catheter in a fixed position and release the lock by sharply pulling the tan mandrel at least 2 cm.
3. At the completion of the lock and release step, the occluder is still loosely attached to the gray control catheter by the retrieval cord. If the occluder position is not acceptable, refer to the section titled “Removing the Occluder With the Retrieval Cord,” later in this chapter. Once the delivery system is withdrawn (next step), the occluder cannot be removed using the delivery system.
4. If position is acceptable, remove the entire delivery system as a single unit, making sure the retrieval cord moves smoothly through the control catheter hub.
Repositioning the Occluder
1. Replace and tighten the red retrieval cord cap and tighten the mandrel Luer-Lok.
2. Repeat steps mentioned in the earlier section “Loading the Occluder Into the Green Delivery Catheter” in order to bring the occluder back into the catheter.
3. Reposition across the defect.
4. Repeat steps listed in the “Deployment” section (earlier in this chapter).
5. During repositioning, if increased force is required to move the catheter components due to abnormal conditions (such as a kinked mandrel, curved mandrel-lock loop or premature lock release), remove the occluder and delivery system entirely and utilize a new device.
Removing the Occluder With the Retrieval Cord
1. If the lock is released and if the retrieval cord is still attached to the gray control catheter, the occluder can be removed by taking up any slack in the retrieval cord and securely reattaching the red retrieval cord cap.
2. Position the green delivery catheter in the right atrium. Withdraw the gray control catheter while pulling the occluder into a linear form and drawing the occluder back into the green delivery catheter.
3. Do not use excessive force in an attempt to withdraw all of the occluder into the green delivery catheter. Doing so could cause the retrieval cord to break or result in occluder fracture.
4. Normal removal practices withdraw 50% to 100% of the occluder into the green delivery catheter. If a portion of the locked or unlocked occluder remains outside of the delivery catheter, the control catheter and delivery catheter should be withdrawn together. If necessary, remove the introducer sheath and occluder together.
Recapture
1. In the event that the occluder is malpositioned or embolized, it can be recaptured with the aid of a loop snare. A long sheath (≥10 F) positioned close to the device is recommended for recapture.
2. Place the loop snare around any portion of the occluder frame.
3. Pull the occluder into the sheath using the snare. If a portion of the occluder frame cannot be retracted into the long sheath, it may be necessary to remove the occluder, loop snare, and long sheath as one unit.
4. Bring the recaptured occluder into the sheath to avoid pulling the unlocked device across valve tissue.
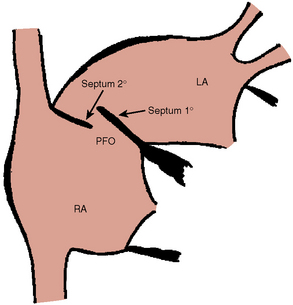
Patent Foramen Ovale
A PFO is part of normal fetal development. Following birth, an increase in pulmonary blood flow, and higher LA relative to RA pressure, the foramen ovale physiologically closes. The foramen is created by the overlap of the septum primum and septum secundum (Fig. 20-12) and fuses closed in latter life. This anatomy can behave like a flap valve, opening if the RA pressure exceeds the LA pressure. Pathologic studies have suggested that the foramen ovale may be probe-patent in 25% of the population.
There are three anatomical types:
Clinical Significance
Transcatheter Closure of PFO
The Amplatzer PFO occluder is a self-expanding, double-disc device made from a nitinol wire mesh (see Fig. 20-1). The nitinol mesh wire is 0.005 to 0.006 inches in diameter.
Step-by-Step Technique: Transcatheter Closure of PFO:
Materials, Equipment, and Personnel
These are the same as for secundum ASD. The preprocedure evaluation is also the same.
Suggested Measurements With TEE/ICE Images
1. Total septal length and edge of the defect to the mitral valve in the four-chamber view
2. SVC to the edge of the defect (long-axis TEE view/caval view by ICE)
3. Edge of defect to the aorta in short-axis view. Do not implant a device if the distance either from the defect to the SVC or from defect to the aortic root is < 9 mm.
Procedure Steps
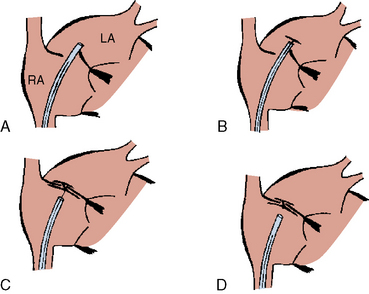
The procedure is identical to that described for secundum ASD except that balloon sizing is not performed. Prior to device release, careful reassessment of the edge of the device along the free atrial wall by TEE/ICE is needed. Do not release the device if it does not conform to its original configuration or if it appears unstable. In this case the operator should recapture and redeploy the device. Figures 20-13 and 20-14 demonstrate the steps of PFO closure.
Postprocedure follow-up is similar to that for secundum ASD closure except that most investigators maintain 81 to 325 mg aspirin per day for 6 months in combination with an antiplatelet agent, usually clopidogrel 75 mg/day, for 1 to 6 months. Follow-up echocardiogram at 3 to 6 months should include assessment for right-to-left atrial level shunt with a venous contrast injection, with Valsalva maneuver. (Figs. 20-15, 20-16)
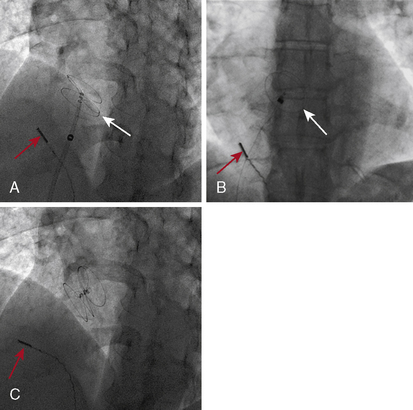
Figure 20-16 Cineangiographic image of the patient whose intracardiac echocardiogram is shown in Figure 20-15. A, The left atrial disc (white arrow) deployed in the left atrium. B, The right atrial disc deployed in the right atrium. C, The device released, demonstrating good position. Red arrow shows position of intracardiac echo transducer.
AGA Medical Corporation. Amplatzer PFO occluder device proctor manual. Golden Valley, MN: AGA Medical Corporation; 2002.
Bove A.A. Risk of decompression sickness with patent foramen ovale. Undersea Hyperb Med. 1998;25:175–178.
Cabanes L., Mas J.L., Cohen A., et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–1873.
Cao Q., Radtke W., Berger F., et al. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transesophageal echocardiography. Eur Heart J. 2000;21:941–947.
Cheng T.O. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis, and management. Catheter Cardiovasc Interv. 1999;47:64–66.
De Belder M.A., Tourkis L., Leech G., Camm A.J. Risk of patent foramen ovale for thromboembolic events in all age groups. Am J Cardiol. 1992;69:1316–1320.
Di Tullio M., Sacco R.L., Gopal A., et al. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–465.
Hagen P.T., Scholz D.G., Edwards W.D. Incidence and size of patent foramen ovale during the first decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20.
Hart R.G., Miller V.T. Cerebral infarction in young adults: a practical approach. Stroke. 1983;14:110–114.
Hausmann D., Mugge A., Becht I., et al. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol. 1992;70:668–672.
Hill S.L., Berul C.I., Patel H.T., et al. Early ECG abnormalities associated with transcatheter closure of atrial septal defects using the Amplatzer septal occluder. J Interv Card Electrophysiol. 2000;4:469–474.
Homma S., Sacco R.L., Di Tullio M.R., et al. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation. 2002;105:2625–2631.
Jones E.F., Calafiore P., Donnan G.A., et al. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol. 1994;74:596–599.
Jones T.K., Latson L.A., Zahn E., et al. Results of the U.S. multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol. 2007;49:2215–2221.
Kim J.J., Hijazi Z.M. Clinical outcomes and cost of Amplatzer transcatheter closure as compared with surgical closure of ostium secundum atrial septal defects. Med Sci Monit. 2002;8:CR787–CR791.
Knauth M., Ries S., Pohimann S., et al. Cohort study of multiple brain lesions in sport divers: role of a patent foramen ovale. Br Med J. 1997;314:701–705.
Lechat P.H., Mas J.L., Lascault G., et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152.
Meier B. Patent foramen ovale—beauty spot or health threat? Cardiol Rounds. 2001;5:1–8.
Mohr J.P., Thompson J.L., Lazar R.M., et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451.
Porter C.J., Feldt R.H., Edwards W.D., et al. Atrial septal defects. In: Emmanouilides G.C., ed. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult. 5th ed. Baltimore: Williams & Wilkins; 1995:687–703.
Sacco R.L., Ellenberg J.H., Mohr J.P., et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–390.
Schwerzmann M., Seiler C., Lipp E., et al. Relation between directly detected patent foramen ovale and ischemic brain lesions in sport divers. Ann Intern Med. 2001;134:21–24.
Steiner M.M., Di Tullio M.R., Rundek T. Patent foramen size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944–948.
Trepels T., Zeplin H., Sievert H. Cardiac perforation following transcatheter PFO closure. Catheter Cardiovasc Interv. 2003;58(1):111–113.
Vick G.W. Defects of the atrial septum including atrioventricular septal defects. In: Garson A., Neish S.R., Bricker J.T., et al. The science and practice of pediatric cardiology. 2nd ed. Baltimore: Williams & Wilkins; 1998:1141–1180.
Webster M.W., Chancellor A.M., Smith H.J. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11–12.
Wilmhurst P.T., Nightingale S., Walsh K.P., et al. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for hemodynamic reasons. Lancet. 2000;356:1648–1651.