Chapter 28 Transcallosal Surgery of Lesions Affecting the Third Ventricle: Basic Principles
Intraventricular tumors represent a relatively small proportion of central nervous system lesions, accounting for approximately 10% of CNS neoplasms.1 Despite this, they represent a diverse range of pathologic entities which pose technical challenges for neurosurgeons. Third ventricular lesions, in particular, are challenging in terms of surgical access and corridor, owing to their central location in the brain. As such, these lesions hold a special place in neurosurgical history and literature.
There is a wide differential diagnosis for third ventricular neoplasms, with the most common entities being colloid cysts, astrocytomas, and craniopharyngiomas. Other lesions include arachnoid cysts, pituitary adenomas, ependymomas, germinomas, metastasis, subependymoma, central neurocytoma, teratoma, dermoid, arteriovenous malformation, meningioma and choroid plexus papilloma (Table 28-1).2,3 The differential can often be refined by location within the third ventricle; for example, astrocytomas and craniopharyngiomas tend to be located in the anterior third ventricle, meningioma and choroid plexus papilloma in the posterior third ventricle, and colloid cysts at the level of the foramen of Monro.4
TABLE 28-1 Frequency of Tumors of the Third Ventricle
| Tumor | Number | Percent |
|---|---|---|
| Astrocytoma | 5 | 21% |
| Craniopharyngioma | 5 | 21% |
| Ependymoma | 4 | 17% |
| Choroid plexus papilloma | 3 | 13% |
| Cystercercosis | 2 | 8.3% |
| Colloid cyst | 1 | 4.2% |
| Epidermoid | 1 | 4.2% |
| Dermoid | 1 | 4.2% |
| Subependymoma | 1 | 4.2% |
| Medulloblastoma | 1 | 4.2% |
| Total | 24 |
Adapted from data in Morrison G, Sobel DF, Kelley WM, Norman D. Intraventricular mass lesions. Radiology. 1984;153:435-442.
Given that the majority of intraventricular lesions are benign, surgery is often the preferred, and potentially curative, option for tumors of this region.5 However, owing to the relative rarity of these lesions, few surgeons have extensive experience with approaches to the ventricular system. Interhemispheric, transcallosal approaches to the third ventricle offer the advantage of potential avoidance of postoperative cortical hemispheric deficits or other long-term complications (e.g., seizures) as compared to transcortical approaches. Partial sectioning of the corpus callosum does not in general lead to significant neurologic impairment. In patients with crossed-dominance, where the hemisphere controlling the dominant hand is contralateral to the hemisphere controlling speech and language, callosal sectioning can lead to severe disability.6–8 This condition can be present in patients with a history a childhood cerebral injury or other dysfunction forcing relocation of language function within the brain. Furthermore, sectioning the splenium of the corpus callosum in patients with a dominant hemisphere hemianopsia may also be contraindicated as this can lead to alexia.8 In modern times, the complications related to transcallosal approaches are generally related to manipulations in the floor of the third ventricle and not the approach.
Historical Notes
Dandy first described the transcallosal approach for accessing the ventricular system in 1922.9 Before that time, localization of tumors of the third ventricle was limited by insufficient diagnostic methods, and the introduction of ventriculography by Dandy in 1918 helped in this regard.10 Dandy accessed the third ventricle primarily through a posterior transcallosal approach. In 1944, Edward Busch described division of the midline forniceal raphe and entrance into the third ventricle by splitting of the plane between the two fornices.11 Several series and reports detailing the use of transcallosal techniques began to appear in the 1950s and 1960s.12–14 In the 1970s and 1980s, series reporting on psychometric and neuropsychological outcomes following transcallosal surgery further elucidated the then growing body of knowledge regarding the physiology of corpus callosum sectioning.15–18
Anatomic Considerations
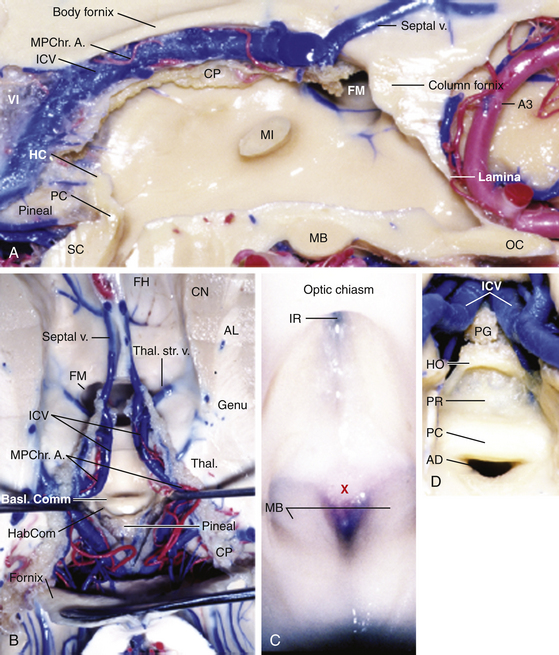
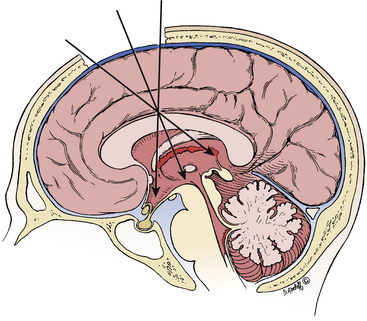
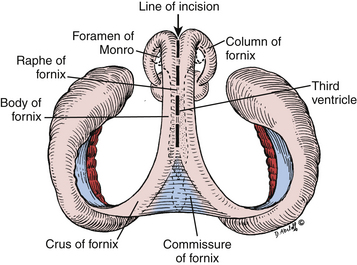
The third ventricle is a narrow, funnel-shaped structure that lies in the center of the brain. It lies below the corpus callosum and body of the lateral ventricles, between the two thalami and walls of hypothalamus, and above the pituitary and midbrain (Fig. 28-1).19 It is a unilocular cavity and communicates with the lateral ventricles at its anterosuperior margin via the foramen of Monro; posteriorly, it communicates with the fourth ventricle via the sylvian aqueduct.19
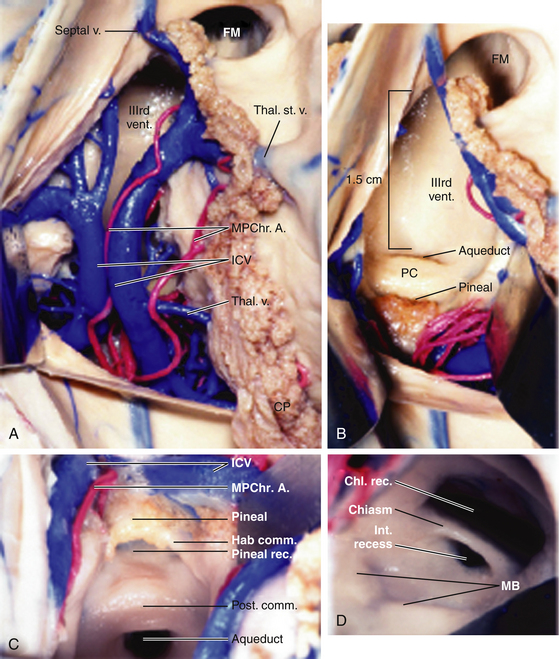
The roof of the third ventricle contains five layers and extends from the foramen of Monro anteriorly to the suprapineal recess posteriorly.19,20 The fornices make up the upper, or neural, layer. The axons of the fornices arise in the temporal horns from the hippocampi and extend around the thalami to reach the mammillary bodies.19 The inferior aspects, or fimbria, arise in the floor of the temporal horn and pass posteriorly to become the posterior portions, or crura of the fornices. These unite at the hippocampal commissure, which covers the posterior portion of the third ventricle; the bodies of the fornices cover the middle portion; the columns of the fornices, along with anterior commissure and lamina terminalis, cover the anterior portion of the third ventricle.19 The second and fourth layers of the roof of the third ventricle are comprised of the superior and inferior layers of the tela choroidea. The superior and inferior layers of the tela choroidea form a space called the velum interpositum, which contains the third, and vascular, layer of the roof of the third ventricle.20 In this vascular layer lie the internal cerebral vein (ICV) and posterior choroidal arteries (PChA). The fifth layer is the choroid plexus of the lateral ventricle, which extends from the foramen of Monro posterosuperolaterally toward the pineal gland.
The choroid plexus runs bilaterally in the choroidal fissures, which are incisuras between the lateral edge of the fornix and the superomedial surface of the thalamus.21 The choroid plexus of the lateral ventricle has an ependymal attachment to the fornix and to the thalamus called the teniae fornicis and the teniae thalami, respectively.22 The teniae fornicies contains no arteries or veins; however, tributaries of the internal cerebral vein, the superior and anterior thalamic veins and the thalamostriate vein, as well as the branches of the medial PChA traverse the teniae thalami.23 Surgically, this is an important point, as opening the choroidal fissure along the teniae fornicis avoids these vascular structures.24
The floor of the third ventricle extends anteriorly from the optic chiasm to the sylvian aqueduct posteriorly.19 The anterior half of the floor is formed by diencephalic structures and the posterior half is formed by those of the mesencephalon.19 From anterior to posterior, structures encountered are the optic chiasm, the infundibular recess, the median eminence, the tuber cinereum, the pair of mammillary bodies, and the ventricular side corresponding to the posterior perforated substance, and part of the tegmentum of the midbrain.19,25 The anterior wall of the third ventricle is formed from superior to inferiorly by the columns of the fornix, the foramina of Monro, the anterior commissure, lamina terminalis, optic recess, and optic chiasm. The posterior wall consists of, from superiorly to inferiorly, the suprapineal recess, the habenular commissure, and the sylvian aqueduct. The lateral walls are formed by the thalamus superiorly and hypothalamus interiorly, which are delineated by the hypothalamic sulcus extending from the foramen of Monro to the sylvian aqueduct.19 The massa intermedia connecting the two thalami is present in roughly three quarters of brains approximately 4 ml posterior to the foramen of Monro.26
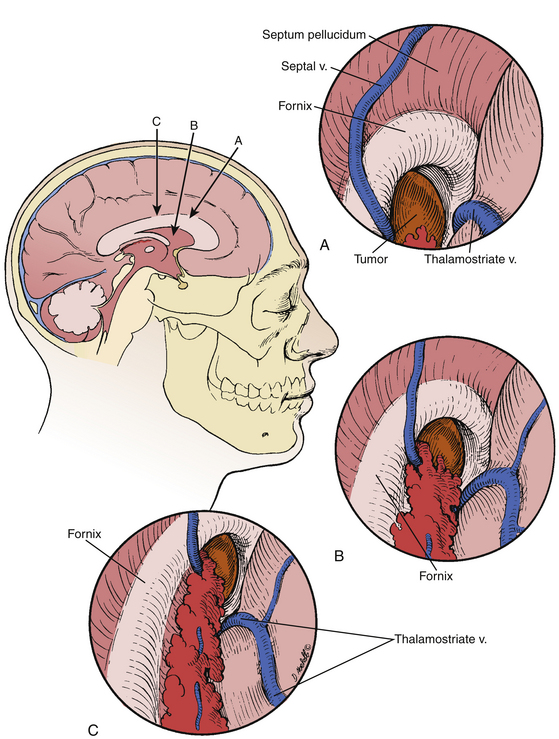
Anatomy of the lateral ventricle and the relationship to the foramen of Monro is key in these approaches as well as these provide a corridor of access to the third ventricle. In the lateral ventricle, medial and lateral groups of veins converge at the level of the foramen of Monro to form the internal cerebral veins. The main medial vein is the septal vein, which crosses the septum pellucidum and the fornix. The lateral veins include the caudate veins, which run from posterolateral to anteromedial to cross the caudate nucleus and the thalamus, and the thalamostriate vein.21 The thalamostriate vein is located in the body of the lateral ventricle. It is the largest tributary of the ICV and runs in the striothalamic sulcus between the caudate nucleus and the thalamus. At the posterior margin of the foramen of Monro, the thalamostriate vein curves medially around the anterior tubercle of the thalamus to form the ICV.20 This U-shaped junction is termed the venous angle.20,27,28
Surgical Technique
After induction of general anesthesia with endotracheal intubation, we typically place the patient in Mayfield pin fixation with the patient supine and the head elevated 15-20 degrees above horizontal (Fig. 28-2). Some surgeons prefer a lateral head position with the head turned 90 degrees laterally with the vertex 30 to 40 degrees above horizontal. Usually in the lateral position, the nondominant hemisphere is placed inferiorly such that it falls away with gravity and opens up the midline corridor; however, based on anatomy of the offending lesion and the patient’s venous drainage, it may be desirable to place the dominant hemisphere down. Advocates for lateral positioning cite that gravity may be used to allow the inferiorly placed hemisphere to fall away, giving midline access with minimal retraction and that lateral positioning of the head allows the surgeon to operate with hands side-by-side versus on top of one another. We have found in our experience that the main disadvantage of lateral positioning is the distortion of midline by displacement of the brain by gravity. This disadvantage is most significant in interforniceal approaches, as identification of the midline is crucial to the prevention of forniceal injury. Furthermore, we have found that by moving slightly to the patient’s side, it is quite easy to operate with hands side-by-side in the supine position. For these reasons, we primarily utilize supine positioning.
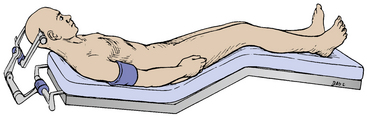
FIGURE 28-2 Patient in supine position with head flexed 15 to 20 degrees in Mayfield head fixation.
(From Apuzzo ML, Amar AP. Transcallosal interforniceal approach. In: Apuzzo ML, ed. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1998:421-452, with permission.)
Consideration is given to placement of preoperative ventriculostomy placement contralateral to the side of dissection. This assists in relaxation of the hemispheres for dissection and should be strongly considered in patients with preoperative ventriculomegaly. Typically, if preoperative ventriculostomy is not performed, we will leave behind a ventricular catheter at the time of closure. Furosemide and mannitol are also administered preoperatively to provide brain relaxation.
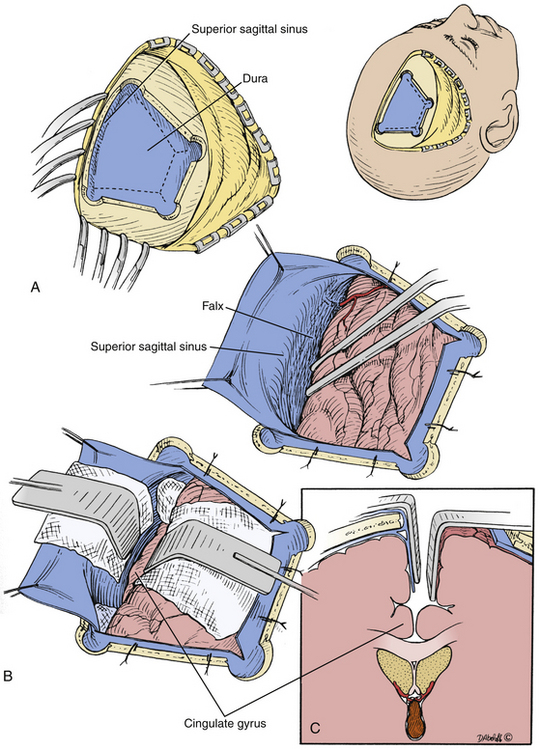
We typically use a two limbed curvilinear scalp flap or horseshoe flap, though other incisions can be tailored to achieve satisfactory cosmesis. A right-sided, 6 cm x 4 cm x 3 cm trapezoidal bone flap with the base at approximately 1 centimeter left of midline is most commonly used, with placement of burr holes at the vertices of the flap to obtain control of the sagittal sinus. For optimal access of the foramen of Monro, anatomy dictates that the bone flap should be placed such that it is bisected by the coronal suture.29 However, the bone flap is classically placed two thirds in front of the coronal suture and one third behind it as generally there is a corridor of relative paucity of parasagittal draining veins at this location.20,29 However, several factors influence craniotomy placement. A slightly more posterior bone flap with a more anteriorly angulated corridor will provide better access to the forniceal body and anterior third ventricle. More anteriorly placed bone flaps will provide better access to the posterior third ventricle (Fig. 28-3). Furthermore, with the advent of high-resolution MRI and frameless stereotaxy, parasagittal draining veins may be identified and a corridor chosen that provides access to the lesion without sacrifice of those veins.
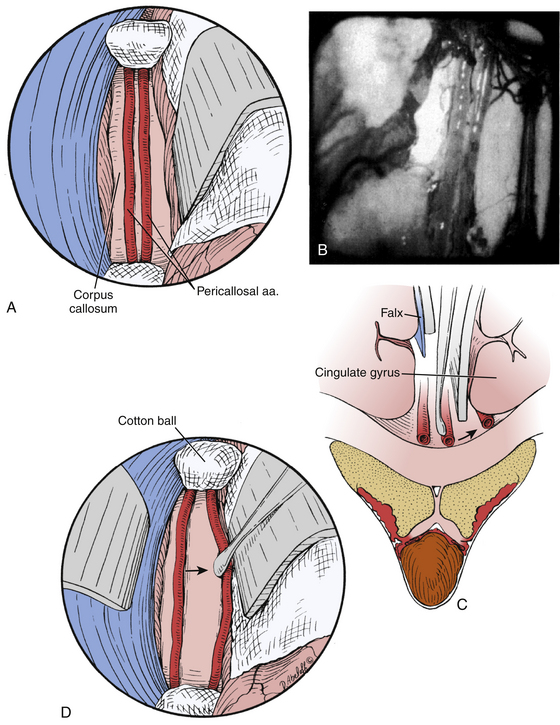
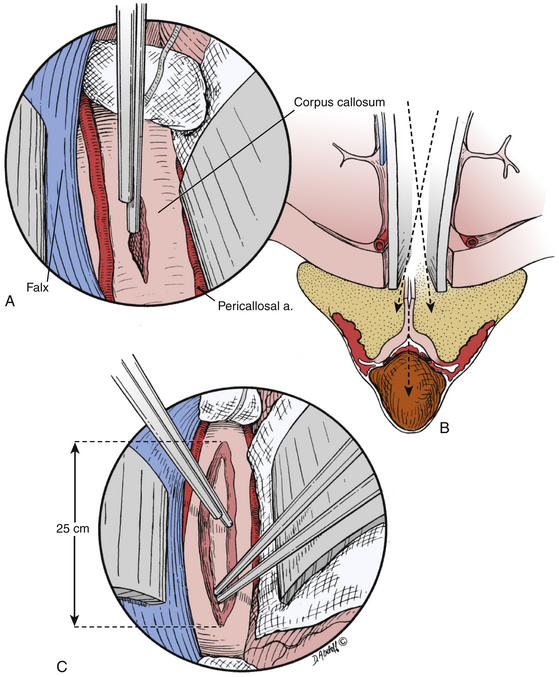
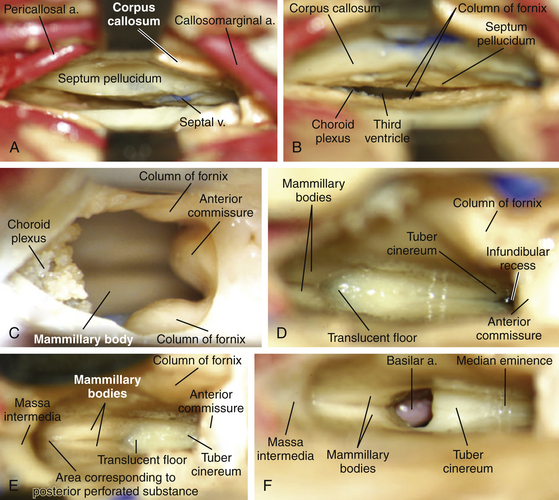
Following craniotomy, a trapezoidal or curvilinear dural opening is made with the base toward the sagittal sinus (Fig. 28-4). Tack-up sutures are placed, and the addition sutures are placed to retract the dura toward the sagittal sinus to tamponade any bleeding from near the structure. Interhemispheric dissection is then undertaken until the corpus callosum is reached. Care should be taken to retract minimally on the exposed hemisphere. Progressively larger cotton balls can be placed at the anterior and posterior extents of the dissection to assist without use of retractor blades.29,30 The cingulate gyri can be mistaken for the corpus callosum if the callosomarginal arteries are mistaken for the pericallosal arteries running over the corpus callosum; however, the corpus callosum has a striking white appearance and is relatively hypovascular compared to the cortical pial color and vascularity of the cingulate (Fig. 28-5).29 The number of A2, pericallosal arteries identified on the corpus callosum can range from one to three.31,32 After the corpus callosum and pericallosal arteries are identified, incision of the corpus callosum is undertaken with a blunt dissector such as a Penfield 1 and 5 F suction. The incision is typically somewhere between 10 mm to 20 mm long, but should be tailored to provide optimal access to the lesion (Fig. 28-6).
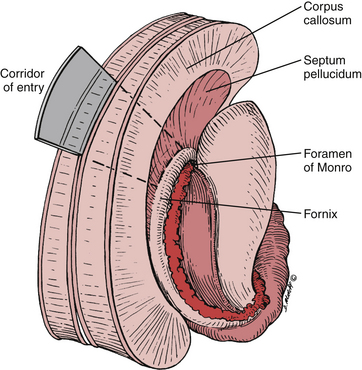
After callosal incision, four possible entries into the ventricular system are possible: right lateral ventricle, left lateral ventricle, septum pellucidum with or without cavum, and forniceal body. In addition, three primary corridors for access to the third ventricle exist: through the foramen of Monro, through the choroidal fissure and velum interpositum, and interforniceal through the midline raphe. Choice of entry and approach taken depend primarily on the location, size, and texture of the lesion (Fig. 28-7). In addition, transcallosal entry into the ventricular system allows for emptying of the ventricular contents and brain relaxation. Furthermore, fenestration of the septum pellucidum will aid in this drainage.
A transforaminal approach is ideally-suited for cystic lesions in the anterior third ventricle and should be the first approach considered as it provides a natural aperture into the third ventricle without further dissection. Often times, the foramen is expanded secondary to the lesion, which allows decompression of the lesion without manipulation of critical structures.8 Forniceal sacrifice is not necessary and should not be considered. From entry into the lateral ventricle, the septal vein medially, thalamostriate vein laterally, and choroid plexus can be identified and followed to the foramen of Monro. The choroid plexus is the most striking landmark leading to the foramen (Fig. 28-8). Again, at the posterior margin of the foramen, the thalamostriate vein joins the septal vein at a U-shaped junction forming the ICV, and this anatomy can be helpful in orienting the surgeon.
If the lesion is not accessible solely through the foramen of Monro, as with lesions in the middle or posterior portions of the third ventricle, other corridors of access should be considered. A detailed review of the three-dimensional radiographic anatomy will typically lend the surgeon the majority of the clues needed to plan an approach. One option as described by Yasargil and Abdulrauf is a combined transcallosal/trans-sylvian approach. This is an elegant approach and is now primarily used in a staged fashion for lesions with origins in the cranial base extending upward, such as craniopharyngiomas.30 Therefore, the two other possible approach for lesions contained in the third ventricle are the transchoroidal approach and the interforniceal approach. The first approach we consider is the transchoroidal approach, as the choroidal fissure can be opened in a straightforward manner along a natural plane extending backward from the foramen of Monro. The choroid plexus of the lateral ventricle runs in the choroidal fissure, which is a C-shaped cleft between the fornix and thalamus extending from the foramen of Monro to the inferior choroidal point in the temporal horn.20 The transchoroidal approach involves opening the choroidal fissure to expose the roof of the third ventricle by mobilization of the fornix to the contralateral side.30 This can be performed supra- or subchoroidally. Classically, in a subchoroidal approach, the taenia thalami is incised for access to the velum interpositum and third ventricle. In this approach, the ipsilateral thalamostriate vein may be injured due to manipulation of the fornix, and as described above, the superior and anterior thalamic veins, the thalamostriate vein, and the branches of the medial PChA pass through the taenia thalami, putting these structures at risk.20,22,30 A suprachoroidal approach traverses the taenia fornicis, where no arteries or veins run, and also has a lesser risk of thalamostriate vein injury.22 A recent anatomical analysis of the transchoroidal approach by Ulm et al.20 found that the choroidal opening was limited to 1.5 cm behind the foramen of Monro due to the expanding width of the fornix (Fig. 28-9). Furthermore, through this approach, they indicated that lesions in the middle third ventricle were most easily accessed, though anteriorly placed craniotomies and callosotomies were ideal for accessing posterior third ventricular pathology from an anterior to posterior angle. Access to the anterior third ventricle was limited by the columns of the fornix, and posteriorly placed craniotomies to achieve more anterior angulation was limited by the presence of parietal parasagittal draining veins.20
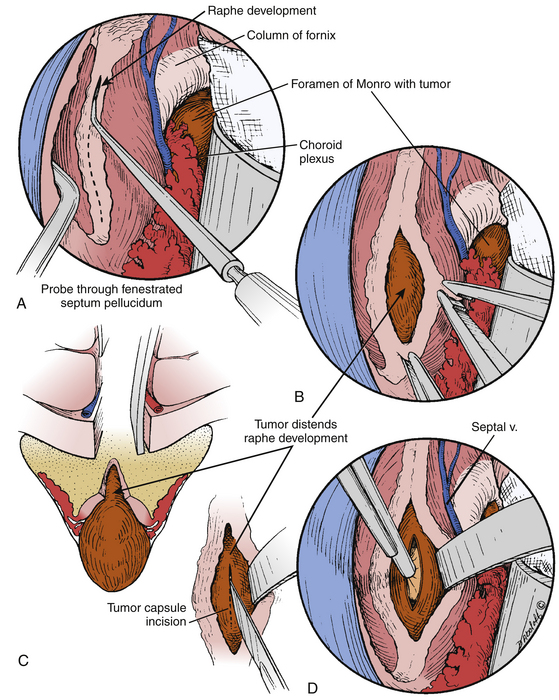
The interforniceal approach was originally described by Busch6,11 in humans and studied in detail at our institution by Apuzzo et al. with the application of modern microsurgical techniques.15,29 In this approach, the midline raphe between the two fornices is opened at the roof of the third ventricle (Fig. 28-10). The interforniceal approach provides access to the anterior and middle third ventricle when the transforaminal approach inadequate and also provides a corridor to the related structures of the hypothalamus, infundibular recess, and mammillary bodies. One key advantage of this approach is that it allows the surgeon to work simultaneously through both foramina of Monro and the midline interforniceal plane. The midline raphe is opened from anterior to posterior beginning at the midpoint foramen of Monro and extending backward no more than two centimeters. Often, the lesion will splay open the plane between the fornices, which aids in dissection (Fig. 28-11). Beginning anterior to the midpoint of the foramen of Monro puts the anterior commissure at risk25,33 and injuries to this structure have been associated with deficits in visual retention.34 Extending this incision beyond 2 cm puts the hippocampal commisure at risk and can lead to memory impairment.29 In addition, injuries to the fornices are related to memory impairments; therefore, strict definition of the midline and natural cleavage planes is required. A variation of this approach, called the anterior interforniceal approach (AIF), has been described by Rosenfeld et al. (Fig. 28-12).25,35 In this approach, a limited splitting of the fornices is performed anterior to the posterior border of the foramen of Monro using stereotactic navigation. This approach has been advocated for removal of hypothalamic hamartomas by Spetzler and Rosenfeld’s groups.25
Closure is performed in the standard fashion, with careful attention to dural closure for the prevention of pseudomeningocele. In cases where the dura cannot be closed to an acceptable degree of “water tightness,” we generally use a dural substitute as an overlay or a suturable underlay. Ventriculostomy catheters are generally placed under direct visualization during closure if not placed preoperatively and either clamped for intracranial pressure monitoring or weaned slowly depending on the perceived risk of postoperative hydrocephalus.
Complications and Avoidance
Due to the delicate and eloquent anatomy traversed in approaching the center of the brain, transcallosal approaches carry a risk of postoperative deficits. However, most of these can be avoided if proper care is taken. Craniotomy placement can lower risk to eloquent structures. Obviously, craniotomies placed primarily behind the coronal suture put the motor cortex at risk, and craniotomies on the dominant side place that hemisphere at risk. Furthermore, careful craniotomy placement can help avoid sacrifice of parasagittal draining veins. Sacrifice of parasagittal veins can lead to postoperative venous infarction and cortical deficits including hemiplegia. As described by Ulm et al.,20 posteriorly placed craniotomies are limited by the presence of parasagittal veins related to the parietal lobes. In addition, preoperative magnetic resonance venography with or without the use of intraoperative navigation can be helpful in planning craniotomy placement and operative corridor so as to avoid major draining veins.
Care should also be taken to avoid other approach-specific complications. Complications related to the transchoroidal approach include infarction in the basal ganglia, mutism, and hemiparesis;29 however, as discussed earlier, a suprachoroidal approach can avoid injury to the ipsilateral thalamostriate vein and fornix. Bilateral cingulate gyrus injuries can lead to akinetic mutism.36 The main drawback to the interforniceal approach is that it puts both fornices at risk, and for that reason, some authors advocated limiting its use to cases where other approaches are inadequate.8 These complications include transient memory loss and hemiparesis; however, definition of the anatomic midline and avoidance of parasagittal veins may mitigate this risk.15
Conclusions
Surgery of the third ventricle represents in many ways one of the great frontiers in intracerebral surgery. The central location in the brain and anatomical and functional complexity of surrounding structures make the third ventricle a structure fitting for the rich history in attempts to reach it. Transcallosal approaches represent a set of highly refined corridors of entry into the third ventricle. The three primary variations of transcallosal approaches are the transforaminal, transchoroidal, and interforniceal approaches (Table 28-2). Preoperative planning is crucial for complication avoidance in these procedures. Venous anatomy should be elucidated with magnetic resonance venography in most cases and operative corridors planned to avoid venous sacrifice. Meticulous definition of critical structures intraoperatively also increases the safety of these procedures.
Apuzzo M.L., Amar A.P. Transcallosal interforniceal approach. In: Apuzzo M.L., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1998:421-452.
Apuzzo M.L., Chikovani O.K., Gott P.S., et al. Transcallosal, interfornicial approaches for lesions affecting the third ventricle: surgical considerations and consequences. Neurosurgery. 1982;10:547-554.
Barris R.W., Schuman H.R. [Bilateral anterior cingulate gyrus lesions; syndrome of the anterior cingulate gyri.]. Neurology. 1953;3:44-52.
Botez-Marquard T., Botez M.I. Visual memory deficits after damage to the anterior commissure and right fornix. Arch Neurol. 1992;49:321-324.
Busch E. A new approach for the removal of tumors of the third ventricle. Acta Psychiatr Scand. 1944;19:57-60.
Dandy W.E. Diagnosis, localization and removal of tumors of the third ventricle. Johns Hopkins Hosp Bull. 1922;33:188-189.
Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237-294.
Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585-644.
Jeeves M.A., Simpson D.A., Geffen G. Functional consequences of the transcallosal removal of intraventricular tumours. J Neurol Neurosurg Psychiatry. 1979;42:134-142.
Ledoux J.E., Risse G.L., Springer S.P., et al. Cognition and commissurotomy. Brain 100 Pt. 1977;1:87-104.
Milhorat T.H., Baldwin M. A technique for surgical exposure of the cerebral midline: experimental transcallosal microdissection. J Neurosurg. 1966;24:687-691.
Morrison G., Sobel D.F., Kelley W.M., et al. Intraventricular mass lesions. Radiology. 1984;153:435-442.
Piepmeier J.M., Spencer D.D., Sass K.J. Lateral ventricular masses. In: Apuzzo M.L.J., editor. Brain surgery: complication avoidance and management. New York: Churchill Livingstone; 1993:581-600.
Rhoton A.L. The lateral and third ventricles: Cranial Anatomy and Surgical Approaches. Schaumburg, IL: Congress of Neurological Surgeons; 2003. 235-298
Rhoton A.L.J. Microsurgical Anatomy of the Third Ventricular Region. In: Apuzzo M.L., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1998:421-452.
Shucart W.A., Stein B.M. Transcallosal approach to the anterior ventricular system. Neurosurgery. 1978;3:339-343.
Siwanuwatn R., Deshmukh P., Feiz-Erfan I., et al. Microsurgical anatomy of the transcallosal anterior interforniceal approach to the third ventricle. Neurosurgery. 2005;56:390-396. discussion 390-396
Ture U., Yasargil M.G., Al-Mefty O. The transcallosal-transforaminal approach to the third ventricle with regard to the venous variations in this region. J Neurosurg. 1997;87:706-715.
Ture U., Yasargil M.G., Friedman A.H., et al. Fiber dissection technique: lateral aspect of the brain. Neurosurgery. 2000;47:417-426. discussion 426-427
Ture U., Yasargil M.G., Krisht A.F. The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery. 1996;39:1075-1084. discussion 1084-1085
Ulm A.J., Russo A., Albanese E., et al. Limitations of the transcallosal transchoroidal approach to the third ventricle. J Neurosurg. 2009;111:600-609.
Wen H.T., Rhoton A.L.Jr., de Oliveira E. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42:1205-1217. discussion 1217-1219
Winkler P.A., Ilmberger J., Krishnan K.G., et al. Transcallosal interforniceal-transforaminal approach for removing lesions occupying the third ventricular space: clinical and neuropsychological results. Neurosurgery. 2000;46:879-888. discussion 888-890
Yamamoto I., Rhoton A.L.Jr., Peace D.A. Microsurgery of the third ventricle: Part I. Microsurgical anatomy. Neurosurgery. 1981;8:334-356.
Yasargil M.G., Abdulrauf S.I. Surgery of intraventricular tumors. Neurosurgery. 2008;62:1029-1040. discussion 1040-1041
1. Greenberg M.S., Arredondo N. Handbook of Neurosurgery. Lakeland, FL/New York: Greenberg Graphics/Thieme Medical Publishers; 2006.
2. Fries G., Perneczky A. Tumors and cysts of the third ventricle. In: Rengachary S.S., Ellenbogen R.G. Principles of Neurosurgery. Edinburgh: Elsevier Mosby; 2005:647-656.
3. Morrison G., Sobel D.F., Kelley W.M., Norman D. Intraventricular mass lesions. Radiology. 1984;153:435-442.
4. Citow JS, Macdonald RL, Refai D: Comprehensive neurosurgery board review. New York, Thieme.
5. Piepmeier J.M., Spencer D.D., Sass K.J. Lateral ventricular masses. In: Apuzzo M.L.J., editor. Brain surgery: complication avoidance and management. New York: Churchill Livingstone; 1993:581-600.
6. Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237-294.
7. Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585-644.
8. Kasowski H., Piepmeier J.M. Transcallosal approach for tumors of the lateral and third ventricles. Neurosurg Focus. 2001;10:E3.
9. Dandy W.E. Diagnosis, localization and removal of tumors of the third ventricle. Johns Hopkins Hosp Bull. 1922;33:188-189.
10. Cohen-Gadol A.A., Geryk B., Binder D.K., Tubbs R.S. Conquering the third ventricular chamber. J Neurosurg. 2009;111:590-599.
11. Busch E. A new approach for the removal of tumors of the third ventricle. Acta Psychiatr Scand. 1944;19:57-60.
12. Baldwin M., Ommaya A.K., Farrier R., Macdonald F. Mesial Cerebral incision. J Neurosurg. 1963;20:679-686.
13. Mc K.W. The surgical treatment of colloid cyst of the third ventricle; a report based upon twenty-one personal cases. Brain. 1951;74:1-9.
14. Milhorat T.H., Baldwin M. A technique for surgical exposure of the cerebral midline: experimental transcallosal microdissection. J Neurosurg. 1966;24:687-691.
15. Apuzzo M.L., Chikovani O.K., Gott P.S., et al. Transcallosal, interfornicial approaches for lesions affecting the third ventricle: surgical considerations and consequences. Neurosurgery. 1982;10:547-554.
16. Jeeves M.A., Simpson D.A., Geffen G. Functional consequences of the transcallosal removal of intraventricular tumours. J Neurol Neurosurg Psychiatry. 1979;42:134-142.
17. Ledoux J.E., Risse G.L., Springer S.P., et al. Cognition and commissurotomy. Brain 100 Pt. 1977;1:87-104.
18. Shucart W.A., Stein B.M. Transcallosal approach to the anterior ventricular system. Neurosurgery. 1978;3:339-343.
19. Rhoton A.L.J. Microsurgical Anatomy of the Third Ventricular Region. In: Apuzzo M.L., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1998:421-452.
20. Ulm A.J., Russo A., Albanese E., et al. Limitations of the transcallosal transchoroidal approach to the third ventricle. J Neurosurg. 2009;111:600-609.
21. Villani R.M., Tomei G. Transcallosal approach to tumors of the third ventricle. In: Schmidek H.H., Roberts D.W. Schmidek & Sweet operative neurosurgical techniques: indications, methods, and results. Philadelphia: Saunders Elsevier; 2006:772-785.
22. Wen H.T., Rhoton A.L.Jr, de Oliveira E. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42:1205-1217. discussion 1209-1217
23. Ture U., Yasargil M.G., Al-Mefty O. The transcallosal-transforaminal approach to the third ventricle with regard to the venous variations in this region. J Neurosurg. 1997;87:706-715.
24. Rhoton A.L. The Lateral and Third Ventricles: Cranial Anatomy and Surgical Approaches. Schaumburg, IL: Congress of Neurological Surgeons; 2003. 235-298
25. Siwanuwatn R., Deshmukh P., Feiz-Erfan I., et al. Microsurgical anatomy of the transcallosal anterior interforniceal approach to the third ventricle. Neurosurgery. 2005;56:390-396. discussion 390-396
26. Yamamoto I., Rhoton A.L.Jr, Peace D.A. Microsurgery of the third ventricle. Part I. Microsurgical anatomy. Neurosurgery. 1981;8:334-356.
27. Johanson C. The central veins and deep dural sinuses of the brain; an anatomical and angiographic study. Acta Radiol Suppl. 1954;107:8-184.
28. Ring B.A. Variations in the striate and other cerebral veins affecting measurements of the “venous angle”. Acta Radiol. 1959;52:433-447.
29. Apuzzo M.L., Amar A.P. Transcallosal interforniceal approach. In: Apuzzo M.L., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1998:421-452.
30. Yasargil M.G., Abdulrauf S.I. Surgery of intraventricular tumors. Neurosurgery. 2008;62:1029-1040. discussion 1040-1041
31. Ture U., Yasargil M.G., Friedman A.H., Al-Mefty O. Fiber dissection technique: lateral aspect of the brain. Neurosurgery. 2000;47:417-426. discussion 417-426
32. Ture U., Yasargil M.G., Krisht A.F. The arteries of the corpus callosum: a microsurgical anatomic study. Neurosurgery. 1996;39:1075-1084. discussion 1075-1084
33. Winkler P.A., Ilmberger J., Krishnan K.G., Reulen H.J. Transcallosal interforniceal-transforaminal approach for removing lesions occupying the third ventricular space: clinical and neuropsychological results. Neurosurgery. 2000;46:879-888. discussion 888-890
34. Botez-Marquard T., Botez M.I. Visual memory deficits after damage to the anterior commissure and right fornix. Arch Neurol. 1992;49:321-324.
35. Rosenfeld J.V., Harvey A.S., Wrennall J. Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery. 2001;48:108-118.
36. Barris R.W., Schuman H.R. [Bilateral anterior cingulate gyrus lesions; syndrome of the anterior cingulate gyri.]. Neurology. 1953;3:44-52.