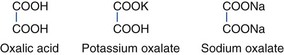Chapter 64 Toxic Plant Ingestions
For online only figures, please go to www.expertconsult.com ![]()
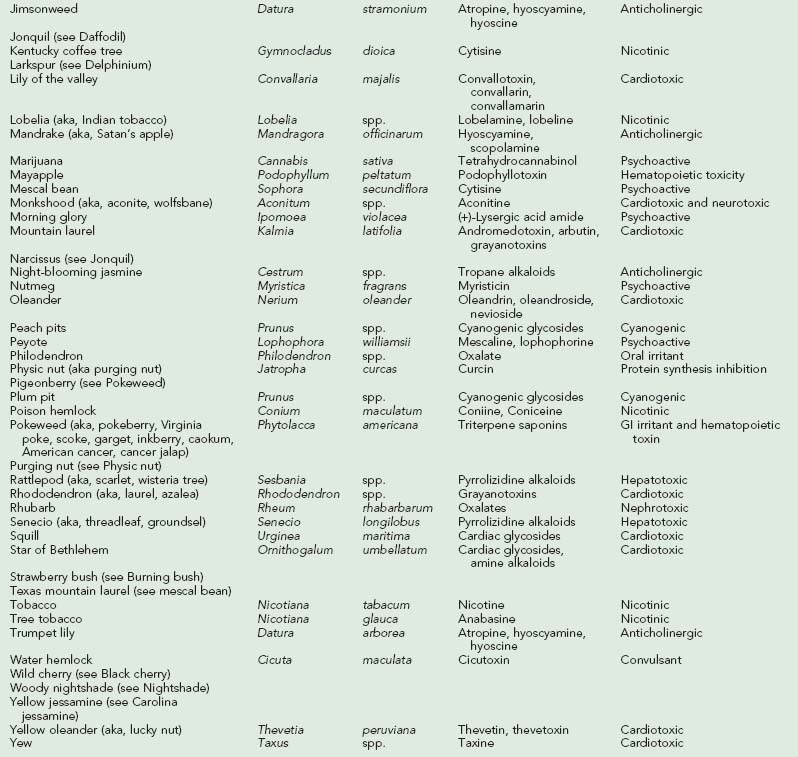
Over 63,000 plant exposures were reported to 61 American poison control centers in 2008, representing 2.5% of all human toxic exposures reported that year. The most frequently reported exposures were peace lily, pokeweed, philodendron, poison ivy and poinsettia. One death due to plant poisoning, out of 1756 deaths due to poisoning, was reported.34 A review of plant poisonings reported to American poison control centers over an 18-year period (1983 to 2000) revealed 30 fatalities, with seven due to Cicuta species (water hemlock) and five due to Datura species (jimsonweed).152 Most plant exposures are unintentional and occur in children under the age of 6 years.168 Intentional ingestions are more common in adults, due to suicidal intent or abuse, and are more likely to produce serious poisonings.34,168 Worldwide, Atropa belladonna (deadly nightshade), Hyoscyamus niger (black henbane), Conium maculatum (poison hemlock), Areca catechu (betel nut), Thevetia peruviana (yellow oleander), Nerium oleander (common oleander), Cerbera manghas (sea mango), Veratrum album (hellebore), Aconitum carmichaeli (aconite), Taxus spp. (yew), Colchicum autumnale (autumn crocus), Gloriosa superba (glory lily), Ricinus communis (castor bean), Atractylis gummifera (bird-lime or blue thistle), and Blighia sapida (ackee fruit) also cause significant morbidity and mortality.*
General Considerations
Prompt and precise identification of the plant causing toxicity may not be feasible.34 Supportive care takes priority over plant identification. Airway, breathing, and circulation are assessed, including hydration status, end-organ perfusion, and urine output. Oral administration of activated charcoal (1g/kg), up to 50 g, may aid gastrointestinal (GI) decontamination; however, recommendations on when to administer activated charcoal are challenging, because this has not been prospectively studied for most plant ingestions. If a patient is actively vomiting, activated charcoal may or may not contribute to decontamination. If the patient has a depressed mental status or is likely to seize, administration of activated charcoal may result in pulmonary aspiration of charcoal. Opinions on when to administer activated charcoal vary between toxicologists. After emergency care has been provided, a history should include time of ingestion, amount and part of plants ingested, initial symptoms, and time between ingestion and onset of symptoms. Method of preparation (e.g., drying, cooking, boiling) and number of persons who ate the same plant are also important considerations. Plant identification may be aided by communication with poison control center personnel and by Internet searches.12,273,274
Laboratory studies depend on clinical presentation and suspected plant exposure. Complications of poisoning include aspiration pneumonia, rhabdomyolysis, and deep vein thrombosis. Differential diagnosis is initially kept broad so that other illnesses, such as infection and trauma, are not missed. Table 64-1 provides signs and symptoms of toxic plant ingestions.
Plant Toxins
Alkaloids
Alkaloids are nitrogen-containing organic compounds that act as bases and form salts with acids. Plant alkaloids are soluble organic acid–alkaloid salts that contain nitrogen in a ring structure that is heterocyclic or aromatic, or both. Alkaloids are generally distributed throughout a given plant, so all ingested parts are toxic. Further subdivision into chemical groups is based on ring structure (Table 64-2).
| Alkaloid Type | Alkaloid Structure | Examples |
|---|---|---|
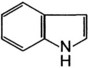
| Indole |
Aconite (monkshood)
Glycosides
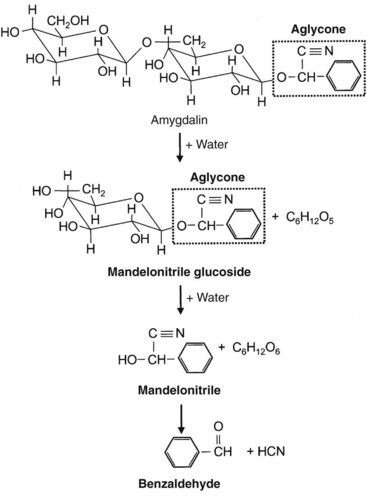
Sugars in the form of acetals are called glycosides. In glycosides, a glycosyl group replaces an alcohol or a hydroxyl group. On hydrolysis, glycosides yield sugars (glycones) and aglycone compounds. The aglycone moiety accounts for most of the toxicity, although the sugar may enhance solubility and absorption (Figure 64-1, online). Glycoside-producing plants include cardioactive, cyanogenic, saponin, anthraquinone, and coumarin glycoside compounds.
Phytotoxins
Phytotoxins, or toxalbumins, are among the most toxic substances of plant origin. They are composed of large protein molecules that resemble bacterial toxins in structure and in their ability to act as antigens (Figure 64-2, online).
Central Nervous System Toxins
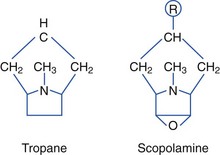
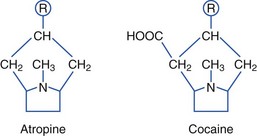
Anticholinergic Plants (Tropane Alkaloids)
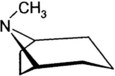
Plants containing tropane alkaloids (often called belladonna alkaloids) include atropine, hyoscyamine (the levorotatory isomer of atropine), and hyoscine (scopolamine).* Structures of the tropane alkaloids follow:
Tropane alkaloids are found in about 25 genera and 2000 species of plants. Plants causing human toxicity include A. belladonna (deadly nightshade), Mandragora species (mandrake), H. niger (black henbane), Datura species (jimsonweed), and Brugmansia species (angel’s trumpet).† The Solanaceae family includes Solanum and Scopolia carniolica, the source of scopolamine.
Anticholinergic Syndrome
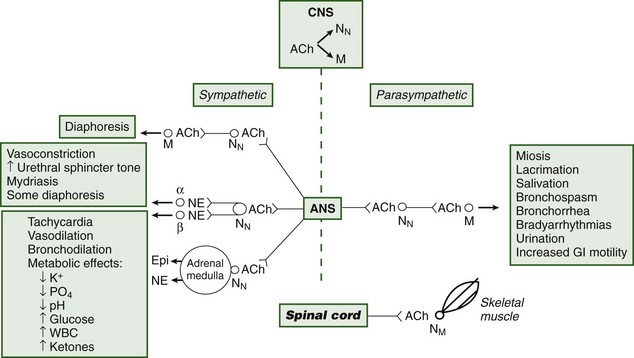
Toxicity may ensue after ingesting or smoking plant parts.45 The tropane alkaloids competitively inhibit postsynaptic muscarinic receptors, producing the classic anticholinergic syndrome (Figure 64-3 and Box 64-1).104,113,203,209,230 A useful clinical sign of anticholinergic toxicity is lack of perspiration in the axillae. Anticholinergic findings suggestive of poisoning may be remembered using the following mnemonic:209,240
Jimsonweed
Datura species are generally known as jimsonweed or thorn apple, and Brugmansia species are generally known as angel’s trumpet (Figure 64-4).112,113,263 Young, thin, and tender stems of jimsonweed contain the highest concentration of tropane alkaloids.179 However, the seeds also contain high concentrations of the alkaloids, and as little as one-half a teaspoonful of seeds may cause death from cardiopulmonary arrest. The word jimsonweed is thought to be derived from Jamestown, Virginia, where British troops reportedly behaved bizarrely after consuming Datura in 1676. D. stramonium has been reportedly used in Haitian zombification rituals.45,67,112,113 Jimsonweed has been used to treat asthma, heralding the current use of ipratropium bromide.
Reports of abuse of Datura and Brugmansia as hallucinogens continue to be reported in the United States and Europe.45,113,217,255 Clusters of poisonings among adolescents are typical.* Frequently, a tea of the plant parts is brewed.138 Report of toxicity after using a homemade Datura toothpaste has also been reported.205 A mass poisoning occurred in Botswana after consumption of porridge made from sorghum flour contaminated with seeds of D. stramonium.197 Symptoms may appear within minutes and may last for days.68,203,255 Tachycardia, dry mouth, agitation, nausea, vomiting, incoherence, disorientation, auditory and visual hallucinations, mydriasis, decreased bowel sounds, slurred speech, hyperthermia, flushed skin, urinary retention, and hypertension have been reported.† Blurred vision and photophobia may be secondary to mydriasis. Isolated mydriasis and cycloplegia may be seen, including anisocoria, after topical contact to the eyes.123,130,263 With severe toxicity, seizures, flaccid paralysis, and coma may ensue. Focal neurologic signs and posturing, as well as death, have also been reported.‡ Death may occur as a result of behavioral changes, leading to trauma, drowning, or environmental exposure associated with hyper- or hypothermia.138 Autopsy has revealed edema of the brain and lungs with focal hemorrhages within the alveolar lumens, ischemic lesions and edema of the heart, and hyperemia of the sinusoidal tracts of the liver.31
Anticholinergic poisoning should be suspected when patients are observed to communicate with imaginary friends with mumbled speech, and to demonstrate repetitive picking behavior.112,242 Symptoms generally begin 30 to 60 minutes after ingestion and continue for 24 to 48 hours, but they may begin within several minutes and persist for several days. Patients are generally amnestic of events during their poisoning. Studies may show leukocytosis, mild transient elevation of liver enzymes, elevated creatine phosphokinase (CPK) levels and other evidence of rhabdomyolysis, and changes on an electrocardiogram (ECG) that are consistent with tachycardia and dysrhythmias.45,68,89,113
Deadly Nightshade
Ingestion of A. belladonna is less common than Datura and Brugmansia ingestion. All parts of deadly nightshade contain tropane alkaloids, but the highest concentrations are in the ripe fruit and green leaves; each berry may contain up to 2 mg of atropine. The berries may be mistaken for bilberries (hurtleberries). A family of eight had varying acute exposures to A. belladonna after eating both raw and cooked berries in a pie. The most severely poisoned patient had anticholinergic symptoms, with hypertonia, hyperthermia, respiratory failure, and coma requiring mechanical ventilation. Urine drug screens detected only atropine and not scopolamine, the latter which is present in much smaller quantities.230,242 A review of 49 children with acute deadly nightshade intoxication found that children most commonly demonstrated meaningless speech, tachycardia, mydriasis, and flushing; lethargy and coma were seen in the more severely poisoned children.37 A patient with seasonal chronic ingestion presented with recurrent tachycardia, mydriasis, inability to concentrate, visual hallucinations, delusions, inappropriate laughter, dizziness, and headache.140
Treatment
Treatment of anticholinergic plant exposure consists primarily of decontamination and supportive care, including airway protection, intravenous (IV) fluids, and vasopressors for hypotension resistant to IV fluids. Hyperthermia should be assessed and treated if significant. Agitation can be treated with careful administration of benzodiazepines. Haloperidol and phenothiazines should not be used because these agents may enhance toxicity. Foley catheterization or nasogastric tube placement may be necessary if there is bladder distention or decreased gut motility, respectively.104,138,140,200,230
Some recommend treating severe central anticholinergic syndrome with carefully titrated physostigmine, which is derived from the calabar bean of Physostigma venenosum. This cholinesterase inhibitor blocks acetylcholine degradation; the resultant accumulation of acetylcholine overcomes the competitive inhibition of atropinic agents, such as those found in jimsonweed. Rapid, though generally transient, reversal of peripheral and central nervous system (CNS) effects can ensue. However, bradycardia, asystole, ventricular arrhythmias, hypotension, bronchospasm, bronchorrhea, and seizures have been reported after rapid IV administration of physostigmine, limiting its routine use. Persons with cardiac conduction abnormalities are particularly susceptible to the cardiac complications. Use of physostigmine generally does not shorten the hospital stay.*
Most cases of anticholinergic poisoning can be managed safely and effectively with supportive care alone.240 Consultation with a toxicologist is recommended before using physostigmine. If physostigmine is used, the recipient should be on a cardiac monitor, with pulse oximetry, and a physician at the bedside should slowly administer graduated doses of the drug.36,104,242
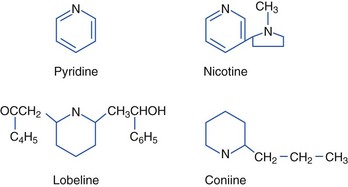
Nicotinic Plants (Pyridine and Piperidine Alkaloids)
Tobacco Plants
Tobacco contains both nicotine and related alkaloids with similar pharmacologic properties, such as the alkaloid anabasine, which is found in Nicotiana glauca (wild tree tobacco) (Figure 64-5). N. glauca has been mistaken for Amaranthus hybridus (marog) and eaten with porridge in South Africa. Ingestion of N. glauca is generally fatal. Anabasine, an isomer of nicotine that appears to be more toxic than nicotine, probably accounts for much of the toxicity of N. glauca, N. debneyi, and N. rotundifolia. Anabasine concentrations can be particularly high in the roots of the plants. Although there are over 60 Nicotiana species, some other more common tobacco family members include N. rustica (Mapacho), N. tabacum (cultivated or common tobacco), N. trigonophylla (desert tobacco) (Figure 64-6), and N. attenuata (coyote tobacco). N. tabacum is the major source of commercial tobacco and contains between 0.5% and 9% nicotine. N. rustica (Mapacho) tends to contain higher concentrations of nicotine than does N. tabacum. Nicotine alkaloids are rapidly absorbed from the oral and GI tracts, the respiratory tract, and the skin. The kidneys excrete nicotine fairly rapidly after biotransformation in the liver and lungs. The half-life is 1 to 2 hours. Although the lethal dose of ingested nicotine is not well established, 2 to 5 mg may cause nausea, and 40 to 60 mg may be lethal in humans. One to two cigarettes, ingested and absorbed, could be lethal in a child.176,180,228,236,247
Nicotinic Syndrome
Peripherally, acetylcholine is a neurotransmitter for autonomic and somatic motor fibers. It is stored in vesicles within the presynaptic neuron and is released by calcium-dependent exocytosis into the synapse, where it binds to receptors and is eventually degraded by acetylcholinesterase. Acetylcholine can bind to two receptor types, nicotinic and muscarinic. Nicotinic receptors are located on postganglionic autonomic neurons (NN receptors) and at skeletal neuromuscular junctions (NM receptors). Direct nicotinic agonists (e.g., arecoline, coniine, cytisine, lobeline, nicotine) prolong depolarization at these receptors and eventually cause blockade of nicotinic receptors. Clinical evidence of stimulation followed by blockade is apparent. Hypertension, tachycardia, pallor, vomiting, diarrhea, abdominal pain, salivation, bronchorrhea, tachypnea, muscle fasciculations, spasms, confusion, agitation, tremor, and convulsions (the stimulation) are followed by hypotension, bradyarrhythmias (occasionally asystole), hyporeflexia, paralysis, coma, and respiratory failure (the blockade). When death occurs, it is generally due to respiratory paralysis (Box 64-2; see also Figure 64-3).*
Green tobacco sickness is a mild form of nicotine poisoning seen in tobacco-naive field workers with dermal exposure to leaves of green tobacco in wet environments. Nicotine, a water-soluble alkaloid, is absorbed dermally. The syndrome is characterized by weakness, salivation, nausea, vomiting, diarrhea, abdominal cramps, headache, dizziness, visual and hearing disturbances, respiratory depression, and occasionally fluctuations in blood pressure and heart rate. Neuromuscular blockade may result in death. A urinary nicotine metabolite, cotinine, may be helpful diagnostically.15,176,228,247
Lobeline, derived from the Indian tobacco plant Lobelia inflata, is a high-affinity nicotinic ligand. It can cause nicotine-like effects, but is generally less toxic than is nicotine.63
Poison Hemlock
C. maculatum (poison hemlock) (Figure 64-7), also known as spotted hemlock, California or Nebraska fern, stinkweed, fool’s parsley, and carrot weed, is often mistaken for an edible plant, such as parsley, parsnip, or anise; however, it has a mousy odor and an unpleasant bitter taste, and it burns the mouth and throat. The stem is hollow with purplish to reddish brown spots. Its long taproot is solid and parsnip-like. Although all plant parts are poisonous, the unripe fruit is especially toxic. Poisonings are more common in the spring and summer. Poisoning may also occur after eating birds that have consumed poison hemlock. Coniine and γ-coniceine, the principal alkaloids in C. maculatum, are pyridine derivatives similar to nicotine. Coniine is more toxic than is γ-coniceine. The alkaloid is volatile and susceptible to drying and heating.25,152,228,268,274
C. maculatum was used in ancient times for capital punishment and murder. The primary action of the toxic alkaloids is activation and then blockade of nicotinic acetylcholine receptors. Initially, stimulation causes sialorrhea, nausea, vomiting, diarrhea, abdominal cramping, tremor, ataxia, confusion, blurred vision, hypertension and tachycardia, followed by dry mucosae, GI hypotonia, lethargy, weakness, diminished cardiac contraction, hypotension, and bradycardia, as with nicotinic syndrome. Muscles rapidly swell and stiffen, with multifocal necrosis of myocytes and associated muscle pain. Muscle fasciculations may be followed by flaccid paralysis, including respiratory paralysis. Rhabdomyolysis and acute tubular necrosis with renal failure may occur. Elevated CPK and liver function tests (LFTs) may be noted. Death is usually from respiratory failure. Autopsy may reveal congestion of the lungs and liver.*
Betel Nut
Areca catechu (areca palm) (Figure 64-8) produces betel nut, a common masticatory drug in the Far East, Asia, India, and the South Pacific; it is also shipped elsewhere. An estimated 10% to 25% of the world’s population chews “betel quid.” It is generally chewed with slaked lime paste (calcium hydroxide) wrapped in leaves of betel pepper (Piper betel). known as “quid,” “punsupari,” and “pan masala.” Occasionally, tobacco is added. Quid is sucked in the lateral gingival pocket. Commercially, areca nut is marketed in the form of sweetened areca nut, known as “supari.” A. catechu contains arecoline and guvacoline, which are hydrolyzed to arecaidine and guvacine, respectively. These are strong inhibitors of gamma-aminobutyric acid (GABA) uptake. These arecal alkaloids also are nicotinic and muscarinic. Betel pepper leaves contain betel oil, which contains psychoactive phenols and cadinene. These possess cocaine-like properties. Clinical effects resemble nicotinic syndrome and cholinergic toxicity, including CNS effects (dizziness, euphoria, subjective arousal, altered mental status, hallucinations, psychosis, convulsions), cardiac effects (tachycardia, hypertension, palpitations, arrhythmias, bradycardia, hypotension, chest discomfort, and acute myocardial infarction in susceptible individuals), pulmonary effects (bronchospasm, tachypnea, dyspnea), GI effects (salivation, vomiting, diarrhea), urogenic effects (urinary incontinence), and musculoskeletal effects (weakness and paralysis). Use with calcium salts may result in hypercalcemia, hypokalemia, and metabolic acidosis with renal potassium wasting and renal insufficiency. Betel nut use is also associated with flushing, diaphoresis, warm sensations, red- or orange-stained oral mucosa and saliva, and teeth stained dark brown or black. Precancerous oral lesions may also occur with chronic use.†
Blue Cohosh
Caulophyllum thalictroides is known as blue cohosh or squaw root. All parts of the plant contain the nicotinic alkaloid n-methylcytisine, which is much less potent than nicotine. Teas made from C. thalictroides have been used to induce labor and have resulted in newborn death and perinatal complications of stroke, congestive heart failure, respiratory failure, and circulatory collapse. The infant toxicity is thought to be due to the toxic saponins, caulosaponin and caulophyllosaponin.228
Golden Chain Tree
All parts of Laburnum anagyroides (golden chain tree) are toxic and contain the nicotinic alkaloids cytisine and n-methylcytisine. Cytisine is a quinolizidine alkaloid but is often classified with the pyridine and piperidine alkaloids. These alkaloids stimulate nicotinic ganglions. The toxins are concentrated in the seeds. Toxicity is generally only mild to moderate; however, an unusual fatality occurred in which 23 seedpods were found in the stomach of an adult at autopsy. Other plants that contain cytisine include Kentucky coffee tree (Gymnocladus dioica), necklace pod sophora (Sophora tomentosa), and mescal bean bush (Sophora secundiflora) (Figure 64-9) (see Hallucinogenic Plants, later). Structures follow:228
Treatment
Treatment of nicotinic syndromes consists of supportive care with particular attention to airway protection and ventilation. Administration of activated charcoal has been used because it adsorbs nicotine in vitro. However, nicotine and related alkaloids are absorbed rapidly and often produce vomiting, which may limit the usefulness of activated charcoal. These alkaloids may also induce altered mental status, which can increase the risks associated with activated charcoal. Benzodiazepines and barbiturates are given for seizures. Adequate urine output is maintained, with consideration of urine alkalinization. Treating initial excessive adrenergic stimulation with adrenergic antagonists is ill advised because this complicates the nicotinic blockade that typically follows. Symptomatic bradycardia is treated with atropine; hypotension can be treated with IV fluids and inotropic agents if needed. Atropine may also help to treat bronchorrhea.228
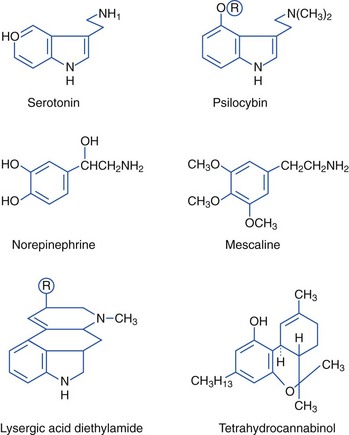
Hallucinogenic Plants (Indoles, Phenylalkylamines)
Ergot
Ergotism has two forms, gangrenous and convulsive. Both begin similarly with a vague illness that may include GI symptoms, progressing to a sensation of ants crawling on the skin, primarily affecting the legs. Hallucinations can occur with both forms. Gangrenous ergotism, a vasospastic disease, was the cause of the ninth-century epidemic disease known as St Anthony’s fire, which consisted of burning limb pain and eventual loss of limb sensation, followed by gangrene and autoamputation of charcoal-black limbs. People migrated to St Anthony’s Order, a society of monks, and began to heal, once they received a diet free of ergot-contaminated grains. Convulsive ergotism is characterized by painful flexion of the fingers and wrists, flexion or extension of the ankles, double vision, altered mental state, hallucinations, and diaphoresis. With progression, the body becomes bent in spasm, appearing to roll up into a ball, or it becomes rigid in extension; this may be followed by seizures.*
Morning Glory
The active component of the naturally occurring hallucinogen found in the seeds of morning glory (Ipomoea violacea) (Figure 64-10) is ergine, or (+)-lysergic acid amide, an indole derivative.112 About 300 seeds, or enough to fill a cupped hand, are equivalent to 200 to 300 mg of LSD, with similar systemic and hallucinatory effects. Ingestion of Hawaiian baby woodrose seeds (Argyrlia nervosa) has similar effects.105
Nutmeg
Myristica fragrans (Figure 64-11) is used to make the spices nutmeg and mace. Mature rinds of the fruit split, revealing a bright-red, fringed, fleshy coating on the outside of its seed. The coating contains mace, and the seed contains nutmeg. Nutmeg contains myristicin, which has an indole-like structure and is metabolized to amphetamine-like compounds. Other alkylbenzene derivatives, such as safrole and elemicin, are also found in nutmeg. Nutmeg has been abused for its alleged hallucinogenic effects. A person who ingested one grated nutmeg (7 g) had weakness, loss of coordination, vertigo, fainting, nausea, and paresthesias but no hallucinations.238,265
Cannabis
Cannabis preparations are largely derived from the female Cannabis sativa plant. The primary psychoactive component is δ-9-tetrahydrocannabinol (THC), which is most concentrated in the flowering tops. Marijuana generally contains 0.5% to 5% THC; however, the Sinsemilla and Netherwood varieties may contain up to 20% THC, and hashish and hashish oils have higher concentrations. Cannabinoids can be smoked or ingested. A typical marijuana cigarette contains 0.5 to 1.0 g of cannabis, and the THC delivered varies from 20% to 70%, with a bioavailability of 5% to 24%. As little as 2 mg of available THC produces effects in occasional users.119
Cannabinoids bind to specific cannabinoid receptors in areas of the brain involved with cognition, memory reward, pain perception, and motor coordination. Cannabinoids act as neuromodulators in the release and action of neurotransmitters (e.g., acetylcholine, glutamate).75,119 Endogenous ligands for these receptors are endocannabinoids. The first endocannabinoid identified was anandamide, named after the Sanskrit word ananda, which means “bliss.”
Desirable effects include mild mood-altering qualities, euphoria, alteration in perceptions, time distortion, and intensification of ordinary sensory experiences (e.g., gustatory, visual, and auditory sensations). Adverse effects include impairment of short-term memory and attention, mydriasis and slowly reactive pupils, impairment of motor skills and reaction times, nausea, vomiting, and anxiety. Psychotic symptoms have been reported in persons vulnerable to psychosis. Clinically, tachycardia may occur within minutes of THC exposure and may last a few hours. Minor changes in blood pressure may also occur. Exposed toddlers may present with lethargy, slurred speech, ataxia, and shaking.27,119
Peyote
The hallucinogenic peyote cactus Lophophora williamsii (Figure 64-12) contains alkaloids that are phenylethylamines or isoquinolines rather than indoles. Mescaline (3,4,5-trimethoxy-β-phenylethylamine), the primary psychoactive component of peyote, is structurally similar to the neurotransmitters norepinephrine and epinephrine and to hallucinogenic amphetamines. Pharmacologically, however, mescaline is similar to hallucinogenic indoles. Mescaline may affect the action of norepinephrine and serotonin, evidenced clinically by sympathomimetic effects, followed by marked visual hallucinations. Mescaline produces slight rises in blood pressure and heart rate, tachypnea, hyperreflexia, mydriasis, ataxia, perspiration, flushing, salivation, and urination. No deaths have been reported from peyote poisoning. Type B botulism was associated with consumption of a ceremonial tea made from peyote that was stored in a jar by members of the Native American Church. Affected members had bilaterally symmetric, flaccid weakness in all extremities, dysphagia, nasal speech, and diplopia.9,126
Mescal Bean Bush
The mescal bean bush or Texas mountain laurel (Sophora secundiflora) of the pea family (Fabaceae) produces hallucinogenic dark-red beans (mescal beans; see Figure 64-9). The beans contain the toxic alkaloid cytisine, which causes nausea, numbing sensations, hallucinations, unconsciousness, convulsions, and death through respiratory failure. The beans may be boiled in water and the mixture consumed, producing a delirium or “visionary trance.” The origin of mescalism in modern peyote religion is debated. Mescal beans are worn during some peyote ceremonies.9
Tabernanthe iboga
Tabernanthe iboga contains indole alkaloids, including ibogaine. The root of this plant is used in West Africa to communicate with ancestors, reportedly producing “visions” and “waking dreams.” It produces altered states of consciousness, delusions, hallucinations, mydriasis, tachycardia, tremor, and ataxia. Convulsions and lethal respiratory arrest have also been reported. A man was found dead after ingesting a powdered root bark of T. iboga shrub mixed with sweet concentrated milk. Autopsy revealed pulmonary edema with hemorrhagic alveolitis and vascular congestion.39,151
Khat
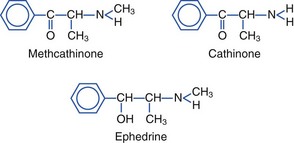
The evergreen khat tree, Catha edulis, grows in East Africa and Arabia (Figure 64-13). Khat is also known as chat, qat, kat, kath, gat, eschat, miraa, murungu, qaad, and jaad. As early as 1237, khat was advocated in Arabic medical literature as a mood-elevating and hunger-suppressing agent. Khat leaves and bark continue to be chewed, with the juice of the masticated plant being swallowed for stimulatory effects. Khat contains cathinone (2-amino-1-phenyl-1-propanone), cathine (norpseudoephedrine), and norephedrine. Cathinone, a phenylalkylamine, is the major psychoactive constituent. Structurally, cathinone is similar to amphetamines, and khat has been referred to as a “natural” amphetamine. Like amphetamines, cathinone is an indirect sympathomimetic, inducing release of dopamine, serotonin, and norepinephrine.*
The structures of methcathinone, cathinone, and ephedrine follow:
Desirable effects of khat include increased energy and alertness, feelings of increased endurance and self-esteem, enhanced imaginative ability, higher capacity to associate ideas, and euphoria. Cathinone has both positive chronotropic and inotropic effects. Tachycardia, increased blood pressure, tachypnea, and mydriasis are seen. Adverse effects include anorexia, hypomania, insomnia, delusions, paranoid psychosis, aggression, depression, anxiety, hyperthermia, stomatitis, oral lesions/cancers, gastritis, and endocrine disturbances. Khat use has also been associated with vasoconstriction, acute myocardial infarction, leukoencephalopathy and an increased incidence of acute cerebral infarction. Khat is known to be habit forming and has been classified as a substance of abuse by the World Health Organization. Cathinone is a Schedule I controlled substance under federal regulations in the United States.†
Sedating Plants (Isoquinoline Alkaloids)
Poppy
Papaver somniferum flowers are large and white with purple stains at the base of each petal. They yield opium, a complex of more than 20 alkaloids, including morphine, codeine, and papaverine. Morphine was the first plant alkaloid isolated, by pharmacist Friederich Wilhelm Adam Serturner in 1806.117 Seeds of P. somniferum are used in various foods and beverages, including bagels, muffins, pastries, curry sauce, rice, and teas. Opiate toxicity from poppy seed exposure occurred in a 6-month-old infant given 75 mL of strained milk made with 200 g of poppy seeds in 500 mL milk, resulting in respiratory arrest requiring ventilation. Opiate toxicity has also occurred after ingestion of a boiled poppy plant and after ingestion of spaghetti with poppy seeds. Poppy dependence has also been described. Ingestion of poppy seeds can result in detectable levels of morphine and codeine by urine drug screen testing.146,158,204,262
Neuromuscular Blocking Plants (Indole Alkaloids)
Yellow Jasmine
Gelsemium sempervirens (Carolina or yellow jessamine) is a woody perennial evergreen vine with fragrant yellow flowers. It contains multiple indole alkaloids, including gelsemine, gelseminine, and gelsemoidin. Gelsemine binds to acetylcholine receptors at the neuromuscular junction (peripheral nicotinic acetylcholine receptors) and, to a lesser extent, at muscarinic receptors. A toddler who ate the blossoms of G. sempervirens experienced neuromuscular blockade with ataxia, dysarthria, facial and extremity weakness, bilateral ptosis, and transient coma. The child recovered without sequelae.26
Convulsant Plants (Indoles, Resins)
Strychnine
Strychnine, an indole found in seeds of the tree Strychnos nux-vomica, is a powerful CNS stimulant. Poisoning may also occur after ingestion of rodent poisons containing strychnine, with the use of illicit drugs contaminated with strychnine, or with the use of herbal remedies contaminated with strychnine. Strychnine is especially concentrated in the seeds and roots of the plant. The bark of S. nux-vomica also contains the alkaloid brucine, which is less potent than strychnine, but produces similar toxicity when consumed. Strychnine is a selective, competitive antagonist of glycine, a major inhibitory neurotransmitter, at its postsynaptic receptors in the spinal cord and brainstem. Poisoning produces an excitatory state, with hyperreflexia, hypersensitivity to stimuli, migratory rippling movements of the muscles, twitching, rigidity, and spinal convulsions (generally, flexor spasm of the upper limbs, extensor spasm of the lower limbs, opisthotonic posturing, and spasms of the jaw muscles, all without loss of consciousness or postictal states). Minimal stimulation elicits diffuse muscle contractions. In between spasms, which last from 30 seconds to 2 minutes, muscles become completely relaxed. Respiratory and secondary cardiac failure may ensue during severe convulsions. Treatment consists of supportive care, benzodiazepines, and barbiturates. Chemical paralysis with a nondepolarizing agent, endotracheal intubation, and mechanical ventilation may be required for severely poisoned patients. Hyperthermia, rhabdomyolysis, renal failure, and acidosis may occur secondary to convulsions. These complications often require treatment. Occasionally, death ensues despite aggressive treatment.*
Some Strychnos species in Africa contain alkaloids that produce curare-like effects. These alkaloids act through nondepolarizing and competitive mechanisms at the neuromuscular junction, competing with acetylcholine for the receptor, hence blocking nerve-to-muscle transmission. This results in paralysis; however, paralysis is not seen after ingestion of these species, because the toxic alkaloids are not absorbed from the gastrointestinal tract.207
Wild Wisteria
The root of Securidacea longepedunculata (violet tree; wild wisteria) has been used as an intravaginal suicidal poison and an abortifacient. The distilled oil of the root is primarily methyl salicylate (discussed latter with essential oils); however, wild wisteria also contains the alkaloid securinine, a GABA-A receptor antagonist, which produces hyperreflexia, hypertonia, and seizures. Death can result within hours of placing the root intravaginally, and is generally preceded by vomiting, diarrhea, and dehydration.246
Water Hemlock
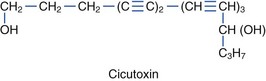
Species within the Umbelliferae (also called Apiaceae) family are divided into the Cicuta and Oenanthe genera. Nine subspecies of Cicuta of the Umbelliferae family are poisonous (Figure 64-14). C. virosa is common European water hemlock, whereas C. maculata and C. douglasii are found in North America. Oenanthe crocata, or hemlock water dropwort, is found in Europe and North America. Common names for Cicuta species include cowbane, five-finger root, snake weed, snake root, wild carrot, dead man’s fingers, death-of-man, poison parsnip, wild parsnip, beaver poison, children’s bane, muskrat weed, spotted hemlock, spotted cowbane, musquash root, false parsley, fever root, mock-eel root, wild dill, spotted parsley, and carotte à Moreau. Roots have a parsnip-like or carrot-like odor, and Cicuta species are often mistaken for edible plants, such as water parsnip. These plants have also been mistaken for pignut, sweet flag, wild carrot, wild celery, wild ginseng, and kvanne. Mature roots have air-filled chambers and are found on the ends of hollow stems. All parts of the plant are toxic, but the roots contain the highest concentration of cicutoxin. Ingestion of as little as 2 to 3 cm of the root may be fatal to an adult. The severity of poisoning and time until onset of symptoms is proportional to the amount of plant ingested. The plant is most toxic in the spring. The principal toxins, cicutoxin and oenanthotoxin, contained in these plants act as noncompetitive GABA antagonists in the central nervous system, resulting in unabated neuronal depolarization that manifiests as seizures, including status epilepticus.104,131,152,229
Water hemlock poisoning should be considered in any patient who presents with cholinergic-like poisoning and abrupt onset of seizures. Early symptoms include muscarinic effects and involve primarily the GI tract: abdominal pain, vomiting, and diarrhea. However, marked diaphoresis, salivation, and respiratory distress may also be seen. Nicotinic effects are less prominent (see Figure 64-3). Tachycardia and hypertension or bradycardia and hypotension may be seen. Dysrhythmias may occur. Ataxia, paresthesias, muscle spasms, weakness, and altered mental status have been reported. With severe poisoning, coma and epileptiform seizure activity, or spastic and tonic movements, including opisthotonus without electroencephalographic seizure activity, may occur. Rhabdomyolysis and renal failure have been reported. Deaths may be associated with persistent seizures, cerebral edema, ventricular fibrillation, pulmonary edema, cardiopulmonary arrest, and disseminated intravascular coagulation. Laboratory abnormalities include metabolic acidosis and elevated CPK and LFTs. Treatment includes securing an airway, ventilation, and treatment of seizures with benzodiazepines and barbiturates. Phenytoin is contraindicated because it is ineffective for seizure control. Anticholinergic agents are not recommended, because these agents do not reduce seizure activity. Continuous EEG monitoring is helpful. Treatment of hypoxia, acidosis, hyperthermia, rhabdomyolysis, and cerebral edema should be provided. Hypotension can be treated with intravenous fluids and vasopressors. Recovery can take up to 4 days, and some patients never completely recover. Although survival has improved with aggressive supportive care, death may still ensue. Autopsies reveal pulmonary and cerebral edema, brain hemorrhages and renal necrosis.104,131,152,229
Myrtle-leaved Coriaria
Coriaria myrtifolia (also known as myrtle-leaved coriaria, Currier’s sumach, or Redoul sumach) grows in the western Mediterranean area. C. myrtifolia contains coriamyrtin, an analogue of picrotoxin. The toxin is found in high concentrations in the berries, which resemble blackberries. Ingestion of only a few fruits can produce significant toxicity. The leaves are also toxic. Rarely, individuals have become poisoned after eating snails acquired off the plant, after drinking the milk of goats that had been eating the plant, and after consuming honey contaminated by its nectar. The leaves have also been mistakenly eaten when thought to be the leaves of senna (Cassia senna L.). Initially, vomiting, abdominal pain and drunken intoxication are seen. This may be followed by twitching, seizures (including status epilepticus), coma and apnea. Death may occur. Treatment with benzodiazepines and barbiturates should be considered.69
Cardiovascular Toxins
Cardiovascular toxins found in plants are listed in Box 64-3.
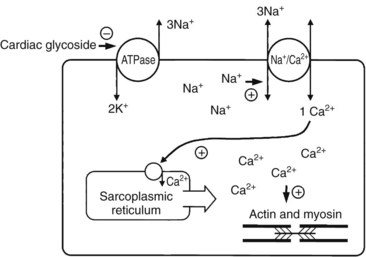
Cardiotoxins that Inhibit Na+/K+ Atpase (Cardiac Glycosides)
More than 200 naturally occurring cardiac glycosides have been identified. Plant cardiac glycosides are composed of a steroid backbone, an attached five-membered unsaturated lactone ring (six-membered for Helleborus), and either a carbohydrate or sugar moiety in glycosidic linkage. The toxic aglycones are released by acid and enzymatic hydrolysis. The attached sugar moiety has no inherent cardiac action but may enhance solubility, absorption, and toxicity of the aglycone moiety. Cardiac glycosides bind to the membrane-bound enzyme Na+,K+ ATPase, increasing intracellular Na+ and Ca2+ levels and automaticity (Figure 64-15).215 Cardiac glycosides are found in Digitalis purpurea (foxglove) (Figure 64-16), Digitalis lanata, N. oleander (common oleander) (Figure 64-17), T. peruviana (yellow oleander) (Figure 64-18), Convallaria majalis (lily of the valley) (Figure 64-19), Urginea maritima (squill or sea onion) (Figure 64-20), U. indica, Cerbera manghas (sea mango), C. odollam (pink-eyed cerbera), Strophanthus gratus (ouabain), Asclepias species (balloon cotton, red-headed cotton-bush milkweeds), Calotropis procera (king’s crown), Carissa spectabilis (wintersweet), C. acokanthera (bushman’s poison), Plumeria rubra (frangipani), Cryptostegia grandifolia (rubber vine), Euonymus europaeus (spindle tree), Cheiranthus, Erysimum (wallflower), and Helleborus niger (henbane).*

FIGURE 64-17 Nerium oleander (common oleander) plants have white or pink flowers (A) and long, narrow seedpods (B).
(Courtesy Kimberlie A. Graeme, MD.)
Foxglove
D. purpurea grows wild in parts of the United States and is cultivated as a garden ornamental plant. D. purpurea contains only digitoxin, not digoxin. Withering reported the medical use of extracts of D. purpurea based on a recipe for treating “dropsy.”129,192 The leaves have been consumed in a risotto when they were mistaken for borage leaves, and in a salad when mistaken for dandelion leaves.38,189 Toxicity has also been reported from consumption of contaminated field water with Digitalis plants growing nearby.189 D. lanata was mistakenly substituted for plantain in herbal products, with resultant human cardiotoxicity.239 D. lanata contains lanatosides A, B, and C, which yield digoxin and digitoxin. Intentional overdoses also occur.157,189
Oleander
All parts of N. oleander and T. peruviana are toxic, but the seeds contain more glycoside than do other parts of the plant. Yellow oleander (T. peruviana), a native plant of tropical America, grows abundantly in the United States. Ingestion of a couple of seeds of yellow oleander, known as “lucky nuts,” can result in death; however, the number of seeds ingested is a poor guide to the degree of poisoning. Yellow oleander contains the cardiac glycosides thevetin A and B, thevetoxin, neriifolin, peruvoside, ruvoside, and others. It is a popular suicidal agent in Sri Lanka and India. Of patients admitted with T. peruviana poisoning in Sri Lanka, 43% had arrhythmias, many requiring temporary pacing; 6% died shortly after admission. Common oleander (N. oleander), a native plant of the Mediterranean, grows abundantly in the United States. Common oleander contains the principal cardiac glycosides oleandrin and neriine, as well as folinerin and digitoxigenin. Severe toxicity has been reported after consumption of unprocessed common oleander leaves and prepared teas. Chronic toxicity has occurred with criminal intentional poisoning. Topical application of homemade N. oleander solutions onto psoriatic wounds has resulted in toxicity.*
Squill
U. maritima was used by ancient Egyptians and Romans as a diuretic, heart tonic, expectorant, emetic, and rat poison. Squill contains several cardiac glycosides, including scillaren A, glucoscillaren A, scillaridin A, and scilliroside.86,260,271
Sea Mango
C. manghas (sea mango) is similar to C. odollam (pink-eyed cerbera, yellow-eyed cerbera, suicide tree). This tree grows in India, Vietnam, Cambodia, Sri Lanka, Burma (Myanmar), Madagascar, and Australia. The plant has large white flowers that smell like jasmine. When the fruit is still green, it looks like a small mango. The inner fruit (kernel) is white, but upon exposure to air turns violet, then dark grey, and then black. The crushed, white fleshy kernel is commonly consumed as a suicidal poison in India and Sri Lanka. The seeds are quite toxic. The leaves are less toxic. These plants contain the toxins cerberoside, cerberin, and odollin. After ingestion, death can occur within hours.83,107,168,259
Clinical Presentation
The onset of symptoms and duration of action are well known for certain glycoside preparations, such as digoxin, digitoxin, and ouabain, but may vary considerably after plant ingestions. For example, oleandrin exhibits protracted binding times to cardiac myocardial tissues.161 Generally, cardiac glycoside toxicity produces nausea, vomiting, diarrhea, abdominal tenderness, visual changes (appearance of yellow and green colors, “halos,” geometric shapes, scintillations, photophobia), mental status changes (disorientation, psychosis, lethargy, stupor, dysarthria, weakness, dizziness, restlessness, seizures), cardiac disturbances (palpitations, premature ventricular beats, bradycardia, atrioventricular block, sinus node block, paroxysmal atrial tachycardia with block, junctional tachycardia, bidirectional ventricular tachycardia, ventricular arrhythmias, myocardial depression with cardiogenic shock, syncope), hyperkalemia, and death. When death occurs, it is generally caused by cardiotoxicity. The ECG may reveal nonspecific ST-segment and T-wave changes, similar to digoxin-induced changes.* Serum digoxin levels may be elevated after exposure to plants containing cardiac glycosides, because antibody-based digoxin assays cross-react nonquantitatively with many cardiac glycosides. Digoxin immunoassays predict only the presence of the glycoside, not the degree of toxicity.82,116,161,260 Conversely, the degree of hyperkalemia often correlates with severity of toxicity.82,107
Treatment
For tachyarrhythmias, lidocaine has been advocated. The role of magnesium is not known, although some have subjectively noted death following yellow oleander poisoning treated with intravenous magnesium. Amiodarone, quinidine, and calcium-channel blockers are contraindicated because they may increase digitalis concentration; beta-blockers are contraindicated because they may worsen heart block. Digoxin-specific Fab antibody fragments may couple to circulating cardiac glycosides and limit binding of cardiac glycosides to Na+,K+ ATPase. Administration of digoxin-specific Fab antibody fragments to reverse cardiotoxicity from plant glycosides has been successful in several animal and human studies. Clinical trials in Sri Lanka revealed that intravenous administration of 1200 mg digoxin-specific antibody fragments reversed life-threatening cardiac arrhythmias and corrected hyperkalemia after yellow oleander poisoning.†
Cardiotoxins that Open Sodium Channels (Steroid Alkaloids, Resins)
Aconite
Aconite poisonings have become more common, because of the use of Aconitum species (A. napellus [monkshood]) (Figure 64-21), A. carmichaeli (chuanwa), A. brachypodium, A. vulparia, and A. kusnezoffii (caowu) in herbal products. Delphinium species (larkspur) demonstrate similar toxicity. All parts of these plants are toxic, with the roots being most toxic. Aconitum species contain diterpenoid-ester alkaloids, particularly aconitine, but also mesaconitine, hypaconitine, and yunaconitine, which are neurotoxins and cardiotoxins. Boiling these plants in water hydrolyzes aconite alkaloids to less toxic benzoylaconine and aconine derivatives. Soaking or boiling, termed decoction, is generally done when these plants are used as herbal medicines. The estimated lethal dose of wild plant is 1 g.*
Aconite alkaloids activate voltage-sensitive sodium channels and affect excitable membranes of neural, cardiac, and muscle tissues. Hypaconitine affects sodium channels of the nerve membrane more selectively than does aconitine and is more potent than aconitine and mesaconitine in producing neuromuscular block. These neurotoxins produce conduction block and paralysis through voltage-sensitive sodium channels in axons. In cardiac tissues, prolonged depolarization prevents repolarization of excitable membranes. Enhancement of the transmembrane inward sodium current during the plateau phase of the action potential prolongs repolarization in cardiac myocytes and induces delayed and early after-depolarizations. Delayed after-depolarizations result in increased automaticity, such as premature ventricular beats. Early after-depolarizations produce lengthening of the QT interval. Hypotension and bradycardia may be induced by activation of the ventromedial nucleus of the hypothalamus.8,46,49,102,165
Symptoms begin within 3 minutes to 6 hours of ingestion and may persist for several days. Nonspecific GI symptoms (nausea, vomiting, diarrhea) are accompanied by neurotoxicity and cardiotoxicity. Visual impairment, dizziness, vertigo, ataxia, paresthesias (e.g., numbness of mouth and extremities), hyporeflexia, weakness, paralysis, coma, restlessness, and convulsions may occur. Cardiac effects are clinically similar to cardiac glycoside toxicity, with enhanced vagal tone, bradycardia, heart block, ectopic beats, supraventricular tachycardia, bundle branch block, junctional escape rhythms, ventricular tachycardia, bifascicular ventricular tachycardia, polymorphic ventricular tachycardia, torsades de pointes, ventricular fibrillation, asystole, and hypotension. Bidirectional ventricular tachycardia, which is generally considered suggestive of cardiac glycoside toxicity, has also been reported with aconite poisoning. Elevated CPK levels, with or without evidence of myocardial infarction, may occur. Hypokalemia, metabolic or respiratory acidosis, respiratory alkalosis, and renal and hepatic impairment may be noted. Occasionally, death ensues, generally from ventricular arrhythmias, such as refractory ventricular fibrillation. Respiratory paralysis may also result in death.*
Veratrum Alkaloids
Veratrum and Zigadenus species belong to the lily family. All parts of these plants are toxic, with the rhizomes being more toxic than the tops. Veratrum species may be found in some sneezing powders and have been accidentally ingested when mistaken for Gentiana lutea (yellow gentian). Gentian wines are usually made from G. lutea. When accidentally made from Veratrum species, these wines can be toxic. V. viride has also been mistaken for skunk cabbage, ramps, and pokeweed (Phytolacca americana), producing toxicity after ingestion. Zigadenus species, also known as foothill camas, death camas, and mountain camas, have been accidentally ingested when mistaken for nontoxic wild onions (Allium macropetalum) and sego lilies (Calochortus nuttallii). Amianthium muscitoxicum and Schoenocaulon officinale (Sabadilla) also contain veratrum alkaloids. Veratrum alkaloids include protoveratrine, veratridine, cevadine, jervine, zygadenine, and zygacine, which are found in the entire plant, although the bulb and flower usually cause toxicity. These alkaloids are extremely toxic and rapidly increase permeability of voltage-sensitive sodium channels in excitable cell membranes. This results in initial depolarization and then subsequent loss of membrane potential. Stimulation of vagal fibers may result in bradycardia and hypotension.†
Symptoms generally occur within 30 minutes to 4 hours and generally resolve within 24 to 48 hours, although symptoms may persist for many days. Toxicity is characterized by headache, diaphoresis, salivation, nausea, vomiting, diarrhea, abdominal pain, hypotension, bradycardia, and shock. Syncope, respiratory depression, scotomata, paresthesias, fasciculations, muscle spasticity, hyperreflexia, vertigo, ataxia, dizziness, coma, seizures, and death may also occur. ECG findings may mimic cardiac glycoside toxicity, including repolarization abnormalities (e.g., abnormal T waves and ST segments), sinoatrial and atrioventricular blocks, and prolonged QT intervals.‡
Grayanotoxins
Resins called grayanotoxins are found in rhododendrons, mountain laurels, and azaleas. They produce toxicity similar to that seen with the steroid alkaloids veratrum and aconite. They act by binding to myocardial sodium channels and increasing their permeability and by increasing vagal tone. Grayanotoxins also inhibit cardiac and respiratory actions within the central nervous system. Poisoning may result from ingestion of leaves, flowers, or nectar. Grayanotoxin-contaminated honey, termed “mad honey,” is the most common source. The nectar of Rhododendron species contains the grayanotoxin o-acetyl-andromedol, formerly andromedotoxin, which is transferred to humans through consumption of honey produced by bees using the plant’s nectar. “Mad honey” was reportedly used as a biologic weapon against the Romans in 67 BC. Poisonings still occur in Asia Minor. “Mad honey” is sometimes taken in hopes of sexual performance enhancement but may result in toxicity. Symptoms include diaphoresis, hypersalivation, nausea, vomiting, bradycardia, arrhythmias (AV blocks, junctional rhythms), chest pain, hypotension, shock, dizziness, syncope, circumoral and extremity paresthesias, incoordination, and muscular weakness. A patient presented with acute myocardial infarction with normal coronary arteries after “mad honey” ingestion.237 Patients generally recover in 1 to 2 days. In Korea, the blossoms of azaleas (R. mucronulatum) are used to make wine, honeyed flower juice, and griddle cakes, with reported toxicity.*
Treatment
Treatment of cardiotoxic steroid alkaloid poisoning is supportive, with attention paid to fluid and electrolyte balance. Atropine for bradycardia and vasopressors for fluid-resistant hypotension are appropriate. Victims may require mechanical ventilation and cardiopulmonary resuscitation. Magnesium may suppress early afterdepolarizations and polymorphic ventricular tachycardia. Lidocaine, procainamide, flecainide, amiodarone, and hemoperfusion have also been used to treat ventricular arrhythmias secondary to aconite poisoning in various case reports. Ventricular dysrhythmias may be resistant to cardioversion and antiarrhythmic agents. Cardiopulmonary bypass, extracorporeal membrane oxygenation, and ventricular assist device placement have been used successfully in the treatment of aconite poisoning.†
Other Cardiotoxins
Taxine Alkaloids
Taxus species include T. baccata (English yew), T. brevifolia (Western yew), T. cuspidata (Japanese yew), and T. sumatrana (Sumatra or Chinese yew). The toxic alkaloids include taxines A and B. Yew plants also contain nitriles, ephedrine, and irritant oils. All parts of Taxus plants are toxic except the pulp, or aril, which contains very little taxine. The seeds, which are surrounded by the aril, may be toxic if chewed sufficiently to allow absorption of taxine. The plant is most toxic in the winter. Taxine B, believed to be the primary cardiotoxic alkaloid, inhibits both calcium and sodium transport across cell membranes.‡
Although GI toxicity is most common, dizziness, pupil dilation, muscle weakness, altered mental status, and convulsions have also been reported. Severe toxicity is characterized by bradycardias, heart blocks, ventricular tachycardia, ventricular fibrillation, widened QRS complexes, hypotension, and cardiac arrest.§ Brugada syndrome pattern has been noted on the ECG during toxicity; it resolved with recovery.267 Hemolysis with multiorgan failure has been reported.168 Autopsies have revealed dilated cardiac ventricles and congestion of the organs with pronounced cerebral and pulmonary edema. Plant parts are often found in the stomach and small bowel on autopsy. 3,5-Dimethoxyphenol demonstrated by gas-chromatography-mass spectrometry of blood or urine is a marker of Taxus poisoning.99,211,272
Reported yew-induced arrhythmias in humans are consistent with arrhythmias induced by blockade of voltage-gated sodium channels. These are described as bradycardia with wide QRS complexes, despite normal electrolytes. Two patients who ingested yew presented with severe cardiotoxicity and a wide QRS complex, which appeared to respond favorably to administration of hypertonic sodium bicarbonate.178,210,222 However, sodium bicarbonate administration did not narrow the widened QRS complex of Taxus-poisoned swine.222 Because there is little risk to administering sodium bicarbonate and possible significant benefit, this treatment should be considered if widened QRS is noted. Lidocaine administration and cardiac pacing have also been reportedly beneficial for treatment of humans poisoned with yew.267
Oral and Gastrointestinal Toxins
Oral Irritants (Glycosides, Oxalates)
Insoluble Oxalates
Plant exposures involving Philodendron, Dieffenbachia (dumb cane), Spathiphyllum species (peace lily) (Figure 64-22), Colocasia species (elephant’s ear, common calla) and Brassaia (octopus tree, Queensland umbrella tree) are frequently reported.153,170,202 In Taiwan, Alocasia macrorrhiza (giant elephant’s ear, taro) commonly produces similar toxicity.168 These plants contain insoluble oxalates arranged in numerous crystalline needles of calcium oxalate (raphides) contained in specialized cells (idioblasts). When stimulated, as by mastication, idioblasts forcefully release raphides that become embedded in exposed tissues, resulting in painful oropharyngeal edema, hypersalivation, vesicle formation, dysphagia, and aphonia. These plants also contain trypsin-like proteases, histamine, and kinin-like substances that may contribute to toxicity.170,254,270

FIGURE 64-22 Plants that are oral irritants. Philodendron (A), Dieffenbachia (B), Spathiphyllum (C).
(Courtesy Kimberlie A. Graeme, MD.)
In a mass food-borne illness in Chicago, victims presented with oral burning and facial edema after lunch in an office cafeteria serving a Chinese vegetable entrée, found to contain raphides.170,202,254,270
Gastrointestinal Irritants (Resins, Alkaloids)
Gastrointestinal toxins found in plants are listed in Box 64-4.
Chinaberry Trees
Melia azedarach plants (Figure 64-23) are found throughout the southern United States and Hawaii. The chinaberry tree, also known as China tree, Texas umbrella tree, or white cedar tree, contains tetranortriterpenes (resins) identified as meliatoxins A1, A2, B1, and B2, which are enterotoxic and neurotoxic. The bark contains toosendanin. The exact mechanisms of these toxins have yet to be determined. Gastroenteritis can occur after ingestion of any part of the plant, but typically occurs after the berries are ingested by children. Immature berries are green but turn yellow and wrinkle with age. After ingestion of as little as one berry, severe gastroenteritis may ensue, often with bloody diarrhea. In Chinese medicine, M. azedarach bark has been boiled and drank with resultant dizziness, muscle soreness and weakness, ataxia, areflexia, coma, blurred vision, ptosis, numbness, headache, nausea, vomiting, abdominal discomfort, and diarrhea that can be bloody. Mild elevation of liver enzymes and CPK, as well as hypokalemia and acidosis, have been reported. Symptoms may be rapid in onset or delayed by several hours after ingestion. Death has been reported in children after the ingestion of berries of the African variety of chinaberry tree. Fatalities are generally due to respiratory arrest. Autopsy has revealed cerebral edema, midbrain necrosis, intracranial hemorrhage, pulmonary congestion, gastrointestinal hemorrhage, yellow discoloration of the liver, and congestion of the kidneys. Treatment is supportive, with replacement of fluids and electrolytes. Tachycardia and hypotension generally respond to IV fluids.124,208
Solanum
The genus Solanum makes up the largest group of steroid alkaloid–containing plants and includes S. tuberosum (potato), S. gracile (wild tomato), S. carolinense (horse nettle), S. pseudocapsicum (Jerusalem cherry), S. dulcamara (woody nightshade), S. americanum (black nightshade), and other nightshade plants. Solanum species are used medicinally in some countries. Solanine, a glycoalkaloid with a steroid-like moiety, has been isolated from more than 1700 different Solanum species. It is found throughout the plants but is most concentrated in unripe fruits. Solanine generally produces gastroenteritis, but bradycardia, weakness, and CNS and respiratory depression may be seen. Treatment is supportive, with replacement of fluids and electrolytes. If hypotension ensues, it generally responds to IV fluids. Atropine may be beneficial if bradycardia develops. Spontaneous recovery usually occurs in 1 to 3 days.44,61,110
Saponin Glycosides (Pokeweed)
Saponin glycosides are found throughout the plant kingdom, for example, in English ivy (Hedera helix), pokeweed (Phytolacca americana), tung tree (Aleurites species), ginseng (Panax ginseng or quinquefolium), and licorice (Glycyrrhiza glabra.)104 Saponins are GI irritants that facilitate their own intestinal absorption. Saponins may induce lysis of erythrocytes, causing hemolytic anemia. In addition, most saponins are found in combination with other toxins, resulting in diverse clinical syndromes.
P. americana, or P. decandra, is most commonly known as pokeweed but is also known as Virginia poke, inkberry, pocan, pigeonberry, American cancer-root, garget, red ink, American nightshade, scoke, jalap, and redwood. It may be mistaken for horseradish, parsnip, or Jerusalem artichoke. P. americana has green leaves with red stalks. Berries are green when immature and turn deep purple with maturity. Pokeberries typically leave a purple stain. The root is the most toxic part of the plant. P. americana is ingested intentionally in pokeweed salad and pokeberry teas. When prepared for ingestion, leaves should be parboiled, which entails boiling the leaves first in water that is discarded before boiling the leaves again and rinsing them with fresh water. However, parboiling does not necessarily offer protection against toxicity.104
The saponin glycosides phytolaccatoxin and phytolaccagenin account for the GI injury, which manifests as fulminant gastroenteritis, with vomiting and diarrhea 2 to 4 hours after ingestion. Diarrhea may appear foamy from the sudsing effect of saponin glycosides. Hypotension may follow significant GI fluid losses. Severe ingestions may result in weakness, loss of consciousness, seizures, and respiratory depression.104
Treatment for acute toxicity includes fluid replacement for dehydration secondary to GI losses. Airway support may be needed. Seizures should be treated with benzodiazepines. Hematologic changes generally resolve within weeks.104
Toxins that Inhibit Protein Synthesis (Phytotoxins)
Toxalbumins (Ricin, Abrin, Curcin, Robin, Phasin)
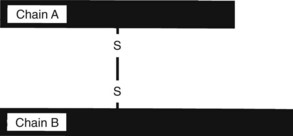
Toxalbumins are found in Abrus precatorius (jequirity bean, rosary pea, prayer bead), which contains abrin, R. communis (castor bean) (Figure 64-24, A), which contains ricin, Jatropha curcas (purging nut) and J. multifida, which contain curcin, and Robinia pseudoacacia (black locust), which contains robin and phasin. These toxins inhibit protein synthesis and cause cell death. These toxins are glycoproteins composed of two peptide chains, designated A and B, connected by a disulfide bond (see Figure 64-2). Chain B binds to the cell membrane. The toxin penetrates the cell by endocytosis. Chain A binds irreversibly and inactivates the ribosomes via glycosidase activity, which disrupts protein synthesis and results in cell death. Much of the toxicity is the result of endothelial cell damage, which causes increased capillary permeability, fluid and protein leakage, and edema.*
Ricin poisoning results from eating or chewing on the nut or seed. Fresh, immature castor beans are encased within a soft, thorny, red seedpod. With age, the seeds and surrounding seedpod become brown, and the seeds develop hard shells that are difficult to penetrate but can be punctured by teeth (see Figure 64-24, B). The oral lethal dose is estimated to be 1 mg/kg, theoretically as little as 1 bean in a child and 8 to 10 in an adult. After a latent period of 1 to 6 hours, nausea, vomiting, diarrhea, hemorrhagic gastritis, abdominal pain, thirst, dehydration, hypotension, coma, hyporeflexia, seizures, respiratory compromise, and shock may occur. Liver disease, with elevated LFTs, is common. Death may occur from dehydration and electrolyte imbalances; convulsions may precede death. Death on the third day or later is usually due to multiorgan failure. Autopsy may reveal GI tract ulcerations, necrosis of the lymph nodes and liver, nephritis, and pulmonary edema.*
A discrepancy between the serious toxicity of isolated ricin exposure and the milder toxicity of ingested seeds of R. communis has been recognized. A young adult consumed 10 to 15 seeds of R. communis and experienced severe cramping abdominal pain and vomiting 4 hours after ingestion, without GI bleeding or abdominal guarding. He received IV fluids. By the third day, he had recovered completely. Other cases of oral ingestion of masticated seeds indicate that GI distress may be followed a few days later by elevated liver enzymes or hyperbilirubinemia, followed by complete recovery. In contrast, a Bulgarian broadcaster who was injected with isolated ricin died from severe gastroenteritis and multiorgan failure. Ingested R. communis is much less toxic than is parenteral ricin. The degree of mastication of castor beans may determine the degree of toxicity seen with oral ingestions. Fresh seeds are likely to be more toxic than are aged seeds that are enveloped by a hard coat. Although deaths have been reported with ingestions of a couple of seeds, toxicity is unlikely if mature seeds are swallowed whole. Furthermore, some of the toxin, which is a protein, is very likely digested in the GI tract.6,32,74,76,199
Similarly, deaths have been reported after ingestion of fresh jequirity beans (A. precatorius) if the soft, immature bean is chewed or if the hard shell of the mature bean is penetrated. The estimated human fatal dose of abrin is 0.1 to 1 mcg/kg. One-half of a seed has been reported to cause toxicity in a child, and one chewed bean could be fatal. However, as with castor beans, the mature bean shell is often not penetrated; therefore significant toxicity is not often seen. For example, a 15-month-old child ingested more than 20 jequirity beans with only minor clinical toxicity, including mild hepatomegaly and mild elevation of aspartate aminotransferase (AST).74,93,212
Symptoms generally begin within a few hours. Mild symptoms involve diarrhea alone. More severe symptoms include persistent vomiting, diarrhea, and abdominal pain, with associated hypovolemia and hypokalemia. Hemorrhagic gastritis with bloody diarrhea may ensue. Dehydration may be associated with shock and hepatorenal dysfunction. Severely ill patients may present with hyperthermia, mental status changes, and seizures. Systemic effects may be delayed as inhibition of protein synthesis occurs. Delayed effects may occur 1 to 5 days after ingestion. Terminally, elevated serum LFTs and creatinine levels may be seen, indicating hepatorenal failure. If death occurs, it is generally 3 to 5 days after ingestion and is associated with seizures, tachycardia, GI distress, mucosal changes of the GI tract (including edema and hemorrhage of the Peyer’s patches), focal necrosis and failure of the liver and kidneys, and retinal hemorrhage. Cerebral and pulmonary edema, hypertension, and pancreatitis have also been observed.6,74,93,212
Jatropha species have attractive, sweet-flavored fruit that may entice children. The presentation of toxicity may be mistaken for organophosphate poisoning, with diaphoresis, vomiting, diarrhea, obtundation, and miosis reported in one case. However, mydriasis has also been reported. Other cases have manifested with hemorrhagic gastroenteritis, hypotension, muscle spasm, and hyperpnea, as well. Leukocytosis may be noted.150
Treatment
Treatment is supportive, including fluid and electrolyte replacement. Patients who remain asymptomatic for 4 to 6 hours after ingestion of seeds may be discharged, with instructions to return if symptoms develop. Laboratory follow-up for delayed liver toxicity may be appropriate. Studies indicate that ricin antibody administered intravenously shortly after exposure could be beneficial. Animal studies indicate that vaccination with abrin and ricin toxoids may offer protection against subsequent abrin and ricin challenges, respectively. However, there does not appear to be cross-immunity between abrin and ricin toxoids. Toxoids and antibodies are not readily available.*
Hepatotoxic Agents
Pyrrolizidine Alkaloids
Plants containing pyrrolizidine alkaloids include Senecio vulgaris (groundsel), S. longilobus (gordolobo), S. jacobaea (tansy ragwort), S. latifolius (Dan’s cabbage or muti), Symphytum officinale (comfrey), Gynura segetum, Ilex paraguanyensis (mate), Heliotropium species, Crotalaria species (rattlebox), Amsinckia intermedia (fiddle neck or tar weed), Baccharius pteronoides, Astragulus lentiginosus, Gnaphalium, Cynoglossum, Echium, Tussilago farfara, and Adenotyles alliariae (alpendost). These plants are consumed in herbal preparations, in breads made with grains that are contaminated with pyrrolizidine-containing weeds (“bread poisoning”), and in teas. For example, Senecio and Crotalaria, which are used to make “bush tea” in Jamaica, have been associated with hepatotoxicity. Less commonly, contaminated animal meat, milk, or honey from exposed animals or bees may be the source of exposure. Pyrrolizidine alkaloids are heterocyclic compounds that act as alkylating agents and are activated in the liver, by cytochrome P450 and mixed-function oxidase systems, to form reactive pyrrolic metabolites that alkylate proteins and DNA, thereby inhibiting protein and nucleic acid synthesis. Toxicity is associated with hepatic venoocclusive disease, hepatomegaly, cirrhosis, and Budd-Chiari syndrome, which is characterized by obstruction of the trunk or large branches of the hepatic vein.100,216,219,243,248
Young victims seem more susceptible than adults. Ingestion of 10 mg of pyrrolizidine alkaloid is probably enough to produce acute or chronic venoocclusive disease. Venoocclusive liver disease is characterized by nonthrombotic occlusion of the central veins of hepatic lobules. Histologic liver findings include central vein dilation, sinusoidal congestion, centrilobular necrosis, and fibrosis. Clinical evidence of intrahepatic portal hypertension may include painful hepatomegaly, ascites, weight gain, and jaundice. Hepatic failure and death may ensue. Neonatal death secondary to hepatotoxicity has been reported after intrauterine exposure to pyrrolizidine alkaloids from an herbal preparation; neonatal ascites, skin edema, hepatomegaly, and anemia were noted. Elevated LFTs and bilirubin, as well as hyponatremia, may be noted. Occasionally, laparoscopic liver biopsy and hepatic and portal decompression are helpful in diagnosis and treatment, respectively. Treatment, including albumin infusions, diuretics, and therapeutic paracentesis, is supportive. N-acetylcysteine may reduce the toxicity. Cirrhosis and portal hypertension may be persistent.†
Kava Kava
Piper methysticum (Kava kava) is used recreationally and medicinally in Polynesia and Micronesia. Traditionally, kava extracts are prepared from kava roots macerated with water or coconut milk. Kava kava is associated with yellowing of the skin and reddening of the eyes, termed kava dermopathy, and may be hepatotoxic.101,171,
Renal Toxins
Soluble Oxalates
Soluble oxalates are found in rhubarb (Rheum rhaponticum) and sorrel (Rumex species) plants. Soluble oxalates are rapidly absorbed via the GI tract. Generalized disturbance of monovalent and divalent cation metabolism occurs. Serum ionized calcium levels may drop rapidly. Weakness, tetany, hypotension, and seizures may develop. Acute renal failure may occur if calcium oxalate precipitates in urine and obstructs the renal tubules.92,225 The structures of oxalates follow:
Other Nephrotoxins
Aloe spp. contains aloins and aloinosides and can cause parenchymatous nephritis and acute renal failure. Initially, inflammation of the gastrointestinal tract with bloody diarrhea is seen, followed by fluid and electrolyte loss. Arthralgias and palpable purpura followed by hematuria, proteinuria, renal failure, and death were reported in a man who ingested the juice extracted from four to five leaves of Aloe vera (Aloe barbadenis).30,246
Aristolocia clematitis (birthwart) contains aristolochic acid, which is nephrotoxic, producing interstitial nephropathy.246
Pithecolobium lobatum (djenkol bean) poisoning is characterized by nausea, vomiting, spasmodic pain, hematuria, and oliguria, with evidence of urinary obstruction and acute renal failure. A sulfurous odor may be noted on the breath and of the urine. Urine may contain needle-like crystals. Animal studies show acute tubular necrosis.246
Cleistanthus collinus (Vadisaaku, Oduvan) is used as a suicidal and homicidal poison in India. Fresh leaves or the extract of boiled leaves are generally consumed. C. collinus contains diphyllin glycosides, cleistanthins A and B, which are thought to be responsible for the toxicity; however, the mechanisms of action remain to be determined. Toxicity is generally not evident for 3 to 4 days. Distal renal tubular acidosis, with urinary potassium wasting and metabolic acidosis, is seen. Renal failure may ensue. Vomiting, dysrhythmias (including bradycardia), hypotension, hypoxia, acute respiratory distress syndrome (ARDS), altered mental status, and muscle weakness are also reported. Muscle weakness may manifest as dysarthria, dysphagia, pstosis, ocular palsies, and weak neck muscles leading to inability to hold up the head. Coagulopathy, elevated liver enzymes, elevated CPK, hypokalemia, hyponatremia, hyperchloremia, and urinary alkalosis have been noted. Treatment is supportive. Neostigmine and atropine have been advocated in case reports and animal studies. Mortality rates of approximately 30% are reported, despite medical care. Death is usually due to cardiac or respiratory failure.21,62,187,251
Hematopoietic Toxins
Plants with Anticoagulant Properties (Lactone Glycosides)
Dipteryx odoratum and Coumarouna (or Dipteryx) oppositofolia (tonka bean) were among the first historically mentioned medical sources of coumarin. Melilotus officinalis (yellow sweet clover, yellow melilot, common melilot), Asperula odorata, and Galium odoratum (woodruff) also contain significant quantities of coumarin. These coumarin-containing products have been consumed in teas. Yellow sweet clover poisoning is caused by dicumarol, a fungal metabolite produced from coumarin substrates in spoiling plants. Dicumarol interferes with the synthesis of vitamin K–dependent coagulation factors, inducing coagulopathy. Generally, this has been a problem in livestock but not humans.155,214,219
Toxins that Inhibit Cell Division and Bone Marrow
Colchicine
Colchicine toxicity can occur after ingestion of Sandersonia aurantiaca (Christmas-bells, Chinese lantern lily), Gloriosa superba (glory lily), and, more commonly, C. autumnale, all of the lily family. C. autumnale is commonly known as autumn crocus, wild saffron, meadow saffron, naked lady, naked boy, and son-before-the-father. It can be mistaken for an edible plant, such as leek, wild garlic or Bear’s garlic (Allium ursinum). C. autumnale is found in Europe, North America, and Asia. All parts of the plant contain colchicine, an amine alkaloid, but the highest concentrations are found in the underground bulb. Colchicine binds selectively and reversibly to microtubules, disrupting cell division, cell shape, mobility, and phagocytosis. Cells with the highest turnover rates, such as those of the GI mucosa and bone marrow, are affected most severely. Cell arrest in metaphase and abnormal nuclear morphology are seen at autopsy; the clumps of chromatin material seen in the nuclei are called colchicine bodies.*
Acute poisoning may occur after a latent period of several hours. Initial GI effects are severe abdominal pain, nausea, vomiting, diarrhea, and hemorrhagic gastroenteritis, which may result in electrolyte abnormalities, volume depletion, acidosis, shock, arrhythmias, and multiorgan failure. Muscular weakness and ascending paralysis may cause respiratory arrest, which may occur with a clear sensorium; however, patients may present comatose or convulsing. Myocardial toxicity, rhabdomyolysis, and pancreatitis have also been reported. Initially, leukocytosis may be seen, but bone marrow depression with pancytopenia may follow and may predispose to hemorrhage and infection. Autopsy reveals hypocellular bone marrow. Autopsy may also reveal diffuse vacuolization in the cytoplasma of hepatocytes and congestion of the liver, kidneys, spleen, lungs, and brain. Occasionally, hemorrhagic edema is seen. Isolated mitotic structures within the epithelium of the colon have also been reported at autopsy. Patients that survive severe toxicity may develop alopecia days to weeks after poisoning.†
Because of the severity of poisoning and not infrequent lethal outcomes, aggressive decontamination should be considered. Colchicine-specific Fab antibody fragments have been developed and used with success in France for management of acute poisoning with the drug form of colchicine, but this treatment is not commercially available; therefore treatment is symptomatic and supportive. Pulmonary function tests can be used to monitor respiratory function, assessing for fatigue and progressive ascending paralysis. Assisted ventilation is used as needed. Parenteral analgesics are given cautiously to relieve severe abdominal pain, because colchicine sensitizes patients to CNS depressants. Fluid and electrolyte replacement and occasionally blood component replacement and granulocyte colony-stimulating factor (G-CSF) may be necessary. Maintain adequate urine output and assess for infection.‡
Podophyllum
Podophyllum peltatum is most commonly known as the mayapple, but has also been called American mandrake. Because of this common name, it has been occasionally confused with true mandrake (Mandragora officinarum), an unrelated plant with anticholinergic properties. Toxicity from P. peltatum exposure is caused by the glucoside podophyllotoxin. Dysosma pleiantha also contains podophyllotoxin.168 Acute toxicity after ingestion of P. peltatum may occur after a latent period of several hours, followed by GI distress and associated hypovolemia. Nervous system effects (confusion, delirium, coma, peripheral neuropathy), bone marrow depression, and multiorgan failure may ensue as a result of the antimitotic effect of podophyllotoxin. Treatment is similar to that for colchicine.98
Plants that Induce Hemolysis
Fava Beans
Fava beans (Vicia faba) contain vicine and convicine, two metabolically inactive glycones that may be cleaved by β-glycosidase to produce divicine and isouramil. Divicine and isouramil may account for the hemolytic crisis seen in response to oxidative stress when individuals deficient in glucose-6-phosphate dehydrogenase ingest fava beans. Favism has been reported in infants who ingest breast milk from mothers who have recently ingested fava beans and in fetuses of mothers who have ingested fava beans. Clinically, favism is characterized by hemoglobinuria, anemia, and jaundice. Patients may present with complaints of malaise, weakness, lethargy, nausea, vomiting, headache, and lumbar or abdominal pain. Renal failure may ensue. Supportive care is the mainstay of treatment. Occasionally, transfusions and hemodialysis are needed.58,122,127
Endocrine and Metabolic Toxins
Plants that Induce Hypoglycemia
Ackee Fruit
B. sapida, or ackee (akee) fruit trees, are indigenous to West Africa but are prevalent in the West Indies, Central America, and southern Florida. Unripe ackee fruit has a closed yellow aril that is toxic. Ripe ackee fruit, with a spontaneously opened red aril, is nontoxic. The seeds contain hypoglycin B and are toxic regardless of the maturation of the aril. Unripe ackee fruit contains hypoglycin A, or L(R,S)-2-amino-3-methylenecyclopropylproprionic acid, a water-soluble amino acid that is converted to methylenecyclopropylacetyl-coenzyme A in vivo. Both compounds are hypoglycemic agents; the second is a suicide inhibitor of β-oxidation of fatty acids. Inhibition of fatty acid metabolism results in microvesicular steatosis of the liver, hyperammonemia, metabolic acidosis, and hypoglycemia. Laboratory and histopathologic findings are indistinguishable from those of Reye’s syndrome, but urine shows increased concentrations of glutaric and ethylmalonic acids after hypoglycin A exposure.18,141,175,234
Ackee fruit constitutes a traditional Jamaican breakfast and has been associated with Jamaican vomiting sickness. Ingestion of unripe ackee fruit caused an outbreak epidemic of fatal encephalopathy in West Africa, primarily affecting children ages 2 to 6 years, who experienced vomiting, hypotonia, convulsions, and coma. All children died within 48 hours of the onset of vomiting. Necropsy revealed massive liver steatosis and severe hypoglycemia. Urine concentrations of dicarboxylic acids were elevated. Similarly, an epidemic of over 100 cases occurred in Haiti, associated with vomiting, abdominal pain, loss of consciousness, convulsions, and many deaths. Hypoglycemia was noted. Carnitine derivatives, octanoylcarnitine and hexanoylcarnitine, were noted in the urine, confirming exposure to hypoglycin. Fatal ackee fruit poisoning is more common in children than in adults; adults are more likely to present with self-resolving cholestatic jaundice.141,175,183,234,235
Treatment is largely supportive and consists of securing an airway and administering IV fluids and dextrose. Before hypoglycemia was recognized, mortality rates of symptomatic unripe ackee fruit exposure approached 80%. Frequent glucose and electrolyte measurements with appropriate replacement are essential. Seizures are treated with benzodiazepines and barbiturates, remembering that serum glucose should be assessed in all seizing patients. Theoretically, riboflavin, clofibrate, glycine, methylene blue, and L-carnitine may be beneficial, although their efficacy is not established. Carnitine may facilitate transport of fatty acids into mitochondria and has been used for other toxins (e.g., valproate) that inhibit β-oxidation of fatty acids.18,156,234,235
Wild Yams
Dioscorea spp. contain dioscorines and dioscines. They are tuberous plants, commonly known as yams, used as a staple food in Africa and Asia. The tubers are usually detoxified in running water, soaking in salt water, boiling for several hours, squeezing out the juice, and roasting. The nondetoxified plant has been used in homicide, suicide, and hunting. Central nervous system irritability with seizures, liver failure, and renal failure are seen. Hypoglycemia is reported.246
Cocklebur
No antidote is known, and treatment is supportive. Phenylbutazone, a nonsteroidal antiinflammatory drug that is generally unavailable because of its toxicity, has been shown to reduce the cytotoxic effects of carboxyatractyloside in rats.245,261
Bird-Lime/Blue Thistle
Atractylis gummifera (bird-lime, blue thistle) is found in North Africa and the Mediterranean. It has a sugary taste and has been confused with edible wild artichoke. A. gummifera contains two poisonous glucosides: atractyloside and carboxyatractyloside. The glucosides inhibit oxidative phosphorylation, block transport of ADP at the mitochondrial membrane, and block conversion of ADP to ATP. The toxins inhibit the actions of P450 and b5 cytochromes. Clinically, headache, abdominal pain, vomiting, hematemesis, diarrhea, and dizziness are followed by liver failure, associated with jaundice and hepatitis. Seizures, coma, dysrhythmias, and renal failure may also be seen. Laboratory studies reveal hypoglycemia, metabolic acidosis, uremia, and hyperkalemia. Death is generally from multiorgan system failure. Autopsy reveals lesions, necrosis, and congestion of the liver and kidneys.120,121,245,246,261
Plants that Interfere with Steroid Metabolism
Licorice
Treatment consists of discontinuing exposure to licorice, maintaining good urine output, and alkalinizing the urine of patients suffering from rhabdomyolysis. Occasionally, potassium-sparing diuretics (e.g., triamterene, spironolactone) are useful. Torsades de pointes may respond to magnesium and potassium infusions.*
Cyanogenic Plants
Glycosides that yield hydrocyanic acid on hydrolysis are known as cyanogenic glycosides (see Figure 64-1, online). Cyanogenic glycosides include amygdalin, prunasin, linamarin, lotaustralin, and triglochinin.50 Amygdalin (D-mandelonitrile-β-D-glucoside-6-β-glucoside), which is abundant in the Rosaceae family, is the cyanogenic compound found in the seeds of Malus species (apples) and pits of Prunus species, including cherries, peaches, plums, and apricots. Deaths have been reported after ingestion of apricot, apple, cherry, and other fruit seeds.226 Black or wild cherries (Prunus serotina) are considered the most dangerous. Poisonings have resulted from milkshakes that contained apricot kernels and from apricot kernels sold in health food stores as snacks.252 Linseeds (Linum usitatissimum) and cycad seeds (Cycas species) are also cyanogenic.50,220 Cassava (Manihot esculenta) contains the cyanogenic glucoside linamarin, which is rapidly hydrolyzed to acetone cyanohydrin, which breaks down into hydrogen cyanide and acetone.88,221 Both chronic and acute cyanide toxicity have been reported after ingestion of cassava. Chronic ingestion produces an upper motor neuron disease known as Konzo, or tropical spastic paraparesis. Konzo occurs after recurrent consumption of improperly prepared cassava during droughts, in areas where cassava is a staple food, such as in Africa. To remove cyanogens, roots should be soaked; however, when water is scarce, this soaking process is limited.16,17,88
Cyanide combines with and inhibits many enzymes. It possesses great affinity for the ferric iron in cytochrome oxidase of the electron transport chain, accounting for most of its toxicity (Figure 64-25). By combining with cytochrome oxidase, cyanide prevents electron transport, thereby preventing ATP production by oxidative phosphorylation. Metabolic acidosis ensues. Humans detoxify cyanide by transferring sulfane sulfur to cyanide to form thiocyanate. Numerous sulfur sources are most likely acted on by various sulfurtransferases to form the sulfane sulfur needed to convert cyanide to thiocyanate. Administration of exogenous sulfane sulfur, such as sodium thiosulfate, can greatly facilitate this detoxification.59
Clinically, GI distress, bitter almond breath, CNS changes (agitation, anxiety, excitement, weakness, numbness, hypotonia, spasticity, coma, seizures), respiratory changes (hyperpnea, dyspnea, apnea, cyanosis), cardiovascular changes (tachycardia and hypertension followed by bradycardia and hypotension, heart block, ventricular arrhythmias, asystole), and metabolic changes (anion gap metabolic acidosis) are seen. Skin color may be pink or cyanotic, and the partial pressure of oxygen may be normal in cyanide poisoning. ECG may reveal T-on-R phenomenon as a result of progressive shortening of the ST segment. Multiorgan failure and death may occur.59,168,221
Treatment
Patients with cyanogenic glycoside poisoning may respond to treatment with 100% oxygen and cyanide antidote kits. The traditional antidote kit (Lily cyanide kit) contains amyl nitrite, sodium nitrite (3% solution given intravenously on the basis of hemoglobin and weight; generally, 300 mg in a nonanemic adult), and sodium thiosulfate (12.5 g in an adult). Red blood cell or plasma cyanide levels can be determined; however, treatment should be initiated promptly, without confirmation of exposure, in patients with evidence of toxicity. Cyanide antidote kits are designed to induce methemoglobinemia through nitrite exposure. Cyanide binds preferentially to the ferric ion of methemoglobin rather than to that of cytochrome oxidase in the electron transport chain. Forming methemoglobin limits inhibition of electron transport by cyanide and restores cellular respiration. Sodium thiosulfate in the cyanide kit provides exogenous sulfane sulfur groups that bind to cyanide and form thiocyanate. Recently available in the United States, hydroxocobalamin (Cyanokit) is also used to treat cyanide toxicity. Cyanide combines with hydroxocobalamin to form cyanocobalamin, which is excreted in the urine and bile. Sodium thiosulfate may be given in conjunction with hydroxocobalamin, with theoretical synergistic therapeutic effects. Supportive care, including mechanical ventilation, intravenous fluids, and vasopressors, may be used.59,221,252
Reproductive Toxins
Plants used as abortifacients include Ruta chalepensis (ruda), Lycopodium saururus (cola de quirquincho), Petroselinum hortense (parsley), Mentha pulegium (pennyroyal), S. longepedunculata (wild wisteria) and T. peruviana (yellow oleander). These plants generally cause significant morbidity and mortality to the women using the plants. Pennyroyal is discussed later. Yellow oleander and wild wisteria were discussed earlier.56,84
Other Toxins
Oils
Clove
The toxic component of clove oil is eugenol. Near fatal hepatic failure has been reported in children ingesting as little as 10 mL of this oil.85
Eucalyptus
The toxic component of eucalyptus oil is 1,8-cineole (eucalyptol), which is derived from leaves of Eucalyptus globulus. Ingestion of eucalyptus oil is associated with diaphoresis, CNS depression (hyporeflexia, weakness, ataxia, slurred speech, agitation, confusion, coma, respiratory depression, convulsions), GI upset (vomiting, diarrhea, abdominal pain), and respiratory effects (bronchospasm, pneumonitis, pulmonary edema). Metabolic acidosis, rhabdomyolysis, hypotension, and death may occur. Fatalities have been reported after ingestion of as little as 3.5 mL and may occur as early as 15 minutes after ingestion. Topical application may also result in significant CNS toxicity. The urine may smell of eucalyptus oil, violets, or fresh-mown hay.11,65,256
Lavender
Lavandula species are used to prepare an essential oil that contains linalool and linalyl acetate, and to a lesser extent, lavandulyl acetate and terpinen-4-ol. This oil can cause central nervous system depression and confusion if ingested. The scent of lavender may be noted on the breath.160
Pennyroyal
Pulegone, a monoterpene, is the primary toxin of pennyroyal oil, which is derived from Hedeoma pulegioides or M. pulegium. Pennyroyal oil also contains several other monoterpenes. Pulegone is oxidized by hepatic cytochrome P450 enzymes to form the toxic metabolite menthofuran and other toxins. Menthofuran induces much of the hepatic and pulmonary damage. Reactive oxidative metabolites of pulegone and menthofuran bind to target cell proteins and induce cellular damage. M. pulegium use has caused death in women using it as an over-the-counter abortifacient and death in infants given mint teas made with the plant. Hepatic and neurologic injuries are generally seen. Hepatic failure followed by shock, multiorgan failure, and death may occur after ingestion. Neurologic manifestations include dizziness, obtundation, hallucinations, seizures, and cerebral edema. Laboratory studies may reveal elevated LFTs, coagulopathies, and metabolic acidosis. Liver histology at autopsy reveals centrilobular hepatic necrosis or confluent hepatocellular necrosis. Treatment includes fluid and electrolyte replacement as needed. N-Acetylcysteine has been used for treatment because the hepatotoxicity is similar to acetaminophen-induced hepatotoxicity and is associated with glutathione depletion. Animal studies indicate that treatment with cimetidine and disulfiram may be helpful.10,13,152,253
Pine
Pine oil is a mixture of highly lipophilic hydrocarbons obtained from the distillation of wood from pine trees. It contains the toxin 1-alpha-terpineol. Pine oil may cause skin irritation, as well as acute respiratory irritation and central nervous system depression. Autopsy has revealed hemorrhagic and necrotic lungs.174
Wintergreen
Methyl salicylate is the methyl ester of salicylic acid, derived from Gaultheria procumbens (wintergreen). Widely used as an antirheumatic agent in the 19th century, it fell into disfavor because of its extreme side effects and availability of the more effective acetylsalicylic acid. Poisoning now occurs most often in children, who are attracted to the color, smell, and flavor of wintergreen oil. As little as 4 mL may result in fatality. Oil of wintergreen is found in herbal products, such as Koong Yick Hung Fa oil, and ingestion has caused significant toxicity. Oil of wintergreen may also produce laryngeal edema. Poisoning is identical to salicylism, including CNS excitation, hyperventilation, hyperthermia, and metabolic acidosis. Treatment is as for salicylism.28,133 Structures of salicylic acid and methyl salicylate follow:
Wormwood
Wormwood oil, from the plants Artemisia absinthium and A. pontica, is used to make the emerald green liqueur absinthe, which has been ingested for its psychoanaleptic effect. Absinthe was banned because it produces neurotoxicity. Wormwood oil contains α- and β-thujone. Alpha-thujone is an antagonist at GABAA receptors. Wormwood oil may cause vomiting, vertigo, delirium, hallucinations, convulsions, coma, respiratory arrest, and death.23,73,134,198,250
Elements and Nitrates
Many plants absorb or accumulate metallic compounds (selenium, molybdenum, arsenic, lead, cadmium), nitrites, and nitrates. They are generally of little importance in humans but constitute a danger to grazing livestock. However, numerous cases of methemoglobinemia in infants ingesting vegetables high in nitrate content have been reported. Vegetables cited include spinach, beets, cabbage, and carrots grown in high-nitrate soils. A prepared fennel puree reportedly caused methemoglobinemia in infants. The infants presented with respiratory distress, tachycardia, depressed mental status, and cyanosis. Methemoglobin levels were as high as 65%. Treatment with methylene blue (2 mg/kg IV) was used successfully. The fennel puree was found to have markedly elevated nitrate levels. Nitrates are converted to nitrites in the infant gut, which then induce methemoglobinemia.143,184
APPENDIX B Nontoxic Plants
Modified from “Your Guide to Plant Safety,” courtesy San Francisco Bay Area Regional Poison Center.
1 Ageely HM. Prevalence of Khat chewing in college and secondary (high) school students of Jazan region, Saudi Arabia. Harm Reduction Journal. 2009;6:11.
2 Akinci S, Arslan U, Karakurt K, et al. An unusual presentation of mad honey poisoning: Acute myocardial infarction. Int J Cardiol. 2008;129:356.
3 Alem A, Kebede D, Kullgren G. The prevalence and socio-demographic correlates of khat chewing in Butajira, Ethiopia. Acta Psychiatr Scand. 1999;100:84.
4 Alem A, Shibre T. Khat induced psychosis and its medico-legal implication: A case report. Ethiop Med J. 1997;35:137.
5 Al-Motarreb A, Al-Kebsi M, Al-Adhi B, et al. Khat chewing and acute myocardial infarction. Heart. 2002;87:279.
6 Alpin PJ, Eliseo T. Ingestion of castor oil plant seeds. Med J Aust. 1997;167:260.
7 Al-Zubairi A, Al-Habori M, Al-Geiry A. Effect of Catha edulis (khat) chewing on plasma lipid peroxidation. J Ethnopharmacol. 2003;87:3.
8 Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol. 1998;56:211.
9 Anderson EF. Peyote: The divine cactus, ed 2. Tucson: University of Arizona Press; 1996.
10 Anderson IB, Mullen WH, Meeker JE. Pennyroyal toxicity: Measurement of toxic metabolite levels in two cases and review of the literature. Ann Intern Med. 1996;124:726.
11 Anpalahan M, Le Couteur DG. Deliberate self-poisoning with eucalyptus oil in an elderly woman. Aust N Z J Med. 1998;28:58.
12 Arachchillage DRJ, Hewapathirana N, Fernando DJS. The role of the Internet in facilitating yellow oleander poisoning and in providing effective treatment. Eur J Intern Med. 2007;18:167.
13 Bakerink JA, Gospe SMJr, Dimand RJ, et al. Multiple organ failure after ingestion of pennyroyal oil from herbal tea in two infants. Pediatrics. 1996;98:944.
14 Balint EE, Falkay G, Balint GA. Khat: A controversial plant. Wien Klin Wochenschr. 2009;121:604.
15 Ballard T, Ehlers J, Freund E, et al. Green tobacco sickness: Occupational nicotine poisoning in tobacco workers. Arch Environ Health. 1995;50:384.
16 Banea-Mayambu JP, Tylleskar T, Gitebo N, et al. Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neuron disease konzo in former Zaire. Trop Med Int Health. 1997;2:1143.
17 Banea-Mayambu JP, Tylleskar T, Rosling H. Konzo and Ebola in Bandundu region of Zaire. Lancet. 1997;349:621.
18 Barennes H, Valea I, Boudat A, et al. Early glucose and methylene blue are effective against unripe ackee apple (Blighia sapida) poisoning in mice. Food Chem Toxicol. 2004;42:809.
19 Baron F, Deprez M, Beguin Y. The veno-occlusive disease of the liver. Haematologica. 1997;82:718.
20 Barrella M, Lauria G, Quatrale R, et al. Hypokalemic rhabdomyolysis associated with liquorice ingestion: Report of an atypical case. Ital J Neurol Sci. 1997;18:217.
21 Benjamin SP, Fernando ME, Jayanth JJ, et al. Cleistanthus collinus poisoning. J Assoc Physicians India. 2006;54:742.
22 Benomran FA, Henry JD. Homicide and strychnine poisoning. Med Sci Law. 1996;36:271.
23 Berlin R, Smilkstein M. Wormwood.oil@toxic.ing. J Toxicol Clin Toxicol. 1996;34:583.
24 Beyer NH, Kogutowska E, Hansen JJ, et al. A mouse model for ricin poisoning and for evaluating protective effects of antiricin antibodies. Clin Toxicol. 2009;47:219.
25 Biberci E, Altuntas Y, Cobanoglu A, et al. Acute respiratory arrest following hemlock (Conium maculatum) intoxication. J Toxicol Clin Toxicol. 2002;40:517.
26 Blaw ME, Adkisson MA, Levin D, et al. Poisoning with Carolina jessamine (Gelsemium sempervirens [L.] Ait.). J Pediatr. 1979;94:998.
27 Bonkowsky JL, Sarco D, Pomeroy SL. Ataxia and shaking in a 2-year-old girl: Acute marijuana intoxication presenting as seizure. Ped Emerg Care. 2005;21:527.
28 Botma M, Colquhoun-Flannery W, Leighton S. Laryngeal oedema caused by accidental ingestion of oil of wintergreen. Int J Pediatr Otorhinolaryngol. 2001;58:229.
29 Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol. 2002;7:103.
30 Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:103.
31 Boumba V, Mitselou A, Vougiouklakis T. Fatal poisoning from ingestion of Datura stramonium seeds. Vet Hum Toxicol. 2004;46:81.
32 Bradberry SM, Dickers KJ, Rice P, et al. Ricin poisoning. Toxicol Rev. 2003;22:65.
33 Brncic N, Viskovic I, Peric R, et al. Accidental plant poisoning with Colchicum autumnale: Report of two cases. Croat Med J. 2001;42:673.
34 Bronstein AC, Spyker DA, Cantilena LR. 2008 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th annual report. Clin Toxicol. 2009;47:911.
35 Brvar M, Kozelj G, Mozina M, et al. Acute poisoning with autumn crocus (Colchicum autumnale L). Wien Klin Wochenschr. 2004;116:205.
36 Burkhart KK, Magalski AE, Donovan JW. A retrospective review of the use of activated charcoal and physostigmine in the treatment of jimson weed poisoning. J Toxicol Clin Toxicol. 1999;37:389.
37 Caksen H, Odabas S, Akbayram S, et al. Deadly nightshade (Atropa belladonna) intoxication: An analysis of 49 children. Hum Exp Toxicol. 2003;22:665.
38 Cardano S, Beldi F, Bignoli C, et al. A dangerous risotto. Recenti Prog Med. 2002;93:245.
39 Carlini EA. Plants and the central nervous system. Pharmacol Biochem Behav. 2003;75:501.
40 Carlton MW, Kunkel DB. St. Anthony’s fire: Eponym misused. Acad Emerg Med. 1995;2:1114.
41 Carlton MW, Kunkel DB. St. Anthony’s fire: An eponym for ergotism, not erysipelas. Am Fam Physician. 1995;52:95.
42 Carvalho F. The toxicological potential of khat. J Ethnopharmacol. 2003;87:1.
43 Castorena JL, Garriott JC, Barnhardt FE, et al. A fatal poisoning from Nicotiana glauca. Clin Toxicol. 1987;25:429.
44 Ceha LJ, Presperin C, Young E, et al. Anticholinergic toxicity from nightshade berry poisoning responsive to physostigmine. J Emerg Med. 1997;15:65.
45 Centers for Disease Control. Jimsonweed poisoning—Texas, New York, and California, 1994. MMWR Morb Mortal Wkly Rep. 1995;44:41.
46 Chan TYK. Aconite poisoning. Clin Toxicol. 2009;47:279.
47 Chan TYK. Herbal medicine causing likely strychnine poisoning. Hum Exp Toxicol. 2002;21:467.
48 Chan TYK. Hidden aconite roots in herbal medicines. Clin Toxicol. 2010;48:92.
49 Chan TYK. Incidence of herb-induced aconitine poisoning in Hong Kong: Impact of publicity measures to promote awareness among the herbalists and the public. Drug Safety. 2002;25:823.
50 Chang SS, Chan YL, Wu ML, et al. Acute Cycas seed poisoning in Taiwan. J Toxicol Clin Toxicol. 2004;42:49.
51 Chappell JS, Lee MM. Cathinone preservation in khat evidence via drying. Forensic Sci Int. 2010;195:108.
52 Chiang WT, Yang CC, Deng JF. Cardiac arrhythmia and betel nut chewing: Is there a causal effect? Vet Hum Toxicol. 1998;40:287.
53 Choi SH, Lee SW, Hong YS, Lim SI. Grayanotoxin poisoning from flower of Rhododendron mucronulatum in humans. Bull Environ Contam Toxicol. 2007;78:11.
54 Chojkier M. Hepatic sinusoidal-obstruction syndrome: Toxicity of pyrrolizidine alkaloids. J Hepatol. 2003;39:437.
55 Choo YK, Kang HY, Lim SH. Cardiac problems in mad-honey intoxication. Circ J. 2008;72:1210.
56 Ciganda C, Laborde A. Herbal infusions used for induced abortion. J Toxicol Clin Toxicol. 2003;41:235.
57 Clark RF, Selden BS, Curry SC. Digoxin-specific Fab fragments in the treatment of oleander toxicity in a canine model. Ann Emerg Med. 1991;20:1073.
58 Corchia C, Balata A, Meloni GF, et al. Favism in a female newborn infant whose mother ingested fava beans before delivery. J Pediatr. 1995;127:807.
59 Curry SC, LoVecchio F. Hydrogen cyanide and inorganic cyanide salts. In: Sullivan JB, Kreiger JR, editors. Clinical environmental health and toxic exposures. ed 2. Philadelphia: Lippincott Williams & Wilkins; 2001:705.
60 Curry SC, Mills KC, Graeme KA. Neurotransmitters. In: Goldfrank LR, Flomenbaum NE, Lewin NA, et al, editors. Toxicologic emergencies. ed 7. Stamford, Conn: Appleton & Lange; 2002:133.
61 Dafni A, Yaniv Z. Solanaceae as medicinal plants in Israel. J Ethnopharmacol. 1994;44:11.
62 Damadaram P, Manohar IC, Kumar DP, et al. Myasthenic crisis-like syndrome due to Cleistanthus collinus poisoning. Indian J Med Sci. 2008;61:62.
63 Damaj MI, Patrick GS, Creasy KR, et al. Pharmacology of lobeline, a nicotinic receptor ligand. J Pharmacol Exp Ther. 1997;282:410.
64 Danel VC, Wiart JF, Hardy GA, et al. Self-poisoning with Colchicum autumnale L. flowers. J Toxicol Clin Toxicol. 2001;39:409.
65 Darben T, Cominos B, Lee CT. Topical eucalyptus oil poisoning. Australas J Dermatol. 1998;39:265.
66 Davies MK, Mayne AJ. Oleander poisoning. Arch Dis Child. 2001;84:9.
67 Davis EW. The ethnobiology of the Haitian zombi. J Ethnopharmacol. 1983;9:85.
68 DeFrates LJ, Hoehns JD, Sakornbu EL, et al. Antimuscarinic intoxication resulting from the ingestion of moonflower seeds. Ann Pharmacother. 2005;39:173.
69 de Haro L, Pommmier P, Tichadou L, et al. Poisoning by Coriaria myrtifolia Linnaeus: A new case report and review of the literature. Toxicon. 2005;46:600.
70 Demircan A, Keles A, Bildik F, et al. Mad honey sex: Therapeutic misadventures from an ancient biological weapon. Ann Emerg Med. 2009;54:824.
71 Deng JF, Ger J, Tsai WJ, et al. Acute toxicities of betel nut: Rare but probably overlooked events. J Toxicol Clin Toxicol. 2001;39:355.
72 de Ridder S, Eerens F, Hofstra L. Khat rings twice: Khat-induced thrombosis in two vascular territories. Netherlands Heart Journal. 2007;15:269.
73 Dettling A, Grass H, Schuff A, et al. Absinthe: Attention performance and mood under the influence of thujone. J Stud Alcohol. 2004;65:573.
74 Dickers K, Bradberry S, Rice P, et al. Abrin poisoning. Toxicol Rev. 2003;22:137.
75 DiMarzo V, Melck D, Bisogno T, et al. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521.
76 Doan LG. Ricin: Mechanism of toxicity, clinical manifestations, and vaccine development: A review. J Toxicol Clin Toxicol. 2004;42:201.
77 Drummer OH, Roberts AN, Bedford PJ, et al. Three deaths from hemlock poisoning. Med J Aust. 1995;162:592.
78 Dwivedi S, Rajpal S, Narang S. Cardiotoxic manifestations of yellow oleander (Thevetia nerifolia) poisoning and its treatment: A case report. Indian Heart J. 2006;58:450.
79 Dyer S. Plant exposures: Wilderness medicine. Emerg Med Clin North Am. 2004;22:299.
80 Eadie MJ. Convulsive ergotism: Epidemics of the serotonin syndrome? Lancet Neurol. 2003;2:429.
81 Eddleston M, Ariaratnam CA, Meyer WP. Epidemic of self-poisoning with seeds of yellow oleander tree (Thevetia peruviana) in northern Sri Lanka. Trop Med Int Health. 1999;4:266.
82 Eddleston M, Ariaratnam CA, Sjostrom L, et al. Acute yellow oleander (Thevetia peruviana) poisoning: Cardiac arrhythmias, electrolyte disturbances, and serum cardiac glycoside concentrations on presentation to hospital. Heart. 2000;83:301.
83 Eddleston M, Haggalla S. Fatal injury in Eastern Sri Lanka, with special reference to cardenolide self-poisoning with Cerbera manghas fruits. Clin Toxicol. 2008;46:745.
84 Eddleston M, Persson H. Acute plant poisoning and antitoxin antibodies. J Toxicol Clin Toxicol. 2003;41:309.
85 Eisenhut M. The toxicity of essential oils. Int J Infect Dis. 2007;11:365.
86 el Bahri LE, Djegham M, Makhlouf M. Urginea maritima L (squill): A poisonous plant of North America. Vet Hum Toxicol. 2000;42:108.
87 Eriksson JW, Carlberg B, Hillorn V. Life-threatening ventricular tachycardia due to liquorice-induced hypokalaemia. J Intern Med. 1999;245:307.
88 Ernesto M, Cardoso P, Nicala D, et al. Persistent konzo and cyanogens toxicity from cassava in northern Mozambique. Acta Tropica. 2002;82:357.
89 Ertekin V, Selimoglu MA, Altinkaynak S. A combination of unusual presentations of Datura stramonium intoxication in a child: Rhabdomyolysis and fulminant hepatitis. J Emerg Med. 2005;28:227.
90 Escoffery CT, Shirley SE. Fatal poisoning in Jamaica: A coroner’s autopsy study from the University Hospital of the West Indies. Med Sci Law. 2004;44:116.
91 Famularo G, Corsi FM, Giacanelli M. Iatrogenic worsening of hypokalemia and neuromuscular paralysis associated with the use of glucose solutions for potassium replacement in a young woman with licorice intoxication and furosemide abuse. Acad Emerg Med. 1999;6:960.
92 Farre M, Xirgu J, Salgado A, et al. Fatal oxalic acid poisoning from sorrel soup. Lancet. 1990;335:233.
93 Fernando C. Poisoning due to Abrus precatorius (jequirity bean). Anaesthesia. 2001;56:1178.
94 Festa M, Andreetto B, Ballaris MA, et al. A case of Veratrum poisoning [Italian]. Minerva Anestesiol. 1996;62:195.
95 Flood RG. Strychnine poisoning. Pediatr Emerg Care. 1999;15:286.
96 Foster PF, McFadden R, Trevino R, et al. Successful transplantation of donor organs from a hemlock poisoning victim. Transplantation. 2003;76:874.
97 Frank BS, Michelson WB, Panter KE, et al. Ingestion of poison hemlock (Conium maculatum). West J Med. 1995;163:573.
98 Frasca T, Brett AS, Yoo SD. Mandrake toxicity: A case of mistaken identity. Arch Intern Med. 1997;157:2007.
99 Froldi R, Croci PR, Dell’Acqua L, et al. Preliminary gas chromatography with mass spectrometry determination of 3,5-dimethoxyphenol in biological specimens as evidence of Taxus poisoning. J Anal Toxicol. 2010;34:53.
100 Fu P, Xia Q, Lin G, et al. Pyrrolizidine alkaloids: Genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 2004;36:1.
101 Fu P, Xia Q, Guo L, et al. Toxicity of kava kava. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:89.
102 Fu P, Wu M, Qiao Y, et al. Toxicological mechanisms of Aconitum alkaloids. Die Pharmazie. 2006;61:735.
103 Fujita K, Miyamoto M, Takata T. Colchicine poisoning by accidental ingestion of the bulbs of Sandersonia aurantiaca: Report of a case [Japanese]. Chudoka Kenkyu. 2002;15:375.
104 Furbee B, Wermuth M. Life-threatening plant poisoning. Crit Care Clin. 1997;13:849.
105 Furbee RB, Curry SC, Kunkel DB. Ingestion of Argyreia nervosa (Hawaiian baby woodrose) seeds. Vet Hum Toxicol. 1991;33:370.
106 Gabrscek L, Lesnicar G, Krivec B, et al. Accidental poisoning with autumn crocus. J Toxicol Clin Toxicol. 2004;42:85.
107 Gaillard Y, Krishnamoorthy A, Bevalot F. Cerbera odollam: A ‘suicide tree’ and cause of death in the state of Kerala, India. J Ethnopharmacol. 2004;95:123.
108 Gaillard Y, Pepin G. LC-EI-MS determination of veratridine and cevadine in two fatal cases of Veratrum album poisoning. J Anal Toxicol. 2001;25:481.
109 Gegax TT. Meet the khat-heads. Newsweek. 2002;140:35.
110 Giron LM, Freire V, Alonzo A, et al. Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala. J Ethnopharmacol. 1991;34:173.
111 Graeme KA, LoVechio FA, Selden BS, et al. Cardiotoxicity from ingestion of unprocessed Nerium oleander leaves treated with Fab fragments. J Toxicol Clin Toxicol. 1998;36:457.
112 Graeme KA, Kunkel DB. Psychoactive plants and mushrooms. Top Emerg Med. 1997;19:64.
113 Greene GS, Patterson SG, Warner E. Ingestion of angel’s trumpet: An increasingly common source of toxicity. South Med J. 1996;89:365.
114 Grobosch T, Binscheck T, Martens F, et al. Accidental intoxication with Veratrum album. J Anal Toxicol. 2008;32:768.
115 Gunnarsdottir S, Johannesson T. Glycyrrhetic acid in human blood after ingestion of glycyrrhizic acid in licorice. Pharmacol Toxicol. 1997;81:300.
116 Gupta A, Joshi P, Jortani SA. A case of nondigitalis cardiac glycoside toxicity. Ther Drug Monit. 1997;19:711.
117 Haas LF. Papaver sominferum (opium poppy). J Neurol Neurosurg Psychiatry. 1995;58:402.
118 Habermehl GG. Secondary and tertiary metabolites as plant toxins. Toxicon. 1998;36:1707.
119 Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611.
120 Hamouda C, Amamou M, Thabet H, et al. Plant poisoning from herbal medication admitted to a Tunisian toxicologic intensive care unit, 1983-1998. Vet Hum Toxicol. 2000;42:137.
121 Hamouda C, Hedhili A, Salah NB, et al. A review of acute poisoning from Atractylis gummifera L. Vet Hum Toxicol. 2004;46:144.
122 Hampl JS, Holland KA, Marple JT, et al. Acute hemolysis related to consumption of fava beans: A case study and medical nutrition therapy approach. J Am Diet Assoc. 1997;97:182.
123 Hansen P, Clerc B. Anisocoria in the dog provoked by a toxic contact with an ornamental plant: Datura stramonium. Vet Ophthalmol. 2002;5:277.
124 Hare WR, Schutzman H, Lee BR, et al. Chinaberry poisoning in two dogs. J Am Vet Med Assoc. 1997;210:1638.
125 Harris R, Marx G, Millett M, et al. Colchicine-induced bone marrow suppression treatment with granulocyte colony-stimulating factor. J Emerg Med. 2000;18:435.
126 Hashimoto H, Clyde VJ, Parko KL. Botulism from peyote. N Engl J Med. 1998;339:203.
127 Hasler J, Lee S. Acute hemolytic anemia after ingestion of fava beans. Am J Emerg Med. 1993;11:560.
128 Hassan NA, Gunaid AA, El-Khally FM, et al. The effect of chewing khat leaves on human mood. Saudi Med J. 2002;23:850.
129 Hauptman PJ, Kelly RA. Digitalis. Circulation. 1999;99:1265.
130 Havelius U, Asman P. Accidental mydriasis from exposure to angel’s trumpet (Datura sauveolens). Acta Ophthalmol Scand. 2002;80:332.
131 Heath KB. A fatal case of apparent water hemlock poisoning. Vet Hum Toxicol. 2001;43:35.
132 Heilpern KL. Zigadenus poisoning. Ann Emerg Med. 1995;25:259.
133 Hofman M, Diaz JE, Martella C. Oil of wintergreen overdose. Ann Emerg Med. 1998;31:793.
134 Hold KM, Siorisoma NS, Ikeda T, et al. Alpha-thujone (the active component of absinthe): Gamma-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc Natl Acad Sci U S A. 2000;97:3826.
135 Huang Z, Wang X, Li Y, et al. Betel nut indulgence as a cause of epilepsy. Seizure. 2003;12:406.
136 Hui A, Marraffa JM, Stork CM. A rare ingestion of the black locust tree. J Toxicol Clin Toxicol. 2004;42:93.
137 Hung YM, Miller M, Haller C, et al. Colchicine related death presenting as an unknown case of multiple organ failure. J Toxicol Clin Toxicol. 2001;39:511.
138 Isbister G, Oakely P, Dawson A, et al. Presumed angel’s trumpet (Brugmansia) poisoning: Clinical effects and epidemiology. Emerg Med. 2003;15:376.
139 Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46:167.
140 Joshi P, Wicks C, Munshi SK. Recurrent autumnal psychosis. Postgrad Med J. 2003;79:239.
141 Joskow R, Belson M, Vesper H, et al. Ackee fruit poisoning: An outbreak investigation in Haiti 2000-2001, and review of the literature. Clin Toxicol. 2006;44:267.
142 Katar S, Kervanciolglu M, Devecioglu C, et al. Colchicine poisoning in a very young child. Saudi Med J. 2006;27:1598.
143 Keating JP, Lell ME, Strauss AW, et al. Infantile methemoglobinemia caused by carrot juice. N Engl J Med. 1973;288:824.
144 Khattab NY, Amer G. Undetected neuropsychophysiological sequelae of khat chewing in standard aviation medical examination. Aviat Space Environ Med. 1995;66:739.
145 Kim JY, Choi SH, Moon SW, et al. Aconitine poisoning from leaves of aconitum pseudo-leave var. erectum, not the root in humans. Clin Toxicol. 2009;47:836.
146 King MA, McDonough MA, Drummer OH, et al. Poppy tea and the baker’s first seizure. Lancet. 1997;350:716.
147 Klintschar M, Beham-Schmidt C, Radner H, et al. Colchicine poisoning by accidental ingestion of meadow saffron (Colchicum autumnale): Pathological and medicolegal aspects. Forensic Sci Int. 1999;106:191.
148 Kne T, Brokaw M, Wax P. Fatality from calcium chloride in a chronic digoxin toxic patient. J Toxicol Clin Toxicol. 1997;35:505.
149 Koca I, Koca A. Poisoning by mad honey: A brief review. Food Chem Toxicol. 2007;45:1315.
150 Koltin D, Uziel Y, Schneidermann D, et al. A case of Jatropha multifida poisoning resembling organophosphate intoxication. Clin Toxicol. 2006;44:337.
151 Kontrimabiciute V, Mathieu O, Mathieu-Daude JC, et al. Distribution of ibogaine and noribogaine in a man following a poisoning involving root bark of the Tabernanthe iboga shrub. J Anal Toxicol. 2006;30:434.
152 Krenzelok EP. Lethal plant exposures reported to poison centers: Prevalence, characterization and mechanisms of toxicity [abstract]. J Toxicol Clin Toxicol. 2002;40:303.
153 Krenzelok EP, Jacobsen TD. Plant exposures … a national profile of the most common plant genera. Vet Hum Toxicol. 1997;39:248.
154 Krenzelok EP, Jacobsen TD, Aronis J. Is the yew really poisonous to you? J Toxicol Clin Toxicol. 1998;36:219.
155 Kresge N, Simoni RD, Hill RL. Hemorrhagic sweet clover disease, dicumarol, and warfarin: The work of Karl Paul Link. J Biol Chem. 2005;208:e5.
156 Kupfer A, Idle JR. Methylene blue and fatal encephalopathy from ackee fruit poisoning. Lancet. 1999;353:1622.
157 Lacassie E, Marquet P, Martin-Dupont S, et al. A non-fatal case of intoxication with foxglove, documented by means of liquid chromatography-electrospray-mass spectrometry. J Forensic Sci. 2000;45:1154.
158 Lachenmeier DW, Sproll C, Musshoff F. Poppy seed foods and opiate drug testing: Where are we today? Ther Drug Monit. 2010;32:11.
159 Lampe KF. Rhododendrons, mountain laurel, and mad honey. JAMA. 1988;259:2009.
160 Landelle C, Francony G, Sam-Lai N, et al. Poisoning by lavandin extract in an 18-month-old boy. Clin Toxicol. 2008;46:279.
161 Langford SD, Boor PJ. Oleander toxicity: An examination of human and animal toxic exposures. Toxicology. 1996;109:1.
162 Lawrence RA. Poison centers and plants: More pollyanna data? J Toxicol Clin Toxicol. 1998;36:225.
163 Le Couteur DG, Fisher AA. Chronic and criminal administration of Nerium oleander. J Toxicol Clin Toxicol. 2002;40:523.
164 Lee SW, Choi SH, Hong YS, et al. Grayanotoxin poisoning from flower of Rhododendron mucronulatum in humans. Bull Environ Contam Toxicol. 2007;78:132.
165 Li L, Sun B, Zhang Q, et al. Metabolic study on the toxicity of Hei-Shun-Pian, the processed lateral root of Aconitum carmichaelii Debx. (Ranunculaceae). J Ethnopharmacol. 2008;116:561.
166 Lin CC, Chou HH, Lin JL. Acute aconitine poisoned patients with ventricular arrhythmias successfully reversed by charcoal hemoperfusion. Am J Emerg Med. 2002;20:66.
167 Lin SH, Lin YF, Cheema-Dhadli S, et al. Hypercalcaemia and metabolic alkalosis with betel nut chewing: Emphasis on its integrative pathophysiology. Nephrol Dial Transplant. 2002;17:708.
168 Lin TJ, Nelson LS, Tsai JL, et al. Common toxidromes of plant poisonings in Taiwan. Clin Toxicol. 2009;47:161.
169 Lindsey T, O’Hara J, Irvine R, et al. Strychnine overdose following ingestion of gopher bait. J Anal Toxicol. 2004;28:135.
170 Loretti AP, da Silva Ilha MR, et al. Accidental fatal poisoning of a dog by Dieffenbachia picta (dumb cane). Vet Hum Toxicol. 2003;45:233.
171 Lude S, Torok M, Dieterle S, et al. Hepatocellular toxicity of kava leaf and root extracts. Phytomedicine. 2008;15:120.
172 Lukandu OM, Neppelberg E, Vintermyr OK. Khat alters the phenotype of in vitro-reconstructed human oral mucosa. J Dent Res. 2010;89:270.
173 Manriquez O, Varas J, Carlos J, et al. Analysis of 156 cases of plant intoxication received in the toxicologic information center at Catholic University of Chile. Vet Hum Toxicol. 2002;44:31.
174 Martz W. A lethal ingestion of a household cleaner containing pine oil and isopropanol. J Anal Toxicol. 2010;34:49.
175 Meda HA, Diallo B, Buchet JP, et al. Epidemic of fatal encephalopathy in preschool children in Burkina Faso and consumption of unripe ackee (Blighia sapida) fruit. Lancet. 1999;353:536.
176 Mellick LB, Makowski T, Mellick GA, et al. Neuromuscular blockade after ingestion of tree tobacco (Nicotiana glauca). Ann Emerg Med. 1999;34:101.
177 Meltem AC, Figen C, Nalan MA, et al. A hypokalemic muscular weakness after licorice ingestion: A case report. Cases J. 2009;2:8053.
178 Miller MB, Eng J, Curry SC. Sodium bicarbonate for Taxus-induced dysrhythmia. J Toxicol Clin Toxicol. 2000;38:572.
179 Miraldi E, Masti A, Ferri S, et al. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia. 2001;72:644.
180 Mizrachi N, Levy S, Goren Z. Fatal poisoning from Nicotiana glauca leaves: Identification of anabasine by gas-chromatography/mass spectrometry. J Forensic Sci. 2000;45:736.
181 Moritz F, Compagnon P, Kaliszczak I, et al. Severe acute poisoning with homemade Aconitum napellus capsules: Toxicokinetic and clinical data. Clin Toxicol. 2005;43:873.
182 Morrish PK, Nicolaou N, Brakkenberg P, et al. Leukoencephalopathy associated with khat misuse. J Neurol Neurosurg Psychiatry. 1999;67:556.
183 Moya J. Ackee (Blighia sapida) poisoning in the Northern Province, Haiti, 2001. Epidemiol Bull. 2001;22:8.
184 Murone AJB, Stucki P, Roback MG, et al. Severe methemoglobinemia due to food intoxication in infants. Ped Emerg Care. 2005;21:536.
185 Mycyk M, Burda A, Tsoustias G, et al. A human fatality from “grazing” on a yew plant. J Toxicol Clin Toxicol. 2001;39:550.
186 Naik BS, Chakrapani M. A rare case of brucine poisoning complicated by rhabdomyolysis and acute renal failure. Malaysian J Pathol. 2009;31:67.
187 Nampoothiri K, Chrispal A, Begum A, et al. A clinical study of renal tubular dysfunction in Cleistanthus collinus (Oduvanthalai) poisoning. Clin Toxicol. 2010;48:193.
188 Nelson BS, Heischober B. Betel nut: A common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Ann Emerg Med. 1999;34:238.
189 Newman LS, Feinberg MW, LeWine HE. A bitter tale. N Engl J Med. 2004;351:594.
190 Nielen RJ, van der Heijden FM, Tuinier S, et al. Khat and mushrooms associated with psychosis. World J Biol Psychiatry. 2004;4:49.
191 Nishioka SA, Resende ES. Transitory complete atrioventricular block associated to ingestion of Nerium oleander. Rev Assoc Med Brasil. 1995;41:60.
192 Norn S, Kruse PR. Cardiac glycosides: From ancient history through Withering’s foxglove to endogenous cardiac glycosides. Dansk Medicinhist Arbog. 2004:119.
193 Numan N. Exploration of adverse psychological symptoms in Yemeni khat users by the Symptoms Checklist-90 (SCL-90). Addiction. 2004;99:61.
194 Oberndorfer S, Grisold W, Hinterholzer G, et al. Coma with focal neurological signs caused by Datura stramonium intoxication in a young man. J Neurol Neurosurg Psychiatry. 2002;73:458.
195 Oberpaur B, Donoso A, Claveria C, et al. Strychnine poisoning: An uncommon intoxication in children. Pediatr Emerg Care. 1999;15:264.
196 Ohuchi S, Izumoto H, Kamata J, et al. A case of aconitine poisoning saved with cardiopulmonary bypass. Kyobu Geka. 2000;53:541.
197 Onen CL, Othol D, Manuel IL. Datura stramonium mass poisoning in Botswana. S Afr Med J. 2002;92:213.
198 Olsen RW. Absinthe and gamma-aminobutyric acid receptors. Proc Natl Acad Sci U S A. 2000;97:4417.
199 Palatnick W, Tennenbein M. Hepatotoxicity from castor bean ingestion in a child. J Toxicol Clin Toxicol. 2000;38:67.
200 Parissis D, Mellidis C, Boutis A. Neurological findings in a case of coma secondary to Datura stramonium poisoning. Eur J Neurol. 2003;10:745.
201 Patel NB. Mechanism of action of cathinone: The active ingredient of khat (Catha edulis). East Afr Med J. 2000;77:329.
202 Pedaci L, Krenzelok EP, Jacobsen TD, et al. Dieffenbachia species exposures: An evidence-based assessment of symptom presentation. Vet Hum Toxicol. 1999;41:335.
203 Pekdemir M, Yanturali S, Akay S, et al. Acute anticholinergic syndrome due to Datura innoxia Miller mixed with lime tea leaves. Vet Hum Toxicol. 2004;45:176.
204 Pelders MG, Ros JJW. Poppy seeds: Differences in morphine and codeine content and variation in inter- and intra-individual excretion. J Forensic Sci. 1996;41:209.
205 Pereira CAL, Nishioka SA. Poisoning by the use of Datura leaves in a homemade toothpaste. J Toxicol Clin Toxicol. 1994;32:329.
206 Peterson MC, Rasmussen GJ. Intoxication with foothill camas (Zigadenus paniculatus). J Toxicol Clin Toxicol. 2003;41:63.
207 Philippe G, Angenot L, Tits M, et al. About the toxicity of some Strychnos species and their alkaloids. Toxicon. 2004;44:405.
208 Phua DH, Tsai WJ, Deng JF, et al. Human Melia azedarach poisoning. Clin Toxicol. 2008;46:1067.
209 Piccillo GA, Mondati EGM, Moro PA. Six clinical cases of Mandragora autumnalis poisoning: Diagnosis and treatment. Eur J Emerg Med. 2002;9:342.
210 Pierog JE, Kane B, Kane K, et al. Management of isolated yew berry toxicity with sodium bicarbonate: A case report in treatment efficacy. J Med Toxicol. 2009;5:84.
211 Pietsch J, Schulz K, Schmidt U, et al. A comparative study of five fatal cases of Taxus poisoning. Int J Legal Med. 2007;121:417.
212 Pillay VV, Bhagyanathan PV, Krishnaprasad R, et al. Poisoning due to white seed variety of Abrus precatorius. J Assoc Physicians India. 2005;53:317.
213 Prince LA, Stork CM. Prolonged cardiotoxicity from poison lily (Veratrum viride). Vet Hum Toxicol. 2000;42:282.
214 Puschner B, Galey FD, Holstege DM, Palazoglu M. Sweet clover poisoning in dairy cattle in California. J Am Vet Med Assoc. 1998;212:857.
215 Rajapakse S. Management of yellow oleander poisoning. Clin Tox. 2009;47:206.
216 Rasenack R, Muller C, Kleinschmidt M, et al. Veno-occlusive disease in a fetus caused by pyrrolizidine alkaloids of food origin. Fetal Diagn Ther. 2003;18:223.
217 Rauber-Luthy CH, Guirguis M, Meier-Abt AST, et al. Lethal poisoning after ingestion of a tea prepared from the angel’s trumpet (Datura suaveolens). J Toxicol Clin Toxicol. 1999;37:414.
218 Rich SA, Libera JM, Locke RJ. Treatment of foxglove extract poisoning with digoxin-specific Fab fragments. Ann Emerg Med. 1993;22:1904.
219 Ridker PM. Health hazards of unusual herbal teas. Am Fam Physician. 1989;39:153.
220 Rosling H. Cyanide exposure from linseed. Lancet. 1993;34:177.
221 Ruangkanchanasetr S, Wananukul V, Suwanjutha S. Cyanide poisoning: 2 case reports and treatment review. J Med Assoc Thai. 1999;82:S162.
222 Ruha AM, Tanen DA, Graeme KA, et al. Hypertonic sodium bicarbonate for Taxus media–induced cardiac toxicity in swine. Acad Emerg Med. 2002;9:179.
223 Salen P, Shih R, Sierzenski P, et al. Effect of physostigmine and gastric lavage in Datura stramonium–induced anticholinergic poisoning epidemic. Am J Emerg Med. 2003;21:316.
224 Sannohe S, Makino Y, Kita T, et al. Colchicine poisoning resulting from accidental ingestion of meadow saffron (Colchicum autumnale). J Forensic Sci. 2002;47:1391.
225 Sanz P, Reig R. Clinical and pathological findings in fatal plant oxalosis: A review. Am J Forensic Med Pathol. 1992;13:342.
226 Sayre JW, Kaymakcalan S. Cyanide poisoning from apricot seeds among children in central Turkey. N Engl J Med. 1964;270:1113.
227 Schep LJ, Schmierer DM, Fountain JS. Veratrum poisoning. Toxicol Rev. 2006;25:73.
228 Schep LJ, Slaughter RJ, Beasley MG. Nicotinic plant poisoning. Cl Toxicol. 2009;47:771.
229 Schep LJ, Slaughter RJ, Becket G, et al. Poisoning due to water hemlock. Clin Toxicol. 2009;47:270.
230 Schneider F, Lutun P, Kintz P, et al. Plasma and urine concentrations of atropine after ingestion of cooked deadly nightshade berries. J Toxicol Clin Toxicol. 1996;34:113.
231 Scully RE, Mark EJ, McNeely WF. Case 12-2001. N Engl J Med. 2001;344:1232.
232 Shadnia S, Molensadat M, Abdollahi M. A case of acute strychnine poisoning. Vet Hum Toxicol. 2004;46:76.
233 Shah S, Merchant AT, Luby SP, et al. Addicted school children: Prevalence and characteristics of areca nut chewers among primary school children in Kaarachi, Pakistan. J Paediatr Child Health. 2002;38:507.
234 Sherrat HSA. Jamaican vomiting sickness. In: DeWolff FA, editor. Handbook of clinical neurology, vol 21. Intoxications of the nervous system, pt 2. Amsterdam: Elsevier Press; 1995:79-113.
235 Sherratt HSA, Turnbull DM. Methylene blue and fatal encephalaopathy from ackee fruit poisoning. Lancet. 1999;353:1623.
236 Sims DN, James R, Christensen T. Another death due to ingestion of Nicotiana glauca. J Forensic Sci. 1999;44:447.
237 Sinan A, Arslan U, Karakurt K, et al. An unusual presentation of mad honey poisoning: Acute myocardial infarction. Int J Cardiol. 2008;129:356.
238 Sjoholm A, Lindberg A, Personne M. Acute nutmeg intoxication. J Intern Med. 1998;243:327.
239 Slifman NR, Obermeyer WR, Aloi BK, et al. Contamination of botanical dietary supplements by Digitalis lanata. N Engl J Med. 1998;339:806.
240 Sopchak CA, Stork CM, Cantor RM, et al. Central anticholinergic syndrome due to jimsonweed physostigmine: Therapy revisited? J Toxicol Clin Toxicol. 1998;36:43.
241 Smith SW, Shah RR, Herzog CA. Bidirectional ventricular tachycardia resulting from herbal aconite poisoning. Ann Emerg Med. 2005;45:100.
242 Southgate HJ, Egerton M, Dauncey EA. Lessons to be learned: A case study approach—Unseasonal severe poisoning of two adults by deadly nightshade (Atropa belladonna). J R Soc Health. 2000;120:127.
243 Sperl W, Stuppner H, Gassner I, et al. Reversible hepatic veno-occlusive disease in an infant after consumption of pyrrolizidine-containing herbal tea. Eur J Pediatr. 1995;154:112.
244 Steen V, Stewart MJ, Zuckerman M. Clinical and analytical aspects of pyrrolizidine poisoning caused by South African traditional medicines. Ther Drug Monit. 2000;22:302.
245 Steenkamp PA, Harding NM, van Heerden FR, et al. Identification of atractyloside by LC-ESI-MS in alleged herbal poisonings. Forensic Sci Int. 2006;163:81.
246 Steenkamp V, Stewart MJ. Nephrotoxicity associated with exposure to plant toxins, with particular reference to Africa. Ther Drug Monit. 2005;27:270.
247 Steenkamp PA, van Heerden FR, van Wyk BE. Accidental fatal poisoning by Nicotiana glauca: Identification of anabasine by high performance liquid chromatography/photodiode array/mass spectrometry. Forensic Sci Int. 2002;127:208.
248 Stewart MJ, Steenkamp V. Pyrrolizidine poisoning: A neglected area of human toxicology. Ther Drug Monit. 2001;23:698.
249 Stoopler ET, Parisi E, Sollecito TP. Betel quid-induced oral lichen planus: A case report. Cutis. 2003;71:307.
250 Strang J, Arnold WN, Peters T. Absinthe: What’s your poison? Though absinthe is intriguing, it is alcohol in general we should worry about. BMJ. 1999;319:1590.
251 Subrahmanyam DK, Mooney T, Raveendran R, et al. A clinical and laboratory profile of Cleistanthus collinus poisoning. J Assoc Physicians India. 2003;51:1052.
252 Suchard JR, Wallace KL, Gerkin RD. Acute cyanide toxicity caused by apricot kernel ingestion. Ann Emerg Med. 1998;32:742.
253 Sztajnkrycer MD, Otten EJ, Bond GR, et al. Mitigation of pennyroyal oil hepatotoxicity in the mouse. Acad Emerg Med. 2003;10:1024.
254 Tagwireyi D, Ball DE. The management of elephant’s ear poisoning. Hum Exp Toxicol. 2001;20:189.
255 Thabet H, Brahml M, Amamou M, et al. Datura stramonium poisoning in humans. Vet Hum Toxicol. 1999;41:320.
256 Tibballs J. Clinical effects and management of eucalyptus oil ingestion in infants and young children. Med J Aust. 1995;163:177.
257 Tiwary AK, Puschner B, Kinde H, et al. Diagnosis of Taxus (yew) poisoning in a horse. J Vet Diagn Invest. 2005;17:252.
258 Toennes SW, Harder S, Schramm M, et al. Pharmacokinetics of cathinone, cathine, and norephedrine after chewing of khat leaves. Br J Clin Pharmacol. 2003;56:125.
259 Tsai YC, Chen CY, Yang NI, et al. Cardiac glycoside poisoning following suicidal ingestion of Cerbera manghas. Clin Toxicol. 2008;46:340.
260 Tuncok Y, Kozan O, Cavdar C, et al. Urginea maritima (squill) toxicity. J Toxicol Clin Toxicol. 1995;33:83.
261 Turgut M, Alhan C, Gurgoze M, et al. Carboxyatractyloside poisoning in humans. Ann Trop Paediatr. 2005;25:125.
262 Unnithan S, Strang J. Poppy tea dependence. Br J Psychiatry. 1993;163:813.
263 Van der Donck I, Mulliez E, Blanckaert J. Angel’s trumpet (Brugmansia arborea) and mydriasis in a child: A case report. Bull Soc Belge Ophtalmol. 2004;292:53.
264 Van Dongen PW, de Groot AN. History of ergot alkaloids from ergotism to ergometrine. Eur J Obstet Gynecol Reprod Biol. 1995;60:109.
265 Van Gils C, Cox PA. Ethnobotany of nutmeg in the Spice Islands. J Ethnopharmacol. 1994;42:117.
266 Van Ingen G, Visser R, Peltenburg H, et al. Sudden unexpected death due to Taxus poisoning: A report of five cases, with review of the literature. Forensic Sci Int. 1992;56:81.
267 Veltmann C, Borggrefe M, Schimpf R, et al. Yew causes Brugada ECG. Circulation. 2009;119:1836.
268 Vetter J. Poison hemlock (Conium maculatum L.). Food Chem Toxicol. 2004;42:1373.
269 von Malottki K, Wiechmann HW. Acute life-threatening bradycardia: Food poisoning by Turkish wild honey. Deutsche Med Wochenschr. 1996;121:936.
270 Watson JT, Jones RC, Siston AM, et al. Outbreak of food-borne illness associated with plant material containing raphides. Clin Toxicol. 2005;1:17.
271 Wax PM. Squill through the ages. J Toxicol Clin Toxicol. 1995;33:86.
272 Wehner F, Gawatz O. Suicidal yew poisoning—from Caesar to today—or suicide instructions on the Internet. Arch Kriminol. 2003;211:19.
273 West PL, Horowitz BZ. Zigadenus poisoning treated with atropine and dopamine. J Med Toxicol. 2009;5:214.
274 West PL, Horowitz BZ, Montanaro MT, et al. Poison hemlock-induced respiratory failure in a toddler. Pediatr Emer Care. 2009;25:761.
275 Wilson CR, Sauer J, Hooser SB. Taxines: A review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon. 2001;39:175.
276 Wojtyna W, Enseleit F. A rare cause of complete heart block after transdermal botanical treatment for psoriasis. Pace. 2004;27:1686.
277 Wollersen K, Erdmann F, Risse M, et al. Accidental fatal ingestion of colchicines-containing leaves: Toxicological and histological findings. Legal Med. 2009;11:S498.
278 Wood DM, Webster E, Martinex D, et al. Case report: Survival after deliberate strychnine self-poisoning, with toxicokinetic data. Crit Care. 2002;6:456.
279 Yeih DF, Chiang FT, Huang SK. Successful treatment of aconitine induced life threatening ventricular tachyarrhythmia with amiodarone. Heart. 2000;84:E8.
280 Zagler B, Zelger A, Salvatore C, et al. Dietary poisoning with Veratrum album: A report of two cases. Wien Klin Wochenschr. 2005;117:106.
281 Zuckerman M, Steenkamp V, Stewart MJ. Hepatic veno-occlusive disease as a result of a traditional remedy: Confirmation of toxic pyrrolizidine alkaloids as the cause, using an in vitro technique. J Clin Pathol. 2002;55:676.
* References 84, 90, 93, 120, 168, 173, 268, 280.
* References 37, 89, 138, 140, 173, 179, 230, 255.
† References 37, 113, 138, 140, 173, 179, 203, 209, 230, 242.
* References 44, 68, 112, 138, 223, 255.
† References 45, 68, 138, 203, 209, 223, 255.
‡ References 89, 194, 197, 200, 203, 255.
* References 44, 104, 138, 200, 209, 223, 242, 255.
* References 43, 60, 176, 180, 228, 236.
* References 25, 77, 96, 97, 104, 118, 228, 268, 274.
† References 29, 52, 71, 135, 167, 168, 188, 233, 249.
* References 40, 41, 45, 80, 118, 264.
* References 3, 5, 7, 39, 42, 128, 190, 193, 201, 258.
† References 1, 3, 4, 5, 7, 14, 51, 72, 109, 128, 144, 172, 182, 190, 193, 201.
* References 22, 47, 95, 169, 186, 195, 207, 231, 232, 278.
* References 81, 86, 104, 116, 118, 129, 161, 189, 260.
* References 66, 81, 82, 104, 111, 161, 163, 191, 215, 276.
* References 78, 111, 163, 189, 215, 239, 276.
† References 57, 82, 83, 84, 107, 111, 148, 161, 189, 215, 218, 239.
* References 8, 46, 48, 49, 104, 165, 181.
* References 46, 49, 102, 104, 145, 165, 168, 181, 241.
† References 94, 104, 108, 114, 132, 206, 213, 227, 273, 280.
‡ References 94, 114, 206, 227, 273, 280.
* References 2, 53, 55, 70, 149, 159, 164, 269.
† References 8, 46, 49, 53, 55, 79, 104, 108, 114, 164, 166, 181, 196, 206, 227, 241, 273, 279.
‡ References 99, 154, 162, 168, 210, 211, 257, 266, 267, 275.
§ References 154, 168, 178, 185, 210, 222, 257, 266, 267, 275.
* References 6, 24, 32, 74, 76, 120, 136, 199, 212.
* References 32, 74, 76, 120, 199, 212.
* References 24, 32, 74, 76, 199, 212.
† References 19, 54, 100, 216, 219, 243, 244, 248, 281.
* References 33, 35, 64, 84, 103, 106, 142, 147, 168, 224, 246, 277.
† References 33, 35, 64, 84, 106, 125, 137, 142, 147, 168, 224, 246, 277.
‡ References 33, 35, 84, 106, 125, 137, 277.
* References 20, 87, 91, 115, 139, 177, 246.
* This species has not been reported to cause illness, but other species may be toxic.