Toxic Keratoconjunctivitis
Introduction
The complication of toxic conjunctivitis from topical preparations for the treatment of ophthalmic conditions has long been recognized. The Ebers papyrus from ancient Egypt mentions eye remedies including red lead, antimony, lead, sea salt, iron and sulphur.1 However, it was not until 1864 that Albrecht von Graefe reported a case of keratoconjunctivitis, following the administration of topical atropine.2 In the last few decades, progress in the understanding of eye diseases’ basic mechanisms has increased the number of therapeutic targets, and the field of ocular pharmacology has grown accordingly, to provide the physician with many more topical drugs.3 Glaucoma therapeutics are of particular importance, since topical therapy may last for decades.4 This chapter analyzes the effects of common ophthalmic treatments on the conjunctiva and cornea.
Pathophysiology
Topical ophthalmic preparations can confuse the diagnosis of toxic conjunctivitis.5 Medications often alleviate the patient’s symptoms initially and only at a later time, sometimes years after initiating therapy, will the patient develop discomfort.6 Medications can be directly toxic to the conjunctival and corneal epithelium, damaging its structure and altering its function with or without an inflammatory response.7 Some preparations are directly cytotoxic due to pH, the osmolarity of the solution, or even due to photosensitization.8 The cellular reaction may reflect the direct effect of the active compound, the accompanying preservative, or the breakdown products. Direct toxic effects usually occur after the first contact and appear after a threshold is reached.8 The chronic nature of these substances may lead to infiltration of the substantia propria, by inflammatory cells and fibroblasts, eventually generating fibrosis that can severely disturb the ocular surface.9
1. Allergic reactions due to type 1 hypersensitivity, triggered by the union of the allergen and initiation of the IgE–mast cell axis, leading to degranulation and release of inflammatory mediators.10
2. Type II–III hypersensitivity, characterized by antibody-specific and immune-complex-mediated effects.11
3. Type IV or delayed hypersensitivity. This type is associated with marked epithelial and subepithelial edema and infiltration by CD4+ lymphocytes and Langerhans cells.12
Ophthalmic treatments may also be toxic through more indirect mechanisms. Antimicrobials modify the ocular external microbiota and may favor bacterial colonization by selecting resistant strains,13,14 while corticosteroids decrease local immune mechanisms. Other indirect effects on the ocular surface may involve direct cytotoxicity to goblet cells (preservatives) or decreased tear production (parasympatholytics and antihistamines).15 Some preservatives (benzalkonium chloride) may have a detergent effect on the lipid layer of the tear film, thus, promoting tear evaporation.7
There is no single mechanism responsible for drug intolerance or toxicity, but a combination of these occur simultaneously or in sequence and are accountable for the patients’ symptoms.16
Clinical Features
Periorbital Skin and Eyelids
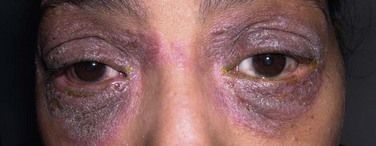
The examination of the ocular adnexa often reveals diagnostic clues. The eyelids and the conjunctiva are contiguous structures that work in synchrony to stabilize and protect the ocular surface. Eczema involving the periorbital region is characteristic of type IV hypersensitivity, specifically if it appears a few days after the initiation of a new drug (Fig. 25.1). Eczema may rarely simulate seborrheic blepharitis. In turn, pruritus, urticaria and edema of the eyelids are characteristic of Type I hypersensitivity (Fig. 25.2). It is important to examine the lacrimal punctum for patency as some drugs may cause fibrosis and obliteration.10
Conjunctiva
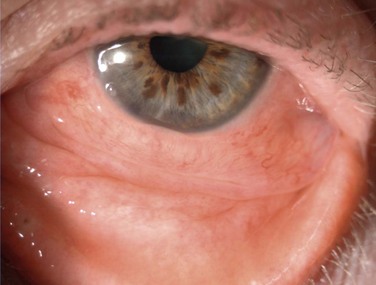
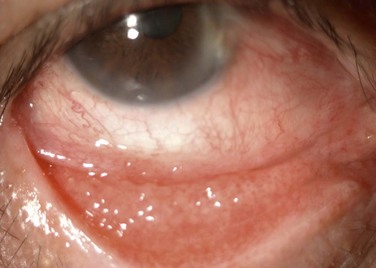
Hyperemia, chemosis, and a clear mucoid discharge are characteristic of an allergic reaction. Pruritus is a strong clinical sign of allergy. Follicles are not seen in pure allergy and they should point to toxicity if supported by clinical history (Fig. 25.3). In these cases, the hyperemia and chemosis are more subtle and difficult to assess. Subconjunctival fibrosis, fornix foreshortening, punctal occlusion and symblepharon formation, should direct the clinician toward drug-induced pemphigoid or pseudopemphigoid (Fig. 25.4).17
Cornea
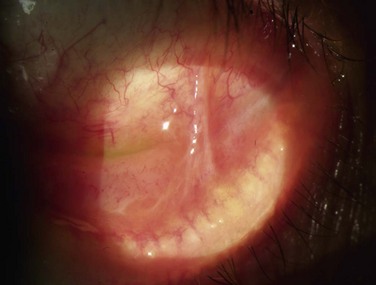
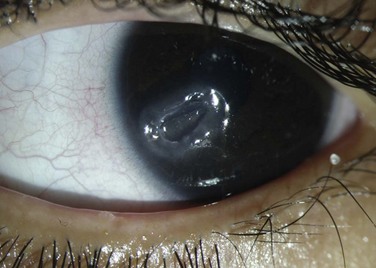
The scope and severity of corneal lesions in toxic keratoconjunctivitis may vary greatly, from very mild punctate epitheliopathy to overt corneal ulceration and necrosis. Toxicity is discovered when epitheliopathy predominates in the inferior-nasal quadrant, where there is maximal contact time between the drug and ocular surface (Fig. 25.5). That is the usual location of corneal ulcers due to anesthetic abuse, which resembles neurotrophic keratitis, in its clinical appearance with rolled edges. Comet’s-impact crater keratitis, intense ciliary flush, and papillary response have also been described in this pathology.5 The corneal epithelium can be opaque and edematous and a hurricane keratopathy can be observed. In the setting of keratoplasty, the latter can be a sign of drug toxicity. Pseudodendrites are also a common feature of drug-induced keratopathy.2
Diagnostic Investigations
Conjunctival Biopsy
Conjunctival biopsies are reserved for confirmation or exclusion of autoimmune diseases and are usually reserved for the diagnosis of cicatrizing conjunctivitis. These biopsies are not well accepted by the patient unless they are absolutely necessary. Regarding findings of toxic damage, previous studies have shown that squamous metaplasia of the conjunctival epithelium and subconjunctival inflammation and fibrosis are present in patients after long-term glaucoma treatment. Pseudopemghigoid may demonstrate findings identical to idiopathic pemphigoid.18
Impression Cytology
Impression cytology can be performed under topical anesthesia and provides a homogenous cell layer for histological studies, with an almost intact architecture and preserved cell junctions. Epithelial cells, goblet cells and inflammatory cells can be differentiated with immunostaining protocols and can even be used for flow cytometry analysis, which permits the measurement of membrane and cytoplasmic inflammatory markers. Some studies have demonstrated that HLA-DR class II antigen and IL-6, IL-8, and IL-10, are strongly expressed in the conjunctival epithelium of patients with history of long-term glaucoma treatment.19
Tear samples can be used to measure levels of IgE or inflammatory cytokines, although given their complex processing, they are rarely used except for research purposes. When positive, skin tests and conjunctival allergen challenge are of great clinical value, but negative findings do not rule out allergy.20
Toxicity of Common Ophthalmic Preparations
Multi-dose eye drops contain antimicrobial preservatives. Many substances have been used for this purpose, including benzalkonium chloride, chlorhexidine, chlorbutanol thimerosal, paraben esters, and mercuric salts. Preservatives are probably the most common cause of toxic conjunctivitis. In a study comprising over 9500 patients, signs and symptoms were more frequent in patients taking preserved medications, compared with those using preservative-free eye drops.21,22
Benzalkonium chloride (BAK) is a quaternary ammonium hapten highly hydrosoluble with surfactant properties. In concentrations ranging from 0.005% to 0.03%, the drug is generally well tolerated. However, it can cause epithelial toxicity and at higher concentrations can even cause irreversible corneal edema. Thimersoal is an organomercurial derivative used in concentrations varying from 0.001% to 0.004%. It is a common cause of hypersensitivity reactions and follicular conjunctivitis, the reported incidence being as high as 8%. Chlorbutanol is an alcohol that increases lipid solubility and its antimicrobial activity is based on its ability to cross the bacterial lipid layer. It is widely used in many pharmaceuticals and cosmetic products. In concentrations of 0.5% it is normally well tolerated and has been found to be less toxic than BAK or thimerosal.7,23
Antivirals, Antibiotics, and Antifungals
Aminoglycosides, such as gentamicin and tobramycin, the latter being the least irritating, can produce conjunctival and corneal hypersensitivity, pruritus and hyperemia.24 The toxic effects of topical fluoroquinolones, even of the first generations, are less common than those generated by aminoglycosides. The most common corneal finding is a white crystalline precipitate after the use of ciprofloxacin.13 The current adoption of the fourth-generation fluoroquinolones has resulted in much less toxicity than the aminoglycosides; however, epithelial toxicity, due to the preservative BAK present in the topical preparation of gatifloxacin, can occur. Sulfonamides are the third most commonly used topical antibiotics; and may produce a mild allergic reaction. The most devastating complication associated with this antibiotic is the induction of Stevens–Johnson syndrome or toxic epidermal necrolysis.14,25 Photosensitization after topical sulfisoxazole ointment use has been reported to cause sunburn of the lid margin.
Antifungals used either in topical commercial preparations or in dilutions prepared from the intravenous forms may impair epithelial healing. Natamycin, the most commonly used topical anti-fungal agent, is generally non-irritating.26
All antivirals may cause toxic keratoconjunctivitis to some degree. On the cornea, IDU produces punctate epitheliopathy, epithelial edema, and cornea; erosions. In the conjunctiva, it causes follicular response, subconjunctival scarring and may occlude the puncta. These same effects but to a lesser degree27 can be caused by trifluorothymidine and vidarabine.
Anesthetics
The indiscriminate use of topical anesthetics is a well known cause of toxic keratoconjunctivitis. Diagnosis is simple when the offending agent is identified, but can be very difficult if the patient hides its misuse. Proparacaine tetracaine, lidocaine, cornecaine, and benoxinate, have all been associated with this entity. The most frequent manifestation is superficial keratopathy, which can be detected even after a single instillation. More severe cases can evolve to corneal ulceration and stromal necrosis. Toxic lesions are due to decreased blinking reflexes, tear film instability with loss of epithelial microvilli, and corneal desiccation. These substances interfere with cell metabolism and cell membrane permeability, alter the function of cytoskeletal proteins, and decrease the rate of epithelial wound healin.28
Glaucoma Medications
Patients with glaucoma frequently require topical treatment for decades. Like all ophthalmic medications, any antiglaucoma drop may initiate a sudden allergic response. These events may occur in about 5% of the cases, are usually easy to diagnose, and respond quickly after discontinuation of the offending drug.4 However, patients may develop a late-onset reaction, appearing months or even decades after treatment. In such cases, local irritation is the rule with foreign body sensation, conjunctival hyperemia, and staining of the conjunctiva with fluorescein and rose bengal.29 Severe cases may induce subconjunctival fibrosis and a pseudopemphigoid syndrome. Furthermore, long-term therapy may induce subclinical inflammation and increased postoperative fibrosis; hence, the long-term use of topical antiglaucoma drugs is considered as an important risk factor for the failure of filtration surgery failure.30 Nurturing of the ocular surface and reduction of inflammation before surgery should be achieved whenever possible.6,17
Many of the toxic effects on disturbing the ocular surface can be linked to preservatives, such as BAK. However, specific glaucoma medications can be associated with certain clinical manifestations. Pilocarpine and beta-blockers have been associated with follicular conjunctivitis and drug-induced pemphigoid; the latter has also been found to produce pseudodendrites and corneal hypoesthesia. Both epinephrine and dipivefrin can also cause a follicular reaction. The former is associated with pigmented adenochrome deposits in the conjunctiva. Alfa-2-adrenergic agonists may produce an acute allergic blepharoconjunctivitis in up to 30% of the cases.31
Others Toxic Agents
Almost all topical treatments used in ophthalmology, given enough time and frequency of use, can be associated with toxicity. Nevertheless, the clinician must be aware that substances other than approved medications may be in contact with the ocular surface. Such is the case of mascara, eyeliner, hair gel, hair sprays, skin preparations, and virtually any substance with direct or indirect contact with the body surface. In many regions of the world, patients still use home-prepared remedies to alleviate common eye complaints, and are often reluctant to disclose their use.32,33
Therapeutic Considerations
On encountering a toxic agent, discontinuation of the offending substance is the primary therapeutic strategy. Treatment will depend on the time of exposure and severity of the ocular surface disease. Attempts should be made to stop medications or to substitute them with preservative-free formulations when possible. Contact lenses should be used cautiously, since they can act as a toxic reservoir. For mild disease, a preservative free artificial tear can be used for symptomatic relief. A topical steroid may be beneficial for more symptomatic patients. More severe pathology, such as persistent epithelial defects may require tarsorrhaphy, amniotic membrane grafting, or conjunctival flaps.34 Keratoplasty may be warranted for corneal ulcerations with impending perforation or necrosis.35
References
1. Magnus, H, Ophthalmology of the ancients. Hirschberg history of ophthalmology, 1st ed. Wayenborgh, J, eds. Hirschberg history of ophthalmology, vol. 4. Wayenborgh: Oostende, 1998:4–12.
2. Wilson, FM, 2nd. Adverse external ocular effects of topical ophthalmic therapy: an epidemiologic, laboratory, and clinical study. Trans Am Ophthalmol Soc. 1983;81:854–965.
3. Baudouin, C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86:716–726.
4. Baudouin, C, Riancho, L, Warnet, JM, et al. In vitro studies of antiglaucomatous prostaglandin analogues: travoprost with and without benzalkonium chloride and preserved latanoprost. Invest Ophthalmol Vis Sci. 2007;48:4123–4128.
5. Schwab, IR, Abbott, RL. Toxic ulcerative keratopathy. An unrecognized problem. Ophthalmology. 1989;96:1187–1193.
6. Baudouin, C, Liang, H, Hamard, P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology. 2008;115:109–115.
7. Chen, W, Li, Z, Hu, J, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. 2011;6:e26103.
8. Wilson, FM, 2nd. Adverse external ocular effects of topical ophthalmic medications. Surv Ophthalmol. 1979;24:57–88.
9. Thorne, JE, Anhalt, GJ, Jabs, DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111:45–52.
10. Guglielmetti, S, Dart, JK, Calder, V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478–485.
11. Kirzhner, M, Jakobiec, FA. Ocular cicatricial pemphigoid: a review of clinical features, immunopathology, differential diagnosis, and current management. Semin Ophthalmol. 2011;26:270–277.
12. Cone, RE, Chattopadhyay, S, O’Rourke, J. Control of delayed-type hypersensitivity by ocular- induced CD8+ regulatory t cells. Chem Immunol Allergy. 2008;94:138–149.
13. Cutarelli, PE, Lass, JH, Lazarus, HM, et al. Topical fluoroquinolones: antimicrobial activity and in vitro corneal epithelial toxicity. Curr Eye Res. 1991;10:557–563.
14. Fine, HF, Kim, E, Eichenbaum, KD, et al. Toxic epidermal necrolysis induced by sulfonamide eyedrops. Cornea. 2008;27:1068–1069.
15. Fraunfelder, FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133–1138.
16. Dart, J. Corneal toxicity: the epithelium and stroma in iatrogenic and factitious disease. Eye (Lond). 2003;17:886–892.
17. Baudouin, C, Pisella, PJ, Fillacier, K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106:556–563.
18. Egbert, PR, Lauber, S, Maurice, DM. A simple conjunctival biopsy. Am J Ophthalmol. 1977;84:798–801.
19. Barbaro, V, Ferrari, S, Fasolo, A, et al. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol. 2010;94:926–932.
20. Van D Meid, KR, Su, SP, Ward, KW, et al. Correlation of tear inflammatory cytokines and matrix metalloproteinases with four dry eye diagnostic tests. Invest Ophthalmol Vis Sci. 2012;54:1512–1518.
21. Jaenen, N, Baudouin, C, Pouliquen, P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17:341–349.
22. Baudouin, C, Labbe, A, Liang, H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334.
23. Beasley, R, Burgess, C, Holt, S. Call for worldwide withdrawal of benzalkonium chloride from nebulizer solutions. J Allergy Clin Immunol. 2001;107:222–223.
24. Lass, JH, Mack, RJ, Imperia, PS, et al. An in vitro analysis of aminoglycoside corneal epithelial toxicity. Curr Eye Res. 1989;8:299–304.
25. Gottschalk, HR, Stone, OJ. Stevens–Johnson syndrome from ophthalmic sulfonamide. Arch Dermatol. 1976;112:513–514.
26. Foster, CS, Lass, JH, Moran-Wallace, K, et al. Ocular toxicity of topical antifungal agents. Arch Ophthalmol. 1981;99:1081–1084.
27. Foster, CS, Pavan-Langston, D. Corneal wound healing and antiviral medication. Arch Ophthalmol. 1977;95:2062–2067.
28. Yagci, A, Bozkurt, B, Egrilmez, S, et al. Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea. 2011;30:571–575.
29. Baudouin, C, Hamard, P, Liang, H, et al. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004;111:2186–2192.
30. Pflugfelder, SC, Baudouin, C. Challenges in the clinical measurement of ocular surface disease in glaucoma patients. Clin Ophthalmol. 2011;5:1575–1583.
31. Noecker, RJ, Herrygers, LA, Anwaruddin, R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–496.
32. Satterfield, D, Mannis, MJ. Episodic bilateral corneal edema caused by hair groom gel. Am J Ophthalmol. 1992;113:107–108.
33. Goto, T, Zheng, X, Gibbon, L, et al. Cosmetic product migration onto the ocular surface: exacerbation of migration after eyedrop instillation. Cornea. 2010;29:400–403.
34. Altinok, AA, Balikoglu, M, Sen, E, et al. Nonpreserved amniotic membrane transplantation for bilateral toxic keratopathy caused by topical anesthetic abuse: a case report. J Med Case Reports. 2010;4:262.
35. Rocha, G, Brunette, I, Le Francois, M. Severe toxic keratopathy secondary to topical anesthetic abuse. Can J Ophthalmol. 1995;30:198–202.