Hepatitis B Until 1982, prevention of hepatitis B was based on passive immunoprophylaxis either with standard Ig, containing modest levels of anti-HBs, or hepatitis B immunoglobulin (HBIG), containing high-titer anti-HBs. The efficacy of standard IG has never been established and remains questionable; even the efficacy of HBIG, demonstrated in several clinical trials, has been challenged, and its contribution appears to be in reducing the frequency of clinical illness, not in preventing infection. The first vaccine for active immunization, introduced in 1982, was prepared from purified, noninfectious 22-nm spherical HBsAg particles derived from the plasma of healthy HBsAg carriers. In 1987, the plasma-derived vaccine was supplanted by a genetically engineered vaccine derived from recombinant yeast. The latter vaccine consists of HBsAg particles that are nonglycosylated but are otherwise indistinguishable from natural HBsAg; two recombinant vaccines are licensed for use in the United States. Current recommendations can be divided into those for pre-exposure and postexposure prophylaxis.
For pre-exposure prophylaxis against hepatitis B in settings of frequent exposure (health workers exposed to blood; first-responder public safety workers; hemodialysis patients and staff; residents and staff of custodial institutions for the developmentally handicapped; injection drug users; inmates of long-term correctional facilities; persons with multiple sexual partners or who have had a sexually transmitted disease; men who have sex with men; persons such as hemophiliacs who require long-term, high-volume therapy with blood derivatives; household and sexual contacts of persons with chronic HBV infection; persons living in or traveling extensively in endemic areas; unvaccinated children under the age of 18; unvaccinated children who are Alaskan natives, Pacific Islanders, or residents in households of first-generation immigrants from endemic countries; persons born in countries with a prevalence of HBV infection ≥2%; patients with chronic liver disease; persons <age 60 with diabetes mellitus [those ≥60 at the discretion of their physicians]; persons with end-stage renal disease; persons with HIV infection), three IM (deltoid, not gluteal) injections of hepatitis B vaccine are recommended at 0, 1, and 6 months (other, optional schedules are summarized in Table 360-8). Pregnancy is not a contraindication to vaccination. In areas of low HBV endemicity such as the United States, despite the availability of safe and effective hepatitis B vaccines, a strategy of vaccinating persons in high-risk groups was not effective. The incidence of new hepatitis B cases continued to increase in the United States after the introduction of vaccines; <10% of all targeted persons in high-risk groups were actually vaccinated, and ~30% of persons with sporadic acute hepatitis B did not fall into any high-risk-group category. Therefore, to have an impact on the frequency of HBV infection in an area of low endemicity such as the United States, universal hepatitis B vaccination in childhood has been recommended. For unvaccinated children born after the implementation of universal infant vaccination, vaccination during early adolescence, at age 11–12 years, was recommended, and this recommendation has been extended to include all unvaccinated children age 0–19 years. In HBV-hyperendemic areas (e.g., Asia), universal vaccination of children has resulted in a marked 10- to 15-year decline in hepatitis B and its complications, including hepatocellular carcinoma.
|
PRE-EXPOSURE HEPATITIS B VACCINATION SCHEDULES |
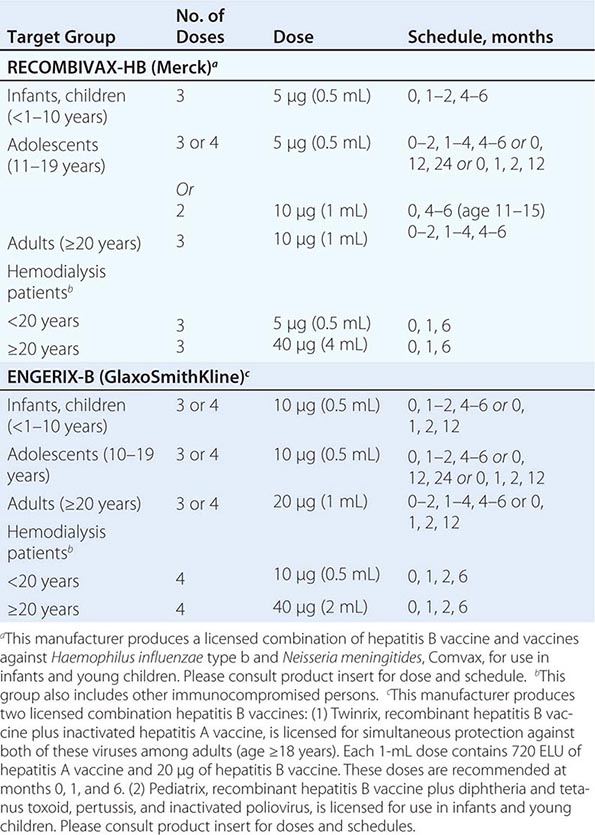
The two available recombinant hepatitis B vaccines are comparable, one containing 10 μg of HBsAg (Recombivax-HB) and the other containing 20 μg of HBsAg (Engerix-B), and recommended doses for each injection vary for the two preparations (Table 360-8). Combinations of hepatitis B vaccine with other childhood vaccines are available as well (Table 360-8).
For unvaccinated persons sustaining an exposure to HBV, post-exposure prophylaxis with a combination of HBIG (for rapid achievement of high-titer circulating anti-HBs) and hepatitis B vaccine (for achievement of long-lasting immunity as well as its apparent efficacy in attenuating clinical illness after exposure) is recommended. For perinatal exposure of infants born to HBsAg-positive mothers, a single dose of HBIG, 0.5 mL, should be administered IM in the thigh immediately after birth, followed by a complete course of three injections of recombinant hepatitis B vaccine (see doses above) to be started within the first 12 h of life. For those experiencing a direct percutaneous inoculation or transmucosal exposure to HBsAg-positive blood or body fluids (e.g., accidental needle stick, other mucosal penetration, or ingestion), a single IM dose of HBIG, 0.06 mL/kg, administered as soon after exposure as possible, is followed by a complete course of hepatitis B vaccine to begin within the first week. For those exposed by sexual contact to a patient with acute hepatitis B, a single IM dose of HBIG, 0.06 mL/kg, should be given within 14 days of exposure, to be followed by a complete course of hepatitis B vaccine. When both HBIG and hepatitis B vaccine are recommended, they may be given at the same time but at separate sites. Testing adults for anti-HBs after a course of vaccine is advisable to document the acquisition of immunity, but, because hepatitis B vaccine immunogenicity is nearly universal in infants, postvaccination anti-HBs testing of children is not recommended.
The precise duration of protection afforded by hepatitis B vaccine is unknown; however, ~80–90% of immunocompetent adult vaccinees retain protective levels of anti-HBs for at least 5 years, and 60–80% for 10 years, and protective antibody has been documented to last for at least two decades after vaccination in infancy. Thereafter and even after anti-HBs becomes undetectable, protection persists against clinical hepatitis B, hepatitis B surface antigenemia, and chronic HBV infection. Currently, booster immunizations are not recommended routinely, except in immunosuppressed persons who have lost detectable anti-HBs or immunocompetent persons who sustain percutaneous HBsAg-positive inoculations after losing detectable antibody. Specifically, for hemodialysis patients, annual anti-HBs testing is recommended after vaccination; booster doses are recommended when anti-HBs levels fall to <10 mIU/mL. As noted above, for persons at risk of both hepatitis A and B, a combined vaccine is available containing 720 enzyme-linked immunoassay units (ELUs) of inactivated HAV and 20 μg of recombinant HBsAg (at 0, 1, and 6 months).
Hepatitis D Infection with hepatitis D can be prevented by vaccinating susceptible persons with hepatitis B vaccine. No product is available for immunoprophylaxis to prevent HDV superinfection in HBsAg carriers; for them, avoidance of percutaneous exposures and limitation of intimate contact with persons who have HDV infection are recommended.
Hepatitis C IG is ineffective in preventing hepatitis C and is no longer recommended for postexposure prophylaxis in cases of perinatal, needle stick, or sexual exposure. Although prototype vaccines that induce antibodies to HCV envelope proteins have been developed, currently, hepatitis C vaccination is not feasible practically. Genotype and quasispecies viral heterogeneity as well as rapid evasion of neutralizing antibodies by this rapidly mutating virus, conspire to render HCV a difficult target for immunoprophylaxis with a vaccine. Prevention of transfusion-associated hepatitis C has been accomplished by the following successively introduced measures: exclusion of commercial blood donors and reliance on a volunteer blood supply; screening donor blood with surrogate markers such as ALT (no longer recommended) and anti-HBc, markers that identify segments of the blood donor population with an increased risk of bloodborne infections; exclusion of blood donors in high-risk groups for AIDS and the introduction of anti-HIV screening tests; and progressively sensitive serologic and virologic screening tests for HCV infection.
In the absence of active or passive immunization, prevention of hepatitis C includes behavior changes and precautions to limit exposures to infected persons. Recommendations designed to identify patients with clinically inapparent hepatitis as candidates for medical management have as a secondary benefit the identification of persons whose contacts could be at risk of becoming infected. A so-called look-back program has been recommended to identify persons who were transfused before 1992 with blood from a donor found subsequently to have hepatitis C. In addition, anti-HCV testing is recommended for persons born between 1945 and 1965, anyone who received a blood transfusion or a transplanted organ before the introduction of second-generation screening tests in 1992, those who ever used injection drugs (or took other illicit drugs by noninjection routes), chronically hemodialyzed patients, persons with clotting disorders who received clotting factors made before 1987 from pooled blood products, persons with elevated aminotransferase levels, health workers exposed to HCV-positive blood or contaminated needles, recipients of blood or organs from a donor found to be positive for hepatitis C, persons with HIV infection, health care and public safety personnel following a needle stick or other nonpercutaneous exposure to HCV-infected material, sexual partners of persons with hepatitis C, and children born to HCV-positive mothers (Table 360-4).
For stable, monogamous sexual partners, sexual transmission of hepatitis C is unlikely, and sexual barrier precautions are not recommended. For persons with multiple sexual partners or with sexually transmitted diseases, the risk of sexual transmission of hepatitis C is increased, and barrier precautions (latex condoms) are recommended. A person with hepatitis C should avoid sharing such items as razors, toothbrushes, and nail clippers with sexual partners and family members. No special precautions are recommended for babies born to mothers with hepatitis C, and breast-feeding does not have to be restricted.
Hepatitis E Whether IG prevents hepatitis E remains undetermined. Safe and effective recombinant genotype 1 vaccines, which protect against other genotypes as well, have been developed and are available in endemic areas but not in the United States.
361 |
Toxic and Drug-Induced Hepatitis |
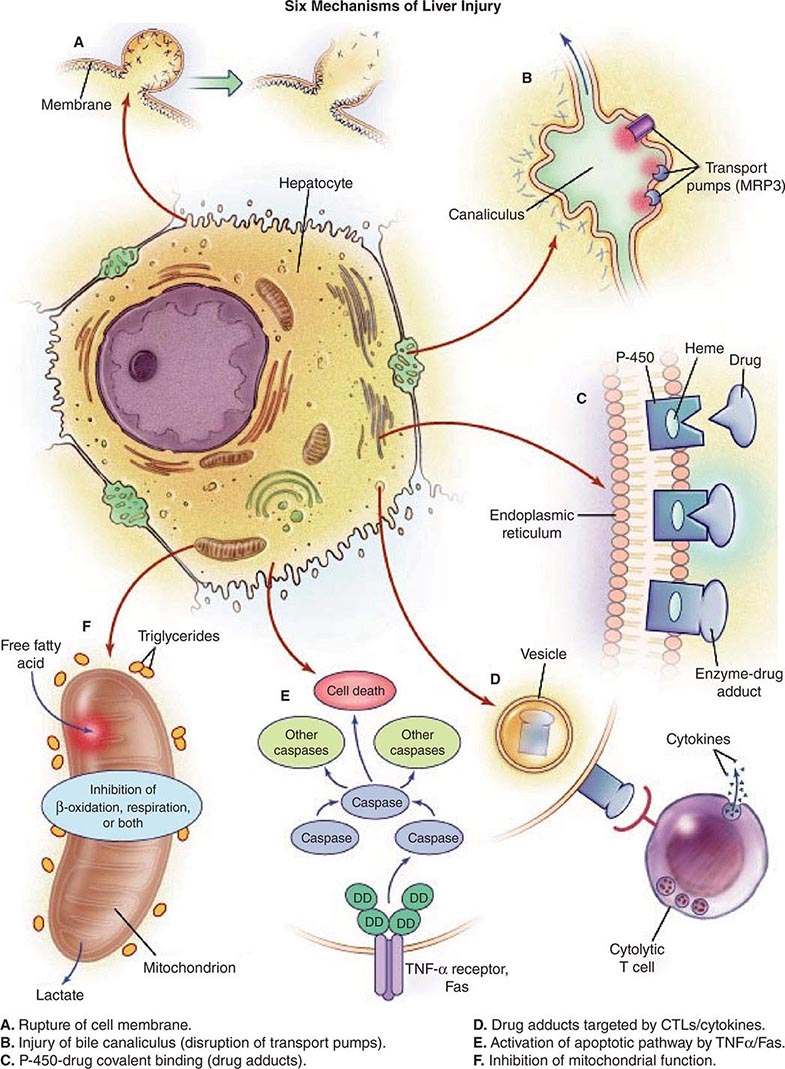
Liver injury is a possible consequence of ingestion of any xenobiotic, including industrial toxins, pharmacologic agents, and complementary and alternative medications (CAMs). Among patients with acute liver failure, drug-induced liver injury is the most common cause, and evidence for hepatotoxicity detected during clinical trials for drug development is the most common reason for failure of compounds to reach approval status. Drug-induced liver injury requires careful history taking to identify unrecognized exposure to chemicals used in work or at home, drugs taken by prescription or bought over the counter, and herbal or dietary supplement medicines. Hepatotoxic drugs can injure the hepatocyte directly, e.g., via a free-radical or metabolic intermediate that causes peroxidation of membrane lipids and that results in liver cell injury. Alternatively, a drug or its metabolite may activate components of the innate or adaptive immune system, stimulate apoptotic pathways, or initiate damage to bile excretory pathways (Fig. 361-1). Interference with bile canalicular pumps can allow endogenous bile acids, which can injure the liver, to accumulate. Such secondary injury, in turn, may lead to necrosis of hepatocytes; injure bile ducts, producing cholestasis; or block pathways of lipid movement, inhibit protein synthesis, or impair mitochondrial oxidation of fatty acids, resulting in lactic acidosis and intracellular triglyceride accumulation (expressed histologically as microvesicular steatosis). In other instances, drug metabolites sensitize hepatocytes to toxic cytokines. The differences observed between susceptible and nonsusceptible drug recipients may be attributable to HLA haplotypes that determine binding of drug-related haptens on the cell surface as well as to polymorphisms in elaboration of competing, protective cytokines, as has been suggested for acetaminophen hepatotoxicity (see below). Immune mechanisms may include cytotoxic lymphocytes or antibody-mediated cellular cytotoxicity. In addition, a role has been shown for activation of nuclear transporters, such as the constitutive androstane receptor (CAR) or, more recently, the pregnane × receptor (PXR), in the induction of drug hepatotoxicity.
FIGURE 361-1 Potential mechanisms of drug-induced liver injury. The normal hepatocyte may be affected adversely by drugs through (A) disruption of intracellular calcium homeostasis that leads to the disassembly of actin fibrils at the surface of the hepatocyte, resulting in blebbing of the cell membrane, rupture, and cell lysis; (B) disruption of actin filaments next to the canaliculus (the specialized portion of the cell responsible for bile excretion), leading to loss of villous processes and interruption of transport pumps such as multidrug resistance–associated protein 3 (MRP3), which, in turn, prevents the excretion of bilirubin and other organic compounds; (C) covalent binding of the heme-containing cytochrome P450 enzyme to the drug, thus creating nonfunctioning adducts; (D) migration of these enzyme-drug adducts to the cell surface in vesicles to serve as target immunogens for cytolytic attack by T cells, stimulating an immune response involving cytolytic T cells and cytokines; (E) activation of apoptotic pathways by tumor necrosis factor α (TNF-α) receptor or Fas (DD denotes death domain), triggering the cascade of intercellular caspases, resulting in programmed cell death; or (F) inhibition of mitochondrial function by a dual effect on both β-oxidation and the respiratory-chain enzymes, leading to failure of free fatty acid metabolism, a lack of aerobic respiration, and accumulation of lactate and reactive oxygen species (which may disrupt mitochondrial DNA). Toxic metabolites excreted in bile may damage bile-duct epithelium (not shown). CTLs, cytolytic T lymphocytes. (Reproduced from WM Lee: Drug-induced hepatotoxicity. N Engl J Med 349:474, 2003, with permission.)
DRUG METABOLISM
Most drugs, which are water-insoluble, undergo a series of metabolic steps, culminating in a water-soluble form appropriate for renal or biliary excretion. This process begins with oxidation or methylation mediated initially by the microsomal mixed-function oxygenases, cytochrome P450 (phase I reaction), followed by glucuronidation or sulfation (phase II reaction) or inactivation by glutathione. Most drug hepatotoxicity is mediated by a phase I toxic metabolite, but glutathione depletion, precluding inactivation of harmful compounds by glutathione S-transferase, can contribute as well.
LIVER INJURY CAUSED BY DRUGS
In general, two major types of chemical hepatotoxicity have been recognized: (1) direct toxic and (2) idiosyncratic. As shown in Table 361-1, direct toxic hepatitis occurs with predictable regularity in individuals exposed to the offending agent and is dose-dependent. The latent period between exposure and liver injury is usually short (often several hours), although clinical manifestations may be delayed for 24–48 h. Agents producing toxic hepatitis are generally systemic poisons or are converted in the liver to toxic metabolites. The direct hepatotoxins result in morphologic abnormalities that are reasonably characteristic and reproducible for each toxin. For example, carbon tetrachloride and trichloroethylene characteristically produce a centrilobular zonal necrosis, whereas yellow phosphorus poisoning typically results in periportal injury. The hepatotoxic octapeptides of Amanita phalloides usually produce massive hepatic necrosis; the lethal dose of the toxin is ~10 mg, the amount found in a single deathcap mushroom. Liver injury, which is often only one facet of the toxicity produced by the direct hepatotoxins, may go unrecognized until jaundice appears.
|
SOME FEATURES OF TOXIC AND DRUG-INDUCED HEPATIC INJURY |
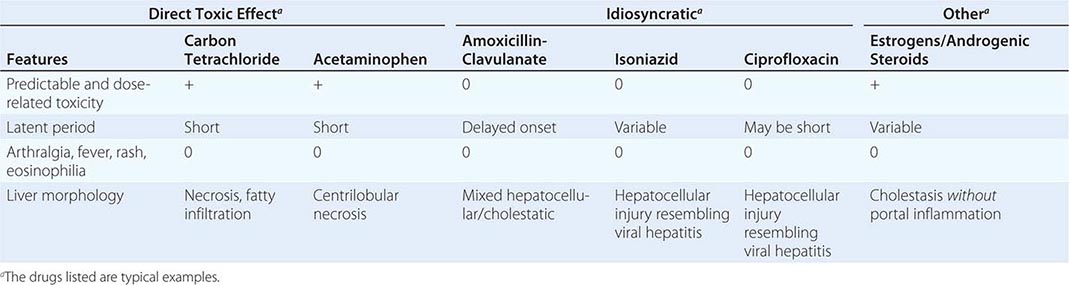
In idiosyncratic drug reactions, the occurrence of hepatitis is usually infrequent (1 in 103–105 patients) and unpredictable; the response is not as clearly dose-dependent as is injury associated with direct hepatotoxins, and liver injury may occur at any time during or shortly after exposure to the drug. That said, recent data suggest that most agents causing idiosyncratic toxicity are given at a daily dose exceeding 100 mg, suggesting a role for dose—drugs with low potency must be given in higher doses that engender greater chances for “off-target” effects. Adding to the difficulty of predicting or identifying idiosyncratic drug hepatotoxicity is the occurrence of mild, transient, nonprogressive serum aminotransferase elevations that resolve with continued drug use. Such “adaptation,” the mechanism of which is unknown, is well recognized for drugs such as isoniazid, valproate, phenytoin, and HMG-CoA reductase inhibitors (statins). Extrahepatic manifestations of hypersensitivity, such as rash, arthralgias, fever, leukocytosis, and eosinophilia, occur in about one-quarter of patients with idiosyncratic hepatotoxic drug reactions but are characteristic for certain drugs and not others. Both primary immunologic injury and direct hepatotoxicity related to idiosyncratic differences in generation of toxic metabolites have been invoked to explain idiosyncratic drug reactions. The most current data appear to implicate the adaptive immune system responding to the formation of immune stimulatory compounds resulting from phase I metabolic activation of the offending drug. Differences in host susceptibility may result from varying kinetics of toxic metabolite generation and genetic polymorphisms in downstream drug-metabolizing pathways or cytokine activation; in addition, certain HLA haplotypes have been associated with hepatotoxicity of certain drugs such as amoxicillin-clavulanate and flucloxacillin. Occasionally, however, the clinical features of an allergic reaction (prominent tissue eosinophilia, autoantibodies, etc.) are difficult to ignore and suggest activation of IgE pathways. A few instances of drug hepatotoxicity are observed to be associated with autoantibodies, including a class of antibodies to liver-kidney microsomes, anti-LKM2, directed against a cytochrome P450 enzyme.
Idiosyncratic reactions lead to a morphologic pattern that is more variable than those produced by direct toxins; a single agent is often capable of causing a variety of lesions, although certain patterns tend to predominate. Depending on the agent involved, idiosyncratic hepatitis may result in a clinical and morphologic picture indistinguishable from that of viral hepatitis (e.g., isoniazid or ciprofloxacin). So-called hepatocellular injury is the most common form, featuring spotty necrosis in the liver lobule with a predominantly lymphocytic infiltrate resembling that observed in acute hepatitis A, B, or C. Drug-induced cholestasis ranges from mild to increasingly severe: (1) bland cholestasis with limited hepatocellular injury (e.g., estrogens, 17,α-substituted androgens); (2) inflammatory cholestasis (e.g., amoxicillin-clavulanic acid [the most frequently implicated antibiotic among cases of drug-induced liver injury], oxacillin, erythromycin estolate); (3) sclerosing cholangitis (e.g., after intrahepatic infusion of the chemotherapeutic agent floxuridine for hepatic metastases from a primary colonic carcinoma); and (4) disappearance of bile ducts, “ductopenic” cholestasis, similar to that observed in chronic rejection (Chap. 368) following liver transplantation (e.g., carbamazepine, levofloxacin). Cholestasis may result from binding of drugs to canalicular membrane transporters, accumulation of toxic bile acids resulting from canalicular pump failure, or genetic defects in canalicular transporter proteins. Clinically, the distinction between a hepatocellular and a cholestatic reaction is indicated by the R value, the ratio of alanine aminotransferase (ALT) to alkaline phosphatase values, both expressed as multiples of the upper limit of normal. An R value of >5.0 is associated with hepatocellular injury, R <2.0 with cholestatic injury, and R between 2.0 and 5.0 with mixed hepatocellular-cholestatic injury.
Morphologic alterations may also include bridging hepatic necrosis (e.g., methyldopa) or, infrequently, hepatic granulomas (e.g., sulfonamides). Some drugs result in macrovesicular or microvesicular steatosis or steatohepatitis, which, in some cases, has been linked to mitochondrial dysfunction and lipid peroxidation. Severe hepatotoxicity associated with steatohepatitis, most likely a result of mitochondrial toxicity, is being recognized with increasing frequency among patients receiving antiretroviral therapy with reverse transcriptase inhibitors for HIV infection (e.g., zidovudine, didanosine), although many of these drugs have been withdrawn because of such hepatotoxicity (Chap. 226). Generally, such mitochondrial hepatotoxicity of these antiretroviral agents is reversible, but dramatic, nonreversible hepatotoxicity associated with mitochondrial injury (inhibition of DNA polymerase γ) was the cause of acute liver failure encountered during early clinical trials of now-abandoned fialuridine, a fluorinated pyrimidine analogue with potent antiviral activity against hepatitis B virus. Another potential target for idiosyncratic drug hepatotoxicity is sinusoidal lining cells; when these are injured, such as by high-dose chemotherapeutic agents (e.g., cyclophosphamide, melphalan, busulfan) administered prior to bone marrow transplantation, venoocclusive disease can result. Nodular regenerative hyperplasia, a subtle form of portal hypertension, may also result from vascular injury to portal venous endothelium following systemic chemotherapy, such as with oxaliplatin, as part of adjuvant treatment for colon cancer.
Not all adverse hepatic drug reactions can be classified as either toxic or idiosyncratic. For example, oral contraceptives, which combine estrogenic and progestational compounds, may result in impairment of hepatic tests and, occasionally, jaundice; however, they do not produce necrosis or fatty change, manifestations of hypersensitivity are generally absent, and susceptibility to the development of oral contraceptive–induced cholestasis appears to be genetically determined. Such estrogen-induced cholestasis is more common in women with cholestasis of pregnancy, a disorder linked to genetic defects in multidrug resistance–associated canalicular transporter proteins.
Any idiosyncratic reaction that occurs in <1:10,000 recipients will go unrecognized in most clinical trials, which involve only several thousand recipients. The U.S. Food and Drug Administration (FDA) and pharmaceutical companies have learned to look for even subtle indications of serious toxicity and monitor regularly the number of trial subjects in whom any aminotransferase elevations develop, as a possible surrogate for more serious toxicity. Even more valid as a predictor of severe hepatotoxicity is the occurrence of jaundice in patients enrolled in a clinical drug trial, so called “Hy’s Law,” named after Hyman Zimmerman, one of the pioneers of the field of drug hepatotoxicity. He recognized that, if jaundice occurred during a phase III trial, more serious liver injury was likely, with a 10:1 ratio between cases of jaundice and liver failure—10 patients with jaundice to 1 patient with acute liver failure. Thus, the finding of such Hy’s Law cases during drug development often portends failure of approval, particularly if any of the subjects sustains a bad outcome. Troglitazone, a peroxisome proliferator-activated receptor γ agonist, was the first in its class of thiazolidinedione insulin-sensitizing agents. Although in retrospect, Hy’s Law cases of jaundice had occurred during phase III trials, no instances of liver failure were recognized until well after the drug was introduced, underlining the importance of postmarketing surveillance in identifying toxic drugs and in leading to their withdrawal from use. Fortunately, such hepatotoxicity is not characteristic of the second-generation thiazolidinedione insulin-sensitizing agents rosiglitazone and pioglitazone; in clinical trials, the frequency of aminotransferase elevations in patients treated with these medications did not differ from that in placebo recipients, and isolated reports of liver injury among recipients are extremely rare.
Proving that an episode of liver injury is caused by a drug is difficult in many cases. Drug-induced liver injury is nearly always a presumptive diagnosis, and many other disorders produce a similar clinicopathologic picture. Thus, causality may be difficult to establish and requires several separate supportive assessment variables to lead to a high level of certainty, including temporal association (time of onset, time to resolution), clinical-biochemical features, type of injury (hepatocellular versus cholestatic), extrahepatic features, likelihood that a given agent is to blame based on its past record, and exclusion of other potential causes. Scoring systems such as the Roussel-Uclaf Causality Assessment Method (RUCAM) yield residual uncertainty and have not been adopted widely. Currently, the U.S. Drug-Induced Liver Injury Network (DILIN) relies on a structured expert opinion process requiring detailed data on each case and a comprehensive review by three experts who arrive at a consensus on a five-degree scale of likelihood (definite, highly likely, probable, possible, unlikely); however, this approach is not practical for routine clinical application.
Generally, drug hepatotoxicity is not more frequent in persons with underlying chronic liver disease, although the severity of the outcome may be amplified. Reported exceptions include hepatotoxicity of aspirin, methotrexate, isoniazid (only in certain experiences), antiretroviral therapy for HIV infection, and certain drugs such as conditioning regimens for bone marrow transplantation in the presence of hepatitis C.
In Table 361-2, several classes of chemical agents are listed together with examples of the pattern of liver injury produced by them. Certain drugs appear to be responsible for the development of chronic as well as acute hepatic injury. For example, nitrofurantoin, minocycline, hydralazine, and methyldopa have been associated with moderate to severe chronic hepatitis with autoimmune features. Methotrexate, tamoxifen, and amiodarone have been implicated in the development of cirrhosis. Portal hypertension in the absence of cirrhosis may result from alterations in hepatic architecture produced by vitamin A or arsenic intoxication, industrial exposure to vinyl chloride, or administration of thorium dioxide. The latter three agents have also been associated with angiosarcoma of the liver. Oral contraceptives have been implicated in the development of hepatic adenoma and, rarely, hepatocellular carcinoma and hepatic vein occlusion (Budd-Chiari syndrome). Another unusual lesion, peliosis hepatis (blood cysts of the liver), has been observed in some patients treated with anabolic or contraceptive steroids. The existence of these hepatic disorders expands the spectrum of liver injury induced by chemical agents and emphasizes the need for a thorough drug history in all patients with liver dysfunction. A helpful LiverTox website that contains up-to-date information on drug-induced liver injury is available through the National Institute of Diabetes and Digestive and Kidney Diseases and the National Library of Medicine (www.livertox.nih.gov).
|
PRINCIPAL ALTERATIONS OF HEPATIC MORPHOLOGY PRODUCED BY SOME COMMONLY USED DRUGS AND CHEMICALSa |
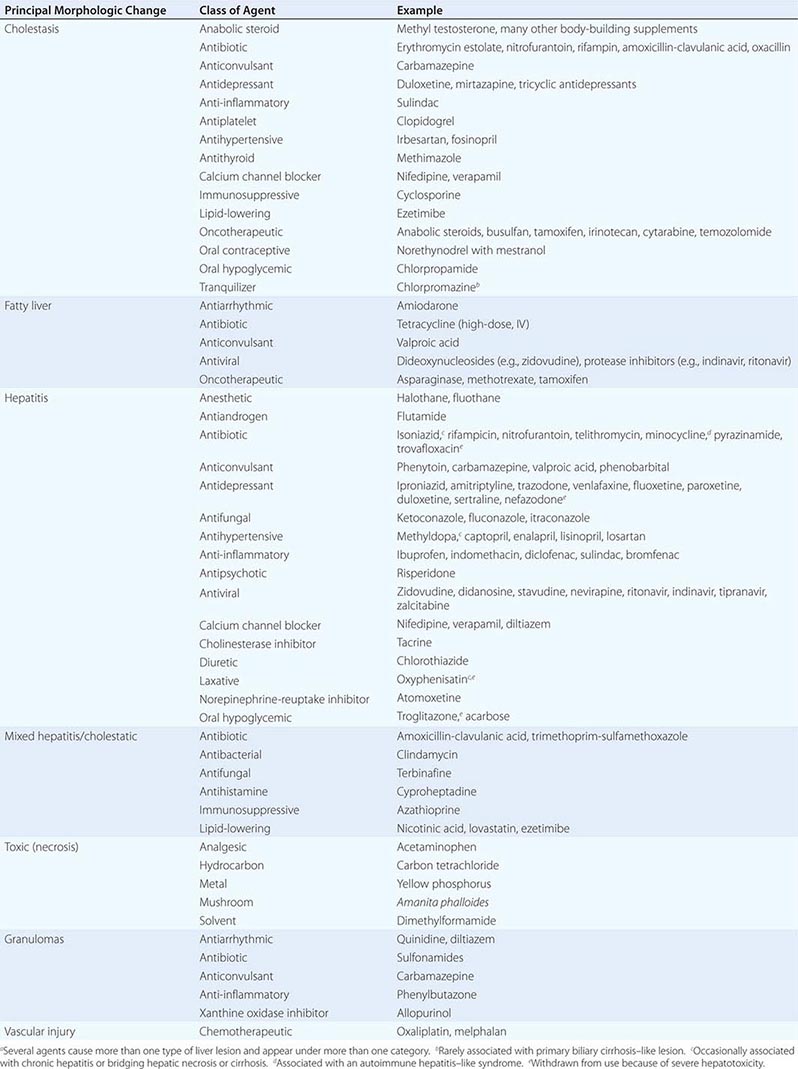
The following are patterns of adverse hepatic reactions for some prototypic agents.
ACETAMINOPHEN HEPATOTOXICITY (DIRECT TOXIN)
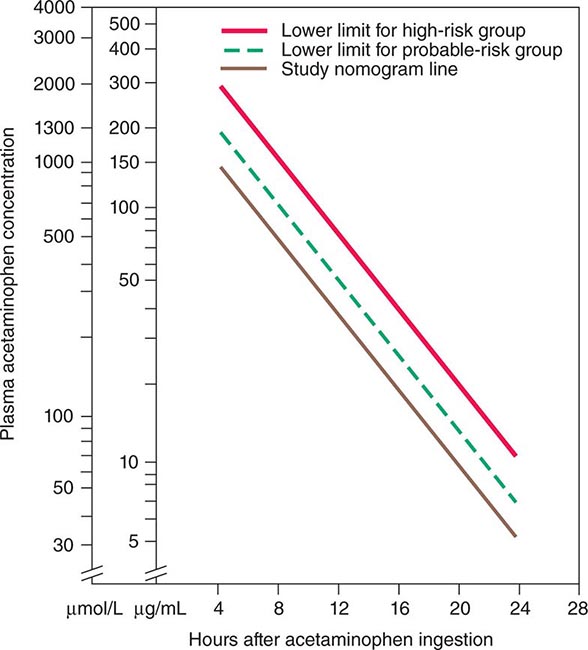
Acetaminophen represents the most prevalent cause of acute liver failure in the Western world; up to 72% of patients with acetaminophen hepatotoxicity in Scandinavia—somewhat lower frequencies in the United Kingdom and the United States—progress to encephalopathy and coagulopathy. Acetaminophen causes dose-related centrilobular hepatic necrosis after single-time-point ingestions, as intentional self-harm, or over extended periods, as unintentional overdoses, when multiple drug preparations or inappropriate drug amounts are used daily for several days, e.g., for relief of pain or fever. In these instances, 8 g/d, twice the daily recommended maximum dose, over several days can readily lead to liver failure. Use of opioid-acetaminophen combinations appears to be particularly harmful, because habituation to the opioid may occur with a gradual increase in opioid-acetaminophen combination dosing over days or weeks. A single dose of 10–15 g, occasionally less, may produce clinical evidence of liver injury. Fatal fulminant disease is usually (although not invariably) associated with ingestion of ≥25 g. Blood levels of acetaminophen correlate with severity of hepatic injury (levels >300 μg/mL 4 h after ingestion are predictive of the development of severe damage; levels <150 μg/mL suggest that hepatic injury is highly unlikely). Nausea, vomiting, diarrhea, abdominal pain, and shock are early manifestations occurring 4–12 h after ingestion. Then 24–48 h later, when these features are abating, hepatic injury becomes apparent. Maximal abnormalities and hepatic failure are evident 3–5 days after ingestion, and aminotransferase levels exceeding 10,000 IU/L are not uncommon (i.e., levels far exceeding those in patients with viral hepatitis). Renal failure and myocardial injury may be present. Whether or not a clear history of overdose can be elicited, clinical suspicion of acetaminophen hepatotoxicity should be raised by the presence of the extremely high aminotransferase levels in association with low bilirubin levels that are characteristic of this hyperacute injury. This biochemical signature should trigger further questioning of the subject if possible; however, denial or altered mentation may confound diagnostic efforts. In this setting, a presumptive diagnosis is reasonable, and the proven antidote, N-acetylcysteine—both safe and presumed to be effective even when injury has already begun to evolve—should be instituted.
Acetaminophen is metabolized predominantly by a phase II reaction to innocuous sulfate and glucuronide metabolites; however, a small proportion of acetaminophen is metabolized by a phase I reaction to a hepatotoxic metabolite formed from the parent compound by the cytochrome P450 CYP2E1. This metabolite, N-acetyl-p-benzoquinone-imine (NAPQI), is detoxified by binding to “hepatoprotective” glutathione to become harmless, water-soluble mercapturic acid, which undergoes renal excretion. When excessive amounts of NAPQI are formed, or when glutathione levels are low, glutathione levels are depleted and overwhelmed, permitting covalent binding to nucleophilic hepatocyte macromolecules forming acetaminophen-protein “adducts.” These adducts, which can be measured in serum by high-performance liquid chromatography, hold promise as diagnostic markers of acetaminophen hepatotoxicity, and a point-of-care assay for acetaminophen-Cys adducts is under development. The binding of acetaminophen to hepatocyte macromolecules is believed to lead to hepatocyte necrosis; the precise sequence and mechanism are unknown. Hepatic injury may be potentiated by prior administration of alcohol, phenobarbital, isoniazid, or other drugs; by conditions that stimulate the mixed-function oxidase system; or by conditions such as starvation (including inability to maintain oral intake during severe febrile illnesses) that reduce hepatic glutathione levels. Alcohol induces cytochrome P450 CYP2E1; consequently, increased levels of the toxic metabolite NAPQI may be produced in chronic alcoholics after acetaminophen ingestion, but the role of alcohol in potentiating acute acetaminophen injury is still debated. Alcohol also suppresses hepatic glutathione production. Therefore, in chronic alcoholics, the toxic dose of acetaminophen may be as low as 2 g, and alcoholic patients should be warned specifically about the dangers of even standard doses of this commonly used drug. In a 2006 study, aminotransferase elevations were identified in 31–44% of normal subjects treated for 14 days with the maximal recommended dose of acetaminophen, 4 g daily (administered alone or as part of an acetaminophen-opioid combination); because these changes were transient and never associated with bilirubin elevation, the clinical relevance of these findings remains to be determined. Although underlying hepatitis C virus (HCV) infection was found to be associated with an increased risk of acute liver injury in patients hospitalized for acetaminophen overdose, generally, in patients with nonalcoholic liver disease, acetaminophen taken in recommended doses is well tolerated. Acetaminophen use in cirrhotic patients has not been associated with hepatic decompensation. On the other hand, because of the link between acetaminophen use and liver injury, and because of the limited safety margin between safe and toxic doses, the FDA has recommended that the daily dose of acetaminophen be reduced from 4 g to 3 g (even lower for persons with chronic alcohol use), that all acetaminophen-containing products be labeled prominently as containing acetaminophen, and that the potential for liver injury be prominent in the packaging of acetaminophen and acetaminophen-containing products. Within opioid combination products, the limit for the acetaminophen component has been lowered to 325 mg per tablet.
Survivors of acute acetaminophen overdose rarely, if ever, have ongoing liver injury or sequelae.
ISONIAZID HEPATOTOXICITY (TOXIC AND IDIOSYNCRATIC REACTION)
Isoniazid (INH) remains central to most antituberculous prophylactic and therapeutic regimens, despite its long-standing recognition as a hepatotoxin. In 10% of patients treated with INH, elevated serum aminotransferase levels develop during the first few weeks of therapy; however, these elevations in most cases are self-limited, mild (values for ALT <200 IU/L), and resolve despite continued drug use. This adaptive response allows continuation of the agent if symptoms and progressive enzyme elevations do not follow the initial elevations. Acute hepatocellular drug-induced liver injury secondary to INH is evident with a variable latency period up to 6 months and is more frequent in alcoholics and patients taking certain other medications, such as barbiturates, rifampin, and pyrazinamide. If the clinical threshold of encephalopathy is reached, severe hepatic injury is likely to be fatal or to require liver transplantation. Liver biopsy reveals morphologic changes similar to those of viral hepatitis or bridging hepatic necrosis. Substantial liver injury appears to be age-related, increasing substantially after age 35; the highest frequency is in patients over age 50, and the lowest is in patients under the age of 20. Even for patients >50 years of age monitored carefully during therapy, hepatotoxicity occurs in only ~2%, well below the risk estimate derived from earlier experiences. Fever, rash, eosinophilia, and other manifestations of drug allergy are distinctly unusual. Recently, antibodies to INH have been detected in INH recipients, but a link to causality of liver injury remains unclear. A clinical picture resembling chronic hepatitis has been observed in a few patients. Many public health programs that require INH prophylaxis for a positive tuberculin skin test or Quantiferon test include monthly monitoring of aminotransferase levels, although this practice has been called into question. Even more effective in limiting serious outcomes may be encouraging patients to be alert for symptoms such as nausea, fatigue, or jaundice, because most fatalities occur in the setting of continued INH use despite clinically apparent illness.
SODIUM VALPROATE HEPATOTOXICITY (TOXIC AND IDIOSYNCRATIC REACTION)
Sodium valproate, an anticonvulsant useful in the treatment of petit mal and other seizure disorders, has been associated with the development of severe hepatic toxicity and, rarely, fatalities, predominantly in children but also in adults. Among children listed as candidates for liver transplantation, valproate is the most common antiepileptic drug implicated. Asymptomatic elevations of serum aminotransferase levels have been recognized in as many as 45% of treated patients. These “adaptive” changes, however, appear to have no clinical importance, because major hepatotoxicity is not seen in the majority of patients despite continuation of drug therapy. In the rare patients in whom jaundice, encephalopathy, and evidence of hepatic failure are found, examination of liver tissue reveals microvesicular fat and bridging hepatic necrosis, predominantly in the centrilobular zone. Bile duct injury may also be apparent. Most likely, sodium valproate is not directly hepatotoxic, but its metabolite, 4-pentenoic acid, may be responsible for hepatic injury. Valproate hepatotoxicity is more common in persons with mitochondrial enzyme deficiencies and may be ameliorated by IV administration of carnitine, which valproate therapy can deplete. Recently, valproate toxicity has been linked to HLA haplotypes (DR4 and B*1502) and to mutations in mitochondrial DNA polymerase gamma 1.
NITROFURANTOIN HEPATOTOXICITY (IDIOSYNCRATIC REACTION)
This commonly used antibiotic for urinary tract infections may cause an acute hepatitis leading to fatal outcome or, more frequently, chronic hepatitis of varying severity but indistinguishable from autoimmune chronic hepatitis. These two scenarios may reflect the frequent use and reuse of the drug for treatment of recurrent cystitis in women. Although most toxic agents manifest injury within 6 months of first ingestion, nitrofurantoin may have a longer latency period, in part perhaps because of its intermittent, recurrent use. Autoantibodies to nuclear components, smooth muscle, and mitochondria are seen and may subside after resolution of infection; however, glucocorticoid or other immunosuppressive medication may be necessary to resolve the autoimmune injury, and cirrhosis may be seen in cases that are not recognized quickly. Interstitial pulmonary fibrosis presenting as chronic cough and dyspnea may be present and resolve slowly with medication withdrawal. Histologic findings are identical to those of autoimmune hepatitis. A similar disease pattern can be observed with minocycline that is used repeatedly for the treatment of acne in teenagers as well as with hydralazine and alpha methyldopa.
AMOXICILLIN-CLAVULANATE HEPATOTOXICITY (IDIOSYNCRATIC MIXED REACTION)
Currently, the most common agent implicated as causing drug-induced liver injury in the United States and in Europe is amoxicillin-clavulanate (most frequent brand name: Augmentin). This medication causes a very specific syndrome of mixed or primarily cholestatic injury. Because hepatotoxicity may follow amoxicillin-clavulanate therapy after a relatively long latency period, the liver injury may begin to manifest at the time of drug withdrawal or after the drug has been withdrawn. The high prevalence of hepatotoxicity reflects in part the very frequent use of this drug for respiratory tract infections, including community-acquired pneumonia. The mechanism of hepatotoxicity is unclear, but the liver injury is thought to be caused by amoxicillin toxicity that is potentiated in some way by clavulanate, which itself appears not to be toxic. Symptoms include nausea, anorexia, fatigue, and jaundice—which may be prolonged—with pruritus. Rash is quite uncommon. On occasion, amoxicillin-clavulanate, like other cholestatic hepatotoxic drugs, causes permanent injury to small bile ducts, leading to the so-called “vanishing bile duct syndrome.” In vanishing bile duct syndrome, initially, liver injury is minimal except for severe cholestasis; however, over time, histologic evidence of bile duct abnormalities is replaced by a paucity and eventual absence of discernible ducts on subsequent biopsies.
PHENYTOIN HEPATOTOXICITY (IDIOSYNCRATIC REACTION)
Phenytoin, formerly diphenylhydantoin, a mainstay in the treatment of seizure disorders, has been associated in rare instances with the development of severe hepatitis-like liver injury leading to fulminant hepatic failure. In many patients, the hepatitis is associated with striking fever, lymphadenopathy, rash (Stevens-Johnson syndrome or exfoliative dermatitis), leukocytosis, and eosinophilia, suggesting an immunologically mediated hypersensitivity mechanism. Despite these observations, evidence suggests that metabolic idiosyncrasy may be responsible for hepatic injury. In the liver, phenytoin is converted by cytochrome P450 to metabolites, including the highly reactive electrophilic arene oxides. These metabolites are normally metabolized further by epoxide hydrolases. A defect (genetic or acquired) in epoxide hydrolase activity could permit covalent binding of arene oxides to hepatic macromolecules, thereby leading to hepatic injury. Hepatic injury is usually manifest within the first 2 months after beginning phenytoin therapy. With the exception of an abundance of eosinophils in the liver, the clinical, biochemical, and histologic picture resembles that of viral hepatitis. In rare instances, bile duct injury may be the salient feature of phenytoin hepatotoxicity, with striking features of intrahepatic cholestasis. Asymptomatic elevations of aminotransferase and alkaline phosphatase levels have been observed in a sizable proportion of patients receiving long-term phenytoin therapy. These liver changes are believed by some authorities to represent the potent hepatic enzyme-inducing properties of phenytoin and are accompanied histologically by swelling of hepatocytes in the absence of necroinflammatory activity or evidence of chronic liver disease.
AMIODARONE HEPATOTOXICITY (TOXIC AND IDIOSYNCRATIC REACTION)
Therapy with this potent antiarrhythmic drug is accompanied in 15–50% of patients by modest elevations of serum aminotransferase levels that may remain stable or diminish despite continuation of the drug. Such abnormalities may appear days to many months after beginning therapy. A proportion of those with elevated aminotransferase levels have detectable hepatomegaly, and clinically important liver disease develops in <5% of patients. Features that represent a direct effect of the drug on the liver and that are common to the majority of long-term recipients are ultrastructural phospholipidosis, unaccompanied by clinical liver disease, and interference with hepatic mixed-function oxidase metabolism of other drugs. The cationic amphiphilic drug and its major metabolite desethylamiodarone accumulate in hepatocyte lysosomes and mitochondria and in bile duct epithelium. The relatively common elevations in aminotransferase levels are also considered a predictable, dose-dependent, direct hepatotoxic effect. On the other hand, in the rare patient with clinically apparent, symptomatic liver disease, liver injury resembling that seen in alcoholic liver disease is observed. The so-called pseudoalcoholic liver injury can range from steatosis to alcoholic hepatitis–like neutrophilic infiltration and Mallory’s hyaline to cirrhosis. Electron-microscopic demonstration of phospholipid-laden lysosomal lamellar bodies can help to distinguish amiodarone hepatotoxicity from typical alcoholic hepatitis. This category of liver injury appears to be a metabolic idiosyncrasy that allows hepatotoxic metabolites to be generated. Rarely, an acute idiosyncratic hepatocellular injury resembling viral hepatitis or cholestatic hepatitis occurs. Hepatic granulomas have occasionally been observed. Because amiodarone has a long half-life, liver injury may persist for months after the drug is stopped.
ERYTHROMYCIN HEPATOTOXICITY (CHOLESTATIC IDIOSYNCRATIC REACTION)
The most important adverse effect associated with erythromycin, more common in children than adults, is the infrequent occurrence of a cholestatic reaction. Although most of these reactions have been associated with erythromycin estolate, other erythromycins may also be responsible. The reaction usually begins during the first 2 or 3 weeks of therapy and includes nausea, vomiting, fever, right upper quadrant abdominal pain, jaundice, leukocytosis, and moderately elevated aminotransferase and alkaline phosphatase levels. The clinical picture can resemble acute cholecystitis or bacterial cholangitis. Liver biopsy reveals variable cholestasis; portal inflammation comprising lymphocytes, polymorphonuclear leukocytes, and eosinophils; and scattered foci of hepatocyte necrosis. Symptoms and laboratory findings usually subside within a few days of drug withdrawal, and evidence of chronic liver disease has not been found on follow-up. The precise mechanism remains ill-defined.
ORAL CONTRACEPTIVE HEPATOTOXICITY (CHOLESTATIC REACTION)
The administration of oral contraceptive combinations of estrogenic and progestational steroids leads to intrahepatic cholestasis with pruritus and jaundice in a small number of patients weeks to months after taking these agents. Especially susceptible seem to be patients with recurrent idiopathic jaundice of pregnancy, severe pruritus of pregnancy, or a family history of these disorders. With the exception of liver biochemical tests, laboratory studies are normal, and extrahepatic manifestations of hypersensitivity are absent. Liver biopsy reveals cholestasis with bile plugs in dilated canaliculi and striking bilirubin staining of liver cells. In contrast to chlorpromazine-induced cholestasis, portal inflammation is absent. The lesion is reversible on withdrawal of the agent. The two steroid components appear to act synergistically on hepatic function, although the estrogen may be primarily responsible. Oral contraceptives are contraindicated in patients with a history of recurrent jaundice of pregnancy. Primarily benign, but rarely malignant, neoplasms of the liver, hepatic vein occlusion, and peripheral sinusoidal dilatation have also been associated with oral contraceptive therapy. Focal nodular hyperplasia of the liver is not more frequent among users of oral contraceptives.
ANABOLIC STEROIDS (CHOLESTATIC REACTION)
The most common form of liver injury caused by complementary and alternative medications is the profound cholestasis associated with anabolic steroids used by body builders. Unregulated agents sold in gyms and health food stores as diet supplements, which are taken by athletes to improve their performance, may contain anabolic steroids. Jaundice in a young male that is accompanied by a cholestatic, rather than a hepatitic, laboratory profile almost invariably will turn out to be caused by the use of one of a variety of androgen congeners. Such agents have the potential to injure bile transport pumps and to cause intense cholestasis; the time to onset is variable, and resolution, which is the rule, may require many weeks to months. Initially, anorexia, nausea, and malaise may occur, followed by pruritus in some but not all patients. Serum aminotransferase levels are usually <100 IU/L and serum alkaline phosphatase levels are generally moderately elevated with bilirubin levels frequently exceeding 342 μmol/L (20 mg/dL). Examination of liver tissue reveals cholestasis without substantial inflammation or necrosis. Anabolic steroids have also been used by prescription to treat bone marrow failure. In this setting, hepatic sinusoidal dilatation and peliosis hepatis have been reported in rare patients, as have hepatic adenomas and hepatocellular carcinoma.
TRIMETHOPRIM-SULFAMETHOXAZOLE HEPATOTOXICITY (IDIOSYNCRATIC REACTION)
This antibiotic combination is used routinely for urinary tract infections in immunocompetent persons and for prophylaxis against and therapy of Pneumocystis carinii pneumonia in immunosuppressed persons (transplant recipients, patients with AIDS). With its increasing use, its occasional hepatotoxicity is being recognized with growing frequency. Its likelihood is unpredictable, but when it occurs, trimethoprim-sulfamethoxazole hepatotoxicity follows a relatively uniform latency period of several weeks and is often accompanied by eosinophilia, rash, and other features of a hypersensitivity reaction. Biochemically and histologically, acute hepatocellular necrosis predominates, but cholestatic features are quite frequent. Occasionally, cholestasis without necrosis occurs, and, very rarely, a severe cholangiolytic pattern of liver injury is observed. In most cases, liver injury is self-limited, but rare fatalities have been recorded. The hepatotoxicity is attributable to the sulfamethoxazole component of the drug and is similar in features to that seen with other sulfonamides; tissue eosinophilia and granulomas may be seen. The risk of trimethoprim-sulfamethoxazole hepatotoxicity is increased in persons with HIV infection.
HMG-COA REDUCTASE INHIBITORS (STATINS) (IDIOSYNCRATIC MIXED HEPATOCELLULAR AND CHOLESTATIC REACTION)
Between 1 and 2% of patients taking lovastatin, simvastatin, pravastatin, fluvastatin, or one of the newer statin drugs for the treatment of hypercholesterolemia experience asymptomatic, reversible elevations (>threefold) of aminotransferase activity. Acute hepatitis-like histologic changes, centrilobular necrosis, and centrilobular cholestasis have been described in a very small number of cases. In a larger proportion, minor aminotransferase elevations appear during the first several weeks of therapy. Careful laboratory monitoring can distinguish between patients with minor, transitory changes, who may continue therapy and those with more profound and sustained abnormalities, who should discontinue therapy. Because clinically meaningful aminotransferase elevations are so rare after statin use and do not differ in meta-analyses from the frequency of such laboratory abnormalities in placebo recipients, a panel of liver experts recommended to the National Lipid Association’s Safety Task Force that liver test monitoring was not necessary in patients treated with statins and that statin therapy need not be discontinued in patients found to have asymptomatic isolated aminotransferase elevations during therapy. Statin hepatotoxicity is not increased in patients with chronic hepatitis C, hepatic steatosis, or other underlying liver diseases, and statins can be used safely in these patients.
TOTAL PARENTERAL NUTRITION (STEATOSIS, CHOLESTASIS)
Total parenteral nutrition (TPN) is often complicated by cholestatic hepatitis attributable to steatosis, cholestasis, or gallstones (or gallbladder sludge). Steatosis or steatohepatitis may result from the excess carbohydrate calories in these nutritional supplements and is the predominant form of TPN-associated liver disorder in adults. The frequency of this complication has been reduced substantially by the introduction of balanced TPN formulas that rely on lipid as an alternative caloric source. Cholestasis and cholelithiasis, caused by the absence of stimulation of bile flow and secretion resulting from the lack of oral intake, is the predominant form of TPN-associated liver disease in infants, especially in premature neonates. Often, cholestasis in such neonates is multifactorial, contributed to by other factors such as sepsis, hypoxemia, and hypotension; occasionally, TPN-induced cholestasis in neonates culminates in chronic liver disease and liver failure. When TPN-associated liver test abnormalities occur in adults, balancing the TPN formula with more lipid is the intervention of first recourse. In infants with TPN-associated cholestasis, the addition of oral feeding may ameliorate the problem. Therapeutic interventions suggested, but not shown, to be of proven benefit, include cholecystokinin, ursodeoxycholic acid, S -adenosyl methionine, and taurine.
ALTERNATIVE AND COMPLEMENTARY MEDICINES (IDIOSYNCRATIC HEPATITIS, STEATOSIS)
Herbal medications that are of scientifically unproven efficacy and that lack prospective safety oversight by regulatory agencies currently account for more than 20% of drug-induced liver injury in the United States. Besides anabolic steroids, the most common category of dietary or herbal products is weight loss agents. Included among the herbal remedies associated with toxic hepatitis are Jin Bu Huan, xiao-chai-hu-tang, germander, chaparral, senna, mistletoe, skullcap, gentian, comfrey (containing pyrrolizidine alkaloids), ma huang, bee pollen, valerian root, pennyroyal oil, kava, celandine, Impila (Callilepis laureola), LipoKinetix, Hydroxycut, herbal nutritional supplements, and herbal teas containing Camellia sinensis (green tea extract). Well characterized are the acute hepatitis-like histologic lesions following Jin Bu Huan use: focal hepatocellular necrosis, mixed mononuclear portal tract infiltration, coagulative necrosis, apoptotic hepatocyte degeneration, tissue eosinophilia, and microvesicular steatosis. Megadoses of vitamin A can injure the liver, as can pyrrolizidine alkaloids, which often contaminate Chinese herbal preparations and can cause a venoocclusive injury leading to sinusoidal hepatic vein obstruction. Because some alternative medicines induce toxicity via active metabolites, alcohol and drugs that stimulate cytochrome P450 enzymes may enhance the toxicity of some of these products. Conversely, some alternative medicines also stimulate cytochrome P450 and may result in or amplify the toxicity of recognized drug hepatotoxins. Given the widespread use of such poorly defined herbal preparations, hepatotoxicity is likely to be encountered with increasing frequency; therefore, a drug history in patients with acute and chronic liver disease should include use of “alternative medicines” and other nonprescription preparations sold in so-called health food stores.
HIGHLY ACTIVE ANTIRETROVIRAL THERAPY (HAART) FOR HIV INFECTION (MITOCHONDRIAL TOXIC, IDIOSYNCRATIC, STEATOSIS; HEPATOCELLULAR, CHOLESTATIC, AND MIXED)
The recognition of drug hepatotoxicity in persons with HIV infection is complicated in this population by the many alternative causes of liver injury (chronic viral hepatitis, fatty infiltration, infiltrative disorders, mycobacterial infection, etc.), but drug hepatotoxicity associated with HAART is an emerging and common type of liver injury in HIV-infected persons (Chap. 226). Although no one antiviral agent is recognized as a potent hepatotoxin, combination regimens including reverse transcriptase and protease inhibitors cause hepatotoxicity in ~10% of treated patients. Implicated most frequently are combinations including nucleoside analogue reverse transcriptase inhibitors zidovudine, didanosine, and, to a lesser extent, stavudine; protease inhibitors ritonavir and indinavir (and amprenavir when used together with ritonavir), as well as tipranavir; and nonnucleoside reverse transcriptase inhibitors nevirapine and, to a lesser extent, efavirenz. These drugs cause predominantly hepatocellular injury but cholestatic injury as well, and prolonged (>6 months) use of reverse transcriptase inhibitors has been associated with mitochondrial injury, steatosis, and lactic acidosis. Indirect hyperbilirubinemia, resulting from direct inhibition of bilirubin-conjugating activity by UDP-glucuronosyltransferase, usually without elevation of aminotransferase or alkaline phosphatase activities, occurs in ~10% of patients treated with the protease inhibitor indinavir. Distinguishing the impact of HAART hepatotoxicity in patients with HIV and hepatitis virus co-infection is made challenging by the following: (1) both chronic hepatitis B and hepatitis C can affect the natural history of HIV infection and the response to HAART, and (2) HAART can have an impact on chronic viral hepatitis. For example, immunologic reconstitution with HAART can result in immunologically mediated liver-cell injury in patients with chronic hepatitis B co-infection if treatment with an antiviral agent for hepatitis B (e.g., the nucleoside analogue lamivudine) is withdrawn or if nucleoside analogue resistance emerges. Infection with HIV, especially with low CD4+ T cell counts, has been reported to increase the rate of hepatic fibrosis associated with chronic hepatitis C, and HAART therapy can increase levels of serum aminotransferases and HCV RNA in patients with hepatitis C co-infection. Didanosine or stavudine should not be used with ribavirin in patients with HIV/HCV co-infection because of an increased risk of severe mitochondrial toxicity and lactic acidosis.
ACKNOWLEDGMENT
Kurt J. Isselbacher, MD, contributed to this chapter in previous editions of Harrison’s.
362 |
Chronic Hepatitis |
Chronic hepatitis represents a series of liver disorders of varying causes and severity in which hepatic inflammation and necrosis continue for at least 6 months. Milder forms are nonprogressive or only slowly progressive, while more severe forms may be associated with scarring and architectural reorganization, which, when advanced, lead ultimately to cirrhosis. Several categories of chronic hepatitis have been recognized. These include chronic viral hepatitis, drug-induced chronic hepatitis (Chap. 361), and autoimmune chronic hepatitis. In many cases, clinical and laboratory features are insufficient to allow assignment into one of these three categories; these “idiopathic” cases are also believed to represent autoimmune chronic hepatitis. Finally, clinical and laboratory features of chronic hepatitis are observed occasionally in patients with such hereditary/metabolic disorders as Wilson’s disease (copper overload), α1 antitrypsin deficiency (Chaps. 365 and 429), and nonalcoholic fatty liver disease (Chap. 367e) and even occasionally in patients with alcoholic liver injury (Chap. 363). Although all types of chronic hepatitis share certain clinical, laboratory, and histopathologic features, chronic viral and chronic autoimmune hepatitis are sufficiently distinct to merit separate discussions. For discussion of acute hepatitis, see Chap. 360.
CLASSIFICATION OF CHRONIC HEPATITIS
Common to all forms of chronic hepatitis are histopathologic distinctions based on localization and extent of liver injury. These vary from the milder forms, previously labeled chronic persistent hepatitis and chronic lobular hepatitis, to the more severe form, formerly called chronic active hepatitis. When first defined, these designations were believed to have prognostic implications, which were not corroborated by subsequent observations. Categorization of chronic hepatitis based primarily on histopathologic features has been replaced by a more informative classification based on a combination of clinical, serologic, and histologic variables. Classification of chronic hepatitis is based on (1) its cause; (2) its histologic activity, or grade; and (3) its degree of progression, or stage. Thus, neither clinical features alone nor histologic features—requiring liver biopsy—alone are sufficient to characterize and distinguish among the several categories of chronic hepatitis.
CLASSIFICATION BY CAUSE
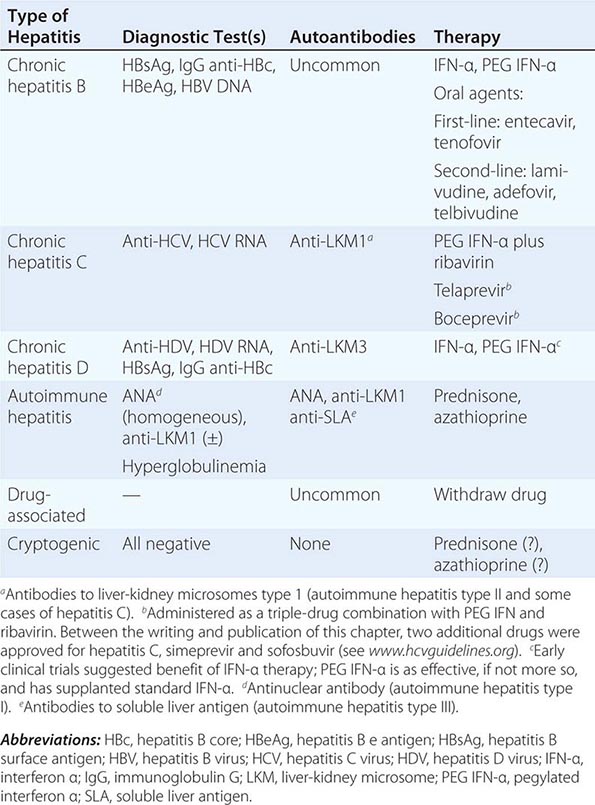
Clinical and serologic features allow the establishment of a diagnosis of chronic viral hepatitis, caused by hepatitis B, hepatitis B plus D, or hepatitis C; autoimmune hepatitis, including several subcategories, I and II (perhaps III), based on serologic distinctions; drug-associated chronic hepatitis; and a category of unknown cause, or cryptogenic chronic hepatitis (Table 362-1). These are addressed in more detail below.
|
CLINICAL AND LABORATORY FEATURES OF CHRONIC HEPATITIS |
CLASSIFICATION BY GRADE
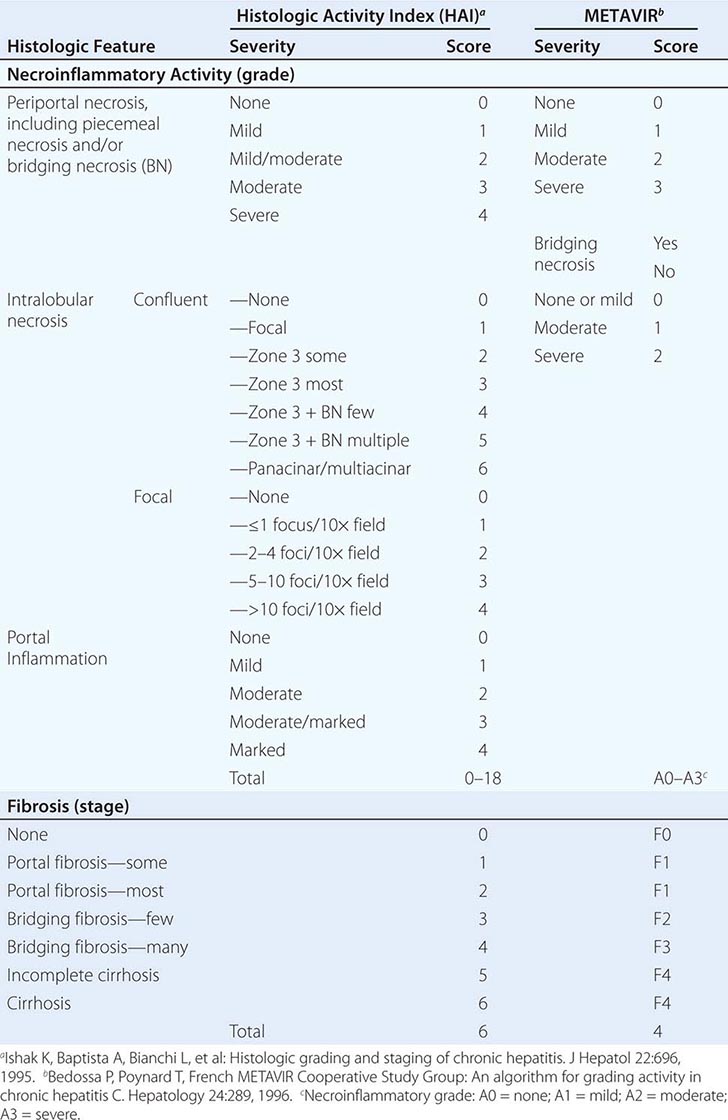
Grade, a histologic assessment of necroinflammatory activity, is based on examination of the liver biopsy. An assessment of important histologic features includes the degree of periportal necrosis and the disruption of the limiting plate of periportal hepatocytes by inflammatory cells (so-called piecemeal necrosis or interface hepatitis); the degree of confluent necrosis that links or forms bridges between vascular structures—between portal tract and portal tract or even more important bridges between portal tract and central vein—referred to as bridging necrosis; the degree of hepatocyte degeneration and focal necrosis within the lobule; and the degree of portal inflammation. Several scoring systems that take these histologic features into account have been devised, and the most popular are the histologic activity index (HAI), used commonly in the United States, and the METAVIR score, used in Europe (Table 362-2). Based on the presence and degree of these features of histologic activity, chronic hepatitis can be graded as mild, moderate, or severe.
|
HISTOLOGIC GRADING AND STAGING OF CHRONIC HEPATITIS |
CLASSIFICATION BY STAGE
The stage of chronic hepatitis, which reflects the level of progression of the disease, is based on the degree of hepatic fibrosis. When fibrosis is so extensive that fibrous septa surround parenchymal nodules and alter the normal architecture of the liver lobule, the histologic lesion is defined as cirrhosis. Staging is based on the degree of fibrosis as categorized on a numerical scale from 0–6 (HAI) or 0–4 (METAVIR) (Table 362-2). Several noninvasive approaches have been introduced to provide approximations of hepatic histologic stage, including serum biomarkers of fibrosis and imaging determinations of liver elasticity.
CHRONIC VIRAL HEPATITIS
Both the enterically transmitted forms of viral hepatitis, hepatitis A and E, are self-limited and do not cause chronic hepatitis (rare reports notwithstanding in which acute hepatitis A serves as a trigger for the onset of autoimmune hepatitis in genetically susceptible patients or in which hepatitis E (Chap. 360) can cause chronic liver disease in immunosuppressed hosts, e.g., after liver transplantation). In contrast, the entire clinicopathologic spectrum of chronic hepatitis occurs in patients with chronic viral hepatitis B and C as well as in patients with chronic hepatitis D superimposed on chronic hepatitis B.
CHRONIC HEPATITIS B
The likelihood of chronicity after acute hepatitis B varies as a function of age. Infection at birth is associated with clinically silent acute infection but a 90% chance of chronic infection, whereas infection in young adulthood in immunocompetent persons is typically associated with clinically apparent acute hepatitis but a risk of chronicity of only approximately 1%. Most cases of chronic hepatitis B among adults, however, occur in patients who never had a recognized episode of clinically apparent acute viral hepatitis. The degree of liver injury (grade) in patients with chronic hepatitis B is variable, ranging from none in inactive carriers to mild to moderate to severe. Among adults with chronic hepatitis B, histologic features are of prognostic importance. In one long-term study of patients with chronic hepatitis B, investigators found a 5-year survival rate of 97% for patients with mild chronic hepatitis, 86% for patients with moderate to severe chronic hepatitis, and only 55% for patients with chronic hepatitis and postnecrotic cirrhosis. The 15-year survival in these cohorts was 77%, 66%, and 40%, respectively. On the other hand, more recent observations do not allow us to be so sanguine about the prognosis in patients with mild chronic hepatitis; among such patients followed for 1–13 years, progression to more severe chronic hepatitis and cirrhosis has been observed in more than a quarter of cases.
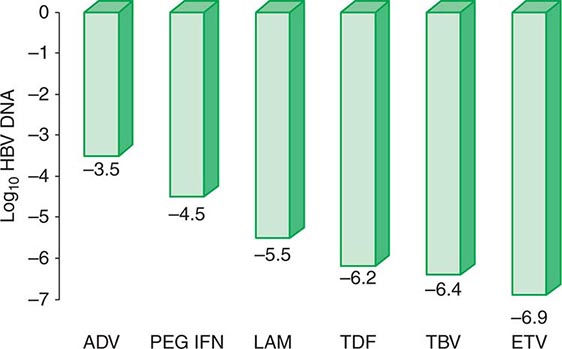
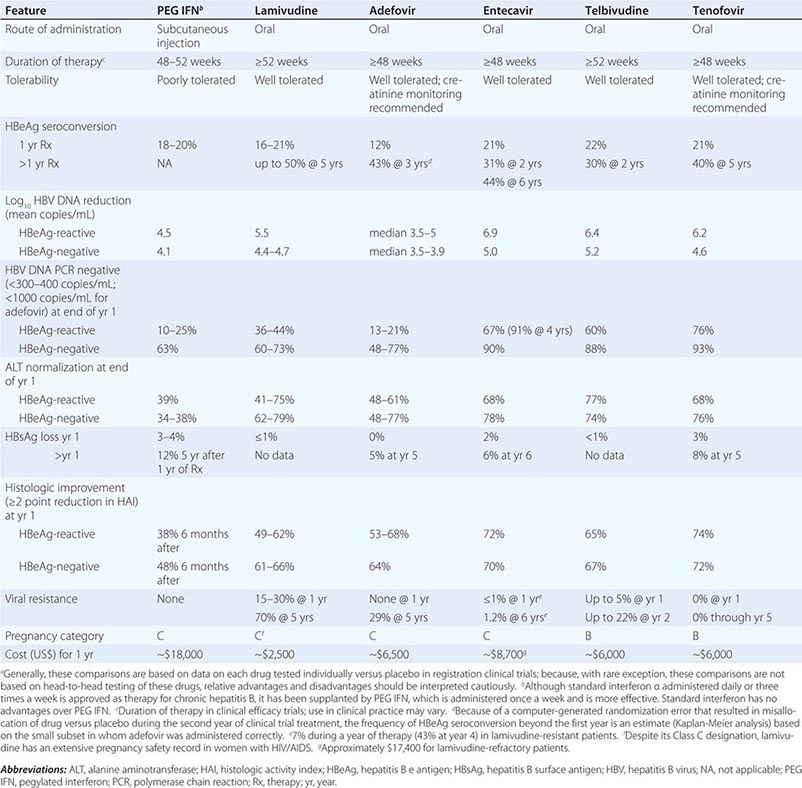
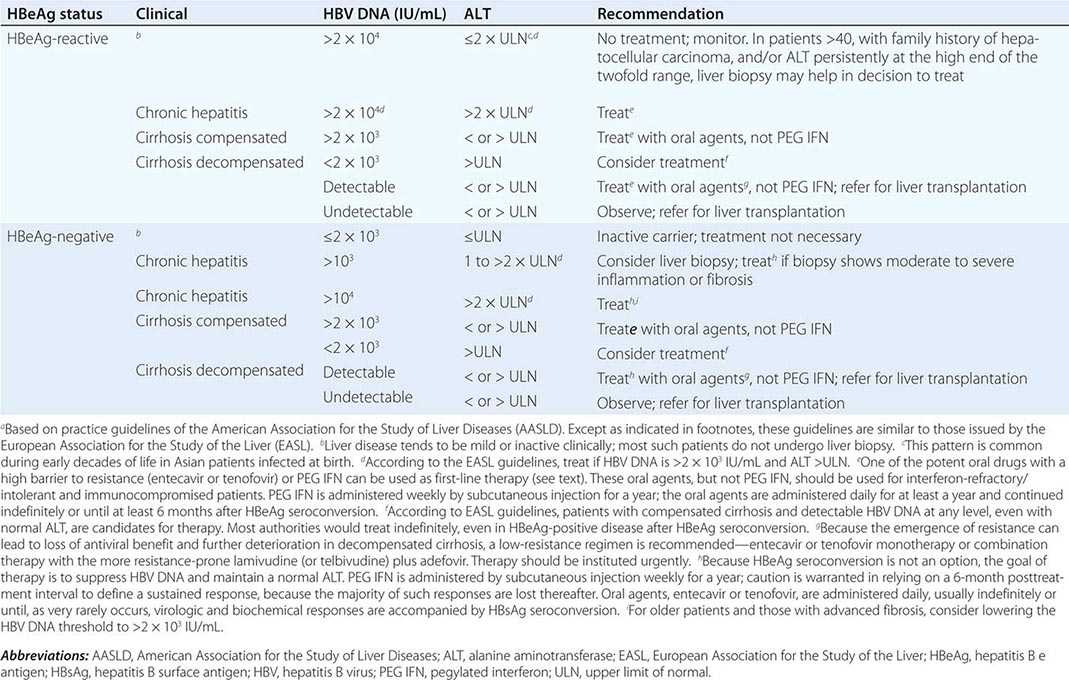
More important to consider than histology alone in patients with chronic hepatitis B is the degree of hepatitis B virus (HBV) replication. As reviewed in Chap. 360, chronic HBV infection can occur in the presence or absence of serum hepatitis B e antigen (HBeAg), and generally, for both HBeAg-reactive and HBeAg-negative chronic hepatitis B, the level of HBV DNA correlates with the level of liver injury and risk of progression. In HBeAg-reactive chronic hepatitis B, two phases have been recognized based on the relative level of HBV replication. The relatively replicative phase is characterized by the presence in the serum of HBeAg and HBV DNA levels well in excess of 103–104 IU/mL, sometimes exceeding 109 IU/mL; by the presence in the liver of detectable intrahepatocyte nucleocapsid antigens (primarily hepatitis B core antigen [HBcAg]); by high infectivity; and by accompanying liver injury. In contrast, the relatively nonreplicative phase is characterized by the absence of the conventional serum marker of HBV replication (HBeAg), the appearance of anti-HBe, levels of HBV DNA below a threshold of ~103 IU/mL, the absence of intrahepatocytic HBcAg, limited infectivity, and minimal liver injury. Patients in the replicative phase tend to have more severe chronic hepatitis, whereas those in the nonreplicative phase tend to have minimal or mild chronic hepatitis or to be inactive hepatitis B carriers. The likelihood in a patient with HBeAg-reactive chronic hepatitis B of converting spontaneously from relatively replicative to nonreplicative infection is approximately 10% per year. Distinctions in HBV replication and in histologic category, however, do not always coincide. In patients with HBeAg-reactive chronic HBV infection, especially when acquired at birth or in early childhood, as recognized commonly in Asian countries, a dichotomy is common between very high levels of HBV replication during the early decades of life (when the level of host tolerance of HBV is relatively high) and negligible levels of liver injury. Yet despite the relatively immediate, apparently benign nature of liver disease for many decades in this population, in the middle decades, activation of liver injury emerges as relative tolerance of the host to HBV declines, and these patients with childhood-acquired HBV infection are ultimately at increased risk later in life of cirrhosis, hepatocellular carcinoma (HCC) (Chap. 111), and liver-related death. A discussion of the pathogenesis of liver injury in patients with chronic hepatitis B appears in Chap. 360.
 HBeAg-negative chronic hepatitis B (i.e., chronic HBV infection with active virus replication, readily detectable HBV DNA but without HBeAg [anti-HBe-reactive]), is more common than HBeAg-reactive chronic hepatitis B in Mediterranean and European countries and in Asia (and, correspondingly, in HBV genotypes other than A). Compared to patients with HBeAg-reactive chronic hepatitis B, patients with HBeAg-negative chronic hepatitis B have levels of HBV DNA that are several orders of magnitude lower (no more than 105–106 IU/mL) than those observed in the HBeAg-reactive subset. Most such cases represent precore or core-promoter mutations acquired late in the natural history of the disease (mostly early-life onset; age range 40–55 years, older than that for HBeAg-reactive chronic hepatitis B); these mutations prevent translation of HBeAg from the precore component of the HBV genome (precore mutants) or are characterized by downregulated transcription of precore mRNA (core-promoter mutants; Chap. 360). Although their levels of HBV DNA tend to be lower than among patients with HBeAg-reactive chronic hepatitis B, patients with HBeAg-negative chronic hepatitis B can have progressive liver injury (complicated by cirrhosis and HCC) and experience episodic reactivation of liver disease reflected in fluctuating levels of aminotransferase activity (“flares”). The biochemical and histologic activity of HBeAg-negative disease tends to correlate closely with levels of HBV replication, unlike the case mentioned above of Asian patients with HBeAg-reactive chronic hepatitis B during the early decades of their HBV infection. An important point worth reiterating is the observation that the level of HBV replication is the most important risk factor for the ultimate development of cirrhosis and HCC in both HBeAg-reactive and HBeAg-negative patients. Although levels of HBV DNA are lower and more readily suppressed by therapy to undetectable levels in HBeAg-negative (compared to HBeAg-reactive) chronic hepatitis B, achieving sustained responses that permit discontinuation of antiviral therapy is less likely in HBeAg-negative patients (see below). Inactive carriers are patients with circulating hepatitis B surface antigen (HBsAg), normal serum aminotransferase levels, undetectable HBeAg, and levels of HBV DNA that are either undetectable or present at a threshold of ≤103 IU/mL. This serologic profile can occur not only in inactive carriers but also in patients with HBeAg-negative chronic hepatitis B during periods of relative inactivity; distinguishing between the two requires sequential biochemical and virologic monitoring over many months.
HBeAg-negative chronic hepatitis B (i.e., chronic HBV infection with active virus replication, readily detectable HBV DNA but without HBeAg [anti-HBe-reactive]), is more common than HBeAg-reactive chronic hepatitis B in Mediterranean and European countries and in Asia (and, correspondingly, in HBV genotypes other than A). Compared to patients with HBeAg-reactive chronic hepatitis B, patients with HBeAg-negative chronic hepatitis B have levels of HBV DNA that are several orders of magnitude lower (no more than 105–106 IU/mL) than those observed in the HBeAg-reactive subset. Most such cases represent precore or core-promoter mutations acquired late in the natural history of the disease (mostly early-life onset; age range 40–55 years, older than that for HBeAg-reactive chronic hepatitis B); these mutations prevent translation of HBeAg from the precore component of the HBV genome (precore mutants) or are characterized by downregulated transcription of precore mRNA (core-promoter mutants; Chap. 360). Although their levels of HBV DNA tend to be lower than among patients with HBeAg-reactive chronic hepatitis B, patients with HBeAg-negative chronic hepatitis B can have progressive liver injury (complicated by cirrhosis and HCC) and experience episodic reactivation of liver disease reflected in fluctuating levels of aminotransferase activity (“flares”). The biochemical and histologic activity of HBeAg-negative disease tends to correlate closely with levels of HBV replication, unlike the case mentioned above of Asian patients with HBeAg-reactive chronic hepatitis B during the early decades of their HBV infection. An important point worth reiterating is the observation that the level of HBV replication is the most important risk factor for the ultimate development of cirrhosis and HCC in both HBeAg-reactive and HBeAg-negative patients. Although levels of HBV DNA are lower and more readily suppressed by therapy to undetectable levels in HBeAg-negative (compared to HBeAg-reactive) chronic hepatitis B, achieving sustained responses that permit discontinuation of antiviral therapy is less likely in HBeAg-negative patients (see below). Inactive carriers are patients with circulating hepatitis B surface antigen (HBsAg), normal serum aminotransferase levels, undetectable HBeAg, and levels of HBV DNA that are either undetectable or present at a threshold of ≤103 IU/mL. This serologic profile can occur not only in inactive carriers but also in patients with HBeAg-negative chronic hepatitis B during periods of relative inactivity; distinguishing between the two requires sequential biochemical and virologic monitoring over many months.
The spectrum of clinical features of chronic hepatitis B is broad, ranging from asymptomatic infection to debilitating disease or even end-stage, fatal hepatic failure. As noted above, the onset of the disease tends to be insidious in most patients, with the exception of the very few in whom chronic disease follows failure of resolution of clinically apparent acute hepatitis B. The clinical and laboratory features associated with progression from acute to chronic hepatitis B are discussed in Chap. 360.
Fatigue is a common symptom, and persistent or intermittent jaundice is a common feature in severe or advanced cases. Intermittent deepening of jaundice and recurrence of malaise and anorexia, as well as worsening fatigue, are reminiscent of acute hepatitis; such exacerbations may occur spontaneously, often coinciding with evidence of virologic reactivation; may lead to progressive liver injury; and, when superimposed on well-established cirrhosis, may cause hepatic decompensation. Complications of cirrhosis occur in end-stage chronic hepatitis and include ascites, edema, bleeding gastroesophageal varices, hepatic encephalopathy, coagulopathy, or hypersplenism. Occasionally, these complications bring the patient to initial clinical attention. Extrahepatic complications of chronic hepatitis B, similar to those seen during the prodromal phase of acute hepatitis B, are associated with deposition of circulating hepatitis B antigen–antibody immune complexes. These include arthralgias and arthritis, which are common, and the more rare purpuric cutaneous lesions (leukocytoclastic vasculitis), immune-complex glomerulonephritis, and generalized vasculitis (polyarteritis nodosa) (Chaps. 360 and 385).
Laboratory features of chronic hepatitis B do not distinguish adequately between histologically mild and severe hepatitis. Aminotransferase elevations tend to be modest for chronic hepatitis B but may fluctuate in the range of 100–1000 units. As is true for acute viral hepatitis B, alanine aminotransferase (ALT) tends to be more elevated than aspartate aminotransferase (AST); however, once cirrhosis is established, AST tends to exceed ALT. Levels of alkaline phosphatase activity tend to be normal or only marginally elevated. In severe cases, moderate elevations in serum bilirubin (51.3–171 μmol/L [3–10 mg/dL]) occur. Hypoalbuminemia and prolongation of the prothrombin time occur in severe or end-stage cases. Hyperglobulinemia and detectable circulating autoantibodies are distinctly absent in chronic hepatitis B (in contrast to autoimmune hepatitis). Viral markers of chronic HBV infection are discussed in Chap. 360.
CHRONIC HEPATITIS D (DELTA HEPATITIS)
Chronic hepatitis D virus (HDV) may follow acute co-infection with HBV but at a rate no higher than the rate of chronicity of acute hepatitis B. That is, although HDV co-infection can increase the severity of acute hepatitis B, HDV does not increase the likelihood of progression to chronic hepatitis B. When, however, HDV superinfection occurs in a person who is already chronically infected with HBV, long-term HDV infection is the rule, and a worsening of the liver disease is the expected consequence. Except for severity, chronic hepatitis B plus D has similar clinical and laboratory features to those seen in chronic hepatitis B alone. Relatively severe and progressive chronic hepatitis, with or without cirrhosis, is the rule, and mild chronic hepatitis is the exception. Occasionally, however, mild hepatitis or even, rarely, inactive carriage occurs in patients with chronic hepatitis B plus D, and the disease may become indolent after several years of infection. A distinguishing serologic feature of chronic hepatitis D is the presence in the circulation of antibodies to liver-kidney microsomes (anti-LKM); however, the anti-LKM seen in hepatitis D, anti-LKM3, are directed against uridine diphosphate glucuronosyltransferase and are distinct from anti-LKM1 seen in patients with autoimmune hepatitis and in a subset of patients with chronic hepatitis C (see below). The clinical and laboratory features of chronic HDV infection are summarized in Chap. 360.
CHRONIC HEPATITIS C
Regardless of the epidemiologic mode of acquisition of hepatitis C virus (HCV) infection, chronic hepatitis follows acute hepatitis C in 50–70% of cases; chronic infection is common even in those with a return to normal in aminotransferase levels after acute hepatitis C, adding up to an 85% likelihood of chronic HCV infection after acute hepatitis C. Few clues had emerged to explain host differences associated with chronic infection until recently, when variation in a single nucleotide polymorphism (SNP) on chromosome 19, IL28B (which codes for IFN-λ3), was identified that distinguished between responders and nonresponders to antiviral therapy (see below). The same variants correlated with spontaneous resolution after acute infection: 53% in genotype C/C, 30% in genotype C/T, but only 23% in genotype T/T. The association with HCV clearance after acute infection is even stronger when IL28B haplotype is combined with haplotype G/G of an SNP near HLA class II DBQ1*03:01.
In patients with chronic hepatitis C followed for 20 years, progression to cirrhosis occurs in about 20–25%. Such is the case even for patients with relatively clinically mild chronic hepatitis, including those without symptoms, with only modest elevations of aminotransferase activity, and with mild chronic hepatitis on liver biopsy. Even in cohorts of well-compensated patients with chronic hepatitis C referred for clinical research trials (no complications of chronic liver disease and with normal hepatic synthetic function), the prevalence of cirrhosis may be as high as 50%. Most cases of hepatitis C are identified initially in asymptomatic patients who have no history of acute hepatitis C (e.g., those discovered while attempting to donate blood, while undergoing lab testing as part of an application for life insurance, or as a result of routine laboratory tests). The source of HCV infection in many of these cases is not defined, although a long-forgotten percutaneous exposure (e.g., injection drug use) in the remote past can be elicited in a substantial proportion and probably accounts for most infections; most of these infections were acquired in the 1960s and 1970s, coming to clinical attention decades later.
Approximately one-third of patients with chronic hepatitis C have normal or near-normal aminotransferase activity; although one-third to one-half of these patients have chronic hepatitis on liver biopsy, the grade of liver injury and stage of fibrosis tend to be mild in the vast majority. In some cases, more severe liver injury has been reported—even, rarely, cirrhosis, most likely the result of previous histologic activity. Among patients with persistent normal aminotransferase activity sustained over ≥5–10 years, histologic progression has been shown to be rare; however, approximately one-fourth of patients with normal aminotransferase activity experience subsequent aminotransferase elevations, and histologic injury can be progressive once abnormal biochemical activity resumes. Therefore, continued clinical monitoring is indicated, even for patients with normal aminotransferase activity.
Despite this substantial rate of progression of chronic hepatitis C, and despite the fact that liver failure can result from end-stage chronic hepatitis C, the long-term prognosis for chronic hepatitis C in a majority of patients is relatively benign. Mortality over 10–20 years among patients with transfusion-associated chronic hepatitis C has been shown not to differ from mortality in a matched population of transfused patients in whom hepatitis C did not develop. Although death in the hepatitis group is more likely to result from liver failure, and although hepatic decompensation may occur in ~15% of such patients over the course of a decade, the majority (almost 60%) of patients remain asymptomatic and well compensated, with no clinical sequelae of chronic liver disease. Overall, chronic hepatitis C tends to be very slowly and insidiously progressive, if at all, in the vast majority of patients, whereas in approximately one-fourth of cases, chronic hepatitis C will progress eventually to end-stage cirrhosis. In fact, because HCV infection is so prevalent, and because a proportion of patients progress inexorably to end-stage liver disease, hepatitis C is the most frequent indication for liver transplantation (Chap. 368). In the United States, hepatitis C accounts for up to 40% of all chronic liver disease, and, as of 2007, mortality caused by hepatitis C surpassed that associated with HIV/AIDS. Moreover, because the prevalence of HCV infection is so much higher in the “baby boomer” cohort borne between 1945 and 1965, three-quarters of the mortality associated with hepatitis C occurs in this age cohort. Referral bias may account for the more severe outcomes described in cohorts of patients reported from tertiary care centers (20-year progression of ≥20%) versus the more benign outcomes in cohorts of patients monitored from initial blood-product-associated acute hepatitis or identified in community settings (20-year progression of only 4–7%). Still unexplained, however, are the wide ranges in reported progression to cirrhosis, from 2% over 17 years in a population of women with hepatitis C infection acquired from contaminated anti-D immune globulin to 30% over ≤11 years in recipients of contaminated intravenous immune globulin.
Progression of liver disease in patients with chronic hepatitis C has been reported to be more likely in patients with older age, longer duration of infection, advanced histologic stage and grade, more complex quasispecies diversity, increased hepatic iron, concomitant other liver disorders (alcoholic liver disease, chronic hepatitis B, hemochromatosis, α1 antitrypsin deficiency, and steatohepatitis), HIV infection, and obesity. Among these variables, however, duration of infection appears to be one of the most important, and some of the others probably reflect disease duration to some extent (e.g., quasispecies diversity, hepatic iron accumulation). No other epidemiologic or clinical features of chronic hepatitis C (e.g., severity of acute hepatitis, level of aminotransferase activity, level of HCV RNA, presence or absence of jaundice during acute hepatitis) are predictive of eventual outcome. Despite the relatively benign nature of chronic hepatitis C over time in many patients, cirrhosis following chronic hepatitis C has been associated with the late development, after several decades, of HCC (Chap. 111); the annual rate of HCC in cirrhotic patients with hepatitis C is 1–4%, occurring primarily in patients who have had HCV infection for 30 years or more.
Perhaps the best prognostic indicator in chronic hepatitis C is liver histology; the rate of hepatic fibrosis may be slow, moderate, or rapid. Patients with mild necrosis and inflammation as well as those with limited fibrosis have an excellent prognosis and limited progression to cirrhosis. In contrast, among patients with moderate to severe necroinflammatory activity or fibrosis, including septal or bridging fibrosis, progression to cirrhosis is highly likely over the course of 10–20 years. The pace of fibrosis progression may be accelerated by such factors as concomitant HIV infection, other causes of liver disease, excessive alcohol use, and hepatic steatosis. Among patients with compensated cirrhosis associated with hepatitis C, the 10-year survival rate is close to 80%; mortality occurs at a rate of 2–6% per year; decompensation at a rate of 4–5% per year; and, as noted above, HCC at a rate of 1–4% per year. A discussion of the pathogenesis of liver injury in patients with chronic hepatitis C appears in Chap. 360.
Clinical features of chronic hepatitis C are similar to those described above for chronic hepatitis B. Generally, fatigue is the most common symptom; jaundice is rare. Immune complex–mediated extrahepatic complications of chronic hepatitis C are less common than in chronic hepatitis B (despite the fact that assays for immune complexes are often positive in patients with chronic hepatitis C), with the exception of essential mixed cryoglobulinemia (Chap. 360), which is linked to cutaneous vasculitis and membranoproliferative glomerulonephritis as well as lymphoproliferative disorders such as B-cell lymphoma and unexplained monoclonal gammopathy. In addition, chronic hepatitis C has been associated with extrahepatic complications unrelated to immune-complex injury. These include Sjögren’s syndrome, lichen planus, porphyria cutanea tarda, type 2 diabetes mellitus, and the metabolic syndrome (including insulin resistance and steatohepatitis).
Laboratory features of chronic hepatitis C are similar to those in patients with chronic hepatitis B, but aminotransferase levels tend to fluctuate more (the characteristic episodic pattern of aminotransferase activity) and to be lower, especially in patients with long-standing disease. An interesting and occasionally confusing finding in patients with chronic hepatitis C is the presence of autoantibodies. Rarely, patients with autoimmune hepatitis (see below) and hyperglobulinemia have false-positive immunoassays for anti-HCV. On the other hand, some patients with serologically confirmable chronic hepatitis C have circulating anti-LKM. These antibodies are anti-LKM1, as seen in patients with autoimmune hepatitis type 2 (see below), and are directed against a 33-amino-acid sequence of cytochrome P450 IID6. The occurrence of anti-LKM1 in some patients with chronic hepatitis C may result from the partial sequence homology between the epitope recognized by anti-LKM1 and two segments of the HCV polyprotein. In addition, the presence of this autoantibody in some patients with chronic hepatitis C suggests that autoimmunity may be playing a role in the pathogenesis of chronic hepatitis C.
Histopathologic features of chronic hepatitis C, especially those that distinguish hepatitis C from hepatitis B, are described in Chap. 360.
AUTOIMMUNE HEPATITIS
DEFINITION
Autoimmune hepatitis is a chronic disorder characterized by continuing hepatocellular necrosis and inflammation, usually with fibrosis, which can progress to cirrhosis and liver failure. When fulfilling criteria of severity, this type of chronic hepatitis, when untreated, may have a 6-month mortality of as high as 40%. Based on contemporary estimates of the natural history of autoimmune hepatitis, the 10-year survival is 80–98% for treated and 67% for untreated patients. The prominence of extrahepatic features of autoimmunity and seroimmunologic abnormalities in this disorder supports an autoimmune process in its pathogenesis; this concept is reflected in the prior labels lupoid and plasma cell hepatitis. Autoantibodies and other typical features of autoimmunity, however, do not occur in all cases; among the broader categories of “idiopathic” or cryptogenic chronic hepatitis, many, perhaps the majority, are probably autoimmune in origin. Cases in which hepatotropic viruses, metabolic/genetic derangements (including nonalcoholic fatty liver disease), and hepatotoxic drugs have been excluded represent a spectrum of heterogeneous liver disorders of unknown cause, a proportion of which are most likely autoimmune hepatitis.
IMMUNOPATHOGENESIS
The weight of evidence suggests that the progressive liver injury in patients with autoimmune hepatitis is the result of a cell-mediated immunologic attack directed against liver cells. In all likelihood, predisposition to autoimmunity is inherited, whereas the liver specificity of this injury is triggered by environmental (e.g., chemical, drug [e.g., minocycline], or viral) factors. For example, patients have been described in whom apparently self-limited cases of acute hepatitis A, B, or C led to autoimmune hepatitis, presumably because of genetic susceptibility or predisposition. Evidence to support an autoimmune pathogenesis in this type of hepatitis includes the following: (1) In the liver, the histopathologic lesions are composed predominantly of cytotoxic T cells and plasma cells; (2) circulating autoantibodies (nuclear, smooth muscle, thyroid, etc.; see below), rheumatoid factor, and hyperglobulinemia are common; (3) other autoimmune disorders—such as thyroiditis, rheumatoid arthritis, autoimmune hemolytic anemia, ulcerative colitis, membranoproliferative glomerulonephritis, juvenile diabetes mellitus, celiac disease, and Sjögren’s syndrome—occur with increased frequency in patients and in their relatives who have autoimmune hepatitis; (4) histocompatibility haplotypes associated with autoimmune diseases, such as HLA-B1, -B8, -DR3, and -DR4 as well as extended haplotype DRB1*0301 and DRB1*0401 alleles, are common in patients with autoimmune hepatitis; and (5) this type of chronic hepatitis is responsive to glucocorticoid/immunosuppressive therapy, effective in a variety of autoimmune disorders.
Cellular immune mechanisms appear to be important in the pathogenesis of autoimmune hepatitis. In vitro studies have suggested that in patients with this disorder, CD4+ T lymphocytes are capable of becoming sensitized to hepatocyte membrane proteins and of destroying liver cells. Molecular mimicry by cross-reacting antigens that contain epitopes similar to liver antigens is postulated to activate these T cells, which infiltrate, and result in injury to, the liver. Abnormalities of immunoregulatory control over cytotoxic lymphocytes (impaired regulatory CD4+CD25+ T cell influences) may play a role as well. Studies of genetic predisposition to autoimmune hepatitis demonstrate that certain haplotypes are associated with the disorder, as enumerated above, as are polymorphisms in cytotoxic T lymphocyte antigens (CTLA-4) and tumor necrosis factor α (TNFA*2). The precise triggering factors, genetic influences, and cytotoxic and immunoregulatory mechanisms involved in this type of liver injury remain incompletely defined.
Intriguing clues into the pathogenesis of autoimmune hepatitis come from the observation that circulating autoantibodies are prevalent in patients with this disorder. Among the autoantibodies described in these patients are antibodies to nuclei (so-called antinuclear antibodies [ANAs], primarily in a homogeneous pattern) and smooth muscle (so-called anti-smooth-muscle antibodies, directed at actin, vimentin, and skeletin), antibodies to F-actin, antibodies to liver-kidney microsomes (anti-LKM, see below), antibodies to “soluble liver antigen” (directed against a uracil-guanine-adenine transfer RNA suppressor protein), antibodies to α-actinin, and antibodies to the liver-specific asialoglycoprotein receptor (or “hepatic lectin”) and other hepatocyte membrane proteins. Although some of these provide helpful diagnostic markers, their involvement in the pathogenesis of autoimmune hepatitis has not been established.
Humoral immune mechanisms have been shown to play a role in the extrahepatic manifestations of autoimmune and idiopathic hepatitis. Arthralgias, arthritis, cutaneous vasculitis, and glomerulonephritis occurring in patients with autoimmune hepatitis appear to be mediated by the deposition of circulating immune complexes in affected tissue vessels, followed by complement activation, inflammation, and tissue injury. While specific viral antigen-antibody complexes can be identified in acute and chronic viral hepatitis, the nature of the immune complexes in autoimmune hepatitis has not been defined.
CLINICAL FEATURES
Many of the clinical features of autoimmune hepatitis are similar to those described for chronic viral hepatitis. The onset of disease may be insidious or abrupt; the disease may present initially like, and be confused with, acute viral hepatitis; a history of recurrent bouts of what had been labeled acute hepatitis is not uncommon. In approximately a quarter of patients, the diagnosis is made in the absence of symptoms, based on abnormal liver laboratory tests. A subset of patients with autoimmune hepatitis has distinct features. Such patients are predominantly young to middle-aged women with marked hyperglobulinemia and high-titer circulating ANAs. This is the group with positive lupus erythematosus (LE) preparations (initially labeled “lupoid” hepatitis) in whom other autoimmune features are common. Fatigue, malaise, anorexia, amenorrhea, acne, arthralgias, and jaundice are common. Occasionally, arthritis, maculopapular eruptions (including cutaneous vasculitis), erythema nodosum, colitis, pleurisy, pericarditis, anemia, azotemia, and sicca syndrome (keratoconjunctivitis, xerostomia) occur. In some patients, complications of cirrhosis, such as ascites and edema (associated with portal hypertension and hypoalbuminemia), encephalopathy, hypersplenism, coagulopathy, or variceal bleeding may bring the patient to initial medical attention.
The course of autoimmune hepatitis may be variable. In patients with mild disease or limited histologic lesions (e.g., piecemeal necrosis without bridging), progression to cirrhosis is limited, but, even in this subset, clinical monitoring is important to identify progression; up to half left untreated can progress to cirrhosis over the course of 15 years. In North America, cirrhosis at presentation is more common in African Americans than in whites. In those with severe symptomatic autoimmune hepatitis (aminotransferase levels >10 times normal, marked hyperglobulinemia, “aggressive” histologic lesions—bridging necrosis or multilobular collapse, cirrhosis), the 6-month mortality without therapy may be as high as 40%. Such severe disease accounts for only 20% of cases; the natural history of milder disease is variable, often accentuated by spontaneous remissions and exacerbations. Especially poor prognostic signs include the presence histologically of multilobular collapse at the time of initial presentation and failure of serum bilirubin to improve after 2 weeks of therapy. Death may result from hepatic failure, hepatic coma, other complications of cirrhosis (e.g., variceal hemorrhage), and intercurrent infection. In patients with established cirrhosis, HCC may be a late complication (Chap. 111) but occurs less frequently than in cirrhosis associated with viral hepatitis.
Laboratory features of autoimmune hepatitis are similar to those seen in chronic viral hepatitis. Liver biochemical tests are invariably abnormal but may not correlate with the clinical severity or histopathologic features in individual cases. Many patients with autoimmune hepatitis have normal serum bilirubin, alkaline phosphatase, and globulin levels with only minimal aminotransferase elevations. Serum AST and ALT levels are increased and fluctuate in the range of 100–1000 units. In severe cases, the serum bilirubin level is moderately elevated (51–171 μmol/L [3–10 mg/dL]). Hypoalbuminemia occurs in patients with very active or advanced disease. Serum alkaline phosphatase levels may be moderately elevated or near normal. In a small proportion of patients, marked elevations of alkaline phosphatase activity occur; in such patients, clinical and laboratory features overlap with those of primary biliary cirrhosis (Chap. 365). The prothrombin time is often prolonged, particularly late in the disease or during active phases.
Hypergammaglobulinemia (>2.5 g/dL) is common in autoimmune hepatitis, as is the presence of rheumatoid factor. As noted above, circulating autoantibodies are also prevalent, most characteristically ANAs in a homogeneous staining pattern. Smooth-muscle antibodies are less specific, seen just as frequently in chronic viral hepatitis. Because of the high levels of globulins achieved in the circulation of some patients with autoimmune hepatitis, occasionally the globulins may bind nonspecifically in solid-phase binding immunoassays for viral antibodies. This has been recognized most commonly in tests for antibodies to hepatitis C virus, as noted above. In fact, studies of autoantibodies in autoimmune hepatitis have led to the recognition of new categories of autoimmune hepatitis. Type I autoimmune hepatitis is the classic syndrome prevalent in North America and northern Europe occurring in young women, associated with marked hyperglobulinemia, lupoid features, circulating ANAs, and HLA- DR3 or HLA-DR4 (especially B8-DRB1*03). Also associated with type I autoimmune hepatitis are autoantibodies against actin and atypical perinuclear antineutrophilic cytoplasmic antibodies (pANCA).
Type II autoimmune hepatitis, often seen in children, more common in Mediterranean populations, and linked to HLA- DRB1 and HLA-DQB1 haplotypes, is associated not with ANA but with anti-LKM. Actually, anti-LKM represent a heterogeneous group of antibodies. In type II autoimmune hepatitis, the antibody is anti-LKM1, directed against cytochrome P450 2D6. This is the same anti-LKM seen in some patients with chronic hepatitis C. Anti-LKM2 is seen in drug-induced hepatitis, and anti-LKM3 (directed against uridine diphosphate glucuronyltransferases) is seen in patients with chronic hepatitis D. Another autoantibody observed in type II autoimmune hepatitis is directed against liver cytosol formiminotransferase cyclodeaminase (anti-liver cytosol 1). More controversial is whether or not a third category of autoimmune hepatitis exists, type III autoimmune hepatitis. These patients lack ANA and anti-LKM1 but have circulating antibodies to soluble liver antigen. Most of these patients are women and have clinical features similar to, perhaps more severe than, those of patients with type I autoimmune hepatitis. Type III autoimmune hepatitis does not appear to represent a distinct category but, instead, is part of the spectrum of type I autoimmune hepatitis; this subcategory has not been adopted by a consensus of international experts.
Liver biopsy abnormalities are similar to those described for chronic viral hepatitis. Expanding portal tracts and extending beyond the plate of periportal hepatocytes into the parenchyma (designated interface hepatitis or piecemeal necrosis) is a mononuclear cell infiltrate that, in autoimmune hepatitis, may include the presence of plasma cells. Necroinflammatory activity characterizes the lobular parenchyma, and evidence of hepatocellular regeneration is reflected by “rosette” formation, the occurrence of thickened liver cell plates, and regenerative “pseudolobules.” Septal fibrosis, bridging fibrosis, and cirrhosis are frequent. In patients with early autoimmune hepatitis presenting as an acute-hepatitis-like illness, lobular and centrilobular (as opposed to the more common periportal) necrosis has been reported. Bile duct injury and granulomas are uncommon; however, a subgroup of patients with autoimmune hepatitis has histologic, biochemical, and serologic features overlapping those of primary biliary cirrhosis (Chap. 365).
DIAGNOSTIC CRITERIA
An international group has suggested a set of criteria for establishing a diagnosis of autoimmune hepatitis. Exclusion of liver disease caused by genetic disorders, viral hepatitis, drug hepatotoxicity, and alcohol are linked with such inclusive diagnostic criteria as hyperglobulinemia, autoantibodies, and characteristic histologic features. This international group has also suggested a comprehensive diagnostic scoring system that, rarely required for typical cases, may be helpful when typical features are not present. Factors that weigh in favor of the diagnosis include female gender; predominant aminotransferase elevation; presence and level of globulin elevation; presence of nuclear, smooth muscle, LKM1, and other autoantibodies; concurrent other autoimmune diseases; characteristic histologic features (interface hepatitis, plasma cells, rosettes); HLA-DR3 or -DR4 markers; and response to treatment (see below). A more simplified, more specific scoring system relies on four variables: autoantibodies, serum IgG level, typical or compatible histologic features, and absence of viral hepatitis markers. Weighing against the diagnosis are predominant alkaline phosphatase elevation, mitochondrial antibodies, markers of viral hepatitis, history of hepatotoxic drugs or excessive alcohol, histologic evidence of bile duct injury, or such atypical histologic features as fatty infiltration, iron overload, and viral inclusions.
DIFFERENTIAL DIAGNOSIS
Early during the course of chronic hepatitis, autoimmune hepatitis may resemble typical acute viral hepatitis (Chap. 360). Without histologic assessment, severe chronic hepatitis cannot be readily distinguished based on clinical or biochemical criteria from mild chronic hepatitis. In adolescence, Wilson’s disease (Chaps. 365 and 429) may present with features of chronic hepatitis long before neurologic manifestations become apparent and before the formation of Kayser-Fleischer rings (copper deposition in Descemet’s membrane in the periphery of the cornea). In this age group, serum ceruloplasmin and serum and urinary copper determinations plus measurement of liver copper levels establish the correct diagnosis. Postnecrotic or cryptogenic cirrhosis and primary biliary cirrhosis (Chap. 365) share clinical features with autoimmune hepatitis, and both alcoholic hepatitis (Chap. 363) and nonalcoholic steatohepatitis (Chap. 367e) may present with many features common to autoimmune hepatitis; historic, biochemical, serologic, and histologic assessments are usually sufficient to allow these entities to be distinguished from autoimmune hepatitis. Of course, the distinction between autoimmune and chronic viral hepatitis is not always straightforward, especially when viral antibodies occur in patients with autoimmune disease or when autoantibodies occur in patients with viral disease. Furthermore, the presence of extrahepatic features such as arthritis, cutaneous vasculitis, or pleuritis—not to mention the presence of circulating autoantibodies—may cause confusion with rheumatologic disorders such as rheumatoid arthritis and systemic lupus erythematosus. The existence of clinical and biochemical features of progressive necroinflammatory liver disease distinguishes chronic hepatitis from these other disorders, which are not associated with severe liver disease. Rarely, hepatic venous outflow obstruction (Budd-Chiari syndrome) may present with features suggestive of autoimmune hepatitis, but painful hepatomegaly, ascites, and vascular imaging provide distinguishing diagnostic clues. Other diagnostic considerations would include celiac disease and ischemic liver disease, which would be readily distinguishable by clinical and laboratory features from autoimmune hepatitis.
Finally, occasionally, features of autoimmune hepatitis overlap with features of autoimmune biliary disorders such as primary biliary cirrhosis, primary sclerosing cholangitis (Chaps. 365 and 369), or, even more rarely, mitochondrial antibody-negative autoimmune cholangitis. Such overlap syndromes are difficult to categorize, and often response to therapy may be the distinguishing factor that establishes the diagnosis.
ACKNOWLEDGMENT
Kurt J. Isselbacher, MD, contributed to this chapter in previous editions of Harrison’s.
*As this chapter was going to press, two additional antiviral drugs, a second-generation protease inhibitor simeprevir and nucleoside analogue polymerase inhibitor sofosbuvir were approved for the treatment of hepatitis C. Simerprevir, which is effective for genotype 1, must be administered, like first-generation protease inhibitors, for 12 weeks with PEG IFN and ribavirin, followed by another 12 weeks of PEG IFN and ribavirin (no response-guided therapy). Sofosbuvir, the more convenient and broadly applicable of the two new drugs, must be administered with PEG IFN and ribavirin but for only 12 weeks in patients with genotyes 1, 4-6; for patients with genotypes 2 and 3, PEG IFN is not required. Sofosbuvir plus ribavirin are administered for 12 weeks in genotype 2 and for 24 weeks in genotype 3. Antiviral therapy is evolving very rapidly; by the end of 2014, all-oral, interferon-free combinations (e.g., sofosbuvir plus the NS5a inhibitor ledipasvir) will supplant earlier treatment regimens. For updated treatment recommendations, please consult www.hcvguidelines.org.