Total Knee Arthroplasty
Julie Wong and Michael D. Ries
Introduction
Osteoarthritis (OA), also called osteoarthroses or degenerative joint disease, is the most common type of arthritis and one of the leading causes of disability worldwide. OA is a chronic condition characterized by the breakdown of the joint’s cartilage. Cartilage is the part of the joint that cushions the ends of the bones and allows easy movement of joints. The breakdown of cartilage causes the bones to rub against each other, causing stiffness, pain, and loss of movement in the joint. In the United States, it is estimated that OA may affect 33 million adults.1 When conservative management fails to decrease pain or restore mobility, surgical intervention becomes the treatment of choice. Arthroscopic surgeries may be beneficial in early stages with mechanical symptoms,2 but when this also fails, a total knee arthroplasty (TKA) is usually recommended.
According to the American Academy of Orthopaedic Surgeons, approximately 581,000 knee replacements are performed each year. Most were performed on people aged 60 to 80,3 reflecting the aging population of Baby Boomers. Understanding the surgical procedure of the TKA and designing appropriate programs for rehabilitation are essential to ensure successful and cost-effective outcomes in anticipation of this future growth. Self-report measures of perceived functional ability indicate that 1 year following TKA, individuals have regained 80% of normal functions. However, despite improvement compared with preoperative status, pain and stiffness can remain a problem for some of these people.4
The scope of this chapter focuses on primary TKA. It is referred to as TKA, rather than total knee replacement (TKR), to distinguish it from total knee revision (which is also referred to as TKR). In addition, minimally invasive surgery (MIS)-TKA is also described.
Surgical Indications And Considerations
TKA is an effective treatment for symptomatic OA or inflammatory arthritis of the knee that is not responsive to conservative therapy. Earlier stages of arthritis may be treated with nonsteroidal antiinflammatory drugs, activity restrictions, exercise, bracing, orthotics, and weight loss. Other treatments include injections of hyaluronic acid or cortisone. However, when conservative measures fail and arthritic symptoms limit functional activity, surgery is a more appropriate treatment option. If symptoms are mechanical and associated with catching or locking more than weight-bearing pain, they may result from a torn or degenerative meniscus. Magnetic resonance imaging (MRI) is useful to delineate meniscal pathology from degeneration of the articular cartilage. Arthroscopic débridement may be beneficial for treatment of meniscal pathology but does not appear to be as helpful for management of articular cartilage degeneration.5 If cartilage degeneration occurs primarily in the medial tibiofemoral (TF) compartment with varus deformity, then valgus osteotomy of the tibia can be effective in relieving medial-sided knee pain and delay the need for total joint replacement.6 Osteotomy is most appropriate for treatment of unicompartmental OA in a young active patient, a knee with adequate range of motion (ROM), and limited varus deformity. Relative contraindications include obesity, flexion contracture, significant lateral compartment or patellofemoral arthritis, TF subluxation, and advanced age. For lateral compartment OA with valgus deformity, distal femoral rather than proximal tibial osteotomy is preferred. However, osteotomy generally requires a longer rehabilitation period, and outcomes are less predictable than for TKA.7
Prosthetic options include metallic interposition hemiarthroplasty, as well as unicompartmental, bicompartmental, and TKA. The McKeever and MacIntosh metallic interposition hemiarthroplasties were used before the development of TKA.8,9 The implant is a metallic spacer placed between the femoral and tibial surfaces. Favorable results may be achieved most commonly in a patient with arthritic changes in one compartment who was not considered an appropriate candidate for osteotomy because of obesity, limited motion, or arthritic involvement of the opposite compartment.10,11 However, pain may develop from articulation of the joint surface with the metallic implant. More recently a mobile metallic Uni spacer* has been used which is intended to distract the medial compartment and transfer loads to the lateral compartment.12 However, results appear less predictable than unicompartmental or TKA.
Unicompartmental, bicompartmental, and TKA resurface both the femoral and tibial articular portions of the joint and are effective in relieving arthritic pain. Unicompartmental arthroplasty is indicated for degenerative arthritis limited to either the medial or lateral TF compartment with preservation of the opposite TF and patellofemoral (PF) compartments. Unicompartmental arthroplasty preserves both cruciate ligaments, the opposite TF compartment, and the PF joint, which is typically associated with more favorable knee kinematics, ROM, and overall joint function than TKA. However, failure of unicondylar arthroplasty may occur from the development of arthritic symptoms in the PF or opposite TF compartment, requiring conversion to TKA. Mechanical failure or polyethylene wear may also limit the longevity of unicondylar replacement. Although the indications for use of unicondylar replacement as an alternative to TKA are controversial, unicondylar replacement is generally considered less predictable in terms of longevity of the arthroplasty, particularly when used in situations in which some arthritic involvement of the opposite TF or PF compartment exists. Recent literature shows favorable functional results and patient satisfaction from unicompartmental knee arthroplasty (UKA), especially in the younger, high-demand, and active patients.13 The advantages of UKA versus TKA include better ROM at discharge and a shorter hospital stay (77° versus 67° and 1.3 to 1.4 days versus 2.2 days). The average arc of motion at initial 6-week follow-up was 116° for the UKA patients and 110° for the TKA patients, with 56% of knees having greater than 120°. Early discharge for the patients appeared to be safe; in 97% of cases, patients were discharged directly to home, but 18% of the cases required home health physical therapy and 76% of the cases required outpatient physical therapy. Only 3% of the patients required a skilled nursing or postdischarge rehabilitation stay.14
Bicompartmental (combined medial and PF) arthroplasty is indicated for treatment of symptomatic medial compartment and PF OA.15 Early results with bicompartmental arthroplasty indicate that satisfactory clinical results and restoration of normal kinematics can be achieved.16 However, since bicompartmental arthroplasty is a relatively new procedure, long-term results are not known.
TKA is an effective treatment for severe arthritic knee pain. Both the medial and lateral TF, and usually the PF compartments, are resurfaced in TKA. After TKA, reliable improvement in pain and function can be expected, and survivorship rates of 90% to 95% after 10 years have frequently been reported.17–22 ![]() Early failures may result from infection, instability, malalignment, stiffness, reflex sympathetic dystrophy, and patellar problems. Relative contraindications include active infection, extensor mechanism disruption, severe loss of bony or ligament support, and uncontrolled cardiac disease or medical comorbidities that substantially increase the risk of perioperative morbidity and mortality. However, using proper surgical technique, implant selection, appropriate postoperative pain management, and rehabilitation can avoid these problems. Recent developments including computer-assisted surgery, more kinematic or high-flexion implant designs, use of MIS, and MRI-derived custom cutting blocks may further improve the results of TKA. TKA performed through a conventional skin incision centered over the rectus tendon proximally and extending distal to the tibial tubercle, with a medial parapatellar arthrotomy, is associated with reliable pain relief, improvement in function, and 90% to 95% 10-year survivorship.17–22 However, many patients experience significant pain and inflammation, which typically occurs to some extent for 6 months after arthroplasty and may limit participation in rehabilitation exercises. Less invasive or MIS permits TKA to be performed with reduced soft tissue trauma, with average skin incision of 9.4 to 10.9 cm in the MIS group and 13.7 to 17.1 cm in the conventional group.23 Reports indicate that MIS is associated with less blood loss, less pain, and earlier return of quadriceps function and ROM.24–26
Early failures may result from infection, instability, malalignment, stiffness, reflex sympathetic dystrophy, and patellar problems. Relative contraindications include active infection, extensor mechanism disruption, severe loss of bony or ligament support, and uncontrolled cardiac disease or medical comorbidities that substantially increase the risk of perioperative morbidity and mortality. However, using proper surgical technique, implant selection, appropriate postoperative pain management, and rehabilitation can avoid these problems. Recent developments including computer-assisted surgery, more kinematic or high-flexion implant designs, use of MIS, and MRI-derived custom cutting blocks may further improve the results of TKA. TKA performed through a conventional skin incision centered over the rectus tendon proximally and extending distal to the tibial tubercle, with a medial parapatellar arthrotomy, is associated with reliable pain relief, improvement in function, and 90% to 95% 10-year survivorship.17–22 However, many patients experience significant pain and inflammation, which typically occurs to some extent for 6 months after arthroplasty and may limit participation in rehabilitation exercises. Less invasive or MIS permits TKA to be performed with reduced soft tissue trauma, with average skin incision of 9.4 to 10.9 cm in the MIS group and 13.7 to 17.1 cm in the conventional group.23 Reports indicate that MIS is associated with less blood loss, less pain, and earlier return of quadriceps function and ROM.24–26
Recent literature shows favorable results in the MIS surgery. In selected patients, the Berger and associates’ study showed that outpatient MIS TKA was safe for discharge on the day of surgery with no short-term readmission or complications in 96% of the patients.27 In Tanavalee’s study, 82% of the MIS patients were able to do active knee extension on day 1 while none were able to do active knee extension in the conventional group. Additionally, patients who could walk on day 1 were 17 versus 2.23 In another study, in the first 12 weeks after surgery, the MIS group had less flexion contracture and better flexion.28 At 1 year postoperative, average passive range of motion was 131° in the MIS group and 121° in the conventional group; while active range of motion was 125° in the MIS group and 115° in the conventional group.29
However, a minimally invasive approach may compromise surgical exposure and result in increased complications. With use of small cutting blocks and avoiding dissection of the suprapatellar pouch, reliable results can be achieved with a complication rate that is not greater than conventional TKA.24–26 Particularly, when combined with a preoperative patient education program and multimodal postoperative pain management, TKA performed through a minimally invasive approach appears to offer advantages compared with conventional TKA. However, more muscular patients, those with prior surgery, stiffness, poor skin vascularity, or significant deformity requiring soft tissue releases may not be appropriate candidates for a minimally invasive approach.
Surgical Procedures—Traditional
Preoperative Evaluation
Preoperative evaluation always includes a thorough history and physical examination, determination of the type of arthritis, other joint involvement and functional status, walking distance, current and expected activity level, and sports involvement. Other significant concerns include history of deep venous thrombosis (DVT) or pulmonary embolus (PE) and previous surgery such as joint replacement, corrective osteotomy, and internal fixation of a hip, femur, or tibial fracture. Close attention is paid to joint alignment (varus or valgus), stability, ROM (especially the presence or absence of flexion contracture), muscle tone, and leg lengths.
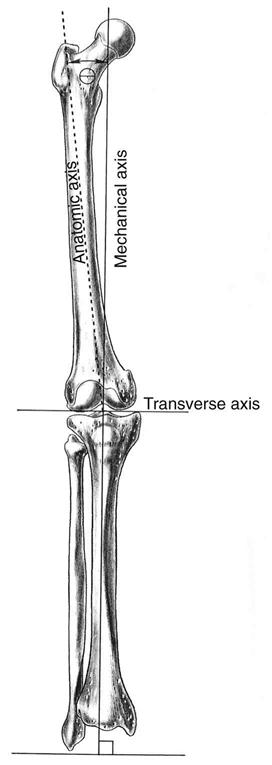
Preoperative radiographs should include long weight-bearing films to demonstrate any femoral or tibial deformity and aid in determining overall lower extremity (LE) alignment. The angle between the mechanical and anatomic axis is measured on the femur to ensure that the distal femoral osteotomy will be perpendicular to the mechanical axis and parallel to the proximal tibial osteotomy (Fig. 27-1). Routine roentgenograms should also include anteroposterior (AP) standing films, as well as lateral and patellar views.
Procedure
Numerous implant and fixation choices are available for TKA:
1 Cemented, uncemented, or hybrid fixation
2 Metal-backed tibia or all-polyethylene tibia
The technique described here is the primary TKA using cemented fixation, a metal-backed tibia, an all-polyethylene patella, and posterior cruciate substitution (Fig. 27-2). Currently, this combination is the most commonly used with consistent long-term results published to date.30–34
Surgical Technique
An antithrombotic stocking or pump is placed on the uninvolved leg. The patient is questioned as to which knee is to be replaced as a final check to avoid the mistake of operating on the wrong knee. An intravenous antibiotic, usually first-generation cephalosporin, is given before the skin incision is made. The patient is placed supine on the operating room table with a tourniquet about the proximal thigh. A general endotracheal or regional anesthetic is required. However, peripheral nerve block of the femoral nerve has been shown to be effective in controlling pain whether through a local infusion (24 hours) or administered via percutaneous insertion of a catheter adjacent to the femoral nerve (a 2- to 3-day ambulatory continuous femoral nerve block).35,36
A sandbag is taped to the operating room table, or a commercial leg-holding device is often used to help stabilize the leg during the procedure. The entire LE is sterilely prepared and draped. The LE is exsanguinated with an Esmarch bandage, and a tourniquet is inflated to an appropriate pressure.
Exposure
A longitudinal midline skin incision is made extending from proximal to the patella to just distal to the tibial tuberosity. Full-thickness skin flaps, including the deep fascia, are developed medially and laterally (Fig. 27-3). A medial arthrotomy is made extending from the quadriceps tendon and ending medial to the tibial tuberosity. The patella is everted or subluxed laterally. After flexing the knee to 90°, the surgeon trims osteophytes from the femoral condyles, intercondylar notch, and tibial plateaus. The cruciate ligaments are excised.
Ligament Balancing
Ligamentous balance is addressed by inserting spreaders in the medial and lateral femoral tibial joints, both in flexion and extension. Equal spacing is then attained by excision of osteophytes and soft tissue releases. The three most common deformities encountered are varus, valgus, and flexion. Release of contracted soft tissues on the concave side of the deformity is achieved either by sequential subperiosteal longitudinal release of individual anatomic soft tissue constraints or use of multiple transverse stab wound incisions (pie crusting) into the contracted soft tissues.
Varus Deformity.
Osteophytes protruding off the medial tibia are removed and the medial capsule is incised. If necessary, then the medial collateral ligament is subperiosteally stripped off the tibia. For deformities with combined varus and flexion contracture, release of the semimembranosus insertion is often necessary.
Valgus Deformity.
A lateral retinacular release is commonly required. If the valgus deformity is more rigid in extension than in flexion, the iliotibial band is released. The popliteus tendon, lateral collateral ligament, and posterolateral capsule may also be released, depending on the severity of the deformity. Peroneal nerve neuropraxia can occasionally occur, especially with correction of flexion contracture in association with valgus deformity.
Flexion Deformity.
Excising posterior femoral osteophytes and releasing posterior capsular adhesions usually address a minor contracture. Further correction requires resection of more bone from the distal femur and posterior capsular release.
Osseous Preparation.
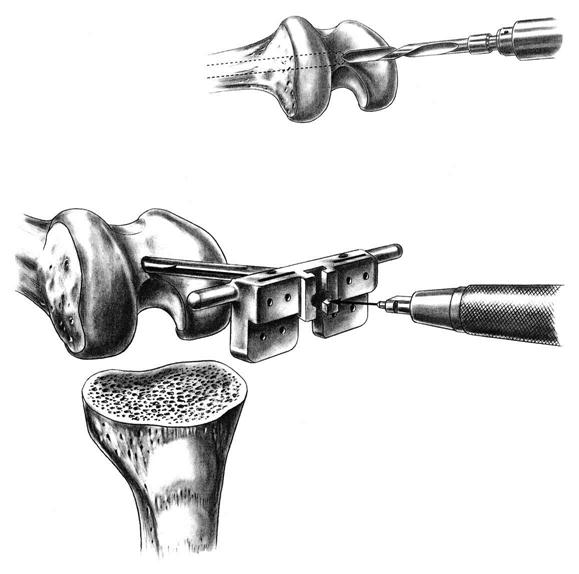
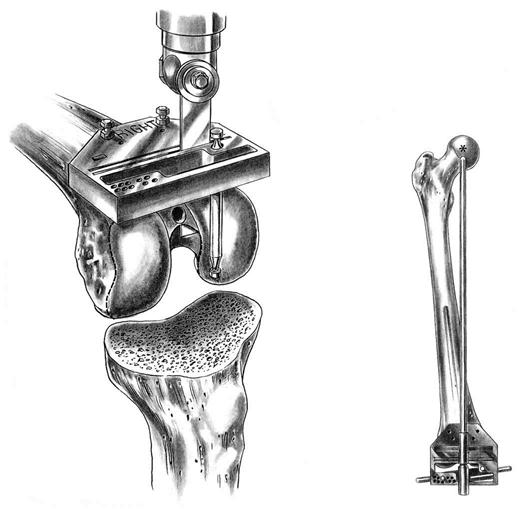
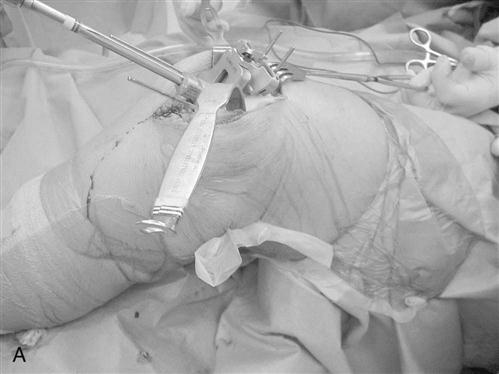
An intramedullary femoral guide is placed through a drill hole in the center of the trochlea (Fig. 27-4). The intramedullary rod must parallel the femoral shaft in both the AP and lateral planes, ensuring placement parallel to the anatomic axis of the femur. Cutting guides are attached to the intramedullary guide to allow precise osteotomies of the anterior and distal femur. The distal femoral osteotomy is usually made 6° to the anatomic axis to produce distal femoral alignment perpendicular to the mechanical axis (Fig. 27-5).
Either intramedullary or extramedullary tibial cutting guides are used. The proximal tibia is osteotomized with a sagittal saw perpendicular to its long axis, approximately 5 mm distal to its articular surface, and angled posteriorly approximately 3° to 5°. Small tibial defects are effectively addressed with cement. Larger defects require either bone grafting or metal augments.
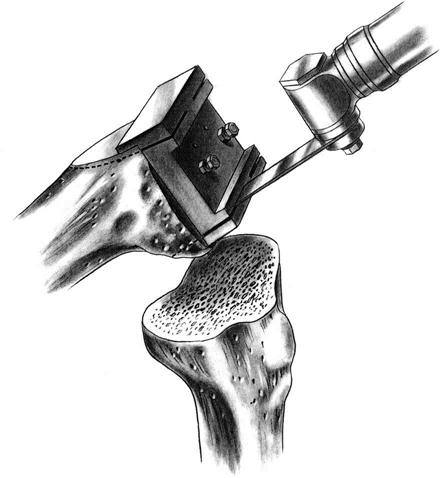
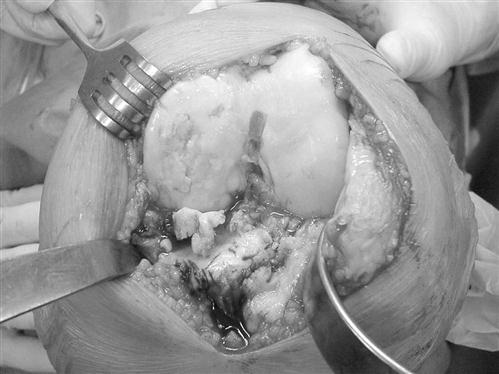
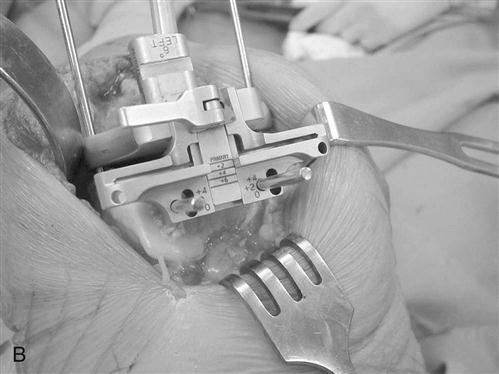
An AP measuring guide is used to determine the appropriate size and position of the femoral component. An AP cutting block is placed to remove the anterior and posterior femoral condyles. This affords excellent visibility and access to remove any remaining meniscus, cruciate ligament, and osteophytes (Fig. 27-6).
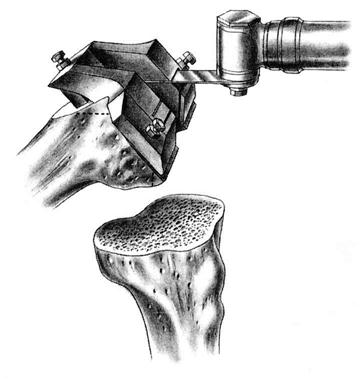
The flexion and extension gaps are measured with standardized spacer blocks. Ideally, the same gap has been produced between the distal femur and tibia in extension and posterior femur and tibia in flexion. This ensures proper soft tissue tension and ligamentous balance. ![]() If full extension is not attained, then further bone is removed from the distal femur. For very severe flexion contractures, such as those encountered in some cases of hemophilic arthropathy or juvenile rheumatoid arthritis, resection of the distal femur to the level of the collateral ligament insertions may be required. Further bone resection is a relative contraindication to conventional TKA if the ligament insertions are compromised, and use of a more highly constrained or revision prosthesis is necessary. A guide is then used to chamfer the anterior and posterior femoral condyles and remove bone from the intercondylar notch (Fig. 27-7).
If full extension is not attained, then further bone is removed from the distal femur. For very severe flexion contractures, such as those encountered in some cases of hemophilic arthropathy or juvenile rheumatoid arthritis, resection of the distal femur to the level of the collateral ligament insertions may be required. Further bone resection is a relative contraindication to conventional TKA if the ligament insertions are compromised, and use of a more highly constrained or revision prosthesis is necessary. A guide is then used to chamfer the anterior and posterior femoral condyles and remove bone from the intercondylar notch (Fig. 27-7).
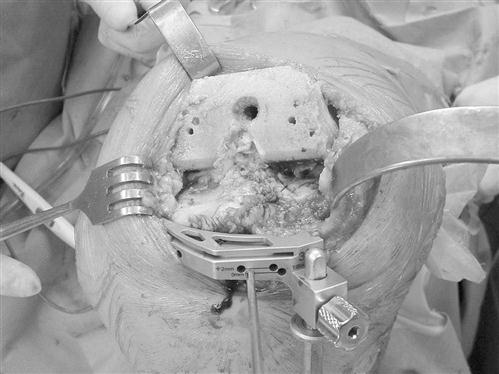
Sizing guides are used to determine the proper-sized tibial component.
After orienting the guide in the AP, medial, and lateral planes, the surgeon ensures proper rotation with the use of an alignment rod extending to the middle of the ankle joint. A bone punch is then used to compress the soft cancellous bone in the tibial metaphysis to accommodate the keel on the tibial component (Fig. 27-8). Trial tibial and femoral components are placed to ensure proper sizing, soft tissue tensioning, ligamentous balance, patellar tracking, and ROM.
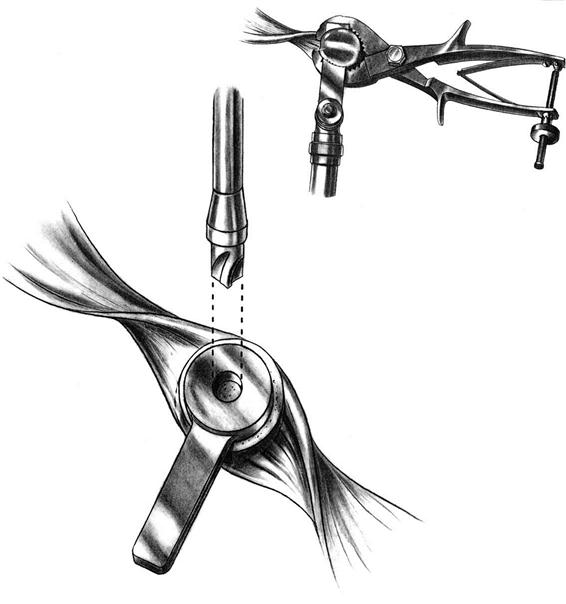
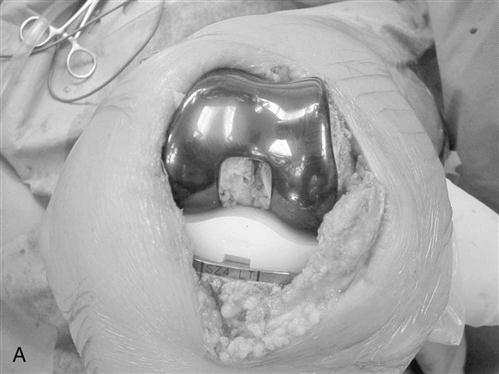
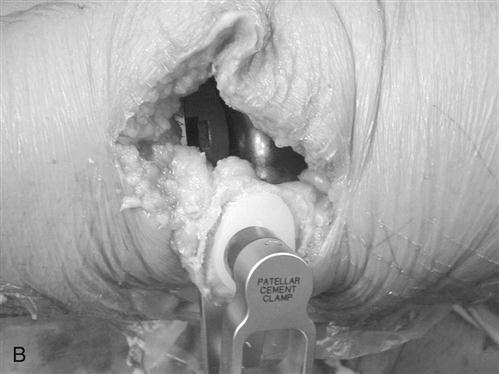
Patellar thickness is measured with a caliper. The articular surface is removed with a power saw or reamer. A guide is used to drill one or more holes in the patella for additional peg fixation (Fig. 27-9). The component is usually placed slightly medial on the patella to assist in patella tracking. The thickness is again checked with the caliper; if the patella is thicker than before resection, then more patella is removed to restore normal patella thickness. Patella tracking is observed (Fig. 27-10) and if the patella tracks laterally, then a lateral retinacular release is performed. Efforts are made to preserve the superior lateral geniculate artery, thereby preserving the blood supply to the patella.
All trial components are removed, bony surfaces are cleansed with pulse lavage, and bone cement is mixed. All components may be cemented at one time, or a second batch of cement may be prepared to allow sequential implantation of the components. All excess cement is trimmed while soft. After the cement has hardened, the tibial spacer may be exchanged to allow final adjustments with regard to ROM and stability. The tourniquet is released and bleeding controlled.
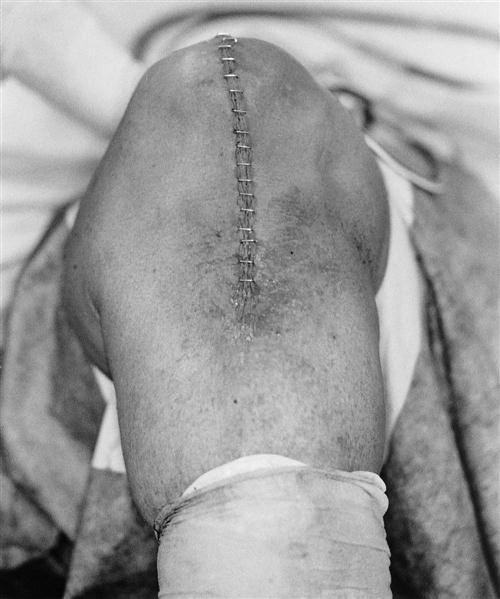
The wound is irrigated thoroughly and closed over a suction drain. A sterile dressing is applied. An antithrombotic stocking or compressive dressing is applied over the sterile dressing (Fig. 27-11).
Minimally Invasive Surgery
Surgical Technique
The minimally or less invasive skin incision extends from the superior pole of the patella to the tibial tubercle (Fig. 27-12). In most patients the length of the incision is approximately 10 to 12 cm, but it may be longer or shorter depending on the size of the patient. Full-thickness skin and subcutaneous tissue flaps are raised to mobilize the skin and subcutaneous layer and permit adequate deep exposure.
Either a medial parapatellar, midvastus, or quadriceps-sparing (subvastus) arthrotomy may be used to expose the knee. Excellent results have been reported using the minimidvastus approach, whereas the quadriceps-sparing approach is more restrictive and may only be appropriate for thin patients with mobile extensor mechanisms and no intraarticular deformity. Typically for muscular male patients, either more proximal dissection of the vastus medialis (in a midvastus approach) or a medial parapatellar arthrotomy is necessary to displace the extensor mechanism laterally. A portion of the fat pad is excised to facilitate exposure. Lateral patellar subluxation is necessary, but eversion of the patella while the knee is flexed does not necessarily increase exposure and may contribute to quadriceps inhibition and postoperative knee pain (Fig. 27-13). The skin and subcutaneous tissue may be considered as a “mobile window.” By extending the knee, the skin incision is moved more proximally and exposure is centered over the distal femur, whereas flexing the knee permits more distal exposure over the proximal tibia (Figs. 27-14 and 27-15). Cutting guides for MIS-TKA are smaller than conventional instruments but permit accurate bone cuts. Alternatively, custom disposable cutting guides can be used. These are derived from preoperative three-dimensional MRI images of the arthritic knee and intended to provide precise orientation of the bone cuts. With the knee in extension, tension in the extensor mechanism is reduced and the patella may be everted for resurfacing without traumatizing the suprapatellar pouch (Fig. 27-16).
Therapy Guidelines For Rehabilitation
Successful postoperative management of the patient ideally begins preoperatively. In many cases, the patient has already been seen by a physical therapist (PT) for a conservative course of treatment. Prescription of high- and low-resistance training exercises is an essential aspect of management for knee OA and both have significantly improved clinical effects.37 Although the goal of rehabilitation at this time may be to avoid surgery, the PT must bear in mind that the treatment plan is similar to those for preoperative care:
1 Patient education regarding the disease process and prognosis
2 Behavioral and health modification for joint protection
3 Cardiovascular conditioning as an adjunct to weight loss
4 An individualized exercise program to address strength and flexibility issues
Much can be done for these patients in this phase of the disease process. A successful outcome for postoperative TKA can be predicted by the patient’s functional ability and status preoperatively.38–40
In the event that a TKA is the intervention of choice, then preoperative treatment is modified to include the assembly of a multidisciplinary team. This group includes the orthopedic surgeon, PT, nursing staff, occupational therapist, and social service worker. Although each team member is responsible for his or her area of expertise, all are committed to the common goal of providing the best possible care to get the maximal benefit and outcome.
During this phase the patient should be educated and familiarized with the surgical procedure and the phases of the rehabilitation process. This serves both to identify and anticipate any special problems or needs that the patient should incur. It will also reinforce the active role of the patient in his or her own long-term care. Recommendations may involve home planning, dental hygiene, and social planning.
In the era of managed care, many hospitals have formed preoperative educational classes for this purpose. In a group atmosphere, patients can begin to understand the rehabilitation process and formulate realistic goals and expectations. Informational pamphlets outlining all pertinent information are also helpful. Good preoperative care and communication between the team and the patient can guarantee a smooth transition through the postoperative process.
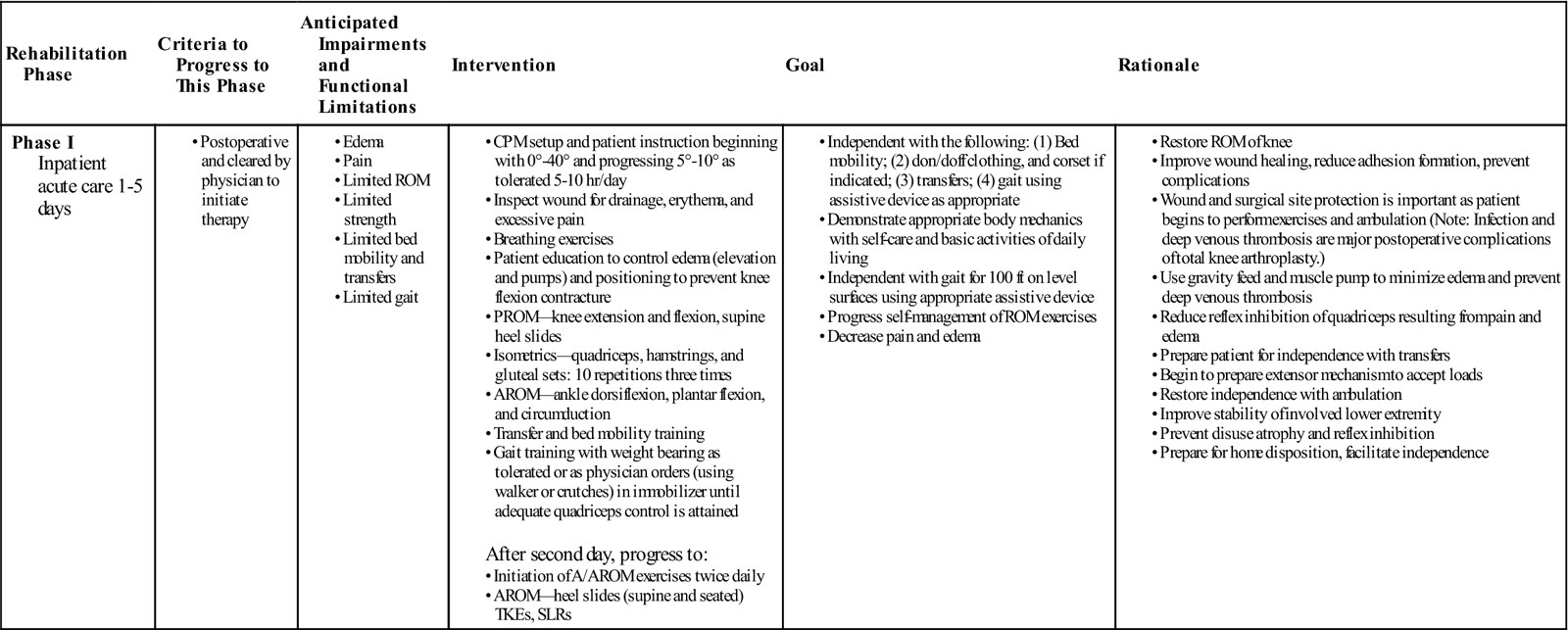
Phase I (Inpatient Acute Care)
TIME: 1 to 5 days after surgery
GOALS: Prevent complications, reduce pain and swelling, promote ROM, restore safety and independence (Table 27-1)
TABLE 27-1
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase I Inpatient acute care 1-5 days |
After second day, progress to: |
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
The goals for this initial period of rehabilitation are standard for any postoperative care. These pertain to the prevention of any possible complications. Medical considerations include (1) the prevention of infection, (2) the prevention of PE, (3) the prevention of DVT, and (4) the reduction of pain and swelling. Functional goals include (1) the promotion of ROM and (2) the restoration of safety and independence in activities of daily living (ADLs) and gait. The treatment plan is formulated with these specific considerations in mind.
Medical Considerations
Intravenous antibiotics are continued for 24 hours. DVT prophylaxis is initiated. This typically consists of antithrombotic pumps, Coumadin, low molecular weight heparin, or a combination of these treatments.
Monitoring the surgical incision for drainage, erythema, excessive pain, or swelling is continued throughout the patient’s stay. ![]() It is vital for the therapist to be aware of the signs and symptoms of wound infection, as well as other complications such as DVT or PE. If signs and symptoms of possible DVT develop, further knee ROM exercises should be restricted until diagnostic testing is completed and DVT is ruled out or an appropriate level of anticoagulation therapy is achieved. Specific signs, symptoms, and tests are discussed in the Troubleshooting section of this chapter. Any symptom must be brought to the immediate attention of the nursing staff and surgeon.
It is vital for the therapist to be aware of the signs and symptoms of wound infection, as well as other complications such as DVT or PE. If signs and symptoms of possible DVT develop, further knee ROM exercises should be restricted until diagnostic testing is completed and DVT is ruled out or an appropriate level of anticoagulation therapy is achieved. Specific signs, symptoms, and tests are discussed in the Troubleshooting section of this chapter. Any symptom must be brought to the immediate attention of the nursing staff and surgeon.
Functional Considerations
Restoration of functional ROM is essential for the success of TKA. Continuous passive motion (CPM) has been used and shown to be beneficial in regaining early mobility, but may not necessarily affect the final ROM achieved. CPM can be initiated immediately after surgery in the recovery room.
In one study, CPM patients were able to achieve 90° of flexion in 9.1 days versus their non-CPM counterparts, who required 13.8 days to reach the same goal.41,42 Unfortunately, CPM was found to be ineffective in the enhancement of knee extension.41,43
Many surgeons choose to begin immediate postoperative CPM.44,45 Because of wound concerns, others choose to begin on postoperative day 2.46 The beneficial effects of CPM include the improvement of wound healing,47 accelerated clearance of hemarthrosis,48 reduced muscle atrophy,49,50 reduced adhesion formation,51–54 reduction in the incident of DVT,55 decreased hospital stay,56 and decreased need for medication.57,58 Although excellent function and ROM can be achieved without the use of CPM, many surgeons and patients find that CPM is useful in reducing the frequency of complications after TKA.45,46,59
The protocol of CPM application varies in the literature. In general, the initial settings range from 0° to 25° to 40° of flexion. The range is then either increased 5° to 10° per day or to patient tolerance. CPM can be used from 4 to 20 hours per day. Its use is discontinued at the end of the acute hospital stay or when maximum knee flexion of the CPM machine is attained.
Another modality that has been shown to be useful in improving ROM and quadriceps strength is neuromuscular electrical stimulation (NMES). ![]() Mitigating quadriceps muscle weakness immediately after TKA using early NMES may improve functional outcomes, because quadriceps weakness has been associated with numerous functional limitations and an increased risk for falls.60 When NMES was added to a voluntary exercise program, deficits in quadriceps muscle strength and activation resolved quickly after TKA.61 In conjunction with the CPM, the application of NMES was shown to reduce extensor lag and the length of stay in the acute care setting.59
Mitigating quadriceps muscle weakness immediately after TKA using early NMES may improve functional outcomes, because quadriceps weakness has been associated with numerous functional limitations and an increased risk for falls.60 When NMES was added to a voluntary exercise program, deficits in quadriceps muscle strength and activation resolved quickly after TKA.61 In conjunction with the CPM, the application of NMES was shown to reduce extensor lag and the length of stay in the acute care setting.59
Exercises are taught at bedside beginning on postoperative day 2 or 3.44,62 Breathing exercises promote full excursion of the rib cage. Ankle ROM exercises (i.e., pumping, circumduction) along with instruction in proper elevation and positioning of the LE is encouraged (Fig. 27-17). The purpose of these exercises is to engage the muscle pump and passive gravity feed to decrease distal edema and avoid DVT.
Isometric gluteal sets, hamstring sets, and quadriceps sets are taught to prevent disuse atrophy and quadriceps reflex inhibition. Patients are encouraged to do these exercises independently, thereby increasing their active participation. It is recommended that this program be initially performed 10 repetitions every hour, and progressed to 20 repetitions, three times daily.62
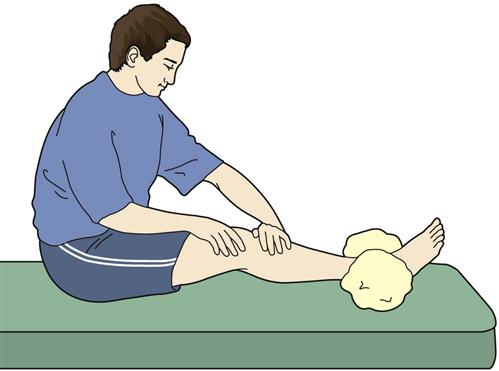
This exercise program is advanced throughout the first week of rehabilitation. Active assistive range of motion (A/AROM) exercises, such as seated heel slides or therapist-assisted knee flexion and passive knee extension, are performed to improve mobility. Straight leg raises (SLRs) and terminal knee extensions (TKEs) further strengthen the quadriceps muscles, thus improving the dynamic stabilizers of the knee.62,63 These exercises will become part of the home program once the patient is discharged.
The patient is also instructed in ADLs, such as dressing, bathing, transfers, reaching, and picking up items. Usually this comes under the supervision of the occupational therapist, although particularly in some smaller hospital settings, the PT may be responsible. The ADLs should be reviewed and performed until the patient can demonstrate safety and independence. The need for any special assistive devices is assessed and they are issued.
Progressive gait training begins with a walker or crutches on postoperative day 2 or 3,62 proceeding throughout the acute care hospitalization. Safety, balance, and patient independence are the primary goals for this intervention. Negotiating level surfaces, ramps, curbs, stairs, or other activities relevant to the patient are practiced.
Current clinical care pathways recommend that the average length of stay in the acute hospital should be approximately 3 to 4 days,62,64 with physical therapy sessions twice daily. The patient is discharged when deemed medically stable. Specifically, from a rehabilitation standpoint, the patient should be able to demonstrate 80° to 90° of motion, 30 transfer supine to sit and sit to stand independently, ambulate 15 to 100 feet, and ascend and descend three steps65 or as the home situation dictates.62 If unable to do these tasks or if any medical postoperative complications occur, then the patient may be transferred to an extended care unit (ECU) or skilled nursing facility for further care.
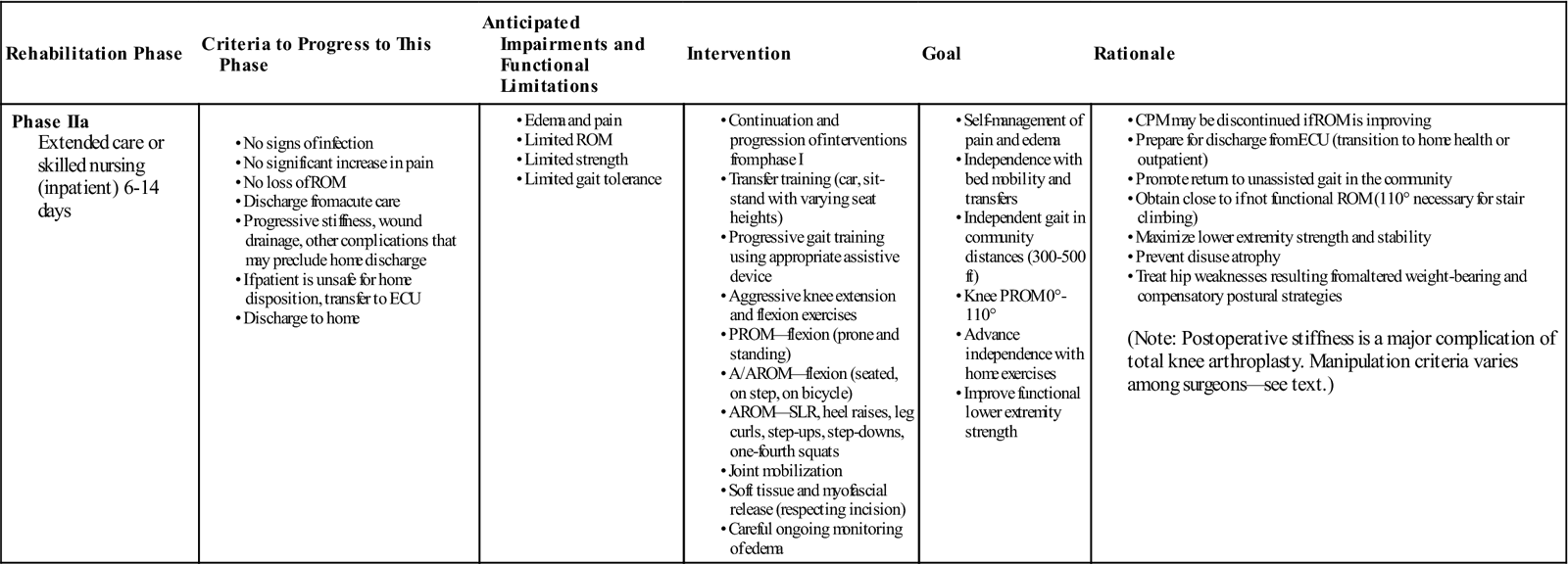
Phase IIa (Inpatient Extended-Care or Skilled Nursing Facility)
TIME: 6 to 14 days after surgery
GOALS: Prevent complications, reduce pain and swelling, promote ROM, restore safety and independence (Table 27-2)
TABLE 27-2
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IIa Extended care or skilled nursing (inpatient) 6-14 days |
|
(Note: Postoperative stiffness is a major complication of total knee arthroplasty. Manipulation criteria varies among surgeons—see text.) |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
The goals of this short-term rehabilitation phase are the same as for in the acute hospital. Treatment efforts continue, with physical therapy scheduled twice a day for a length of stay of approximately 3 to 7 days or until goals are met. Sometimes it becomes necessary to begin training family members or caregivers in assisting the patient during gait and transfers. Social services are often necessary to assist in planning home care needs or placement in long-term care facilities.
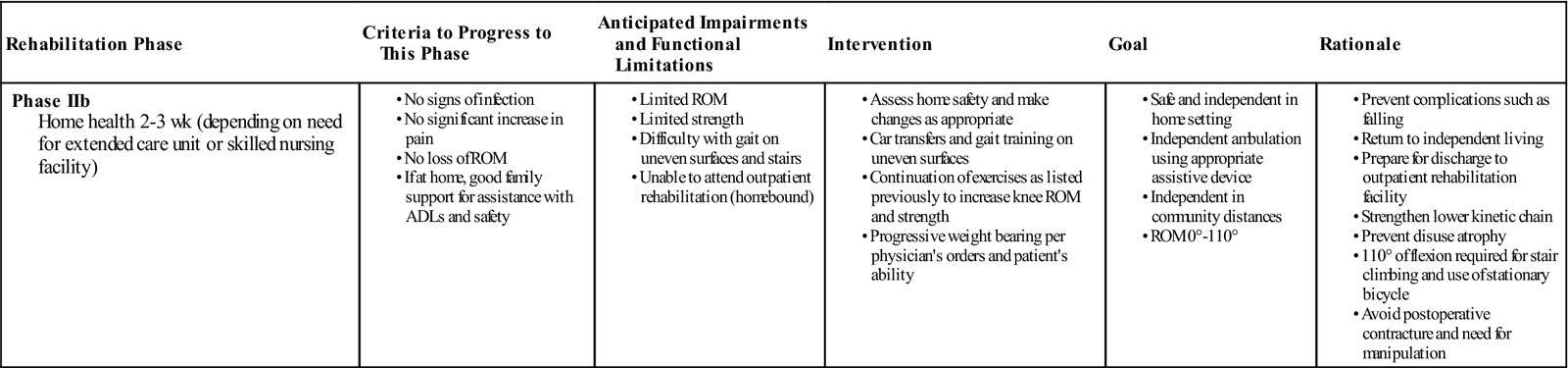
Phase IIb (Outpatient Home Health)
TIME: 2 to 3 weeks after discharge to home
GOALS: Become safe in home environment with transfers, gait, and most ADLs (Table 27-3)
TABLE 27-3
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Once discharged home, physical therapy treatments are reduced to three times weekly. During this phase of rehabilitation, the goals are expanded to facilitate functional ROM, endure safe and independent ADLs, transfers, and gait in the community. It is important for the therapist to assess the home for safety and make changes as appropriate. Recommendations may include but are not limited to the installation of nonskid rugs, safety rails and ramps, and the elimination of potential obstacles around the house.
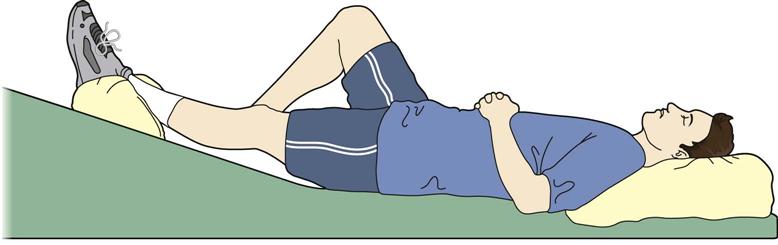
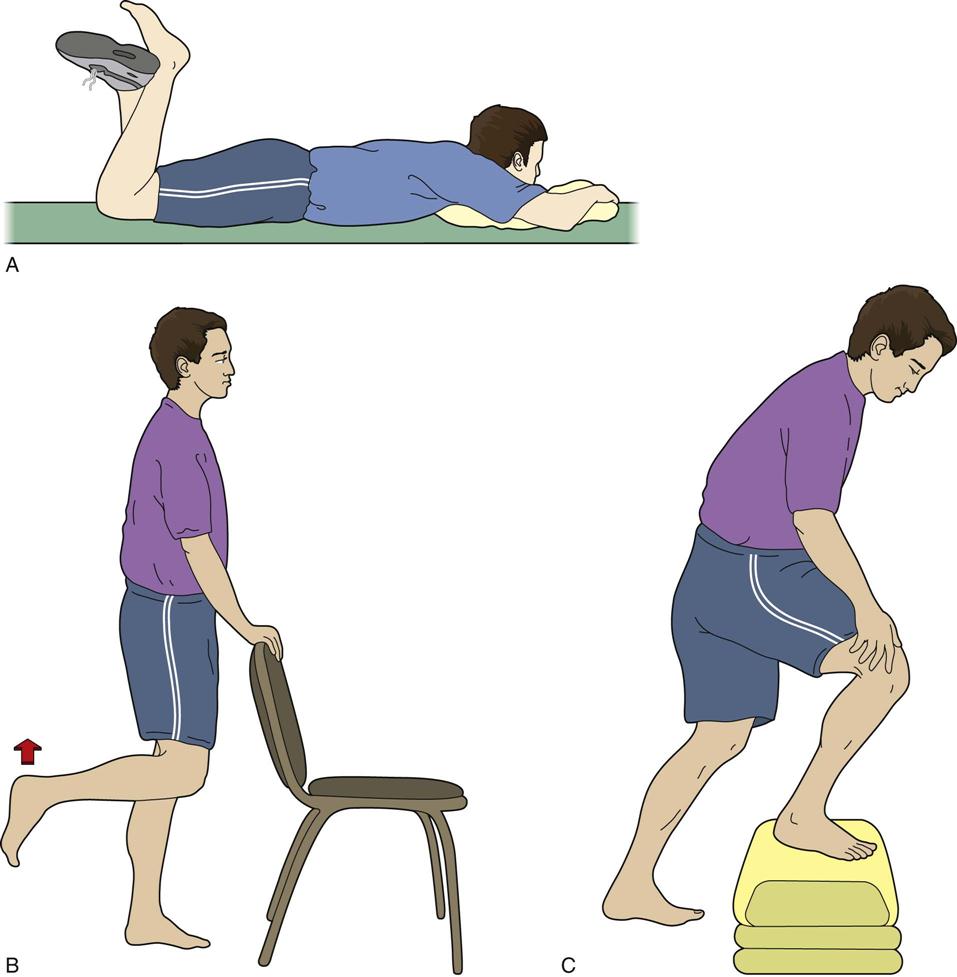
The home exercise program initiated in the inpatient setting is reviewed and refined. ![]() If extension mobility is lacking, then more aggressive knee extension exercises are instructed (Fig. 27-18). Postoperative pain may limit a patient’s ability to perform knee ROM exercises. If postoperative pain is not adequately controlled, then more effective pain management strategies should be considered, including inpatient pain management for very severe cases. If knee flexion is limited, then more progressive active and active assistive exercises are given (Fig. 27-19, A to C). Functional strengthening exercises are progressed in both the open- and closed-chain positions. Examples of closed-chain exercises are bilateral toe raises, sit-to-stand exercises (Fig. 27-20, A and B), one-quarter squats, and progressive step-ups and step-downs (Fig. 27-21, A and B). Closed-chain exercises have been shown to be highly effective in recruiting the vastus medialis oblique and vastus lateralis as compared with open-chain isometric exercises.66–68 Specific transfers in the home and the car are practiced. Progression in gait includes advancing the patient to crutches or a cane as his or her balance dictates. Ambulation on uneven, ramped, and outdoor surfaces is also reviewed. Home physical therapy is discontinued when the patient is no longer home bound.
If extension mobility is lacking, then more aggressive knee extension exercises are instructed (Fig. 27-18). Postoperative pain may limit a patient’s ability to perform knee ROM exercises. If postoperative pain is not adequately controlled, then more effective pain management strategies should be considered, including inpatient pain management for very severe cases. If knee flexion is limited, then more progressive active and active assistive exercises are given (Fig. 27-19, A to C). Functional strengthening exercises are progressed in both the open- and closed-chain positions. Examples of closed-chain exercises are bilateral toe raises, sit-to-stand exercises (Fig. 27-20, A and B), one-quarter squats, and progressive step-ups and step-downs (Fig. 27-21, A and B). Closed-chain exercises have been shown to be highly effective in recruiting the vastus medialis oblique and vastus lateralis as compared with open-chain isometric exercises.66–68 Specific transfers in the home and the car are practiced. Progression in gait includes advancing the patient to crutches or a cane as his or her balance dictates. Ambulation on uneven, ramped, and outdoor surfaces is also reviewed. Home physical therapy is discontinued when the patient is no longer home bound.
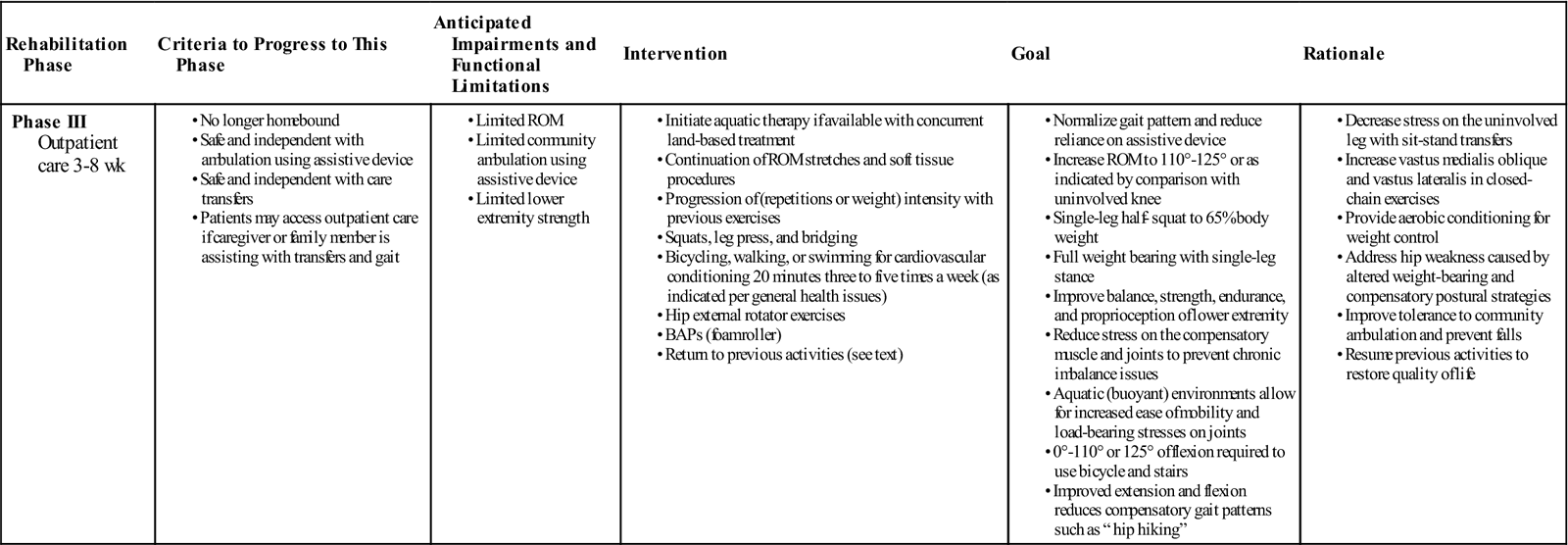
Phase III (Outpatient Care)
TIME: 3 to 12 weeks after surgery
GOALS: Normalize gait; reduce reliance on assistive devices; increase ROM; improve weight bearing, balance, strength, endurance, and proprioception (Table 27-4)
TABLE 27-4
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase III Outpatient care 3-8 wk |
|
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
BAPs, Balance and proprioception exercises; ROM, range of motion.
This phase of rehabilitation may begin on postoperative week 2 to 4 for the highly advanced and active patients or week 4 to 6 for those proceeding more routinely. The length of this stage is also dependent on factors such as the patient’s goals and potential functional abilities. The therapist should review the insurance benefits and explain to the patient all options for rehabilitation if there are any insurance limitations.
Common difficulties encountered with TKAs include patellar instability and lack of motion. Routine functional activities usually require ROM from 0° to 110°. ![]() Full extension is necessary to normalize the gait cycle69–71 and to facilitate quadriceps strength.64 Full normal ROM may not be a realistic goal for all patients. Postoperative ROM may be restricted, particularly if preoperative and intraoperative ROM is restricted. Stair climbing, sitting on a regular toilet seat or chair (17-inch height), and stationary bike riding requires 110° of knee flexion.72 If motion is limited, then a manipulation under anesthesia (MUA) may be warranted.
Full extension is necessary to normalize the gait cycle69–71 and to facilitate quadriceps strength.64 Full normal ROM may not be a realistic goal for all patients. Postoperative ROM may be restricted, particularly if preoperative and intraoperative ROM is restricted. Stair climbing, sitting on a regular toilet seat or chair (17-inch height), and stationary bike riding requires 110° of knee flexion.72 If motion is limited, then a manipulation under anesthesia (MUA) may be warranted.
General indications for a manipulation include less than 90° at postoperative week 673 or a progressive loss of flexion.73,74 ![]() Manipulation carries a small risk of fracture or other complications that may further compromise the outcome of the TKA.73 Relative contraindications to manipulation include severe osteoporosis and markedly restricted intraoperative ROM.
Manipulation carries a small risk of fracture or other complications that may further compromise the outcome of the TKA.73 Relative contraindications to manipulation include severe osteoporosis and markedly restricted intraoperative ROM.
Muscle strength and flexibility imbalances of the hip, knee, ankle, and foot can occur after knee injury or surgery.75–78 Reflex inhibition, faulty joint mechanics, altered gait, presurgical disuse atrophy, immobilization, or nerve injury have been shown to cause altered function of the muscles in the lower kinetic chain.79–82 Therefore it is essential for the PT to fully assess the entire LE for any loss of ROM or muscle strength and then develop a comprehensive rehabilitation program to address the findings.
A successful plan must include aerobic conditioning and, for overweight patients, weight reduction.83 Because obesity (defined as body mass index of more than 30) is often associated with OA, many patients with a TKA are overweight and deconditioned.
Increased forces such as those found in obesity can be the cause of wear to the weight-bearing surfaces.84,85 One study found that subjects were 12 to 13 kg (25 to 30 lbs) heavier and had 4% to 6% more body fat 1 year after TKA.120 On the other hand, after total joint replacement, many patients have been shown to resume routine walking and recreational activities that improved maximum oxygen consumption 1 year after the surgery.86 Nonimpact activities such as stationary bicycling, distance walking, and swimming are suggested for cardiovascular conditioning.85
Aquatic therapy programs have proven to be effective in rehabilitation of total joint replacements.87–89 The buoyant and warm environment can provide pain relief, increase circulation, and decrease weight bearing for the patient. Many can immediately start to work on ROM, strengthening, and normalizing gait without the assistance of a walker. The therapist can challenge the patient by progressing him or her from shoulder-deep water (approximately 24% weight bearing) to waist-deep water (50% weight bearing).
Changes in equilibrium after LE injuries have been cited in the medical literature.90–94 Balance activities and single-leg exercises should be incorporated to offset any compensatory postural changes that have occurred as a result of decreased weight bearing, altered gait, and pain. The use of rocker boards, half foam rollers, and other balance apparatuses can be helpful to improve the patient’s proprioception, balance, and postural control strategies.
With regard to long-term rehabilitation goals, the patient’s primary concern should be to maintain a pain-free functional activity level for as long a period as possible. With a TKA, certain restrictions in activities are warranted. Generally those recreational activities and sports that involve high repetitive compression or impact loading are not encouraged because of the possibility of loosening or osteolysis of the joint implant.76,95,96 Joint forces at the TF interface are 1.5 to 4.0 times body weight when walking,38 1.2 times body weight when cycling, and increase to 2.0 to 8.0 times body weight when running.97 Patellofemoral joint forces also show comparable increases. During walking, these are 0.5 times body weight98 and with running they increase to 3.0 to 4.0 times body weight.99 Failures of TKAs may occur because of mechanical loosening or wear of the implant. Therefore patients with joint replacement are encouraged to participate in those activities that maintain cardiovascular fitness while subjecting the implant to reduced impact-loading stresses.
In the gym, using a treadmill (walking only), ski machine, stair-climbing machine, elliptical machine, or stationary bicycle is acceptable. Outdoor sports that are allowable include golfing, hiking, cycling, cross-country skiing, swimming, fishing, hunting, scuba diving, sailing, and occasional light doubles tennis.85 Baseball, basketball, football, martial arts, parachuting, singles tennis, racquetball, running, soccer, and volleyball are generally discouraged.
Rehabilitation For Minimally Or Less Invasive Surgery Total Knee Arthroplasty
Minimally invasive surgery is one of the most recent advancements in primary TKA. Because of the smaller incision, quadriceps muscle and tendon sparing, less intraoperative blood loss, deletion of patella eversion procedure, and reduction of pain, rehabilitation can occur on an accelerated timeline. Shorter hospital stays; less overall use of analgesics; and more rapid return of ROM, strength, and function are the benefits.100–102
In one observation, hospital length of stay was decreased from 7 days to 2 to 3 days.89 Physical therapy can begin on postoperative day 1, with ROM, ADLs, and gait training. It has been shown that the average MIS-TKA patient regained 90° of flexion within 3.2 days after surgery.101
Depending on the patient’s age, motivation, previous function, and fitness level, gait and ADL training is progressed immediately. Early studies after MIS-TKA show that patients used crutches or walkers for 1 to 2 weeks, then advanced to a cane for 1 to 2 weeks thereafter. Many ambulated without the use of an assistive device 4 weeks after surgery. Most were independent with bed and bath transfers and all other ADLs after 2 weeks. Many were climbing stairs at 1 month and descending stairs at 2 months. Driving is allowed at 3 to 6 weeks.
As with traditional TKAs, light-impact activities such as hiking, swimming, and cycling are permitted when comfortable. High-level activities such as running and singles tennis remain unadvisable in the long term.100,103
MIS-TKA outcomes show that a large percentage of patients were satisfied with the cosmetic benefit as well as the quicker recovery and excellent early function. Patients achieved discharge goals 18% faster than those who had traditional TKA. Good function and knee scores were achieved after 3 to 4 months as compared with the usual 1-year result.100,101,103
Troubleshooting
Although the treatment guidelines given here provide a framework for postoperative rehabilitation of TKA, they should not be used as strict protocols. In fact, modifications are expected because each patient has individual needs, abilities, and goals. Continual reassessment of the patient’s status and subsequent alterations in the treatment plan are necessary to achieve optimal results. The therapist must have the necessary abilities and skills to anticipate any potential complications and implement appropriate changes in the treatment plan. The following section describes additional procedures and modalities that can be used to refine the therapy to meet the patient’s individual needs.
Medical Complications
During the initial stage of postoperative care, the most serious complications are wound infection, PE, DVT, persistent joint effusion, and lymphedema. ![]() As previously stated, the therapist must know all symptoms and signs that may indicate any of these medical problems. Any of these complications can severely deter rehabilitation progress in terms of time frame and overall prognosis.
As previously stated, the therapist must know all symptoms and signs that may indicate any of these medical problems. Any of these complications can severely deter rehabilitation progress in terms of time frame and overall prognosis.
Wound Infection
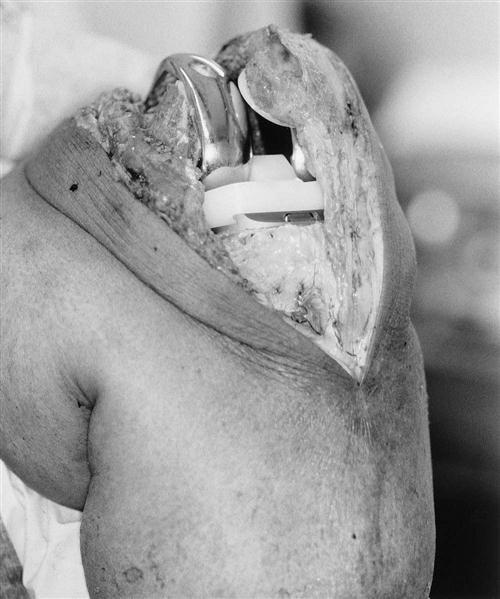
![]() Systemic signs of a potential wound infection include patient complaints of fever or chills. Knee pain can be severe and not relieved with rest. Knee flexion can increase skin tension. If skin necrosis develops, then ROM exercises should be limited until adequate soft tissue healing has occurred. Other symptoms of infection include increased warmth and erythema about the surgical site and wound drainage.104 Wound dressings should be checked and changed daily until the staples are removed.72 Although uncommon (less than 1%), infection remains a serious problem, usually requiring additional surgery and intravenous antibiotic therapy.
Systemic signs of a potential wound infection include patient complaints of fever or chills. Knee pain can be severe and not relieved with rest. Knee flexion can increase skin tension. If skin necrosis develops, then ROM exercises should be limited until adequate soft tissue healing has occurred. Other symptoms of infection include increased warmth and erythema about the surgical site and wound drainage.104 Wound dressings should be checked and changed daily until the staples are removed.72 Although uncommon (less than 1%), infection remains a serious problem, usually requiring additional surgery and intravenous antibiotic therapy.
Methods to reduce the risk of infection include proper preoperative skin preparation; prophylactic antibiotics and local antibiotics in cement, particularly for immune-compromised patients (e.g., rheumatoid arthritis, diabetes mellitus, human immunodeficiency virus, cancer); and minimizing the length of surgery and tissue trauma.105
Pulmonary Embolus
![]() PE is a potentially fatal, but rare, complication. PE can be life threatening and requires prompt diagnostic testing and treatment. Patients at risk for PE are those who may have proximal LE DVT. Proximal LE DVTs are located at or proximal to the trifurcation of the popliteal vein and considered the more dangerous form of LE DVT because the thrombi are larger than those associated with calf DVT and are more likely to embolize.106
PE is a potentially fatal, but rare, complication. PE can be life threatening and requires prompt diagnostic testing and treatment. Patients at risk for PE are those who may have proximal LE DVT. Proximal LE DVTs are located at or proximal to the trifurcation of the popliteal vein and considered the more dangerous form of LE DVT because the thrombi are larger than those associated with calf DVT and are more likely to embolize.106
Clinical signs of PE include tachycardia, distended cervical veins, hypotension, and chest pain. Pulmonary symptoms include tachypnea, rales, wheezing, and pleural effusion.104 Sudden dyspnea is the most frequent symptom. Pleuritic pain is also common in patients with severe embolization. If any of these signs or symptoms occur, then the PT should immediately notify the nursing staff and physician.
Deep Venous Thrombosis
Because of decreasing lengths of hospitalization, patients are seen in outpatient settings with elevated risks of DVT. Significant signs and symptoms of DVT include complaints of pain and swelling in the involved extremity, calf tenderness, and a positive Homans sign. Specifically, the patient may report a dull ache, tight feeling, or frank pain in the calf or entire leg. The signs and symptoms include slight swelling in the involved calf; distention of the superficial venous collaterals; tenderness, induration, or spasm of the calf muscles, with or without pain, produced by dorsiflexion of the foot (Homans sign); warmth of the affected leg when both legs are exposed to room temperature; and a slight fever or tachycardia.92 However, DVTs are also often asymptomatic. When signs and symptoms of possible DVT develop, further ROM exercises should be restricted until definitive diagnostic testing is performed.
Additionally, improved assessment of risk for DVT can be obtained by the use of a clinical decision rule (CDR). A series of studies found that patients could be categorized into low-, moderate-, and high-risk groups based on their CDR scores. The use of the CDR may aid in improving the accuracy of probability estimates of DVT and subsequent referral decisions.106
Persistent Joint Effusion
A patient sometimes complains of postoperative stiffness, pain, and swelling. The patient should be reassured that this usually decreases within the first few weeks after surgery. ![]() Persistent joint effusion can forestall the rehabilitation process. Knee ROM exercises can be continued if an effusion is present, but the effusion may limit motion and should be treated with ice before and after exercise. Soft tissue mobilization is an effective procedure that can enhance muscle recovery and reduce soreness after intense physical activity.107,108 Increasing blood flow may increase oxygen delivery to the injured tissue, enhance healing, and restore homeostasis.109 If edema, swelling, and inflammation are significant factors in muscle soreness sensation,110,111 then massage may be able to reduce soreness in the involved muscles. Persistent effusion may be aspirated, not only to relieve pressure and stiffness but also to rule out an indolent infection.
Persistent joint effusion can forestall the rehabilitation process. Knee ROM exercises can be continued if an effusion is present, but the effusion may limit motion and should be treated with ice before and after exercise. Soft tissue mobilization is an effective procedure that can enhance muscle recovery and reduce soreness after intense physical activity.107,108 Increasing blood flow may increase oxygen delivery to the injured tissue, enhance healing, and restore homeostasis.109 If edema, swelling, and inflammation are significant factors in muscle soreness sensation,110,111 then massage may be able to reduce soreness in the involved muscles. Persistent effusion may be aspirated, not only to relieve pressure and stiffness but also to rule out an indolent infection.
Lymphedema and Total Knee Arthroplasty
Lymphedema is abnormal swelling caused by the presence of excess lymphatic fluid within the tissues. This swelling occurs when the lymphatic system malfunctions or is damaged from a decrease in developmental transport capacity of the lymph vessels, trauma, surgery, radiation, or infection. ![]() Although lymphedema is a minor complication, persistent swelling can occur.112
Although lymphedema is a minor complication, persistent swelling can occur.112
Lymphedema that is present before surgery should be minimized with use of support stockings and, if necessary, diuretic medications. If lymphedema develops after surgery, rehabilitation can be continued, but compressive stockings should be used to limit the amount of swelling. Because abundant lymph vessels are found at the medial aspect of the knee, trauma to this area or surgery can lead to lymphedema. Lymph flow swelling in this area of bottleneck occurs as a result of tissue trauma in this area. A patient who has venous insufficiency and undergoes TKA also has an increased risk of developing lymphedema after surgery.112,113
In treating lymphedema, therapy consists of manual lymph drainage, special compressive bandaging, exercise, and skin care. With proper treatment, edema can be reduced by as much as 60% and in some cases up to 74%. Once the limb is reduced, the patient graduates from the compressive bandages to a proper compression class stocking. Antiembolism stockings, such as TED hose stockings114 (12 to 20 mm Hg), are worn postsurgery as DVT prophylaxis from bed rest, mild edema, or mild varicosities. Class I (20 to 30 mm Hg) are used for mild lymphedema, mild venous insufficiency, moderate varicose veins, or DVT prevention in individuals with clotting disorders. Higher grades of class II to IV (30 to ≥ 50 mm Hg) are available for individuals with moderate to severe lymphedema or cardiovascular insufficiency or for prevention of DVT in postthrombotic syndrome.113,115
Functional Complications
Peroneal Nerve Neuropraxia
Peroneal nerve neuropraxia may arise more frequently in patients with (1) flexion contractures associated with valgus deformity, (2) those who had epidural anesthesia for postoperative control of pain or previous laminectomy, and (3) those who had a previous proximal tibial osteotomy.116
In peroneal nerve neuropraxia, the common peroneal nerve and its branches are tethered at the fibular neck. Clinical findings of nerve entrapment or injury include local tenderness around the fibular neck with pain, diminished sensation, or paresthesia radiating over the lateral surface of the lower leg and the dorsum of the foot.11,117 Nerve conduction velocity testing and electromyographic studies will be positive for neuropathic dysfunction in the motor distribution of the common peroneal nerve distal to the injury or entrapment site at the fibular neck. Clinically, the patient displays an inability to walk or stand on the heel because of the weakness of the ankle dorsiflexors. Additionally, instability when attempting toe walking results from the muscle imbalance and associated sensory abnormalities at the ankle joint. The muscles affected are those in the anterior and lateral compartments of the leg.
When the superficial peroneal nerve is compressed, a decrease in sensation is noted over the dorsum of the foot, with the exception of the first web space. The involvement of the deep peroneal nerve produces a diminution of sensation in the first web space of the foot and affects the muscles of the anterior compartment, including the extensor hallucis brevis and the extensor digitorum brevis.
Complete common peroneal nerve palsy results in a severely affected gait pattern. Without an ankle-foot orthosis, the patient suffers from a foot drop with associated steppage gait in profoundly affected cases or foot slapping in milder ones. The ankle is unstable and vulnerable to ankle inversion sprains. The functional result of partial peroneal nerve palsy depends on which nerve components are the most affected. Loss of the peroneal muscles in the lateral compartment results in a chronically inverted foot, with weight bearing occurring more laterally than normal and invariably affecting the position and stability of the foot and ankle throughout the stance phase of the gait cycle. Loss of the anterior compartment muscles, especially the tibialis anterior, affects the entire gait cycle. The loss of the dorsal intrinsic muscles of the foot has a relatively minor effect on basic weight-bearing functions.
Physical therapy interventions for nerve entrapment include examination of the joint mechanics of the proximal and distal tibiofibular joints and determination of the specific muscle weakness and sensory loss. Manual techniques include joint mobilization as appropriate, facilitation of the recruitment of the affected muscles, and dural nerve root stretches for the sciatic and peroneal nerves.
Muscle Imbalance
A detailed evaluation of the entire lower kinetic chain is necessary for the successful treatment of patients with TKA. Altered gait, faulty mechanics, and muscle imbalances have more than likely existed before the TKA. These factors contribute greatly to the eventual surgical outcome. Most of the present understanding regarding muscle imbalances and neuromotor retraining comes from the work of Janda,118,119 Lewit,120 and Sahrmann.82 Muscle imbalance is a multifactorial problem and can be highly complex. In simplistic terms, the result of muscle imbalance is that the tight muscles become tighter, weak muscles become weaker, and motor control becomes asymmetric.121
According to Janda,118,119 muscle balance is continually adapting the body’s posture to gravity. When an injury occurs, faulty posture and weight bearing alter the body’s center of gravity, which initiates mechanical responses requiring muscle adaptation. Change in the mechanical behavior of a joint causes neuroreflexive alteration of muscle function through aberrant afferent mechanoreceptor stimulation of articular reflexes.122 Postural-tonic muscles respond to dysfunction with facilitation, hypertonicity, and shortening. Dynamic-phasic muscles respond with inhibition, hypotonicity, and weakness. In the lower quadrant, Janda118,119 identified a common pattern of muscle imbalance. Hyperactive muscles include the iliopsoas, rectus femoris, tensor fascia latae, quadratus lumborum, the thigh adductors, piriformis, hamstrings, and the lumbar erector spinae musculature. Muscles that display inhibition or reflexive weakness include the gluteus maximus, medius, and minimus; quadriceps (vasti); rectus abdominis; and external and internal obliques. Sahrmann,82 Dorman and associates,123 and Bullock-Saxton, Janda, and Bullock,79 similarly identified weakness in the entire LE in the presence of knee dysfunction.
Quadriceps weakness, especially in the early stages of TKA rehabilitation, must be addressed. Failure to adequately address the chronic muscle impairments has the potential to limit the long-term functional gains that may be possible following TKA. Postoperative rehabilitation addressing quadriceps strength should mitigate these impairments and ultimately result in improved functional outcomes.124 These impairments with significant worsening of knee ROM, quadriceps strength, and performance on functional tests occurred 1 month after surgery. Quadriceps strength went through the greatest decline of all the physical measures assessed and never matched the strength of the uninvolved limb. The high correlation between quadriceps strength and functional performance suggests that improved postoperative quadriceps strengthening could be important to enhance the potential benefits of TKA.125 A knee immobilizer may be needed for ambulation in the hospital setting if quadriceps strength is not great enough to stabilize the knee. Biofeedback or NMES can be beneficial in “jump starting” the recruitment of the quadriceps. In cases of patella instability, quadriceps strength should be restored as soon as possible. Soft tissue work, friction massage, and assisted stretches to the iliotibial band may be beneficial.
Faulty joint mechanics at the hip, knee, and ankle affect the overall surgical result. A study by Dorman and associates123 found that inhibition or facilitation of the gluteus medius is influenced by the position of the sacroiliac joint. An anterior rotation (an apparent long leg) manifested a significantly weaker muscle than one in posterior rotation (an apparent short leg). Exercises that address gluteus medius weakness include hip abduction and lateral hip rotation (Fig. 27-22).
Altered ankle movements and instability disturb the overall sense of balance and influence gait safety accordingly. Joint mobilization techniques to correct associated dysfunctions in the joints of the LE can be helpful. A stiff knee can be helped with contraction and relaxation techniques to the muscles that may be guarding or fatigued.73,87
Kneeling
Kneeling is an important functional activity frequently not performed after knee replacement, thus affecting a patient’s ability to carry out basic daily tasks. Kneeling ability before surgery was poor in osteoarthritic patients but improves with knee arthroplasty surgery.126 Despite no clinical reason preventing kneeling, many patients fail to resume this activity. Patients avoided kneeling because of uncertainties or recommendations from third parties (doctors, nursing staff, or friends). Inability to kneel may also be caused by scar position, skin hypoaesthesia, restricted range of flexion, involvement of other joints, or pain. The solution to this problem is to incorporate kneeling activity with PT intervention at 6 weeks after surgery because there was significant improvement in patient reported kneeling 1 year after surgery in patients who received kneeling intervention.125,127
Outcomes
TKA is a method to improve “quality” of life. This is accomplished primarily through pain relief, resulting in increased functional mobility. Results are generally reported using the Hospital for Special Surgery128 or the Knee Society129 rating systems. Haas’ patients24 from the Hospital for Special Surgery who had MIS showed favorable outcomes (Table 27-5) compared with the traditional TKA. They were able to do an SLR on postoperative day 1, walk without a cane on postoperative day 8, alternate stair climbing by week 6, and perform full knee ROM by week 8.
TABLE 27-5
Functional Outcomes—Minimally Invasive Surgery Compared with Traditional Total Knee Arthroplasty
< ?comst?>
< ?comen?>< ?comst1?>

< ?comst1?>
< ?comen1?>
It should be noted that despite extensive rehabilitation efforts, various studies have shown outcomes of diminished functional capacity. Self-report measures of perceived abilities indicate that at 1 year after TKA, most individuals have regained 80% of normal function. However, stiffness and pain occasionally still remained a problem.93,130,131 Lingard’s study39,132 showed that the most improvement in pain reduction and functional improvement was made in the first 3 months after TKA, with little change after 12 months.133,134 Walsh and associates83 also showed that at 1 year after TKA, little pain was noted during activities of walking, stair climbing, and concentric muscle testing. However, after implantation of a total knee prosthesis, it only partly improved performance from sit to stand movement. After TKA, patients were able to fully load their operated leg, but they could not generate enough knee angular velocity during rising.135 Additionally, the minimum 96° of hip and knee flexion is required at initial sit to stand movement. Without effective hip and knee joint extension post lift off from the chair, people were not able to do this task.136 Sled and associates found in their study that following implementation of an 8-week home strengthening program for the hip abductor muscles, participants with knee OA had a decrease in knee pain and demonstrated a significant improvement in hip abductor strength and improved function in sit to stand.137 Other functional deficits, including slower walking speeds for both males and females, were reported (62% and 25% decrease at normal pace and 31% and 6% decrease at fast pace, respectively). Clinical relevance points to the fact that 17% of these individuals were not able to cross safely at a typical city intersection. Other recent studies have shown slower sit-to-stand and up-and-go tests,138 quadriceps weakness,46,47 and smaller girth circumference47 for individuals at greater than 1 year after surgery. Lastly, the first study that demonstrated patients with TKA had impaired balance and movement control because of lack of exercise interventions for this problem was done by Piva and associates. In this study, exercise programs that target balance and movement control improved functional performance, stiffness, and pain in patients after TKA.139
With these studies in mind, the development and implementation of a well-designed, comprehensive treatment plan is paramount. Addressing the entire lower quadrant with an appropriate exercise program will enhance the course of rehabilitation and ensure a more successful outcome.
Clinical Case Review
1John is 69 years old. He had a left TKR 8 days ago. He is now semireclined in his hospital bed. When the therapist helps him out of bed during transfer, John complains of feeling light-headed. He usually requires a few moments for his head to clear, but today he needs a little more time to adjust. Gait training is initiated, and suddenly John has difficulty breathing. What do these symptoms indicate?
Rapid heart rate, hypotension, and dyspnea are signs of a possible PE—a potentially fatal complication. Cardiovascular signs may include tachycardia, distended jugular veins, hypotension, and chest pain. Pulmonary symptoms include tachypnea, rales, wheezing, and pleural effusion. Sudden dyspnea is the most common symptom.
2Randy is 52 and had a TKA 2 weeks ago. He presented for his initial evaluation with −30° extension and 75° flexion. What should be considered and addressed during his initial evaluation?
Prior functional level and preoperative ROM
Prior response to surgery and potential to develop adhesions
General health status (e.g., diabetes, PVD, CHF).
Current state of incision (infection potential) and edema (girth measurements)
Discussion with his home health PT will also give you an indication of his current response to treatment thus far.
All of the above are important to consider when evaluating and making a treatment plan.
3What exercises should be emphasized with Randy?
Passive stretches (prone and supine hangs) should be performed regularly and, as appropriate and pain allows, weight should be added to the ankle and knee to provide increased compliance into extension. Passive flexion stretches should include supine heel slides with a towel or strap to allow the patient to provide overpressure as able. Also supine wall slides (with weight as appropriate). Duration of stretches should be progressed as able taking into account the response of the knee to stretches (minimal—no increase in residual pain, edema, or weight-bearing tolerance).
4Sharon is a 60-year-old patient who had TKA 4 weeks ago. She complains that her operative leg is shorter than the other leg. No preoperative leg length difference was noted, and the physician noted that there were no relative changes made that would account for decreased leg length. What possible explanation could there be for this?
Upon further evaluation it was found that the patient had a posteriorly rotated ilium, creating an apparent leg length difference. Faulty joint mechanics at the hip, knee, and ankle affect the overall surgical result. Manual techniques and exercises were used to address the posterior rotation. Exercises that address gluteus medius weakness include hip abduction and lateral hip rotation (see Fig. 27-22).
5Noreen is a 55-year-old patient who had TKR 7 weeks ago. Her pain levels have prevented ROM gains beyond −8° to 95°. Her preoperative ROM was −5° to 120°. Her ROM in the last week has plateaued and may have even decreased. What should the therapist’s next course of care be?
A phone call to the orthopedist is warranted to explore if she is appropriate for an MUA. After consultation with the physician, an MUA was scheduled and performed. The patient returned to therapy and gained full extension and 125° of flexion. The patient’s post-MUA physical therapy should also include taking medication before her treatments to manage pain and maintain and improve the gains in ROM made from the MUA.
6Gemma is 55 years old. She had severe degenerative joint disease in her right knee and underwent a TKR 8 weeks ago. She says the pain around the knee has considerably decreased. However, she complains of pain around the area of the fibular neck. The area also is sensitive to palpation. Gemma has intermittent pain radiating down the lateral surface of the lower leg. What is the probable cause of these symptoms?
The peroneal nerve travels around the fibular neck. The symptoms described indicate peroneal nerve irritation. On further investigation, the PT noted altered sensation over the dorsum of the foot, with the exception of the first web space. In addition, right dorsiflexion and eversion strength both equaled 4/5 to 5/5 (5/5 is “normal” strength on a manual muscle test [MMT]). Normal strength was demonstrated throughout the left LE. Dural nerve root stretches for the sciatic and peroneal nerve were initiated. After the first treatment using nerve tissue mobilization to target these nerves, strength returned to normal for dorsiflexion and eversion. After three treatments using dural mobilization, the pain was decreased by 90% and the sensation tested normal.
7Ginny is 12 weeks postoperation, and while she demonstrates excellent ROM, she complains of medial knee pain with single-leg squat (SLS) exercises. What can be done to address this issue?
Up to this point in her rehabilitation she had not complained of any pain until SLS resistance increased. In observing her technique with SLS, it was noted that she began to allow her hip to internally rotate, causing a “valgus” collapse at the knee. With reinstruction in technique and hip abduction/external rotation strengthening, her symptoms subsided. It is important that strengthening exercises continue to progress inclusive of the entire LE.140
8Nancy is 3 months postop and continues to complain of knee pain that has not improved and limits her weight bearing tolerance. What should be considered as a potential explanation of her symptoms?
Chronic knee pain is unfortunately a side effect for some patients. Differential diagnosis of continuing extra articular knee pain should consider:
Neurologic (lumbar origin, neuroma, complex regional pain syndrome)
Vascular claudication
Musculoskeletal (Hip arthritis, tendinitis, bursitis, stress fracture, and periprosthetic fracture)141