Chapter 18 Total Body Irradiation
History of Total Body Irradiation
Only a decade after Roentgen described the “x ray,” German biophysical engineer Friedrich J. Dessauer1,2 first described a “new technique of radiotherapy” that involved homogenous irradiation of the entire body. In his initial report describing the technique in 1905, he proposed irradiating a supine patient using three simultaneously active, low-voltage roentgen-ray sources (Fig. 18-1). In 1907, Aladár Elfer,3 a medical professor in Hungary, reported his experience using a TBI technique that spared the head in three patients with leukemia. Although there is a paucity of data regarding the early use of the technique, some have speculated that untoward hematologic toxicity probably limited its application.4
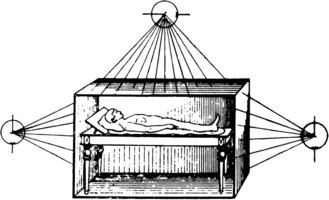
Figure 18-1 Diagram demonstrating the total body irradiation technique proposed by Dessauer in 1905.
Reprinted from Wetterer J (ed): Handbuch der Röntgentherapie nebst Anhang. Die Radium Therapie. Leipzig, Otto Nemnich, 1908.
Early success using TBI to treat hematopoietic and lymphoid malignant tumors in Europe (there named the Teschendorf method) prompted development of the technique in the United States.5–7 Arthur C. Heublein, in collaboration with Gioacchino Failla, is credited with the development of the first TBI unit in North America, located at Memorial Hospital in New York City. In the United States, the technique became known as “Heublein therapy.”8 A specially constructed treatment ward was designed to treat four patients at extended distance (5 to 7 m) simultaneously at an exposure rate of 0.7 roentgen (R)/hour, for about 20 hours/day, typically over 1 to 2 weeks, using a 185-kV x-ray tube at 3 mA, with a 2-mm copper filter. The goal was to deliver 25% of the erythema dose (750 R).
Remarkably, in Heublein’s initial report, no hematopoietic toxicity was noted with this treatment schedule. Seven of 12 patients (58%) with advanced lymphomas and leukemias and 2 of 8 patients (25%) with metastatic breast, melanoma, and kidney cancers were noted to demonstrate some form of improvement after treatment.9,10 A later report of the experience with 270 cancer patients from Memorial Hospital treated with TBI between 1931 and 1940 confirmed that the technique was more successful in patients with hematopoietic and lymphoid cancers compared with those with carcinomas or sarcomas, for whom it was ineffective. The authors emphasized that the technique was safe if doses were prescribed cautiously. They did not recommend exposures greater than 300 R and noted hematopoietic and gastrointestinal toxicity with exposures as low as 50 to 100 R.8
In the early 1940s, World War II prompted an initiative to develop nuclear weapons, called the Manhattan Project. Part of this endeavor sponsored research into the human biologic response to ionizing radiation, including TBI. The military’s interest in TBI was primarily to help understand human tolerance for radiation exposure during occupational duties and warfare and to develop radiation biodosimetric assays. Several research studies coordinated through the Manhattan Project were initiated in patients with advanced cancers,11–13 as well as patients with benign diseases.13 For example, studies of dose escalation, radiation biologic dosimetry, and cognitive and psychomotor function were carried out at the M.D. Anderson Hospital for Cancer Research.14 A detailed report of 30 patients treated at the maximum exposure level (200 R) in the initial study concluded that side effects primarily consisted of nausea, vomiting, and myelosuppression, and that intervention was necessary in 10% of patients treated with this dose of TBI.15 At Baylor University College of Medicine, studies using 25 to 250 R of TBI with 250 kV to 2 MV photons were performed to find a biologic dosimeter, as well as to study acute effects of radiotherapy.16 The Sloan-Kettering Institute for Cancer Research also participated in similar studies of at least 20 patients, although the results were never published. The military conducted similar studies at the Naval Hospital in Bethesda, Maryland, and reported palliation of patients with radiosensitive diseases treated with fractionated TBI.17 ![]()
The most recent research study of TBI sponsored by the U.S. Department of Defense was at the University of Cincinnati. It focused on identifying biochemical markers in the urine that predicted response to TBI. Later, studies of the neuropsychiatric effects of TBI were initiated. Ultimately, only results regarding the palliation of advanced cancers were reported.18 Patients with advanced metastatic radioresistant malignant tumors, for whom chemotherapy was unavailable, were often treated with TBI in the absence of any clear anticipated benefit. Patients treated with TBI in this manner were included in research studies, often without consenting to participate. The ethics of this practice was called into question by a report written in 1995 by the U.S. Department of Energy’s Advisory Committee on Human Radiation Experiments,19 which may have contributed to the public’s general uneasiness regarding radiation.20
TBI was not only used in malignant diseases, but it was considered the critical immunomodulator in the first successful solid organ transplant. In 1959, a kidney was successfully transplanted between dizygotic twins after TBI at exposures of up to 450 R (given to the recipient).21 Around the same time in France, successful kidney transplants after TBI were being reported.22,23 Of the first seven patients who underwent kidney transplant following TBI and/or pharmacologic immunomodulation worldwide between 1959 and 1962, the two who did not experience kidney failure were treated with TBI alone (without chemical immunosuppression) before transplant, and each survived for more than 20 years after transplant.21,22,23 However, successful preclinical studies with pharmacologic therapy prompted the use of chemical immunosuppressants (corticosteroids, 6-mercaptopurine, and azathioprine) for solid organ transplantation after 1963.24
With an increased understanding of the human response to TBI and a rapidly growing body of preclinical in vivo studies of TBI, therapeutic protocols were developed to maximize benefit in patients with malignant diseases. In 1957, Nobel laureate E. Donnall Thomas25,26 first reported the use of bone marrow infusion in humans following whole body irradiation or chemotherapy, and less than 1 year later he published his experience in using TBI with exposures up to 600 R followed by bone marrow transplantation. In the series of the first five patients with leukemia treated with TBI, who then received intravenous infusion of normal donor marrow, Thomas and colleagues26 noted the difficulty of acute myelosuppression and resultant hemorrhage and infection during the period leading up to engraftment. The report also commented that low dose rates (delivery over 2 to 3 days) appeared preferable to higher dose rates, for metabolic and immunologic reasons. In addition, patients receiving 200 to 300 R fared better than those receiving 400 to 600 R. The problem of delivering an adequately homogenous dose was raised, and suggestions about using higher-energy photons were proposed. Thomas and associates27 later reported on syngeneic bone marrow transplantation in two children after 850 to 1140 R was delivered in a single fraction over 22 to 25 hours, using cobalt-60 (60Co) sources. The authors concluded that 1000 R of TBI did not produce “troublesome” acute radiation sickness, did produce remission of leukemia, but did not cure the disease. The first report of successful cure of a patient with leukemia with allogeneic transplantation after TBI was reported in 1969. The technique involved opposed 60Co sources, which operated at 5.8 R/minute, to a total exposure of 1620 R, calculated to be 954 rad at midline. With appropriate supportive care, no major acute radiation sickness was noted, but the patient died of overwhelming cytomegalovirus infection, without evidence of leukemia.28
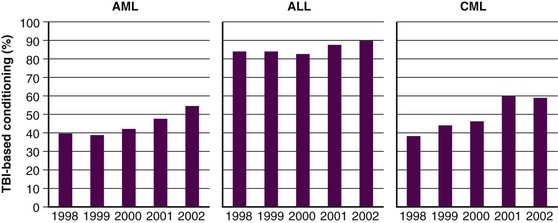
Over the next several years, techniques of combining chemotherapy and TBI were developed and refined, with promising results.29,30 Success in the treatment of advanced leukemias and severe aplastic anemia was achieved. Departure from the use of TBI alone was primarily fostered by the development of more effective cytotoxic chemotherapeutics and immunologic therapies, which when combined with TBI, yielded fewer leukemic recurrences.29 Although the use of TBI without HSCT has largely been abandoned, primarily for fear of inducing secondary malignant tumors and limiting later therapeutic options, some question the validity of this fear, and still contend that very low dose TBI (1.5 to 2 Gy in 10 to 20 fractions over several weeks) is a viable option for initial therapy in advanced indolent lymphomas.31 Figure 18-2 demonstrates how the use of TBI during HSCT has remained constant or increased over the past few years.32
Hematopoietic Stem Cell Transplant
Hematopoietic stem cell transplant (HSCT) has evolved into a highly complex clinical discipline, firmly rooted in immune system and cancer biology, the details of which are beyond the scope of this chapter. When HSCT was initially performed (see History of Total Body Irradiation), bone marrow was extracted from the donor, filtered, and infused intravenously into the recipient. Later, peripheral blood stem cells, instead of bone marrow, were collected from the donor as an alternative to bone marrow grafting. For this reason, “bone marrow transplants” are now more appropriately termed “hematopoietic stem cell transplants” because the critical component of the graft is the hematopoietic stem cell (HSC), independent of the source. The peripheral blood stem cells are often “mobilized” from the donor using hematopoietic growth-stimulating factors and are removed from the donor by apheresis. In addition, recent success using HSCs derived from human umbilical cord blood has been described. Depending on the source of HSCs, various postcollection processing measures (e.g., cell selection and depletion) may be undertaken to optimize the outcome of the transplant.
When transplant occurs between different individuals, the hematopoietic graft is said to be an allogeneic graft. This is in contradistinction to reinfusion of native HSCs back into the donor in an autologous transplant, more appropriately termed an autoplant, because nothing is being transferred between different individuals.33 A rare but alternative situation is when an organ from a genetically identical twin of a patient is transplanted (syngeneic transplant). Of these three methods of HSCT, autologous and syngeneic transplantations are generally associated with less risk because issues related to immunocompatibility are minimized. Allogeneic transplantations require the “matching” of donor and recipient and are typically carried out through identification of human leukocyte antigen (HLA) compatibility. The donor may be related to the recipient or may be identified through registries of volunteers, such as the National Marrow Donor Program.
Before undergoing HSCT, most patients will require intensive antineoplastic and/or immunomodulatory therapy, often referred to as conditioning, in preparation for HSCT. Conditioning can involve cytotoxic chemotherapy, immunomodulators, antibody therapy, or radiation therapy. The nature of the conditioning regimen can be referred to as of high or reduced intensity or as myeloablative, submyeloablative, or nonmyeloablative, to carry out conventional or mini-(ature) transplants.33 Although agreed-on formal definitions of these regimens do not exist,34 the goal of high-intensity/myeloablative/conventional transplant is to completely eliminate the recipient’s native HSC compartment, which necessitates HSCT (autologous or allogeneic) for survival. High-intensity/myeloablative/conventional transplants may or may not involve TBI to high doses (>5 Gy in a single fraction, >8 Gy in multiple fractions). Reduced-intensity/nonmyeloablative/mini-transplant conditioning regimens are often used for older patients or those with medical problems, for whom a high-intensity/myeloablative/conventional transplant would cause excessive morbidity or mortality, and may or may not involve TBI to lower doses. During and immediately after conditioning, the transplant recipient is at significant risk for infections and other hematologic complications. For this reason, the supportive care of HSCT recipients is complex and should only be undertaken in specialized facilities. Nonetheless, some groups have developed reduced-intensity and myeloablative HSCT regimens, including TBI, which have been safely undertaken on an outpatient basis.35,36
According to data summarized by the Center for International Blood and Marrow Transplant Research (CIBMTR) in 2006, the diseases most commonly treated with HSCT in North America are (in decreasing order of frequency) multiple myeloma (MM), non-Hodgkin’s lymphoma (NHL), acute myelogenous leukemia (AML), Hodgkin’s disease (HD), acute lymphoid leukemia (ALL), myelodysplastic and myeloproliferative syndrome (MDS), chronic myelogenous leukemia (CML), aplastic anemia (AA), and various other leukemias, cancers, and nonmalignant diseases.37 The 2009 National Comprehensive Cancer Network (NCCN) guidelines for therapy indicate that allogeneic or autologous HSCT may be a treatment option for testicular cancer, AML, MM, MDS, CML, HD, and NHL, depending on the clinical situation. HSCT with or without TBI-based conditioning has also been described in the treatment of solid tumors, including breast cancer, germ cell tumors, renal cell carcinoma, melanoma, neuroblastoma, and other pediatric cancers. Detailed discussion of these diseases and their management is beyond the scope of this chapter, and the reader is referred to other appropriate chapters in this text and other texts for further review.38–41 The role of TBI in nonmalignant diseases will be discussed further in subsequent sections of this chapter.
Radiobiology
Preclinical studies have helped define some of the fundamental radiobiologic properties of the normal lymphocytes. The D0 (see Chapter 5) of normal lymphocytes has been reported to be 0.5 to 1.4 Gy,42–45 depending on the in vitro or in vivo model used to calculate this parameter. This D0 suggests that normal lymphocyte cells are very sensitive to ionizing radiation. A very small shoulder on the radiation cell survival curve has been noted,46,47 suggesting little repair between fractions of radiation. Clinical data have revealed similar findings in patients undergoing hyperfractionated TBI, with lymphocyte survival demonstrating an effective D0 of 3.8 Gy, according to one study.48 Other radiobiologic phenomena have been ill defined in other normal hematopoietic cells. Radiobiologically relevant levels of hypoxia are unlikely in the hematopoietic compartment. Repopulation is not likely to influence hematopoietic cell survival, given the short duration of most TBI regimens (1 to 5 days), although given the variable life span of leukocytes (days to years), it may be of some relevance. Redistribution would appear to be of significance, given the time scale for TBI; however, this has been difficult to assess.49
The radiobiology of malignant hematopoietic cells has been described. The D0 of leukemic cells generally ranges from 0.8 to 1.5 Gy; however, compared with normal hematopoietic cells, a wider range of radiosensitivities have been described.50,61 Many have cited the technical nuances and variations in assay technique for this great range.49,62 Similar to normal hematopoietic cells, their malignant counterparts are thought to demonstrate little sublethal damage repair,63,64,65–67 although split-dose-rate and low-dose-rate experiments have demonstrated the capacity of leukemic cells to repair radiation-induced damage.55–59,68,69 Generally, leukemic cells are thought to have a cell survival curve with a minimal shoulder or no shoulder, although this varies across cell types and cell lines.47,51–54 For example, Cosset and colleagues62,70 summarized preclinical and clinical findings, concluding that AML demonstrates little repair, whereas CML does demonstrate repair; ALL, myeloma, and lymphomas have not been well studied but appear to demonstrate a wide range of repair capacity. Similar to normal hematopoietic cells, reoxygenation is unlikely to be radiobiologically relevant to malignant hematopoietic cells during TBI. Redistribution and repopulation, however, may be relevant but have not been systematically studied. ![]()
In vivo preclinical research laid the foundation for the first successful HSCT in humans. Studies in rats,71 dogs,72 and nonhuman primates73 demonstrated that reconstitution of the hematopoietic system was possible after TBI with supralethal doses of radiation. Later work in animals revealed that delivering TBI in several fractions required a higher total dose relative to the biologically isoeffective dose given in a single fraction.74–76 Another model demonstrated no significant difference in the effect of a low-dose-rate (0.04 Gy/minute), single-fraction of TBI compared with a hyperfractionated course of TBI given three times a day to the same total dose.77
Although the hematopoietic system is the target of TBI, normal tissues effectively limit the dose that can be safely delivered. The sparing of normal tissues with fractionated TBI was proposed by Peters and colleagues63,64 and was subsequently supported by preclinical data in mice78,79 and dogs80 that showed that less lung injury occurred with fractionated TBI regimens.
Immediate Toxicity and Management in Total Body Irradiation
Although a good deal of what has been learned about the acute in vivo biologic effects of TBI has been derived from laboratory-based animal studies, whole body irradiation also has been studied in people exposed during accidental or wartime nuclear events.81,82 These large-scale studies are valuable because they deal with apparently normal subjects; however, the retrospective nature limits the quality of the data. The reader is referred to several excellent texts for a review of acute and fatal radiation syndromes (gastrointestinal, hematopoietic, and cerebrovascular syndromes) that can be caused by TBI in an uncontrolled setting.83–85
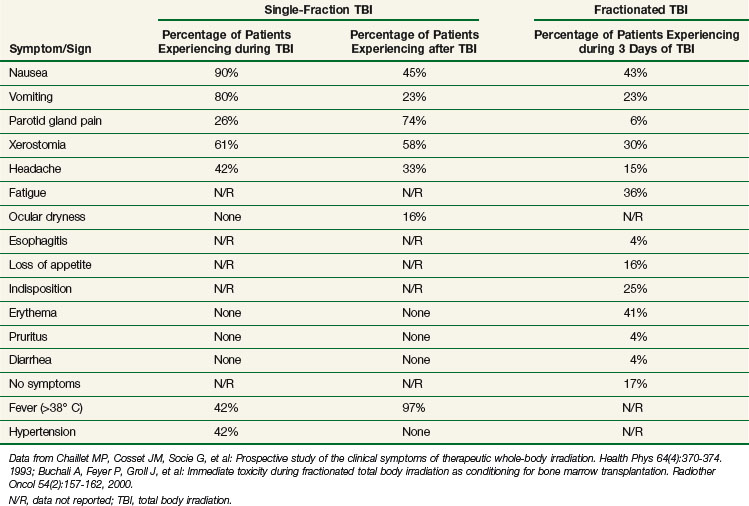
Acute side effects of therapeutic TBI can be difficult to distinguish from other HSCT-related morbidities. However, Chaillet and colleagues86 conducted an informative prospective clinical study of the symptoms and signs that occur in patients after TBI, before the initiation of any other HSCT-related therapy. Thirty-one patients, 4.5 to 55 years of age, were treated using parallel-opposed anteroposterior 18-MV photons from a linear accelerator. Shielding was used to limit the lung dose to 8 Gy. A total dose of 10 Gy was given as a “single dose” as six discrete fractions of 1.6 Gy each given over 15 minutes, with a 30-minute break between fractions, for a mean dose rate of 0.04 Gy/minute and an instantaneous dose rate of 0.11 to 0.12 Gy/minute. Symptoms and signs were assessed regularly during the 4-hour TBI and for 20 hours after the completion of TBI. Antiemetics, but no chemotherapy or steroids, were given before the start of TBI. Table 18-1 displays the symptoms and signs experienced by patients during the 4 hours of TBI and within 24 hours of starting TBI. ![]()
The results of a similar study conducted by Buchali and colleagues87 of patients who were treated with a fractionated course of TBI delivered mostly to a total dose of 12 Gy using 2 Gy per fraction, twice daily, 8 hours apart, with lung doses limited to 10 Gy, are also summarized in Table 18-1.
A prospective clinical study showed that fractionation of TBI can reduce acute nausea, vomiting, mucositis, diarrhea, and parotitis, although the differences were not statistically significant. Late cutaneous eruptions were more common in patients undergoing fractionated TBI, although the numbers were not statistically significant. The same study, which randomized patients to either high- or low-dose-rate TBI, revealed no differences in the acute toxicities mentioned when comparing dose rate.88 Another randomized controlled trial (RCT) reported that fractionating TBI revealed “no apparent difference in acute toxicity” compared with single-fraction TBI, with both regimens being “well-tolerated.”89
Older studies cite nausea and vomiting as frequent side effects of TBI. These symptoms have been substantially minimized with the advent of more effective antiemetics, such as the 5-hydroxytryptamine (serotonin) receptor-3 (5-HT3) antagonists. Several small but high-quality controlled clinical studies support the prophylactic use of 5-HT3 antagonists to reduce nausea and vomiting during TBI90–94; they are summarized in web-only Table 18-2, available ![]() on the Expert Consult website. The use of corticosteroids in conjunction with 5-HT3 antagonists is supported by a trial listed in Table 18-2. However, given the toxicity associated with this approach, consensus regarding routine administration in conjunction with TBI is lacking.95–97 Of note, less nausea and vomiting have been noted in myeloablative conditioning regimens involving TBI compared with those that use chemotherapy alone, even with modern antiemetics.98
on the Expert Consult website. The use of corticosteroids in conjunction with 5-HT3 antagonists is supported by a trial listed in Table 18-2. However, given the toxicity associated with this approach, consensus regarding routine administration in conjunction with TBI is lacking.95–97 Of note, less nausea and vomiting have been noted in myeloablative conditioning regimens involving TBI compared with those that use chemotherapy alone, even with modern antiemetics.98
TABLE 18-2 Randomized Controlled Trials of Prophylactic Antiemetics in Patients Undergoing Total Body Irradiation
Oral mucositis is a side effect of TBI in up to 75% of patients undergoing myeloablative TBI, causing mouth pain and odynophagia and necessitating intensive supportive care such as total parenteral nutrition and opioid analgesics.99 In one study, intensive dental hygiene conferred a reduction in the rate of moderate and severe mucositis, although the authors thought the rate to be clinically insignificant.100 Topical oral agents, such as chlorhexidine digluconate and neutral calcium phosphate in conjunction with topical fluoride treatments can decrease pain duration and severity of oral mucositis, as well as pain and need for opioid analgesics.101–103 Similarly, prophylactic oral sucralfate and clarithromycin have reduced moderate and severe oral mucositis rates.104,105 One study showed that when given prophylactically, amifostine limited the duration of mucositis, with an associated decrease in the rate of moderate and severe infections, with no effect on HSCT outcome.106
In one study, researchers noted that short-term intravenous recombinant granulocyte-macrophage colony–stimulating factor decreased rates of moderate to severe mucositis,107 but in another study, they found no effect when this agent was delivered topically.108 Recently, Spielberger and associates109 reported the results of a trial of the recombinant human keratinocyte growth factor palifermin, given before and after conditioning with 12 Gy of fractionated TBI. Palifermin reduced the rate and duration of moderate and severe mucositis by 35% and 3 days, respectively, and decreased mouth and throat pain, as reflected in reduced morphine usage and decreased need for total parenteral nutrition (by 24%). This study dealt only with patients undergoing autologous HSCT; however, in the setting of TBI for allogeneic HSCT, palifermin may also confer a protective effect on the mucosa, although this has not been studied in an RCT.110
Skin erythema may also be noted toward the end of a course of TBI; desquamation is rare. Hyperpigmentation may be noted in the long term. Alopecia typically occurs 7 to 14 days after TBI is complete, and hair typically returns 3 to 6 months after treatment.111 Changes in the color or texture of regrown hair have been noted by some. Of note, myeloablative conditioning regimens using chemotherapy alone have noted a significantly higher incidence of permanent alopecia.112
Later Toxicity and Its Management in Total Body Irradiation
Hematopoietic Toxicity
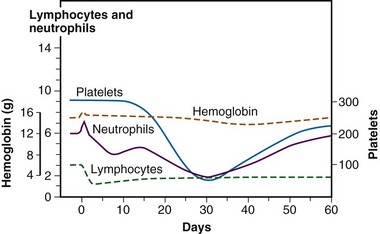
As previously mentioned (see the previous section on Radiobiology), the hematopoietic system is particularly sensitive to TBI, and lymphopenia is often seen with doses of 0.5 Gy and can be seen with doses of 0.3 Gy. Lymphopenia is typically followed by neutropenia, thrombocytopenia, and finally anemia. Soon after a TBI dose of 4 to 6 Gy has been given, lymphocytosis can be seen, but it typically is followed by neutropenia within 1 week. Three to 4 weeks after TBI, neutrophils fall to their minimum113 (Fig. 18-3). Regeneration of the HSC compartment depends on the total dose used because higher doses cause more rapid myelosuppression of greater duration. Administration of hematopoietic growth factors after TBI have the theoretical potential to alter hematopoietic system reconstitution, although reports in the setting of allogeneic HSCT have demonstrated an increased risk of GVHD and compromised survival114 and, therefore, routine use is controversial.115,116 Of note, hematopoietic growth factors have only been used in the period following TBI, given the concerns raised by a trial in lung cancer, where growth factors increased pulmonary toxicity and thrombocytopenia when given concurrently with chemoradiation therapy.117
Oral Toxicity
As noted before, the salivary glands frequently are affected by TBI. Although acute parotitis is typically self-limited and can be managed with anti-inflammatory medicines, long-term salivary gland dysfunction can result in xerostomia, which may lead to dental caries. In a study of children who underwent allogeneic HSCT, the risk of developing impaired salivary function was 22% in those who received TBI as part of conditioning versus 1% in those who did not.118 Studies have shown that salivary flow can improve up to 1 year after the completion of TBI.119 Fractionating TBI was shown to reduce salivary dysfunction by 54% in one study.120
Myeloablative conditioning regimens with and without TBI have been associated with abnormalities in tooth development in children.120–122 In one series, myeloablative conditioning regimens using chemotherapy alone were associated with significantly higher rates of tooth developmental abnormalities than those involving TBI, although rates of salivary gland dysfunction were highest amongst the patients treated with single-fraction TBI.123 Because of the increased risk of oral pathology associated with TBI, careful pretransplant evaluation by a dental specialist is recommended to minimize the risk of serious morbidity.124 Pilocarpine has been noted to help relieve symptoms of xerostomia in patients treated with TBI.125
Pulmonary Toxicity
The major dose-limiting toxicity of TBI is pneumonopathy (restrictive or obstructive lung disease), which can manifest early as pneumonitis and/or later as pulmonary fibrosis. In the setting of HSCT, radiation pneumonopathy can be difficult to distinguish from other causes of lung pathology; moreover, lung damage is likely multifactorial, with the risk of acute lung complications estimated to be 30% to 60%, depending on factors such as infection, conditioning regimen, GVHD, age, and diagnosis.126 Likewise, late pneumonopathy occurs in 10% to 26% of patients and is associated with underlying lung dysfunction, type of conditioning regimen, acute and chronic GVHD and prophylaxis, donor and recipient age and immunocompatibility, stage of disease, and genetic predisposition.127 TBI has been shown to be a risk factor for idiopathic pneumonia syndrome,128 as well as for diffuse alveolar hemorrhage.129 Although rates of pneumonopathy in patients receiving TBI vary widely (10% to 84%),130 some series have reported pneumonitis in up to 20% of HSCT patients who never received TBI.131 In the modern era, with appropriate TBI techniques, the risk of pneumonopathy in patients treated with TBI may not be increased at all.132 Nevertheless, the significance of the problem is clear, given that mortality related to interstitial pneumonitis in patients treated with TBI can be 60% to 80%.131,133,134
Several TBI-specific factors (e.g., total dose, fractionation, dose rate, and use of lung shielding) have been shown to have a significant bearing on the development of pulmonary complications. The total dose used during TBI has frequently been cited as a major factor influencing lung complications.130,135,136 In two prospective RCTs using 12 Gy versus 15.75 Gy, higher rates of mortality were noted within the first 6 months in patients treated with 15.75 Gy, although pulmonary complications were not specifically cited as the excess cause of deaths.137–139,140 In a retrospective dosimetric study, a mean lung dose of more than 9.4 Gy was found to be an independent predictor for lethal pulmonary complications in patients receiving TBI to a total dose of 10 Gy in three daily fractions, at 0.055 Gy/minute using parallel opposed lateral fields.141 Two RCTs have demonstrated that fractionated TBI can reduce pneumonitis compared with single-fraction TBI, although only one study showed differences that were statistically significant.89,142,143 A retrospective study found no difference in pneumonitis rates when comparing a single fraction of 6 Gy and three daily fractions of 3.33 Gy, suggesting that total doses of less than 10 Gy may not require fractionation to prevent toxicity, although no randomized data support this.144 The necessity of hyperfractionation to prevent lung toxicity is unclear: A comparison of two prospective single-arm trials at the same institution revealed that conventional fractionation given with anteroposterior fields and lung blocks to a total dose of 12 Gy in daily 3-Gy fractions may not be any different than hyperfractionated TBI given twice daily with 1.7 Gy per fraction to a total dose of 10.2 Gy over 3 days, using parallel opposed lateral fields and no blocks.145
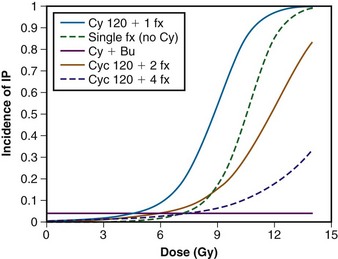
Sampath and colleagues146 recently reviewed 26 studies involving 1096 patients to create a dose-response model for predicting the risk of pneumonitis from TBI while taking other factors into consideration. Although unable to estimate the risk of pneumonitis for hyperfractionated regimens, they were able to determine the effect of fractionation and cyclophosphamide and busulfan on the risk of developing pneumonitis, in a dose-response model, as seen in Figure 18-4.
Pneumonitis rates were not significantly different in a trial that randomized patients to high- or low-dose-rate TBI.88 However, there is an abundance of retrospective clinical data suggesting that lowering the dose rate (<0.025 to 0.09 Gy/minute) does decrease the likelihood of pulmonary complications,130,136,140,147,148 especially if TBI is delivered as a single fraction.149 If TBI is fractionated, some report that a low dose rate (<0.069 Gy/minute) is not necessary,134 whereas others found a beneficial effect.150,151 Studies of patients receiving fractionated TBI with lung shielding have demonstrated a reduction in pneumonopathy.152,153 however, one RCT found no difference in pneumonopathy rates if the shielding allowed a lung dose of either 6 or 8 Gy in a single fraction.154
Pulmonary function tests (PFTs) (e.g., spirometry and diffusion capacity) are often helpful in the assessment of patients with pulmonary symptoms or radiographic abnormalities. Studies of pulmonary function tests in patients treated with HSCT have demonstrated a deleterious effect on spirometry and diffusion capacity, which often resolves in the absence of other complicating factors155–157 and is related to the TBI dose.158 A retrospective study found that lung shielding had a small but significantly beneficial effect on pulmonary function tests 1 year after HSCT, especially in patients with abnormal function before HSCT.159 In one study, busulfan and not TBI, was associated with a negative effect on PFTs.160 There is no evidence that PFTs improve pulmonary outcome after HSCT in adulthood, and for this reason they are not recommended.161 However, some groups recommend baseline PFTs as part of long-term follow-up care for children treated with TBI.162 Counseling regarding smoking cessation is of critical importance for all patients, especially those who are at increased risk of developing lung injury. In the event of acute pneumonitis, high-dose steroids (30 to 60 mg of prednisone/day) typically alleviate symptoms within 24 to 48 hours.
Cardiovascular Toxicity
Cardiovascular toxicity in adults as a result of HSCT has been relatively rare, given the stringent selection criteria for patients treated with this aggressive therapeutic modality. Nevertheless, in adult patients who survived autologous or allogeneic HSCT, cardiac events were responsible for 2.4% and 3% of deaths, respectively; this finding represents a greater than expected occurrence.163,164 Most of the recent literature has identified no association between TBI and the development of cardiovascular disease in adults.165,166 In several detailed prospective analyses using plasma cardiac troponin and brain natriuretic peptide levels, electrocardiography and echocardiography revealed no evidence of cardiac dysfunction in previously healthy individuals treated with TBI.167,168 However, a prospective study of children who underwent allogeneic HSCT found a 12% and 26% cumulative incidence of abnormalities in ejection fraction (<30%) on echocardiography before HSCT and 5 years after HSCT, respectively. This study revealed that TBI was associated with abnormalities in cardiac function on univariate analysis but not multivariate analysis; the 5-year cumulative incidence of cardiac abnormality was 26% or 2% in children treated with TBI, with or without prior anthracycline therapy, respectively.169 Recent data from large studies of survivors of childhood cancer suggest that the risk of cardiac mortality is significantly elevated for children who receive heart doses of 5 Gy, although it should be noted that anthracycline doses of over 360 mg/m2 similarly increased the risk.170 Importantly, the rate of death from recurrence or progression of cancer exceeds the rate of death from cardiac disease by an approximate factor of 7.171 The reason for excess late cardiovascular toxicity in TBI in children is probably because of the association of TBI with the premature development of cardiovascular disease risk factors (e.g., hypertension, dyslipidemia, diabetes) that result in deleterious consequences rather than direct radiation cardiotoxicity.172,173 Therefore it appears prudent to screen patients who have undergone HSCT for cardiovascular disease for risk factors, whether or not treatment involved TBI, to minimize late morbidity and mortality.
Hepatic Toxicity
Hepatotoxicity from TBI manifests primarily as veno-occlusive disease (VOD), also known as sinusoidal obstructive syndrome (SOS), of the liver. This clinicopathologic phenomenon was first described in 1977 by Shulman and colleagues,174 who noted the onset of weight gain from ascites, painful hepatomegaly, and jaundice from centrilobular liver acinus necrosis 1 to 4 weeks after HSCT. Overall, up to 70% of patients who undergo HSCT can be affected by VOD. TBI, along with many other risk factors, has been implicated in the development of VOD.175 An RCT and a meta-analysis found that patients treated with busulfan instead of TBI were significantly more likely to develop VOD.176,177 Two RCTs have concluded that fractionated TBI reduces the incidence of VOD compared with single-dose TBI.142,143 Another RCT found no difference in the rate of VOD when the dose rate was either 0.06 Gy/minute or 0.15 Gy/minute,88 although a retrospective study of single-dose TBI found that dose rates of 0.07 Gy/minute were associated with less VOD than dose rates of 0.18 to 1.2 Gy/minute.178 A TBI dose greater than 13.2 Gy has been reported to be associated with higher rates of VOD on univariate analysis,179 although a dose greater than 12 Gy was not associated with VOD in another retrospective study.180 Lawton and associates181 reported a nonstatistically significant 10% decrease in the rate of fatal VOD in patients treated with TBI as part of HSCT when a 10% attenuation liver block was employed. Ursodeoxycholic acid was effective in preventing VOD in an RCT of patients undergoing TBI followed by HSCT,182 although another RCT did not support this finding.183 Reduced-intensity conditioning regimens may also prevent VOD. Treatment of VOD can include the fibrinolytic antithrombotic agent defibrotide; decompressing the sinusoids by a transjugular intrahepatic portosystemic shunt and liver transplantation is another, more invasive option for the management of severe disease.184
Visual Toxicity
Cataracts are one of the most common complications of TBI. Patients may present with painless vision loss and may be noted to have opacification of the lens on examination. In one series of patients treated with TBI in one or two fractions, severe visual impairment was noted in about half of patients.185 This problem has been noted to arise in a large proportion of patients treated with TBI, depending on the total dose, use of fractionation, and the dose rate. When considering risk factors associated with HSCT, steroid use,186–188 prior cranial irradiation,189,190 and the development of GVHD188 have been shown to predispose to cataractogenesis, whereas heparin use appears to be protective.191 Delivering TBI in a single fraction is the single biggest risk factor for developing a cataract after HSCT.191–196 High-dose-rate (>0.035 to 0.048 Gy/minute) TBI also appears to increase the risk of cataract formation.189,191,194,197 A prospective study that randomized patients to high- or low-dose-rate TBI found that the incidence of cataract 5 years after treatment was 12% and 34% in the low- and high-dose-rate arms, respectively, with 13% and 39% of the cataracts, respectively, occurring in patients who received fractionated or single-dose TBI.198 Kal199 and van Kempen-Harteveld200 recently reviewed the subject of TBI-induced cataracts and concluded that a biologically equivalent dose of 40 Gy yields a 10% chance of developing cataracts, using linear-quadratic modeling that included corrections for dose rate, with an α/β of 0.65 for late effects on the lens. On the basis of this, they suggest considering lens shielding for single-fraction TBI regimens,199 although this is controversial in the setting of malignant disease.200 Given the frequency of cataracts occurring after TBI, patients should be monitored for the development of this complication.201 Management of cataracts that impair vision or degrade quality of life may involve phacoemulsification and extraction; recent data suggest that these procedures are safe, with an adverse event rate of 0.1% for experienced surgeons,202 and effective, with a 90% chance of 20/40 vision postoperatively.203
Renal Toxicity
Kidney dysfunction occurs in approximately 17% of survivors of HSCT204 and can manifest in a number of ways, with the most to least frequent syndromes being idiopathic chronic kidney disease, nephrotic syndrome, thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome), and acute renal failure.205 The syndrome most associated with TBI is thrombotic microangiopathy, which can manifest as nephritis, hypertension, proteinuria, and/or anemia 6 to 12 months after HSCT. HSCT-related risk factors for nephropathy can include GVHD, infections with cytomegalovirus or BK virus, nephrotoxic medications such as cytotoxic chemotherapeutic agents (cytarabine, cyclophosphamide, ifosfamide, cisplatin, retinoic acid, carmustine, actinomycin D, melphalan), antibiotics (acyclovir, ganciclovir, foscarnet, vancomycin, amphotericin, aminoglycosides), and immunosuppressants (cyclosporine, tacrolimus, methotrexate). The larger and more contemporary studies of chronic kidney disease after HSCT have not demonstrated an association with TBI.206–210
Total dose has been implicated as the most important factor in predicting renal morbidity from TBI; a retrospective study found that GVHD and high-dose TBI (13.5 Gy) were associated with elevated serum creatinine levels.211 A prospective evaluation of renal function using radioisotopes found that early nephropathy was associated with age of less than 40 years, use of kidney blocks (possibly related to nephrotoxic contrast media given during simulation), and nephrotoxic drug use, whereas late nephropathy was associated with nephrotoxic drug use but not TBI dose.212 The benefit of kidney shielding has been assessed in two retrospective studies, both of which demonstrated significant improvement in long-term kidney function, with no evidence of dysfunction when the hyperfractionated dose was limited to 9.8 to 10 Gy.213,214 Two recent dose-effect modeling studies have demonstrated that nephropathy is unlikely after a biologically equivalent total dose of 16 Gy (calculated using a linear quadratic model, with corrections for dose rate and an α/β of 2.5 Gy) and that fractionating TBI and delivery of a low dose rate (<0.10 Gy/minute) prevent kidney dysfunction.199,215 Monitoring blood chemistry and counts, as well as blood pressure and urine studies, is advised given the prevalence of kidney disease after HSCT.
Although no therapies have been proven to treat HSCT-related nephropathy, medication therapies (antihypertensives, corticosteroids), plasma exchange, hemodialysis, and renal transplant are possible options for management.205 The angiotensin-converting enzyme inhibitor captopril prevents chronic nephropathy in patients with diabetes216 and may reduce the risk of radiation nephropathy from TBI in preclinical studies.217,218 A small RCT in which captopril was given to mitigate chronic renal failure in patients undergoing 12 Gy of fractionated TBI (with a kidney dose of 9.8 Gy) before HSCT revealed less nephropathy; however, the results were not statistically significant.219
Endocrine Toxicity
Hypothyroidism is the most common endocrinopathy after HSCT.220 Most cases are overt and primary in nature, but subclinical and autoimmune thyroid dysfunction have also been noted. Hypothyroidism has been reported in up to 90% of patients after TBI delivered with a single dose of 10 Gy221 and in up to 15% of patients treated with hyperfractionated TBI to 15 Gy.222 These findings demonstrate the benefit of fractionation in avoiding this complication. One of the largest studies with a lengthy follow-up of children treated with or without TBI before HSCT revealed that TBI was not a risk factor for developing hypothyroidism when compared with equivalent conditioning with busulfan. In this study, the risk of hypothyroidism was higher in children undergoing HSCT before 10 years of age, and the risk continued to increase until 28 years after HSCT.223 A recent study demonstrated that reduced-intensity conditioning with lower doses of radiation during TBI was not associated with lower rates of hypothyroidism,224 suggesting that there is no dose threshold effect. Nevertheless, this complication should be monitored with assessment of thyroid hormones and can be easily managed with thyroid hormone replacement and monitoring.
Gonadal and reproductive endocrine functions can be altered by HSCT and TBI, as reviewed by Chemaitilly and Sklar.220 In males, Leydig cell function is typically preserved, given the dose of radiation delivered during full-dose TBI.225 However, in patients who have received prior testicular radiation or who will have a testicular boost during conditioning, Leydig cell function may be threatened. Germ cells are typically thought to be exquisitely sensitive to radiation. A recent study demonstrated that fertility is significantly reduced among boys treated with a radiation dose of more than 7.5 Gy to the gonads, although it should be kept in mind that other antineoplastics, such as alkylating agents, also profoundly reduce fertility.226 Although there have been case reports of men being treated with TBI and later having children, most men are rendered sterile by HSCT with full-dose TBI; most will experience azoospermia with reduced-dose TBI, but some may also be rendered sterile. With this in mind, male patients should be counseled regarding gamete cryopreservation before initiation of therapy. Likewise, postpubertal women are likely to experience ovarian failure as a result of intensive conditioning, including TBI, and should be counseled about this before treatment. However, about half of prepubertal girls who are treated with fractionated TBI will experience normal reproductive development.227 As reviewed by Schmidt and colleagues,228 several fertility-preserving options may be available to women who wish to bear children after HSCT.
Hypothalamic-pituitary function appears to be unaffected by TBI of adults to doses of 12 Gy.229 However, if additional brain radiotherapy has been or will be delivered, this complication could be encountered. Growth dysfunction may be caused by TBI through a variety of mechanisms, including growth hormone deficiency and skeletal dysplasia; other factors, including GVHD, liver dysfunction, and busulfan-based conditioning, may also contribute. Younger patients are more likely to be affected by growth impairment. In addition, children treated with single-fraction TBI (as opposed to fractionated TBI) are more likely to experience growth impairment.230 Growth hormone replacement therapy has been recommended to correct this deficiency,231 although RCTs have not shown proven benefit232 and treatment is associated with several significant toxicities.
Bone Toxicity
Bone health is another concern in patients that have undergone HSCT. Although TBI has been shown to be a risk factor for avascular necrosis of bone,233 a prospective study found no association between bone metabolism and conditioning with TBI; corticosteroid use is probably the major factor in HSCT causing bone health problems.234 Monitoring bone health with growth assessments, biochemical hormone assessments, and dual-energy x-ray absorptiometry (DEXA) scans is appropriate, and consideration of counseling, weight-bearing exercise, and use of calcium and vitamin D supplementation or antiresorptive agents (bisphosphonates) should be given in patients with evidence of abnormalities.
Nervous System Toxicity
Mild to moderate neurocognitive impairment has been noted in up to 60% of adults undergoing HSCT with TBI.235 However, these findings have not been consistent, because several studies have documented no impairment at all.236,237 TBI-based conditioning (compared with non–TBI-based conditioning) has not been associated with neurocognitive impairment in adults who received full-dose238 or reduced-intensity conditioning.239 Children treated with TBI may experience mild neuropsychologic effects.240 The effects are more prominent in young children,241 especially those under 3 years of age.242 Similar to adults, no difference in neurocognitive function has been associated with TBI and non–TBI-based conditioning regimens in older children.243 However, in young children, TBI-based regimens appear to exert more of a negative effect than non–TBI-based regimens.244 The magnitude of the deficit has been described as statistically, but not clinically, significant (i.e., a deficit of 3 points in IQ) in recent investigations.245
Beyond generally mild cognitive effects of TBI on the nervous system, other types of toxicity are rare. Myelopathy is uncommon, but it has been reported to occur with TBI in conjunction with involved radiation therapy fields, even at cumulative doses considered to be “tolerable” (e.g., 45 Gy).246 Similarly, severe and fatal neurologic toxicity was reported in a series of children treated with TBI and was generally associated with prior whole brain radiotherapy to 18 Gy; therefore the cumulative radiation dose in this approach must be considered carefully.247 Other neurologic toxicity has not been associated with TBI in adults248 or children.249
Risk of Secondary Malignant Tumors
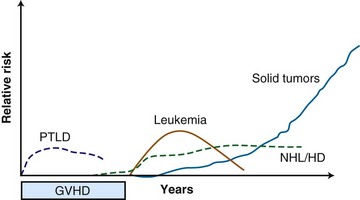
Beyond organ-specific toxicities, the risk of developing secondary malignant neoplasms is increased in patients who have undergone HSCT. Typically, three groups of secondary malignant neoplasms after HSCT are described, and they follow a distinct pattern of development250 (Fig. 18-5): myelodysplastic disease (MDS) and acute myelogenous leukemia (AML), posttransplant lymphoproliferative disorder (PTLD), and solid tumors.251 There are multiple risk factors that may predispose to development of a secondary malignant tumor after HSCT, including, but not limited to, genetic aberrations, therapy given before HSCT, conditioning regimen, graft source and processing, posttransplantation immunosuppression, and GVHD38; only the association of TBI with secondary malignant neoplasms will be discussed here.
Secondary MDS or AML after HSCT has been reported to occur at rates between 1.1% at 20 months and 24.3% at 43 months. The World Health Organization classifies secondary MDS and AML as alkylating-agent and/or radiation-related disease, typically occurring 4 to 7 years after treatment, or topoisomerase-II inhibitor–related disease, typically occurring 6 months to 5 years after treatment.252 Several studies have attempted to quantify the risk of developing secondary MDS or AML after undergoing TBI, with conflicting results. Studies from research groups from Minnesota,253 Newcastle,254 the City of Hope,255 and France256 have demonstrated no increase in rates of MDS or AML after myeloablative HSCT using TBI. Other studies from Nebraska,257 the European Group for Bone Marrow Transplantation (EBMT),258 Paris,259 Barcelona,260 and France261 have found weak associations of borderline or no statistical significance. One study found an association of secondary MDS or AML with TBI on multivariate analysis when TBI was used with etoposide and cyclophosphamide.262 A recent, detailed study of this subject found that among 4000 patients treated with autologous HSCT for lymphoma, 57 developed MDS or AML, with a cumulative incidence rate of 3.7%, 7 years after HSCT. A case-control analysis revealed a weak association of TBI and the subsequent development of MDS or AML that was not statistically significant. In a small subgroup analysis, a TBI dose of 13.2 Gy was associated with MDS or AML, but the authors cautioned that this group of patients may have shared other non–TBI-related factors that could have contributed to leukemogenesis. Older age also appeared to increase the risk.263 The dose threshold effect was not supported by a more recent study that found no differences in the rate of MDS or AML after TBI, whether 12 or 14 Gy of TBI was given.264
The association of TBI with the subsequent development of a PTLD has been described in several studies. Because it is thought to result from immune system dysfunction, PTLD occurs primarily in patients following allogeneic, not autologous, HSCT. The cumulative incidence of PTLD at 12 years varied by the number of risk factors the patient had, and ranged from 0.2% to 8.1%.265 Small early studies from Seattle251 and the CIBMTR266 suggested an association between TBI use during HSCT and subsequent development of a PTLD. However, later research from Minnesota,253 Vancouver,267 and Nebraska268 suggested no association between TBI and PTLD. A follow-up study from the CIBMTR, the largest and most comprehensive study available, involving 26,000 patients who underwent allogeneic HSCT over a 30-year period, clearly demonstrated no association between PTLD and TBI, in contrast to their previous findings.265
The risk of solid tumors after HSCT involving TBI has been studied extensively.269–279 The largest and most recently updated analysis from the CIMBTR suggests that among patients who undergo allogeneic HSCT, the cumulative incidence of developing a solid malignant tumor (including carcinomas in situ that are not of the skin) is 1%, 2.2%, and 3.3% at 10, 15, and 20 years after transplant, respectively, based on the competing risk analysis method.280 The number of secondary solid tumors was double what would have been expected in this population; significantly higher rates of oral cavity and pharyngeal, liver, central nervous system, thyroid, bone, and soft tissue cancers and melanomas were observed. TBI was found to be a risk factor for developing a secondary solid tumor, with the risk increased in those who survived more than 5 years from the HSCT and in those less than 30 years old at the time of HSCT. Although an earlier study found that TBI doses of less than 10 Gy were associated with fewer secondary solid tumors,269 this dose association was not maintained in the subsequent analysis.280 There was no difference in the rate of secondary solid tumors in patients treated with fractionated or single-dose TBI. There was no increased risk of squamous cell carcinomas in patients treated with TBI and no increased risk of secondary solid tumors in patients treated with TBI after age 30. Chronic GVHD and male sex were associated with the development of secondary solid tumors and squamous cell carcinoma, respectively.280
Given the increased risk of secondary malignant neoplasms after TBI, careful surveillance of long-term survivors is a desirable (but not yet proven effective) strategy for minimizing secondary morbidity and mortality. Guidelines published by the American Cancer Society,281 the National Comprehensive Cancer Network (NCCN) (www.nccn.org), and the Children’s Oncology Group162 provide important references regarding assessing patients at higher risk of developing a secondary solid tumor.
Physical Principles of Total Body Irradiation
Although irradiating the entire body may seem like a simple exercise, TBI is a highly specialized technique fraught with unique challenges. Nevertheless, the physical aspects of TBI have not changed significantly over the last three decades. The American Association of Physicists in Medicine (AAPM) Report 17, Task Group 29, “The Physical Aspects of Total and Half Body Photon Irradiation,” is still considered the authoritative reference for methodology and recommendations regarding the physical aspects of TBI.282 More recently, the American College of Radiology created a practice guideline for TBI treatment delivery.283 The reader is referred to these resources and others284 for detailed information on this subject.
Comparison of Techniques of Total Body Irradiation
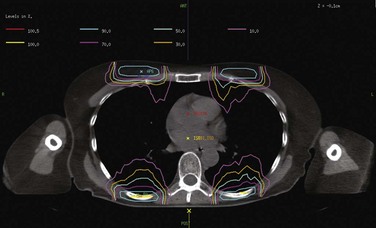
A report from 1980 highlighted several approaches that have been explored in TBI.285 The common factor in the different ways of delivering TBI is the goal, which is to deliver the prescribed dose of ionizing radiation to the entire body in a uniform manner (±10% of the prescription dose). An opposed-parallel anterior-posterior (AP/PA) horizontal field setup was developed at Memorial Sloan-Kettering Cancer Center (MSKCC) many years ago.286 In this technique, the patient is positioned upright several meters from the source with the support of a custom-designed stand. In this technique, high-energy photons are used to increase dose homogeneity throughout the patient. As mentioned previously, high-energy photons will reduce the dose in superficial structures (skin and subcutaneous tissues), and therefore a beam spoiler is used to produce electrons that will increase the surface dose. The beam spoilers are affixed to the stand, which can also accommodate blocks for shielding, imaging equipment for field and patient position verification, and placement of dosimeters (Fig. 18-6). When using shielding, compensatory electron boosts can be delivered to superficial tissues such as those of the chest wall (Fig. 18-7).
An alternative to the AP/PA field arrangement is a lateral field arrangement, pioneered by Khan.287 In this approach, the patient is able to recline in a comfortable position during treatment, typically with the knees drawn toward the chest and the arms by the side. As with the AP/PA technique, the patient is typically positioned several meters from the radiation source. In this technique, the arms can provide shielding to the lungs from the lateral beams. However, as the lateral body thickness is quite disparate over the head-to-toe length of a patient, compensators (usually fixed to the gantry head) will often be required to reduce the dose to the head, neck, and legs. Positioning with this technique is critical; therefore performing and confirming several measurements as well as determining symmetry using in-room light fields and lasers are essential.
Although the AP/PA and lateral field arrangements are the most commonly employed TBI techniques, other methods exist. Children undergoing TBI may require conscious sedation during radiation therapy, making the previously described techniques impossible. In this situation, TBI can be delivered with the child lying on or near the floor. When a patient is in this position, the gantry is pointed down. The child’s smaller body size often will facilitate this arrangement; however, in larger children it may be necessary to match two fields. An alternative to this would be translating the patient or radiation source during treatment.286 Logistic considerations largely prohibit this type of approach.
In recent years, several novel approaches to delivering radiation therapy to the entire body have been described. One of these approaches involves rotational intensity-modulated radiation therapy (delivered by a TomoTherapy unit). This approach appears dosimetrically advantageous because it could allow targeting and radiation dose escalation in the bone marrow, and for this reason, it is often referred to as total marrow irradiation (TMI).288 However, few clinical data exist to support its use. Moreover, sparing the circulating hematopoietic cells from radiation therapy might defeat the purpose of delivering radiation to the entire body. Another alternative approach of delivering systemic radiation therapy is using radionuclides conjugated to monoclonal antibodies, commonly referred to as radioimmunotherapy, instead of external beam radiotherapy. The feasibility of this concept has been demonstrated by several groups, with encouraging results in allogeneic289,290 and autologous HSCT.291,292 The uncertainty in biodistribution and clearance of this form of radiotherapy is the major drawback.
Simulation and Planning for AP/PA Total Body Irradiation
While the patient is in the treatment position, radiographs of the thoracic cavity can be obtained to create lung shielding or other blocks. Careful attention should be paid to the size of the blocks so that they are produced in a manner proportional to the treatment field size and distance from the source; this often requires a magnification correction factor. For example, lung shielding is typically designed so that block edges are 1 to 2 cm from the bony thoracic periphery, diaphragm, and vertebral bodies. When shielding is used, some institutions choose to perform compensatory electron boosts matched to the blocks. In these situations, CT can be helpful for planning purposes (Fig. 18-7), although the obvious limitation is that CT cannot be performed when the patient is in the treatment position. Alternative imaging modalities (e.g., ultrasound) performed in the treatment position may be helpful in specific situations (e.g., chest wall boosts in patients with large, pendulous breasts).
Role of Total Body Irradiation in Contemporary HSCT
Necessity of Using Total Body Irradiation in HSCT Conditioning
Although the earliest studies of HSCT for malignant hematopoietic diseases involved both chemotherapy and radiotherapy (see earlier section on history), in the 1970s several regimens using only chemotherapy (without TBI) were developed, largely for logistic reasons, because facilities adequate for performing TBI were not widely available.293 Soon thereafter, the availability of conditioning regimens for HSCT without TBI (primarily involving busulfan and cyclophosphamide),294 gave rise to several important clinical studies that investigated the necessity of TBI in HSCT in various hematopoietic diseases.
Acute Myelogenous Leukemia (AML)
There have been two RCTs comparing TBI-based conditioning regimens with non–TBI-based conditioning regimens for AML. In 1987, the Groupe d’Etude des Greffes de Moelle Osseuse (GEGMO) initiated a prospective multicenter RCT to evaluate whether TBI could be replaced by busulfan, in an effort to limit toxicity in allogeneic HSCT.295 A similar single-institution study was initiated at the University of Minnesota, for patients receiving autologous HSCT, but accrual was poor and the study was terminated early.296 As noted in web-only Table 18-3, available ![]() on the Expert Consult website, superior rates of overall survival, disease-free survival, and survival in general and fewer relapses were noted with the TBI conditioning regimen in both trials, with only the French trial demonstrating a statistically significant difference.295,297 An updated combined analysis of three trials of allogeneic HSCT for AML that randomized patients to conditioning with or without TBI found marginally significant improvements in overall survival and disease-free survival rates. In patients treated with TBI, the estimated 10-year survival rate was 63%, and in those not treated with TBI, the rate was 51% (p = .068); TBI-treated patients had a 10-year disease-free survival rate of 57%, and untreated patients had a rate of 47% (p = .051). On multivariate analysis, TBI was associated with significantly less hair loss and no difference in the rates of GVHD, cataracts, avascular necrosis, or pulmonary complications.298 A retrospective analysis by the CIBMTR revealed that TBI-based conditioning yielded fewer relapses (especially in extramedullary and central nervous system sites) and less hepatic VOD than busulfan-based conditioning.299
on the Expert Consult website, superior rates of overall survival, disease-free survival, and survival in general and fewer relapses were noted with the TBI conditioning regimen in both trials, with only the French trial demonstrating a statistically significant difference.295,297 An updated combined analysis of three trials of allogeneic HSCT for AML that randomized patients to conditioning with or without TBI found marginally significant improvements in overall survival and disease-free survival rates. In patients treated with TBI, the estimated 10-year survival rate was 63%, and in those not treated with TBI, the rate was 51% (p = .068); TBI-treated patients had a 10-year disease-free survival rate of 57%, and untreated patients had a rate of 47% (p = .051). On multivariate analysis, TBI was associated with significantly less hair loss and no difference in the rates of GVHD, cataracts, avascular necrosis, or pulmonary complications.298 A retrospective analysis by the CIBMTR revealed that TBI-based conditioning yielded fewer relapses (especially in extramedullary and central nervous system sites) and less hepatic VOD than busulfan-based conditioning.299
Chronic Myelogenous Leukemia (CML)
Two RCTs have compared the efficacy and toxicity of allogeneic HSCT in patients with CML, preceded by conditioning with either TBI and cyclophosphamide or busulfan and cyclophosphamide300–302 (see web-only Table 18-3, available ![]() on the Expert Consult website). Both studies showed no significant difference in rates of overall survival, event-free survival, or transplant-related mortality. The French study found more relapses in patients treated with TBI (especially fractionated TBI), but this was not seen in the study from Seattle, which used fractionated TBI exclusively. The Seattle trial did show significantly longer episodes of fever, more blood cultures revealing bacteria or fungi, more hospitalizations in the 100 days after transplantation, and a higher incidence of grade 2 to 4 GVHD in patients treated with TBI. An updated, combined analysis of three studies that randomized patients with CML to different conditioning regimens before allogeneic HSCT confirmed no significant difference in rates of survival (65% and 63%) and disease-free survival (52% and 42%) for patients treated with busulfan and cyclophosphamide or cyclophosphamide and TBI, respectively. There were no differences in the 5-year cumulative incidence of chronic GVHD (37% and 39%). However, cataracts were more common among patients treated with TBI.298 Retrospective data also suggest that busulfan-cyclophosphamide–conditioning regimens before allogeneic HSCT for CML may be favored because of lower relapse rates,303 although higher rates of GVHD, hepatotoxicity, and hemorrhagic cystitis have been reported with chemotherapy alone.304
on the Expert Consult website). Both studies showed no significant difference in rates of overall survival, event-free survival, or transplant-related mortality. The French study found more relapses in patients treated with TBI (especially fractionated TBI), but this was not seen in the study from Seattle, which used fractionated TBI exclusively. The Seattle trial did show significantly longer episodes of fever, more blood cultures revealing bacteria or fungi, more hospitalizations in the 100 days after transplantation, and a higher incidence of grade 2 to 4 GVHD in patients treated with TBI. An updated, combined analysis of three studies that randomized patients with CML to different conditioning regimens before allogeneic HSCT confirmed no significant difference in rates of survival (65% and 63%) and disease-free survival (52% and 42%) for patients treated with busulfan and cyclophosphamide or cyclophosphamide and TBI, respectively. There were no differences in the 5-year cumulative incidence of chronic GVHD (37% and 39%). However, cataracts were more common among patients treated with TBI.298 Retrospective data also suggest that busulfan-cyclophosphamide–conditioning regimens before allogeneic HSCT for CML may be favored because of lower relapse rates,303 although higher rates of GVHD, hepatotoxicity, and hemorrhagic cystitis have been reported with chemotherapy alone.304
Acute Lymphoid Leukemia (ALL)
The Pediatric Blood and Marrow Transplant Consortium conducted the only RCT comparing the effect of pre-allogeneic HSCT conditioning using busulfan or TBI, in children with ALL. A central nervous system boost of 6 Gy was given to patients with a history of prior central nervous system disease but no prior central nervous system irradiation if randomized to TBI; if randomized to busulfan, patients with a history of central nervous system disease received 18 Gy of central nervous system radiation therapy before receiving busulfan. The study showed no difference in overall survival rates; however, longer event-free survival times were noted for patients treated with TBI. Patients 6 years old or younger, as well as those in their first complete remission, appeared to experience the most benefit from the TBI regimen.305 A retrospective study by Davies306 using the CIBMTR data of 627 children with ALL who underwent HSCT after conditioning with cyclophosphamide and TBI (CY/TBI) or busulfan and cyclophosphamide (Bu/CY) found that the risk of relapse was not significantly different in the two cohorts. However, the risk of treatment-related mortality was significantly greater in the Bu/CY group. The use of CY/TBI was associated with superior rates of overall survival and leukemia-free survival. Based on these data, the authors concluded that less treatment-related mortality in the CY/TBI group led to greater rates of overall survival and leukemia-free survival. A multivariate analysis of adults with ALL who underwent HSCT preceded by conditioning with fractionated TBI to 12 Gy (lung dose of 9 Gy) or busulfan found that shorter times of event-free survival and disease relapse were more likely among patients who did not receive TBI on multivariate analysis. There were no differences in the rates of transplant-related mortality, hepatic VOD, or GVHD.307
Multiple Myeloma
The Intergroup Francophone du Myelome conducted a trial (9502) that randomized 399 patients with newly diagnosed multiple myeloma to high-dose therapy in preparation for autologous HSCT, after three cycles of vincristine-Adriamycin-dexamethasone chemotherapy. High-dose therapy consisted of melphalan 200 mg/m2 (HDM200) or melphalan 140 mg/m2 and 8 Gy of TBI delivered in four daily fractions without lung shielding (HDM140). Overall survival rates were longer in the HDM200 group (p = .05); however, event-free survival rates were not different in the two groups. The authors speculated that the improved overall survival rate was attributed to the better salvage regimens available after initial therapy with HDM200 in the protocol and suggested that the new standard conditioning regimen for autologous HSCT for MM should be without TBI.308
Mixed Diseases
Two prospective trials of conditioning have been carried out in groups of patients with leukemias and lymphomas. Because these trials included a heterogeneous group of diseases, results should be interpreted with caution. Nevertheless, in 1988, the Nordic Bone Marrow Transplantation Group initiated a multicenter RCT of 167 patients (27 children and 140 adults); 68 patients had AML, 38 had ALL, 57 had CML and 4 had lymphoma before allogeneic HSCT.176 With 7 years of follow-up, the authors found a survival benefit in the TBI group among patients with “advanced” disease, with less toxicity compared with busulfan regimens; no differences were identified in the subset analysis of the different diseases that were treated.112 The Southwest Oncology Group’s RCT (SWOG 8612) also investigated the role of TBI before allogeneic HSCT for ALL (n = 48), AML (n = 40), and CML (n = 34) patients who were not in their first remission. This regimen substituted etoposide for cyclophosphamide in the TBI group only, making a meaningful comparison difficult. Although rates of overall survival and disease-free survival appeared to be superior for “good-risk” patients in the TBI group, the differences were not statistically significant.309
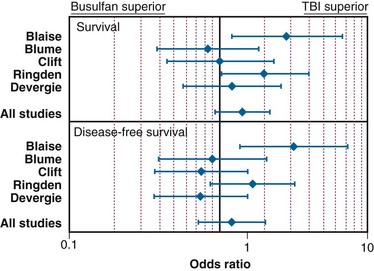
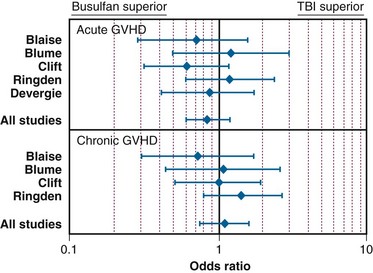
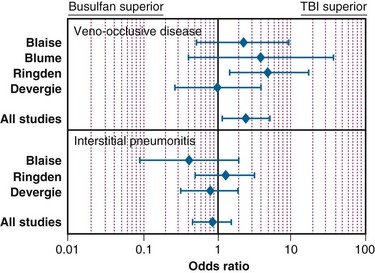
In the setting of allogeneic HSCT, a meta-analysis of the RCTs by Hartman and colleagues177 summarized how TBI influences the outcome of conditioning regimens before allogeneic HSCT. As illustrated in Figure 18-8, overall survival and disease-free survival rates are slightly better with TBI-based regimens, although the results were not statistically significant. As shown in Figures 18-9 and 18-10, rates of GVHD were roughly equivalent in the studies, whereas hepatic VOD was clearly more common with busulfan-based regimens. The occurrence of interstitial pneumonitis also appeared to be similar in both groups. Another recent meta-analysis of 18 prospective and retrospective studies comparing TBI-based conditioning regimens with those not involving TBI (busulfan-based regimens) found that TBI improved disease-free survival rates in AML and ALL but not in CML. Growth and development problems, interstitial pneumonitis, and cataracts were more common with TBI-based regimens, but transplant-related mortality, VOD, and hemorrhagic cystitis were more common with busulfan-based regimens.310
Dose of Total Body Irradiation
The appropriate radiation dose to use during conditioning with TBI has been investigated by several groups. In 1985, researchers in Seattle conducted an RCT of two TBI regimens as part of the HSCT for AML. Seventy-one patients were randomized to receive 12 Gy in six daily fractions (2 Gy/fraction/day) or 15.75 Gy in seven daily fractions (2.25 Gy/fraction/day). TBI was delivered using opposed 60Co sources, at 0.06 to 0.07 Gy/minute. The use of shielding was not described. Higher-dose TBI (15.75 Gy) was associated with higher rates of mortality in patients who did not relapse, although the authors note that this was limited to the first 6 months after transplant and to higher rates of grade 2 to 4 acute GVHD (p = .02). Patients receiving higher-dose TBI were less likely to receive adequate immunosuppression. Nonetheless, patients treated with lower-dose TBI (12 Gy) appeared to be more likely to relapse, although this finding was only marginally significant (p <.06).137 A report of the long-term results (minimum follow-up of 7.5 years) of the trial revealed no difference in overall survival rates between the two TBI groups.139
In a similar trial, also from Seattle, 116 patients with CML were randomized to receive either 12 Gy or 15.75 Gy, as described above. Higher-dose TBI (15.75 Gy) was associated with a superior relapse-free survival rate and with more grade 2 to 4 acute GVHD, although this was not statistically significant (p = 0.15). Initially, survival rates appeared to be superior in patients receiving lower-dose TBI (12 Gy). However, further follow-up revealed no significant difference in survival rates. Similar to the experience with AML, the authors concluded that higher-dose TBI was associated with greater relapse-free survival but at the cost of more acute GVHD, which likely led to transplant-related mortality that offset the survival benefit.138
Retrospective data suggest a benefit to higher-dose (>12 Gy) TBI in certain clinical scenarios. The highest doses of TBI reported in the literature are 20 Gy before autologous HSCT,311 but at least one study has reported lower relapse rates and less transplant-related mortality in a subset of patients with ALL who received a TBI dose of more than 13 Gy.312 Although modeling studies suggest that higher biologically effective doses are associated with lower relapse rates and longer overall survival times,313 the finding of excess toxicity with higher doses in clinical dose-escalation studies does warrant caution.314,315,316,317 For these reasons, fractionated TBI doses used before myeloablative HSCT typically range between 12 and 15 Gy.
Reduced-intensity conditioning regimens have been used more often over the last decade for patients who might not be able to tolerate a myeloablative HSCT (because of advanced age or comorbidities) but could benefit from the graft-versus-tumor effects of allogeneic HSCT. The role of TBI as part of reduced-intensity regimens is ill defined because prospective RCTs have not been completed. Likewise, the optimal dose of TBI as part of reduced-intensity autologous HSCT has not been defined in an RCT. Several notable studies of allogeneic HSCT have employed TBI doses of 2 to 8 Gy, often in conjunction with chemotherapy.318–333 Web-only Table 18-4, available![]() on the Expert Consult website, lists some representative allogeneic HSCT regimens using low-dose TBI.
on the Expert Consult website, lists some representative allogeneic HSCT regimens using low-dose TBI.
Fractionation and the Total Body Irradiation Dose Rate
Laboratory-based radiobiologic data (see the previous section on Radiobiology) and clinical observations prompted trials investigating fractionation and dose-rate delivery in TBI. Thomas and colleagues were the first to conduct an RCT of single-dose or fractionated TBI in patients with AML. In this study, 53 patients with AML were randomized to receive TBI in a single 10-Gy dose or in a dose of 12 Gy in six daily fractions after receiving cyclophosphamide. TBI was delivered using opposed 60Co sources, at a rate of 80 R/minute in air at midpoint (~0.06 Gy/minute). Disease-free survival times were significantly longer in the patients who were treated with fractionated TBI. There was no apparent difference in acute toxicity in the two treatment groups.89 A follow-up report from the same institution described similar results using a slightly lower dose of single-fraction TBI (9.2 Gy using opposed 60Co sources at 0.04 Gy/minute). Amongst the patients randomized, those who received fractionated TBI survived significantly longer than those receiving single-fraction TBI. Patients receiving single-fraction TBI experienced twice as many leukemic recurrences compared with those receiving fractionated TBI, although this was not statistically significant (p = .09). VOD of the liver was significantly more common in patients receiving single-fraction TBI (p = .02).142
Ozsahin and colleagues88 conducted a study investigating the effect of dose rate in patients undergoing single-dose or fractionated TBI as part of autologous or allogeneic HSCT for a variety of hematologic and lymphoid cancers. One-hundred fifty-seven patients receiving single-dose or multiple-fraction TBI were randomized to a low dose rate (0.06 Gy/minute or 0.03 Gy/minute, respectively) or a high dose rate (0.15 Gy/minute or 0.06 Gy/minute, respectively). Radiotherapy was delivered with 60Co or 6-MV photons. Single-dose TBI was delivered to a total dose of 10 Gy with the lung dose limited to 8 Gy. Fractionated TBI was delivered to a total dose of 12 Gy in six fractions over 3 days, with the lung dose limited to 9 Gy. A variety of chemotherapies were used. The authors found no significant differences in rates of overall survival or relapse-free survival, pneumonitis, VOD, or GVHD. Cataracts were significantly more common amongst patients receiving single-fraction, high-dose-rate TBI. In a follow-up report on this study, in which the median follow-up time was 50 months, it was demonstrated that TBI in a single fraction or at a high dose rate was associated with the development of cataracts.198
Girinsky and colleagues143 conducted an RCT of 160 patients older than 15 years who were undergoing autologous or allogeneic HSCT for a variety of hematologic and lymphoid cancers. Patients were treated with a single fraction of TBI (STBI) with 10 Gy over 4 hours (average dose rate of 0.045 Gy/minute; instantaneous dose rate of 0.125 Gy/minute) or with hyperfractionated TBI (HTBI) with 14.85 Gy in 11 fractions over 5 days at a dose rate of 0.25 Gy/minute. A linear accelerator was used to deliver 18-MV photons with lung shielding; patients receiving STBI received a lung dose of 8 Gy, and HTBI patients received a lung dose of 9 Gy. After TBI, cyclophosphamide or melphalan was given, followed by HSCT. With a median follow-up of 8 years in 147 assessable patients, rates of overall survival and cause-specific survival were higher in the HTBI group; however, the differences were not statistically significant. A multivariate analysis that controlled for prognostic variables revealed greater cause-specific survival rates but not greater overall survival rates in patients treated with HTBI (p = .04). There was no significant difference in the rates of lethal pneumonitis, hepatic VOD, GVHD, graft failure, infection, or organ failure. However, the rate of hepatic VOD was significantly higher in the STBI group.
The necessity of hyperfractionation versus conventional daily fractionation has never been tested in a prospective trial. Investigators from the M.D. Anderson Cancer Center compared the outcome of patients in different prospective HSCT protocols and found that fewer patients in the conventionally fractionated group had recurrences of disease. These results should be interpreted with caution because this study was not randomized and other factors not related to TBI may have influenced the results.145 Investigators in Seattle have conducted phase I/II studies of twice- and thrice-daily hyperfractionation protocols that did not suggest a significant benefit with hyperfractionation versus conventional fractionation when they were compared with historical results.316,334
Organ Shielding during Total Body Irradiation
The necessity of shielding to prevent normal tissue toxicity has been investigated in several studies; because pneumonopathy has been considered the dose-limiting toxicity of TBI, the studies have primarily been concerned with lung shielding. Labar and associates152 conducted a trial in 64 patients with AML, ALL, and CML who were undergoing TBI and cyclophosphamide conditioning. Patients were treated with 12 Gy of TBI in three fractions given once daily and were randomized to TBI with or without lung shielding after stratifying by age group (<20, 21 to 30, 31 to 40, >40 years), sex, underlying disease, GVHD prophylaxis, and donor-recipient sex combination. TBI was delivered using a 60Co source, and blocks were used to limit the estimated lung dose to 9 Gy in the group of patients treated with lung shields. There was no difference in rates of leukemia-free survival or relapse. Although interstitial pneumonitis was more common in the group of patients treated without lung blocks (12%) compared with those treated with lung blocks (4%), this difference was not statistically significant.
Girinsky and colleagues154 conducted a similar study in a group of 85 patients with various lymphoid or hematopoietic cancers. Each patient received TBI with a dose of 10 Gy given in a single fraction over 4 hours (average dose rate of 0.04 Gy/minute; instantaneous dose rate of 0.125 Gy/minute). Patients were randomly assigned to receive a 6- or 8-Gy lung dose. The authors observed no differences in rates of overall survival or lung complications. However, chronic GVHD and infections were significantly less common in the low lung dose group. Interestingly, there were significantly more leukemia relapses in the low lung dose group. The authors speculated that the additional lung shielding may have fostered survival of leukemic cells, which led to higher rates of relapse.
Retrospective data support the benefit of shielding. Weshler and colleagues153 reported a series of 44 consecutive patients who received 12 Gy of TBI; the first 23 patients were treated with opposed lateral fields and had no lung shielding in place, whereas the subsequent 21 patients had half-value layer transmission blocking after 6 Gy. Pneumonitis occurred in 26% of patients without lung shielding, whereas no pneumonitis was noted in those who had lung shielding. Investigators from Wisconsin have demonstrated sparing of renal and hepatic dysfunction with shielding.181,213
Boosting in Total Body Irradiation
There is scant high-level evidence for integrating a boost into the radiotherapy plan involving TBI. The EBMT conducted an RCT to assess the benefit of splenic boosting before allogeneic HSCT in patients with CML in their first complete remission. Two-hundred twenty-nine participants were enrolled and stratified based on age (25 years) and T-cell depletion. Patients were randomized to receive a 10-Gy boost to the spleen (given in one, two or three fractions) within 14 days of HSCT. Overall, there was no difference in the groups. However, in subgroup analysis, it was determined that patients who did not undergo T-cell depletion and who had more than 3% circulating basophils before transplant demonstrated a statistically significant improvement in overall survival rates.335 At MSKCC, the testes of males with leukemia undergoing myeloablative HSCT are boosted with 4 Gy in one fraction, based on the results of a retrospective series that revealed significantly lower rates of testicular relapse with this treatment strategy.336 These findings have never been validated in a prospective study, but the other institutions have incorporated the findings in their radiotherapy strategies, as discussed by Quaranta and colleagues.337 In other situations, investigators have reported success in delivering an involved field boost to the central nervous system (in leukemia)338 or bulky nodal disease (in lymphoma),339 although the efficacy of these approaches has never been validated in prospective trials. ![]()
Total Body Irradiation in Nonmalignant Diseases
Although no RCT data exist to support the use of TBI in the conditioning regimen of patients undergoing autologous HSCT for autoimmune conditions, several pilot studies have prompted the execution of phase III studies incorporating TBI. The basis for the role of TBI lies in preclinical studies of a rat model of autoimmune arthritis treated with pseudo-autologous HSCT. When the highest tolerable doses of TBI, cyclophosphamide, or busulfan were given, TBI was the most effective single conditioning agent.340 Saccardi and associates341 summarized the results of phase I and II trials carried out by the EBMT using HSCT for multiple sclerosis (MS), which revealed stabilization or improvement of neurologic symptoms in 63% of patients, who generally had severe or progressive disease. Collectively, TBI was part of the conditioning regimen in 16 of the 178 patients. Statistical analysis revealed that busulfan, and not TBI, was associated with transplant-related mortality. Interestingly, Nash and colleagues342 studied 26 patients in a pilot study using high-dose myeloablative conditioning before autologous HSCT, including 8 Gy of TBI, and found that the 3-year progression-free survival rate was 73%, with a 91% overall survival rate, with minimal toxicity. Concerns about the use of TBI in HSCT for MS were brought up by Samijin and associates343 as well as Burt and colleagues,344 who speculated that radiotherapy may have contributed to axonal injury or caused dysfunction of neural precursor cells, although direct evidence of this was lacking. For these reasons, the ongoing RCT to compare autologous HSCT (ASCT) with mitoxantrone drug therapy for severe MS will not involve TBI (www.astims.org/). Similar concerns regarding the toxicity of radiotherapy in patients with inflammatory bowel disease have prompted investigators to omit TBI from ongoing RCTs of autologous HSCT in Crohn’s disease.345
As in studies of MS and Crohn’s disease, autologous HSCT has been explored recently for patients with systemic sclerosis (SSc, scleroderma) in several pilot studies. Binks and colleagues346 reported the results of a phase I/II trial of autologous HSCT for poor-prognosis systemic sclerosis, using a variety of conditioning regimens, some of which included TBI. In their initial report, two of nine patients who received 8 Gy of TBI died of interstitial pneumonitis 1 and 3 months after TBI. This prompted investigators to minimize the TBI dose during further investigations of HSCT in this disease.347 After two of the first eight patients in a study by Nash and associates348 died of pneumonitis, these investigators shielded the lungs with five half-value layers after patients received the first 2-Gy fraction. With shielding in place, no further TBI-related pulmonary mortality has occurred; however, two patients developed myelodysplastic syndrome and a former smoker was diagnosed with stage 1 lung cancer.349 Autologous HSCT for systemic sclerosis is currently being studied in two RCTs; in the United States, the Scleroderma Cyclophosphamide or Transplantation (SCOT) trial, NCT00114530, will incorporate 8 Gy of TBI, whereas in the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial in Europe (www.astistrial.com), TBI will not be used.
Several inherited diseases have been successfully treated with HSCT using TBI for conditioning; however, this approach is not desirable for children, who frequently need HSCT at a very young age. HSCT for thalassemia has been carried out in several Italian centers and generally does not involve TBI. However, reduced-intensity regimens involving low doses of TBI have been used in a few small series of patients undergoing HSCT for thalassemia and sickle cell disease with favorable results.350,351 Immunodeficiency syndromes are often treated with HSCT, but TBI is not commonly employed during conditioning, probably because these syndromes are commonly caused by impaired DNA damage repair by nonhomologous end-joining. Phagocytic disorders have been treated with HSCT using myeloablative doses of TBI.352 HSCT is considered the only curative therapy for osteopetrosis, and the first successful case involved TBI.353 Likewise, hundreds of patients have been successfully treated for lysosomal or peroxisomal storage diseases (i.e., mucopolysaccharidoses, mucolipidoses, leukodystrophies, glycoprotein and other disorders) with HSCT, often using TBI, although this approach is undesirable in younger children.354–356 Recent studies have focused on non–TBI-based approaches in these diseases.357 Fanconi’s anemia is a disease commonly treated with allogeneic HSCT. In favorable patients with an HLA-matched sibling donor, TBI can usually be avoided. However, in patients with evidence of MDS or AML, use of TBI should be considered. TBI is typically delivered in a single fraction, given the underlying defects in DNA repair and cell-cycle checkpoint regulation in patients with Fanconi’s anemia. Prospective dose-escalation studies have found no benefit in doses beyond 4.5 Gy.358
Full list of cited references (black and red numbers) is published online at www.expertconsult.com. The full list for this chapter contains 358 references.
1 Dessauer FJ. Beitrage zur Bestrahlung tiefliegender Prozesse. Medizinischen Klinik. 1905:21.
8 Medinger FG, Craver LF. Total body irradiation with review of cases. Am J Roentgenol Radium Ther Nucl Med. 1942;48(5):651-671.
9 Heublein AC. A preliminary report on continuous irradiation of the entire body. Radiology.. 1932;18(6):1051-1062.
21 Merrill JP, Murray JE, Harrison JH, et al. Successful homotransplantation of the kidney between nonidentical twins. N Engl J Med. 1960;262(25):1251-1260.
25 Thomas ED, Lochte HLJr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491-496.
29 Thomas ED, Storb R, Clift RA, et al. Bone marrow transplantation. 1. N Engl J Med. 1975;292(16):832-843.
30 Thomas ED, Storb R, Clift RA, et al. Bone marrow transplantation. 2. N Engl J Med. 1975;292(17):895-902.
32 Heinzelmann F, Ottinger H, Müller CH, et al. Total body irradiation: role and indications: Results from the German Registry for Stem Cell Transplantation (DRST). Strahlentherapie und Onkologie. 2006;182(4):222-230.
49 Wheldon TE. The radiobiological basis of total body irradiation. Br J Radiol. 1997;70(840):1204-1207.
62 Cosset JM. Radiobiological and clinical bases for total body irradiation in the leukemias and lymphomas. Semin Radiat Oncol. 1995;5(4):301-315.
63 Peters L. Discussion. The radiobiological bases of TBI. Int J Radiat Oncol Biol Phys. 1980;6(6):785-787.
64 Peters LJ. Radiobiological considerations in the use of total body irradiation for bone marrow transplantation. Radiology. 1979;131(1):243-247.
86 Chaillet MP, Cosset JM, Socie G, et al. Prospective study of the clinical symptoms of therapeutic whole body irradiation. Health Physics. 1993;64(4):370-374.
87 Buchali A, Feyer P, Groll J, et al. Immediate toxicity during fractionated total body irradiation as conditioning for bone marrow transplantation. Radiother Oncol. 2000;54(2):157-162.
88 Ozsahin M, Pène F, Touboul E, et al. Total body irradiation before bone marrow transplantation. Results of two randomized instantaneous dose rates in 157 patients. Cancer. 1992;69(11):2853-2865.
89 Thomas ED, Clift RA, Hersman J, et al. Marrow transplantation for acute nonlymphoblastic leukemia in 1st remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8(5):817-821.
90 Tiley C, Powles R, Catalano J, et al. Results of a double-blind placebo controlled study of ondansetron as an antiemetic during total-body irradiation in patients undergoing bone-marrow transplantation. Leuk Lymphoma. 1992;7(4):317-321.
91 Spitzer TR, Bryson JC, Cirenza E, et al. Randomized double-blind, placebo-controlled evaluation of oral ondansetron in the prevention of nausea and vomiting associated with fractionated total-body irradiation. J Clin Oncol. 1994;12(11):2432-2438.
92 Prentice HG, Cunningham S, Gandhi L, et al. Granisetron in the prevention of irradiation-induced emesis. Bone Marrow Transplant. 1995;15(3):445-448.
93 Okamoto S, Takahashi S, Tanosaki R, et al. Granisetron in the prevention of vomiting induced by conditioning for stem cell transplantation. A prospective randomized study. Bone Marrow Transplant. 1996;17(5):679-683.
94 Matsuoka S, Okamoto S, Watanabe R, et al. Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation. A prospective randomized study. Int J Hematol. 2001;77(1):86-90.
109 Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351(25):2590-2598.
112 Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation. Long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Blood. 1999;93(7):2196-2201.
137 Clift RA, Bruckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid-leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867-1871.
138 Clift RA, Bruckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid-leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660-1665.
139 Clift RA, Bruckner CD, Appelbaum FR, et al. Long-term follow-up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92(4):1455-1456.
142 Deeg HJ, Sullivan KM, Buckner CD, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission: toxicity and long-term follow-up of patients conditioned with single dose or fractionated total-body irradiation. Bone Marrow Transplant. 1986;1(2):151-157.
143 Girinsky T, Benhamou E, Bourhis JH, et al. Prospective randomized comparison of single-dose versus hyperfractionated total body irradiation in patients with hematologic malignancies. J Clin Oncol. 2000;18(5):981-986.
146 Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys. 2005;63(3):876-884.
152 Labar B, Bogdanić V, Nemet D, et al. Total body irradiation with or without lung shielding for allogeneic bone-marrow transplantation. Bone Marrow Transplant. 1992;9(5):343-347.
154 Girinsky T, Socie G, Ammarguellat H, et al. Consequences of two different doses to the lungs during a single dose of total body irradiation: Results of a randomized study on 85 patients. Int J Radiat Oncol Biol Phys. 1994;30(4):821-824.
176 Ringdén O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total-body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia. A report from the Nordic-Bone-Marrow-Transplantation-Group. Blood. 1994;83(9):2723-2730.
177 Hartman AR, Williams S, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation. A meta-analysis. Bone Marrow Transplant. 1998;22(5):439-443.
198 Ozsahin M, Belkacemi Y, Pène F, et al. Total body irradiation and cataract incidence. A randomized comparison of two instantaneous dose rates. Int J Radiat Oncol Biol Phys. 1994;28(2):343-347.
250 Ades L, Guardiola P, Socie G. Second malignancies after allogeneic hematopoietic stem cell transplantation. New insight and current problems. Blood Rev. 2002;16(2):135-146.
282 American Association of Physicists in Medicine (AAPM). Report 17, Task Group 29. The Physical Aspects of Total and Half Body Photon Irradiation. American Institute of Physics, New York, 1986, www.aapm.org/pubs/reports.RPT_17.pdf.
295 Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid-leukemia in first remission. A randomized trial of a busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen. A report from the Groupe-Detudes-De-La-Greffe-De-Moelle-Osseuse. Blood. 1992;79(10):2578-2582.
296 Dusenbery KE, Daniels KA, McClure JS, et al. Randomized comparison of cyclophosphamide total body irradiation versus busulfan cyclophosphamide conditioning in autologous bone-marrow transplantation for acute myeloid-leukemia. Int J Radiat Oncol Biol Phys. 1995;31(1):119-128.
297 Blaise D, Maraninchi D, Archimbaud E, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97(11):3669-3671.
300 Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid-leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84(6):2036-2043.
301 Clift RA, Radich J, Appelbaum FR, et al. Long-term follow-up of a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide for patients receiving allogeneic marrow transplants during chronic phase of chronic myeloid leukemia. Blood. 1999;94(11):3960-3962.
302 Devergie A, Blaise D, Attal M, et al. Allogeneic bone-marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total-body irradiation as preparative regimen. A report from the French-Society-of-Bone-Marrow-Graft (SFGM). Blood. 1995;85(8):2263-2268.
305 Bunin N, Aplenc R, Kámani N, et al. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia. A Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32(6):543-548.
308 Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma. Final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99(3):731-735.
309 Blume KG, Kopecky KJ, Henslee-Downey JP, et al. A prospective randomized comparison of total-body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone-marrow transplantation in patients with leukemia who were not in first remission. A Southwest Oncology Group Study. Blood. 1993;81(8):2187-2193.
312 Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as patients conditioning regimens for undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438-453.
316 Demirer T, Petersen FB, Appelbaum FR, et al. Allogeneic marrow transplantation following cyclophosphamide and escalating doses of hyperfractionated total-body irradiation in patients wtih advanced lymphoid malignancies. A phase I/II trial. Int J Radiat Oncol Biol Phys. 1995;32(4):1103-1109.
1 Dessauer FJ. Beitrage zur Bestrahlung tiefliegender Prozesse. Medizinischen Klinik. 1905:21.
2 Dessauer FJ. Eine neue Anordnung zur Rontgenbestrahlung. Archiv fur physikalische Medizin und medizinische Technik. 1907;2:218-223.
3 Elfer A. Orvosi Hetilap. 1907;51:587.
4 del Regato JA. Total body irradiation in treatment of chronic lymphogenous leukemia. Janeway Lecture, 1973. AJR Am J Roentgenol. 1974;120(3):504-520.
5 Teschendorf W. Uber Bestrahlungen des ganzen menshlichen Korpers bei Blutkrankenheiten. Strahlentherapie. 1925;26:720-728.
6 Sluys F. La roentgenisation totale dans la lymphogranulomatose maligne. J Belge de Radiol. 1930;19:297-317.
7 Sluys F. La roentgentherapie totale dans la lymphogranulomatose maligne. Scalpel. 1930;2(27):733-740.
8 Medinger FG, Craver LF. Total body irradiation with review of cases. Am J Roentgenol Radium Ther Nucl Med. 1942;48(5):651-671.
9 Heublein AC. A preliminary report on continuous irradiation of the entire body. Radiology.. 1932;18(6):1051-1062.
10 Craver LF, MacComb WS. Heublein’s method of continuous irradiation of entire body for generalized neoplasms. Am J Roentgenol Radium Ther Nucl Med. 1934;32:654-674.
11 Craver LF. Tolerance to whole body irradiation of patients with advanced cancer. In: Stone RS, editor. Industrial Medicine on the Plutonium Project: Survey and Collected Papers. New York: McGraw-Hill; 1951:485-498.
12 Nickson JJ. Blood changes in human beings following total body irradiation. In: Stone RS, editor. Industrial Medicine on the Plutonium Project: Survey and Collected Papers. New York: McGraw-Hill; 1951:308-337.
13 Low-Beer BVA, Stone RS. Hematological studies on patients treated by total-body exposure to x-rays. In: Stone RS, editor. Industrial Medicine on the Plutonium Project: Survey and Collected Papers. New York: McGraw-Hill; 1951:338-418.
14 Miller LS, Fletcher GH, Gerstner HB. Systemic and clinical effects induced in 263 cancer patients by whole body X-irradiation with nominal air doses of 15 to 200 R. School of Aviation Medicine, USAF report 57-92. Texas: Randolph AFB; 1957. p 22
15 Miller LS, Fletcher GH, Gerstner HB. Radiobiologic observations on cancer patients treated with whole-body x-irradiation. radiation research. Radiat Res. 1958;8(2):150-165.
16 Collins VP, Loeffler RK. The therapeutic use of single doses of total body radiation. Am J Roentgenol Radium Ther Nucl Med. 1956;75(3):542-547.
17 King ER. Use of total body radiation in treatment of far-advanced malignancies. JAMA. 1961;177(9):610.
18 Saenger EL, et al. Whole body and partial body radiotherapy of advanced cancer. AJR Am J Roentgenol. 1973;117(3):670-685.
19 Advisory Committee on Human Radiation Experiments. Final Report. U.S. Department of Energy. Washington, D.C.: U.S. Government Printing Office; 1995.
20 Thomas J. Rebuilding trust in established institutions. Health Physics. 2001;80(4):379-383.
21 Merrill JP, Murray JE, Harrison JH, et al. Successful homotransplantation of the kidney between nonidentical twins. N Engl J Med. 1960;262(25):1251-1260.
22 Hamburger J, et al. Transplantation Dun Rein Entre Jumeaux Non Monozygotes. Apres Irradiation Du Receveur Bon Fonctionnement Au Quatrieme Mois. Presse Medicale. 1959;67(48):1771-1775.
23 Hamburger J, et al. Renal homotransplantation in man after radiation of recipient. Experience with 6 patients since 1959. Am J Med. 1962;32(6):854.
24 Murray JE, et al. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268(24):1315.
25 Thomas ED, Lochte HLJr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257(11):491-496.
26 Thomas ED, Lochte HL, Ferrebee JW. Irradiation of the entire body and marrow transplantation. Some observations and comments. Blood. 1959;14(1):1-23.
27 Thomas ED, et al. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38(10):1709-1716.
28 Buckner CD, et al. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood. 1970;35(6):741.
29 Thomas ED, Storb R, Clift RA, et al. Bone marrow transplantation. 1. N Engl J Med. 1975;292(16):832-843.
30 Thomas ED, Storb R, Clift RA, et al. Bone marrow transplantation. 2. N Engl J Med. 1975;292(17):895-902.
31 Haas RL. Low dose radiotherapy in indolent lymphomas. Enough is enough. Hematol Oncol. 2009;27(2):71-81.
32 Heinzelmann F, Ottinger H, Müller CH, et al. Total-body irradiation: role and indications: Results from the German Registry for Stem Cell Transplantation (DRST). Strahlentherapie und Onkologie. 2006;182(4):222-230.
33 Deeg HJ. Transplantation conditioning regimens. Can we say it better? Biol Blood Marrow Transplant. 2009;15(6):653-655.
34 Giralt S, et al. Reduced-Intensity Conditioning Regimen Workshop. Defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367-369.
35 Bredeson C, et al. Outpatient total body irradiation as a component of a comprehensive outpatient transplant program. Bone Marrow Transplant. 2002;29(8):667-671.
36 Stiff P, et al. Autologous hematopoietic stem cell transplants that utilize total body irradiation can safely be carried out entirely on an outpatient basis. Bone Marrow Transplant. 2006;38(11):757-764.
37 Pasquini MC, Wang Z, Schneider L. Current use and outcome of hematopoietic stem cell transplantation. Part I. CIBMTR summary slides, 2007. CIBMTR Newsletter [serial online]. 2007;13:5-9.
38 DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology, ed 8. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
39 Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for children with sickle cell disease. Cochrane Database Syst Rev. 2009;1:1-11.
40 Michlitsch JG, Walters MC. Recent advances in bone marrow transplantation in hemoglobinopathies. Curr Molec Med. 2008;8(7):675-689.
41 Prasad V, Kurtzberg J. Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant. 2008;41(2):99-108.
42 Testa NG, Hendry JH, Molineux G. Long-term bone marrow damage in experimental systems and in patients after radiation or chemotherapy. Anticancer Res. 1985;5(1):101-110.
43 Uckun FM, Song CW. Radiobiological features of human pluripotent bone marrow progenitor cells (Cfu-Gemm). Int J Radiat Oncol Biol Phys. 1989;17(5):1021-1025.
44 Vriesendorp HM, van Bekkum DW. Role of total body irradiation in conditioning for bone marrow transplantation. In: Thierfelder S, Rodt H, Koldb HJ, editors. Haematology and Blood Transfusion. Berlin: Springer-Verlag; 1980:349.
45 Vriesendorp HM, van Bekkum DW. Susceptibility to total body irradiation. In: Broerse TJ, MacVittie TJ, editors. Response of Different Species to Total Body Irradiation. Boston: Nijhoff; 1984:43.
46 Kwan DK, Norman A. Radiosensitivity of human lymphocytes and thymocytes. Radiat Res. 1977;69(1):143-151.
47 Szczylik C, Wiktorjedrzejczak W. The effects of x-irradiation in vitro on sub-populations of human lymphocytes. Int J Radiat Biol. 1981;39(3):253-263.
48 Shank B, Andreeff M, Li D. Cell-survival kinetics in peripheral-blood and bone marrow during total body irradiation for marrow transplantation. Int J Radiat Oncol Biol Phys. 1983;9(11):1613-1623.
49 Wheldon TE. The radiobiological basis of total body irradiation. Br J Radiol. 1997;70(840):1204-1207.
50 Uckun FM, Aeppli D, Song CW. Radiation-resistance of primary clonogenic blasts from children with acute lymphoblastic-leukemia. Int J Radiat Oncol Biol Phys. 1993;27(4):899-906.
51 Steel GG, Wheldon TE. The radiation biology of paediatric tumors. In: Pinkerton PN, editor. Paediatric Oncology. London: Wiley, 1991.
52 O’Donoghue JA. The implications of in-vitro radiation-survival curves for the optimal scheduling of total body irradiation with bone marrow rescue in the treatment of leukaemia. Br J Radiol. 1987;60(711):279-283.
53 Song CW. Radiobiological basis of total body irradiation with different dose rate and fractionation. Repair capacity of hemopoietic cells. Int J Radiat Oncol Biol Phys. 1981;7(12):1695-1701.
54 Weichselbaum RR, et al. In vitro radiosensitivity of human leukemia cell-lines. Radiology. 1981;139(2):485-487.
55 Rhee JG, et al. Effect of fractionation and rate of radiation-dose on human-leukemic cells, Hl-60. Radiat Res. 1985;101(3):519-527.
56 FitzGerald TJ. Effect of x-irradiation dose rate on the clonagenic survival of human and experimental animal hematopoietic tumor cell lines. Evidence for heterogeneity. Int J Radiat Oncol Biol Phys. 1986;12(1):69-73.
57 FitzGerald TJ. Radiosensitivity of human bone marrow granulocyte-macrophage progenitor cells and stromal colony-forming cells. Effect of dose rate. Radiat Res. 1986;107(2):205-215.
58 Lehnert S, et al. Radiation response of hematopoietic-cell lines of human-origin. Int J Radiat Biol. 1986;49(3):423-431.
59 Laver J. Effects of low dose rate irradiation on human marrow hematopoietic and microenvironmental cells. Sparing effect upon survival of stromal and leukemic cells. Bone Marrow Transplant. 1987;2(3):271-278.
60 FitzGerald TJ. Radiosensitivity of permanent human bone marrow stromal cell lines. Effect of dose rate. Int J Radiat Oncol Biol Physi. 1988;15(5):1153-1159.
61 FitzGerald TJ. Radiosensitivity of cloned permanent murine bone marrow stromal cell lines. Nonuniform effect of low dose rate. Exp Hematol. 1988;16(10):820-826.
62 Cosset JM. Radiobiological and clinical bases for total body irradiation in the leukemias and lymphomas. Semin Radiat Oncol. 1995;5(4):301-315.
63 Peters L. Discussion. The radiobiological bases of tbi. Int J Radiat Oncol Biol Phys. 1980;6(6):785-787.
64 Peters LJ. Radiobiological considerations in the use of total body irradiation for bone-marrow transplantation. Radiology. 1979;131(1):243-247.
65 Dutreix J. Time factors in total body irradiation. Pathol Biol. 1979;27(6):365-369.
66 Dutreix J. Biological problems of total body irradiation. Journal Européen de Radiothérapie. 1982;3(4):165-173.
67 Kimler BF, et al. Radiation response of human normal and leukemic hemopoietic cells assayed by in vitro colony formation. Int J Radiat Oncol Biol Phys. 1985;11(4):809-816.
68 Shank B. Hyperfractionation versus single-dose irradiation in human acute lymphocytic-leukemia cells. Application to TBI for marrow transplantation. Radiother Oncol. 1993;27(1):30-35.
69 Uckun FM, et al. Intrinsic radiation-resistance of primary clonogenic blasts from children with newly diagnosed B-cell precursor acute lymphoblastic-leukemia. J Clin Invest. 1993;91(3):1044-1051.
70 Cosset JM, et al. Single-dose versus fractionated total body irradiation before bone marrow transplantation. Radiobiological and clinical considerations. Int J Radiat Oncol Biol Phys. 1994;30(2):477-492.
71 Odell TT, et al. The homotransplantation of functional erythropoietic elements in the rat following total body irradiation. Ann NY Acad Sci. 1957;64(5):811-825.
72 Alpen EL, Baum SJ. Acute radiation protection of dogs by bone marrow autotransfusion. Radiat Res. 1957;7(3):298-299.
73 Crouch BG, Overman RR. Whole body radiation protection in primates. Fed Proc. 1957;16(1):27.
74 Storb R, et al. Comparison of fractionated to single-dose total body irradiation in conditioning canine littermates for dla-identical marrow grafts. Blood. 1989;74(3):1139-1143.
75 Storb R, et al. Fractionated versus single-dose total body irradiation at low and high-dose rates to condition canine littermates for Dla-identical marrow grafts. Blood. 1994;83(11):3384-3389.
76 Salomon O, et al. Induction of donor-type chimerism in murine recipients of bone marrow allografts by different radiation regimens currently used in treatment of leukemia patients. Blood. 1990;76(9):1872-1878.
77 Girinski T, et al. Similar effects on murine hematopoietic compartment of low-dose-rate single-dose and high-dose-rate fractionated total body irradiation. Preliminary results after a unique dose of 750 Cgy. Br J Cancer. 1990;61(6):797-800.
78 Vegesna V, et al. Multifraction radiation response of mouse lung. Int J Radiat Biol. 1985;47(4):413-422.
79 Penney DP, et al. Morphological correlates of fractionated radiation of the mouse lung. Early and late effects. Int J Radiat Oncol Biol Phys. 1994;29(4):789-804.
80 Mcchesney SL, Gillette E, Powers BE. Response of the canine lung to fractionated irradiation. Pathologic-changes and isoeffect curves. Int J Radiat Oncol Biol Phys. 1989;16(1):125-132.
81 Andrews GA. Criticality accidents in Vinca, Yugoslavia, and Oak Ridge, Tennessee. Comparison of radiation injuries and results of therapy. JAMA. 1962;179(3):191-193.
82 Cosset JM. ESTRO Breur Gold Medal Award Lecture 2001. Irradiation accidents, Lessons for oncology? Radiother Oncol. 2002;63(1):1-10.
83 International Atomic Energy Agency. Safety reports series. In: World Health Organization: Diagnosis and Treatment of Radiation Injuries. Vienna: International Atomic Energy Agency; 1998. p 49
84 Waselenko JK, et al. Medical management of the acute radiation syndrome. Recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140(12):1037-1051.
85 Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2006.
86 Chaillet MP, Cosset JM, Socie G, et al. Prospective study of the clinical symptoms of therapeutic whole body irradiation. Health Physics. 1993;64(4):370-374.
87 Buchali A, Feyer P, Groll J, et al. Immediate toxicity during fractionated total body irradiation as conditioning for bone marrow transplantation. Radiother Oncol. 2000;54(2):157-162.
88 Ozsahin M, Pène F, Touboul E, et al. Total body irradiation before bone-marrow transplantation. Results of two randomized instantaneous dose rates in 157 patients. Cancer. 1992;69(11):2853-2865.
89 Thomas ED, Clift RA, Hersman J, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8(5):817-821.
90 Tiley C, Powles R, Catalano J, et al. Results of a double-blind placebo controlled study of ondansetron as an antiemetic during total body irradiation in patients undergoing bone marrow transplantation. Leuk Lymphoma. 1992;7(4):317-321.
91 Spitzer TR, Bryson JC, Cirenza E, et al. Randomized double-blind, placebo-controlled evaluation of oral ondansetron in the prevention of nausea and vomiting associated with fractionated total body irradiation. J Clin Oncol. 1994;12(11):2432-2438.
92 Prentice HG, Cunningham S, Gandhi L, et al. Granisetron in the prevention of irradiation-induced emesis. Bone Marrow Transplant. 1995;15(3):445-448.
93 Okamoto S, Takahashi S, Tanosaki R, et al. Granisetron in the prevention of vomiting induced by conditioning for stem cell transplantation. A prospective randomized study. Bone Marrow Transplant. 1996;17(5):679-683.
94 Matsuoka S, Okamoto S, Watanabe R, et al. Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation. A prospective randomized study. Int J Hematol. 2001;77(1):86-90.
95 Maranzano E, et al. Evidence-based recommendations for the use of antiemetics in radiotherapy. Radiother Oncol. 2005;76(3):227-233.
96 NCCN Clinical Practice Guidelines in Oncology. Antiemesis. V.3.2009 www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf, 2009. [cited December 28, 2010]. Available from
97 Kris MG, et al. American Society of Clinical Oncology Guideline for Antiemetics in Oncology. Update 2006. J Clin Oncol. 2006;24(18):2932-2947.
98 Orchard PJ, et al. A prospective randomized trial of the anti-emetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biol Blood Marrow Transplant. 1999;5(6):386-393.
99 Woo SB, et al. A longitudinal-study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer. 1993;72(5):1612-1617.
100 Borowski B, et al. Prevention of oral mucositis in patients treated with high-dose chemotherapy and bone marrow transplantation: A randomized controlled trial comparing 2 protocols of dental-care. Oral Oncol Eur J Cancer. 1994;30B(2):93-97.
101 Ferretti GA, et al. Chlorhexidine for prophylaxis against oral infections and associated complications in patients receiving bone-marrow transplants. J Am Dent Assoc. 1987;114(4):461-467.
102 Ferretti GA, et al. Control of oral mucositis and candidiasis in marrow transplantation: A prospective, double-blind trial of chlorhexidine digluconate oral rinse. Bone Marrow Transplant. 1988;3(5):483-493.
103 Papas AS, et al. A prospective, randomized trial for the prevention of mucositis in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31(8):705-712.
104 Castagna L, et al. Prevention of mucositis in bone marrow transplantation. A double blind randomised controlled trial of sucralfate. Ann Oncol. 2001;12(7):953-955.
105 Yuen KY, et al. Effects of clarithromycin on oral mucositis in bone marrow transplant recipients. Haematologica. 2001;86(5):554-555.
106 Hwang WYK, et al. A randomized trial of amifostine as a cytoprotectant for patients receiving myeloablative therapy for allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34(1):1-56.
107 Ifrah N, et al. Intensive short term therapy with granulocyte-macrophage-colony stimulating factor support, similar to therapy for acute myeloblastic leukemia, does not improve overall results for adults with acute lymphoblastic leukemia. Cancer. 1999;86(8):1496-1505.
108 van der Lelie H, et al. Effect of locally applied GM-CSF on oral mucositis after stem cell transplantation:.a prospective placebo-controlled double-blind study. Ann Hematol. 2001;80(3):150-154.
109 Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351(25):2590-2598.
110 Hensley ML, et al. American Society of Clinical Oncology 2008 Clinical Practice Guideline Update. Use of Chemotherapy and Radiation Therapy Protectants. J Clin Oncol. 2009;27(1):127-145.
111 Thomas ED, et al. Marrow transplantation for patients with acute lymphoblastic leukemia in remission. Blood. 1979;54(2):468-476.
112 Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation. Long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Blood. 1999;93(7):2196-2201.
113 Andrews GA. Radiation accidents and their management. Radiat Res. 1967;S:390.
114 Trivedi M, et al. Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant. 2009;43(12):895-908.
115 Smith TJ, et al. 2006 update of recommendations for the use of white blood cell growth factors. An evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187-3205.
116 Crawford J, et al. Hematopoietic growth factors. ESMO recommendations for the applications. Ann Oncol. 2009;20:162-165.
117 Bunn PA, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: A prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol. 1995;13(7):1632-1641.
118 Dahllof G, et al. Risk factors for salivary dysfunction in children 1 year after bone marrow transplantation. Oral Oncol. 1997;33(5):327-331.
119 Bagesund M, et al. Longitudinal scintigraphic study of parotid and submandibular gland function after total body irradiation in children and adolescents. Int J Paediatr Dent. 2007;17(1):34-40.
120 Dahllof G. Oral and dental late effects after pediatric stem cell transplantation. Biol Blood Marrow Transplant. 2007;14(1):81-83.
121 Holtta P, et al. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer. 2005;103(1):181-190.
122 Holtta P, et al. Disturbed root development of permanent teeth after pediatric stem cell transplantation: Dental root development after SCT. Cancer. 2005;103(7):1484-1493.
123 Nasman M, Forsberg CM, Dahllof G. Long-term dental development in children after treatment for malignant disease. Eur J Orth. 1997;19(2):151-159.
124 Jones LR, Toth BB, Keene HJ. Effects of total-body irradiation on salivary-gland function and caries-associated oral microflora in bone-marrow transplant patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont. 1992;73(6):670-676.
125 Singhal S, et al. Pilocarpine hydrochloride for symptomatic relief of xerostomia due to chronic graft-versus-host disease or total body irradiation after bone-marrow transplantation for hematologic malignancies. Leuk Lymphoma. 1997;24(5-6):539-543.
126 Peters SG, Afessa B. Acute lung injury after hematopoietic stem cell transplantation. Clin Chest Med. 2005;26(4):561.
127 Patriarca F, et al. Clinical presentation, outcome and risk factors of late-onset non-infectious pulmonary complications after allogeneic stem cell transplantation. Curr Stem Cell Res Ther. 2009;4:161-167.
128 Fukuda T, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102(8):2777-2785.
129 Robbins RA, et al. Diffuse alveolar hemorrhage in autologous bone-marrow transplant recipients. Am J Med. 1989;87(5):511-518.
130 Weiner RS, et al. Interstitial pneumonitis after bone marrow transplantation. Assessment of risk factors. Ann Intern Med. 1986;104(2):168-175.
131 Pino y Torres JL, et al. Risk factors in interstitial pneumonitis following allogenic bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1982;8(8):1301-1307.
132 Savani BN, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(12):1261-1269.
133 Shank B, et al. Hyperfractionated total body irradiation for bone marrow transplantation .1. Early results in leukemia patients. Int J Radiat Oncol Biol Phys. 1981;7(8):1109-1115.
134 Gogna NK, et al. Lung dose rate and interstitial pneumonitis in total body irradiation for bone marrow transplantation. Australas Radiol. 1992;36(4):317-320.
135 Keane TJ, Vandyk J, Rider WD. Idiopathic interstitial pneumonia following bone-marrow transplantation: the relationship with total body irradiation. Int J Radiat Oncol Biol Phys. 1981;7(10):1365-1370.
136 Bortin M, et al. Factors associated with interstitial pneumonitis after bone-marrow transplantation for acute leukaemia. Lancet. 1982;319(8269):437-439.
137 Clift RA, Bruckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid-leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867-1871.
138 Clift RA, Bruckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid-leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660-1665.
139 Clift RA, Bruckner CD, Appelbaum FR, et al. Long-term follow-up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92(4):1455-1456.
140 Vandyk J, et al. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to lung. Int J Radiat Oncol Biol Phys. 1981;7(4):461-467.
141 Della Volpe A, et al. Lethal pulmonary complications significantly correlate with individually assessed mean lung dose in patients with hematologic malignancies treated with total body irradiation. Int J Radiat Oncol Biol Phys. 2002;52(2):483-488.
142 Deeg HJ, Sullivan KM, Buckner CD, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. Toxicity and long-term follow-up of patients conditioned with single dose or fractionated total-body irradiation. Bone Marrow Transplant. 1986;1(2):151-157.
143 Girinsky T, Benhamou E, Bourhis JH, et al. Prospective randomized comparison of single-dose versus hyperfractionated total-body irradiation in patients with hematologic malignancies. J Clin Oncol. 2000;18(5):981-986.
144 Morgan TL, et al. A comparison of single-dose and fractionated total-body irradiation on the development of pneumonitis following bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1996;36(1):61-66.
145 Gopal R, et al. Comparison of two total body irradiation fractionation regimens with respect to acute and late pulmonary toxicity. Cancer. 2001;92(7):1949-1958.
146 Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys. 2005;63(3):876-884.
147 Beyzadeoglu M, et al. Effect of dose rate and lung dose in total body irradiation on interstitial pneumonitis after bone marrow transplantation. Tohoku J Exp Med. 2004;202(4):255-263.
148 Belkacemi Y, et al. Total-body irradiation before bone marrow transplantation for acute leukemia in first or second complete remission: Results and prognostic factors in 326 consecutive patients. Strahlentherapie und Onkologie. 1998;174(2):92-104.
149 Ozsahin M, et al. Interstitial pneumonitis following autologous bone marrow transplantation conditioned with cyclophosphamide and total body irradiation. Int J Radiat Oncol Biol Phys. 1996;34(1):71-77.
150 Carruthers SA, Wallington M. Total body irradiation and pneumonitis risk. A review of outcomes. Br J Cancer. 2004;90(11):2080-2084.
151 Corvo R, et al. Total body irradiation correlates with chronic graft versus host disease and affects prognosis of patients with acute lymphoblastic leukemia receiving an HLA identical allogeneic bone marrow transplant. Int J Radiat Oncol Biol Phys. 1999;43(3):497-503.
152 Labar B, Bogdanić V, Nemet D, et al. Total body irradiation with or without lung shielding for allogeneic bone-marrow transplantation. Bone Marrow Transplant. 1992;9(5):343-347.
153 Weshler Z, et al. Interstitial pneumonitis after total body irradiation: Effect of partial lung shielding. Br J Haematol. 1990;74(1):61-64.
154 Girinsky T, Socie G, Ammarguellat H, et al. Consequences of two different doses to the lungs during a single dose of total body irradiation. Results of a randomized study on 85 patients. Int J Radiat Oncol Biol Phys. 1994;30(4):821-824.
155 Gore EM, et al. Pulmonary function changes in long-term survivors of bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1996;36(1):67-75.
156 Tait RC, et al. Subclinical pulmonary-function defects following autologous and allogeneic bone marrow transplantation. Relationship to total-body irradiation and graft-versus-host disease. Int J Radiat Oncol Biol Phys. 1991;20(6):1219-1227.
157 Gandola L, et al. Prospective evaluation of pulmonary-function in cancer-patients treated with total body irradiation, high-dose melphalan, and autologous hematopoietic stem-cell transplantation. Int J Radiat Oncol Biol Phys. 1990;19(3):743-749.
158 Chien JW, et al. Comparison of lung function after myeloablative and 2 Gy of total body irradiation-based regimens for hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(4):288-296.
159 Soule BP, et al. Pulmonary function following total body irradiation (with or without lung shielding) and allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant. 2007;40(6):573-578.
160 Beinert T, et al. Late pulmonary impairment following allogeneic bone marrow transplantation. Eur J Med Res. 1996;1(7):343-348.
161 Carver JR, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors. Cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991-4008.
162 Childrens Oncology Group, C. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, version 3.0. Radiation reference guide www.survivorshipguidelines.org, October, 2008. [cited August 27, 2009.]. Available from
163 Bhatia S, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation. Report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215-4222.
164 Bhatia S, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors. Report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784-3792.
165 Tichelli A, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation. A retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203-1210.
166 Armenian SH, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26(34):5537-5543.
167 Auner HW, et al. Monitoring of cardiac function by serum cardiac troponin T levels, ventricular repolarisation indices, and echocardiography after conditioning with fractionated total body irradiation and high-dose cyclophosphamide. Eur J Haematol. 2002;69(1):1-6.
168 Snowden JA, et al. Assessment of cardiotoxicity during haemopoietic stem cell transplantation with plasma brain natriuretic peptide. Bone Marrow Transplant. 2000;26(3):309-313.
169 Uderzo C, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation. A prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007;39(11):667-675.
170 Tukenova M, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308-1315.
171 Mertens AC, et al. Cause-specific late mortality among 5-year survivors of childhood cancer. The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368-1379.
172 Meacham LR, et al. Cardiovascular risk factors in adult survivors of pediatric cancer. A report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170-181.
173 Baker KS, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation. A report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765-1772.
174 Shulman HM, et al. An analysis of hepatic venooclusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980;79(6):1178-1191.
175 Wadleigh M, et al. Hepatic veno-occlusive disease. Pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10(6):451-462.
176 Ringdén O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total-body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia. A report from the Nordic-Bone-Marrow-Transplantation-Group. Blood. 1994;83(9):2723-2730.
177 Hartman AR, Williams S, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation. A meta-analysis. Bone Marrow Transplant. 1998;22(5):439-443.
178 Shulman HM, Hinterberger W. Hepatic venoocclusive disease. Liver toxicity syndrome after bone-marrow transplantation. Bone Marrow Transplant. 1992;10(3):197-214.
179 McDonald GB, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation. A cohort study of 355 patients. Ann Intern Med. 1993;118(4):255-267.
180 Rozman C, et al. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia. Bone Marrow Transplant. 1996;17(1):75-80.
181 Lawton CA, et al. Technical modifications in hyperfractionated total body irradiation for lymphocyte-t deplete bone marrow transplant. Int J Radiat Oncol Biol Phys. 1989;17(2):319-322.
182 Ohashi K, et al. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64(1):32-38.
183 Park SH, et al. A randomized trial of heparin plus ursodiol vs heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29(2):137-143.
184 Senzolo M, et al. Veno occlusive disease. Update on clinical management. World J Gastroenterol. 2007;13(29):3918-3924.
185 van Kempen-Harteveld ML, et al. Cataract after total body irradiation and bone marrow transplantation. Degree of visual impairment. Int J Radiat Oncol Biol Phys. 2002;52(5):1375-1380.
186 van Kempen-Harteveld ML, et al. Cataract-free interval and severity of cataract after total body irradiation and bone marrow transplantation. Influence of treatment parameters. Int J Radiat Oncol Biol Phys. 2000;48(3):807-815.
187 Dunn JP, et al. Bone marrow transplantation and cataract development. Arch Ophthalmol. 1993;111(10):1367-1373.
188 Deeg HJ, et al. Cataracts after total body irradiation and marrow transplantation. A sparing effect of dose fractionation. Int J Radiat Oncol Biol Phys. 1984;10(7):957-964.
189 Fife K, et al. Risk-factors for requiring cataract-surgery following total body irradiation. Radiother Oncol. 1994;33(2):93-98.
190 Zierhut D, et al. Cataract incidence after total body irradiation. Int J Radiat Oncol Biol Phys. 2000;46(1):131-135.
191 Belkacemi Y, et al. Cataractogenesis after total body irradiation. Int J Radiat Oncol Biol Phys. 1996;35(1):53-60.
192 Aristei C, et al. Cataracts in patients receiving stem cell transplantation after conditioning with total body irradiation. Bone Marrow Transplant. 2002;29(6):503-507.
193 Schneider RA, et al. Long-term outcome after static intensity-modulated total body radiotherapy using compensators stratified by pediatric and adult cohorts. Int J Radiat Oncol Biol Phys. 2008;70(1):194-202.
194 Belkacemi Y, et al. Cataracts after total body irradiation and bone marrow transplantation in patients with acute leukemia in complete remission, A study of the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys. 1998;41(3):659-668.
195 Ferry C, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007;40(3):219-224.
196 Fahnehjelm KT, et al. Visual outcome and cataract development after allogeneic stem-cell transplantation in children. Acta Ophthalmol Scand. 2007;85(7):724-733.
197 Beyzadeoglu M, et al. Evaluation of fractionated total body irradiation and dose rate on cataractogenesis in bone marrow transplantation. Haematologica. 2002;32(1):25-30.
198 Ozsahin M, Belkacemi Y, Pène F, et al. Total body irradiation and cataract incidence. A randomized comparison of two instantaneous dose rates. Int J Radiat Oncol Biol Phys. 1994;28(2):343-347.
199 Kal HB, van Kempen-Harteveld ML. Induction of severe cataract and late renal dysfunction following total body irradiation: Dose-effect relationships. Anticancer Res. 2009;29(8):3305-3309.
200 van Kempen-Harteveld ML, et al. Eye shielding during total body irradiation for bone marrow transplantation in children transplanted for a hematological disorder. Risks and benefits. Bone Marrow Transplant. 2003;31(12):1151-1156.
201 Gurney JG, et al. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood. Results from the Bone Marrow Transplant Survivor study. Cancer. 2006;106(6):1402-1408.
202 Bell CM, et al. Surgeon volumes and selected patient outcomes in cataract surgery. A population-based analysis. Ophthalmology. 2007;114(3):405-410.
203 Powe NR, et al. Synthesis of the literature on visual-acuity and complications following cataract-extraction with intraocular-lens implantation. Arch Ophthalmol. 1994;112(2):239-252.
204 Ellis MJ, et al. Chronic kidney disease after hematopoietic cell transplantation. A systematic review. Am J Transplant. 2008;8(11):2378-2390.
205 Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17(7):1995-2005.
206 Choi M, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer. 2008;113(7):1580-1587.
207 Weiss AS, et al. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6(1):89-94.
208 Hingorani SR, et al. Risk factors for chronic kidney disease (CKD) after hematopoietic cell transplantation (HCT). Biol Blood Marrow Transplant. 2005;11(2):72-73.
209 Kist-van Holthe JE, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr Nephrol. 2002;17(12):1032-1037.
210 Hingorani S, et al. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39(4):223-229.
211 Miralbell R, et al. Renal toxicity after allogeneic bone marrow transplantation. The combined effects of total-body irradiation and graft-versus-host disease. J Clin Oncol. 1996;14(2):579-585.
212 Miralbell R, et al. Renal insufficiency in patients with hematologic malignancies undergoing total body irradiation and bone marrow transplantation. A prospective assessment. Int J Radiat Oncol Biol Phys. 2004;58(3):809-816.
213 Lawton CA, et al. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;20(12):1069-1074.
214 Igaki H, et al. Renal dysfunction after total body irradiation. Significance of selective renal shielding blocks. Strahlentherapie und Onkologie. 2005;181(11):704-708.
215 Cheng JC, Schultheiss TE, Wong JYC. Impact of drug therapy, radiation dose, and dose rate on renal toxicity following bone marrow transplantation. Int J Radiat Oncol Biol Phys. 2008;71(5):1436-1443.
216 Lewis EJ, et al. The effect of angiotensin-converting enzyme-inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456-1462.
217 Moulder JE, Fish BL, Cohen EP. Noncontinuous use of angiotensin converting enzyme inhibitors in the treatment of experimental bone marrow transplant nephropathy. Bone Marrow Transplant. 1997;19(7):729-735.
218 Cohen EP, Fish BL, Moulder JE. Successful brief captopril treatment in experimental radiation nephropathy. J Lab Clin Med. 1997;129(5):536-547.
219 Cohen EP, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation. A randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70(5):1546-1551.
220 Chemaitilly W, Sklar CA. Endocrine complications of hematopoietic stem cell transplantation. Endocrinol Metab Clin North Am. 2007;36(4):983.
221 Borgstrom B, Bolme P. Thyroid-function in children after allogeneic bone-marrow transplantation. Bone Marrow Transplant. 1994;13(1):59-64.
222 Boulad F, et al. Thyroid-dysfunction following bone-marrow transplantation using hyperfractionated radiation. Bone Marrow Transplant. 1995;15(1):71-76.
223 Sanders JE, et al. Thyroid function following hematopoietic cell transplantation in children. 30 years’ experience. Blood. 2009;113(2):306-308.
224 Al-Hazzouri A, et al. Similar risks for hypothyroidism after allogeneic hematopoietic cell transplantation using TBI-based myeloablative and reduced-intensity conditioning regimens. Bone Marrow Transplant. 2009;43(12):949-951.
225 Sklar CA, et al. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic-leukemia. A report from the Children’s Cancer Study Group. J Clin Oncol. 1990;8(12):1981-1987.
226 Green DM, et al. Fertility of male survivors of childhood cancer. A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(2):332-339.
227 Mertens AC, et al. Patterns of gonadal dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1998;22(4):345-350.
228 Schmidt KT, et al. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG Int J Obstet Gynaecol. 2010;117(2):163-174.
229 Kauppila M, et al. Long-term effects of allogeneic bone marrow transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adults. Bone Marrow Transplant. 1998;22(4):331-337.
230 Thomas BC, et al. Growth following single fraction and fractionated total body irradiation for bone marrow transplantation. Eur J Pediatr. 1993;152(11):888-892.
231 Saenger P. Growth hormone in growth hormone deficiency. Start treatment early and give it for long enough. Br Med J. 2002;325(7355):58-59.
232 Rizzo JD, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT). Bone Marrow Transplant. 2006;37(3):249-261.
233 Fink JC, et al. Avascular necrosis following bone marrow transplantation. A case-control study. Bone. 1998;22(1):67-71.
234 Stern JM, et al. Bone density loss after allogeneic hematopoietic stem cell transplantation. A prospective study. Biol Blood Marrow Transplant. 2001;7(5):257-264.
235 Harder H, et al. Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer. 2002;95(1):183-192.
236 Peper M, et al. Neurobehavioral toxicity of total body irradiation. A follow-up in long-term survivors. Int J Radiat Oncol Biol Phys. 2000;46(2):303-311.
237 Wenz F, et al. Prospective evaluation of delayed central nervous system (CNS) toxicity of hyperfractionated total body irradiation (TBI). Int J Radiat Oncol Biol Phys. 2000;48(5):1497-1501.
238 Syrjala KL, et al. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104(10):3386-3392.
239 Barba P, et al. Early and late neurological complications after reduced-intensity conditioning allogeneic stem cell transplantation. Biol Blood Marrow Transplantat. 2009;15(11):1439-1446.
240 Smedler AC, Nilsson C, Bolme P. Total body irradiation. A neuropsychological risk factor in pediatric bone-marrow transplant recipients. Acta Paediatr. 1995;84(3):325-330.
241 Smedler AC, Bolme P. Neuropsychological deficits in very young bone-marrow transplant recipients. Acta Paediatr. 1995;84(4):429-433.
242 Phipps S, et al. Cognitive and academic functioning in survivors of pediatric bone marrow transplantation. J Clin Oncol. 2000;18(5):1004-1011.
243 Simms S, et al. Neuropsychological outcome of children undergoing bone marrow transplantation. Bone Marrow Transplant. 1998;22(2):181-184.
244 Smedler AC, Winiarski J. Neuropsychological outcome in very young hematopoietic SCT recipients in relation to pretransplant conditioning. Bone Marrow Transplant. 2008;42(8):515-522.
245 Phipps S, et al. Cognitive and academic consequences of stem-cell transplantation in children. J Clin Oncol. 2008;26(12):2027-2033.
246 Schwartz DL, et al. Radiation myelitis following allogeneic stem cell transplantation and consolidation radiotherapy for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2000;26(12):1355-1359.
247 Faraci M, et al. Severe neurologic complications after hematopoietic stem cell transplantation in children. Neurology. 2002;59(12):1895-1904.
248 Baker KS, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation. Results from the Bone Marrow Transplant Survivor Study. Blood. 2004;104(6):1898-1906.
249 Rubin J, et al. Acute neurological complications after hematopoietic stem cell transplantation in children. Pediatr Transplant. 2005;9(1):62-67.
250 Ades L, Guardiola P, Socie G. Second malignancies after allogeneic hematopoietic stem cell transplantation. New insight and current problems. Blood Rev. 2002;16(2):135-146.
251 Witherspoon RP, et al. Secondary cancers after bone-marrow transplantation for leukemia or aplastic-anemia. N Engl J Med. 1989;321(12):784-789.
252 Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292-2302.
253 Bhatia S, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633-3639.
254 Taylor PRA, et al. Low incidence of myelodysplastic syndrome following transplantation using autologous non-cryopreserved bone marrow. Leukemia. 1997;11(10):1650-1653.
255 Krishnan A, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma. An assessment of risk factors. Blood. 2000;95(5):1588-1593.
256 Sebban C, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma. The GELF-94 randomized study from the Groupe d’Etude des Lymphomes de l’Adulte (GELA). Blood. 2006;108(8):2540-2544.
257 Darrington DL, et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol. 1994;12(12):2527-2534.
258 Milligan DW, et al. Secondary leukaemia and myelodysplasia after autografting for lymphoma. Results from the EBMT. Br J Haematol. 1999;106(4):1020-1026.
259 Park S, et al. Myelodysplasias and leukemias after autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2000;26(3):321-326.
260 Sureda A, et al. Autologous stem-cell transplantation for Hodgkin’s disease. Results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autolog de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395-1404.
261 Beauchamp-Nicoud A, et al. Therapy-related myelodysplasia and/or acute myeloid leukaemia after autologous haematopoietic progenitor cell transplantation in a prospective single centre cohort of 221 patients. Br J Haematol. 2003;122(1):109-117.
262 Hosing C, et al. Risk of therapy-related myelodysplastic syndrome/acute leukemia following high-dose therapy and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. Ann Oncol. 2002;13(3):450-459.
263 Metayer C, et al. Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma. A multicenter case-control study. Blood. 2003;101(5):2015-2023.
264 Brown JR, et al. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total body irradiation and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(10):2208-2214.
265 Landgren O, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113(20):4992-5001.
266 Curtis RE, et al. Risk of lymphoproliferative disorders after bone marrow transplantation. A multi-institutional study. Blood. 1999;94(7):2208-2216.
267 Micallef INM, et al. Lymphoproliferative disorders following allogeneic bone marrow transplantation. The Vancouver experience. Bone Marrow Transplant. 1998;22(10):981-987.
268 Gross TG, et al. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation. Risk factors, treatment and outcome. Bone Marrow Transplantat. 1999;23(3):251-258.
269 Curtis RE, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897-904.
270 Kolb HJ, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Ann Intern Med. 1999;131(10):738-744.
271 Socie G, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348-357.
272 Bhatia S, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464-471.
273 Baker KS, et al. New malignancies after blood or marrow stem-cell transplantation in children and adults. Incidence and risk factors. J Clin Oncol. 2003;21(7):1352-1358.
274 Friedman DL, et al. Second malignant neoplasms following hematopoietic stem cell transplantation. Int J Haematol. 2004;79(3):229-234.
275 Shimada K, et al. Solid tumors after hematopoietic stem cell transplantation in Japan. Incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36(2):115-121.
276 Leisenring W, et al. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24(7):1119-1126.
277 Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109(1):84-92.
278 Cohen A, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation. An EBMT late effects working party study. J Clin Oncol. 2007;25(17):2449-2454.
279 Friedman DL, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation. A report from the FHCRC and the EBMT Late Effect Working Party. Blood. 2008;111(2):939-944.
280 Rizzo JD, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175-1183.
281 Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009. A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59(1):27-41.
282 American Association of Physicists in Medicine (AAPM). Report 17, Task Group 29. The Physical Aspects of Total and Half Body Photon Irradiation. New York: American Institute of Physics; 1986. p 55. Available at www.aapm.org/pubs/reports.RPT_17.pdf
283 American College of Radiology. American College of Radiology (ACR) Practice Guideline for the Performance of Total Body Irradiation. Reston, Virginia: American College of Radiology; 2006. pp 975-979
284 Khan FM. Total body irradiation. In: Kahn FM, editor. The Physics of Radiation Therapy. Philadelphia: Lippincott Williams & Wilkins; 2010:405-412.
285 Kim TH, Khan FM, Galvin JM. A report of the work party. Comparison of total-body irradiation techniques for bone-marrow transplantation. Int J Radiat Oncol Biol Phys. 1980;6(6):779-784.
286 Shank B. Techniques of magna-field irradiation. Int J Radiat Oncol Biol Phys. 1983;9(12):1925-1931.
287 Khan FM, et al. Basic data for dosage calculation and compensation. Int J Radiat Oncol Biol Phys. 1980;6(6):745-751.
288 Zhuang AH, Liu A, Schultheis TE, Wong JY. Dosimetric study and verification of total body irradiation using helical tomotherapy and its comparison to extended SSD technique. Med Dosim. 2009;35(4):243-249.
289 Pagel JM, et al. Allogeneic hematopoietic cell transplantation after conditioning with I-131-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114(27):5444-5453.
290 Koenecke C, et al. Radioimmunotherapy with [Re-188]-labelled anti-CD66 antibody in the conditioning for allogeneic stem cell transplantation for high-risk acute myeloid leukemia. Int J Haematol. 2008;87(4):414-421.
291 Krishnan A, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(1):90-95.
292 Vose JM, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(3):461-467.
293 Copelan EA, Deeg HJ. Conditioning for allogeneic marrow transplantation in patients with lymphohematopoietic malignancies without the use of total-body irradiation. Blood. 1992;80(7):1648-1658.
294 Santos GW. Busulfan (Bu) and cyclophosphamide (Cy) for marrow transplantation. Bone Marrow Transplant. 1989;4:236-239.
295 Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid-leukemia in first remission. A randomized trial of a busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen. A report from the Groupe-Detudes-De-La-Greffe-De-Moelle-Osseuse. Blood. 1992;79(10):2578-2582.
296 Dusenbery KE, Daniels KA, McClure JS, et al. Randomized comparison of cyclophosphamide total body irradiation versus busulfan cyclophosphamide conditioning in autologous bone-marrow transplantation for acute myeloid-leukemia. Int J Radiat Oncol Biol Phys. 1995;31(1):119-128.
297 Blaise D, Maraninchi D, Archimbaud E, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97(11):3669-3671.
298 Socie G, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia. Long-term follow-up of 4 randomized studies. Blood. 2001;98(13):3569-3574.
299 Litzow MR, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119(4):1115-1124.
300 Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid-leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84(6):2036-2043.
301 Clift RA, Radich J, Appelbaum FR, et al. Long-term follow-up of a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide for patients receiving allogeneic marrow transplants during chronic phase of chronic myeloid leukemia. Blood. 1999;94(11):3960-3962.
302 Devergie A, Blaise D, Attal M, et al. Allogeneic bone-marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan–total body irradiation as preparative regimen. A report from the French-Society-of-Bone-Marrow-Graft (SFGM). Blood. 1995;85(8):2263-2268.
303 Kim I, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia. A retrospective study of busulfan-cytoxan versus total body irradiation-cytoxan as preparative regimen in Koreans. Clin Transplant. 2001;15(3):167-172.
304 Kroger N, et al. Comparison of total body irradiation vs busulfan in combination with cyclophosphamide as conditioning for unrelated stem cell transplantation in CML patients. Bone Marrow Transplant. 2001;27(4):349-354.
305 Bunin N, Aplenc R, Kámani N, et al. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia. A Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32(6):543-548.
306 Davies S. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18(2):340-347.
307 Granados E, et al. Hematopoietic cell transplantation in acute lymphoblastic leukemia. Better long-term event-free survival with conditioning regimens containing total body irradiation. Haematologica. 2000;85(10):1060-1067.
308 Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma. Final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99(3):731-735.
309 Blume KG, Kopecky KJ, Henslee-Downey JP, et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone-marrow transplantation in patients with leukemia who were not in first remission. A Southwest Oncology Group Study. Blood. 1993;81(8):2187-2193.
310 Shi-Xia X, et al. Total body irradiation plus cyclophosphamide versus busulphan with cyclophosphamide as conditioning regimen for patients with leukemia undergoing allogeneic stem cell transplantation. A meta-analysis. Leuk Lymphoma. 2010;51(1):50-60.
311 McAfee SL, et al. Dose-escalated total body irradiation and autologous stem cell transplantation for refractory hematologic malignancy. Int J Radiat Oncol Biol Phys. 2002;53(1):151-156.
312 Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as patients conditioning regimens for undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438-453.
313 Kal HB, et al. Biologically effective dose in total body irradiation and hematopoietic stem cell transplantation. Strahlentherapie und Onkologie. 2006;182(11):672-679.
314 Alyea E, et al. Effect of total body irradiation dose escalation on outcome following T-cell-depleted allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2002;8(3):139-144.
315 Bieri S, et al. Total body irradiation before allogeneic bone marrow transplantation: Is more dose better? Int J Radiat Oncol Biol Phys. 2001;49(4):1071-1077.
316 Demirer T, Petersen FB, Appelbaum FR, et al. Allogeneic marrow transplantation following cyclophosphamide and escalating doses of hyperfractionated total body irradiation in patients wtih advanced lymphoid malignancies. A phase I/II trial. Int J Radiat Oncol Biol Phys. 1995;32(4):1103-1109.
317 Sobecks RM, et al. A dose escalation study of total body irradiation followed by high-dose etoposide and allogeneic blood stem cell transplantation for the treatment of advanced hematologic malignancies. Bone Marrow Transplant. 2000;25(8):807-813.
318 Hegenbart U, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24(3):444-453.
319 Stelljes M, et al. Conditioning with 8-Gy total body irradiation and fludarabine for allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Blood. 2005;106(9):3314-3321.
320 Hallemeier C, et al. Outcomes of adults with acute myelogenous leukemia in remission given 550 cGy of single-exposure and total body irradiation, cyclophosphamide, unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2004;10(5):310-319.
321 Sorror ML, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23(16):3819-3829.
322 Kerbauy FR, et al. Hematopoietic cell transplantation from HLA-identical sibling donors after low-dose radiation-based conditioning for treatment of CML. Leukemia. 2005;19(6):990-997.
323 Khoury H, et al. Low incidence of transplantation-related acute complications in patients with chronic myeloid leukemia undergoing allogeneic stem cell transplantation with a low-dose (550 cGy) total body irradiation conditioning regimen. Biol Blood Marrow Transplant. 2001;7(6):352-358.
324 McSweeney PA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies. Replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390-3400.
325 Baron F, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23(9):1993-2003.
326 Tomblyn M, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(5):538-545.
327 Maris MB, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104(12):3535-3542.
328 Schmid C, et al. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675-5687.
329 Laport GG, et al. Reduced-intensity conditioning follow by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246-255.
330 Hallemeier CL, et al. Long-term remissions in patients with myelodysplastic syndrome and secondary acute myelogenous leukemia undergoing allogeneic transplantation following a reduced intensity conditioning regimen of 550 cGy total body irradiation and cyclophosphamide. Biol Blood Marrow Transplant. 2006;12(7):749-757.
331 Maloney DG, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102(9):3447-3454.
332 Badros A, et al. Improved outcome of allogeneic transplantation in high-risk multiple myeloma patients after nonmyeloablative conditioning. J Clin Oncol. 2002;20(5):1295-1303.
333 Luznik L, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650.
334 Petersen FB, et al. Marrow transplantation following escalating doses of fractionated total-body irradiation and cyclophosphamide. A phase I trial. Int J Radiat Oncol Biol Phys. 1992;23(5):1027-1032.
335 Gratwohl A, et al. Role of splenic irradiation in patients with chronic myeloid leukemia undergoing allogeneic bone marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2000;6(2A):211-213.
336 Shank B, et al. Total body irradiation for bone marrow transplantation. The Memorial Sloan Kettering Cancer Center experience. Radiother Oncol. 1990;18(Suppl 1):68-81.
337 Quaranta BP, et al. The incidence of testicular recurrence in boys with acute leukemia treated with total body and testicular irradiation and stem cell transplantation. Cancer. 2004;101(4):845-850.
338 Alexander BM, et al. Utility of cranial boost in addition to total body irradiation in the treatment of high risk acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 2005;63(4):1191-1196.
339 Hoppe BS, et al. Involved-field radiotherapy before high-dose therapy and autologous stem-cell rescue in diffuse large-cell lymphoma. Long-term disease control and toxicity. J Clin Oncol. 2008;26(11):1858-1864.
340 van Bekkum DW. Conditioning regimens for the treatment of experimental arthritis with autologous bone marrow transplantation. Bone Marrow Transplant. 2000;25(4):357-364.
341 Saccardi R, et al. Autologous stem cell transplantation for progressive multiple sclerosis. Update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Multi Scler. 2006;12(6):814-823.
342 Nash RA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102(7):2364-2372.
343 Samijn JPA, et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77(1):46-50.
344 Burt RK, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis. Failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102(7):2373-2378.
345 Fortun PJ, Hawkey CJ. The role of stem cell transplantation in inflammatory bowel disease. Autoimmunity. 2008;41(8):654-659.
346 Binks M, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis. Procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60(6):577-584.
347 Farge D, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis. Report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004;63(8):974-981.
348 Nash RA, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis. Long-term follow-up of the US multicenter pilot study. Blood. 2007;110(4):1388-1396.
349 McSweeney PA, et al. High-dose immunosuppressive therapy for severe systemic sclerosis. Initial outcomes. Blood. 2002;100(5):1602-1610.
350 Iannone R, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9(8):519-528.
351 Hsieh MM, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361(24):2309-2317.
352 Hale GA, et al. Allogeneic bone marrow transplantation for children with histiocytic disorders. Use of TBI and omission of etoposide in the conditioning regimen. Bone Marrow Transplant. 2003;31(11):981-986.
353 Coccia PF, et al. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med. 1980;302(13):701-708.
354 Peters C, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87(11):4894-4902.
355 Peters C, et al. Cerebral X-linked adrenoleukodystrophy. The international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104(3):881-888.
356 Grewal SS, et al. Effective treatment of alpha-mannosidosis by allogeneic hematopoietic stem cell transplantation. J Pediatr. 2004;144(5):569-573.
357 Staba SL, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350(19):1960-1969.
358 Macmillan ML, et al. Haematopoietic cell transplantation in patients with Fanconi anaemia using alternate donors. Results of a total body irradiation dose escalation trial. Br J Haematol. 2000;109(1):121-129.