Tissue Engineering for Reconstruction of the Corneal Epithelium
Introduction
Over the past several years tissue engineering has become a rapidly growing field of research with strong translational potential into clinical practice through application of adult stem cells (SC) and biomaterial sciences. It is believed that SC, with their inherent plasticity, could be utilized to regenerate the natural complexity present in native tissue, while providing factors required for lineage maintenance and differentiation. Thus, the basic strategy of tissue engineering is the construction of a biocompatible scaffold that in combination with SC and bioactive molecules replaces and regenerates damaged cells or tissues.1
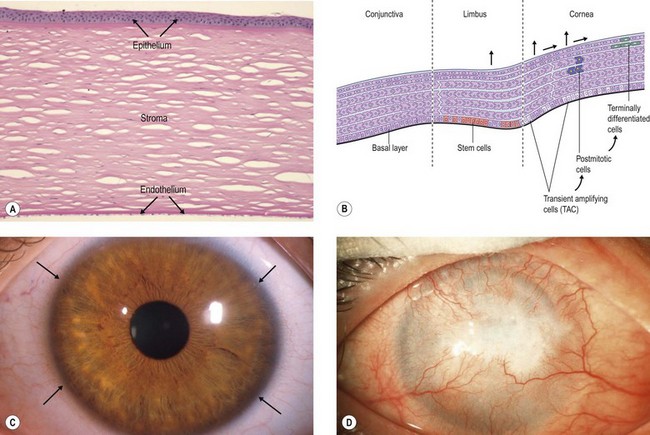
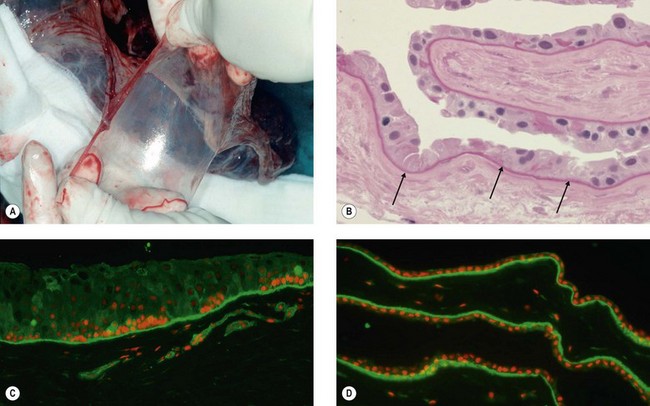
The cornea is composed of three layers, an outer stratified, rapidly regenerating epithelium, the underlying stroma, and an inner single-layered endothelium. Homeostasis of corneal epithelial cells is an important prerequisite not only for the integrity of the ocular surface, but also for corneal transparency and visual function (Fig. 43.1A,C). The continuous renewal of the corneal epithelium is provided by a population of adult SC located in the basal epithelium of the transitional zone between cornea and conjunctiva, the limbus (Fig. 43.1B).2 Stem cell maintenance and function are controlled by various intrinsic and extrinsic factors provided by a unique local microenvironment or niche.3 Limbal stem cells (LSC) and their progeny reside within small clusters in the basal epithelium in close relationship with specific extracellular matrix components, stromal fibroblasts, blood vessels, and nerves providing increased levels of growth and survival factors. Limbal SC can divide both symmetrically to self-renew and asymmetrically to produce daughter transiently amplifying cells that migrate centripetally to populate the basal layer of the corneal epithelium and eventually become post-mitotic terminally differentiated epithelial cells (Fig. 43.1B). Limbal SC can be identified by positive expression of putative stem/progenitor cell markers, including ABCG2, ΔNp63α, Bmi1, CEBPδ, OCT4, Lgr5, cytokeratin K15, and N-Cadherin, and the absence of corneal epithelial differentiation markers, such as cytokeratins K3 and K12.4
Dysfunction or loss of the LSC population in combination with an impairment of their niche, due to different inherited or acquired conditions may result in partial or total limbal stem cell deficiency (LSCD), which has severe consequences for ocular surface integrity and visual function (Fig. 43.1D). A range of inflammatory eye conditions (e.g. Stevens – Johnson syndrome, mucous membrane pemphigoid), degenerative processes (e.g. recurrent pterygia), hereditary causes (e.g. congenital aniridia), and chemical or thermal trauma, can lead to chronic epithelial healing defects associated with conjunctival epithelial ingrowth, neovascularization, inflammation, ulceration and scarring, and ultimately to functional blindness.5,6 These conditions are contraindications for conventional corneal transplantation, i.e., a full-thickness corneal graft, because the ocular surface will not re-epithelialize properly without replenishment of the depleted stem cell pool. Therefore, corneal repair may be possible only by addressing the epithelial disorder through layer-specific stem cell-based approaches to corneal surface reconstruction. Reconstruction of the stratified ocular surface epithelium, particularly in patients with bilateral LSCD, is critical to restore vision and represents one of the most challenging problems in clinical ophthalmology.
Current tissue engineering approaches for reconstruction of the corneal epithelium utilize adult SC, usually LSC derived from a small tissue biopsy from either the patient (autologous) or a donor (allogeneic), followed by their ex vivo expansion in culture on a natural scaffold, usually human amniotic membrane, and generation of three-dimensional epithelial constructs for transplantation.7 Differences in culture techniques include the use of explant or single-cell suspension systems, the presence or absence of a mouse 3T3 fibroblast feeder layer, the type of substrate, and the optional application of airlifting to promote epithelial differentiation and stratification. In their attempt to improve and standardize this therapeutic approach, researchers have focused on the optimization of culture conditions in order to replicate the in vivo stem cell niche and to preserve stemness, on the introduction of safer culture procedures to avoid the use of xenobiotics, on the exploration of alternative autologous stem cell sources for treatment of bilateral surface disorders, and on the evaluation of novel scaffolds to aid SC expansion and enhance transplantation efficacy. The challenges to create a tissue-engineered corneal epithelial equivalent include generating a biocompatible, mechanically stable, and optically transparent construct, which supports SC growth and maintenance in culture and after transplantation.
Stem Cell Sources for Corneal Epithelial Reconstruction
Limbal SC have been extensively investigated for their ex vivo culture and subsequent transplantation efficacy in clinical application. In addition, cultured oral mucosal epithelial SC have been used for the treatment of LSCD in patients with bilateral ocular surface disease.8 Cell types other than LSC or oral mucosal epithelial SC, including conjunctival epithelial SC,9–11 hair follicle SC,12,13 mesenchymal SC,14–16 immature dental pulp SC,17 umbilical cord SC,18 and embryonic SC19 have been additionally proposed as alternative autologous SC sources for tissue engineering and already provided promising results in vitro and in vivo preclinical animal studies. It is clearly desirable to use autologous cells for tissue engineering, as this avoids the risk of allogeneic immune rejection and the need for immunosuppression.
Conditions mimicking the in vivo LSC niche, such as limbal fibroblast conditioned media, have been used to induce a corneal epithelial-like phenotype in constructs derived from non-corneal SC sources.12,16,20 The suitability of epithelial constructs as a corneal epithelium replacement is usually evaluated by expression of typical corneal epithelial differentiation markers (cytokeratins K3 and K12) and the ability to reconstruct the damaged ocular surface by providing sufficient mechanical stability and optical transparency.
Limbal Epithelial Stem/Progenitor Cells
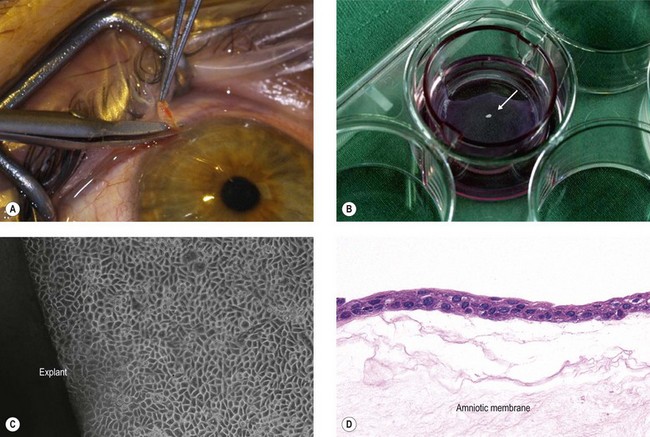
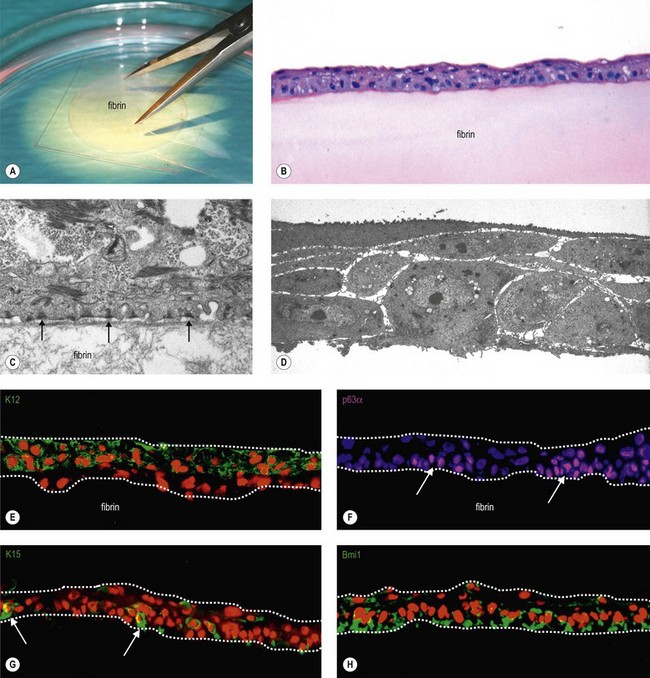
A major tissue engineering strategy for reconstruction of the corneal epithelium is based on ex vivo expansion of autologous SC taken from the limbus of the patient’s contralateral healthy eye in unilaterally affected patients.21,22 This cultured limbal epithelial transplantation (CLET) appears to be a promising treatment modality for LSCD with an overall success rate of 75% up to 119 months follow-up.23–26 The most widely used expansion method is the explant culture system, in which a small limbal biopsy (1–2 mm2) is placed on a carrier, usually amniotic membrane, and the limbal epithelial cells then migrate out of the biopsy and proliferate to form an epithelial sheet (Fig. 43.2). However, outgrowths from human limbal explants show a rapid decline in proliferative potential,27 and use of limbal epithelial cell suspensions appears to increase the proportion of SC in the culture system.28
An essential prerequisite of epithelial grafts for long-term restoration of the ocular surface is the presence of an adequate number of stem cells.4,29,30 Since the pioneering work by Rheinwald and Green,31 studies have confirmed that long-term survival of epithelial SC is possible if co-cultured with embryonic fibroblast feeder cells, which appear to reproduce aspects of the SC niche in vitro. This strategy led to the development of a culture system that involves enrichment of LSC by clonal growth on an inactivated mouse 3T3 fibroblast feeder layer before being seeded onto transplantable carriers to produce epithelial sheets.22,32 Preoperative demonstration of ΔNp63-positive cells within epithelial grafts has been considered as a means of quality control for transplantation.25,33–35
Over the past years there have been various modifications of culture techniques. Important parameters that influence cell proliferation and differentiation include growth factors, serum and calcium concentrations, type of matrix coating, and substrate stiffness as indicated by the elastic modulus.32,36 Culture of epithelial cells at the air – liquid interface (airlifting) has addressed the in situ environment of the cornea thereby promoting epithelial cell differentiation and stratification.37 Hypoxic conditions coupled with air exposure have been shown to further enhance LSC proliferation.38 Moreover, co-culture of LSC and adherent mesenchymal niche cells expressing embryonic SC markers has been reported to promote the preservation of a LSC phenotype.39 Recent approaches aim at establishing genetically modified cells, such as telomerase immortalized corneal epithelial cells,40 and at replacing potentially hazardous xenobiotics, such as fetal calf serum and murine feeder cells, by materials of human origin41–43 or using serum- and feeder-free culture systems.44
Non-Corneal Epithelial Stem/Progenitor Cells
For treatment of patients with bilateral ocular surface disease, strategies may utilize autologous SC from stratified epithelia of other areas of the body. Recent progress in this field suggests that conjunctival epithelium,9–11 oral mucosal epithelium,8,45,46 epidermis,47 and hair follicle12,32 may serve as alternative sources of autologous adult SC of related lineage, which can be used to construct an artificial corneal epithelium and to reconstruct the ocular surface in animal models and patients with LSCD.
Oral mucosal epithelium has attracted much attention as an autologous epithelial stem cell source and cultured oral mucosal epithelial transplantation (COMET) has already been used for restoration of the corneal surface in patients with bilateral LSCD.8,45,46,47,48 A recent study by Nakamura and coworkers showed promising long-term clinical results of COMET with an improved visual acuity in 10 eyes (53%) at 36 months postoperatively.49 However, peripheral neovascularization is commonly seen in COMET, and the long-term clinical success of this technique still requires detailed investigation. It has also been reported that oral mucosal epithelial cell grafts rarely transdifferentiate to a corneal epithelial phenotype, as indicated by lacking expression of cytokeratin K12.8
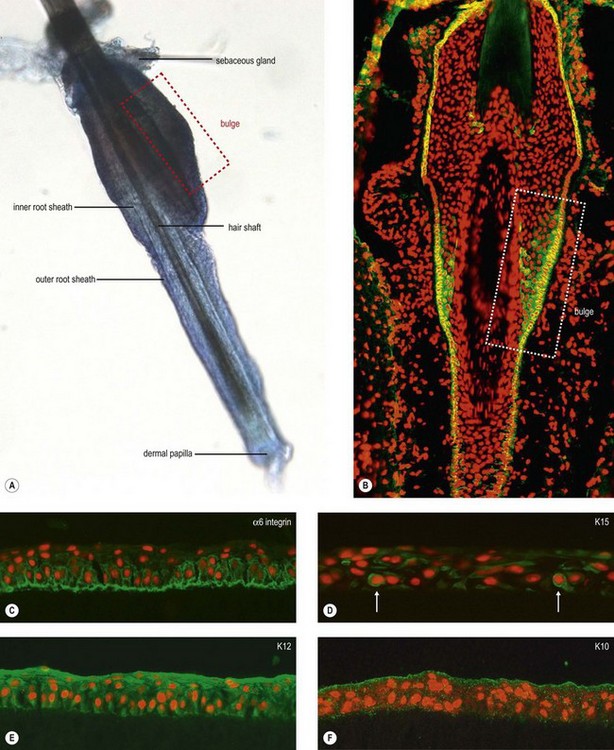
The discovery of various, not yet completely defined SC populations in the epithelial and mesenchymal compartment of the hair follicle, has encouraged research into utilizing the hair follicle as a source of adult multipotent SC for regenerative medicine.50 The hair follicle bulge region represents a major repository of multipotent keratinocyte SC, which have the potential to differentiate into hair follicle, sebaceous gland, and epidermis (Fig. 43.3A,B). The potential of murine hair follicle-derived adult SC to transdifferentiate into corneal epithelial-like cells has been recently shown by Blazejewska et al.12 When exposed to a limbus-specific environment using conditioned medium from limbal stromal fibroblasts and laminin V-coated culture dishes, hair follicle-derived SC could be reprogrammed in vitro into a corneal epithelial phenotype expressing corneal epithelial differentiation markers K12 and Pax6 (Fig. 43.3C–F). Using a transgenic reporter mouse model, which allowed for the detection of K12 expression in vivo, the transplanted corneal epithelial constructs provided evidence of corneal epithelial transdifferentiation and were able to reconstruct the ocular surface of LSCD mice (Fig. 43.4).13 These data highlight the promising therapeutic potential of these plastic and readily accessible SC to treat bilateral LSCD.
Mesenchymal Stem Cells
Other proposed sources of SC for reconstruction of the corneal epithelium include bone marrow-derived mesenchymal SC,14,15,51,52 adipose tissue-derived mesenchymal SC53 and dental pulp SC.17
Studies by Ye et al.15 have implied that locally recruited mesenchymal SC may play a role in corneal epithelial healing following alkali injury in a rabbit model and justified the idea that transplantation of mesenchymal SC could be used to treat corneal epithelial defects. Whereas injection of human mesenchymal SC under transplanted amniotic membrane did not provide an improved corneal surface, compared to controls,52 the ocular surface of chemically burned rat eyes was repaired when mesenchymal SC were transplanted as an intact sheet.14 However, their differentiation into corneal epithelial cells was not confirmed, leading these and other authors to suggest that the therapeutic effect of mesenchymal SC transplantation was rather associated with the inhibition of inflammation and angiogenesis than with mesenchymal to epithelial transdifferentiation.54 Though co-culture with corneal fibroblasts stimulated mesenchymal SC to express K3 and K12 and to transdifferentiate into a corneal epithelial phenotype in vitro and in vivo.16,51 Adipose tissue-derived mesenchymal SC, isolated from human orbital fat tissue, were also shown to differentiate into a corneal epithelial lineage when exposed to proper environmental stimuli, such as co-culture with corneal epithelial cells.53 Finally, human dental pulp stem cells, which represent a type of mesenchymal SC from dental pulp of deciduous teeth, have been shown to share key features with LSC and to have the capacity to differentiate into and reconstruct the corneal epithelium in rabbits after chemical burn.17
Embryonic Stem Cells
Embryonic SC remain largely unexplored for corneal regenerative applications. In one study, mouse embryonic SC cultured on collagen type IV were found to transdifferentiate into corneal epithelial cells and to re-epithelialize the chemically injured corneas of mice within 24 hours of application.19 In a follow-up study, Ueno et al.55 transplanted Pax6-transfected embryonic SC to damaged corneas with even better efficacy. Corneal restoration was achieved and no teratomas were observed. The group also used embryonic SC obtained from cynomolgous monkeys, which are more similar to human cells.56 Similarly, human embryonic SC cultured on type IV collagen using limbal fibroblast conditioned medium were found to express corneal epithelial markers, such as K3 and K12, in vitro.19 These studies provide initial data to support further investigation of embryonic SC in corneal epithelial tissue engineering.
Scaffolds for Corneal Epithelial Reconstruction
Biological Scaffolds
The most widely used substrate for corneal epithelial reconstruction is the human amniotic membrane (HAM), i.e., the innermost membrane of the fetal sac, which can be obtained from healthy volunteers during routine caesarean sections (Fig. 43.5A).57 It is composed of a single-layered epithelium, which rests on a thick basement membrane and an avascular stroma (Fig. 43.5B). In addition to its structural stability and elasticity, it has anti-immunogenic, antiangiogenic and anti-inflammatory properties, and contains a variety of growth factors and cytokines promoting epithelialization.58 HAM basement membrane composition shows extensive similarities with that of the human cornea and limbus (Fig. 43.5C,D),59 supporting the notion of HAM serving as a surrogate niche for ex vivo expansion of LSC.35,60 However, the preparation of HAM is not standardized, and it has been used fresh or frozen, and as an intact or epithelially denuded membrane. Epithelially denuded HAM exposing its BM appears to provide a superior niche for LSC proliferation and phenotypic maintenance.61 On the other hand, a progressive decline in the number of epithelial progenitor cells was observed during ex vivo expansion on HAM, suggesting limitations in its suitability as a surrogate limbal niche.3
Despite its extensive and successful clinical use in ocular surface reconstruction, HAM has several disadvantages, such as great intra- and interdonor tissue variability and poor standardization. Regional variations in growth factor concentrations and differences in protein expression profiles, dependent on donor age, race, length of pregnancy, and HAM processing and storage, affect not only composition and physical structure of the membrane but also clinical outcome.62 Additional shortcomings, such as low transparency, donor-associated risk of infections, inconsistent supply, high costs, and wrinkling during culture and transplantation, have encouraged efforts to develop alternative substrates for LSC expansion and ocular surface reconstruction.63 Still, HAM serves as a gold standard in all studies exploring the potential of novel scaffolds with respect to their mechanical and cell growth-supporting properties. In addition, attempts have been made to modify the HAM itself, e.g. by freeze-drying, coating with fibrin, or cross-linking with a polyvinyl alcohol (PVA) hydrogel, to enhance mechanical stability.63
Lens Capsule
Human anterior lens capsules obtained from patients undergoing cataract surgery were used as substrate for cultivation of LSC after removal of the lens epithelium in one study.64
Acellular and Cellular Corneal Tissue
Although the main field of application of decellularized corneas may be the development of artificial corneas, they have also been used as biological scaffolds to support the growth and differentiation of cultured limbal epithelial cells and to reconstruct the corneal epithelium and anterior stroma (‘hemicornea’).65 Such tissue-derived scaffolds provide an appropriate extracellular matrix for the functional reconstruction of tissues, such as the stratified corneal epithelium. For decellularization of animal, i.e. bovine or porcine, or human cadaver corneas, research groups have used various processing methods, including detergent- and nondetergent based approaches.65,66 Such tissue-processing methods can, in fact, be applied to xenografts because the essential components of the remaining extracellular matrix are often conserved between species. Consistently, transplantation experiments in rabbit eyes showed these biological constructs to be immunologically inert with mechanical and optical properties of native corneal tissue.
Reconstruction of a tissue-engineered human hemicornea using human keratoplasty lenticules as a biological scaffold resulted in natural corneal grafts for clinical applications.67 The lenticule stroma revealed an intact collagen architecture and viable keratocytes which maintained the phenotypic appearance of quiescent fibroblasts. Limbal SC expanded onto keratoplasty lenticules gave rise to a stratified keratinized epithelium morphologically similar to that of normal cornea without any alterations of their clonogenic potential.
Biosynthetic Scaffolds
Fibrin sealant or fibrin ‘glue’ is a natural serum-derived component of wound repair that is being utilized with increasing frequency in surgical applications. It is commercially available as a two-component system, which is prepared by diluting defined volumes of stock solutions of fibrinogen and thrombin of human origin in order to form a gel of standardized thickness.12 Aprotinin, a protease inhibitor, is added to the culture medium in order to prevent fibrinolysis by cultured cells.
Fibrin-based scaffolds have been used as suitable substrate for cultivating LSC and have been shown to promote epithelial adhesion, proliferation and migration, and to support the maintenance of a SC phenotype (Fig. 43.6).25,32,68,69 The fibrin-based epithelial constructs offer mechanical stability, resiliency, and good transparency, and appear to be completely degraded within few days after transplantation. Hence, fibrin-based epithelial constructs have been successfully used in the treatment of LSC deficiency.25,70 In their large series, Rama and colleagues25 reported that 82/107 eyes (76.6%) were considered as a success based on their ability to maintain a stable, avascular and transparent corneal surface for a mean follow-up period of 2.91 years (range 1–10 years).
In essence, distinct advantages of this biomatrix include its commercial availability, clinical approval, standardization, easy manipulation, complete and rapid bioadsorbance, and good mechanical and optical qualities. In view of these favorable properties, it is surprising that fibrin gels have not been used more widely for reconstruction of the corneal epithelium.26
Collagen Scaffolds
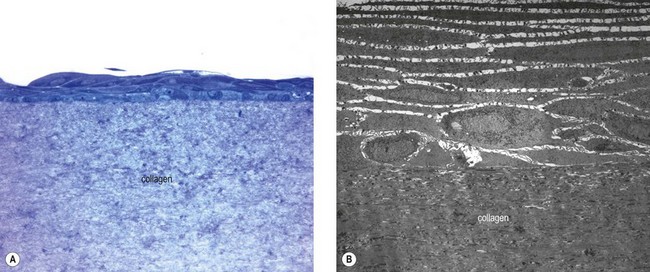
In view of the major structural roles played by collagen in different tissues, such as the corneal stroma, research has focused on developing novel biomaterials to mimic the fibrillar architecture as a scaffold for replacing the native collagenous extracellular matrix or for supporting adhesion and growth of overlying cells. Animal-derived and recombinant collagens, especially type I, are acknowledged as one of the most useful biomaterials available and are widely used for tissue engineering. They are used either in their native fibrillar form or after denaturation in variously fabricated forms, such as sheets of variable thickness.71 Thus, this natural biopolymer has been a commonly used constituent of bioengineered corneal tissue replacements.63 Collagen gels used as a cellular substrate are suitable carriers for corneal epithelial reconstruction to replace HAM (Fig. 43.7). For example, a novel source of collagen from fish scales was used to fabricate collagen scaffolds of good mechanical strength and optical transparency for ex vivo expansion of LSC.72
However, hydrated collagen gels contain a large proportion of water and are inherently weak. Collagen can be cross-linked using different methods to produce a rather stable hydrogel with improved mechanical properties. Carbodiimide-crosslinked recombinant human collagen type III was used as a substrate for LSC in culture.73 The hydrogels revealed a tensile strength, refractive index, transmission and backscatter properties that were similar to that of the native cornea, and supported growth, stratification, and stemness of LSC in culture. Similarly, type I collagen gels cross-linked with riboflavin and UV light were found to support LSC adhesion, proliferation, and formation of multilayered epithelial sheets, while preserving a stem cell phenotype in the basal epithelial layers. Preliminary transplantation experiments in a rabbit model confirmed their biocompatibility and optical transparency (Petsch et al., manuscript in preparation). However, chemical cross-linking may reduce biocompatibility and cell-based remodeling of the collagen scaffold, and may lower the rate of degradation.
Vitrification is a potential alternative method to chemical cross-linking, and consists of evaporation of fluid from the collagen gel at low temperatures to yield a thin (about 20 µm thick), rigid, glass-like platelet, which can be rehydrated to obtain a transparent collagen membrane, with improved mechanical properties.74 These vitrigel membranes are composed of high-density collagen fibrils, equivalent to connective tissues in vivo, e.g. Bowman’s layer of the corneal stroma. They have superior optical properties to other collagen substrates and have been used successfully as a matrix for keratinocyte and limbal epithelial cell culture and transplantation of stratified epithelial sheets.75,76 Recently, this group has used collagen vitrigel membranes for developing a corneal epithelial model for an ocular irritancy evaluation as an alternative to the Draize eye irritation test.77
Another elegant strategy to improve the mechanical quality of collagen scaffolds is plastic compression of collagen hydrogels, which results in thin, semitransparent membranes with enhanced mechanical strength. Collagen fibers within compressed gels were found to be densely packed and more evenly arranged than those of conventional collagen gels, resembling collagen fibers within the normal corneal stroma. Incorporated corneal fibroblasts obviously remain viable after compression and exert a beneficial microenvironmental effect on cultivated surface epithelial cells. Consistently, these cellular constructs have been shown to offer suitable substrates for reconstituting a stratified corneal epithelial cell sheet with phenotypic features closely resembling that of a normal corneal epithelium78,79 In addition, an increased mechanical stiffness of the collagen substrates, induced by compression, has been shown to promote cell growth and to influence the phenotype of cultured LSC by increasing expression of the differentiation marker K3 compared with uncompressed gels.80 The elastic modulus, which is a measure of tissue stiffness, was found to be 2900 Pa of compressed collagen gels, compared to 3 Pa of conventional collagen gels. Compressed collagen gels were thus suggested to represent a significant improvement over conventional collagen gels and to serve as an excellent biomimetic scaffold for the construction of an artificial corneal epithelium.
Collagen hydrogels are composed of a randomly arranged network of collagen fibrils. However, aligned sheets of collagen fibers allow for the construction of scaffolds that better mimic the dense and regularly structured connective tissue, such as the corneal stroma. The process of electrospinning, which is commonly used to produce synthetic polymer nanofibers, can also be used to achieve an alignment of type I collagen fibers. This method uses an electric field to control the deposition of collagen fibers from solutions that are combined with synthetic polymers, which may, however, not be applicable for cell culture purposes.81 Recently, electrospun aligned collagen I fibers have been produced using a less toxic solvent and have been used for cultivation of corneal fibroblasts.82,83 Such a construct appears to provide an appropriate viable scaffold material with improved optical properties for corneal stroma replacement, but its suitability for LSC expansion remains to be investigated.
Keratin Films
Keratins are a group of cysteine-rich structural proteins formed in epithelial cells of vertebrates. Keratin obtained from hair or wool has been proposed as an appropriate material for producing films or scaffolds for cell cultivation and tissue engineering.84 Transparent keratin films were engineered by a multistep procedure, including keratin extraction, neutral and alkaline dialysis, drying and a curing process, and were composed of a nanoparticulate keratin structure. The film characteristics could be varied by changing the protein composition, adding softening agents or varying the curing temperature and duration. Films based on human hair keratin showed improved biomechanical strength and light transmission as compared with HAM, but provided comparable conditions for corneal epithelial cell adhesion and proliferation. It has been suggested that keratin films may represent a new, promising alternative for ocular surface reconstruction, but further investigations need to be undertaken.
Silk Fibroin
Silk fibroin is a structural protein obtained from the cocoon of the domesticated silkworm (Bombyx mori). It is a particularly useful material in corneal bioengineering as it can be prepared as membranes of defined thickness and material characteristics, which are mechanically robust, highly transparent, porous, degradable, and easy to handle.85,86 Their biocompatibility was confirmed in rabbit corneas for up to 6 months.87 Silk fibroin films have also been shown to support the adhesion and growth of LSC in vitro, and epithelial multilayers constructed on silk fibroin retained comparable numbers of progenitor cells to those grown on HAM.88,89 Surface modifications, such as collagen coating or RGD-coupling appear to improve cell attachment and proliferation. It may also be used as a depot for biologically active molecules. Due to its fibrous structure, this biomaterial offers important benefits not only for corneal epithelial reconstruction but also for regeneration of the corneal stroma.90
Chitosan
Chitosan is a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine. Chitosan is produced commercially by deacetylation of chitin, which is the structural element in the exoskeleton of crustaceans and cell walls of fungi. Novel polymeric hydrogel scaffolds based on blends of chitosan and other biopolymers, such as hydroxypropylcellulose, polycaprolactone, collagen, and elastin, have been fabricated for corneal epithelial tissue engineering.91 Blending two polymers provides the chance to develop novel biomaterials with superior properties to that of the individual components. Comparing various combinations, hydrogels prepared from chitosan – collagen blend, cross-linked with genipin, provided adequate mechanical properties and the most promising scaffolds for corneal epithelial cell culture by supporting the formation of a regular stratified epithelial layer.
Three-dimensional porous collagen-GAG-chitosan matrices have been used as a scaffold for the combined cultivation of epithelial cells and fibroblasts enabling the reconstruction of a ‘hemicornea’ composed of a surface epithelium in combination with a connective tissue equivalent.92
Synthetic Scaffolds
Electrospinning is a widely used technique for the production of nanofibers from various natural or synthetic polymers. By adjusting the processing conditions and the composition of the polymer solution, the properties of the resultant nanofibrous networks, including fiber diameter, porosity, mechanical properties and surface topography, can be tightly controlled. Electrospun nanofibers have been proposed as a biocompatible cell culture substrate. The structure of the nanofibrous network mimics the fibrillar architecture of the extracellular matrix, and the high surface area to volume ratio has been shown to support adhesion, proliferation and differentiation of various cell types.93
Currently, electrospun nanofibers fabricated from poly-ε-caprolactone (PCL) dissolved in trifluoroethanol have been studied as a potential scaffold for LSC expansion.94 The polymer, consisting of fibers about 130 nm in diameter with a pore size of 0.2–4.0 µm and a tensile strength of 1.75 MPa, proved to be biocompatible and to support LSC attachment, proliferation, and differentiation into a normal corneal epithelial phenotype. Epithelial cells infiltrated the nanofibers and formed a three-dimensional corneal epithelium, which was viable for two weeks. Similarly, 3D-nanofiber scaffolds prepared from polyamide 6/12 (PA6/12) by electrospun technology, have been shown to represent a suitable scaffold for expansion of LSC, and were able to reconstruct the ocular surface of mouse eyes. Co-transfer with mesenchymal SC, which have immunosuppressive properties, significantly inhibited local inflammatory reactions and supported the healing process.95 A comparison of randomly oriented and aligned nanofiber scaffolds showed better corneal epithelial cell growth on randomly arranged networks.96
Therapeutic Contact Lens
In 2007, Di Girolamo and coworkers provided proof-of-concept that human limbal epithelial cells could be directly expanded on a standard siloxane-hydrogel contact lens used as a bandage for patients after pterygium surgery.97 This material (Lotrafilcon A) sustained proliferation, migration and differentiation of limbal cells in the presence of autologous serum and without 3T3 feeder cells. In a follow-up pilot study, autologous limbal or conjunctival SC were expanded and cell-laden contact lenses were transferred to the damaged corneal surface of three patients with LSCD. After removal of the contact lenses 2–3 weeks later, a stable corneal epithelial surface was restored in all patients.98 One year after transplantation, clinical results showed establishment of a healthy corneal surface and significant improvement in visual acuity. The authors suggested that autologous SC transfer using a FDA-approved contact lens may provide a safe and convenient therapeutic strategy for patients with LSCD.
Other researchers have used acrylic acid-coated contact lenses to improve cell attachment and facilitate the delivery of cultured limbal epithelial cells in animal models.99
Carrier-Free Epithelial Cell Sheets
Enzymatic Substrate Degradation
Higa and colleagues24,100 have developed a technique for generating carrier-free corneal epithelial sheets using a commercial biodegradable fibrin sealant as culture substrate. They seeded corneal epithelial cells onto a fibrin matrix that was later enzymatically digested by intrinsic proteases to create a stratified epithelial sheet, which could be transplanted onto the corneal surface of rabbit eyes without sutures. These epithelial sheets seemed to contain more differentiated epithelial cells than those cultivated on HAM, while retaining similar levels of colony-forming progenitor cells. This technique was subsequently adopted for the cultivation and transplantation of carrier-free oral mucosal epithelial cell sheets.101 The results showed that the success rates of COMET at 12 months after surgery were 62.5% in the substrate-free sheet group and only 43.8% in the HAM control group.
Carrier-free epithelial cell sheets can be also produced by cultivating corneal epithelial cells in culture dishes coated with biodegradable type I collagen and subsequent release of the multilayered cell sheet by using collagenase digestion.102
Temperature-Responsive Substrates
Temperature-responsive synthetic polymers, e.g. poly-N-isopropylacrylamide, chemically immobilized in thin films on cell culture surfaces, exhibit reversible hydration and dehydration of their polymer chains in response to temperature changes. They facilitate cell adhesion and growth at normal cell culture conditions at 37°C, whereas, by lowering the temperature below 30°C, they can reversibly alter their hydration properties, prompting complete detachment of adherent cells, without the use of proteolytic enzymes.103 Taking advantage of this elegant technique, Nishida et al.104 have developed carrier-free corneal epithelial cell sheets that have been transplanted directly to the ocular surface of rabbit eyes without sutures. Subsequent preclinical studies demonstrated the great potential of this method for carrier-free transplantation, which ensures optimal preservation of cell-cell junctions and basement membrane components produced during cultivation.105,106
Similar temperature-responsive culture wells, the UpCell®-Inserts (CellSeed Inc, Tokyo, Japan), have been used to manufacture Cultured Autologous Oral Mucosal Epithelial Cell-Sheets (CAOMECS), transparent, resistant and rapidly bioadhesive cell sheets.107,108 Suture-free transplantation of CAOMECS has been proposed for the treatment of total bilateral LSCD, in patients with moderate or severe symptoms, for whom any other treatment options are not applicable. The safety and efficacy of CAOMECS transplantation has been recently confirmed in a clinical study, showing a successful clinical outcome, defined by a stable avascular ocular surface, in 16/25 patients (64%) one year after grafting.108
Mebiol gel, a synthetic copolymer comprised of thermoresponsive polymer poly-N-isopropylacrylamide-co-n-butyl methacrylate and hydrophilic polymer polyethylene glycol (PEG), is hydrophilic below 20°C and forms a hydrophobic hydrogel at 37°C.109 The sol-gel transition temperature can be controlled by altering the chemical composition of the thermoreversible gelation polymer. Human LSC cultured on Mebiol gel in the absence of a 3T3 feeder layer, showed a high proliferative capacity and a limbal SC phenotype, as indicated by expression of ABCG2 and p63.110 Transplantation of hydrogel-based epithelial cell sheets showed high transparency of the construct and restoration of a nearly normal ocular epithelial surface in rabbit eyes.111
Centrifugation-Constructed Corneal Epithelial Sheet (CCCES)
Zhang and coworkers developed a centrifugal cell seeding method for the rapid and efficient construction of corneal epithelial sheets for reconstruction of the ocular surface in a rabbit model of LSCD.112 Corneal epithelial sheets were constructed by applying centrifugal driving force, and orthogonal design experiments were used to optimize centrifugation parameters for cell seeding. In vitro experiments showed the rapid construction of three-layered epithelial sheets, which displayed the characteristics of a normal corneal epithelium. In vivo, CCCES successfully reconstructed the cornea epithelium in a rabbit model of LSCD.
References
1. Polak, JM, Bishop, AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366.
2. Cotsarelis, G, Cheng, SZ, Dong, G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209.
3. Li, W, Hayashida, Y, Chen, YT, et al. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36.
4. Pellegrini, G, Rama, P, Mavilio, F, et al. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J Pathol. 2009;217:217–228.
5. Dua, HS, Saini, JS, Azuara-Blanco, A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48:83–92.
6. Burman, S, Sangwan, V. Cultivated limbal stem cell transplantation for ocular surface reconstruction. Clin Ophthalmol. 2008;2:489–502.
7. Selvam, S, Thomas, PB, Yiu, SC. Tissue engineering: current and future approaches to ocular surface reconstruction. Ocul Surf. 2006;4:120–136.
8. Nishida, K, Yamato, M, Hayashida, Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196.
9. Tanioka, H, Kawasaki, S, Yamasaki, K, et al. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci. 2006;47:3820–3827.
10. Ono, K, Yokoo, S, Mimura, T, et al. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol Vis. 2007;13:1138–1143.
11. Ang, LP, Tanioka, H, Kawasaki, S, et al. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2010;51:758–764.
12. Blazejewska, EA, Schlötzer-Schrehardt, U, Zenkel, M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–652.
13. Meyer-Blazejewska, EA, Call, MK, Yamanaka, O, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells. 2011;29:57–66.
14. Ma, Y, Xu, Y, Xiao, Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–321.
15. Ye, J, Yao, K, Kim, JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye. 2006;20:482–490.
16. Jiang, TS, Cai, L, Ji, WY, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304–1316.
17. Gomes, JA, Geraldes Monteiro, B, Melo, GB, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51:1408–1414.
18. Reza, HM, Ng, BY, Gimeno, FL, et al. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev. 2011;7:935–947.
19. Homma, R, Yoshikawa, H, Takeno, M, et al. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Ophthalmol Vis Sci. 2004;45:4320–4326.
20. Ahmad, S, Stewart, R, Yung, S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–1155.
21. Lindberg, K, Brown, ME, Chaves, HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34:2672–2679.
22. Pellegrini, G, Traverso, CE, Franzi, AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993.
23. Shortt, AJ, Secker, GA, Notara, MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52:483–502.
24. Higa, K, Shimazaki, J. Recent advances in cultivated epithelial transplantation. Cornea. 2008;27(Suppl. 1):S41–S47.
25. Rama, P, Matuska, S, Paganoni, G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155.
26. Baylis, O, Figueiredo, F, Henein, C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112:993–1002.
27. Li, W, Hayashida, Y, He, H, et al. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:605–613.
28. Koizumi, N, Cooper, LJ, Fullwood, NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–2121.
29. De Luca, M, Pellegrini, G, Green, H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med. 2006;1:45–57.
30. Pellegrini, G, Rama, P, De Luca, M. Vision from the right stem. Trends Mol Med. 2010;17:1–7.
31. Rheinwald, JG, Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343.
32. Meyer-Blazejewska, EA, Kruse, FE, Bitterer, K, et al. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Invest Ophthalmol Vis Sci. 2010;51:765–774.
33. Pellegrini, G, Dellambra, E, Golisano, O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161.
34. Di Iorio, E, Barbaro, V, Ruzza, A, et al. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–9528.
35. Tsai, RJ, Tsai, RY. Ex vivo expansion of corneal stem cells on amniotic membrane and their outcome. Eye Contact Lens. 2010;36:305–309.
36. Eberwein, P, Steinberg, T, Set, Schulz. al. Expression of keratinocyte biomarkers is governed by environmental biomechanics. Eur J Cell Biol. 2011;90:1029–1040.
37. Ban, Y, Cooper, LJ, Fullwood, NJ, et al. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003;76:735–743.
38. Li, C, Yin, T, Dong, N, et al. Oxygen tension affects terminal differentiation of corneal limbal epithelial cells. J Cell Physiol. 2011;226:2429–2437.
39. Chen, SY, Hayashida, Y, Chen, MY, et al. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548.
40. Robertson, DM, Kalangara, JP, Baucom, RB, et al. A reconstituted telomerase-immortalized human corneal epithelium in vivo: a pilot study. Curr Eye Res. 2011;36:706–712.
41. Chen, YT, Li, W, Hayashida, Y, et al. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005.
42. Notara, M, Haddow, DB, MacNeil, S, et al. A xenobiotic-free culture system for human limbal epithelial stem cells. Regen Med. 2007;2:919–927.
43. Omoto, M, Miyashita, H, Shimmura, S, et al. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci. 2009;50:2109–2115.
44. Shortt, AJ, Secker, GA, Rajan, MS, et al. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115:1989–1997.
45. Nakamura, T, Kinoshita, S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea. 2003;22:S75–S80.
46. Inatomi, T, Nakamura, T, Koizumi, N, et al. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141:267–275.
47. Yang, X, Moldovan, NI, Zhao, Q, et al. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol Vis. 2008;14:1064–1070.
48. Ang, LP, Nakamura, T, Inatomi, T, et al. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124:1543–1551.
49. Nakamura, T, Takeda, K, Inatomi, T, et al. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–946.
50. Tiede, S, Kloepper, JE, Bodò, E, et al. Hair follicle stem cells: walking the maze. Eur J Cell Biol. 2007;86:355–376.
51. Gu, S, Xing, C, Han, J, et al. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107.
52. Reinshagen, H, Auw-Haedrich, C, Sorg, RV, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011;89:741–748.
53. Ho, JH, Ma, WH, Tseng, TC, et al. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A. 2011;17:255–266.
54. Oh, JY, Kim, MK, Shin, MS, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055.
55. Ueno, H, Kurokawa, MS, Kayama, M, et al. Experimental transplantation of corneal epithelium-like cells induced by Pax6 gene transfection of mouse embryonic stem cells. Cornea. 2007;26:1220–1227.
56. Kumagai, Y, Kurokawa, MS, Ueno, H, et al. Induction of corneal epithelium-like cells from cynomolgus monkey embryonic stem cells and their experimental transplantation to damaged cornea. Cornea. 2010;29:432–438.
57. Gomes, JA, Romano, A, Santos, MS, et al. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–240.
58. Tseng, SCG, Espana, EM, Kawakita, T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–187.
59. Dietrich-Ntoukas, T, Hofmann-Rummelt, C, Kruse, FE, et al. Comparative analysis of the basement membrane composition of the human limbus epithelium and amniotic membrane epithelium. Cornea. 2012;31:564–569.
60. Grueterich, M, Espana, EM, Tseng, SCG. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646.
61. Koizumi, N, Rigby, H, Fullwood, NJ, et al. Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:123–134.
62. Dua, HS, Rahman, I, Miri, A, et al. Variations in amniotic membrane: relevance for clinical applications. Br J Ophthalmol. 2010;94:963–964.
63. Levis, H, Daniels, JT. New technologies in limbal epithelial stem cell transplantation. Curr Opin Biotechnol. 2009;20:593–597.
64. Galal, A, Perez-Santonja, JJ, Rodriguez-Prats, JL, et al. Human anterior lens capsule as a biologic substrate for the ex vivo expansion of limbal stem cells in ocular surface reconstruction. Cornea. 2007;26:473–478.
65. Shafiq, MA, Gemeinhart, RA, Yue, BY, et al. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng Part C Methods. 2012;18:340–348.
66. Ponce Márquez, S, Martínez, VS, McIntosh Ambrose, W, et al. Decellularization of bovine corneas for tissue engineering applications. Acta Biomater. 2009;5:1839–1847.
67. Barbaro, V, Ferrari, S, Fasolo, A, et al. Reconstruction of a human hemicornea through natural scaffolds compatible with the growth of corneal epithelial stem cells and stromal keratocytes. Mol Vis. 2009;15:2084–2093.
68. Han, B, Schwab, IR, Madsen, TK, et al. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea. 2002;21:505–510.
69. Talbot, M, Carrier, P, Giasson, CJ, et al. Autologous transplantation of rabbit limbal epithelia cultured on fibrin gels for ocular surface reconstruction. Mol Vis. 2006;12:65–75.
70. Rama, P, Bonini, S, Lambiase, A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485.
71. Cen, L, Liu, W, Cui, L, et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492–496.
72. Krishnan, S, Sekar, S, Katheem, MF, et al. Fish scale collagen–a novel material for corneal tissue engineering. Artif Organs. 2012;36:829–835.
73. Dravida, S, Gaddipati, S, Griffith, M, et al. A biomimetic scaffold for culturing limbal stem cells: a promising alternative for clinical transplantation. J Tissue Eng Regen Med. 2008;2:263–271.
74. Takezawa, T, Ozaki, K, Nitani, A, et al. Collagen vitrigel: a novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004;13:463–473.
75. McIntosh Ambrose, W, Salahuddin, A, So, S, et al. Collagen Vitrigel membranes for the in vitro reconstruction of separate corneal epithelial, stromal, and endothelial cell layers. J Biomed Mater Res B Appl Biomater. 2009;90:818–831.
76. McIntosh Ambrose, W, Schein, O, Elisseeff, J. A tale of two tissues: stem cells in cartilage and corneal tissue engineering. Curr Stem Cell Res Ther. 2010;5:37–48.
77. Takezawa, T, Nishikawa, K, Wang, PC. Development of a human corneal epithelium model utilizing a collagen vitrigel membrane and the changes of its barrier function induced by exposing eye irritant chemicals. Toxicol In Vitro. 2011;25:1237–1241.
78. Levis, HJ, Brown, RA, Daniels, JT. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials. 2010;31:7726–7737.
79. Mi, S, Chen, B, Wright, B, et al. Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J Biomed Mater Res A. 2010;95:447–453.
80. Jones, RR, Hamley, IW, Connon, CJ. Ex vivo expansion of limbal stem cells is affected by substrate properties. Stem Cell Res. 2012;8:403–409.
81. Buttafoco, L, Kolkman, NG, Engbers-Buijtenhuijs, P, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–734.
82. Wray, LS, Orwin, EJ. Recreating the microenvironment of the native cornea for tissue engineering applications. Tissue Eng Part A. 2009;15:1463–1472.
83. Phu, D, Wray, LS, Warren, RV, et al. Effect of substrate composition and alignment on corneal cell phenotype. Tissue Eng Part A. 2011;17:799–807.
84. Reichl, S, Borrelli, M, Geerling, G. Keratin films for ocular surface reconstruction. Biomaterials. 2011;32:3375–3386.
85. Lawrence, BD, Marchant, JK, MAet, Pindrus. al. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30:1299–1308.
86. Lawrence, BD, Pan, Z, Weber, MD, et al. Silk film culture system for in vitro analysis and biomaterial design. J Vis Exp. 2012;24:62.
87. Higa, K, Takeshima, N, Moro, F, et al. Porous silk fibroin film as a transparent carrier for cultivated corneal epithelial sheets. J Biomater Sci Polym Ed. 2011;22:2261–2276.
88. Chirila, T, Barnard, Z, Zainuddin, A, et al. Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng Part A. 2008;14:1203–1211.
89. Bray, LJ, George, KA, Ainscough, SL, et al. Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials. 2011;32:5086–5091.
90. Bray, LJ, George, KA, Hutmacher, DW, et al. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials. 2012;33:3529–3538.
91. Grolik, M, Szczubiałka, K, Wowra, B, et al. Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering. J Mater Sci Mater Med. 2012;23:1991–2000.
92. Auxenfans, C, Builles, N, Andre, V, et al. Porous matrix and primary-cell culture: a shared concept for skin and cornea tissue engineering. Pathol Biol. 2009;57:290–298.
93. Kubinová, S, Syková, E. Nanotechnologies in regenerative medicine. Minim Invasive Ther Allied Technol. 2010;19:144–156.
94. Sharma, S, Mohanty, S, Gupta, D, et al. Cellular response of limbal epithelial cells on electrospun poly-ε-caprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Mol Vis. 2011;17:2898–2910.
95. Zajicova, A, Pokorna, K, Lencova, A, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19:1281–1290.
96. Yan, J, Qiang, L, Gao, Y, et al. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J Biomed Mater Res A. 2011. [[Epub ahead of print]].
97. Di Girolamo, N, Chui, J, Wakefield, D, et al. Cultured human ocular surface epithelium on therapeutic contact lenses. Br J Ophthalmol. 2007;91:459–464.
98. Di Girolamo, N, Bosch, M, Zamora, K, et al. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87:1571–1578.
99. Deshpande, P, Notara, M, Bullett, N, et al. Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng Part A. 2009;15:2889–2902.
100. Higa, K, Shimmura, S, Kato, N, et al. Proliferation and differentiation of transplantable rabbit epithelial sheets engineered with or without an amniotic membrane carrier. Invest Ophthalmol Vis Sci. 2007;48:597–604.
101. Hirayama, M, Satake, Y, Higa, K, et al. Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci. 2012;53:1602–1609.
102. Ke, Q, Wang, X, Gao, Q, et al. Carrier-free epithelial cell sheets prepared by enzymatic degradation of collagen gel. J Tissue Eng. Regen Med. 2011;5:138–145.
103. Schmaljohann, D, Oswald, J, Jørgensen, B, et al. Thermo-responsive PNiPAAm-g-PEG films for controlled cell detachment. Biomacromolecules. 2003;4:1733–1739.
104. Nishida, K, Yamato, M, Hayashida, Y, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385.
105. Hayashida, Y, Nishida, K, Yamato, M, et al. Transplantation of tissue-engineered epithelial cell sheets after excimer laser photoablation reduces postoperative corneal haze. Invest Ophthalmol Vis Sci. 2006;47:552–557.
106. Yang, J, Yamato, M, Nishida, K, et al. T. Cell delivery in regenerative medicine: the cell sheet engineering approach. J Control Release. 2006;116:193–203.
107. Yamato, M, Utsumi, M, Kushida, A, et al. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473–480.
108. Burillon, C, Huot, L, Justin, V, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53:1325–1331.
109. Vemuganti, GK, Fatima, A, Madhira, SL, et al. Limbal stem cells: application in ocular biomedicine. Int Rev Cell Mol Biol. 2009;275:133–181.
110. Sudha, B, Madhavan, HN, Sitalakshmi, G, et al. Cultivation of human corneal limbal stem cells in Mebiol gel–A thermo-reversible gelation polymer. Indian J Med Res. 2006;124:655–664.
111. Sitalakshmi, G, Sudha, B, Madhavan, HN, et al. Ex vivo cultivation of corneal limbal epithelial cells in a thermoreversible polymer (Mebiol Gel) and their transplantation in rabbits: an animal model. Tissue Eng Part. 2009;15:407–415.
112. Zhang, W, Xiao, J, Li, C, et al. Rapidly constructed scaffold-free cornea epithelial sheets for ocular surface reconstruction. Tissue Eng Part C Methods. 2011;17:569–577.