Thyroidectomy and Parathyroidectomy
Thyroidectomy
Surgical Anatomy for Thyroidectomy
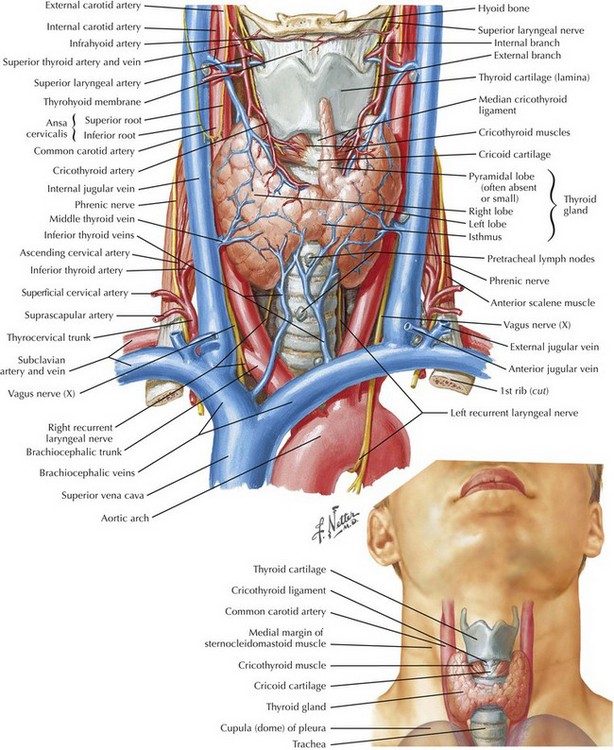
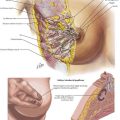
The thyroid gland consists of two lobes and an isthmus (Fig. 3-1). Between 30% and 60% of patients also have a pyramidal lobe that forms as a result of the persistence of the embryologic thyroglossal duct. The principal blood supply to the thyroid gland comes from the inferior thyroid artery, a branch of the thyrocervical trunk and the superior thyroid artery, which is the first branch of the external carotid artery. The venous drainage of the thyroid gland is from the superior and middle thyroid veins, which empty into the internal jugular vein, and the inferior thyroid veins, which empty into the brachiocephalic veins.
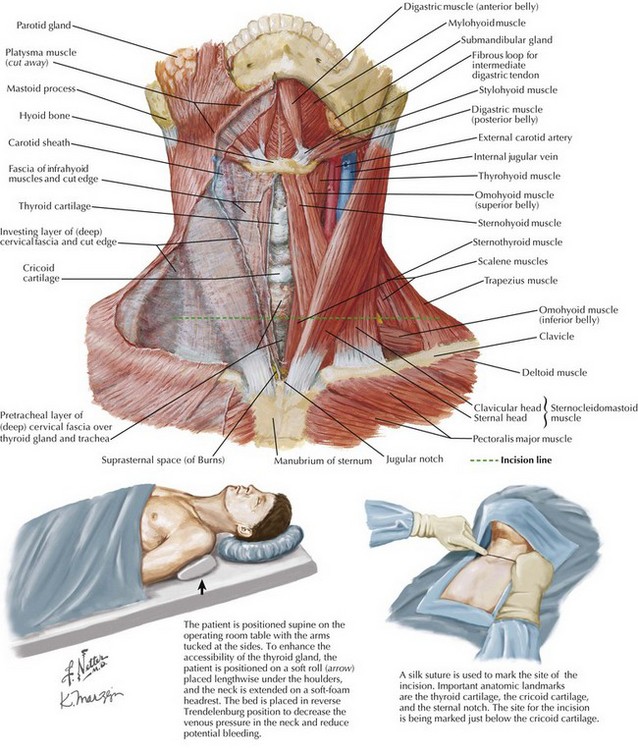
Determining the surface anatomy of the neck is necessary for proper placement of the skin incision (Figs. 3-1 and 3-2). The patient is positioned supine on the operating room table with the arms tucked at the side, a soft roll placed lengthwise beneath the shoulders, and the head in a soft-foam headrest with the neck extended (Fig. 3-2). Important landmarks that should be palpated include the prominence of the thyroid cartilage, the cricoid cartilage, and the sternal notch. The isthmus of the thyroid gland lies immediately below the cricoid cartilage. A transverse skin incision is made in a normal skin crease below the cricoid cartilage for an optimal cosmetic result.
Anatomy for Exposing the Thyroid Gland
It is necessary to create a working space to remove the thyroid gland. This is accomplished first by dividing the subcutaneous tissue and platysma muscle to expose the sternal head of the sternocleidomastoid muscles laterally and the sternothyroid muscles in the midline of the incision (Figs. 3-2 and 3-3, A). Superior and inferior skin flaps are raised in the subplatysmal plane, anterior to the anterior jugular veins. The skin flaps are raised superior to the prominence of the thyroid cartilage, inferior to the sternal notch, and lateral to the sternal head of the sternocleidomastoid muscles.
Anatomy for Mobilization of the Thyroid Lobe
Anteromedial traction is applied to the lobe of the thyroid gland, and the middle thyroid vein is divided (Fig. 3-3, B). The areolar tissue between the thyroid gland and the common carotid artery is separated using a combination of blunt and sharp dissection, allowing for further anteromedial mobilization of the thyroid gland. The superior thyroid artery and the superior thyroid veins are exposed by applying caudal and lateral traction to the thyroid parenchyma at the superior pole. This helps expose the cricothyroid space and facilitates the individual ligation of the superior-pole vessels close to the thyroid gland, staying lateral to the muscles of the pharynx and the larynx to avoid injury to the external branch of the superior laryngeal nerve (EBSLN).
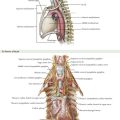
Anatomy of the Superior Laryngeal Nerve
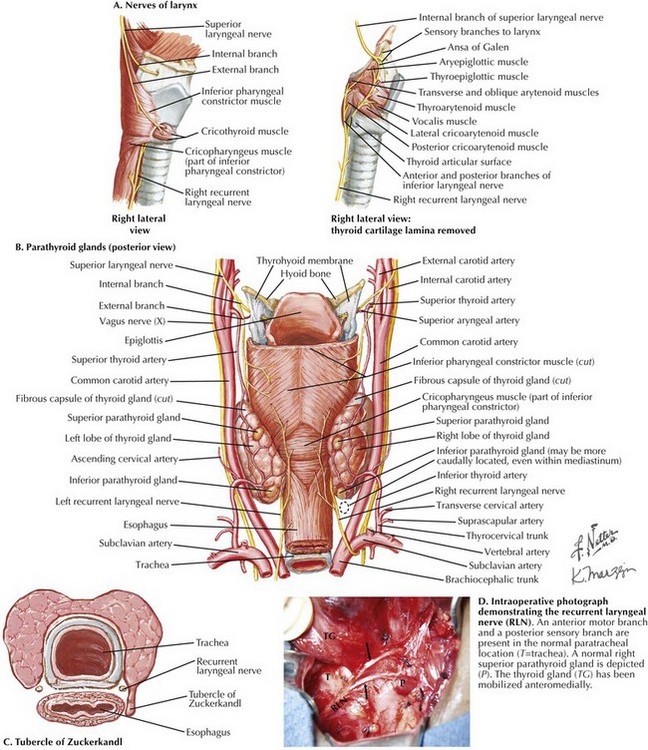
The superior laryngeal nerve is a branch of the vagus nerve from high in the neck (Fig. 3-4, A and B). At 2 to 3 cm above the superior-pole vessels, the superior laryngeal nerve divides into an internal branch that provides sensory innervations to the supraglottic area of the larynx and the base of the tongue and an external branch that provides motor innervation to the cricothyroid muscle.
Anatomy of the Recurrent Laryngeal Nerve
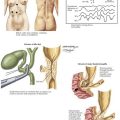
The recurrent laryngeal nerves (RLNs) are branches of the vagus nerve from within the thorax (Fig. 3-4). The right RLN “recurs” around the right subclavian artery, and the left RLN recurs around the arch of the aorta. The RLNs ascend into to the neck from the thoracic inlet, passing from lateral to medial into the tracheoesophageal groove. The left RLN enters the neck in a more medial location than the right RLN, which has a more oblique course.
The RLN is identified inferior to the inferior thyroid artery, where its location is most constant. In the neck the RLN crosses the inferior thyroid artery and then maintains a paratracheal location for approximately 1 cm before passing posterior to the inferior edge of the inferior pharyngeal constrictor muscle to enter the larynx (see Fig. 3-3, B).
In 85% to 90% of patients, thyroid parenchyma tissue protrudes from the posterolateral margin of the lateral lobe of the thyroid gland, known as the tubercle of Zuckerkandl (Fig. 3-4, C). This is an important anatomic landmark because the RLN passes in the tracheoesophageal groove beneath the inferior portion of the tubercle of Zuckerkandl before it turns posteriorly to enter the larynx. Before entering the larynx, the RLN divides into two or more branches in 40% to 80% of patients (Fig. 3-4, D). The RLN innervates all the muscles of the larynx except the cricothyroid muscle.
Parathyroidectomy
Photographs
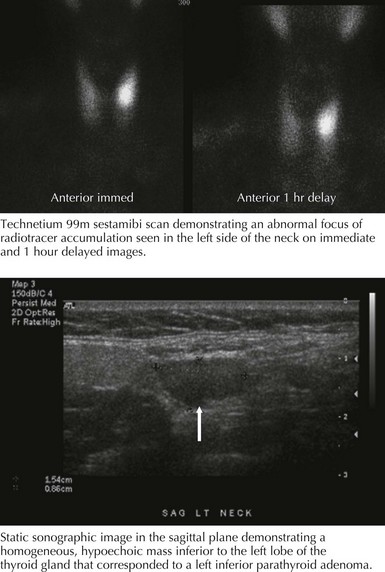
Accurate preoperative parathyroid localization is a prerequisite for performing MIP. Technetium 99m sestamibi scintigraphy and high-resolution ultrasonography are the localizing procedures of choice (Fig. 3-5). Intraoperative parathyroid hormone (IOPTH) measurement is used to determine whether additional hyperfunctioning parathyroid tissue remains. A greater than 50% decline in IOPTH levels 10 minutes after excision of all hyperfunctioning parathyroid tissue is indicative of a curative parathyroidectomy. Failure of IOPTH levels to decline by more than 50% indicates that residual hyperfunctioning tissue remains and that further exploration is necessary.
Anatomy and Embryology of Parathyroid Glands
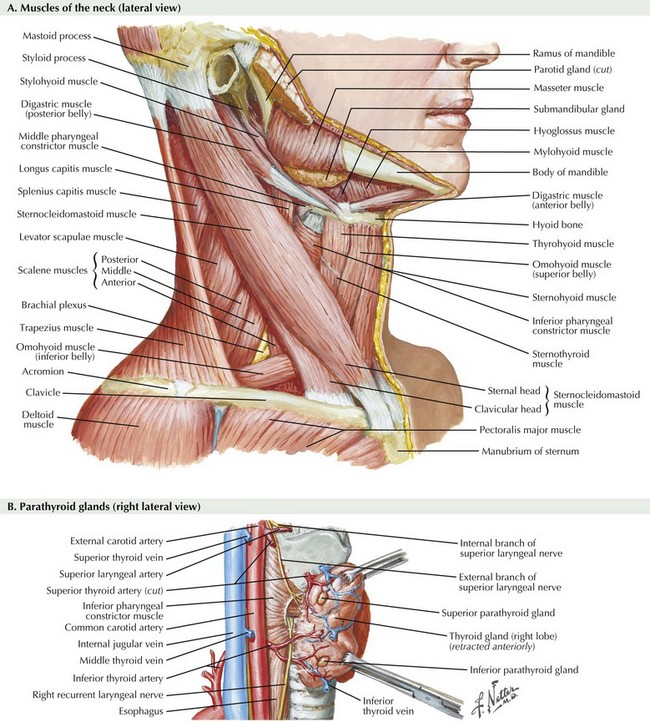
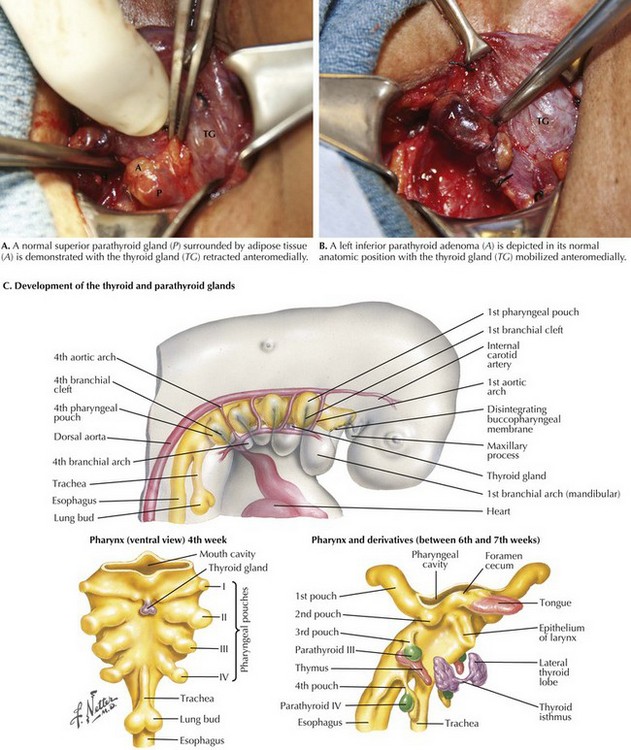
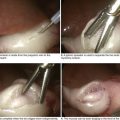
A normal parathyroid gland is oval, bean shaped or spherical, yellow-tan in color, 5 mm in maximum dimension, and weighs 35 mg or less. It is usually surrounded by adipose tissue (Fig. 3-6, A). A parathyroid gland can be flattened in appearance, especially when it is within the capsule or adherent to the thyroid gland.
The superior parathyroid glands are usually posterior and superior to the RLN, approximately 1 cm cephalad from the junction of the RLN and inferior thyroid artery at the level of the cricoid cartilage, where the RLN enters posterior to the inferior pharyngeal constrictor muscle (see Fig 3-3, B). The superior glands are often found in a subcapsular location. The inferior parathyroid glands are usually anterior to the RLN along the posterolateral aspect of the inferior pole to the thyroid gland, approximately 1 cm caudal to the junction to the RLN and inferior thyroid artery. The inferior parathyroid glands are less often subcapsular. The inferior thyroid artery is the principal blood supply to the parathyroid glands, although they may also obtain blood supply from the superior thyroid artery and small vessels from the capsule of the thyroid gland.
The steps necessary to expose the parathyroid glands are similar to those used for thyroidectomy, except a smaller incision is used (2.5 to 4.0 cm). Superior and inferior skin flaps are raised; the strap muscles are separated in the midline; and the lobe of the thyroid gland is mobilized and retracted anteromedially. The search for an enlarged parathyroid gland begins with an exploration of the normal anatomic locations for the superior and inferior parathyroid glands (Fig. 3-6, B). Division of the middle thyroid vein and the superior-pole vessels is generally unnecessary, but this may be helpful in exposing parathyroid glands, especially in ectopic locations.
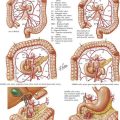
The inferior parathyroid glands and the thymus develop from the third bronchial pouch (Fig. 3-6, C). The superior parathyroid glands and the lateral lobes of the thyroid gland develop from the fourth bronchial pouch. Inferior parathyroid glands undergo extensive migration with the primordium of the thymus and eventually become located on the dorsolateral surface of the inferior pole of the thyroid lobe. These superior parathyroid glands undergo minimal migration and become located on the dorsomedial surface of the superior pole of the thyroid gland.
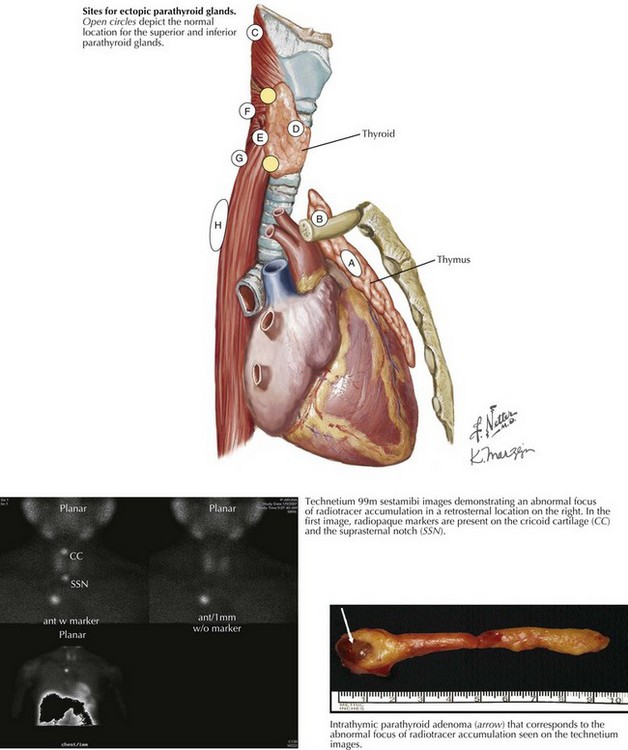
The inferior parathyroid glands, because of their extensive embryologic migration, are more often present in ectopic locations. The most common location for an ectopic inferior parathyroid gland is within the thymus (Fig. 3-7, top, circled A). Other sites include the thyrothymic ligament and the anterosuperior mediastinum (B), undescended in a submandibular location (C), or within the thyroid gland (D). The most common ectopic location for a superior parathyroid gland is in the tracheoesophageal groove (E). Ectopic superior parathyroid glands may also be found in a retropharyngeal (F), retroesophageal (G), posterior mediastinal (H), or intrathyroidal (D) location. Preoperative localization is important to identify ectopic glands, particularly the retrosternal, intrathymic adenoma (Fig. 3-7, bottom scans and photo) or the undescended submandibular adenoma.
Ander, S, Johansson, K, Lennquist, S, Smed, S. Human parathyroid blood supply determined by laser Doppler flowmetry. World J Surg. 1994;18(3):417–420.
Irvin, GL, 3rd., Prudhomme, DL, Deriso, GT, Sfakianakis, G, Chandarlapaty, SK. A new approach to parathyroidectomy. Ann Surg. 1994;219:574–579.
McHenry, CR. Thyroidectomy for nodules and small cancers. In: Duh QY, Clark OH, Kebebew E, eds. Atlas of endocrine surgery techniques. Surgical Techniques Atlas Series. Philadelphia: Saunders; 2010:3–24.
Phitayakorn, R, McHenry, CR. Parathyroidectomy: overview of the anatomic basis and surgical strategies for parathyroid operations. Clin Rev Bone Miner Metab. 2007;5:89–102.
Phitayakorn, R, McHenry, CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg. 2006;191(3):418–423.