68 Thyroid Disease
Thyroid Hormone Production
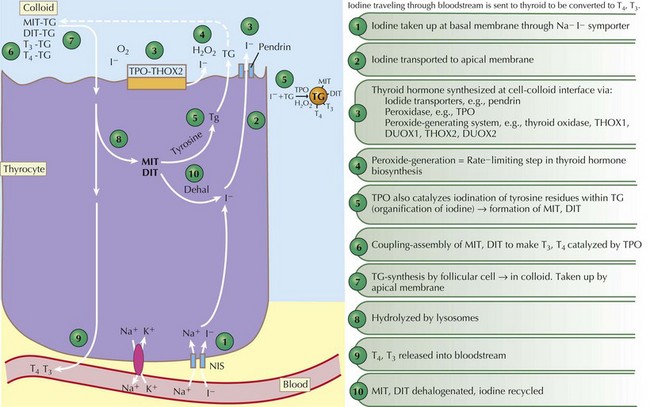
Thyroid hormones are produced by the coupling of iodine molecules to the amino acid tyrosine (Figure 68-1). The principal secreted thyroid hormone is T4. The normal thyroid secretes only small amounts of T3, and most circulating T3 (≈70%-90%) is derived from peripheral deiodination of T4. Thyroid hormones circulate bound to carrier proteins, including thyroid-binding globulin (TBG), transthyretin (thyroxine-binding prealbumin), and albumin. Less than 1% of circulating T4 and T3 are unbound or “free.”
Congenital Hypothyroidism
Etiology and Pathogenesis
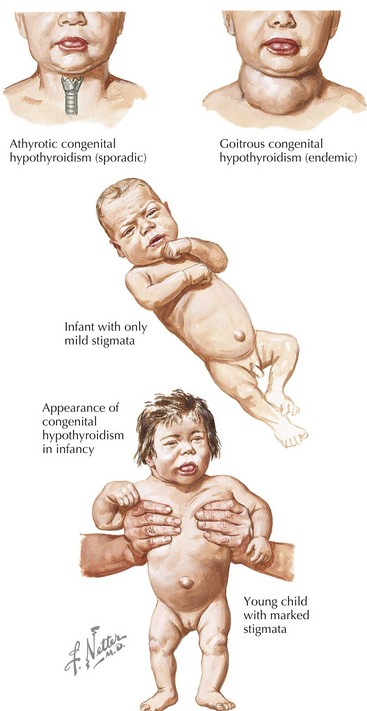
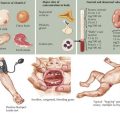
Congenital hypothyroidism (CH) is one of the most common causes of preventable mental retardation (Figure 68-2). Fortunately, early identification and rapid treatment lead to normal neurocognitive development. Although approximately 10% of cases of CH are transient and caused by factors such as iodine exposure, prematurity, or maternal transfer of antithyroid antibodies, in most cases, hypothyroidism is permanent. Worldwide, iodine deficiency is the most common cause of CH. However, in areas of the world where iodine deficiency is uncommon, CH most commonly results from thyroid dysgenesis (≈75% of cases); thyroid dyshormonogenesis (≈10%), TSH deficiency (5%), and genetic defects in the TSH receptor are much less common. The incidence of CH is approximately one in 3000 to 4000 births. In most cases, CH is sporadic, but mutations in genes encoding transcription factors that are required for normal development of the thyroid gland are present in about 10% to 15% of cases. Circulating levels of TSH are elevated and thyroid hormone levels are low in those with CH.
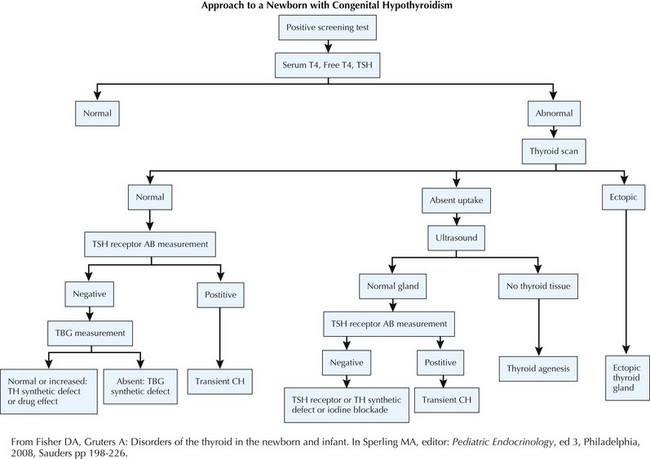
Evaluation (Figure 68-3)
Acquired Hypothyroidism
Acquired Hyperthyroidism
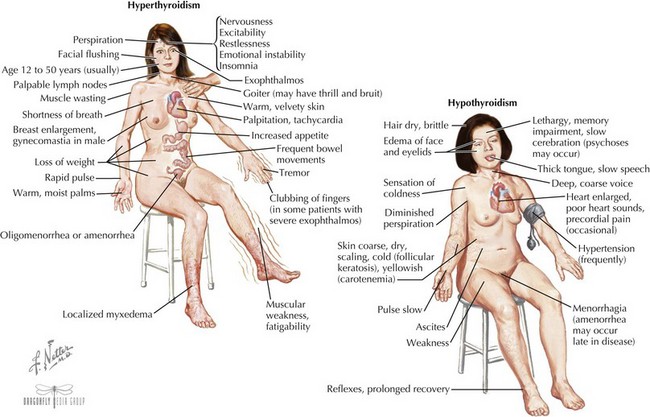
Clinical Presentation (see Figure 68-4)
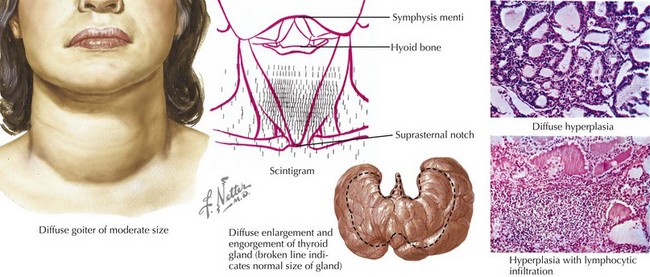
The eyes may reveal a “lid lag” or prominent eyes, with or without proptosis (exophthalmos) in which the eyes are pushed forward (may be asymmetric). In Graves’ disease, the more severely hyperthyroid patients have the largest goiters (Figure 68-5). Mild polyuria may be seen. Frequent bowel movements, rather than diarrhea, are occasionally present.
Evaluation
Thyroid TPO and TG antibodies can be present in both Graves’ disease and chronic lymphocytic thyroiditis. Measuring TSI can be helpful when the diagnosis of Graves’ disease is uncertain in a child with hyperthyroidism. An I-123 scan will show diffusely high uptake of iodine in patients with Graves’ disease (see Figure 68-5) and patchy areas of uptake in those with chronic lymphocytic thyroiditis. The thyroid gland is hypervascular in Graves’ disease, and this increased blood flow can be assessed by color-flow Doppler ultrasonography.
American Academy of PediatricsRose SR, Section on Endocrinology and Committee on GeneticsAmerican Thyroid AssociationBrown RS, Public Health Committee, Lawson Wilkins Pediatric Endocrine SocietyFoley T, Kaplowitz PB, Kaye CI, et al. Update on newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290-2303.
Fisher DA, Gruters A. Disorders of the thyroid in the newborn and infant. In: Sperling MA, editor. Pediatric Endocrinology. ed 3. Philadelphia: Saunders; 2008:198-226.
Gruters A, Krude H. Update on the management of congenital hypothyroidism. Horm Res. 2007;68(Suppl 5):107-111.
Kratzsch J, Pulzer F. Thyroid gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22:57-75.
Ng SM, Anand D, Weundling AM: High versus low dose initial thyroid hormone replacement for congenital hypothyroidism. Cochrane Database Syst Rev (1):CD006972, 2009.
Osborn DA, Hunt R: Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database Sys Rev(1):CD005948, 2007.