Chapter 36 Thyroid Cancer
Thyroid tumors are the most common endocrine neoplasms. They usually manifest as anterior neck nodules, which in most cases can be localized to the thyroid gland by palpation. Most nodules are benign hyperplastic (or colloid) nodules, but 5% to 20% of nodules that come to medical attention are true neoplasms: benign follicular adenomas or carcinomas of follicular or parafollicular cell (C cell) origin. Differentiating true neoplasms from hyperplastic nodules and distinguishing benign from malignant tumors are major diagnostic challenges for clinical endocrinologists. High-resolution ultrasound studies assessing large groups of normal volunteers suggested that the prevalence of incidentally discovered nodular thyroid disease in healthy adults is more than 60%.1 In the United States during 2010, however, only 44,670 new cases of thyroid cancer were expected.2 Given that the prevalence of clinical thyroid cancer in most populations is much less than 1%, most so-called thyroid incidentalomas must be benign.3
In the current era, when patients are increasingly being advised on the advantages of self-examination to detect cancer at an early stage, the finding of a palpable mass in such a superficial and visibly obvious location as the thyroid gland can be disconcerting, and the patient is likely to seek prompt medical evaluation. At the conclusion of an appropriate investigation, the patient can usually be reassured that the nodule is benign. If the discovered lesion is suspected to be malignant, the patient can be advised that the management of typical thyroid cancer is effective4 and usually consists of surgical resection,5 followed by medical therapy6 and regular postoperative surveillance.7 In recent years, a degree of consensus has been achieved with regard to the initial evaluation of nodular thyroid disease8,9 and the management of differentiated thyroid cancer,8,10,11 but important biologic and clinical questions remain unanswered.12–14
From a radiation oncologic standpoint, follicular cell–derived cancer (FCDC) of the thyroid has some historic interest. In 1940, Hamilton and associates15 at the University of California, San Francisco, first reported evidence of uptake of radioactive iodine (RAI) in FCDC. In 1942 at Columbia University, Keston and colleagues16 gave a patient with an iodophilic femoral metastasis a 10-mCi therapeutic dose of RAI. Since that time, RAI therapy has become the primary treatment for iodophilic distant metastases in the papillary or follicular histologic type of FCDC.17 The concept of radioiodine remnant ablation (RRA) to “complete” a thyroidectomy in FCDC, derived from pioneering work by Blahd and colleagues18 at the University of California, Los Angeles, in the late 1950s, has been increasingly employed in the routine management of FCDC.19 The use of external beam radiotherapy (EBRT) in managing thyroid cancer has its origin in the 1960s.20 Subsequently, the role of EBRT generally has been restricted to the treatment of locoregional advanced FCDC, but it has also been employed as primary therapy in the management of two rare malignancies: undifferentiated (anaplastic) thyroid carcinoma and primary malignant lymphoma of the thyroid.21 In this chapter, we discuss the more common FCDCs, but where indicated, less common tumors derived from nonfollicular cell origin are considered.
Etiology and Epidemiology
The annual incidence of thyroid cancer is 0.5 to 10 per 100,000 people in the general population in most countries; a global estimate suggested 87,000 new cases worldwide each year.22 Clinical thyroid malignancy is relatively uncommon, accounting for only about 2% of human malignancies.2,23 Nonetheless, it was estimated that thyroid cancers would account for 95% of endocrine malignancies in the United States in 2010.2 It was estimated by the American Cancer Society that 1690 patients with thyroid cancer would die during 2010, accounting for 66% of deaths from endocrine malignancies.2
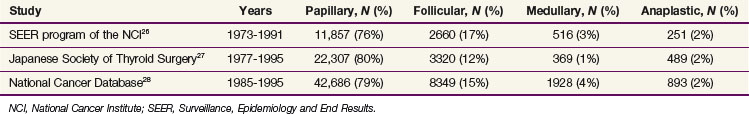
Most thyroid cancers are derived from follicular epithelium. In most countries, incidence rates for papillary thyroid carcinoma (PTC) generally exceed the incidence rates for follicular thyroid carcinoma (FTC), and either PTC or FTC is far more common than the usually lethal anaplastic (undifferentiated) thyroid carcinoma.24,25 Very similar data on the frequency of the various histologic types are contained in three large series of more than 97,000 cases of thyroid cancer reported from the United States and Japan.26–28 These data are summarized in Table 36-1.
Anaplastic carcinomas and FTCs tend to be relatively more common in areas endemic for goiter, and numerous case-control studies have strongly suggested that dietary iodine content is responsible for the increased incidence rates in these areas.29 This hypothesis is supported by the fact that dietary iodine supplementation has been shown to increase the relative proportion of PTC and to decrease the frequency of FTC.30
PTC and FTC are more than twice as common in women as men and tend to occur much more commonly in middle age and later, although patients with PTC are younger than patients with FTC.24,25 The preponderance of women with FCDC has led to speculation about the role of estrogens as a risk factor. Other putatively estrogen-dependent tumors, particularly breast cancer, and thyroid cancer occur more frequently in the same individual than expected by chance.31 Case-control studies have suggested a correlation between pregnancy, a high estrogen state, and the onset of thyroid cancer.32 Other studies have suggested that pregnancy per se, rather than the associated estrogen levels, may be associated with increased thyroid cancer risk.33 The role of female sex hormones as a risk factor for thyroid cancer development must still be considered unresolved.
The most firmly established risk factor for development of thyroid cancer is prior exposure to ionizing radiation, particularly to the head and neck region during childhood, which used to be a common problem in some exposed populations in Japan and the Pacific during the 1960s and 1970s in the aftermath of atomic bomb use at the end of World War II and in areas exposed to atmospheric nuclear bomb tests during the 1950s and 1960s.34,35 In most other countries, radiation treatments for benign medical conditions, such as acne vulgaris, thymic enlargement, tinea capitis, or inflammatory connective tissue disorders, contributed to increasing numbers of patients with thyroid cancer.36,37 These practices have largely been abandoned, and radiation exposure as a risk factor for the most part has ceased to be of significant importance.37 Exceptions are areas of high natural background radiation; radiotherapy used for malignant conditions38; and areas where radioactive contamination of the environment from military or civilian sources is a notable problem, particularly in many countries in the southern part of the former Soviet Union that were heavily contaminated in the wake of the Chernobyl nuclear reactor accident.39 The contamination in parts of Belarus and Ukraine was significant and prolonged, and there is mounting clinical evidence that this contamination has led to increased rates of thyroid malignancies, often of an unexpectedly aggressive nature.40–42 In the United States, it has been disclosed that a significant segment of the U.S. population was exposed to radioiodine during a series of nuclear bomb explosions at the Nevada test site in the 1950s.43 Predictions of excess relative risk of thyroid cancer vary but may prove to be significant. However, it is impossible to determine whether individual thyroid cancers arose as a result of radiation exposure or as sporadic events.43
Although most cases of thyroid cancer are sporadic occurrences, a small proportion of thyroid cancers may be familial, and in the case of C cell–derived malignancy, medullary thyroid carcinoma (MTC) may be associated with multiple endocrine neoplasia (MEN) type II (MEN-II) syndrome and its associated adrenal medullary tumors (pheochromocytomas). In patients with the MEN-II syndrome, a strong and typical family history can often be obtained. PTC may also be associated with other nonthyroid malignancies and with premalignant conditions such as Cowden’s syndrome and familial adenomatous polyposis coli (i.e., Gardner’s syndrome).44 Several cases of familial PTC have been described.45 No such distinct associations exist for FTC, but aggregation of FTC cases in families with dyshormonogenesis has been described.46
Prevention and Early Detection
In contrast, early detection of the often more aggressive, familial MTC is possible when there is evidence within the family of an inherited mutation of the RET proto-oncogene in the pericentromeric region of the short arm of chromosome 10. Such mutations have been found in more than 90% of familial MTC cases, and since the successful cloning and sequencing of the RET gene, asymptomatic members of affected families can be tested for the presence of a mutation at this locus.47 A positive test result obviates the need for any further testing. The present recommendation for persons shown to harbor the mutation is to undergo prophylactic total thyroidectomy, which completely prevents the development of the invariably multicentric MTC associated with these conditions.11,48 If such a mutation is found in infancy, the current practice is to perform a total thyroidectomy when the child is 3 to 5 years old.11,49
Pathology and Pathways of Spread
PTC most often occurs in patients 30 to 50 years old; the mean age at diagnosis is about 45 years. Most primary tumors are 1 to 4 cm in diameter; the average is about 2 to 3 cm in the greatest dimension. Of tumors, 95% are classified on the basis of degree of differentiation as histologic grade 1 (of 4); 80% of primary PTC tumors are assessed to be DNA diploid by flow cytometry.50 Extrathyroidal invasion of adjacent soft tissues is present in about 15% (range 5% to 34%) at primary surgery, and about one-third of PTC patients have clinically evident lymphadenopathy at presentation.51 About 35% to 50% of excised neck nodes have histologic evidence of involvement, and in patients 17 years old or younger, nodal involvement may be present in up to 90%.52 The primary disease is confined to the neck in 93% to 99% of PTC patients at diagnosis.50,51 Spread to superior mediastinal nodes is usually associated with extensive neck nodal involvement. Distant metastases are diagnosed in only 1% to 7% of patients with PTC before or within 30 days of primary treatment.50,53
FTC occurs in older patients, and the mean age in most studies is more than 50 years, about 10 years older than for typical PTC.25,54 Women affected by FTC outnumber men by more than 2 : 1. FTC patients rarely (4% to 6%) have clinically evident lymphadenopathy at presentation.55 In most series, the average tumor size in FTC is larger than in PTC.54 When tumor grading is performed, higher grade tumors are more common than with PTC.54 DNA aneuploidy is present in about 60% of FTC tumors and in up to 90% of patients with oxyphilic or Hürthle cell variant tumors.25,56 Direct extrathyroidal extension into adjacent soft tissues does not occur in the common “minimally invasive” FTC, but it is not unusual in the rare patient with “widely invasive” FTC. Of patients with FTC, 5% to 20% may have distant metastases at presentation, and the most common sites of distant spread are lung and bone.25,57
MTC arises from the C cells of the thyroid rather than the follicular epithelium; secretes a characteristic hormone, calcitonin; is frequently associated with one or more paraendocrine manifestations; and provides an early biochemical signal (i.e., hypersecretion of calcitonin) that permits its early detection, treatment, and cure.11,58 The tumor occurs in sporadic and familial forms, with the latter constituting about 20% of the total. The familial variety usually appears at a younger age, is almost invariably bilateral, is less likely to have associated cervical metastases at presentation, and has a better prognosis.11,58 Most importantly, the familial variety is preceded by a premalignant C cell hyperplasia that can be cured by total thyroidectomy.59
ATC is the least common FCDC and typically constitutes less than 5% of most reported series.60 It usually occurs after age 60 years, and it is only slightly more common in women than men (1.3 : 1 to 1.5 : 1). It is highly malignant, rapidly invading adjacent structures and metastasizing throughout the body. Pathologic examination of tumor biopsy specimens may reveal evidence of PTC or FTC, which may represent a precursor of ATC. Thorough biopsy sampling may be necessary to detect residual well-differentiated thyroid tissue. On histologic examination, the tumor is usually composed of atypical cells that exhibit numerous mitoses and form a variety of patterns. Spindle-shaped cells and multinucleated giant cells usually predominate, but in a third histologic pattern described as squamoid, the cells are undifferentiated but retain an epithelial appearance. It was formerly thought that there was a small cell ATC, but most of these tumors have been classified as malignant thyroid lymphomas.
Biologic Characteristics, Prognostic Factors, and Staging
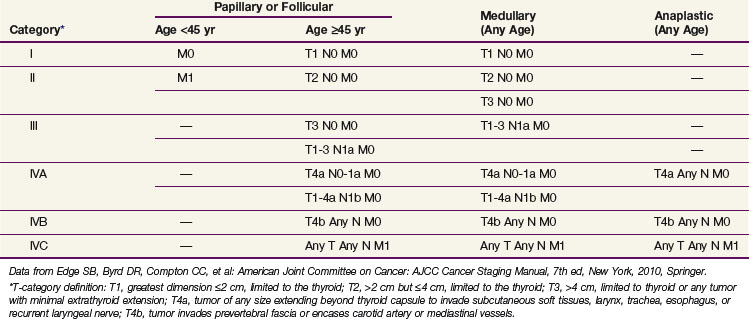
The seventh edition of the TNM (primary tumor, regional nodes, metastases) staging scheme for thyroid carcinoma, approved in 2009 by the International Union Against Cancer61 and in 2010 by the American Joint Committee on Cancer (AJCC),62 is presented in Table 36-2. The primary tumor status is defined in this scheme on the basis of the size of the primary lesion (diameter in centimeters) and the presence of extrathyroidal extension. A T1 tumor is 2 cm or smaller and limited to the thyroid. T1a refers to tumors of 1 cm or less, and T1b refers to a tumor more than 1 cm but not more than 2 cm in greatest dimension. A T2 tumor is 2.1 to 4.0 cm in diameter and limited to the thyroid. A T3 tumor is larger than 4 cm and limited to the thyroid or any tumor with minimal extrathyroidal extension (i.e., to sternothyroid muscles or perithyroid soft tissues). A T4a tumor represents moderately advanced disease, which extends beyond the thyroid capsule and invades subcutaneous soft tissue, larynx, trachea, esophagus, or recurrent laryngeal nerve, and a T4b tumor represents very locally advanced disease, typically invading prevertebral fascia or encasing carotid artery or mediastinal vessels.
Papillary Thyroid Carcinoma
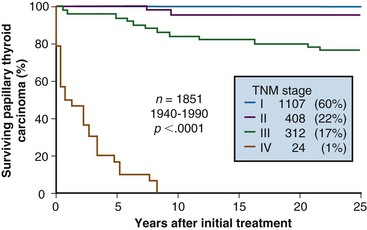
Most patients with PTC present with localized, node-negative disease.28,50,65 Patients 45 years old or older with nodal metastases or extrathyroidal extension account for less than 20% of cases.50 Only about 1% to 3% of older PTC patients present with distant metastases. Figure 36-1 shows cause-specific survival according to pathologic TNM (pTNM) stage in a cohort of 1851 patients who underwent surgical treatment at Mayo Clinic during the period 1940-1990.63
Three types of tumor relapse may occur with PTC: postoperative regional nodal metastasis, local recurrences, and postoperative distant metastases. Local recurrence has been defined as “histologically confirmed tumor occurring in the resected thyroid bed, thyroid remnant, or other adjacent tissues of the neck (excluding lymph nodes)” after complete surgical removal of the primary tumor.64 Nodal or distant spread is considered postoperative if the metastases are discovered within 180 days or 30 days, respectively. In a cohort of 2370 patients with PTC who did not have initial distant metastases and had complete surgical resection of their primary tumors during treatment at Mayo Clinic from 1945-2000, relapse rates at nodal, local, and distant sites were 9.8%, 5.5%, and 4.6% after 25 years of follow-up.65 In a larger cohort of 2512 PTC patients treated during the period 1940-1990 at Mayo Clinic, the cause-specific survival rates at 5, 10, and 20 postoperative years were 98%, 96%, and 95%.65 Of patients with lethal PTC, 20% of deaths occurred within the first year after diagnosis, and 80% of the deaths occurred within 10 postoperative years.50,51,65
Only a fraction (≈15%) of PTC patients have relapse of disease, and even fewer (≈5%) have a lethal outcome.50–52,65 The exceptional patient who experiences an aggressive course tends to relapse early, and the rare fatalities usually occur within 5 to 10 years of initial diagnoses.50,51,65
Multivariate analyses have been used to identify variables predictive of cause-specific mortality.66,67,68,69 Increasing patient age and presence of extrathyroidal invasion are independent prognostic factors in all such studies.66,67,68,69 The presence of initial distant metastases and large size of the primary tumor are also significant variables in most studies,66,68 and some groups26,28,50,66,67 have reported that histopathologic grade (i.e., degree of differentiation) is an independent variable. The completeness of initial tumor resection (i.e., postoperative status) is also a predictor of mortality.50,69 The presence of initial neck nodal metastasis, although relevant to future nodal relapse, does not apparently influence cause-specific mortality.50,51,69
From a multivariate analysis of more than 14,200 patient-years of experience, a prognostic scoring system was devised and named the AGES system after five independent variables: patient’s age, tumor grade, tumor extent (e.g., local invasion, distant metastasis), and tumor size.50,66 With the use of such a scoring system, 86% of PTC patients were in the minimal-risk group (AGES score <4), and they had a cause-specific mortality rate of only 1%.50 In contrast, patients with AGES scores of 4 or greater (i.e., high-risk group is 14% of the total) had a 20-year cause-specific mortality rate of 40%. Based on the description of the Mayo Clinic–derived AGES system,66 Cady and Rossi,70 working at the Lahey Clinic, devised a simplified version of the AGES system, which they called the AMES multifactorial system. The AMES system disregarded tumor grade because this information was not readily available to the authors, but they took advantage of the other four variables: age, metastasis, extent, and size. The details of the AMES system are presented in Table 36-3. Of the 1961-1980 cohort of patients studied by Cady and Rossi, 89% were deemed low risk and had a death rate of 1.8%—outcome results virtually identical to results defined by the AGES prognostic scores.50,66
TABLE 36-3 Classification of Risk Group Categories According to the AMES System
| Low Risk |
AMES, age, metastasis, extent, and size.
Data from Cady B, Rossi R: An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947-953, 1988.
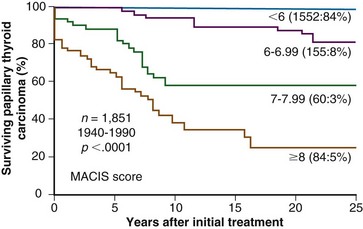
Although the AGES scheme had the potential for universal application, some academic centers could not include the differentiation variable (tumor grade [G]) because their surgical pathologists did not recognize higher grade PTC tumors.71 Another prognostic scoring system for predicting PTC mortality rates was devised with the use of candidate variables that included completeness of primary tumor resection but excluded histologic grade.69 Cox model analysis and stepwise variable selection led to a final prognostic model that included five variables: metastasis, age, completeness of resection, invasion, and size (MACIS). The final score was defined as MACIS = 3.1 (if age is ≤39 years) or 0.08 × age (if age is ≥40 years), +0.3 × tumor size (in centimeters), +1 (if tumor not completely resected), +1 (if locally invasive), +3 (if distant metastases are present). As illustrated by Figure 36-2, the MACIS scoring system permitted identification of patient groups with a broad range of risk of death from PTC. Twenty-year cause-specific survival rates for patients with MACIS scores of less than 6, 6 to 6.99, 7 to 7.99, and 8 or more were 99%, 84%, 56%, and 24%. When cumulative mortality from all causes of death was considered, approximately 85% of PTC patients (who had AGES scores <4 or MACIS scores <6) experienced no excess mortality over rates predicted for control subjects.50,65,66,69
Follicular Thyroid Carcinoma
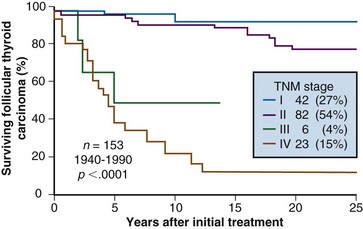
When more than 75% of cells in an FTC exhibit Hürthle cell or oncocytic features, the tumor is classified as a Hürthle cell carcinoma (HCC), oncocytic carcinoma, or oxyphilic variant FTC.25 Most patients with FTC or HCC present with tumors 2 cm or larger and confined to the neck. Patients 45 years old or older with nodal metastases or extrathyroidal extension account for only about 4% to 7% of FTCs and 8% to 10% of HCCs.28,63 In contrast to PTC patients, among whom only 1% to 3% present with distant metastases, about 4% to 6% of HCC patients and 7% to 15% of non–oxyphilic variant FTC patients have distant metastases at the time of initial diagnosis.25,28,63 Figure 36-3 shows cause-specific survival according to pTNM stage in a cohort of 153 patients with nonoxyphilic FTC surgically treated at Mayo Clinic during the period 1940-1990.63
Nodal metastases are rare in typical FTC, and nodal relapse rates at 10 and 20 postoperative years are 1% and 2%. About 6% of patients with HCC have nodal involvement at presentation,55 but at 20 and 30 years after primary surgery, 18% and 24% of these patients have nodal relapse.25 When relapse at neck or distant sites is considered, patients with HCC have the highest rates of tumor relapse after 10 or 20 years. Local recurrences account for most of these postoperative events because the numbers of distant metastases in patients with HCC are comparable with the numbers of metastases found in nonoxyphilic FTC (i.e., about 20% after 20 postoperative years).25
Cause-specific mortality rates vary with the presenting TNM stage for patients with FTC or HCC. The death rates tend to parallel the curves for development of distant metastases. In 5 decades of Mayo Clinic experience, the mortality rate for FTC initially exceeded that of HCC, but by 20 and 30 postoperative years, there were no significant differences in cause-specific survival (CSS) rates between FTC and HCC,25 with both survival rates about 80% at 20 postoperative years and 70% at 30 postoperative years.
The risk factors that predict outcome of patients with FTC are largely the same as for patients with PTC: distant metastases at presentation, increased patient age, large tumor size, and presence of local (extrathyroidal) invasion.25 To a lesser degree, increased mortality is associated with male sex and higher grade tumors. Vascular invasiveness, lymphatic involvement at presentation, DNA aneuploidy, and oxyphilic histology are potential prognostic variables unique to FTC.25 The importance of vascular invasion is underscored by a study showing that FTC patients with minimal capsular invasion and no evidence of vascular invasion had 0% cause-specific mortality after 10 years of postoperative follow-up.72
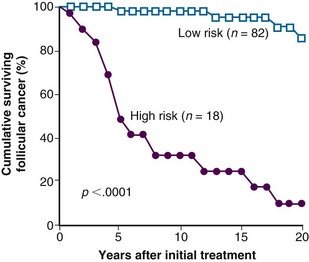
Prognostic scoring systems for FTC allow stratification of patients into high-risk and low-risk categories.25,73 A multivariate analysis at the Mayo Clinic found that distant metastases, patient age older than 50 years, and marked vascular invasion predicted a poor outcome.54 As illustrated by Figure 36-4, if two or more of these factors are present, the 5-year survival rate is 47%, and the 20-year survival rate is 8%. If only one of these factors is present, the 5-year survival rate is 94%, and the 20-year survival rate is 86%.54
Systems developed to predict outcomes for PTC or FTC have been applied to patients with FTC. Specifically, the AMES risk-group categorization by Cady and Rossi has proved useful in FTC.28,70 From a multivariate analysis of 228 patients with FTC treated at Memorial Sloan-Kettering Cancer Center, the independent adverse prognostic factors were identified as age older than 45 years, Hürthle cell histologic type, extrathyroidal extension, tumor size exceeding 4 cm, and presence of distant metastasis.74 The prognostic importance in FTC of histologic grade has also been confirmed74,75 by the Memorial Sloan-Kettering group, who have included this factor in their assignment of risk groups to low-risk, intermediate-risk, and high-risk categories, with 20-year survival rates of 97%, 87%, and 49%.74 The AGES and MACIS prognostic scoring systems, originally developed for PTC, have also been successfully applied to FTC.76,77 It seems that scoring systems used in PTC can be cautiously applied in FTC as long as some of the unique features of this tumor, such as vascular invasiveness and the remarkable significance of DNA aneuploidy in HCC, are considered.25
Medullary Thyroid Carcinoma
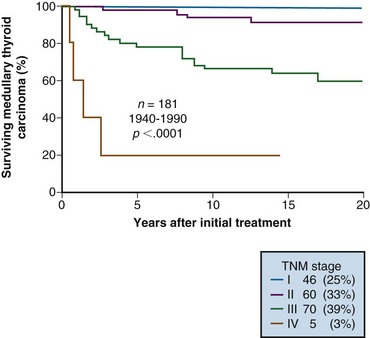
In reported studies of treated MTC, the proportion of patients with intrathyroidal node-negative tumors of 2 cm in diameter or smaller (TNM stage I) varies, depending on the number of familial cases detected by biochemical testing or DNA screening. The number of patients who present with TNM stage I MTC ranges from 5% to 25%, with the lower numbers representing the older series. Of patients, 25% to 50% present with positive neck nodes; the proportion of patients presenting with distant metastases usually exceeds the proportion with PTC but is typically less than the proportion with FTC. Stage IV cases constitute 3% to 10% of most MTC series. Figure 36-5 illustrates CSS according to pTNM stage in a cohort of 181 patients with MTC surgically treated at Mayo Clinic during the period 1940-1990.63
Other prognostic factors relevant to outcome in MTC include age at diagnosis, male gender, vascular invasion, calcitonin immunoreactivity, amyloid staining, presence or absence of postoperative gross residual disease, and abnormal postoperative plasma calcitonin levels.11,78–80 In a multivariate analysis from Toronto, only the presence of extrathyroidal invasion and postoperative gross residual disease were significant in the prediction of CSS.80 In another multivariate study from Mayo Clinic, however, the only factors remaining in the final Cox model were pTNM stage III or IV disease, negative Congo red staining for amyloid, and postoperative gross residual disease.79 Based on these three independent prognostic variables, a scoring system was devised to define four risk groups with 10-year mortality rates ranging from 5% to 100%.79
Anaplastic Carcinoma
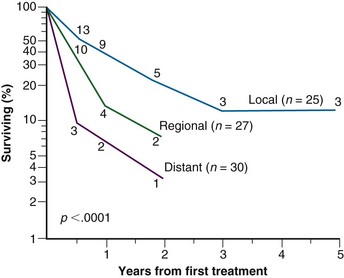
Patients with ATC have a very poor prognosis, and survival beyond 1 year is rare. There is no real use for an elaborate staging system for these patients, although distant and lymphatic spread at presentation, advanced age, and extremely poor differentiation have been identified as factors that make an already grim picture worse.60,81 Figure 36-6 shows survival curves for 82 patients with ATC, stratified by extent of disease at presentation.60
Clinical Manifestations and Patient Evaluation
At initial assessment, most patients with thyroid cancer have a palpable neck mass, which may represent the primary intrathyroidal tumor or metastatic regional lymphadenopathy. In some patients, the tumor may be clinically occult, and the impalpable lesion may first be recognized on high-resolution neck imaging3 or during the course of a neck exploration for presumed benign thyroid disease. In patients with a family history of MTC or MEN-II syndrome, the finding of a RET oncogene mutation (identical to the proband) or abnormal calcitonin (or stimulated calcitonin) levels, or both, may necessitate elective prophylactic thyroidectomy in a patient who may prove to have early MTC visible only under the surgical pathologist’s microscope.48,49
Features of the history and physical examination rarely provide convincing evidence for a diagnosis of thyroid malignancy.8,9 The diagnosis of thyroid cancer necessitates pathologic confirmation from cytologic or histologic material. It is generally recognized that fine-needle aspiration (FNA) biopsy is the most effective method available of preoperatively distinguishing between benign and malignant thyroid nodules.8,9 All cancer diagnoses should be verified, however, by careful examination of histologic material after surgical excision of affected tissues. This approach is particularly relevant to the problem of the cellular follicular lesions described by cytologists as “suspicious” for follicular or Hürthle cell neoplasm. The diagnosis of FTC or HCC depends on the demonstration of invasion of the thyroid capsule or the adjacent blood vessels (i.e., angioinvasion), a process that typically necessitates careful evaluation of serial sections from the excised specimen for the presence or absence of such microinvasion. Even if FTC or HCC is apparently excluded at intraoperative frozen section, the resected specimen must be carefully reviewed in multiple sections from paraffin-embedded material.25
FNA biopsy can usually allow a confident diagnosis of PTC, which typically represents more than 75% of clinically recognized thyroid cancers in most contemporary series.12,26,28,50 Some authorities claim that the characteristic nuclear abnormalities diagnostic for PTC may be best seen in cytologic preparations from an FNA biopsy specimen, rather than in frozen sections or in paraffin-embedded histologic material. MTC may be readily diagnosed by FNA biopsy, but in equivocal cases, amyloid stained by Congo red or immunoperoxidase labeling of intracytoplasmic calcitonin may allow a definitive preoperative diagnosis.11,58 ATC may often be diagnosed by FNA biopsy, but it sometimes may be difficult to distinguish from carcinoma metastatic to the thyroid.60 When the biopsy specimen is found to be suspicious for ATC, immunostaining for thyroglobulin can help confirm the diagnosis of ATC. FNA diagnosis of thyroid lymphoma is difficult to make, and verification may necessitate examination of open biopsy material and specific immunostaining for clonal B-cell and T-cell populations.82
Scanning with RAI has little role to play in the preoperative evaluation of thyroid malignancy. However, a postoperative whole body scan has become the gold standard for identification of iodophilic distant metastasis in patients with PTC or FTC.8,10
Primary Therapy
Surgery
Surgery is the standard primary treatment for differentiated thyroid cancers, but the extent of initial surgery is an area of considerable controversy.5,66,83,84 Various investigators have advocated ipsilateral thyroid lobectomy with isthmectomy, bilateral subtotal thyroidectomy, and near-total or total thyroidectomy. Most institutions favor the initial resection of both lobes of the thyroid. Clinical guidelines promote near-total or total thyroidectomy as the recommended operative procedure for FCDC and total thyroidectomy for MTC.8,10,11 Even thyroid carcinomas that are locally extensive, involving extrathyroidal structures, may be most effectively treated by a more aggressive surgical approach.85,86 Most studies that have evaluated the impact of surgical treatment in thyroid cancers have compared total or near-total thyroidectomy with less aggressive surgical procedures.5,64,66,87 These reports suggest that survival rates in higher risk cases are significantly improved after the more extensive surgery.5,66
An often-cited reason for removing all or nearly all of the thyroid gland is reducing the risk of future recurrence of FCDC in the contralateral thyroid lobe. This approach may be most appropriate in cases of PTC, in which multicentricity is a frequent phenomenon. Some investigators claim, however, that a subsequent local recurrence in the contralateral lobe also can be managed with a secondary operative procedure.88 Such “completion” thyroidectomies carry a higher morbidity except in the hands of highly skilled surgeons.64,89 One advantage of initial resection of the entire gland is the subsequent ability to follow serum thyroglobulin levels and to perform meaningful radioiodine scanning more easily.7,8,10 Whole body scans may be difficult to interpret in the setting of residual thyroid remnant, as would be found after unilateral or bilateral subtotal resection.
In addition to the initial resection of the thyroid, the question of the extent of neck node removal remains controversial. When clinically evident nodal metastases are identified by palpation or ultrasound examination, surgical removal is performed. The role of prophylactic or prognostic lymph node dissections is unclear.* For patients undergoing total or near-total thyroidectomy, removal of the central compartment lymph nodes may not add significantly to the morbidity of the procedure and should be included routinely with the primary resection.8,89,90–92 Modified radical neck dissection is indicated for gross nodal metastases found before or during surgery, and it may improve the outcome of older PTC patients with larger tumors or evidence of extrathyroidal invasion.93,94
The advantage of total thyroidectomy over near-total thyroidectomy may be nonexistent or limited to patients with more advanced malignancies.64,66,95 The use of more limited surgery, such as unilateral total lobectomy, is probably appropriate only for a selected population of low-risk patients, such as patients with papillary microcarcinoma.8,10,96
Adjuvant Therapy
Thyroid Hormone
Postoperative thyroid suppression therapy for FCDC is based on administration of supraphysiologic oral doses of levothyroxine. This therapy has been widely used for more than 40 years, and it is assumed that suppression of endogenous TSH deprives TSH-dependent differentiated FCDC cells of an important growth-promoting influence. Traditionally, the goal of thyroxine therapy has been complete suppression of pituitary TSH secretion, as indicated by undetectable levels of serum TSH when measured in sensitive immunometric assays. Uncertainty surrounds the level to which the serum TSH level must be suppressed to maximize benefit while avoiding potential long-term complications.4,6,8,10
With the increasing availability of sensitive TSH assays, meticulous titration of the level of TSH suppression has become possible. If patients with FCDC are deemed to be at high risk for relapse or mortality on the basis of scoring or staging systems,62,69,70 or if they have persistent or recurrent carcinoma that cannot be eradicated by surgery, RAI, or other therapeutic measures, the goal would be to maintain the TSH level at less than 0.1 mU/L indefinitely in the absence of specific contraindications. In most patients with PTC who would be classified as low risk by prognostic scoring systems,66,69,70,75 it is generally accepted that the degree of TSH suppression would be less stringent, and the goal for basal serum TSH should be in the readily detectable, barely subnormal range of 0.1 to 0.5 mU/L.6,8,10,63,95
Radioactive Iodine Therapy
Radioiodine remnant ablation (RRA) has been defined as “the destruction of residual macroscopically normal thyroid tissue after surgical thyroidectomy.”95,97 Typically, RRA is used to complete the initial therapy in a patient whose FCDC has been completely resected (i.e., no gross residual disease is reported at the conclusion of the primary neck exploration). RRA is a procedure that is offered to patients with FCDC who have undergone “potentially curative” surgical treatment, and it should not be confused with RAI therapy, in which larger doses of iodine-131 (131I) are used in an attempt to destroy persistent neck disease or distant metastatic lesions.82,95,97,98,99
Proponents of RRA describe at least three advantages for this adjunctive therapy. First, by being actively trapped by normal thyroid cells, RRA is thought to destroy occult microscopic carcinoma cells within the thyroid remnant. Second, the later detection of persistent or recurrent disease, particularly in the neck by RAI scanning, is facilitated by the destruction of remaining normal tissue. Third, the performance of RRA is thought to increase the value of serum thyroglobulin measurement during follow-up. This last benefit has convinced many physicians to consider RRA in patients with FCDC who are, by prognostic scoring or staging, deemed to be at low risk for tumor relapse or cause-specific mortality.19,97 Other physicians have not advocated RRA in these low-risk patients, however, because of a lack of evidence of improved outcome.94,95,97,98
Investigators have reported benefit and a lack of benefit from the use of RRA.* It is unclear from these reports whether outcome is improved with the routine use of RRA in the initial therapy of all patients with differentiated thyroid carcinoma, largely because of difficulties obtaining a comparable control group and the potential for selection biases, which may account for the outcomes reported. Sawka and colleagues97 systematically reviewed 1543 English references to determine whether RRA decreases the risk of thyroid cancer–related death or relapse after bilateral thyroidectomy for PTC or FTC. In 18 cohort studies not adjusted for prognostic factors or interventions, the benefit of RRA in decreasing thyroid cancer–related mortality and any relapse by 10 years was inconsistent among centers. However, pooled analyses suggested a statistically significant treatment effect of RRA in terms of 10-year outcomes for locoregional recurrence (relative risk 0.31) and distant metastases (absolute decrease in risk 3%). The conclusion of this meta-analysis was that RRA might be beneficial in decreasing relapse of well-differentiated thyroid cancer; however, Sawka and colleagues97 said that “the incremental benefit of remnant ablation in low-risk patients treated with bilateral thyroidectomy and thyroid hormone suppressive therapy is unclear.”
The National Thyroid Cancer Treatment Cooperative Study Group (NTCTCSG) was founded in 1986 and has maintained over more than two decades a registry contributed to by 11 North American institutions prospectively following a large nonrandomized cohort of patients with FCDC, with the objective of assessing the effects of initial and longitudinal management on outcomes. By June 2001, 2936 patients were registered, and the more recently published results of outcome showed that “no treatment modality, including lack of radioactive iodine, was associated with altered survival in stage I patients.” Jonklaas and colleagues4 “were unable to show any impact, positive or negative, of specific therapies in stage I patients.” These authors concluded that “postoperative RAI (RRA) therapy does not provide significant benefit in stage I patients, and could even be harmful,” and suggested that “further evaluation of the potential risks and benefits of treatment in stage I patients is, therefore, indicated.”4
Use of prognostic scoring schemes may help to define patients at significant risk for relapse and who are more appropriate for consideration of remnant ablation. At the Mayo Clinic, patients with PTC who have MACIS scores greater than 6.0 or are found to have persistent elevation of serum thyroglobulin levels after 6 to 8 postoperative weeks are considered for an ablative procedure. Use of such a scheme is applicable only if the patient undergoes a near-total or total thyroidectomy. If the goal is to minimize the use of RAI, more extensive surgery such as near-total or total thyroidectomy is more appropriate in low-risk patients. However, if RRA is planned, more extensive surgery may not give any advantage. In high-risk patients, the use of RAI in addition to more aggressive surgery seems warranted.94,98,100
Further treatment with RAI ideally should reflect the local or distant uptake of iodine in residual differentiated thyroid cancer tissue. Adequate ability of thyroid cancer cells to concentrate radioiodine is variable, and this approach may not be appropriate in all patients. Patients with FTC may concentrate RAI better than patients with PTC.99 In a multicenter Canadian study, Simpson and associates99 reviewed the outcomes of more than 1500 patients with PTC or FTC and studied 321 patients who were treated with RAI. The use of RAI resulted in significantly improved local control in patients with microscopic residual disease. Cause-specific survival was also significantly better for PTC patients with microscopic residual disease who received RAI. Most patients received only thyroid remnant ablation doses (50 to 125 mCi), but total (cumulative) therapeutic doses given in one to seven treatments ranged from 75 to 1000 mCi.
The definitive role of RRA continues to evolve.100,101 Multiple studies have suggested that RRA be used routinely in high-risk patients. Although the definition of “high risk” varies, most investigators favor using RAI in patients with large tumors or extensive extrathyroidal invasion and in patients with incompletely resected disease or distant metastases. Robbins and Schlumberger100 recommended RRA for “any individual with a carcinoma larger than 1.5 cm or with a thyroid carcinoma of any size with obvious lymph node involvement, extrathyroidal extension, or multicentricity.” The clinical guidelines proposed by the European Thyroid Association and the American Thyroid Association should allow some greater uniformity of treatment approach.8,10,101
External Beam Irradiation
The appropriate application of EBRT in PTC and FTC is controversial, and this modality is not uniformly used.101,102 A better understanding of prognostic factors that are specific to local recurrence may help identify an appropriate population of patients who would be candidates for EBRT. Most prognostic schemes consider only the overall risk of relapse and CSS.
Historically, indications for irradiation have included residual macroscopic or microscopic disease, extrathyroidal extension, multiple lymph node involvement, Hürthle cell histology, older age of patients, large tumor size, and residual disease that does not take up RAI. Several investigators have retrospectively reviewed their experience with EBRT and suggested improved local control or survival benefit or both.* The patient populations in these studies are not uniform with respect to other treatments that also effect local control, making interpretation difficult.
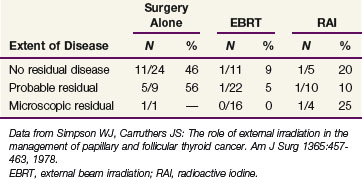
Most series support the use of adjuvant irradiation for patients with microscopic residual disease or narrow surgical margins of resection. An early Canadian study of follicular and papillary carcinomas evaluated the role of EBRT according to completeness of surgical resection.103 Table 36-4 summarizes these findings. Although the overall numbers are small, they suggest that patients with surgically narrow or microscopically positive margins may benefit from the addition of EBRT in moderate doses. These results were achieved with minimal morbidity.
A larger synopsis of 13 Canadian institutions in which EBRT was used predominantly in patients with high-risk features, such as extrathyroidal invasion, high-grade malignancies, and older age, revealed a significant improvement in local control for patients who had microscopically positive or narrow surgical margins.101 In this study, the overall survival (OS) of PTC patients with presumed or microscopic residual was significantly improved with the addition of adjuvant EBRT. These improvements were seen with or without the use of RAI. Results from Germany for patients with pathologic T4 disease (based on the older staging system) with or without lymph node involvement also suggest a benefit from EBRT with respect to local control and distant failure rates, especially in patients older than 40 years.105 In a review from Princess Margaret Hospital in Toronto of 207 patients with microscopic residual, the addition of EBRT did not significantly improve the CSS or the local control (p = .38 and p = .14).106 However, a more recent review with more patients and longer follow-up revealed an improved 10-year CSS in high-risk patients (age >60 years, extrathyroid extension, microscopic residual disease, no gross residual disease) with the addition of EBRT.118,119 A review from Mount Sinai Hospital in Toronto found that the use of EBRT was the only predictor of significantly improved disease-specific survival and OS in patients with well-differentiated thyroid cancer with extrathyroidal extension.122
Benker and associates108 described 932 patients with differentiated thyroid malignancies and found no OS benefit with the addition of EBRT. On subset analysis, however, patients older than 40 years with T3 or T4 disease experienced improved survival that approached statistical significance (p = .09). All patients in this series received thyroxine-suppressive therapy and RAI.
The role of EBRT in the setting of gross residual disease has been evaluated by multiple investigators, with a wide range of outcomes. A study from the Royal Marsden Hospital showed complete response in 37% of patients and partial response in an additional 25%.121 The Princess Margaret Hospital study had 33 patients with macroscopic disease treated with EBRT with or without RAI; the 5-year local recurrence-free rate was 62%.106 Tubiana and associates104 from Institut Gustav-Roussy evaluated 97 patients with macroscopic residual based on the surgeon’s clinical assessment. Local recurrence after EBRT was only 15% compared with 32% in a similar surgical category without postoperative EBRT. None of these patients received RAI. With the ability to deliver higher doses of EBRT using techniques such as intensity-modulated radiotherapy (IMRT), it may be feasible to give higher doses than were used in these studies and perhaps increase the long-term control of disease in this difficult patient population.
Although the definitive role of EBRT for differentiated thyroid carcinoma remains controversial, retrospective data support its use in a selected population of patients who have microscopic or presumed microscopic residual cancer.123 Evidence exists not only for PTC and FTC but also for Hürthle cell carcinoma and MTC.124–126 The only randomized trial evaluating the role of EBRT was initiated in 2000 in patients with pathologic T4, N0-1, M0 disease after surgical resection and ablative 131I therapy.127 The study closed because of inadequate accrual in March 2003 despite acceptable toxicity.128 The study authors indicated that at early follow-up, there are lower than expected relapse rates in the nontreatment arm. The improvements in surgical management of these patients and the appropriate application of RAI may have accounted for the decreased relapse and mortality rates compared with historical studies.
The current American Thyroid Association guidelines suggest that EBRT should be considered in patients older than age 45 with grossly visible extrathyroid extension at the time of surgery and a high likelihood of microscopic residual disease and patients with gross residual tumor in whom further surgery or RAI would likely be ineffective.117 Changes in the staging of locally advanced thyroid cancers in 2002 by categorizing minimal extrathyroidal extension as T3 disease and creating two T4 categories should help delineate further the highest risk population. In the clinical setting of T4b disease, it is unlikely that complete surgical resection can be achieved, and use of EBRT in addition to RAI seems to help control gross disease. For patients with locally recurrent disease, early use of EBRT as a component of treatment should be considered.
Treatment of the thyroid bed or gland and its draining lymph node areas has historically been a challenging undertaking. Advances in treatment modalities include three-dimensional conformal multiple-beam treatments and IMRT (and intensity-modulated arc or volumetric therapy) treatments. Typical total doses are 50 to 60 Gy administered as 2 Gy per treatment, 5 days per week, over 5 to 6 weeks for microscopic disease (adjuvant treatment). Total doses of 60 to 70 Gy over 6 to 7 weeks are employed for gross residual disease. Treatment volumes typically encompass all lymph node regions “at risk” (typically from the hyoid bone to the carina, levels II, III, IV, V, and VI). Although there is no definitive evidence of a dose-response relationship between radiation dose and local control probability, there is some suggestion that higher doses are associated with lower local recurrence rates.109,116 With the increasing availability of IMRT and proton radiotherapy, dose escalation may be more readily achievable in the adjuvant setting without increasing toxicity.129–131 Stereotactic body radiotherapy (SBRT) has been proposed as a highly targeted technique that is efficient (one to three treatments) and effective in controlling cervical lymph node recurrence of nonanaplastic thyroid cancer (100% tumor control) with no serious adverse events.132
Chemotherapy
The Eastern Cooperative Oncology Group (ECOG) reported the only randomized study of the use of chemotherapy in advanced thyroid cancer.133 Patients with locally advanced or metastatic FTC, PTC, MTC, and ATC were eligible. In this study, patients were randomly assigned to chemotherapy with doxorubicin alone or doxorubicin with cisplatin. Of the 84 eligible patients, the investigators reported a complete response rate of 6% and a partial response rate of 15%. A partial or complete response to chemotherapy occurred in 8 (23%) of 35 patients with advanced differentiated thyroid carcinomas. Overall, there was no advantage to the combination-drug regimen over doxorubicin alone. All five complete responders were in the combination arm, however.
Thyroid Lymphoma and Anaplastic Thyroid Carcinoma
Primary Thyroid Lymphoma
Since the development of immunocytochemical staining, this distinct entity has become more accurately recognized.134 Most thyroid lymphomas are diffuse, large B-cell lymphomas (DLBCLs), but significant numbers are in the category of lymphomas known as mucosa-associated lymphoid tissue (MALT) lymphomas or mixed tumors containing elements of DLBCL and MALT. Rarely, other types of lymphoma, including follicular lymphomas and Hodgkin’s lymphomas, have been reported.134,135 Thyroid lymphomas usually manifest with a rapidly enlarging mass that is sometimes associated with respiratory and esophageal symptoms. These lymphomas typically occur in middle-aged and older women and are often associated with chronic thyroiditis.134,135 MALT lymphomas have a more indolent natural history, whereas DLBCLs tend to be more aggressive.
An early review by Doria and colleagues136 before the widespread use of immunohistochemical staining suggests that the distant failure rate with thyroid lymphomas was seen primarily in patients with non-MALT lymphomas. Their review included 211 patients in 11 series and showed an 11% distant relapse rate for patients with MALT lymphomas compared with 45% for patients with localized non-MALT lymphomas. This review suggests that local therapy alone in patients with DLBCL is inadequate and that systemic therapy is warranted.
Surgery has a diagnostic role only in the management of thyroid lymphomas, and this role is diminishing with the increasing accuracy of FNA.137 Some investigators contend that surgery can provide prognostic information, influence treatment options, and improve outcome.135,138–140 They assert that the use of surgery may obviate the need for chemotherapy in some patients or identify a population requiring local therapy only. One investigator reported five patients with MALT lymphoma or small lymphocytic lymphoma treated with surgery alone who had no relapses.135 Surgery may have a palliative role in selected patients with obstructive symptoms.141 In the absence of prospective or randomized data, the improved outcome of patients who are able to undergo resection may indicate only selection of a favorable subgroup and not provide clear evidence of survival advantage associated with the surgery itself, and it does not eliminate the consideration of irradiation or chemotherapy. The infiltrative nature of this disease can lend itself to a higher rate of surgical complications involving the parathyroid glands and recurrent laryngeal nerves. Some investigators have failed to show any advantage to more aggressive surgery, provided that the patient receives adequate irradiation with or without chemotherapy.142,143
The use of irradiation alone in the treatment of thyroid lymphomas has been reported by multiple institutions.138,142–146 The reports indicate that the OS for patients with Ann Arbor stage I and II disease ranges from 40% to 93%. Patients with stage I disease have a better outcome. Some of these reports indicate improved outcome with neck and mediastinal irradiation over neck treatment alone.142,147–149 Other authors have not found this association.138,140 In one report of 27 patients treated with irradiation alone or in combination with chemotherapy, all four local failures occurred in patients treated solely with irradiation.146
The role of EBRT alone in the treatment of thyroid lymphomas is limited, and given the randomized clinical data for DLBCL with combined chemoradiation, EBRT as the sole treatment modality should be given only when chemotherapy is not feasible or tolerated. With the increased risk of distant relapse reported by Doria and colleagues,136 survival was improved with the use of systemic chemotherapy in addition to local EBRT. Other authors have concluded that chemotherapy improves the outcome in these patients.137,144
Two large prospective randomized studies have evaluated the use of combined-modality therapy in thyroid lymphomas. Studies by ECOG and Southwestern Oncology Group (SWOG) evaluated the use of CHOP (cyclophosphamide, hydroxydaunomycin, Oncovin [vincristine], prednisone) chemotherapy with or without involved-field irradiation for stage I or II non-Hodgkin’s lymphomas, including thyroid lymphomas.150,151 The number of thyroid lymphoma patients enrolled in these studies is not published, but 18% of the patients in the ECOG study had stage IE disease (i.e., disease confined to one extranodal site). Both studies indicated a survival benefit for combined-modality therapy. Doses of 30 Gy were used in the ECOG study after eight cycles of CHOP chemotherapy except when there was only a partial response to chemotherapy. In that circumstance, a dose of 40 Gy was delivered using standard fractionation. In the SWOG study, the combined-modality arm received only three cycles of CHOP along with 40 to 55 Gy. The OS at 6 years in the ECOG study was 84% for combined-modality therapy compared with 70% with chemotherapy alone (p <.05).150 Further data from this study await publication. The SWOG report estimated an 82% overall survival rate compared with 72% for CHOP alone (p = .02).151 Based on these randomized studies, it seems that the use of combined-modality therapy offers the most appropriate management of this disease process.
Further improvement in outcomes for DLBCLs has been shown with the addition of monoclonal antibodies, especially rituximab, to combination chemotherapy.152,153 Other immunologic and radiolabeled agents are being evaluated.
There is minimal information about the use of chemotherapy alone in this particular disease.154 Data from the SWOG and ECOG studies show a high response rate with CHOP chemotherapy alone. Given the locally aggressive nature of this extranodal site and the bulky disease that can extend into the mediastinum, however, the use of combined-modality therapy is preferable over chemotherapy alone.
Anaplastic Thyroid Carcinoma
The optimal role of surgery has not been well defined. Surgery may be performed initially in some patients, but this probably reflects the selection of a favorable population of patients. The M.D. Anderson Cancer Center reported that more extensive surgery by itself did not provide a survival advantage.81 Overall, most patients present with unresectable or metastatic disease, and the initial surgery in these situations is primarily for diagnostic purposes. In most reports, however, the only long-term survivors were patients who had undergone surgical resection.155,157–160 For this reason, surgery should be strongly considered as a component of treatment. Surgery has been performed before or after irradiation, chemotherapy, or both.81,161 The sequence of surgery relative to radiation and chemotherapy may be primarily a reflection of the extent of disease at time of patient presentation to the treating physicians.162
Radiation has been used in the treatment of these advanced tumors, but because of the high metastatic rate and high rate of local failure with irradiation alone, the use of chemotherapy in addition to irradiation is warranted.163 Multiple studies have investigated the use of multimodality therapy.156–158161 The use of hyperfractionated irradiation has been examined because of the rapid proliferation rate of these malignancies.156,158,161,164 Various approaches have been reported, but most studies use doxorubicin-based chemotherapy given concurrently with local irradiation.133,156,157,165
Although long-term survival (>1 year) after combined-modality adjuvant therapy in ATC has been so rare as to provoke reports of single cases,166 subsequent series support the benefit of combined-modality approaches. Swaak-Kragten and colleagues167 conducted a study of 30 ATC patients treated with an adjuvant approach combining surgery, radiotherapy, and single-agent chemotherapy with doxorubicin administered concurrently with radiotherapy and continued after radiotherapy completion and reported an encouraging improvement in 1-year survival from 9% (historical controls) to 23% (new patient cohort). Haigh and associates168 reported a 75% 2-year survival in eight patients undergoing complete surgical resection and adjuvant radiotherapy and chemotherapy. Tan and coworkers169 reported an estimated 5-year survival of 60% for five patients who underwent complete resection. Four patients had received postoperative radiotherapy, three with postradiotherapy doxorubicin-based chemotherapy. Pudney and colleagues170 reported a median OS of 13 months for five patients with stage IVB ATC treated with either radiotherapy concurrent with doxorubicin or induction docetaxel, doxorubicin, and cyclophosphamide. Three patients developed progression of local disease, and one developed distant metastases. Higashiyama and associates171 reported a 12-month survival rate of 44% in nine patients with stage IVb ATC treated with induction paclitaxel. This survival rate compared favorably with a historical control group of 50 patients treated without induction paclitaxel; their 12-month survival was just 5.9%.
The optimal EBRT fractionation schedule has not yet been determined. Of patients treated with moderate-dose (57.6 Gy) slightly accelerated radiotherapy, given twice daily 3 days per week, combined with concurrent weekly doxorubicin, 70% required a 1-week interruption in treatment because of pharyngoesophagitis and tracheitis.156 Moderate-dose (60.8 Gy) accelerated fractionation even without concurrent chemotherapy may be too toxic with a median OS of just 10 weeks.172 Low-dose (40 Gy) hyperfractionated accelerated radiotherapy with sequential but not concurrent chemotherapy (doxorubicin) seems to be well tolerated with 1-year and 3-year OS of 50% and 35%.165 Moderate-dose (60 Gy) hyperfractionated accelerated radiotherapy without concurrent chemotherapy seems to be well tolerated with a median OS of 13.6 months and 1-year and 5-year OS of 66.7% and 0%.173 However, others have reported less than 10% 1-year survival with hyperfractionated accelerated radiotherapy to a median dose of 57 Gy in patients with nonmetastatic disease.174
In a report of the Surveillance, Epidemiology and End Results database from the National Cancer Institute, the only factors predicting for lower cause-specific mortality were age younger than 60 years, intrathyroidal tumor, and combined use of surgery and irradiation.175 However, the overall cause-specific mortality rate was 80.7% at 1 year. The use of multimodality therapy, including surgery, irradiation, and chemotherapy, is appropriate and offers the best chance for long-term survival, but more active systemic agents need to be found to improve survival significantly.
Irradiation Techniques and tolerance
Treatment Volumes and Doses
Treatment of the thyroid bed or gland and the regional lymph nodes is challenging because of the contour of the body; the potential extension of disease to the upper mediastinum; involvement of lymph nodes in the neck and mediastinum; and the close proximity of the lungs, spinal cord, esophagus, larynx, pharyngeal constrictors, and brachial plexus. Historically, several approaches have been reported for the initial extended fields, including a single anterior electron field,106 an anterior field with a posterior supplemental field for the mediastinum, and lateral fields.104
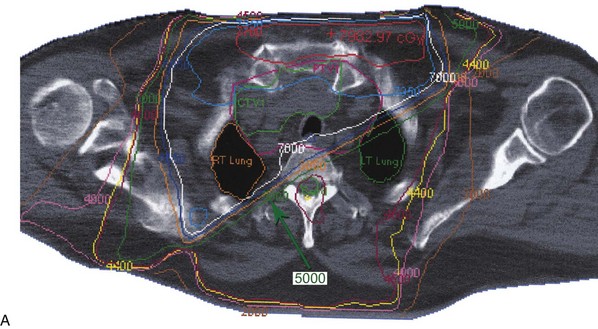
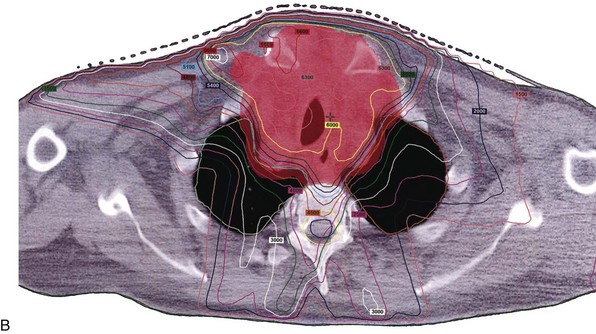
At Mayo Clinic, we have chosen to use IMRT or intensity-modulated arc therapy in the treatment of these patients, which allows us to administer a more conformal high dose (60 to 70 Gy) with improved homogeneity to the thyroid bed or gross disease and high-risk areas (lymph nodes and tracheoesophageal groove), while lowering the dose to normal organs at risk, including the salivary glands, epiglottis, false vocal cords, true vocal cords, arytenoids, central and posterior aspects of the pharyngeal constrictors and esophagus, brachial plexus, and spinal cord. The use of IMRT has allowed for more elegant treatment of this disease site compared with conventional and three-dimensional treatment planning (Fig. 36-7). IMRT improves the minimum and mean dose to the planning tumor volume and significantly reduces the dose to the spinal cord.129,131,176,177 This is true for treatment of the thyroid tumor bed or the thyroid region and locoregional nodal sites.
The radiation dose used in the postoperative setting for microscopic residual disease should be 60 Gy in 6 weeks. However, some centers have suggested 40 Gy given over 3 to 3.5 weeks is adequate.103 For known gross residual disease, a dose escalation to 66 to 70 Gy is reasonable. One retrospective study from the United Kingdom suggested a possible dose-response effect at greater than 50 Gy for patients treated with curative intent.102 The use of higher doses without added morbidity may be feasible with IMRT. Although there is no definitive evidence of a dose-response relationship between radiation dose and local control probability, there is some suggestion that higher doses are associated with lower local recurrence rates.109,116
Treatment Tolerance
With the increasing availability of IMRT, intensity-modulated arc therapy, and proton radiotherapy, dose escalation may be more readily achievable in the primary setting without increasing toxicity and lowering toxicity in the adjuvant setting.129–131 Ideal planning target volumes and dose remain inadequately characterized, however. Early experiences with IMRT seem to suggest that escalation of dose to subclinical sites of possible involvement (nodal regions outside the thyroid bed to 54 Gy), the margin-negative thyroid bed (60-63 Gy), margin-positive regions (66 Gy), and gross disease (70 Gy) is technically feasible without increasing toxicity.129,131 One treatment is given each day, 5 days a week, 1.8 to 2.0 Gy per treatment. Acute radiation reactions include dermatitis, laryngitis, pharyngitis, tracheitis, and esophagitis. Late sequelae are uncommon but may include laryngeal edema, cartilage necrosis, esophageal stenosis, myelitis, brachial plexopathy, and pulmonary fibrosis.
Treatment Algorithm, Conclusions, and Future Possibilities
Treatment Algorithm
Knowledge of patient age, cell type, pTNM stage, and postoperative status permits classification of patients into risk groups, which may influence the selection of subsequent postoperative adjuvant therapy. The treatment of patients with different tumor histologies is outlined in the following paragraphs and summarized as four steps in the accompanying treatment algorithm (Table 36-5).
| Step I | Initial neck exploration/thyroid resection |
| FCDC: usually near-total or total thyroidectomy | |
| MTC: total thyroidectomy | |
| ATC: open biopsy or subtotal thyroidectomy | |
| Lymphoma: FNA or open biopsy | |
| Lymph nodes: removal of central compartment nodes in FCDC and MTC; modified radical neck dissection for involved lateral nodes | |
| Step II | Thyroid hormone therapy |
| Replacement doses for MTC, ATC, lymphoma | |
| TSH-suppressive doses for FCDC except microcarcinoma | |
| Step III | Outcome prediction by risk-group classification |
| Gauged according to age, stage, histologic type, and cancer type-specific scoring systems (e.g., AMES, MACIS) | |
| Step IV | Patient selection for RAI therapy, EBRT, chemotherapy, or combinations |
| FCDC: RAI therapy indicated for distant spread, unresectable or residual neck tumor, possibly invasive disease in PTC and most cases of FTC or HCC; EBRT for local or metastatic tumor nonresponsive to RAI therapy; almost no role for chemotherapy in differentiated FCDC | |
| MTC: residual or recurrent neck disease considered for EBRT; octreotide or chemotherapy considered for palliation only | |
| ATC: postbiopsy EBRT and concurrent chemotherapy | |
| Lymphoma: CHOP chemotherapy and radiotherapy |
AMES, age, metastasis, extent, and size; ATC, anaplastic thyroid cancer; CHOP, cyclophosphamide, hydroxydaunomycin, Oncovin (vincristine), prednisone; EBRT, external beam radiotherapy; FNA, fine-needle aspiration, FCDC, follicular cell–derived cancer; FTC, follicular thyroid carcinoma; HCC, Hürthle cell carcinoma; MACIS, metastasis, age, completeness of resection, invasion, and size; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma; RAI, radioactive iodine; TSH, thyroid-stimulating hormone.
1 Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901-911.
4 Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229-1242.
5 Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375-384.
8 Cooper DS, Doherty GM, Haugen BR, et al. Revised management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
9 Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazone Medici Endocrinologi, and European Thyroid Association medical guidelines for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16:468-475.
10 Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787-803.
11 Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565-612.
12 Ward EM, Jemal A, Chen A. Increasing incidence of thyroid cancer: is diagnostic scrutiny the sole explanation? Future Oncol. 2010;6:185-188.
13 Mazzaferri EL. What is the optimal treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology. 2009;23:579-588.
14 Antonelli A, Fallahi P, Ferrari SM, et al. Dedifferentiated thyroid cancer: A therapeutic challenge. Biomed Pharmacother. 2008;62:559-563.
21 Brierley JD, Tsang RW. External radiation therapy in the treatment of thyroid malignancy. Endocrinol Metab Clin North Am. 1996;25:141-157.
24 Ain KB. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1995;24:711-760.
25 Grebe SKG, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am. 1995;24:761-801.
26 Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma: a population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) Program 1973-1991. Cancer. 1997;79:564-573.
27 Ebihara S, Saikawa M. Survey and analysis of thyroid carcinoma by the Japanese Society of Thyroid Surgery. Thyroidol Clin Exp. 1998;10:89-95.
28 Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Database report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83:2638-2648.
39 Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster: pathomorphologic study of 84 cases (1991-92) from the Republic of Belarus. Cancer. 1994;74:748-766.
43 Hundahl SA. National Cancer Institute report on iodine-131 exposure from fallout following Nevada atmospheric nuclear bomb tests. CA Cancer J Clin. 1998;48:285-298.
48 Skinner MA, Moley JA, Dilley WG, et al. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353:1105-1113.
49 Frank-Raue K, Buhr H, Dralle H, et al. Long-term outcome in 46 gene carriers of hereditary medullary thyroid carcinoma after prophylactic thyroidectomy: impact of individual RET genotype. Eur J Endocrinol. 2006;155:229-236.
50 Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990;19:545-576.
51 McConahey WM, Hay ID, Woolner LB, et al. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy and outcome. Mayo Clin Proc. 1986;61:978-996.
52 Hay ID, Gonzalez-Losada T, Reinalda MS, et al. Long-term outcome in 215 children and adolescents with papillary thyroid carcinoma treated during 1940 through 2008. World J Surg. 2010;34:1192-1202.
53 Dinneen SF, Valimaki MJ, Bergstralh EJ, et al. Distant metastases in thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80:2041-2045.
54 Brennan MD, Bergstralh EJ, van Heerden JA, et al. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11-19.
55 Grebe SKG, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5:43-63.
60 McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028-1034.
61 Greene FL, Sobin LH. A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol. 2009;99:269-272.
62 Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer: AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010.
63 Larsen PR, Davies TF, Hay ID. The thyroid gland. In: In Wilson JD, Foster DW, Kronenberg HM, et al, editors. Williams Textbook of Endocrinology. 9th ed. Philadelphia: WB Saunders; 1998:389-515.
66 Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088-1095.
69 Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1,779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050-1058.
72 van Heerden JA, Hay ID, Goellner JR, et al. Follicular thyroid carcinoma with capsular invasion alone: a non-threatening malignancy. Surgery. 1992;112:1130-1136.
76 D’Avanzo A, Ituarte P, Treselar P, et al. Prognostic scoring systems in patients with follicular thyroid cancer: a comparison of different staging systems in predicting the patient outcome. Thyroid. 2004;14:453-458.
82 Mack LA, Pasieka JL. An evidence-based approach to the treatment of thyroid lymphoma. World J Surg. 2007;31:978-986.
87 Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124:958-966.
89 DeGroot LJ, Kaplan EL. Second operations for “completion” of thyroidectomy in treatment of differentiated thyroid cancer. Surgery. 1991;110:936-939.
96 Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980-987.
98 Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006;94:692-700.
101 Wartofsky L. Highlights of the American Thyroid Association Guidelines for patients with thyroid nodules or differentiated thyroid carcinoma: the 2009 Revision. Thyroid. 2009;19:1139-1143.
116 Meadows KM, Amdur RJ, Morris CG, et al. External beam radiotherapy for differentiated thyroid cancer. Am J Otolaryngol Head Neck Med Surg. 2006;27:24-28.
117 Brierley JD, Tsang RW. External beam radiation therapy for thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:497-509.
123 Terezakis SA, Lee KS, Ghossein RA, et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-Kettering Cancer Center Experience. Int J Radiat Oncol Biol Phys. 2009;73:795-801.
129 Rosenbluth BD, Serrano V, Happersett L, et al. Intensity-modulated radiation therapy for the treatment of nonanaplastic thyroid cancer. Int J Radiat Oncol Biol Phys. 2005;63:1419-1426.
130 Ask A, Bjork-Eriksson T, Zackrisson B, et al. The potential of proton beam radiation therapy in head and neck cancer. Acta Oncol. 2005;44:876-880.
131 Schwartz DL, Lobo MJ, Ang KK, et al. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys. 2009;74:1083-1091.
132 Kim JH, Kim MS, Yoo SY, et al. Stereotactic body radiotherapy for refractory cervical lymph node recurrence of nonanaplastic thyroid cancer. Otolaryngol Head Neck Surg. 2010;142:338-343.
150 Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032-3038.
176 Nutting CM, Convery DJ, Cosgrove VP, et al. Improvements in target coverage and reduced spinal cord irradiation using intensity-modulated radiotherapy (IMRT) in patients with carcinoma of the thyroid gland. Radiother Oncol. 2001;60:173-180.
177 Posner MD, Quivey JM, Akazawa PF, et al. Dose optimization for the treatment of anaplastic thyroid carcinoma: a comparison of treatment planning techniques. Int J Radiat Oncol Biol Phys. 2000;48:475-483.
1 Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901-911.
2 Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
3 Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226-231.
4 Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229-1242.
5 Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375-384.
6 Hovens GC, Stokkel MP, Kievit J, et al. Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:2610-2615.
7 Pacini F, Elisei R, Fugazzola L, et al. Post-surgical follow-up of differentiated thyroid cancer. J Endocrinol Invest. 1995;18:165-166.
8 Cooper DS, Doherty GM, Haugen BR, et al. Revised management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
9 Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazone Medici Endocrinologi, and European Thyroid Association medical guidelines for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16:468-475.
10 Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787-803.
11 Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565-612.
12 Ward EM, Jemal A, Chen A. Increasing incidence of thyroid cancer: is diagnostic scrutiny the sole explanation? Future Oncol. 2010;6:185-188.
13 Mazzaferri EL. What is the optimal treatment of low-risk papillary thyroid cancer (and why is it controversial)? Oncology. 2009;23:579-588.
14 Antonelli A, Fallahi P, Ferrari SM, et al. Dedifferentiated thyroid cancer: A therapeutic challenge. Biomed Pharmacother. 2008;62:559-563.
15 Hamilton JG, Soley MH, Eichorn KB. Deposition of radioactive iodine in human thyroid tissue. Univ Calif Publ Pharmacol. 1940;1:330-346.
16 Keston AS, Bali RP, Frantz VK, et al. Storage of radioactive iodine in a metastasis from thyroid carcinoma. Science. 1942;95:362-366.
17 Freitas JE. Treatment of thyroid carcinoma with radioiodine. Curr Concept Diag Nucl Med. 1986;3:8-29.
18 Blahd WH, Nordyke RD, Bauer FK. Radioactive iodine (131I) in the postoperative treatment of thyroid cancers. Cancer. 1960;13:745-753.
19 Sweeney DC, Johnston GS. Radioiodine therapy for thyroid cancer. Endocrinol Metab Clin North Am. 1995;24:803-839.
20 Sheline GE, Galante M, Lindsay S. Radiation therapy in the control of persistent thyroid cancer. Am J Roentgenol Radium Ther Nucl Med. 1966;97:923-930.
21 Brierley JD, Tsang RW. External radiation therapy in the treatment of thyroid malignancy. Endocrinol Metab Clin North Am. 1996;25:141-157.
22 Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64.
23 Robbins J, Merino MJ, Boice JDJr, et al. Thyroid cancer: a lethal endocrine neoplasm. Ann Intern Med. 1991;115:133-147.
24 Ain KB. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1995;24:711-760.
25 Grebe SKG, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am. 1995;24:761-801.
26 Gilliland FD, Hunt WC, Morris DM, et al. Prognostic factors for thyroid carcinoma: a population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) Program 1973-1991. Cancer. 1997;79:564-573.
27 Ebihara S, Saikawa M. Survey and analysis of thyroid carcinoma by the Japanese Society of Thyroid Surgery. Thyroidol Clin Exp. 1998;10:89-95.
28 Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Database report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83:2638-2648.
29 Williams ED, Doniach I, Bjarnason O, et al. Thyroid cancer in an iodide rich area: a histopathological study. Cancer. 1977;39:215-222.
30 Harach HR, Williams ED. Thyroid cancer and thyroiditis in the goitrous regions of Salta, Argentina, before and after iodine prophylaxis. Clin Endocrinol. 1995;43:701-706.
31 McTiernan A, Weiss NS, Daling JR. Incidence of thyroid cancer in women in relation to known or suspected risk factors for breast cancer. Cancer Res. 1987;47:292-295.
32 Ron E, Kleinerman RA, Boice JD, et al. A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987;79:1-12.
33 Kravdal O, Glattre E, Haldorsen T. Positive correlation between parity and incidence of thyroid cancer: new evidence based on complete Norwegian birth cohorts. Int J Cancer. 1991;49:831-836.
34 Hamilton TE, van Belle G, LoGerfo JP. Thyroid neoplasia in Marshall Islanders exposed to nuclear fallout. JAMA. 1987;258:629-636.
35 Prentice RL, Kato H, Yoshimoto K, et al. Radiation exposure and thyroid cancer incidence among Hiroshima and Nagasaki residents. Natl Cancer Inst Monogr. 1982;62:207-212.
36 Schneider AB, Recant W, Pinsky SM, et al. Radiation-induced thyroid carcinoma: clinical course and results of therapy in 296 patients. Ann Intern Med. 1986;105:405-412.
37 Mahta MP, Goetowski PG, Kindella TJ. Radiation-induced thyroid neoplasms, 1920 to 1987: a vanishing problem? Int J Radiat Oncol Biol Phys. 1989;16:1471-1475.
38 McHenry C, Jarosz H, Calundra D, et al. Thyroid neoplasia following radiation therapy for Hodgkin’s lymphoma. Arch Surg. 1987;122:684-686.
39 Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster: pathomorphologic study of 84 cases (1991-92) from the Republic of Belarus. Cancer. 1994;74:748-766.
40 Robbins J. Lessons from Chernobyl: the event, the aftermath fallout: radioactive, political, social. Thyroid. 1997;7:180-192.
41 Becker DV, Robbins J, Beebe GW, et al. Childhood thyroid cancer following the Chernobyl accident: a status report. Endocrinol Metab Clin North Am. 1995;25:197-211.
42 Nikiforov YE, Fagin JA. Radiation induced thyroid cancer in children after the Chernobyl accident. Thyroid Today. 1998;21:1-11.
43 Hundahl SA. National Cancer Institute report on iodine-131 exposure from fallout following Nevada atmospheric nuclear bomb tests. CA Cancer J Clin. 1998;48:285-298.
44 Farid NR, Shi Y, Kou M. Molecular basis of thyroid cancer. Endocr Rev. 1994;15:202-232.
45 Capezzone M, Marchisotta S, Cantara S, et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Rel Cancer. 2008;15:1075-1081.
46 Cooper DS, Axelrod L, DeGroot LJ, et al. Congenital goiter and the development of metastatic follicular carcinoma with evidence for a leak of non-hormonal iodine: clinical, pathological, kinetic and biochemical studies and a review of the literature. J Clin Endocrinol Metab. 1981;52:294-306.
47 Ledger GA, Khosla S, Lindor NM, et al. Genetic testing in the diagnosis and management of multiple endocrine neoplasia type II. Ann Intern Med. 1995;122:118-126.
48 Skinner MA, Moley JA, Dilley WG, et al. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353:1105-1113.
49 Frank-Raue K, Buhr H, Dralle H, et al. Long-term outcome in 46 gene carriers of hereditary medullary thyroid carcinoma after prophylactic thyroidectomy: impact of individual RET genotype. Eur J Endocrinol. 2006;155:229-236.
50 Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990;19:545-576.
51 McConahey WM, Hay ID, Woolner LB, et al. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy and outcome. Mayo Clin Proc. 1986;61:978-996.
52 Hay ID, Gonzalez-Losada T, Reinalda MS, et al. Long-term outcome in 215 children and adolescents with papillary thyroid carcinoma treated during 1940 through 2008. World J Surg. 2010;34:1192-1202.
53 Dinneen SF, Valimaki MJ, Bergstralh EJ, et al. Distant metastases in thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80:2041-2045.
54 Brennan MD, Bergstralh EJ, van Heerden JA, et al. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11-19.
55 Grebe SKG, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5:43-63.
56 Hay ID. Cytometric DNA ploidy analysis in thyroid cancer. Diagn Oncol. 1991;1:181-185.
57 Ruegemer JJ, Hay ID, Bergstralh EJ, et al. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;63:960-967.
58 Moley JF. Medullary thyroid cancer. Surg Clin N Am. 1995;75:405-420.
59 Lips CJM, Landsvater RM, Hoppener JWM, et al. Clinical screening as compared with DNA analysis in families with multiple endocrine neoplasia type 2A. N Engl J Med. 1994;331:828-835.
60 McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028-1034.
61 Greene FL, Sobin LH. A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol. 2009;99:269-272.
62 Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer: AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2010.
63 Larsen PR, Davies TF, Hay ID. The thyroid gland. In: In Wilson JD, Foster DW, Kronenberg HM, et al, editors. Williams Textbook of Endocrinology. 9th ed. Philadelphia: WB Saunders; 1998:389-515.
64 Grant CS, Hay ID, Gough IR, et al. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988;104:954-962.
65 Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241-260.
66 Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088-1095.
67 Simpson WJ, McKinney SE, Carruthers JS, et al. Papillary and follicular thyroid cancer: prognostic factors in 1,578 patients. Am J Med. 1987;83:474-488.
68 Shah JP, Loree TR, Dharker D, et al. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164:658-661.
69 Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1,779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050-1058.
70 Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947-953.
71 DeGroot LJ, Kaplan EL, Straus FH, et al. Does the method of management of papillary thyroid carcinoma make a difference in outcome? World J Surg. 1994;18:123-130.
72 van Heerden JA, Hay ID, Goellner JR, et al. Follicular thyroid carcinoma with capsular invasion alone: a non-threatening malignancy. Surgery. 1992;112:1130-1136.
73 Mueller-Gaertner HW, Brzac HT, Rehpenning W. Prognostic indices for tumor relapse and tumor mortality in follicular thyroid carcinoma. Cancer. 1991;67:1903-1908.
74 Shaha AR, Loree TR, Shah JP. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery. 1995;118:1131-1138.
75 Loree TR. Therapeutic implications of prognostic factors in differentiated carcinoma of the thyroid gland. Semin Surg Oncol. 1995;11:246-255.
76 D’Avanzo A, Ituarte P, Treselar P, et al. Prognostic scoring systems in patients with follicular thyroid cancer: a comparison of different staging systems in predicting the patient outcome. Thyroid. 2004;14:453-458.
77 Davis NL, Bugis JD, McGregor GI, et al. An evaluation of prognostic scoring systems in patients with follicular thyroid cancer. Am J Surg. 1995;170:476-480.
78 Gharib H, McConahey WM, Tiegs RD, et al. Medullary thyroid carcinoma: clinicopathologic features and long-term follow-up of 65 patients treated during 1946 through 1970. Mayo Clin Proc. 1992;67:934-940.
79 Pyke CM, Hay ID, Goellner JR, et al. Prognostic significance of calcitonin immunoreactivity amyloid staining, and flow cytometric DNA measurements in medullary thyroid carcinoma. Surgery. 1991;110:964-970.
80 Brierley J, Tsang R, Simpson WJ, et al. Medullary thyroid cancer: analyses of survival and prognostic factors and the role of radiation therapy in local control. Thyroid. 1996;6:305-310.
81 Venkatesh YS, Ordonez NG, Schultz PN, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 121 cases. Cancer. 1990;66:321-328.
82 Mack LA, Pasieka JL. An evidence-based approach to the treatment of thyroid lymphoma. World J Surg. 2007;31:978-986.
83 Famakinwa OM, Roman SA, Wang TS, et al. ATA practice guidelines for the treatment of differentiated thyroid cancer: were they followed in the United States? Am J Surg. 2010;199:189-198.
84 Panigrahi B, Roman SA, Sosa JA. Medullary thyroid cancer: are practice patterns in the United States discordant from American Thyroid Association Guidelines? Ann Surg Oncol. 2010;17:1490-1498.
85 Friedman M, Danielzadeh JA, Caldarelli DD. Treatment of patients with carcinoma of the thyroid invading the airway. Arch Otolaryngol Head Neck Surg. 1994;120:1372-1381.
86 Ballantyne AJ. Resections of the upper aerodigestive tract for locally invasive thyroid cancer. Am J Surg. 1994;168:636-639.
87 Hay ID, Grant CS, Bergstralh EJ, et al. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124:958-966.
88 Cady B. Our AMES is true: how an old concept still hits the mark: or, risk group assignment points the arrow to rational therapy selection in differentiated thyroid cancer. Am J Surg. 1997;174:461-468.
89 DeGroot LJ, Kaplan EL. Second operations for “completion” of thyroidectomy in treatment of differentiated thyroid cancer. Surgery. 1991;110:936-939.
90 Grant CS, Stulak JM, Thompson GB, et al. Risks and adequacy of an optimized surgical approach to the primary surgical management of papillary thyroid carcinoma treated during 1999-2006. World J Surg. 2010;95:1328-1332.
91 Mazzaferri EL. A vision for the surgical management of papillary thyroid carcinoma: extensive lymph node compartmental dissections and selective use of radioiodine. J Clin Endocrinol Metab. 2009;94:1086-1088.
92 Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683-689.
93 Noguchi S, Murakami N, Yamashita H, et al. Papillary thyroid carcinoma: modified radical neck dissection improves prognosis. Arch Surg. 1998;133:276-280.
94 Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419-425.
95 Grebe SKG, Hay ID. Follicular cell-derived thyroid carcinomas. Cancer Treat Res. 1997;89:91-140.
96 Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980-987.
97 Sawka AM, Thephamongkhol K, Brouwers M, et al. Clinical review 170. A systemic review and meta-analysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3668-3676.
98 Hay ID. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J Surg Oncol. 2006;94:692-700.
99 Simpson WJ, Panzarella I, Carruthers JS, et al. Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys. 1988;14:1063-1071.
100 Robbins RJ, Schlumberger MJ. The evolving role of I-131 for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46:28S-37S.
101 Wartofsky L. Highlights of the American Thyroid Association Guidelines for patients with thyroid nodules or differentiated thyroid carcinoma: the 2009 Revision. Thyroid. 2009;19:1139-1143.
102 Ford D, Giridharan S, McConkey C, et al. External beam radiotherapy in the management of differentiated thyroid cancer. Clin Oncol (R Coll Radiol). 2003;15:337-341.
103 Simpson WJ, Carruthers JS. The role of external irradiation in the management of papillary and follicular thyroid cancer. Am J Surg. 1978;1365:457-463.
104 Tubiana M, Haddad E, Schlumberger M, et al. External radiotherapy in thyroid cancers. Cancer. 1985;55:2062-2071.
105 Farahati J, Reiners C, Stuschke M, et al. Differentiated thyroid cancer: impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration. Cancer. 1996;77:177-180.
106 Tsang RW, Brierley JD, Simpson WJ, et al. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375-388.
107 Sheline GE, Galante M, Lindsay S. Radiation therapy in the control of persistent thyroid cancer. Am J Roentgenol Radium Ther Nucl Med. 1966;97:923-930.
108 Benker G, Olbricht T, Reinwein D, et al. Survival rates in patients with differentiated thyroid carcinoma: influence of postoperative external radiotherapy. Cancer. 1990;65:1517-1520.
109 Tubiana M, Haddad E, Schlumberger M, et al. External radiotherapy in thyroid cancers. Cancer. 1985;55:2062-2071.
110 Simpson WJ, McKinney SE, Carruthers JS, et al. Papillary and follicular thyroid cancer: prognostic factors in 1,578 patients. Am J Med. 1987;83:479-488.
111 Esik O, Nemeth G, Eller J. Prophylactic external irradiation in differentiated thyroid cancer: a retrospective study over a 30-year observation period. Oncology. 1994;51:372-379.
112 Phlips P, Hanzen C, Andry G, et al. Postoperative irradiation for thyroid cancer. Eur J Surg Oncol. 1993;19:399-404.
113 Brierley JD, Tsang RW. External-beam radiation therapy in the treatment of differentiated thyroid cancer. Semin Surg Oncol. 1999;16:42-49.
114 Mazzarotto R, Cesaro MG, Lora O, et al. The role of external beam radiotherapy in the management of differentiated thyroid cancer. Biomed Pharmacother. 2000;54:345-349.
115 Keum KC, Suh YG, Koom SW, et al. The role of postoperative external-beam radiotherapy in the management of patients with papillary thyroid cancer invading the trachea. Int J Radiat Oncol Biol Phys. 2006;65:474-480.
116 Meadows KM, Amdur RJ, Morris CG, et al. External beam radiotherapy for differentiated thyroid cancer. Am J Otolaryngol Head Neck Med Surg. 2006;27:24-28.
117 Brierley JD, Tsang RW. External beam radiation therapy for thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:497-509.
118 Brierley J, Tsang R, Panzarella T, et al. Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin Endocrinol. 2005;63:418-427.
119 Wilson PC, Millar BM, Brierley JD. The management of advanced thyroid cancer. Clin Oncol (R Coll Radiol). 2004;16:561-568.
120 Brierley JD, Tsang RW. External radiation therapy in the treatment of thyroid malignancy. Endocrinol Metab Clin North Am. 1996;25:141-157.
121 O’Connell MEA, A’Hern RP, Harmer CL. Results of external beam radiotherapy in differentiated thyroid carcinoma: a retrospective study from the Royal Marsden Hospital. Eur J Cancer. 1994;30A:733-739.
122 Hu A, Clark J, Payne RJ, et al. Extrathyroidal extension in well-differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. 2007;133:644-649.
123 Terezakis SA, Lee KS, Ghossein RA, et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-Kettering Cancer Center Experience. Int J Radiat Oncol Biol Phys. 2009;73:795-801.
124 Foote RL, Brown PD, Garces YI, et al. Is there a role for radiation therapy in the management of Hürthle cell carcinoma? Int J Radiat Oncol Biol Phys. 2003;56:1067-1072.
125 Besic N, Hocevar M, Zgajnar J, et al. Aggressiveness of therapy and prognosis of patients with Hürthle cell papillary thyroid carcinoma. Thyroid. 2006;16:67-72.
126 Schwartz DL, Rana V, Shaw S, et al. Postoperative radiotherapy for advanced medullary thyroid cancer—local disease control in the modern era. Head Neck. 2008;30:883-888.
127 Biermann M, Pixberg MK, Schuck A, et al. Multicenter study differentiated thyroid carcinoma: diminished acceptance of adjuvant external beam radiotherapy. Nuklearmedizin. 2003;9:244-250.
128 Schuck A, Bierman M, Pixberg MK, et al. Acute toxicity of adjuvant radiotherapy in locally advanced differentiated thyroid carcinoma. Strahlenther Onkol. 2003;179:832-839.
129 Rosenbluth BD, Serrano V, Happersett L, et al. Intensity-modulated radiation therapy for the treatment of nonanaplastic thyroid cancer. Int J Radiat Oncol Biol Phys. 2005;63:1419-1426.
130 Ask A, Bjork-Eriksson T, Zackrisson B, et al. The potential of proton beam radiation therapy in head and neck cancer. Acta Oncol. 2005;44:876-880.
131 Schwartz DL, Lobo MJ, Ang KK, et al. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys. 2009;74:1083-1091.
132 Kim JH, Kim MS, Yoo SY, et al. Stereotactic body radiotherapy for refractory cervical lymph node recurrence of nonanaplastic thyroid cancer. Otolaryngol Head Neck Surg. 2010;142:338-343.
133 Shimaoka K, Schoenfeld DA, De Wys WD, et al. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155-2161.
134 Derringer GA, Thompson LDR, Frommelt RA, et al. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol. 2000;24:623-639.
135 Thieblemont C, Mayer A, Dumontet C, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab. 2002;87:105-111.
136 Doria R, Jekel JF, Cooper D. Thyroid lymphoma: the case for combined modality therapy. Cancer. 1994;73:200-206.
137 Matsuzuka F, Miyauchi A, Katayama S, et al. Clinical aspects of primary thyroid lymphoma: diagnosis and treatment based on our experience of 119 cases. Thyroid. 1993;3:93-99.
138 Tupchong L, Hughes F, Harmer CL, et al. Primary lymphoma of the thyroid gland: clinical features, prognostic factors, and results of treatment. Int J Radiat Oncol Biol Phys. 1986;12:1813-1821.
139 Rosen IB, Sutcliffe SB, Gospodarowicz MK, et al. The role of surgery in the management of thyroid lymphoma. Surgery. 1988;104:1095-1099.
140 Junor EJ, Paul J, Reed NS. Primary non-Hodgkin’s lymphoma of the thyroid. Eur J Surg Oncol. 1992;18:313-321.
141 Sippel RS, Gauger PG, Angelos P, et al. Palliative thyroidectomy for malignant lymphoma of the thyroid. Ann Surg Oncol. 2002;9:907-911.
142 Blair TJ, Evans RG, Buskirk SJ, et al. Radiotherapeutic management of primary thyroid lymphoma. Int J Radiat Oncol Biol Phys. 1985;11:365-370.
143 Logue JP, Hale RJ, Stewart AL, et al. Primary malignant lymphoma of the thyroid: a clinicopathologic analysis. Int J Radiat Oncol Biol Phys. 1992;22:929-933.
144 Tsang R, Gospodarowicz M, Sutcliffe S, et al. Non-Hodgkin’s lymphoma of the thyroid gland: prognostic factors and treatment outcome. Int J Radiat Oncol Biol Phys. 1993;27:594-604.
145 Vigliotti A, Kong JS, Fuller LM, et al. Thyroid lymphomas stages IE and IIE: comparative results for radiotherapy only, combination chemotherapy only, and multimodality treatment. Int J Radial Oncol Biol Phys. 1986;12:1807-1812.
146 DiBiase SJ, Grigsby PW, Guo C, et al. Outcome analysis for stage IE and IIE thyroid lymphoma. Am J Clin Oncol. 2004;27:178-184.
147 Burke JS, Butler JJ, Fuller LM. Malignant lymphomas of the thyroid: a clinicopathologic study of 35 patients including ultra-structural observations. Cancer. 1977;39:1587-1602.
148 Souhami L, Simpson W, Carruthers J. Malignant lymphoma of the thyroid gland. Int J Radiat Oncol Biol Phys. 1980;6:1143-1147.
149 Harrington KJ, Michalaki VJ, Nutting CM, et al. Management of non-Hodgkin’s lymphoma of the thyroid: the Royal Marsden Hospital experience. Br J Radiol. 2005;78:405-410.
150 Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032-3038.
151 Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21-26.
152 Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346:235-242.
153 Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2 associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2003;101:4279-4284.
154 Leedman PJ, Sheridan WP, Downey WF, et al. Combination chemotherapy as single modality therapy for stage 1E and IIE thyroid lymphoma. Med J Aust. 1990;152:40-43.
155 McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1029-1034.
156 Kim JH, Leeper RD. Treatment of anaplastic giant and spindle cell carcinoma of the thyroid gland with combination Adriamycin and radiation therapy: a new approach. Cancer. 1983;52:954-963.
157 Schlumberger M, Parmentier C, Delisle MJ, et al. Combination therapy for anaplastic giant cell thyroid carcinoma. Cancer. 1991;67:564-566.
158 Tallroth E, Wallin G, Lundell G, et al. Multimodality treatment in anaplastic giant cell thyroid carcinoma. Cancer. 1987;60:1428-1431.
159 Sugino K, Ito K, Mimura T, et al. The important role of operations in the management of anaplastic thyroid carcinoma. Surgery. 2002;131:245-248.
160 Goutsouliak V, Hay JH. Anaplastic thyroid cancer in British Columbia 1985-1999: a population-based study. Clin Oncol. 2005;17:75-87.
161 Tennvall J, Lundell G, Wahlberg P, et al. Anaplastic thyroid carcinoma. Br J Cancer. 2002;86:1848-1853.
162 Besic N, Auersperg M, Us-Krasovec M, et al. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001;27:260-264.
163 Levendag PC, De Porre PM, van Putten WL. Anaplastic carcinoma of the thyroid gland treated by radiation therapy. Int J Radiat Oncol Biol Phys. 1993;26:125-128.
164 Simpson WJ. Anaplastic thyroid carcinoma: a new approach. Can J Surg. 1980;23:25-31.
165 De Crevoisier R, Baudin E, Bachelot A, et al. Combined treatment of anaplastic thyroid carcinoma with surgery, chemotherapy, and hyperfractionated accelerated radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:1137-1143.
166 Haddad R, Mahadevan A, Posner MR, et al. Long term survival with adjuvant carboplatin, paclitaxel, and radiation therapy in anaplastic thyroid cancer. Am J Clin Oncol. 2005;28:104.
167 Swaak-Kragten AT, de Wilt JH, Schmitz PI, et al. Multimodality treatment for anaplastic thyroid carcinoma: treatment outcome in 75 patients. Radiother Oncol. 2009;92:100-104.
168 Haigh PI, Ituarte PHG, Wu HS, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91:2335-2342.
169 Tan RK, Finley RK, Driscoll D, et al. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995;17:41-48.
170 Pudney D, Lau H, Ruether JD, et al. Clinical experience of the multimodality management of anaplastic thyroid cancer and literature review. Thyroid. 2007;17:1243-1250.
171 Higashiyama T, Ito Y, Hirokawa M, et al. Induction chemotherapy with weekly paclitaxel administration for anaplastic thyroid carcinoma. Thyroid. 2010;20:7-14.
172 Mitchell G, Huddart R, Harmer C. Phase II evaluation of high dose accelerated radiotherapy for anaplastic thyroid carcinoma. Radiother Oncol. 1999;50:33-38.
173 Wang Y, Tsang R, Asa S, et al. Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer. 2006;107:1786-1792.
174 Dandekar P, Harmer C, Barbachano Y, et al. Hyperfractionated accelerated radiotherapy (HART) for anaplastic thyroid carcinoma: toxicity and survival analysis. Int J Radiat Oncol Biol Phys. 2009;74:518-521.
175 Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma: treatment outcome and prognostic factors. Cancer. 2005;103:1330-1335.
176 Nutting CM, Convery DJ, Cosgrove VP, et al. Improvements in target coverage and reduced spinal cord irradiation using intensity-modulated radiotherapy (IMRT) in patients with carcinoma of the thyroid gland. Radiother Oncol. 2001;60:173-180.
177 Posner MD, Quivey JM, Akazawa PF, et al. Dose optimization for the treatment of anaplastic thyroid carcinoma: a comparison of treatment planning techniques. Int J Radiat Oncol Biol Phys. 2000;48:475-483.