Chapter 17 Thyroid abnormalities
AETIOLOGY
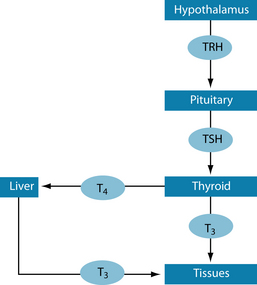
The main function of the thyroid is to produce hormones that regulate the metabolic rate of all cells, energy, cell growth and tissue differentiation.1,2 This is regulated by thyrotropin-releasing hormone (TRH), secreted by the hypothalamus, which activates thyroid-stimulating hormone (TSH), secreted by the pituitary gland, to produce thyroxine (T4) and triiodothyronine (T3) (Figure 17.1).1
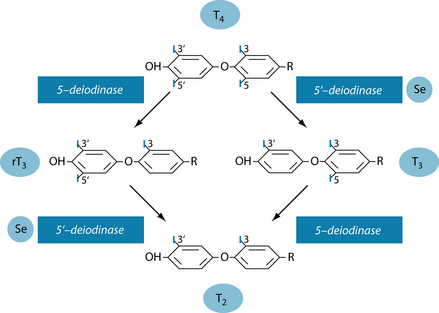
The precursors for thyroid hormone production are tyrosine and iodine. The thyroid gland actively accumulates iodine (~ 60 mg/day) in the form of iodide (I–), which leads to a much higher concentration in this gland than elsewhere in the body in order to produce T4 and some T3. These hormones are then released into the circulation where most are bound to transport proteins. Less than 0.1% are unbound (free T3 and T4) and active as hormone. Although the amount of T4 in the blood exceeds that of T3 nearly 50-fold, it is T3 that has much higher biological activity.3,4 Zinc seems to be needed to facilitate the uptake of the hormone by the cells5 and favourably affects T3.6 Some organs and tissues can convert T4 to T3 (mainly liver, kidneys, brain, muscle and brown adipose tissue), with most of the circulating T3 having been produced peripherally7 or in the liver. The conversion of T4 to T3 is accomplished by 5’-deiodinase which is selenium dependent.4,8 In the absence of selenium, reverse T3 (rT3) is produced; this is metabolically inactive.9 Figure 17.2 shows the metabolic pathways of T4 conversion.4
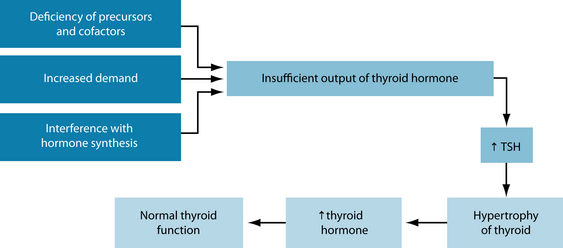
Thyroid function not only depends on sufficient precursors (such as iodine and tyrosine) but also on hormone synthesis regulation and the demand for these hormones. Disturbances in any of these can lead to increased TSH production by the pituitary gland with subsequent (non-toxic) goitre formation. This increased thyroid tissue production is an attempt to produce sufficient thyroid hormones in order to maintain normal blood levels. Figure 17.3 illustrates this. However, if the enlarged thyroid gland overproduces thyroid hormones, leading to hyperthyroidism, it is called a ‘toxic goitre’.10
Thyroid antibodies (to thyroglobulin, peroxidase or thyroid receptor) are only raised in the autoimmune forms of hypothyroidism (Hashimoto’s disease) and hyperthyroidism (Grave’s disease). Gastrointestinal pathogens, through ‘molecular mimicry’, could be responsible for this.1,11 Refer to Chapter 28 on autoimmunity for further details.
In hypothyroidism a disturbance in glucose tolerance has been found to be due to decreased sensitivity to insulin, and raised cortisol and free fatty acids.12 This is not surprising considering that insulin resistance, diabetes type 2, stress (hypercortisolism and hyperadrenalism) and hypothyroidism share a number of common signs and symptoms, taking into account the link between the hormones involved in these conditions. Raised cortisol levels with impaired glucagon response were noted when hypoglycaemia was induced in hypothyroid patients.13 Insulin and glucose control was regained when these patients were given thyroid hormone replacement therapy.14 Further, hypothyroidism, like insulin resistance and diabetes type 2 leads to inhibition of delta 5 and 6 desaturase, impeding the utilisation of dietary omega-3 and omega-6 fatty acids.1 A rise in blood pressure has been noted in the hypothyroid state which normalised on thyroid treatment.15 Conversely, impaired extrathyroidal conversion of T4 to T3 has been found in diseases such as heart and liver disease and diabetes type 2, where there is high plasma free fatty acid concentration with no obvious thyroid pathology.1
Sick euthyroid syndrome, an underfunctioning thyroid with apparently normal test results, can occur as part of a number of health problems. It has been found, for a variety of reasons, to be part of virtually all severe systemic illnesses, fasting and major operations, affecting T3, T4 and rT3.16 Treating obese individuals with hypocaloric diets leads to similar thyroid hormone changes as in anorexia, namely decreases in T3 and T4 and increases in rT3. These hormones will normalise with weight normalisation.17 Certain medications also influence thyroid hormone status by either increasing or decreasing T4.7 It is therefore essential to test for these hormones in these situations in order to determine treatment options.
Testing for thyroid function
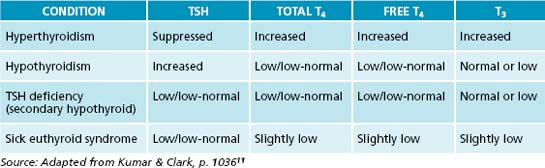
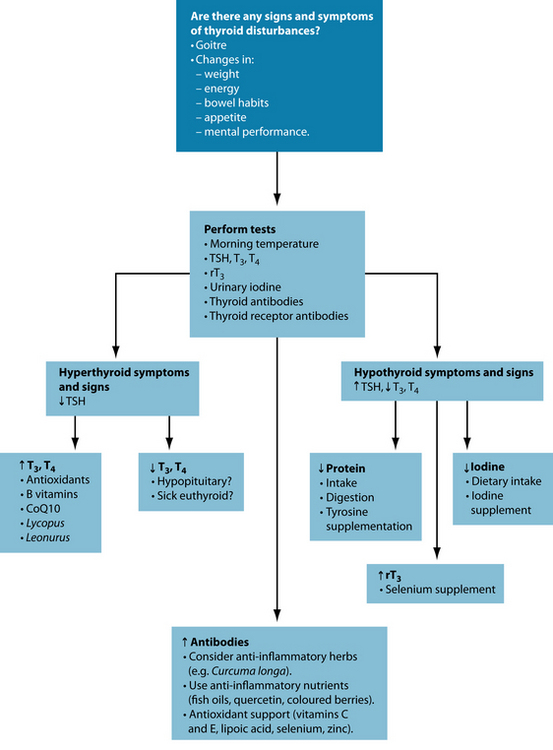
In hyperthyroidism, T4 and T3 levels are elevated, suppressing TSH. In contrast, in primary hypothyroidism TSH tends to be high with T3 and T4 being low. In secondary hypothyroidism, however, TSH as well as T3 and T4 are low. This latter condition arises from an inability of the pituitary gland to secrete TSH, possibly due to low TRH. By measuring TSH only, this condition can be missed or misinterpreted. Therefore, when there is indication from the symptom picture that thyroid function could be compromised, not only TSH but also T3 and T4 should be measured.18 Table 17.1 gives an overview of the laboratory parameters for each condition, and Table 17.2 shows the reference ranges (which vary somewhat between laboratories) and the ideal ranges of these parameters. In addition, the thyroid should be checked for goitre and nodules by palpation and the latter, if needed, by ultrasound or biopsy.7
Table 17.2 Reference ranges and ideal ranges of thyroid parameters2
| PARAMETER | REFERENCE RANGE | IDEAL RANGE |
|---|---|---|
| TSH | 0.3–5 mU/L | 0.4–2.5 mU/L |
| T3 | 2.6–6.0 pmol/L | 4–5 pmol/L |
| T4 | 9–19 pmol/L | 14–19 pmol/L |
| rT3 | 140–540 pmol/L | < 240 pmol/L |
| Thyroglobulin AB | < 4 IU/mL | < 1 IU/mL |
| Peroxidase AB | < 6 IU/mL | < 1 IU/mL |
| TRAB | < 1 IU/L | < 1 IU/L |
AB = antibodies, TRAB = thyroid receptor antibodies
Laboratory reference values for these parameters encompass 95% of the ‘healthy’ population, and only when individual values are outside this range will further investigations and possibly therapy be instigated by the doctor. However, in a well-functioning thyroid TSH should be between 0.4 and 2.5 mU/L (otherwise the system is labouring—subclinical hypothyroidism (SCH)—see below), with a therapeutic target range of 0.5 to 2 mU/L.19,20 The actual hormones should ideally be in the high-normal range. The ratio of T4 to T3 has been estimated as 4:1 in healthy thyroid function.1 Antibodies should be absent and rT3 should be low.
EPIDEMIOLOGY
Thyroid disorders are much more frequent in women than in men. The overall incidence in Australia is estimated at 5 in 1000 in males and 27 in 1000 in females. No specific figures for hypothyroidism in Australia could be found but in the US populations this is estimated to be 0.3%.21 Approximately 10–12 million people in the USA have hypothyroidism.22 In the UK the annual incidence of primary hypothyroidism is estimated to be 0.6 in 1000 for men and 3.5 in 1000 for women. In total, 3% of the population is taking medication for hypothyroidism.23 In contrast, it is stated that in the US 1–2% of the population is hypothyroid, with a tenfold higher occurrence in women than in men and increasing with age.3 Including SCH, the figures increase to 4.3–9.5%.3 Considering that this condition is generally underdiagnosed, at least in its milder form, the incidence would be much higher.
RISK FACTORS
Subclinical hypothyroidism
SCH has been characterised by elevated TSH with normal T3 and T4.24,25 There is still debate regarding the significance and possible treatment of SCH. Although treatment with T4 and/or T3 has apparently not been shown to provide benefits,26 withholding treatment may be ill advised. Subtle improvements with early hormonal intervention have been observed, thus warranting thyroid hormone replacement in subclinical manifestations.27 Most at risk are the elderly and women over the age of 60 years, with increased risk of developing overt hypothyroidism.25 It is therefore recommended that this population group, people with autoimmune disease and anybody with suggestive symptoms of hypothyroidsism be screened.28 TSH values ranging between 4.5 and 10 mU/L, particularly if antibodies or symptoms are present, may indicate the need for commencement of low-dose T4 treatment.29 However, some researchers consider a TSH range up to 20 μU/L (well above the upper limit of the reference range) as the cut-off before instigating therapy.22
It is interesting to note that the upper limit of normal for TSH has been reduced from 10 μU/L to between 4 and 5 μU/L during the last decade,22 with suggestions that this may be further reduced to 2.5 μU/L in the near future. This is based on the observation that 95% of people with normal thyroid function have values under 2.5 μU/L.20 Suboptimal thyroid function may be due to latent Hashimoto’s disease, which could lead, if not addressed early, to overt hypothyroidism.19,20,22,25 Further, patients with SCH tend to have higher readings of blood pressure, C-reactive protein, homocysteine, cholesterol and LDL, and thus may be presenting with cardiovascular risk factors or disease, including congestive heart failure. Early treatment is therefore recommended;22 this will also improve long-term life quality by preventing the development of overt hypothyroidism and also reducing the risk factors for cardiovascular disease, not to mention the benefits to the health-care system.
Further, a low T3 syndrome has been described with increased proinflammatory cytokines, notably IL6, which inhibits 5’-deiodinase. This is commonly seen in congestive heart failure, and T3 has been shown to be a stronger indicator of all-cause and congestive heart failure mortality than age or dyslipidaemia.22
Stress
Stress seems to play a crucial part in thyroid dysregulation and interference with hormone synthesis by triggering the release of corticotropin-releasing hormone, noradrenaline and cortisol. These hormones have an inhibitory influence on TSH secretion and suppress 5’-deiodinase, thus contributing to the suppression of thyroid function30 (see Chapter 15 on adrenal exhaustion also). The thyroid as well as the adrenal glands requires tyrosine for thyroid hormone and catecholamine synthesis, respectively. Low protein intake and/or impaired protein digestion/use may lead to low tyrosine stores, resulting in low thyroid hormones, especially if this amino acid is required to preferentially produce stress hormones (dopamine, noradrenaline and adrenaline). Urinary cortisol metabolite levels have also been linked to thyroid disorders, both in hypo- and hyperthyroidism, indicative of the influence of stress on thyroid function.31
Iodine
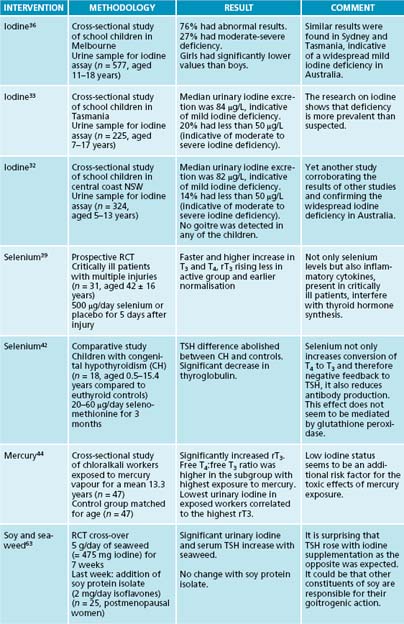
Iodine is a common deficiency worldwide, leading to hypothyroidism.11 It has been found in various parts of Australia (notably Tasmania)32–36 and New Zealand.37,38 Often the first visible sign is the development of a goitre. Congenital hypothyroidism, caused by maternal iodine deficiency, leads to mental and physical retardation in infants known as cretinism.9,39 Iodine reference values, according to the World Health Organization/International Council for the Control of Iodine Deficiency Disorders (WHO/ICCIDD), are given in Table 17.3.
| URINARY IODINE (µG/L) | IODINE NUTRITIONAL STATUS |
|---|---|
| < 20 | Severe deficiency |
| 20–49 | Moderate deficiency |
| 50–99 | Mild deficiency |
| 100–199 | Optimal status |
| ≥ 200 | Risk of adverse effects (e.g. hyperthyroidism) |
Selenium
Selenium is needed for the conversion of T4 to T3 (Figure 17.2). If it is low, the result is not only reduced active thyroid hormone (T3), but the resultant accumulation of rT3. Further, rT3 is also known to block the action of thyroid hormone, thus contributing to hypothyroidism. It is therefore important to measure rT3, especially with normal T4 and low T3 results. Hence, when T3 is low selenium deficiency should be considered as rT3 could be elevated.16 Trauma from injury affects thyroid metabolism with lowered selenium levels, and supplementation with selenium has led to faster normalisation of T4 and reductions in rT3.41 In congenital hypothyroidism, selenium (as selenomethionine) was found to lower TSH and thyroglobulin. It was suggested that the mechanism involved feedback to the hypothalamus–pituitary, thus reducing the stimulation of thyroid tissue and increasing intracellular conversion of T4 to T3.42
Similarly, in critically ill patients low selenium and T3 and elevated rT3 have been found, in addition to low TSH and T4. However, not only low selenium but also increased cytokine production during inflammation in these patients are responsible for low 5’-deiodinase; this may explain the elevated rT3. The abnormalities in these parameters have been found to correlate with the severity of the disease.43 Low T4 has been attributed to decreases in thyrotropin as well as T4-binding globulins. Supplementation with T3, not T4, has resulted in improvements. The effect of severe systemic illness on thyroid function has been termed ‘nonthyroidal illness syndrome’ (NTIS).16
Mercury
Workers exposed to mercury vapours had a higher risk of hypothyroidism with decreased T3 and increased rT3 values, especially if they were also low in iodine.44
Molecular mimicry
In hyperthyroidism thyroid hormones are high, suppressing TSH. The increased T3 and T4 is evidently due to an antithyroid autoantibody thyroid-stimulating immunoglobulin, which acts like TSH but is not controlled by the same negative feedback mechanism.10 Certain gram-negative bacteria, such as Yersinia enterocolitica and Escherichia coli, have been shown to contain TSH binding sites. It could therefore be possible that infection with these organisms could initiate hyperthyroidism through ‘molecular mimicry’.11
CONVENTIONAL TREATMENT
Hyperthyroidism
Depending on the severity of the disease, hyperthyroidism is treated with drugs that suppress the production of thyroid hormones, radioactive iodine to destroy part or all of the thyroid gland, or surgery to remove some or all of the thyroid. The latter two are likely to require thyroid hormone replacement for life.11
In order to make treatment effective but not detrimental to health, in total thyroidectomy a replacement dose of T4 is usually given at 1.6 μg/kg body weight, with an absorption rate of approximately 65%, resulting in a daily dose of 100–200 μg. It is best taken 1 hour prior to or 2–3 hours after meals. It takes 4–6 weeks for hormone levels to adjust to treatment.22
Hypothyroidism
The aim of treatment is to normalise thyroid function, and the treatment of choice for hypothyroidism is levothyroxine sodium (l-T4). In primary hypothyroidism, optimisation is achieved when TSH levels are 0.5–2 mU/L,22,45 and in secondary hypothyroidism (where TSH is low) with T4 and T3 levels in the upper normal range. When two doses of l-T4 were compared, one to bring T4 into the middle and the other into the upper range of normal, the higher dose resulted in lower BMI, cholesterol and LDL. When the high dose of l-T4 was combined with liothyronine (l-T3) at a ratio of 9:1, further benefits were obtained.22
This indicates that in certain situations l-T4 alone is insufficient to return thyroid function to normal. It is estimated that in 10–20% of hypothyroid patients symptoms will persist. This could be due to impaired conversion of T4 to T3 (see above under ‘Selenium’), a polymorphism in the enzyme 5’-deiodinase,22 plus several others.46 Treatment with liothyronine (l-T3) should therefore be considered when T4 alone does not improve symptoms or laboratory parameters.16,22 This is particularly the case in secondary hypothyroidism due to other ill health where there is impaired conversion from T4 to T3. In vivo, however, T3 has a short half-life, leading to supraphysiological peaks without normalisation of TSH. Slow-release formulations are therefore needed.22
To overcome the problem regarding when to give T4 and when T3, combination preparations are available. However, apart from using the l-T3 with its half-life problems, the studies done on this have not included measures that indicated the need for T3.47 To overcome the instability of T3, desiccated extract of beef or pork thyroid have been used.22,45 (Desiccated bovine or porcine thyroid extract is contained in some natural formulations currently on the market and which are available to naturopaths.) This was the standard treatment before l-T4 was available commercially, but reproducible results were difficult, if not impossible, to obtain due to the variability of thyroid hormone content in these preparations.22
In younger people diagnosed with hypothyroidism, treatment with T4 should be instigated at the highest calculated dose, whereas in older people the dose should be conservative and slowly titrated up to avoid complications, particularly in those people with coronary artery disease. However, long-term treatment above the optimal dose can lead to osteoporosis and atrial fibrillation and should therefore be avoided.22,45 In autoimmune hypothyroid women planning pregnancy, TSH levels should be kept below 2.5 μU/L, with a 30% increase in l-T4 dosage once pregnancy has been confirmed.22
Table 17.4 lists the treatments currently employed to treat hypo- and hyperthyroidism.
Table 17.4 Conventional treatment for hyper- and hypothyroidism
| CONDITION | DRUG | ACTION |
|---|---|---|
| Hyperthyroidism | Propylthiouracil Carbamidazole (active metabolite: methimazole) | Antithyroid drugs: inhibit formation of thyroid hormone; immunosuppressive |
| Propranolol |
KEY TREATMENT PROTOCOLS
Diagnostic aids
Temperature regulation
NATUROPATHIC TREATMENT AIMS
One such marker is body temperature. Ideally, a temperature reading needs to be taken at the same time each day in the morning, straight after waking and before getting out of bed. The reason for taking the temperature in the morning is the influence melatonin exerts on thyroid hormones.48 The thermometer should be placed under the tongue until it beeps (for digital thermometers) or for a minimum of 5 minutes (if using a mercury one). Ear thermometers or taking the temperature under arm is too inaccurate for this purpose (although the latter has been advocated for decades). This should be repeated for 4 consecutive days and an average taken of the readings. For a woman of childbearing age, the temperature should be taken in the first half of her cycle, ideally the days straight after menses, as there is a natural rise in temperature at ovulation and throughout the second half of the cycle.
A normal reading is between 36.5 and 37.0°C. Low readings have been linked to thyroid underfunction, and values above this range to hyperthyroidism. Research in elderly hospitalised patients revealed a link between low core (rectal) temperature, low T3 and high rT3, and mortality. Further, low serum albumin and weight loss (as indicators of malnutrition) have also been linked to hypothermia (defined as a core temperature between 35.0 and 36.5°C). Interestingly, TSH did not make a significant difference in these patients.49 However, when healthy males were subjected to different sleep temperatures a significant increase was found in plasma cortisol and TSH with lowered body temperature.50 Likewise, a rise in TSH and drop in T3 and T4 were noted in cold climates and winter months, especially in people above the age of 40 years.51 In some subjects with fever, rT3 was found to be directly correlated whereas T3 was inversely correlated to body temperature, at 40oC reducing to levels seen only in severe hypothyroidism.52 It seems that the thyroid gland is very sensitive to either heat or cold stress as a result of the hypothalamus–pituitary–thyroid axis dysregulation, and reacts by reducing its hormone production.48,51
A number of factors can interfere with the accuracy of the temperature method. Late nights, lack of sleep, infections and acute illnesses will alter the readings. Antidepressant medication has also been linked to lower body temperature. A significant rise in TSH and a drop in T3 and T4 have been noted after administration of antidepressants such as tricyclics and selective serotonin reuptake inhibitors (SSRIs), but also after lithium and electroshock treatment. In some patients a blunted TSH response to TRH has been found.53 This begs the question whether these treatments interfere with negative feedback loops (such as between T3 and TSH or TSH and TRH) or whether tyrosine was needed preferentially to produce adrenalin?
Physical and pathology tests
Since goitre is one of the earliest symptoms of iodine deficiency, the thyroid gland should be palpated to check for enlargement.40 Further tests that are recommended are a 24-hour urinary iodine excretion,40 and red blood cell (solidus and or 24-hour urinary excretion of) selenium and zinc. If any of these are low, appropriate supplementation should be instigated. These tests will confirm or deny the results of a dietary analysis with regard to these elements. Protein status should be evaluated by investigating dietary protein intake and digestive capacity, and possibly by body composition analysis. If protein is found to be low, then it is likely that tyrosine will also be low. If stress is suspected to play a major part in the aetiology of the thyroid disorder then it would be prudent to also assess cortisol.
Addressing underlying pathology
Treatment depends on the findings as a result of case taking, dietary analysis and pathology tests. If stress is a major contributor, adaptogens (for example, Withania somnifera, Rhodiola rosea, Panax ginseng and Glycyrrhiza glabra) or anxiolytics (for example, Piper methysticum, Valeriana spp., Passiflora incanata) could be of use.54–57 If autoimmunity is present anti-inflammatory (for example bioflavonoids,58 enzymes58 and Curcuma longa)54 and immunosuppressant (for example, Tylophora indica and Hemidesmus indicus)54 agents may be indicated.
Hyperthyroidism
Managing increased metabolic rate
In hyperthyroidism, metabolic rate and, with this, energy production are increased. The overactive metabolism needs to be supported through nutrient-dense foods with ample amounts of fruit and vegetables for antioxidant support. Further, supplementation with Krebs cycle and oxidative phosphorylation nutrients, notably B vitamins, magnesium, coenzyme Q10 and carnitine (Table 17.5), is highly advisable.1,4
| SUPPLEMENT | AMOUNT | RATIONALE |
|---|---|---|
| B complex | 1 b.i.d. | High quality, high dose, preferably in their activated (phosphorylated) forms, for ready use in energy production in the Krebs cycle |
| Coenzyme Q10 | 100 mg/day | Essential for energy production in the electron transport chain |
| Magnesium | 100–400 mg/day | Needed in Krebs cycle and for any ATP-dependent reactions |
| Antioxidants | 1 b.i.d. | Broad-spectrum, high dose, to dampen the oxidative damage occurring through the higher metabolic rate and autoimmunity (if present).60 |
| Vitamin C | 1000 mg/day | Shown to benefit hyperthyroidism. Best taken in small frequent doses.61 |
| Leonurus cardiaca | 15–40 mL/wk | To reduce iodine metabolism and thyroid hormone production54,56 |
| Lycopus virginicus | 15–25 mL/wk | Adjuvant therapy for thyroid hyperfunction. Inhibition of peripheral deiodination of T4 to T3.54,56 |
An increased metabolic rate brings with it increases in inflammation and oxidative stress, particularly if autoimmunity is also present.59 The best cellular defence includes broad-spectrum antioxidants (including the vitamins A, C and E, the minerals zinc and selenium, and the bioactive substances α-lipoic acid, N-acetyl cysteine and coenzyme Q10), as well as intracellular antioxidant support (notably glutathione and superoxide dismutase).60 Essential fatty acids are also needed. Vitamin C at 1000 mg per day has shown beneficial effects in hyperthyroidism.61
Herbal and nutritional treatment
In order to dampen thyroid hormone production herbs such as Lycopus virginicus and Leonurus cardiaca are useful.54,56 In a rat model, oral administration of L.virginicus has reduced T3 levels; this is thought to be the result of decreased peripheral T4 conversion to T3.65 L. cardiaca is used for nervous cardiac disorders such as palpitations,56 and since these symptoms also occur in hyperthyroidism this herb has been used as an adjuvant for this condition.54 Other herbs with thyroid-blocking action include Melissa officinalis and Lithospermum spp.66,67
Dietary goitrogens39,40 (see the box below) or smoking can have an inhibitory effect on thyroid function by interfering with iodine uptake and thyroid hormone production. Paradoxically, excess iodine intake can also impede thyroid hormone production.39,40
Hypothyroidism
Cofactors for hormone production
For hypothyroidism, hormone precursors and cofactors, such as combinations of tyrosine, iodine, selenium and zinc, are recommended to support the thyroid (Table 17.6). However, hyperthyroidism can develop as a result of recent supplementation with iodine to correct a hypothyroid state. Commonly, nodules develop on the thyroid gland, secreting thyroid hormones that are not regulated by TSH. If this should occur iodine supplementation needs to cease and treatment as for hyperthyroidism instigated.40
| NUTRIENT | AMOUNT | RATIONALE |
|---|---|---|
| Iodine39,40,74 | 150 μg/day | Constituent of thyroid hormone |
| Tyrosine74 | 1000–3000 mg/day | Constituent of thyroid hormone. Needs to be taken between meals. |
| Selenium39,74 | 100–200 μg/day | Facilitates the conversion from T4 to the active form T3. |
| Zinc5,6 | 25 mg 1–2/day | Supports thyroid hormone regulation |
| Withania somnifera72,73∗ |
Iodine
Nutritionally, goitrogens (see above) in large amounts, particularly raw, should be avoided, especially if iodine status is already compromised.39,40 Food rich in iodine includes seaweed products, seafood, eggs and products where iodine has been added to the feed. In the past, iodine has been used to sterilise milking equipment, resulting in iodine-rich milk. But in recent years iodine has been replaced by other methods of sterilisation (notably chlorine), so dairy is not a good source of iodine any longer.35 In certain parts of the world, such as Australia and New Zealand where goitre used to be prevalent, iodine has been added to table salt.39 However, the decreasing use of salt has further contributed to lowered iodine status.35
Available iodine supplements include potassium iodide and potassium iodate, iodised vegetable oil, nascent (atomic) iodine and Lugol’s solution. When treating iodine deficiency it was noted that simultaneously correcting other nutrient deficiencies, notably iron and vitamin A, produced significantly better results.4,40
In pregnancy the requirement for thyroid hormone increases; this needs to be met by adequate iodine status. Therefore, additional iodine at about 125 μg/day should be supplied to allow for the increase in metabolic demand.68–70
Herbal and nutritional treatment
Certain herbs, such as Withania somnifera, Bacopa monnieri and Fucus vesiculosus, may be beneficial in hypothyroidism.54,71–73 W. somnifera has also been shown to cause a decrease in hepatic lipid peroxidation and may thus be useful in concomitant heart and liver disease.73
Comprehensive diagnosing
In both hyper- and hypothyroidism, despite the best treatment measures, symptoms can persist, leading to compromised quality of life. It would therefore be prudent to not only assess thyroid function through laboratory testing and temperature readings, but also through a comprehensive interview or questionnaire that takes the patient’s symptoms and overall health status into account.75
INTEGRATIVE MEDICAL CONSIDERATIONS
Any therapies that help reduce stress are of value, such as bodywork, mind therapies and relaxation techniques. Homoeopathy and traditional Chinese medicine can help balance body energies, thus setting the stage for healing to take place. The homoeopathic remedy Thyroidinum has been given in cases of thyroid dysfunction,76 however individualisation of remedies may yield better results long-term.
Example treatment
Hyperthyroidism
In the first consultation, the diagnosis of hyperactive thyroid was made on the basis of the laboratory results and her symptom of weight loss (confirmed by medical diagnosis). Her diet was reviewed and she was encouraged to eat nutrient-dense food and to
avoid refined carbohydrates and processed food in order to provide the best nutrition to her overactive metabolism. Iodine in the form of iodised salt and seaweed products were best avoided, too, so that the thyroid was deprived of one of the materials from which to make thyroid hormones. Raw brassica, such as coleslaw, was advised to increase iodine binding for elimination.
Herbal prescription (hyperthyroidism)
| Rhodiola rosea 1:1 | 30 mL |
| Lycopus virginicus 1:2 | 40 mL |
| Leonurus cardiaca 1:2 | 30 mL |
| 7 mL b.d. | 100 mL |
Nutritional prescription
| Activated vitamin B complex | 1 tablet b.d. |
| Coenzyme Q10 | 100 mg/day |
| Magnesium citrate | 300 mg/day |
| Broad-spectrum antioxidant | 1 tablet b.d. |
To dampen thyroid function and increase resilience, she was prescribed a herbal formula containing Rhodiola rosea to counteract stress and to improve stamina, and Lycopus virginicus and Leonurus cardiacato dampen thyroid hyperactivity. She was also treated with nutritional supplements to support her increased metabolism: activated vitamin B complex to provide cofactors for the Krebs cycle in their coenzyme form, coenzyme Q10 to support energy production in the electron transport chain, magnesium citrate to support Krebs cycle function
and ATP-dependent reactions, and a broad-spectrum antioxidant to minimise oxidative damage due to an overactive metabolism.
Further, she was advised to practise a relaxation technique such as guided imagery or meditation. This patient needed time out – time for herself after years of emotional and physical stress. Informal counselling was used as part of the therapeutic relationship. Intensive exercise (other than hatha yoga, a gentle walk or swim) was discouraged to prevent further weight loss.
Hypothyroidism (3 years later)
Herbal prescription (hypothyroidism)
| Rhodiola rosea 1g | 1 capsule b.d. |
| Curcuma longa 2g | 1 tablet/day |
| Fucus vesiculosis 1 g | 1 tablet/day |
Nutritional prescription
| Thyroid and adrenal support nutrients | 2 capsules b.d. |
| Bioflavonoid formula | 1 tablet b.d. |
| Omega-3 fish oils | 3 g t.d.s. |
| Digestive support | 1 with each meal |
Expected outcomes and follow-up protocols
After 3 months, the patient is expected to report improvement regarding hypothyroidism symptoms, in addition to balancing her weight. This should reflect on her blood test with antibodies and TSH readings normalising. If no beneficial effect occurs after 4–6 weeks of treatment, the prescription should be altered to address a potential underlying autoimmune pathology (see Chapter 28 on autoimmunity for treatment details). If after 3 months of treatment no change had occurred on follow-up blood tests (and symptoms), she would be advised to start thyroxine at 50 μg/day. The prescription can still be repeated and use adjuvantly, with the exception of the thyroid and adrenal support formula, which can be reduced to 1 capsule b.d. Regular blood tests are advised and the prescription modified for the patient’s current health status. After the thyroid parameters have normalised, she can be advised to consider slowly reducing thyroxine (with medical support), while continuing with her herbal and nutriceutical precription. Some patients may have the hyperthyroidism return, and thereby may need to titrate up the dose of thyroxine; however, others may find that they stay euthyroid. The key factor for practioners to note in treating thyroid conditions is that in some instances people may alternate from hyperthyroidism to hypothyroidism and vice versa. Due to this, regular monitoring of signs and symptoms and blood tests is advised.
Danzi S., Klein I. Recent considerations in the treatment of hypothyroidism. Curr Opin Investig Drugs.. 2008;9(4):357-362.
Vaidya B., Pearce S.H.S. Management of hypothyroidism in adults. BMJ. 2008;337(7664):284-289.
Woolever D.R., Beutler A.I. Hypothyroidism: a review of the evaluation and management. Family Practice Recertification. 2007;29(4):45-52.
1. Lord R.S., Alexander B.J. Laboratory evaluations for integrative and functional medicine, 2nd edn. Duluth: Georgia: Metametrix Institute; 2008.
2. Thibodeau G., Patton K. Anatomy and physiology, 5th edn. St. Louis: CV Mosby; 2003.
3. Woolever D.R., Beutler A.I. Hypothyroidism: a review of the evaluation and management. Family Practice Recertification. 2007;29(4):45-52.
4. Gropper S., et al. Advanced nutrition and human metabolism, 5th edn. Australia: Wadsworth, Cengage Learning; 2009.
5. Ganapathy S., Volpe S.L. Zinc, exercise, and thyroid hormone function. Crit Rev Food Sci Nutr. 1999;39(4):369-390.
6. Maxwell C., Volpe S.L. Effect of zinc supplementation on thyroid hormone function. A case study of two college females. Ann Nutr Metab. 2007;51(2):188-194.
7. Tierney L.M., et al. Medical diagnosis and treatment, 44th edn. New York: McGraw-Hill; 2005.
8. Brown K.M., Arthur J.R. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4(2B):593-599.
9. Leonard J.L. Non-genomic actions of thyroid hormone in brain development. Steroids. 2008;73(9–10):1008-1012.
10. Crowley L.V. An introduction to human disease: pathology and pathophysiology correlations, 6th edn. Sudbury: Jones and Bartlett Publishers; 2004.
11. Kumar P., Clark M. Clinical medicine, 5th edn. London: W. B. Saunders; 2002.
12. Kosovskiĭ M.I., et al. [Glucose tolerance disorders in patients with hypothyroidism]. Probl Endokrinol. 1992;38(2):26-29.
13. Clausen N., et al. Counterregulation of insulin-induced hypoglycaemia in primary hypothyroidism. Acta Endocrinol. 1986;111(4):516-521.
14. Stanická S., et al. Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy. Clin Chem Lab Med. 2005;43(7):715-720.
15. Fommei E., Lervasi G. The role of thyroid hormone in blood pressure homeostasis: evidence from short-term hypothyroidism in humans. J Clin Endocrinol Metab. 2002;87(5):1996-2000.
16. Chopra I.J. Nonthyroidal illness syndrome or euthyroid sick syndrome? Endocr Pract. 1996;2(1):45-52.
17. Douyon L., Schteingart D.E. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol Metab Clin North Am. 2002;31(1):173-189.
18. Ochi Y., Kajita Y. [Determination of thyroid hormone]. Nippon Rinsho. 1999;57(8):1794-1799.
19. Dickey R.A., et al. Optimal thyrotropin level: normal ranges and reference intervals are not equivalent. Thyroid. 2005;15(9):1035-1039.
20. Wartofsky L., Dickey R.A. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90(9):5483-5488.
21. Brown B. Moving towards balance—hypothyroidism. Online. Available: http://www.moving-towards-balance.com.au/MTB_Hypothyroidism.html. Accessed 19 April 2009.
22. Danzi S., Klein I. Recent considerations in the treatment of hypothyroidism. Curr Opin Investig Drugs. 2008;9(4):357-362.
23. Vaidya B., Pearce S.H.S. Management of hypothyroidism in adults. Br Med J. 2008;337(7664):284-289.
24. Krysiak R., et al. [Subclinical hypothyroidism]. Wiad Lek. 2008;61(4–6):139-145.
25. Papi G., et al. Subclinical hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2007;14(3):197-208.
26. Villar H., et al. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Systemat Rev. (3):2007. CD003419
27. Gharib H. Commentary: review: Available evidence does not support a benefit for thyroid hormone replacement in adult with subclinical hypothyroidism. ACP J Club. 2008. Sect. 6
28. Tchong L., et al. Hypothyroidism: management across the continuum. J Clin Outcomes Manag. 2009;16(5):231-235.
29. Arrigo T., et al. Subclinical hypothyroidism: the state of the art. J Endocrinol Invest. 2008;31(1):79-84.
30. Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865-871.
31. Taniyama M., et al. Urinary cortisol metabolites in the assessment of peripheral thyroid hormone action: application for diagnosis of resistance to thyroid hormone. Thyroid. 1993;3(3):229-233.
32. Guttikonda K., et al. Iodine deficiency in urban primary school children: a cross-sectional analysis. Med J Aust. 2003;179(7):346-348.
33. Guttikonda K., et al. Recurrent iodine deficiency in Tasmania, Australia: a salutary lesson in sustainable iodine prophylaxis and its monitoring. J Clin Endocrinol Metab. 2002;87(6):2809-2815.
34. Gunton J.E., et al. Iodine deficiency in ambulatory participants at a Sydney teaching hospital: is Australia truly iodine replete? [see comment]. Med J Aust. 1999;171(9):467-470.
35. Li M., et al. Re-emergence of iodine deficiency in Australia. Asia Pac J Clin Nutr. 2001;10(3):200-203.
36. McDonnell C.M., et al. Iodine deficiency and goitre in schoolchildren in Melbourne. Med J Aust. 2003;178(4):159-162.
37. Mann J.I., Aitken E. The re-emergence of iodine deficiency in New Zealand? N Z Med J. 2003;116(1170):U351.
38. Thomson C.D. Selenium and iodine intakes and status in New Zealand and Australia. Br J Nut. 2004;91(5):661-672.
39. Higdon J. An evidence-based approach to vitamins and minerals. New York: Thieme; 2003.
40. Shils M.E., et al. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2006.
41. Berger M.M., et al. Influence of selenium supplements on the post-traumatic alterations of the thyroid axis: a placebo-controlled trial. Intensive Care Med. 2001;27(1):91-100.
42. Chanoine J.P., et al. Selenium decreases thyroglobulin concentrations but does not affect the increased thyroxine-to-triiodothyronine ratio in children with congenital hypothyroidism. J Clin Endocrinol Metab. 2001;86(3):1160-1163.
43. Gärtner R. Selenium and thyroid hormone axis in critical ill states: an overview of conflicting view points. J Trace Elem Med Biol. 2009;23(2):71-74.
44. Ellingsen D.G., et al. Effects of low mercury vapour exposure on the thyroid function in chloralkali workers. J Appl Toxicol. 2000;20(6):483-489.
45. Clarke N., Kabadi U.M. Optimizing treatment of hypothyroidism. Treat Endocrinol. 2004;3(4):217-221.
46. Paoletti J. Differentiation and treatment of hypothyroidism, functional hypothyroidism, and functional metabolism. International Journal of Pharmaceutical Compounding. 12(6), 2008. 489–487
47. Joffe R.T., et al. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. Psychosomatics. 2007;48(5):379-384.
48. Mazzoccoli G., et al. The hypothalamic-pituitary-thyroid axis and melatonin in humans: possible interactions in the control of body temperature. Neuro Endocrinol Lett. 2004;25(5):368-372.
49. Nogues R., et al. Influence of nutrition, thyroid hormones, and rectal temperature on in-hospital mortality of elderly patients with acute illness. Am J Clin Nutr. 1995;61(3):597-602.
50. Beck U., et al. Temperature and endocrine activity during sleep in man. Activation of cortisol and thyroid-stimulating hormone, inhibition of human growth hormone secretion by raised or decreased ambient and body temperatures. Arch Psychiatr Nervenkr. 1976;222(2–3):245-256.
51. Reed H.L. Circannual changes in thyroid hormone physiology: the role of cold environmental temperatures. Arctic Med Res. 1995;54(Suppl 2):9-15.
52. Ljunggren J.G., et al. The effect of body temperature on thyroid hormone levels in patients with non-thyroidal illness. Acta Med Scand. 1977;202(6):459-462.
53. Höflich G., et al. Thyroid hormones, body temperature, and antidepressant treatment. Biol Psychiatry. 1992;31(8):859-862.
54. Bone K. A clinical guide to blending liquid herbs. USA: Elsevier; 2003.
55. Blumenthal M. The ABC clinical guide to herbs. Austin: American Botanical Council; 2003.
56. Blumenthal M. The complete German Commission E monographs—therapeutic guide to herbal medicines [CD-ROM]. Austin: American Botanical Council; 1999.
57. Mills S., Bone K. Principles and practice of phytotherapy. Sydney: Churchill Livingstone; 2000.
58. Pizzorno J., Murray M. Textbook of natural medicine, 3rd edn. USA: Churchill Livingstone; 2006.
59. Gershwin M., et al. Handbook of nutrition and immunity. Totowa, New Jersey: Humana Press; 2004.
60. Ma A., et al. Antioxidant therapy for prevention of inflammation, ischemic reperfusion injuries and allograft rejection. Cardiovasc Hematol Agents Med Chem. 2008;6(1):20-43.
61. Seven A., et al. Biochemical evaluation of oxidative stress in propylthiouracil treated hyperthyroid patients. Effects of vitamin C supplementation. Clin Chem Lab Med. 1998;36(10):767-770.
62. Doerge D.R., Sheehan D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;3:349-353.
63. Teas J., et al. Seaweed and soy: companion foods in Asian cuisine and their effects on thyroid function in American women. J Med Food. 2007;10(1):90-100.
64. Messina M., Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16(3):249-258.
65. Winterhoff H., et al. Endocrine effects of Lycopus europaeus L. following oral application. Arzneimittelforschung. 1994;44(1):41-45.
66. Yarnell E., Abascal K. Botanical medicine for thyroid regulation. Alternative and Complementary Therapies. 2006;12(3):107.
67. Auf’mkolk M., et al. Extracts and auto-oxidized constituents of certain plants inhibit the receptor-binding and the biological activity of Graves’ immunoglobulins. Endocrinology. 1985;116(5):1687-1693.
68. Glinoer D. Feto-maternal repercussions of iodine deficiency during pregnancy. An update. Ann Endocrinol. 2003;64(1):37-44.
69. Glinoer D. Pregnancy and iodine. Thyroid. 2001;11(5):471-481.
70. Glinoer D. What happens to the normal thyroid during pregnancy? Thyroid. 1999;9(7):631-635.
71. Kar A., et al. Relative efficacy of three medicinal plant extracts in the alteration of thyroid hormone concentrations in male mice. J Ethnopharmacol. 2002;81(2):281.
72. Panda S., Kar A. Changes in thyroid hormone concentrations after administration of ashwagandha root extract to adult male mice. J Pharm Pharmacol. 1998;50(9):1065-1068.
73. Panda S., Kar A. Withania somnifera and Bauhinia purpurea in the regulation of circulating thyroid hormone concentrations in female mice. J Ethnopharmacol. 1999;67(2):233-239.
74. Braun L., Cohen M. Herbs and natural supplements, 2nd edn. Sydney: Churchill Livingstone; 2007.
75. Razvi S., et al. Instruments used in measuring symptoms, health status and quality of life in hypothyroidism: a systematic qualitative review. Clin Endocrinol. 2005;63(6):617-624.
76. Tarkas P.I. Thyroidinum. Indian Journal of Homoeopathic Medicine. 1989;24(1):67.