Thrombophilia
Which patients should be investigated for thrombophilia?
Testing for heritable thrombophilia is not indicted in unselected patients presenting with venous thrombosis. Table 39.1 summarises factors which should prompt consideration of thrombophilia. Accurate history taking is essential; particular attention should be given to the nature of the recent thrombotic event, the presence of known risk factors (Table 39.2), a previous history of thrombosis and the family history. Definition of a ‘positive’ family history of thrombosis is problematic. If we use the simple definition of a history of deep vein thrombosis (DVT) or pulmonary embolus (PE) in a first or second degree relative, then approximately 25% of all patients will have a positive family history. Even among those with a strong family history only a small minority will have a cause of inherited thrombophilia identified.
Table 39.1
Table 39.2
Major risk factors for thrombosis
| Venous | Arterial |
| Increasing age | Increasing age |
| Immobility | Smoking |
| Obesity | Male sex |
| Oral contraceptive pill | Hypertension |
| Trauma/surgery | Strong family history |
| Thrombophilia (see text) | Hyperlipidaemia |
| Pregnancy | Diabetes mellitus |
| Malignancy | Raised fibrinogen |
Familial thrombophilia
In theory, familial thrombophilia could be caused by any genetically determined defect of the coagulation or fibrinolytic systems that causes accelerated thrombin formation or impaired fibrin dissolution. In practice, the well-defined causes are associated with accelerated thrombin formation either due to a shortage or failure of activation of one of a number of circulating inhibitors of coagulation (Fig 39.1). Inherited thrombophilia defects are only important in venous thrombosis.
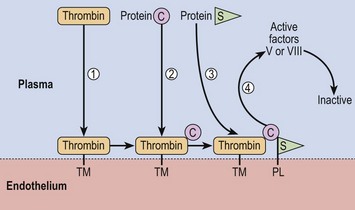
Fig 39.1 Actions of proteins C and S.
Thrombin and protein C bind to thrombomodulin (TM), an endothelial membrane protein (steps 1 and 2). Protein S then binds to this complex and also endothelial phospholipid (PL) (step 3). The resulting complex proteolytically degrades activated factors V and VIII (step 4). Protein C is activated by proteolytic cleavage by thrombin. In APCR, factor V is relatively resistant to inactivation by the protein C complex.
Acquired forms of thrombophilia
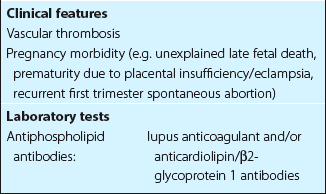
Antiphospholipid antibody syndrome
Diagnosis of this syndrome requires either venous and/or arterial thromboembolism or adverse outcomes in pregnancy in the presence of a persisting antiphospholipid antibody (Table 39.3). The syndrome can be ‘primary’, where the patient has no obvious autoimmune disease, or ‘secondary’ if the patient also has systemic lupus erythematosus (SLE) or a lupus-like disease. About half of all patients have the primary form of the disorder. Up to 2% of the general population have detectable antiphospholipid antibodies – the probability of clinical problems is greatest where the antibody titre is high.