202 Thrombolytics
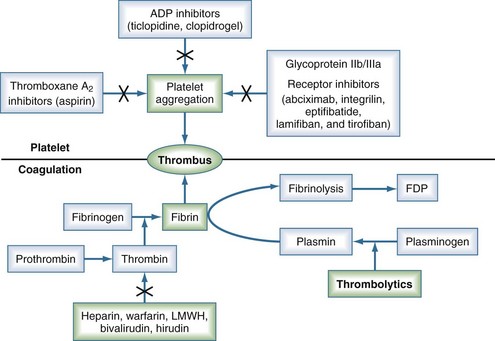
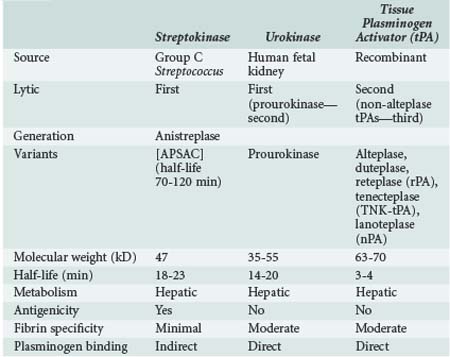
Thrombolytic agents comprise a diverse group of compounds that indirectly initiate lysis of thrombi. In the formation of a thrombus, fibrin forms the molecular scaffolding. After initiation of the coagulation cascade, fibrinolytic mechanisms are concomitantly activated to prevent unchecked thrombosis. The fibrinolytic process begins with cleavage of the proenzyme plasminogen to plasmin, an enzyme that hydrolyzes key bonds within the fibrin clot matrix, resulting in clot lysis (Figure 202-1). Intravenous (IV) or intraarterial thrombolytic agents function by promoting the conversion of plasminogen to plasmin. The different thrombolytic agents vary in their specificity for plasminogen, metabolic half-life, and antigenicity (Table 202-1).
 Drugs
Drugs
Streptokinase
Streptokinase, a protein produced by β-hemolytic streptococci, was first identified as having fibrinolytic properties in the 1930s1 and was the first compound used clinically as a thrombolytic drug.2 Despite its name, streptokinase itself is not an enzyme. Rather, it complexes with plasminogen in a 1 : 1 stoichiometric relationship. The streptokinase-plasminogen complex then converts both circulating and fibrin-bound plasminogen to plasmin. One of the major drawbacks to clinical use of streptokinase is its antigenicity, as antibodies may have formed during prior streptococcal infection. Allergic reactions occur in 2% to 5% of patients receiving the drug and are generally mild, but severe anaphylactic reactions can occur.3 It has a short half-life of approximately 20 minutes. Anistreplase (APSAC [anisoylated plasminogen streptokinase activator complex]) is a modified form of streptokinase that has a substantially longer half-life but still can cause allergic reactions (see Figure 202-1).
Urokinase
Urokinase is a thrombolytic protein that was initially isolated from human urine and has been used clinically for over 30 years. It is isolated from human fetal renal tissue cultures and, unlike streptokinase, enzymatically cleaves plasminogen. During the 1980s and early 1990s, urokinase was the primary thrombolytic agent used clinically for treatment of graft thrombosis and peripheral arterial occlusion. In 1999, urokinase was removed from the U.S. market after questions were raised by the Food and Drug Administration (FDA) regarding the safety of this product.4 It was reintroduced in the United States in late 2002 after rigorous testing showed that the preparations were free of human pathogens, but its only current indication is for pulmonary embolism. Prourokinase, also known as single-chain urokinase-type plasminogen activator (scu-PA), is a single-chain precursor molecule of urokinase that is converted into two-chain urokinase by hydrolysis. It is relatively fibrin specific and has low antigenicity.
Tissue Plasminogen Activator
Tissue plasminogen activator (tPA) was first isolated in 1981.5 It is a naturally occurring protein synthesized by human vascular endothelial cells. Commercially available preparations are manufactured using recombinant technologies, as first described by Pennica and colleagues.6 A number of different recombinant variants are available, including alteplase (rtPA, approved by the FDA in 1987) and duteplase, as well as other forms of the tissue-type plasminogen activators: reteplase (rPA), tenecteplase (TNK-tPA), and lanoteplase (nPA). Recombinant tPAs have the advantage of being nonantigenic and specific for fibrin-bound plasminogen and avoid the infectious risks associated with products isolated from cultured human tissues. Newer recombinant tPAs have improved pharmacokinetics, allowing for more convenient administration such as bolus dosing.
 Clinical Indications
Clinical Indications
Myocardial Infarction
Acute myocardial infarction (AMI) represents a significant healthcare burden in industrialized countries, with trends estimating the growing impact of this disease on the world. Modern management of AMI focuses on rapidly restoring perfusion to optimize salvage of myocardium. To this end, primary percutaneous coronary interventions (PCIs) have been shown to be superior to thrombolytic therapy when employed as an early reperfusion strategy after AMI and are thus recommended as first-line therapy when available.7 However, logistical barriers exist hindering access to early PCI for all patients with AMI, whereas fibrinolysis can be used in nearly all hospitals.
The use of lytic therapy for the treatment of AMI was first attempted in the 1950s.8 The rationale for this therapy was that reestablishing coronary blood flow in an acutely thrombosed vessel would reduce infarct size and mortality. A meta-analysis of 33 randomized trials in 1985 demonstrated a 22% reduction in mortality with the use of thrombolytics in AMI, and these findings prompted further investigation into lytic therapy for MI.9 The Fibrinolytic Therapy Trialists’ Collaborative Group (FTT) performed a meta-analysis in 1994 with aggregated results from over 58,000 patients treated with thrombolytics.10 This analysis revealed a nearly 25% reduction in mortality in those patients with ST-segment elevation or bundle branch block. Numerous studies since have been performed to evaluate the efficacy of different lytic agents, dosing strategies, routes of administration, and adjunctive therapies for rapid restoration of antegrade flow in thrombosed coronary arteries.
Early studies focused on the use of streptokinase, demonstrating 18% and 25% reductions in mortality at 3 and 5 weeks, respectively, in the ISIS-23 and GISSI studies.11 These short-term findings in the fibrinolytic group were maintained out to 1- to 10-year follow-ups.12 The efficacy of tPA was studied in the GUSTO-1 trial, which examined 4 dosing regimens for the treatment of MI in 41,021 patients.13 This study utilized “accelerated” tPA dosing, wherein two-thirds of the total dose was administered in the first 30 minutes rather than over 3 hours. This dosing regimen resulted in a modest but significant reduction in 30-day mortality (6.3%) compared to streptokinase (7.4%) or a combination of tPA and streptokinase (7.0%). The GUSTO angiographic substudy demonstrated that differing patency rates between patients treated with either agent accounted for this difference in clinical efficacy. A subsequent meta-analysis of this approach, however, failed to validate the survival advantage.14 The recombinant deletion mutant of tPA, reteplase, was compared to accelerated tPA in 15,059 patients in the GUSTO III trial. No survival advantage was observed with reteplase, and rates of intracranial hemorrhage were similar (0.91% and 0.87% with reteplase and tPA, respectively).15 The ASSENT-2 trial showed that mortality at 30 days and ICH rates were identical between use of tenecteplase and accelerated tPA.16 While reteplase and tenecteplase have not surpassed tPA in terms of efficacy, their pharmacokinetics translate to simplified administration versus accelerated tPA.
Adjunctive therapies such as aspirin or clopidogrel and antithrombin agents improve the results of lytic therapy. As fibrinolysis strips fibrin from the occluding thrombus, the exposed thrombin initiates platelet aggregation and subsequent rethrombosis.17 Therefore some form of antithrombin strategy is warranted. Heparin can be infused to keep the activated partial thromboplastin time (APTT) between 50 and 70 seconds. If heparin-induced thrombocytopenia is suspected, direct thrombin inhibitors (hirudin or bivalirudin) can be used.18 Another development has been the introduction of glycoprotein IIb/IIIa receptor blockers such as abciximab (ReoPro), eptifibatide (Integrilin), and tirofiban (Aggrastat).19–21 Despite some promising early results,22 no randomized trial has yet to show an impact on mortality with combination therapy.23–25
The timing of diagnosis and institution of thrombolytic therapy is critical.26,27 Patients with AMI treated with thrombolytic agents more than 4 hours after the onset of symptoms have 30-day and 6-month mortality rates that are 2 to 3 times higher than patients who were treated within 2 hours after the onset of symptoms.28 Eighty-two percent of patients treated within 2 hours had return of normal cardiac wall motion, whereas only 46% of those treated within 2 to 5 hours after the onset of symptoms have return of normal wall motion.29 The LATE (Late Assessment of Thrombolytic Efficacy) study reported 1-year mortality rates of 17.6% versus 15.8% in those patients treated with rtPA at greater than 3 hours versus less than 3 hours, respectively, after the onset of symptoms.30 So critical is the timing of the initiation of treatment that prehospital administration of thrombolytics has been advocated in selected patients with ST-segment elevations on an electrocardiogram.31,32
Currently accepted guidelines for lytic therapy in AMI are outlined by the American College of Chest Physicians in the 2008 8th edition of the Evidence-Based Clinical Practice Guidelines and by the American College of Cardiology/American Heart Association in their 2004 guidelines.33,34 The treatment algorithm is summarized in Figure 202-2. Unfortunately, the value of thrombolytic agents for the management of unstable angina remains unproven. Currently there is no role for lytic therapy in acute coronary syndrome in the absence of ST-segment elevation in two or more contiguous leads or without new-onset bundle branch block. Contraindications to lytic therapy in the setting of AMI are also summarized in Table 202-2.
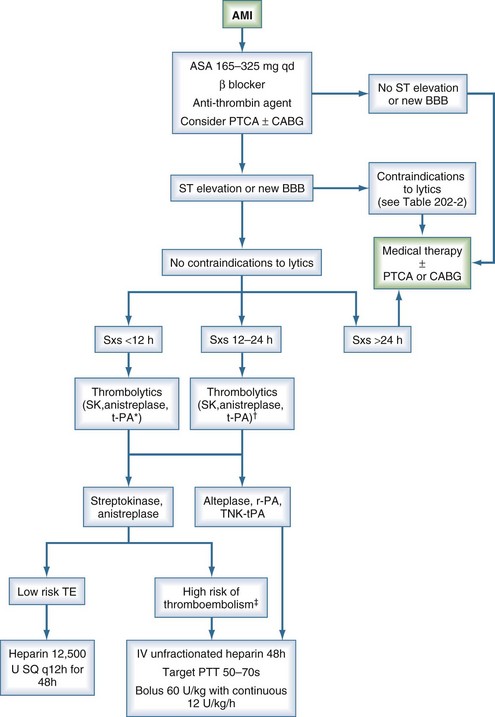
Figure 202-2 Algorithm for treatment of acute myocardial infarction. *, preferred for symptoms <6 h; †, grade 2b data11; ‡, anterior myocardial infarction, existing heart failure, previous embolus, atrial fibrillation, left ventricular thrombus; AMI, acute myocardial infarction; BBB, bundle branch block; CABG, coronary artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty; PTT, partial thromboplastin time; SK, streptokinase; SQ, subcutaneous; Sxs, signs and symptoms; TE, thromboembolism.
(Adapted from 1999/2002 ACC/AHA Guideline Update and 2001 ACCP Consensus Conference.11–12)
TABLE 202-2 Contraindications to Thrombolytic Therapy in the Setting of Acute Myocardial Infarction*
| Absolute Contraindications | Relative Contraindications |
|---|---|
| >24 hours since onset of symptoms | 12 to 24 hours since onset of symptoms |
| Prior intracranial hemorrhage | Age > 75 years |
| Stroke within past year | Systolic blood pressure > 180 mm Hg or diastolic blood pressure > 110 mm Hg |
| Intracranial neoplasm | Bleeding disorder |
| Active bleeding/bleeding diathesis | Prior allergic reaction to thrombolytics |
| Suspected aortic dissection | Pregnant or lactating |
| Significant closed-head or facial trauma within 3 months | Prolonged cardiopulmonary resuscitation (>10 min) Recent internal bleeding (<2-4 wk) Active peptic ulcer |
* With ST-segment elevation and/or new bundle branch block.
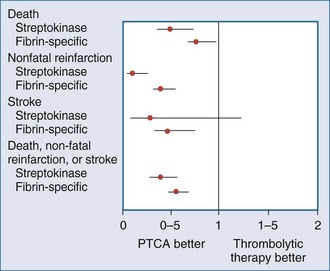
Recently, a large meta-analysis was performed that examined 7739 patients with ST-segment elevation randomized to either thrombolytic agents (76% receiving fibrin-specific lytics) or primary percutaneous transluminal coronary angioplasty (PTCA).7 Short-term (4-6 weeks) mortality in the PTCA group was 7%, compared to 9% in the group that received lytic therapy (P = .0003). The group treated with primary PTCA had lower rates for nonfatal reinfarction (3% versus 7%) and stroke (1% versus 2%) as part of a follow-up to a smaller study.35 The short-term results of this meta-analysis are summarized in Figure 202-3.
Potential advantages of angioplasty over thrombolysis36–39 as primary therapy for AMI must be tempered by the recognition that angioplasty results are highly dependent on the volume of cases at a given treatment center. Moreover, comparisons to lytic therapy often use historical data before the era of accelerated dosing regimens and adjunctive treatment with antiplatelet and antithrombin agents. Lytic therapy also may be advantageous in critically ill patients who are unable to be transported to cardiac catheterization facilities or in those who have other contraindications to PTCA. Ultimately, the ideal treatment for some patients may involve combinations of angioplasty, reduced-dose thrombolytic therapy, antithrombotic agents, and antiplatelet agents.
Stroke
Stroke is the third leading cause of death in the United States, affecting over 700,000 people per year. Strokes are a major source of morbidity and mortality among hospitalized patients.40 The majority of strokes are ischemic in nature, resulting from sudden occlusion of arteries delivering blood supply to the brain. These occlusions often are caused by thromboemboli from a variety of sources.41 Traditional therapy for ischemic stroke has focused on the use of anticoagulation and antiplatelet agents for medical support and then rehabilitation after the acute event. More recently, thrombolytic therapy has emerged as a mode of intervention in ischemic stroke patients. Similar to the treatment of AMI, the efficacy of thrombolytic agents is highly time dependent owing to the characteristics of the ischemic penumbra.42 Indeed, efficacy is greater when lytic treatment is administered within 90 minutes of the event.43
The concept of utilizing “clot-busting” therapies for ischemic stroke blossomed in 1995 when the National Institute of Neurological Disorders and Stroke (NINDS) published a study on rtPA in acute ischemic stroke.44 This trial consisted of two parts. Part I enrolled 291 patients and examined the clinical efficacy of IV tPA given within 3 hours after symptom onset. This treatment failed to improve neurologic function after 24 hours versus placebo. By 3 months, however, patients treated with tPA showed significant improvement in 4 different functional outcome measurements as assessed by the National Institutes of Health Stroke Scale (NIHSS). Part II of this study assessed the long-term outcomes of tPA treatment. Patients who received tPA were 30% more likely to have minimal residual disability or to have returned to baseline functional status at 3, 6, and 12 months.45 Unfortunately, patients treated with tPA suffered a greater incidence of intracerebral hemorrhage (6.4% versus 0.6% in the placebo group; P < .001) at 36 hours. Subsequent studies have shown similar rates of intracranial hemorrhage after lytic therapy. Nevertheless, mortality at 3 months was not significantly different (17% versus 21%; P = .30). Intravenous tPA was approved by the FDA for treatment of acute ischemic stroke within a 3-hour window as a result of this study. Other early randomized trials of IV tPA in acute stroke treatment included the European Cooperative Acute Stroke Study (ECASS-I),46 ECASS-II,47 and the ATLANTIS trials.42,48 These trials, while each failing to reach significance for their primary outcome measure, did show significant benefit in favor of tPA usage between 0 and 6 hours for alternative outcome measures, lending some additional support for its use. A subsequent analysis of these trials showed that the odds ratio of benefit of IV tPA decreased as time from stroke onset increased.49
More recently, the ECASS-III trial did demonstrate a lesser, albeit significant, benefit of tPA compared to placebo when administered in the 3- to 4.5-hour window, with no difference in mortality.50 Rates of symptomatic intracranial hemorrhage (based on the NINDS definition) were slightly higher than in the NINDS trial (7.9% versus 3.5% in the placebo group; P = 0.006). It is important to note that this trial exercised more stringent inclusion criteria than the NINDS trial. Based on the results of this trial, Lansberg et al. calculated that the number of patients deriving benefit per 100 treated are 28, 23, and 17 for 0- to 1.5-hour, 1.5- to 3-hour, and 3- to 4.5-hour windows, respectively.51 It is estimated that in the 3- to 4.5-hour window, 1 in 6 patients will have a better outcome and 1 in 35 a worse outcome.52 As a result of the ECASS III trial, IV tPA usage for treatment of stroke has been supported by the Scientific Advisory from the American Heart Association Stroke Council, although the FDA has not expanded its approval to date.53
Currently, the only drug and route of administration currently approved by the FDA for treatment of ischemic stroke is tPA through an IV route. However, prourokinase as well as intraarterial fibrinolytic administration are also used in the setting of established clinical protocols. Intravenous delivery has both practical and theoretical disadvantages. One disadvantage is the inability to effectively lyse the internal carotid artery or the middle cerebral artery.54 After confirmation by head computed tomography (CT) of ischemic stroke (i.e., not associated with hemorrhage), patients are typically treated by IV tPA at a dose of 0.9 mg/kg, with 10% of the total dose given as an initial bolus, and the remainder infused over 60 minutes.55
Intraarterial administration of thrombolytics is gaining popularity for treating ischemic stroke. This method requires that a neurointerventionalist obtain arterial images and place an intraarterial catheter into the thrombosed vessel. The thrombolytic agent is then infused through the catheter directly into the target vessel, achieving high local concentration at the site of occlusion while decreasing systemic drug levels. Direct intraarterial delivery has been shown to be effective in limited studies. The Prolyse in Acute Cerebral Thromboembolism (PROACT II) study56 randomized 180 patients with middle cerebral artery occlusion to either intraarterial prourokinase plus heparin or heparin alone. Intraarterial prourokinase improved the modified Rankin score to 2 or less in over 40% of patients, whereas heparin infusion alone improved the score in only 25%. Also, prourokinase was associated with significantly higher vessel recanalization rates (66% versus 18%; P < .0001). The MELT trial was a Japanese trial of intraarterial urokinase which was halted early due to external reasons and was thus underpowered to reach significance of the primary endpoint.57 Nevertheless, secondary analysis from that study and combined analyses with the PROACT trials suggested benefit to arterial urokinase.58 Prourokinase is not available for general use, but tPA and its variants are frequently administered in intraarterial fashion.59,60
Additionally, the Interventional Management of Stroke (IMS) trialists recently began a phase III trial comparing standard-dose IV tPA with a reduced-dose IV tPA bridge to an endovascular treatment consisting of either mechanical thrombectomy, arterial tPA infusion, or a combination of low-intensity ultrasound with tPA infusion.61 Although growing evidence supports use of intraarterial revascularization methods, they are obviously restricted to those centers with skilled neurointerventionalists. Standard contraindications to IV thrombolytic therapy in acute ischemic stroke are similar to the exclusion criteria used in the NINDS study (Table 202-3). Blood pressure should be tightly controlled, ideally maintained below 180/105 mm Hg. Antithrombotic agents should also be withheld for 24 hours owing to the risk of intracranial hemorrhage. A number of adjunctive therapies including mechanical thrombectomy,62 glycoprotein IIb/IIIa inhibitors,63 and ultrasound64,65 have shown promise in improving recanalization rates.
TABLE 202-3 Contraindications for Thrombolytic Therapy in Ischemic Stroke
| Contraindications | Relative Contraindications |
|---|---|
| Symptom duration >6 hours | Symptom duration of 3 to 6 hours |
| History of intracranial hemorrhage | Witnessed seizure |
| Evidence of active bleeding | Gastrointestinal or urinary hemorrhage within 3 weeks |
| Platelet count <100,000/mm3 | Recent lumbar puncture, noncompressible arterial puncture site |
| Prior stroke, head trauma, or intracranial surgery within 3 months | Systolic blood pressure >185 mm Hg or diastolic blood pressure >110 mm Hg |
| Rapidly improving or only minor symptoms | Mass effect or hypodensity of greater than one-third middle cerebral artery distribution on head CT |
| Major surgery within 14 days | |
| Known arteriovenous malformation or intracranial aneurysm | Glucose <50 mg/dL or >400 mg/dL |
| Elevated partial thromboplastin time or international normalized ratio (>1.7) |
Pulmonary Embolism
Pulmonary embolism (PE) is not only a major source of morbidity and mortality in hospitalized patients, accounting for up to 15% of in-hospital deaths, but is also a surprisingly underrecognized source of cardiovascular collapse.66–68 Anticoagulation has been a critical component in the treatment of PE since first shown to be beneficial in 1960.69 This may be achieved using IV unfractionated heparin (UFH), with an initial bolus of 80 units/kg followed by 18 units/kg/h, with monitored dose adjustment to maintain the APTT prolongation at a level that corresponds to 0.3 to 0.7 IU/mL anti-Xa activity. Alternatively, subcutaneous (SQ) low-molecular-weight heparin (LMWH), SQ fondaparinux, or monitored or fixed-dose SQ UFH may be used.70 Unfortunately, despite the proven efficacy of anticoagulation in the setting of acute PE, a significant proportion of patients will have incomplete resolution of their occlusion, with subsequent organization of the thrombus and obliteration of the pulmonary artery.71–73 Accordingly, thrombolytic therapy for pulmonary embolism may offer more rapid and complete resolution of thrombus burden.
One of the initial studies that evaluated thrombolytic therapy for PE was the Urokinase Pulmonary Embolism Trial (UPET).71 This prospective trial randomized 160 patients to either urokinase followed by heparin or heparin alone. Although transient hemodynamic improvement was achieved, no differences were evident with regard to mortality or perfusion scan past 5 days. Nonetheless, the UPET and the subsequent Urokinase-Streptokinase Embolism Trial (USET) demonstrated improvements in small vessel patency at 2 weeks and 1 year compared with anticoagulation alone.74–75 Seven-year follow-up of this cohort of patients suggested the risk of pulmonary hypertension was decreased by thrombolysis, presumably because lytic therapy achieved superior clot dissolution and decreased the risk of subsequent pulmonary embolism.76
Currently, the only patients for whom thrombolytics are widely accepted are those with hemodynamic instability due to massive PE. Other patients who may benefit are those with right ventricular dysfunction or refractory hypoxemia due to PE in the setting of preserved systemic arterial blood pressure. These cases of “sub-massive” PE may achieve an improved clinical course with thrombolytic therapy than with anticoagulation alone, although mortality rate has not been shown to be improved.77
The IV route of administration is most commonly used in PE. The only trial to compare direct pulmonary artery infusion to IV infusion was by Verstraete et al.78 The study failed to show a benefit of pulmonary artery infusion. In addition, the time required to place a pulmonary artery catheter can delay treatment and increase the risk of bleeding from a central venous puncture. On the other hand, local catheterization permits mechanical lysis, which has been shown to benefit selected patients.79 FDA-approved regimens for acute PE are listed in Table 202-4.
TABLE 202-4 FDA-Approved Regimens for Treatment of Pulmonary Embolism
| Drug | Systemic Administration |
|---|---|
| Streptokinase | 250,000 units over 30 minutes followed by 100,000 units/h for 24 hours |
| Urokinase | 4400 units/kg over 10 minutes followed by 4400 units/kg/h for 12-24 hours |
| tPA (alteplase) | 100 mg over 2 hours |
Although no difference in thrombolytic regimens has been shown to be significant to date,80 most agree that the drug of choice is IV tPA81 because of its short infusion time. Unlike patients treated for AMI, patients treated with thrombolytic agents for acute PE are generally not heparinized during thrombolytic administration. However, systemic anticoagulation should begin upon completion of thrombolysis. After the acute treatment of PE, patients should be maintained on anticoagulation for a minimum of 3 months, keeping the International Normalized Ratio between 2.0 and 3.0.82 Major hemorrhagic complications occur in approximately 12% of patients irrespective of the lytic agent used.83
Deep Venous Thrombosis
The formation of deep venous thrombosis (DVT) is surprisingly common in acutely ill patients, occurring in as many as 30% of ICU patients, despite prophylaxis with pneumatic compression devices and/or various prophylactic anticoagulation regimens.84 ICU patients are at especially high risk for DVT because they often have indwelling central venous catheters. Central venous catheterization can increase the incidence of DVT by 5% to 30% depending on the site of insertion, type of catheter, duration of placement, and presence or absence of infection.85–89 Acute occlusion of the deep venous system can lead to severe sequelae such as venous gangrene (phlegmasia cerulea dolens), as well as long-term consequences including recurrent venous thrombosis and post-phlebitic syndrome.90–93 Additionally, patients with iliofemoral DVT experience greater postthrombotic morbidity than those with infrainguinal DVT. Although venous gangrene is rare, patients with acute DVT have a nearly 26-fold increase in the risk of developing chronic venous disease,94 with reported incidence ranging from 16% to 82%.95–97 Traditional therapy for DVT, consisting of anticoagulation alone, allows for stabilization of the thrombus and prevents PE. However, anticoagulation is not effective for restoration and preservation of venous function.
Thrombolytic treatment of DVT, therefore, focuses both on prevention of PE and dissolution of the clot to prevent development of post-phlebitic syndrome, which is characterized by persistent pain, edema, discoloration, and ulceration. A number of randomized clinical studies have demonstrated the efficacy of both streptokinase and tPA compared with heparin alone in this setting.98–101 These studies reported partial lysis in 70% and complete lysis in 28% of patients who underwent thrombolysis, versus only 24% and 4%, respectively, in patients treated with heparin alone.81 Meta-analyses of the available data indicated that systemic streptokinase is 3.7 times more likely102 and tPA is 7 times more likely103 to result in thrombus resolution than anticoagulation alone.
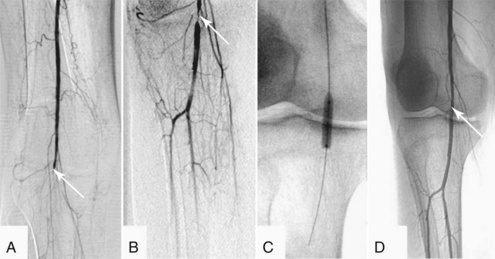
Exact indications for thrombolysis are unclear. Patients who are most likely to benefit include those with a first occurrence of iliofemoral DVT (<10 days old) who are at low risk for major bleeding complications.104 Indeed, the AACP guidelines for the application of thrombolytics weakly recommend (grade 2B, 2C) that they may be used in selected patients with extensive acute proximal DVT.105 Others have advocated lytic therapy for young patients with primary upper extremity DVT either due to effort thrombosis (Paget-Schroetter syndrome) or idiopathic factors.106 Most investigations have used a locoregional approach with infusions from a distal peripheral vein (e.g., a pedal vein). Another approach involves catheter-directed infusions that offer the advantage of requiring lower total doses of thrombolytic agents with fewer systemic side effects, as well as the ability to use angioplasty to dilate underlying venous stenoses (Figure 202-4).107,108 Both systemic109 and catheter-directed thrombolysis110,111 have been shown to be effective. With the completion of the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial in the near future, the level 1 evidence needed to elucidate the optimal role of thrombolytic therapy in the treatment of DVT may be provided.112
Acute Peripheral Arterial Occlusion
Acute peripheral arterial occlusion (APAO) is a highly morbid condition that leads to amputation in 10% to 30% of cases and is associated with a mortality rate as high as 15% at 30 days.113 Occlusive events generally arise from dissection, trauma, local thrombosis, or embolus. Traumatic occlusion or disruption of the vessel almost always warrants surgical exploration and repair. A variety of noninvasive maneuvers, however, have been developed for treatment of thromboembolic disease. Differentiating between in situ thrombosis and embolus as the etiology of the arterial occlusion can be extremely challenging if not impossible in up to 15% of cases.113 In the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial, thrombosis (85%) was found to be more common than embolism (15%).114,115
Thrombolysis has become a popular means of treating acute arterial occlusion in certain settings. This approach has been performed since the 1950s.3 Formerly, relatively high-dose lytic therapy was administered IV to achieve therapeutic levels at the site of occlusion but was associated with prohibitively high bleeding risks and generally poor clinical outcome. Since the early 1970s, however, catheter-directed infusion has become the standard of care, permitting higher local thrombolytic concentrations at the treatment site while effectively reducing the systemic burden of the drug.116 Recent advances in development of infusion catheters and wires have contributed to the effectiveness of intraarterial lytic therapy. Differing infusion methods have been developed and studied, such as low-dose infusion regimes, high dose, and high-pressure forced infusion (“pulse spray”). Although duration of treatment may be shorter with the high-dose and pulse spray infusion techniques, bleeding complications are increased. Regardless, no infusion method has been shown to achieve genuine benefit in terms of clinical outcome.117–118
Although streptokinase was the first agent used for APAO, multiple studies indicate that urokinase and tPA are more effective for this indication and have fewer bleeding complications.119–121 Before its removal from the U.S. market, urokinase was the predominant thrombolytic agent used to treat acute arterial occlusion. Currently, tPA and its derivatives have supplanted urokinase as the drug of choice for APAO; tPA has been shown to have similar safety and efficacy profiles as urokinase when using “low-dose” (<2 mg/h, usually beginning at 0.5 mg/h) regimens with adjunctive heparin infusions to maintain the APTT at 1.5 times baseline. With this regimen, over 60% of patients have complete resolution, and 30% have partial resolution within 24 hours of initiating treatment.122 An advisory panel recommended either weight-based dosing (0.001-0.02 mg/kg/h) or non-weight-based dosing (0.12-2 mg/h), with total doses not to exceed 40 mg.123 They also recommended subtherapeutic heparin infusions to maintain the APTT at between 1.25 and 1.5 times control values.
Use of thrombolytics in the setting of APAO is part of a multifaceted approach often involving additional endovascular techniques and/or surgical intervention. There are several well-controlled trials examining initial surgical versus thrombolytic therapy. The Rochester trial compared initial surgery with urokinase in severely threatened limbs (mean symptom duration 2 days) in 114 patients.124 Limb salvage rates in the 2 groups were identical (82%) at 12 months, whereas mortality was significantly lower in the patients treated with urokinase (16% versus 42% with surgery). The Surgery or Thrombolysis for the Ischemic Lower Extremity (STILE) trial examined 393 patients randomized to either primary surgery or one of two lytic therapies (rtPA or urokinase).125 At 30 days, limb loss rates (5% with lysis versus 6% with surgery) and mortality rates were similar (4% versus 5%, respectively). One of the major contributions of this study involved subgroup analyses126,127 that revealed a greater benefit of thrombolysis in patients with graft occlusion rather than native vessel occlusion and in patients with acute ischemia of less than 2 weeks’ duration. Finally, the TOPAS trial compared recombinant urokinase therapy to surgery in 544 patients.114 Although it failed to demonstrate an amputation-free survival benefit at 1 year (68% for urokinase, 69% for surgery), it did show that over 30% of the patients treated with urokinase were not only alive without amputation but also had nothing more than a percutaneous procedure at 6 months. Therefore a significant number of patients were able to avoid surgical intervention with the use of thrombolytic therapy.
Thrombolytic therapy is still not considered to be the standard of care for treatment of APAO. However, it may prove to be a useful tool for treating patients who are poor surgical candidates. These include patients who are too sick to safely undergo extremity revascularization and those with distal thromboemboli not amenable to surgical extraction or bypass. Thrombolysis may also benefit selected patients who present with less than 2 weeks of symptoms. It may be the best approach for patients with occlusion of bypass grafts.125,126 Furthermore, thrombolysis can aid in recanalization of small distal vessels that are not patent at initiation of treatment, permitting subsequent revascularization via bypass surgery. Thrombolysis may uncover an arterial lesion as the inciting factor for the thrombosis, which then may be treated endovascularly or surgically (Figure 202-5). Finally, thrombolysis may allow a more gradual reperfusion of an ischemic limb and thus reduce the metabolic derangements associated with ischemia/reperfusion. Lytic agents are contraindicated for treating early postoperative thrombosis, thrombosis following penetrating or multiple traumas, or in limbs with irreversible ischemia.
Other Applications
In addition to the indications for thrombolytic therapy noted earlier, other common indications include treatment of thrombosed dialysis grafts or central venous catheters. Vascular access complications are the single greatest source of morbidity among hemodialysis patients, accounting for 15% of all hospitalizations.128 Whereas the ultimate goal is to recognize and treat a graft before it clots, thrombolytics can play an important role once the graft has occluded. A number of techniques have been used for the acutely thrombosed graft, including mechanical thrombectomy, surgical revision, and pharmacologic thrombolysis. A technique that has gained popularity recently is called “lyse and wait.” This method avoids the need for mechanical devices or pulse-spray catheters and shortens lysis times to approximately 45 minutes, as compared with 65 minutes for the pulse spray technique.129 It involves placing a mixture of urokinase (250,000 International Units) and heparin (5000 units) into the graft (or alternatively, injecting 2-5 mg of rtPA into the graft and administering 5000 units of heparin systemically).130 After lysis, the arterial plug is removed, and the venous anastomosis is dilated. With this technique, Cynamon et al. reported that 98% of patients have successful restoration of graft flow and function, with 1- and 3-month patency rates of 80% and 55%, respectively.129 Nevertheless, surgical thrombectomy remains the standard of care and achieved superior patency rates in a recent meta-analysis,131 presumably because anastomotic revision is performed concurrently. As for occluded central venous catheters, the Advisory Panel on Catheter-Directed Thrombolytic Therapy in 2000 recommended a 2-mg (1 mg/mL) aliquot of alteplase for each occluded lumen for up to 2 hours.123 This therapy can be repeated a second time if necessary. This regimen has proven to be both safe (≤1% bleeding risk) and efficacious (≈90% patency after two treatments) in multiple studies.132–135
 Management/Laboratories
Management/Laboratories
During administration of thrombolytic agents, circulating plasminogen and fibrinogen concentrations decrease. Fibrinogen is degraded as part of the fibrinolytic process, reaching nadir values between 5 and 7 hours after the institution of therapy.136 These values return to baseline in most patients within 48 hours of discontinuation of therapy. Likewise, circulating plasminogen levels begin to decrease immediately after initiating streptokinase and urokinase infusions; the decrease is greater with streptokinase, as it complexes with plasminogen in a 1 : 1 relationship. Despite being relatively fibrin specific, the second- and third-generation lytic agents (recombinant tPAs, APSAC, and prourokinase) still interact with circulating fibrinogen and therefore decrease circulating fibrinogen levels. Fibrin degradation products are a reliable indicator of the activity of the fibrinolytic system because the only source in humans is the degradation of fibrinogen or fibrin by plasmin.
Most advocate monitoring fibrinogen levels every 6 to 8 hours during lytic therapy, decreasing the dose or discontinuing the infusion if levels drop below 100 mg/dL. In addition to monitoring the fibrinolytic system, daily or every-other-day monitoring of the platelet count should be performed. Use of rtPA has been associated with thrombocytopenia in as many as 10% of patients, whereas thrombocytopenia occurred in less than 1% of patients receiving streptokinase.137–139 Selected patients should be typed for blood products. If bleeding does occur, thrombolytic infusion should be discontinued and blood products (fresh frozen plasma or cryoprecipitate) administered as necessary to correct the patient’s hypocoagulable state.
Key Points
Arcasoy SM, Vachani A. Local and systemic thrombolytic therapy for acute venous thromboembolism. Clin Chest Med. 2003;24:73-91.
Hilleman DE, Tsikouris JP, Seals AA, et al. Fibrinolytic agents for the management of ST-segment elevation myocardial infarction. Pharmacotherapy. 2007;11:1558-1570.
2003 Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. Lancet. 2008;359:1317-1329.
Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340:1781-1787.
Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105-1111.
1 Tillet WS, Garner RL. The fibrinolytic activity of hemolytic streptococci. J Exp Med. 1933;58:485-502.
2 Tillet WS, Johnson AJ, McCarthy WR. The intravenous infusion of the streptococcal fibrinolytic principle (streptokinase) into patients. J Clin Invest. 1955;34:169-185.
3 Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349-360.
4 Hartnell GG, Gates J. The case of Abbokinase and the FDA: The events leading to the suspension of Abbokinase supplies in the United States. J Vasc Interv Radiol. 2000;11:841-847.
5 Rijken DC, Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981;256:7035-7041.
6 Pennica D, Holmes WE, Kohr WJ, et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983;301:214-221.
7 Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361:13-20.
8 Fletcher AP, Alkjaersig N, Southgate KM. Treatment of patients suffering from early myocardial infarction with massive and prolonged streptokinase therapy. Trans Assoc Am Physicians. 1958;71:287-296.
9 Yusuf S, Collins R, Peto R, et al. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: Overview of results on mortality, reinfarction and side-effects from 33 randomized controlled trials. Eur Heart J. 1985;6:556-585.
10 Indications for fibrinolytic therapy in suspected acute myocardial infarction: Collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311-322.
11 Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI). Lancet. 1986;1:397-402.
12 Franzosi MG, Santoro E, DeVita C, et al. Ten-year follow-up of the first megatrial testing thrombolytic therapy in patients with acute myocardial infarction: results of the Gruppo Italiano per lo Studio della Soprawivenza nell’Infarto-1 study. The GISSI Investigators. Circulation. 1998;98:2659-2665.
13 An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673-682.
14 Dundar Y, Hill R, Dickson R, Walley T. Comparative efficacy of thrombolytics in acute myocardial infarction: A systematic review. Q J Med. 2003;96:103-113.
15 The Global Use of Strategies to Open Occluded Coronary Arteries—GUSTO III Investigators. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337:1118-1123.
16 Assessment of the Safety and Efficacy of a New Thrombolytic Investigators. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: ASSENT-2 double-blind randomized trial. Lancet. 1999;354(9180):716-722.
17 Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326:242-250.
18 White H. Thrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: The HERO-2 randomised trial. Lancet. 2001;358:1855-1863.
19 Trial of abciximab with and without low-dose reteplase for acute myocardial infarction. Strategies for Patency Enhancement in the Emergency Department (SPEED) Group. Circulation. 2000;101:2788-2794.
20 Ohman EM, Kleiman NS, Gacioch G, et al. Combined accelerated tissue-plasminogen activator and platelet glycoprotein IIb/IIIa integrin receptor blockade with Integrilin in acute myocardial infarction: Results of a randomized, placebo-controlled, dose-ranging trial. IMPACT-AMI Investigators. Circulation. 1997;95:846-854.
21 Combining thrombolysis with the platelet glycoprotein IIb/IIIa inhibitor lamifiban: Results of the Platelet Aggregation Receptor Antagonist Dose Investigation and Reperfusion Gain in Myocardial Infarction (PARADIGM) trial. J Am Coll Cardiol. 1998;32:2003-2010.
22 Antman EM, Giugliano RP, Gibson CM, NS, et al. Abciximab facilitates the rate and extent of thrombolysis: Results of the thrombolysis in myocardial infarction (TIMI) 14 trial. The TIMI 14 Investigators. Circulation. 1999;99:2720-2732.
23 Topol EJ. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: The GUSTO V randomised trial. Lancet. 2001;357:1905-1914.
24 Lincoff AM, Califf RM, Van de WF, et al. Mortality at 1 year with combination platelet glycoprotein IIb/IIIa inhibition and reduced-dose fibrinolytic therapy vs. conventional fibrinolytic therapy for acute myocardial infarction: GUSTO V randomized trial. JAMA. 2002;288:2130-2135.
25 Sinnaeve PR, Alexander JH, Bogaerts K, et al. Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: The ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605-613.
26 Cannon CP, Antman EM, Walls R, Braunwald E. Time as an adjunctive agent to thrombolytic therapy. J Thromb Thrombolysis. 1994;1:27-34.
27 Newby LK, Rutsch WR, Califf RM, et al. Time from symptom onset to treatment and outcomes after thrombolytic therapy. GUSTO-1 Investigators. J Am Coll Cardiol. 1996;27:1646-1655.
28 Zijlstra F, Patel A, Jones M, et al. Clinical characteristics and outcome of patients with early (<2 h), intermediate (2-4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J. 2002;23:550-557.
29 Mathey DG, Sheehan FH, Schofer J, Dodge HT. Time from onset of symptoms to thrombolytic therapy: A major determinant of myocardial salvage in patients with acute transmural infarction. J Am Coll Cardiol. 1985;6:518-525.
30 Langer A, Goodman SG, Topol EJ, et al. Late assessment of thrombolytic efficacy (LATE) study: Prognosis in patients with non-Q wave myocardial infarction. (LATE Study Investigators). J Am Coll Cardiol. 1996;27:1327-1332.
31 Wallentin L, Goldstein P, Armstrong PW, et al. Efficacy and safety of tenecteplase in combination with the low-molecular-weight heparin enoxaparin or unfractionated heparin in the prehospital setting: The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 PLUS randomized trial in acute myocardial infarction. Circulation. 2003;108:135-142.
32 Bonnefoy E, Lapostolle F, Leizorovicz A, et al. Primary angioplasty versus prehospital fibrinolysis in acute myocardial infarction: A randomised study. Lancet. 2002;360:825-829.
33 Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA 2004 Guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the management of patients with acute myocardial infarction). www.acc.org/clinical/guidelines/stemi/index.pdf, 2004. Available at
34 Goodman SG, Menon V, Cannon CP, et al. Acute ST-segment elevation myocardial infarction: American College of Chest Physicians evidence-based clinical practice guidelines. 8th ed. Chest. 2008;133:708S-775S.
35 Weaver WD, Simes RJ, Betriu A, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review. JAMA. 1997;278:2093-2098.
36 Cucherat M, Bonnefoy E, Tremeau G. Primary angioplasty versus intravenous thrombolysis for acute myocardial infarction. Cochrane Database Syst Rev 2003;(3):CD001560.
37 Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328:673-679.
38 Zijlstra F, de Boer MJ, Hoorntje JC, et al. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680-684.
39 A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. N Engl J Med. 1997;336:1621-1628.
40 Benesch C. Antithrombotic and thrombolytic therapy for ischemic stroke. Curr Atheroscler Rep. 2003;5:267-275.
41 Fieschi C, Argentino C, Lenzi GL, et al. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. J Neurol Sci. 1989;91:311-321.
42 Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: A randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019-2026.
43 Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. 2000;55:1649-1655.
44 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587.
45 Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340:1781-1787.
46 Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017-1025.
47 Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245-1251.
48 Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): Results of a double-blind, placebo-controlled, multicenter study. Thrombolytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31:811-816.
49 Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768-774.
50 Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329.
51 Lansberg MG, Schrooten M, Bluhmki E, et al. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin scale. Stroke. 2009;40:2079-2084.
52 Saver JL, Gombein J, Grotta J, et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: joint outcome table analysis of the ECASS 3 trial. Stroke. 2009;40:2433-2437.
53 Del Zoppo GJ, Saver JL, Jauch EC, et al. On behalf of the American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945-2948.
54 del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78-86.
55 Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke. Chest. 2001;119(1 Suppl):300S-320S.
56 Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011.
57 Ogawa A, Mori E, Minematsu K, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke. 2007;38:2633-2639.
58 Saver JL. Intra—arterial fibrinolysis for acute ischemic stroke: the message of MELT. Stroke. 2007;38:2627-2628.
59 Qureshi AI, Suri MF, Shatla AA, et al. Intraarterial recombinant tissue plasminogen activator for ischemic stroke: an accelerating dosing regimen. Neurosurgery. 2000;47:473-476.
60 Qureshi AI, Ali Z, Suri MF, et al. Intra-arterial third-generation recombinant tissue plasminogen activator (reteplase) for acute ischemic stroke. Neurosurgery. 2001;49:41-48.
61 Khatri P, Hill MD, Palesch YY, et al. Methodology of the Interventional Management of Stroke III Trial.
62 Qureshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: A prospective study. Neurosurgery. 2002;51:1319-1327.
63 Seitz RJ, Hamzavi M, Junghans U, et al. Thrombolysis with recombinant tissue plasminogen activator and tirofiban in stroke: Preliminary observations. Stroke. 2003;34:1932-1935.
64 Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke. 2000;31:610-614.
65 Eggers J, Koch B, Meyer K, et al. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797-800.
66 Rubinstein I, Murray D, Hoffstein V. Fatal pulmonary emboli in hospitalized patients: An autopsy study. Arch Intern Med. 1988;148:1425-1426.
67 Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: Are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203-205.
68 Lilienfeld DE, Chan E, Ehland J, et al. Mortality from pulmonary embolism in the United States: 1962 to 1984. Chest. 1990;98:1067-1072.
69 Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: A controlled trial. Lancet. 1960;1:1309-1312.
70 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians evidence-based clinical practice guidelines. 8th ed. Chest. 2008;133:454s-545s.
71 Urokinase pulmonary embolism trial. Phase 1 results: A cooperative study. JAMA. 1970;214:2163-2172.
72 Dalen JE, Banas JSJr, Brooks HL, et al. Resolution rate of acute pulmonary embolism in man. N Engl J Med. 1969;280:1194-1199.
73 Paraskos JA, Adelstein SJ, Smith RE, et al. Late prognosis of acute pulmonary embolism. N Engl J Med. 1973;289:55-58.
74 Urokinase-streptokinase embolism trial. Phase 2 results. A cooperative study. JAMA. 1974;229:1606-1613.
75 Sharma GV, Burleson VA, Sasahara AA. Effect of thrombolytic therapy on pulmonary-capillary blood volume in patients with pulmonary embolism. N Engl J Med. 1980;303:842-845.
76 Sharma GV, Folland ED, McIntyre KM, Sasahara AA. Long-term benefit of thrombolytic therapy in patients with pulmonary embolism. Vasc Med. 2000;5:91-95.
77 Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-1150.
78 Verstraete M, Miller GA, Bounameaux H, et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988;77:353-360.
79 De Gregorio MA, Gimeno MJ, Mainar A, et al. Mechanical and enzymatic thrombolysis for massive pulmonary embolism. J Vasc Interv Radiol. 2002;13(2 pt 1):163-169.
80 Capstick T, Henry MT. Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J. 2005;26:864-874.
81 Arcasoy SM, Vachani A. Local and systemic thrombolytic therapy for acute venous thromboembolism. Clin Chest Med. 2003;24:73-91.
82 Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 2001;119(1 Suppl):176S-193S.
83 Arcasoy SM, Kreit JW. Thrombolytic therapy of pulmonary embolism: A comprehensive review of current evidence. Chest. 1999;115:1695-1707.
84 Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335-337.
85 Martin C, Viviand X, Saux P, Gouin F. Upper-extremity deep vein thrombosis after central venous catheterization via the axillary vein. Crit Care Med. 1999;27:2626-2629.
86 Bozzetti F, Scarpa D, Terno G, et al. Subclavian venous thrombosis due to indwelling catheters: A prospective study on 52 patients. JPEN J Parenter Enteral Nutr. 1983;7:560-562.
87 Becker DM, Philbrick JT, Walker FB. Axillary and subclavian venous thrombosis: Prognosis and treatment. Arch Intern Med. 1991;151:1934-1943.
88 Timsit JF, Farkas JC, Boyer JM, et al. Central vein catheter-related thrombosis in intensive care patients: Incidence, risks factors, and relationship with catheter-related sepsis. Chest. 1998;114:207-213.
89 RaadII, Luna M, Khalil SA, et al. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA. 1994;271:1014-1016.
90 Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
91 Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of post-phlebitic syndrome: Results of a 3-part study. Arch Intern Med. 2001;161:2105-2109.
92 Bernardi E, Prandoni P. The post-thrombotic syndrome. Curr Opin Pulm Med. 2000;6:335-342.
93 Comerota AJ, Gravett MH. Iliofemoral venous thrombosis. J Vasc Surg.. 2007;46:1065-1076.
94 Scott TE, LaMorte WW, Gorin DR, et al. Risk factors for chronic venous insufficiency: a dual case-control study. J Vasc Surg. 1995;22:622-628.
95 Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 suppl 1):I22-I30.
96 Mohr DN, Silverstein MD, Heit JA, et al. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: a population-based study. Mayo Clin Proc. 2000;75:1249-1256.
97 Peden E, Zhou W, Bush RL, et al. The case for thrombolysis for iliofemoral venous thrombosis. Semin Vasc Surg. 2005;18:139-147.
98 Schweizer J, Kirch W, Koch R, et al. Short- and long-term results after thrombolytic treatment of deep venous thrombosis. J Am Coll Cardiol. 2000;36:1336-1343.
99 Goldhaber SZ, Meyerovitz MF, Green D, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am J Med. 1990;88:235-240.
100 Turpie AG, Levine MN, Hirsh J, et al. Tissue plasminogen activator (rt-PA) vs heparin in deep vein thrombosis: Results of a randomized trial. Chest. 1990;97(4 Suppl):172S-175S.
101 Schulman S, Granqvist S, Juhlin-Dannfelt A, Lockner D. Long-term sequelae of calf vein thrombosis treated with heparin or low-dose streptokinase. Acta Med Scand. 1986;219:349-357.
102 Forster A, Wells P. Tissue plasminogen activator for the treatment of deep venous thrombosis of the lower extremity: A systematic review. Chest. 2001;119:572-579.
103 Goldhaber SZ, Buring JE, Lipnick RJ, Hennekens CH. Pooled analyses of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am J Med. 1984;76:393-397.
104 Meissner MH. Thrombolytic therapy for acute deep vein thrombosis and the venous registry. Rev Cardiovasc Med. 2002;3(Suppl 2):S53-S60.
105 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: ACCP Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133:454S-545S.
106 Joffe HV, Goldhaber SZ. Upper-extremity deep vein thrombosis. Circulation. 2002;106:1874-1880.
107 Grossman C, McPherson S. Safety and efficacy of catheter-directed thrombolysis for iliofemoral venous thrombosis. AJR Am J Roentgenol. 1999;172:667-672.
108 Semba CP, Dake MD. Iliofemoral deep venous thrombosis: Aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487-494.
109 Comerota AJ, Aldridge SC. Thrombolytic therapy for deep venous thrombosis: a clinical review. Can J Surg. 1993;36:359-364.
110 AbuRahma AF, Perkins SE, Wulu JT, et al. Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752-760.
111 Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis: A randomized clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209-214.
112 Comerota AJ. The ATTRACT Trial: rationale for early intervention for iliofemoral DVT. Perspect Vasc Surg Endovasc Ther. 2009;21:221-224.
113 Dormandy J, Heeck L, Vig S. Acute limb ischemia. Semin Vasc Surg. 1999;12:148-153.
114 Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: Phase I results. TO PAS Investigators. J Vasc Surg. 1996;23:64-73.
115 Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105-1111.
116 Dotter CT, Rosch J, Seaman AJ. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31-37.
117 Robertson I, Kessel DO, Berridge DC. Fibrinolytic agents for peripheral arterial occlusion. Cochrane Database Syst Rev 2010; Issue 3 Art. No.: CD001099.
118 Kessel DO, Berridge DC, Robertson I. Infusion techniques for peripheral arterial thrombolysis. Cochrane Database Syst Rev 2004; Issue 1 Art. No.: CD000985.
119 van Breda A, Graor RA, Katzen BT, et al. Relative cost-effectiveness of urokinase versus streptokinase in the treatment of peripheral vascular disease. J Vasc Interv Radiol. 1991;2:77-87.
120 Berridge DC, Gregson RH, Hopkinson BR, Makin GS. Randomized trial of intra-arterial recombinant tissue plasminogen activator, intravenous recombinant tissue plasminogen activator and intra-arterial streptokinase in peripheral arterial thrombolysis. Br J Surg. 1991;78:988-995.
121 van Breda A, Katzen BT, Deutsch AS. Urokinase versus streptokinase in local thrombolysis. Radiology. 1987;165:109-111.
122 Sugimoto K, Hofmann LV, Razavi MK, et al. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg. 2003;37:512-517.
123 Semba CP, Bakal CW, Calis KA, et al. Alteplase as an alternative to urokinase. Advisory Panel on Catheter-Directed Thrombolytic Therapy. J Vasc Interv Radiol. 2000;11:279-287.
124 Ouriel K, Shortell CK, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021-1030.
125 Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251-266.
126 Weaver FA, Comerota AJ, Youngblood M, et al. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: Results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. J Vasc Surg. 1996;24:513-521.
127 Comerota AJ, Weaver FA, Hosking JD, et al. Results of a prospective, randomized trial of surgery versus thrombolysis for occluded lower extremity bypass grafts. Am J Surg. 1996;172:105-112.
128 Feldman HI, Held PJ, Hutchinson JT, et al. Hemodialysis vascular access morbidity in the United States. Kidney Int. 1993;43:1091-1096.
129 Cynamon J, Pierpont CE, Vogel P, Novick AS. Multicenter prospective randomized comparison of “lyse and wait” versus pulse-spray pharmacomechanical thrombolysis for treating thrombosed hemodialysis access grafts. J Vasc Interv Radiol. 2000;11(Suppl 2):253.
130 Cynamon J, Lakritz PS, Wahl SI, et al. Hemodialysis graft declotting: Description of the “lyse and wait” technique. J Vasc Interv Radiol. 1997;8:825-829.
131 Green LD, Lee DS, Kucey DS. A metaanalysis comparing surgical thrombectomy, mechanical thrombectomy, and pharmacomechanical thrombolysis for thrombosed dialysis grafts. J Vasc Surg. 2002;36:939-945.
132 Deitcher SR, Fesen MR, Kiproff PM, et al. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: Results of the cardiovascular thrombolytic to open occluded lines trial. J Clin Oncol. 2002;20:317-324.
133 Ponec D, Irwin D, Haire WD, et al. Recombinant tissue plasminogen activator (alteplase) for restoration of flow in occluded central venous access devices: A double-blind placebo-controlled trial—the Cardiovascular Thrombolytic to Open Occluded Lines (COOL) efficacy trial. J Vasc Interv Radiol. 2001;12:951-955.
134 Haire WD, Atkinson JB, Stephens LC, Kotulak GD. Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: A double-blinded, randomized trial. Thromb Haemost. 1994;72:543-547.
135 Semba CP, Deitcher SR, Li X, et al. Treatment of occluded central venous catheters with alteplase: Results in 1,064 patients. J Vasc Interv Radiol. 2002;13:1199-1205.
136 Bell WR. Present-day thrombolytic therapy: Therapeutic agents—pharmacokinetics and pharmacodynamics. Rev Cardiovasc Med. 2002;3(Suppl 2):S34-S44.
137 The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932-936.
138 Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial—phase I: Hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1-11.
139 Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction: Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med. 1991;115:256-265.