Chapter 121 Thoracoscopic Sympathectomy for Hyperhidrosis
Axillary and palmar hyperhidrosis are relatively common disorders that affect approximately 0.5% to 1% of the population, with possibly higher percentages in those of Asian descent.1 Hyperhidrosis is an idiopathic overactivation of sweat glands that results in secretion of sweat in excess of that needed for typical autonomically controlled thermoregulation. It is often overlooked or untreated and can cause significant distress that may lead to a negative impact on social and professional quality of life.2 Simple tasks such as handshaking, writing, and handling daily objects can become daunting problems. It can become so severe that sweat can drip off a person’s fingertips and can even require multiple changes of clothing daily. Patients with untreated hyperhidrosis have an increased risk for cutaneous skin infection if the abnormal physiology is not adequately corrected. Walling determined the overall odds ratio for infection of untreated hyperhidrosis is 3.2, for dermatophyte fungus is 9.8, and for bacteria is 2.6.3
Diagnosis criteria stratify severity into four classes, according to the Hyperhidrosis Disease Severity Scale: never noticeable, tolerable but interferes, barely tolerable and frequently interferes, and intolerable and always interferes.4 Additional criteria include duration in the previous 6 months; bilateral with symmetry; onset before 25 years of age; family history; and asymptomatic during sleep.5
History
While the earliest surveys of the sympathetic nervous system were conducted by du Petit in 1727, the first report of surgical sympathectomy was at the level of the neck for treatment of epilepsy by Alexander in 1889.6,7 Since then, it has been used for numerous clinical presentations with limited success. For example, sympathectomy has been indicated in treatment of angina,8 Raynaud phenomenon,9 exophthalmic goiter,10 glaucoma,11 various pain conditions,12 and spastic paralysis of lower extremities.13
Bernard and Horner independently published a series of reports in the latter half of the 19th century describing sectioning of the sympathetic chain and associated clinical effects.13a13b Since the 1920s, when Kotzareff described anhidrosis with sectioning of the sympathetic chain,14 sympathectomy has been commonly used for hyperhidrosis. The adaptation of endoscopic surgery was introduced in the 1940s when Hughes described an endoscopic approach for thoracic sympathectomy.15
Anatomy and Physiology
The sweat response is under hypothalamic thermoregulatory control via the preoptic sweat area.16 Autonomic output to eccrine glands arise both from input responding to thermoregulatory and from emotional state. Therefore, heightened emotions trigger a sweat response, such as sweaty palms with anxiety or nervousness.
Importantly, approximately 10% of the population carry the nerve of Kuntz, an additional aberrant division of the sympathetic chain arising from T1, T2, or T3.17 If present, the nerve of Kuntz must be sectioned to assure that sympathectomy will be effective.
Operative Technique
Patient Selection
Careful patient selection is critical for success. Prior to consideration for surgery, patients should have completed and failed a range of appropriate medical therapies. The mainstay of medical therapy includes over-the-counter (OTC) antiperspirants and topical 20% AlCl compounds. Chronic use of such antiperspirants can be limited by skin irritation, however. Medications that block alpha-adrenergic receptors such as phenoxybenzamine may also help symptoms. Often, these medications are poorly tolerated, as frequent side effects include hypotension and sexual dysfunction. Another more invasive option consists of a series of topical injections of botulinum toxin A (Botox). For palmar hyperhidrosis, an average of 26 Botox injections were required for determination of success or failure.18 Typically, even successfully treated patients need repeated injections after several months. Also, iontophoresis treatment can be tried prior to consideration of surgical treatment. Iontophoresis includes electrical stimulation, often with tap water, and can be conducted at home. However, it requires a significant daily time commitment. Lastly, in selected patients, imaging with computed tomography or magnetic resonance can be useful in excluding alternative pathologies, including infection, metabolic disorders, and neoplastic processes. If hyperhidrosis remains debilitating despite some of these interventions, surgery should be considered.
Anesthesia
Endoscopic thoracic sympathectomy typically requires placement of a double-lumen endotracheal tube. This allows for deflation of the lung on the operative side while allowing for ventilation of the contralateral lung. Alternatively, single-lumen endotracheal tube may be used with positive pressure carbon dioxide insufflation.19
Instruments
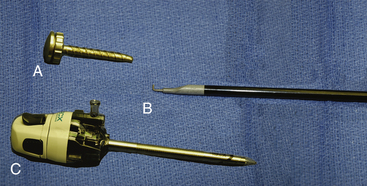
We typically use a 5-mm rigid endoscope for thoracoscopic procedures. Lens angle depends upon surgeon preference. We typically prefer a 30-degree angled scope. During the procedure, a dissection tool such as a cautery hook (Fig. 121-1) or scissors is necessary, as well as cotton pledgets and a suction irrigator.
Positioning
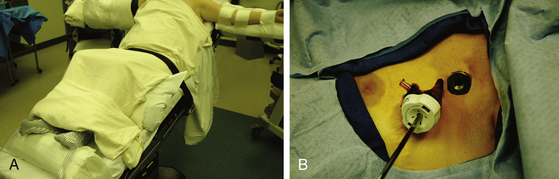
The patient should be positioned supine with the arms on arm boards perpendicular to the bed (Fig. 121-2). A reverse Trendelenburg position to approximately 40 degrees enables the deflated lung to fall away by gravity and minimizes the need for retraction. A footboard attached to the surgical table may be necessary to prevent the patient from sliding along the inclined bed.
Procedure
In the multiport method, two 5-mm thoracoscopic ports are placed in the third and fourth intercostal spaces (Fig. 121-2). The endoscope is introduced through one, and the second serves as a working port for sectioning the sympathetic chain. Alternatively, the procedure may be performed through a single port.20
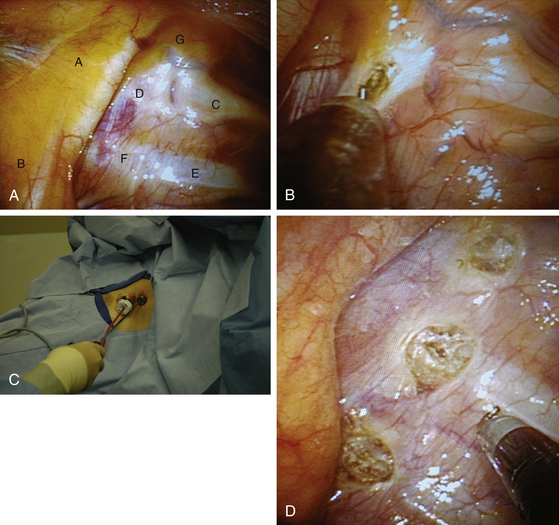
The sympathetic chain is identified using the rib heads as landmarks. The first rib is uniquely curved like a sickle (Fig. 121-3A). It is associated with a prominent fat pad containing the first sympathetic ganglion, otherwise known as the stellate ganglion. The white sympathetic chain is seen coursing over the rib heads with the swelling of the ganglia close to the respective rib. Following adequate identification, the T2 to T4 portions of the sympathetic chain can be disconnected or clipped or excised.
We prefer to disconnect the ganglia from the chain. A cotton pledget can be used to push the lung down and away from the upper thoracic spine if needed. A cautery hook is then used to open the pleura on both sides of the respective ganglion. It is typically easiest to open the pleura directly over the bone of the rib (Fig. 121-3B). The hook is dissected under the chain and used to elevate it into view. Cautery is then applied to cut the chain above and below the ganglion (Fig. 121-3C). These steps are then repeated as many times as needed to disconnect the appropriate ganglia. Anecdotal association of T2 with facial, T3 with palmar, and T4 with axillary hyperhidrosis has been observed.21 However, contemporary practice includes sectioning the T2-T3 ganglia for palmar hyperhidrosis and T2-T4 ganglia for palmar and axillary hyperhidrosis. It is important to be sure any accessory fibers, including the nerve of Kuntz, if present, are disconnected or excised as well.
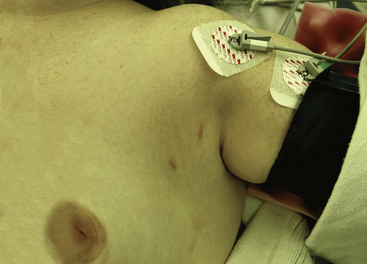
Once sympathectomy is completed, a red rubber catheter is inserted through one port site while the other is removed and the wound is closed (Fig. 121-3D). The catheter is submerged in saline while positive-pressure ventilation by the anesthesiologist re-expands the lung. Bubbles of air can be seen in the saline as the lung inflates and air representing pneumothorax decreases. The red rubber catheter is removed when the lung is fully inflated, and incisions are closed with absorbable sutures and dressed with Steri-Strips (Fig. 121-4). Postoperative chest x-ray is obtained to confirm sufficient lung expansion.
Outcomes
Patients typically notice significant improvement almost immediately. This improvement remains fairly stable, as long-term outcome studies show a range of efficacy of endoscopic sympathectomy for hyperhidrosis of 93.4% to 100% at 6 years.22,23 Moreover, surgery has been shown to have a significant reduction in symptoms at both 6 and 12 months when compared to botulinum toxin injection.24
Myriad studies have investigated efficacy of treatment, comparing ablation level (T2-T4, T2, T3, or T4) and method (resection, electrocautery, transection, or clipping).25–29 Reduced incidence or severity of compensatory hyperhidrosis was noted in isolated T3 ablation in comparison to T2 or T2-T4.27 Ablation of T4 alone was found to have similar frequency of success but decreased incidence or severity of overdryness following surgery.30 One report advised complete excision with histologic confirmation.31 A systemic review in 2008 concluded that there was no significant difference in outcome with respect to resection method, total ganglion resection, nerve transection, or ablation.32 Indeed, the authors further concluded inclusion of T2 and the adjacent sympathetic chain is important for success, in comparison to ablation of T3 or T4 alone, and may result in lower incidence of compensatory hyperhidrosis. Overall, the literature presents a mixed picture of which level or levels are necessary for resection to ensure success; thus, further studies are warranted.
Complications
Thoracoscopic sympathectomy is typically well tolerated. However, some adverse events are well-known risks of surgery. Pneumothorax is among the most common complication, with incidence ranging from 7% to 17%.2,23 We routinely obtain a recovery room chest x-ray. If a substantial pneumothorax is seen, the patient remains in the hospital with another follow-up film within 24 hours. In our experience, a chest tube is rarely needed and is reserved for symptomatic cases. Other centers prophylactically place a chest tube postoperatively and remove it shortly thereafter.
Subcutaneous emphysema is caused by the collection of air in the subcutaneous tissues of the thorax and neck. Recent reports indicate the rate of subcutaneous emphysema associated with thoracoscopic sympathectomy to be about 2%.33,34
Horner syndrome can result from sympathectomy. This consists of miosis, ptosis, enophthalmos, hyperemia of the eye, and anhidrosis of half the face. It is the result of injury to the first cervical ganglion (stellate), and incidence is reported to range from 0% to 24% in the initial postoperative period. When Horner syndrome occurs, symptoms typically resolve without further treatment. Studies suggest a 0% to 8% long-term rate for persistent Horner syndrome.2 This complication can be avoided by carefully avoiding direct injury to the stellate ganglion, excessive traction of sympathetic chain, or thermal injury to adjacent structures.35
Compensatory hyperhidrosis is an increase in perspiration occurring in areas other than the hands or axilla following a sympathectomy procedure. It is quite common in the initial few weeks following surgery and then typically subsides. Current literature suggest compensatory hyperhidrosis can occur in 17% to 75% of patients following sympathectomy and has been related to both level and method of ablation.32,36–38 Fortunately, compensatory hyperhidrosis is only severe in approximately 5% to 10% of patients and tends to improve or resolve within 6 months after surgery. Severity, location, and prevalence of occurrence with respect to level of sympathectomy vary highly in reports, and no conclusive determination has been made. Reconstruction of the sympathetic chain as treatment has been described; however, substantiated claims have yet to be reported.
Lastly, even successfully treated hyperhidrosis can recur over time. Freeman et al. suggested the recurrence rate of intolerable palmar hyperhidrosis and/or reoperation ranges from 6.6% to 12.5% for thoracoscopic sympathomectomy.39 The success rate with reoperation was comparable to that with original surgery, with 95% experiencing improved symptoms in the reoperative group versus 98% in initial group, without significant increase in morbidity. However, compensatory sweating did significantly increase in those with a second procedure, 53% versus 31% (reoperation vs. initial, respectively). The authors did not comment on the ratio of patients with symptomatic recurrence to those who required reoperation.
Ambrogi V., Campione E., Mineo D., et al. Bilateral thoracoscopic T2 to T3 sympathectomy versus botulinum injection in palmar hyperhidrosis. Ann Thorac Surg. 2009;88:238-245.
Baumgartner F.J., Bertin S., Konecny J. Superiority of thoracoscopic sympathectomy over medical management for the palmoplantar subset of severe hyperhidrosis. Ann Vasc Surg. 2009;23:1-7.
Chung I.H., Oh C.S., Koh K.S., et al. Anatomic variations of the T2 nerve root (including the nerve of Kuntz) and their implications for sympathectomy. J Thorac Cardiovasc Surg. 2002;123:498-501.
Doolabh N., Horswell S., Williams M., et al. Thoracoscopic sympathectomy for hyperhidrosis: indications and results. Ann Thorac Surg. 2004;77:410-414. discussion 414
Dumont P., Denoyer A., Robin P. Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg. 2004;78:1801-1807.
Freeman R.K., Van Woerkom J.M., Vyverberg A., Ascioti A.J. Reoperative endoscopic sympathectomy for persistent or recurrent palmar hyperhidrosis. Ann Thorac Surg. 2009;88:412-416. discussion 416-417
Gossot D., Galetta D., Pascal A., et al. Long-term results of endoscopic thoracic sympathectomy for upper limb hyperhidrosis. Ann Thorac Surg. 2003;75:1075-1079.
Hornberger J., Grimes K., Naumann M., et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51:274-286.
Kopelman D., Hashmonai M. The Correlation Between the Method of Sympathetic Ablation for Palmar Hyperhidrosis and the Occurrence of Compensatory Hyperhidrosis: A Review. World J Surg. 2008;32:2343-2356.
Solish N., Bertucci V., Dansereau A., et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908-923.
Solomon B.A., Hayman R. Botulinum toxin type A therapy for palmar and digital hyperhidrosis. J Am Acad Dermatol. 2000;42:1026-1029.
Vanaclocha V., Saiz-Sapena N., Panta F. Uniportal endoscopic superior thoracic sympathectomy. Neurosurgery. 2000;46:924-928.
Walling H.W. Primary hyperhidrosis increases the risk of cutaneous infection: a case-control study of 387 patients. J Am Acad Dermatol. 2009;61:242-246.
1. Baumgartner F.J., Bertin S., Konecny J. Superiority of thoracoscopic sympathectomy over medical management for the palmoplantar subset of severe hyperhidrosis. Ann Vasc Surg. 2009;23:1-7. doi:S0890-5096(08)00185-4 [pii]. 10.1016/j.avsg.2008.04.014
2. Adar R., Kurchin A., Zweig A., Mozes M. Palmar hyperhidrosis and its surgical treatment: a report of 100 cases. Ann Surg. 1977;186:34-41.
3. Walling H.W. Primary hyperhidrosis increases the risk of cutaneous infection: a case-control study of 387 patients. J Am Acad Dermatol. 2009;61:242-246. doi:S0190-9622(09)00246-1 [pii]. 10.1016/j.jaad.2009.02.038
4. Solish N., Bertucci V., Dansereau A., et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908-923. doi:DSU33192 [pii]. 10.1111/j.1524-4725.2007.33192.x
5. Hornberger J., Grimes K., Naumann M., et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51:274-286. doi:10.1016/j.jaad.2003.12.029. S0190962204005158 [pii]
6. Hashmonai M., Kopelman D. History of sympathetic surgery. Clin Auton Res. 2003;13(Suppl 1):I6-9. doi:10.1007/s10286-003-1103-5
7. Alexander W. The Treatment of Epilepsy. Edinburgh: Pentland; 1889. 228
8. Jonnesco T. Angine De Poitrine Guérie Par La Résection Du Sympatique Cervicothoracique. Bull Acad Med Paris. 1920;84:93-102.
9. Brüning F. Zur Technik der kombinierten Resektionsmethode sämtlicher sympathischen Nervenbahnen am Halse. Zentralbl Chir. 1923;5:1056-1059.
10. Jaboulay M. Chirurgie du Grand Sympathique et du Corps Thyroïde (Les Différents Goîtres). Articles originaux et observations réunis et publiés par le Dr. Etienne Martin. Paris: Lyon A. Storck & Cie; 1900.
11. François-Franck M. Signification physiologique de la résection du sympathique dans la maladie de Basedow, l’épilepsie, l’idiotie et le glaucome. Bull Acad Med Paris. 1899;41:565-594.
12. Leriche R. The Surgery of Pain. London: Baillière, Tindall and Cox; 1939.
13. Royle N. A new operative procedure in the treatment of spastic paralysis and its experimental basis. Med J Aust. 1924;1:77-86.
13a. Bernard C. Des phénomènes oculo-pupillaires produits par la section du nerf sympathique cervical: ils sont indépendants des phénomènes vasculaires calorifiques de la tête. Comptes rendus de l’Académie des sciences, Paris. 1852;55:381-388.
13b. Horner J.F. Über eine Form von Ptosis. Klinische Monatsblätter für Augenheilkunde. Stuttgart. 1869;7:193-198.
14. Kotzareff A. Resection partielle de trone sympathetique cervical droit pour hyperhidrose unilaterale. Rev Med Suisse Romande. 1920;40:111-113.
15. Hughes J. Endothoracic Sympathectomy. Proc R Soc Med. 1942;35:585-586.
16. Lowe N., Campanati A., Bodokh I., et al. The place of botulinum toxin type A in the treatment of focal hyperhidrosis. Br J Dermatol. 2004;151:1115-1122. doi:BJD6317 [pii]. 10.1111/j.1365-2133.2004.06317.x
17. Chung I.H., Oh C.S., Koh K.S., et al. Anatomic variations of the T2 nerve root (including the nerve of Kuntz) and their implications for sympathectomy. J Thorac Cardiovasc Surg. 2002;123:498-501. doi:S002252230273955X [pii]
18. Solomon B.A., Hayman R. Botulinum toxin type A therapy for palmar and digital hyperhidrosis. J Am Acad Dermatol. 2000;42:1026-1029. doi:S0190-9622(00)90298-6 [pii]
19. Wong R.Y., Fung S.T., Jawan B., et al. Use of a single lumen endotracheal tube and continuous CO2 insufflation in transthoracic endoscopic sympathectomy. Acta Anaesthesiol Sin. 1995;33:21-26.
20. Vanaclocha V., Saiz-Sapena N., Panta F. Uniportal endoscopic superior thoracic sympathectomy. Neurosurgery. 2000;46:924-928.
21. Doolabh N., Horswell S., Williams M., et al. Thoracoscopic sympathectomy for hyperhidrosis: indications and results. Ann Thorac Surg. 2004;77:410-414. discussion 414, doi:10.1016/j.athoracsur.2003.06.003. S0003497503016886 [pii]
22. Gossot D., Galetta D., Pascal A., et al. Long-term results of endoscopic thoracic sympathectomy for upper limb hyperhidrosis. Ann Thorac Surg. 2003;75:1075-1079.
23. Dumont P., Denoyer A., Robin P. Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg. 2004;78:1801-1807. doi:S000349750400548X [pii]. 10.1016/j.athoracsur.2004.03.012
24. Ambrogi V., Campione E., Mineo D., et al. Bilateral thoracoscopic T2 to T3 sympathectomy versus botulinum injection in palmar hyperhidrosis. Ann Thorac Surg. 2009;88:238-245. doi:S0003-4975(09)00631-6 [pii]. 10.1016/j.athoracsur.2009.04.003
25. Imhof M., Zacherl J., Plas E.G., et al. Long-Term Results of 45 Thoracoscopic Sympathicotomies for Primary Hyperhidrosis in Children. J. Ped. Surg. 1999;34:1839-1842.
26. Kopelman D., Hashmonai M. The Correlation Between the Method of Sympathetic Ablation for Palmar Hyperhidrosis and the Occurrence of Compensatory Hyperhidrosis: A Review. World J Surg. 2008;32:2343-2356.
27. Li X., Tu Y.R., Lin M., et al. Endoscopic thoracic sympathectomy for palmar hyperhidrosis: a randomized control trial comparing T3 and T2-4 ablation. Ann Thorac Surg. 2008;85:1747-1751. doi:S0003-4975(08)00201-4 [pii]. 10.1016/j.athoracsur.2008.01.060
28. Walles T., Somuncuoglu G., Steger V., et al. Long-term efficiency of endoscopic thoracic sympathicotomy: survey 10 years after surgery. Interact Cardiovasc Thorac Surg. 2009;8:54-57. doi:icvts.2008.185314 [pii]. 10.1510/icvts.2008.185314
29. Weksler B., Pollice M., Souza Z.B., Gavina R. Comparison of ultrasonic scalpel to electrocautery in patients undergoing endoscopic thoracic sympathectomy. Ann Thorac Surg. 2009;88:1138-1141. doi:S0003-4975(09)01288-0 [pii]. 10.1016/j.athoracsur.2009.06.052
30. Chang Y.T., Li H.P., Lee J.Y., et al. Treatment of palmar hyperhidrosis: T4 level compared with T3 and T2. Ann Surg. 2007;246:330-336. doi:10.1097/SLA.0b013e3180caa466. 00000658-200708000-00024 [pii]
31. Rathinam S., Nanjaiah P., Sivalingam S., Rajesh P.B. Excision of sympathetic ganglia and the rami communicantes with histological confirmation offers better early and late outcomes in Video assisted thoracoscopic sympathectomy. J Cardiothorac Surg. 2008;3:50. doi:1749-8090-3-50 [pii]. 10.1186/1749-8090-3-50
32. Kopelman D., Hashmonai M. The correlation between the method of sympathetic ablation for palmar hyperhidrosis: A Review. World Journal of Surgery. 2008;32:2343-2356.
33. Imhof M., Zacherl J., Plas E.G., et al. Long-term results of 45 thoracoscopic sympathicotomies for primary hyperhidrosis in children. J Pediatr Surg. 1999;34:1839-1842. doi:S0022-3468(99)90326-3 [pii]
34. Zacherl J., Huber E.R., Imhof M., et al. Long-term results of 630 thoracoscopic sympathicotomies for primary hyperhidrosis: the Vienna experience. Eur J Surg Suppl. 43-46, 1998.
35. Singh B., Moodley J., Allopi L., Cassimjee H.M. Horner syndrome after sympathectomy in the thoracoscopic era. Surg Laparosc Endosc Percutan Tech. 2006;16:222-225. doi:00129689-200608000-00005 [pii]
36. Lee K.H., Hwang P.Y. Video endoscopic sympathectomy for palmar hyperhidrosis. J Neurosurg. 1996;84:484-486. doi:10.3171/jns.1996.84.3.0484
37. Shelley W., Florence R. Compensatory hyperhidrosis of sympathectomy. N J Med. 1960;263:1056-1058.
38. Shih C.J., Wang Y.C. Thoracic sympathectomy for palmar hyperhidrosis: report of 457 cases. Surg Neurol. 1978;10:291-296.
39. Freeman R.K., Van Woerkom J.M., Vyverberg A., Ascioti A.J. Reoperative endoscopic sympathectomy for persistent or recurrent palmar hyperhidrosis. Ann Thorac Surg. 2009;88:412-416. discussion 416-417, doi:S0003-4975(09)00770-X [pii]10.1016/j.athoracsur.2009.03.101