CHAPTER 28 Thoracoscopic Sympathectomy
Introduction
Thoracoscopic sympathectomy has been used for the treatment of sympathetic dysfunction since it was first described by Kux and colleagues in the 1940s.1 With the advent of video-assisted thoracic surgery (VATS), the procedure has become more widely applied.2 VATS allows excellent visual acuity and the possibility of doing the procedure more quickly and with fewer complications. It is the most effective treatment for palmar hyperhidrosis.
Nomenclature
Approach to Video-Assisted Thoracoscopic Sympathectomy
Choice of Transection Level
Video-Assisted Thoracoscopic Sympathectomy
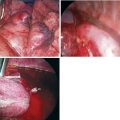
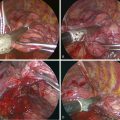
Step 1. Setup for Sympathectomy
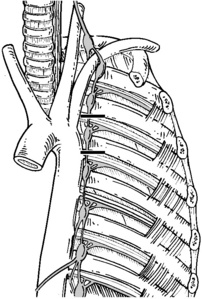
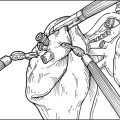
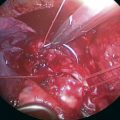
Step 2. Exposure of the Sympathetic Chain
Step 3. Sympathectomy
Step 4. Lung Reexpansion
Results
In a series of 396 consecutive procedures (synchronous bilateral in 388 patients, right side alone in 6, left side alone in 1, staged in 1) included 191 (48%) men and 206 (52%) women. The mean age was 29 years (range, 9 to 65 years). Median hospital stay was 0.5 day (range, 0.5 to 3 days). Median follow-up was 2.6 years (range, 2 months to 9 years). The indications were hyperhidrosis in 370 patients, facial blushing in 21, Raynaud’s in 3, digital ischemia in 2, and reflex sympathetic dystrophy in 1 patient. Compensatory sweating occurred in 40% (n = 81 of 202).5
A series of 453 patients showed excellent (74.2%) or good (19.6%) quality of life at 5 years of follow-up.6 In another series of 222 patients, excellent results were reported for more than 90% and compensatory sweating for 85% of patients.7 Results of these two studies suggested that division of the R2 ganglion should be avoided because compensatory sweating developed in 48% of patients when R2 was transected but in only 16% when R2 was not transected. When severe compensatory sweating developed, more than 50% of patients wished that they had not undergone the procedure.
Miller and Force8 described a novel approach to thoracoscopic sympathectomy. They devised a method to screen patients who would be more likely to develop compensatory sweating. They injected the nerve at the planned operative level and reassessed patients after they emerged from anesthesia.8 If the patients benefitted from the injection and did not develop intolerable compensatory sweating, they subsequently underwent sympathectomy.
Tools have been devised to evaluate quality of life (QOL) in terms of objective response, satisfaction, and complications after thoracoscopic sympathectomy. In 1998, Krasna and colleagues proposed a 4-point scoring system of the severity and social impact after thoracoscopic sympathectomy.9 Kwong described a QOL index,10 and Ribas and associates used a standard short-form health survey (SF-36).11 Each of these systems attempts to quantitate the positive and negative results of this procedure for making decisions and assessing costs.
Complications and Repeat Operations
An unusual complication that should be discussed with the patient preoperatively is Horner’s syndrome, which occurs in less than 5% of patients. Decreased baseline heart rate and a decrease in the response to stress are expected to some degree in all patients.12 This is a possible cause of postoperative dysfunction and should be cautiously investigated. All of these complications should be discussed with the patient before surgery.
Reoperation after prior thoracic procedures is not contraindicated, as reported by Kim and colleagues.13 In this series, even patients with previous open and thoracoscopic surgery did not have adhesions that prevented successful thoracoscopic sympathectomy.
1 Kux M. Thoracic endoscopic sympathectomy in palmar and axillary hyperhidrosis. Arch Surg. 1978;113:264-266.
2 Göthberg C., Drott, Claes G. Thoracoscopic sympathicotomy for hyperhidrosis—surgical technique, complications and side effects. Eur J Surg Suppl. 1994;572:51-53.
3 Lin T.S., Huang L.C., Wang N.P., Chang C.C. Video-assisted thoracoscopic T2 sympathetic block by clipping for palmar hyperhidrosis: analysis of 52 cases. J Laparoendosc Adv Surg Tech A. 2001;11:59-62.
4 Wolfer R.S., Krasna M.J., Hasnain J.U., McLaughlin J.S. Hemodynamic effects of carbon dioxide insufflation during thoracoscopy. Ann Thorac Surg. 1994;58:404-408.
5 Kwong K., Krasna M. Clinical experience in 397 consecutive thoracoscopic sympathectomies. Ann Thorac Surg. 2005;80:1063-1066.
6 Milanez de Campos J., Kauffman P., et al. Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg. 2003;76:886-891.
7 Dewey T.M., Herbert M.A., Hill S.L., et al. One-year follow-up after thoracoscopic sympathectomy for hyperhidrosis: outcomes and consequences. Ann Thorac Surg. 2006;81:1227-1232.
8 Miller D.L., Force S.D. Temporary thoracoscopic sympathetic block for hyperhidrosis. Ann Thorac Surg. 2008;85:1211-1214.
9 Krasna M., Demmy T., McKenna R., Mack M. Thoracoscopic sympathectomy: the U.S. experience. Eur J Surg Suppl. 1998;580:19-21.
10 Krasna M.J., Jiao X., Sonett J., et al. Thoracoscopic sympathectomy. Surg Laparosc Endosc Percutan Tech. 2000;10:314-318.
11 Ribas Milanez de Campos J., Kauffman P., Wolosker N., et al. Axillary hyperhidrosis: T3/T4 versus T4 thoracic sympathectomy in a series of 276 cases. J Laparoendosc Adv Tech A. 2006;16:598-603.
12 Drott C., Claes G., Paszkowski P. Cardiac effects of endoscopic electrocautery of the upper sympathetic chain. Eur J Surg. 1994;572(Suppl):65-70.
13 Kim D.H., Paik H.C., Lee D.Y. Video assisted thoracoscopic re-sympathetic surgery in the treatment of re-sweating hyperhidrosis. Eur J Cardiothorac Surg. 2005;27:741-744.