CHAPTER 25 Thoracoscopic Approach for Spinal Conditions
Thoracoscopic Anterior Release and Fusion
Mack and colleagues1 first introduced endoscopic spine techniques in 1993. Since that time, thoracoscopy, also known as video-assisted thoracic surgery (VATS), has evolved to become a valuable tool in the treatment of spinal deformity and other spinal conditions. The goals of thoracoscopic anterior spinal surgery are essentially the same as the goals of open surgery, but they are accomplished with less invasive techniques. Specifically, the goal of VATS in the surgical management of idiopathic scoliosis is to perform a safe, reproducible, and effective procedure that results in improvement in spinal alignment and balance in all planes and axial derotation comparable to, or better than, that obtained with an open procedure.2 In addition to idiopathic scoliosis, thoracoscopy has been used for anterior releases in kyphosis, hemiepiphysiodeses and hemivertebrectomies, excision of spinal tumors, and treatment of spinal trauma.
Indications
Scoliosis
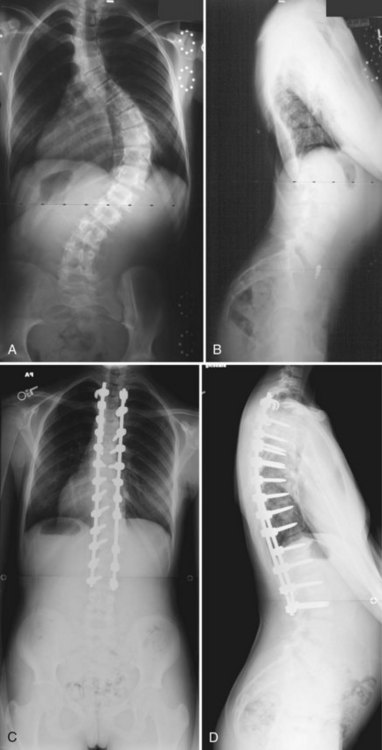
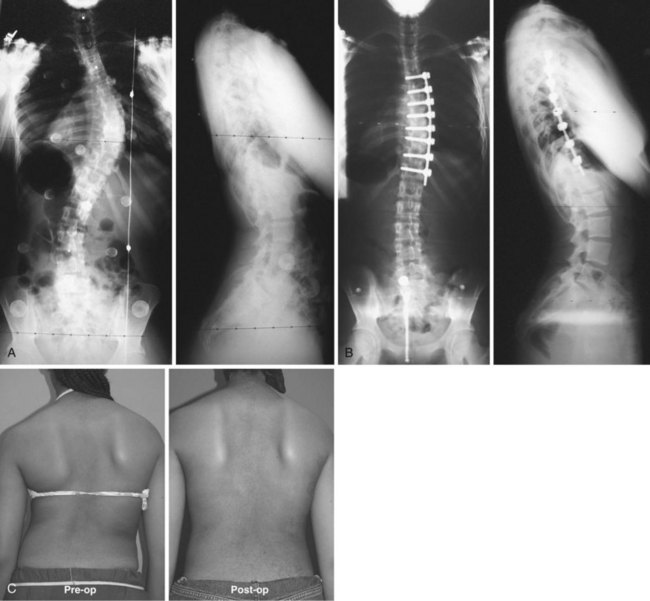
Thoracic scoliosis has various etiologies (idiopathic, neuromuscular, syndrome related) that are frequently not diagnosed and treated until the curve is relatively large and stiff. Anterior surgery has been most frequently used as a means to achieve a complete discectomy and release of the anterior spine; this results in greater curve flexibility and prevents the “crankshaft” phenomenon in young patients. Although no strict guidelines on the magnitude and flexibility of the spinal curvature that requires release have been established, generally curves with a Cobb angle greater than 70 to 75 degrees and a bend correction less than 50% are considered appropriate for release. When sufficient segmental mobility of the released vertebra has been achieved, posterior instrumentation is placed to correct the deformity (Fig. 25–1).
Lenke3 reported on a combined anterior VATS release and fusion followed by posterior instrumentation in the treatment of adolescent idiopathic scoliosis that had an average preoperative curve of 82 degrees (range 41 to 125 degrees) with postoperative correction of 70% to 28 degrees (range 5 to 60 degrees). Similarly, Newton and colleagues4 reported a series of 112 pediatric spinal deformity cases with an average preoperative curve of 80 degrees that received an anterior release combined with posterior instrumentation and found a 67% correction in idiopathic scoliosis and a 52% correction in neuromuscular scoliosis. More recent studies have called into question the utility of the anterior release, however, in the age of modern segmental pedicle screw instrumentation.5,6 Suk and colleagues6 found an average correction of 66% when posterior pedicle screws alone were used in preoperative thoracic curves of 80 degrees with a flexibility of 45%. Although modern pedicle screw constructs offer a similar correction, an anterior release may still be indicated to optimize coronal and axial plane correction, improve sagittal alignment by increasing thoracic kyphosis, and prevent crankshaft growth.
Children and young adolescents (Risser 0, open triradiate cartilage) with progressive scoliosis are known to be at risk for crankshaft deformity when treated with a posterior fusion alone.7 These results were reported for children using hook and wire fixation, however, and not modern pedicle screw instrumentation. Although there has been concern regarding adequate pedicle size in children to accommodate pedicle screws, Catan and colleagues8 performed a magnetic resonance imaging (MRI) analysis of thoracic pedicle morphology in preadolescent patients with idiopathic scoliosis and found that the anatomic measurements were compatible with pedicle screw instrumentation. Sarlak and colleagues9 reported more recently a series of seven children (average age 7.4 years) with scoliosis with an average preoperative thoracic curve of 56 degrees who were treated with posterior segmental pedicle screw instrumentation and 5 years of follow-up. These authors found a 57% correction rate with no evidence of crankshaft phenomenon in four patients but found a slight increase in Cobb angle and a significant increase in angle of trunk rotation (ATR) suggesting crankshaft phenomenon in two patients.9 Given the lack of clear evidence on the appropriate treatment of these cases, an anterior fusion may be a viable option to limit anterior growth and prevent this late increasing deformity.10 Thoracoscopic disc excision and fusion provides a minimally invasive option and minimizes the risk of pulmonary complications in these young patients with progressive deformity.11–13
Patients with spinal deformity associated with Marfan syndrome, neurofibromatosis 1, and prior spinal irradiation may have an increased risk of pseudarthrosis after an isolated posterior scoliosis correction. In cases such as these, an anterior fusion procedure may improve the odds of successful arthrodesis, especially when autogenous bone graft is used.4
Kyphosis
Controversy exists regarding the need for anterior procedures in cases of thoracic kyphosis.14–16 In previous studies, Papagelopoulos and colleagues17 and Sturm and colleagues18 found that posterior correction with hook or hybrid fixation alone did not provide adequate strength to maintain correction in patients with progressive kyphosis. Because of the lack of satisfactory results with posterior hook or hybrid instrumentation, combined anterior and posterior approaches to treatment of kyphotic deformity have been investigated. In a retrospective analysis of 32 patients with Scheuermann kyphosis treated with a combined anterior release followed by posterior segmental hybrid instrumentation, Lowe and Kasten19 found that a combined approach resulted in a 51% correction of the deformity with no major postoperative complications. Herrera-Soto and colleagues20 specifically investigated the use of thoracoscopy in these patients and found similar benefits to scoliosis patients, including decreased blood loss and less morbidity compared with an open thoracotomy.
In a series of 39 patients with Scheuermann kyphosis, Lee and colleagues15 compared posterior-only thoracic segmental pedicle screw constructs with combined anterior and posterior constructs. These authors found a similar correction rate between both techniques, with an increase in complications in the combined anterior and posterior group. Although posterior segmental pedicle instrumentation seems to offer a similar correction rate to a combined anterior and posterior approach, no recommendations can be made based on the current evidence.14 The optimal treatment approach to progressive kyphosis must be based on the surgeon’s judgment and experience for each individual patient.
Congenital Deformity
Operative management of congenital scoliosis depends on the type of vertebral anomaly, its location, the age of the patient, and the potential continued growth of the child. Although various techniques are available, thoracoscopy also has been applied to the treatment of congenital scoliosis.4,12,21 Several methods of surgical treatment have been employed in these patients with limited results. In a study with 12 years of follow-up in patients with congenital scoliosis, Kesling and colleagues22 found that 15% of 54 patients who received a posterior arthrodesis developed crankshaft phenomenon.
Given the lack of satisfactory results with previous posterior-only techniques, anterior surgery may be considered in these patients The anterior portion of circumferential arthrodesis and growth-modifying hemiepiphysiodesis are theoretically possible via the endoscopic approach. First reported by Roaf,23 hemiepiphysiodesis has been described more recently by Samdani and Storm,24 who reported that a convex hemiepiphysiodesis is best performed on children 5 years of age with a short curve less than 40 degrees and scoliosis involving five or fewer segments using a combined anterior and posterior approach. In this combined anterior and posterior hemiepiphysiodesis procedure, the convex halves of the discs are removed anteriorly, followed by a posterior arthrodesis and casting.25 Although a lower thoracic level hemivertebra occasionally may be indicated for excision, doing so thoracoscopically is challenging. Many patients undergoing treatment for congenital scoliosis are younger than 5 years of age and require anterior fusion over very few levels of the spine and may not benefit from a thoracoscopic approach. The use of VATS in these patients must be decided on a case-by-case basis.
Contraindications
As suggested earlier, the small size of the patient is a relative contraindication to thoracoscopy. Lung deflation is more difficult in these cases because standard size double-lumen and bronchial blocking endotracheal tubes are too large. Another contraindication is the presence of a markedly reduced working distance between the chest wall and the spine. In severe cases of scoliosis, the reduced distance limits the field of vision (the endoscope is too close to the spine to obtain any perspective) and the maneuverability of the working instruments. A minimum distance of 2 to 3 cm of working space between the rib cage and the spinal column is required to provide adequate visualization. If the distance is less than 2 cm, thoracoscopy is not advised. Body weight of less than 30 kg is a relative contraindication because the relative benefits of thoracoscopy seem to be reduced in small patients.26
Visualization of the surgical field is mandatory in all surgical approaches, and it may be compromised in thoracoscopic surgery by incomplete deflation of the lung or pleural adhesions that prevent collapse away from the chest wall and spine. Pleural adhesions (Fig. 25–2) can be anticipated in patients with a history of prior ipsilateral thoracic surgery or significant pulmonary infection, both of which should discourage the surgeon from using the thoracoscopic approach.
Surgical Technique
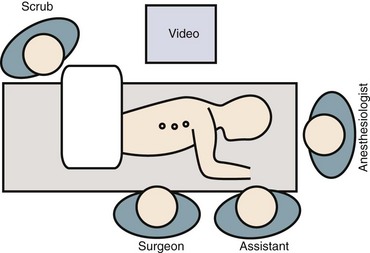
Patient positioning has traditionally been in the lateral decubitus position (Fig. 25–3). Some studies have suggested that in select cases prone positioning may be possible, avoiding the need to reposition the patient for the posterior procedure or even allowing simultaneous anterior release and posterior instrumentation.27–29 Although the ability to convert to an open approach may be restricted, it has been shown that the prone position does not adversely affect postoperative pulmonary function.30 This approach necessitates a more posterior portal placement, however, and may limit the anterior extent of spinal exposure and disc excision.
The role of the anesthesiologist is crucial in the success and safety of thoracoscopic surgery.31 Spinal cord monitoring is advised using somatosensory and transcranial motor evoked potentials. Complete ipsilateral lung deflation is essential to prevent lung parenchymal injury from passing instruments and to allow visualization of the spine. Double-lumen endotracheal tubes are preferred in patients large enough (>45 kg) to accept these devices. In children (<45 kg), selective intubation of a single lung is often required as an alternative. A small balloon advanced into the main stem bronchus blocks ventilation to the lung on the operative side. In nearly all patients with normal preoperative pulmonary function, single-lung ventilation can be tolerated. The surgeon and anesthesiologist should be aware of the increased risk of developing postoperative mucous plugs as a result of single-lung ventilation.
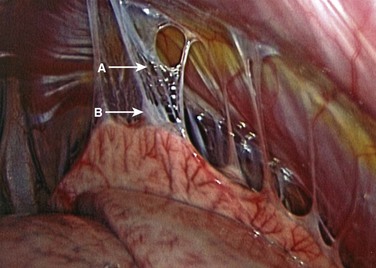
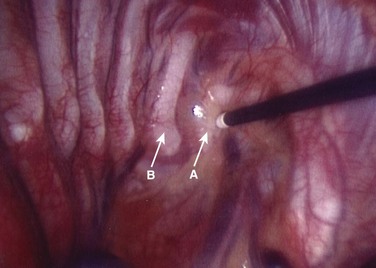
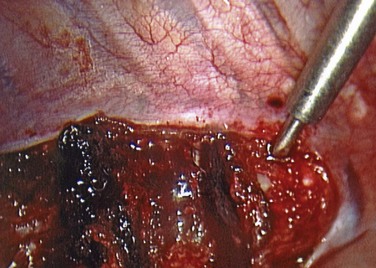
After lung deflation, portals are established through the chest wall (Fig. 25–4). The orientation of these portals may vary depending on the pathology, although in most cases of deformity release and fusion they are best placed in a linear relationship along the anterior axillary line. Owing to the site of diaphragm insertion, the inferior portals require a slightly more posterior placement to maintain an intrathoracic position. Initial exposure of the spine often requires gentle retraction of the lung, at least until it becomes completely atelectatic (Fig. 25–5). The vasculature including the azygos vein and subclavian artery is identified before the introduction of surgical instruments to prevent inadvertent injury (Fig. 25–6). The vertebral levels are confirmed by identifying the first rib partially hidden beneath the subclavian artery and counting down distally (Fig. 25–7). Division of the pleura overlying the spine may be performed either longitudinally, over the length of the spine to be fused, or transversely, at each disc space.

FIGURE 25–4 Proper placement of portals is necessary for multilevel discectomies with the patient in lateral position.
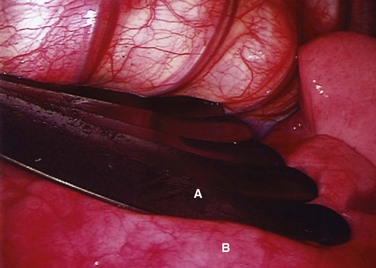
FIGURE 25–5 Intraoperative endoscopic view of fan retractor (A) placed on lung (B) to aid in complete deflation.
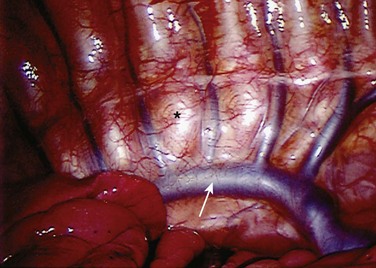
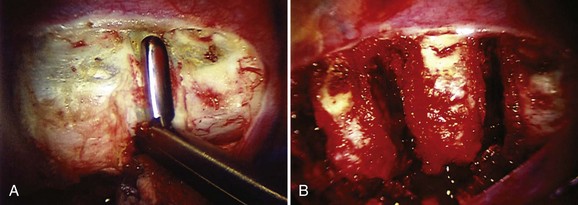
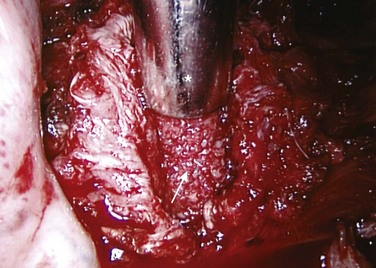
Treatment of the segmental vessels (Fig. 25–8) may be similarly individualized with either division or preservation, depending on the needs of the case or preference of the surgeon. In most cases, the authors prefer a longitudinal pleural exposure with division of the segmental vessels using Harmonic laparoscopic coagulating sheers (Ethicon Endo-Surgery, Cincinnati, Ohio). Division of the segmental vessels allows greater anterior spinal exposure for more complete annular release. Blunt dissection of the pleura to the contralateral side of the spine is performed exposing approximately 270 degrees of the disc perimeter. After division of the pleura, any remaining areolar tissue is divided, and packing sponges are used to create a space between the anterior spine and the pleura.
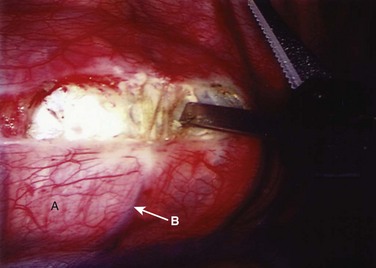
FIGURE 25–8 After division of pleura (A), Harmonic laparoscopic coagulating sheers are applied to segmental vessel (B).
Possible levels that can be accessed thoracoscopically are T2-L1. Exposure of the T12-L1 disc and L1 vertebral body requires division of a small segment of the diaphragm insertion, which can be accomplished by extending the pleural incision distally into the diaphragm. The proximal thoracic spine in the right chest is often covered by the confluence of the segmental veins, which may appear daunting at the T3 and T4 levels. With slow cautious use of the ultrasonic devices, these vessels can be sealed and divided safely, however, exposing the upper thoracic spine. Disc excision techniques are similar to techniques used in open surgery. An annulotomy is performed with the electrocautery or Harmonic scalpel. A rongeur is an excellent tool for most of the disc excision. Specially designed endoscopic rongeurs are available in extended lengths with various angles (straight, up, right, left) to reach the depths of each disc space (Fig. 25–9).
When the discectomy is complete, either allogeneic cancellous or autogenous (rib or iliac crest) bone graft is placed into the disc space with an endoscopic tubular plunger (Fig. 25–10). The method and type of bone grafting also seems to be important to the success of arthrodesis. This may be crucial only in selected cases; however, all patients are at some risk for pseudarthrosis after posterior instrumentation and fusion procedures. In a study of 112 patients treated with an anterior release followed by posterior instrumentation, Newton and colleagues4 compared the grade of arthrodesis between patients who received autogenous versus allogeneic bone graft. These authors found the disc space was fused in 88% of the autograft group compared with 72% of the allograft group at 2-year follow-up.4 When autograft is not available, either allograft bone or demineralized bone matrix may be used because they have been shown to result in similar fusion rates.4,32 Although autogenous bone graft is optimal for patients at greatest risk for pseudarthrosis, the risk-to-benefit ratio must be analyzed on a case-by-case basis.
Outcomes
The thoracoscopic approach has several advantages compared with an open thoracotomy approach. These advantages, including pulmonary function, reduced recovery period, less pain, and improved cosmesis, are realized, however, only if the efficacy of the spinal procedure equals that of open surgery. Experimental animal and clinical studies suggest comparable efficacy in experienced hands. Several studies have been conducted to evaluate the learning curve necessary to be experienced in this technique.12,33,34 Newton and colleagues12 found that there was a slight decrease in operative time throughout the course of the first 65 patients treated at their institution and concluded that thoracoscopy had a steep but not prohibitive learning curve. In a more recent study by Son-Hing and colleagues,33 the learning curve was found to be short with appropriate training and resulted in an excision of a greater amount of disc tissue and a decrease in operative time, while providing similar curve correction to an open thoracotomy.
Several experimental studies have been done to analyze the extent of disc excision possible with thoracoscopic techniques. Biomechanical evaluations of the instability resulting from discectomy were equivalent between open and endoscopic approaches in various animal models.34–37 The extent of endplate bony exposure has also been shown to be similar with the two approaches experimentally.34,38 In a histomorphometric study of 32 pigs (160 discs), Zhang and colleagues34 found that there was not a learning curve associated with the amount of disc material excised (>50% in 94% of the discs in this study), but a learning curve was present for the thoroughness of the endplate excision in thoracoscopic discectomy. Because the purpose of the endplate excision is to remove the growth potential and expose a cancellous bony surface for fusion, added care must be taken during the endplate removal step of this procedure.
The clinical results of thoracoscopic anterior release and fusion in patients with spinal deformity have been generally favorable although poorly controlled.39 Although there was an increase in the use of this method during the 1990s, it has since declined in popularity with the widespread adoption of posterior segmental pedicle screw instrumentation. Despite more recent studies that have called the necessity of this technique into question for large, stiff curves, there may be a subset of patients who benefit from this procedure.5,6,40 Studies by Luhmann and colleagues5 and by Suk and colleagues6 looked at curves with an average of 80 degrees main thoracic Cobb angle and an average flexibility of 45%. Both groups of investigators concluded that a similar coronal correction can be achieved with posterior segmental pedicle screw fixation as with a combined anterior and posterior procedure. These studies did not examine deformity on the extreme end of curves greater than 100 degrees with less than 25% flexibility, however. In a more recent study of 21 patients with an average preoperative Cobb angle greater than 110.5 degrees and flexibility of 13%, Yamin and colleagues40 found a staged procedure to provide a safe and effective treatment with a mean correction rate of 65.2%. These authors recommended that patients with a Cobb angle greater than 80 degrees and flexibility less than 20% should be treated with a staged anterior release and posterior pedicle screw instrumentation with the addition of halo-pelvic traction to correct the deformity.40
Evaluation of VATS perioperative and postoperative data reveals an increase in operative time compared with open thoracotomy but a decrease in blood loss, chest tube drainage, and pulmonary morbidity and an increase in patient satisfaction. The time to perform thoracoscopic surgery has ranged from 90 minutes to 4 hours with a decrease in operative time as experience is gained. The total operative time per disc level excised averages 20 to 40 minutes. Studies on the learning curve for VATS have been performed by Newton and colleagues12 as well as by Son-Hing and colleagues.33 VATS operative time decreased 26% to 30% and operative time per disc decreased 15% to 24% between the first seven and last seven patients in both series.12,33 As surgeons gain experience with improved thoracoscopic techniques, instrumentation, and training, the operative time has been shown to be less for VATS compared with an open approach.33
The reported blood loss and chest tube drainage have been comparable to open procedures with blood loss generally averaging less than 300 mL.12,33,41,42 In cases of excessive blood loss, conversion to open thoracotomy may be required. The incidence of major complications for either VATS or open thoracotomy is less than 1%.12,33,43 The most common complications associated with VATS are pulmonary, such as atelectasis, pleural effusions, pneumothorax, and excessive chest tube drainage.12,33,42,43 Faro and colleagues44 compared pulmonary function after an open versus a thoracoscopic anterior procedure and found that pulmonary function recovered more quickly with VATS, and this difference was maintained after 2 years of follow-up. As with all anterior spinal surgery, these risks exist and must be minimized. Although the instrumentation, techniques, and support for VATS continue to improve, this approach is technically demanding, and proper training is essential to the success of this procedure.
Thoracoscopic Anterior Scoliosis Instrumentation
Over the past decade, spinal surgical techniques have evolved to minimize soft tissue disruption in an effort to improve functional outcomes and accelerate the rehabilitation process. Since the first thoracoscopic instrumentation and fusion of idiopathic scoliosis in 1996, this technique has been developed based on the principles of open thoracic anterior scoliosis instrumentation. The rationale for anterior spinal fusion compared with posterior fusion for idiopathic scoliosis is the potential to preserve vertebral motion segments, to reduce intraoperative blood loss, to reduce muscle disruption, to achieve greater coronal plane correction and increased thoracic kyphosis restoration, and to improve spontaneous correction of the unfused lumbar and cephalad thoracic curves.4,43,45,46 The goal of anterior thoracoscopic instrumentation in the surgical management of idiopathic scoliosis is to perform a safe, reproducible, and effective procedure that results in improvement in spinal alignment and balance in all planes and axial rotation comparable to, or better than, that obtained with an open procedure.2 Although early studies described high rates of pseudarthrosis, implant failure, and loss of fixation, more recent studies have shown comparable results between thoracoscopic anterior instrumentation and fusion and posterior and open anterior techniques.45–49
Indications
Anterior instrumentation and fusion has been used in various spinal conditions and studied extensively in adolescent patients with idiopathic scoliosis. Although the curve patterns amenable to this approach continue to be elucidated, in general, structural adolescent or adult (with normal bone density) thoracic curves may be considered for selective thoracic anterior instrumentation (Fig. 25–11).50 A review of the pediatric spine literature reveals that anterior instrumentation and fusion has been primarily evaluated in moderate thoracic, thoracolumbar, and lumbar curves between 40 and 70 degrees with flexibility greater than 50%.51,52 Anterior instrumentation and fusion is kyphogenic and may be a valuable tool in patients with significant hypokyphosis. Eight or fewer vertebrae may be included in the arthrodesis, and the fusion may extend from T4 to L1.52
Contraindications
Contraindications for thoracoscopic instrumentation are similar to the contraindications for anterior release and fusion. The procedure is contraindicated if the patient has severe or acute respiratory insufficiency. Previous severe respiratory infections and ipsilateral thoracic surgery are also relative contraindications because pleural adhesions complicate thoracoscopic visualization. In addition to general thoracoscopic contraindications, large stiff curves and hyperkyphosis measuring 40 degrees or greater (from T5 to T12) are relative contraindications. Patients with osteopenia or poor bone stock are also not good candidates for this technique. Additionally, in patients in whom a single rod construct is being considered, a seizure disorder and a suspicion that the patient would be noncompliant with postoperative orders are also contraindications because of concerns of implant loosening. Given that thoracoscopic visualization extends from T4 to L1, thoracoscopic instrumentation should not be attempted for double major curves or curves that extend beyond these end points.52–54
Surgical Technique
Either a single-rod or dual-rod system may be used depending on the size of the vertebrae, the concerns for rod breakage, and the experience of the surgeon. Single-rod systems offer a greater ease of implantation; however, biomechanical studies have suggested that dual-rod constructs are more stable than single-rod constructs.55,56 Also, in their series of 60 patients with 2 to 5 years of follow-up, Hurford and colleagues57 reported that dual-rod constructs provided a similar curve correction rate to single-rod constructs but with a significantly improved SRS questionnaire outcome and no reported cases of pseudarthrosis. The length of the cephalad-most screw is templated on the preoperative posteroanterior radiograph (usually 27.5 or 30 mm), and this screw is placed first. The screws are optimally positioned 1 to 2 mm anterior to the rib head in the middle of the vertebral body with approximately 10 degrees of subtle anterior angulation.
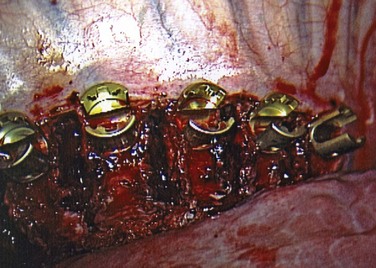
The path of each screw is approximately parallel to the endplates (Fig. 25–12). Penetration of the far cortex greatly enhances the fixation and is mandatory at the proximal levels to reduce the risk of screw pullout. Care must be taken to avoid placing successive screws increasingly anterior in the vertebral body because this may negatively affect fixation and restoration of kyphosis and potentially place the aorta at increased risk of iatrogenic injury.58 Although there have been concerns regarding vertebral body screws placed thoracoscopically, Qiu and colleagues59 showed that there is no statistically significant difference in the accuracy of vertebral body screw placement in thoracoscopic versus mini-open thoracotomy approaches. When the proper screw length has been selected, a pilot hole is created with an awl and the tapped advanced under fluoroscopic guidance. A bicortical screw (5.5 to 7.5 mm in diameter) is placed into the vertebra. Screw position is confirmed again fluoroscopically, and the remaining screws are placed in a similar fashion.
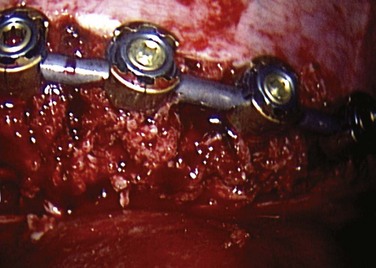
After all the screws are placed (Fig. 25–13), the length of the rod is determined with a calibrated rod-measuring device. A rod of appropriate length is cut and contoured to the desired level of postoperative scoliosis and kyphosis. The rod is sequentially loaded into the screws and secured with locking nuts (Fig. 25–14). Fully seating the rod into the screws may be accomplished with either a rod pusher or the use of a reduction device. Several styles of compressors have been developed to compress between levels. This compression is an important component to the anterior correction of scoliosis but must be performed in a cautious manner, particularly at the upper levels where screw fixation may be tenuous.
Bone grafting is crucial to the success of this procedure. As mentioned previously in the section on thoracoscopic release, autogenous graft provides the optimal base for a solid fusion and is recommended.4,32 At the lower levels of the thoracic spine, distal to T11, structural anterior support may be required to aid in maintaining proper sagittal alignment.
Outcomes
In 1998, Picetti and colleagues60 were the first to report a clinical case of multilevel anterior scoliosis correction performed thoracoscopically. Early studies reported high rates of pseudarthrosis, implant failure, and loss of fixation.54,61,62 Since that time, the initial learning curve has been overcome,12,63 and comparable results have been reported between thoracoscopic anterior procedures and open anterior and posterior techniques.* Early and midterm results of thoracoscopic anterior instrumentation have been favorable. In a series of 45 patients with adolescent idiopathic scoliosis, Norton and colleagues2 found an overall curve correction 87.3% (51.6 degrees preoperatively to 6.6 degrees postoperatively), an average hospital stay of 2.9 days, and a return to school after 2 to 4 weeks. Newton and colleagues46 reported that radiographic findings, pulmonary function, and clinical measures remained stable between 2 and 5 years’ follow-up in their series of 23 patients treated with VATS instrumentation. In the Norton series,2 three patients had transient pulmonary complications, and in the Newton series,46 three patients had an implant failure that necessitated a surgical revision in one patient. Both series found no significant loss of correction after an average 4.6-year (Norton series) and 5.3-year (Newton series) follow-up.
Although operative time is longer in VATS cases compared with an open anterior or posterior procedure, operative time does decrease with experience. Lonner and colleagues63 reported a significant reduction in operative time from 6 hours in their first 28 cases to 4 hours 30 minutes in the last 15 cases. Studies by Lonner and colleagues13 and Wong and colleagues65 compared VATS with standard posterior procedures and found a decrease in intraoperative blood loss and a reduced hospital stay in the Lonner study and a reduced intensive care unit stay in the Wong study. When pulmonary function is evaluated, VATS has been shown to result in better recovery of pulmonary function compared with open techniques and similar return to function as in standard posterior techniques.13,44 Similarly, in regard to shoulder girdle function, Ritzman and colleagues66 found that VATS was comparable to posterior instrumentation, which were both better than open anterior surgery. Further comparison by Lonner and colleagues13 of the impact of VATS versus posterior spinal instrumentation and fusion on the patients’ quality of life using the SRS-22 instrument revealed that patients who underwent VATS scored higher in the self-image, mental health, and total domains despite similar curve corrections.
In all of the aforementioned studies, VATS was compared with a mix of posterior spinal fusion instrumentation anchors (wires, hooks, screws). Although many of these past studies focused on the outcomes of thoracoscopic instrumentation compared with open anterior or posterior hybrid instrumentation, more recently VATS has been directly compared with posterior segmental pedicle screw constructs. Lonner and colleagues45 performed a matched-pair analysis of 34 patients with adolescent idiopathic scoliosis with single thoracic curves of approximately 50 degrees. The patients had equivalent radiographic results, patient-based clinical outcomes, and complication rates with the exception that the posterior thoracic pedicle screw group had a slightly better major curve correction (63.8% vs. 57.3%). These authors concluded that VATS offers the advantages of reduced blood loss (371 mL vs. 1018 mL), fewer total levels fused (5.9 vs. 8.9), and preservation of nearly one caudal fusion level. The disadvantages included increased operative time (325 minutes vs. 246 minutes) and slightly less improved pulmonary function.
Treatment of Other Thoracic Spine Conditions
Tumor
Approximately 70% of all spinal tumors are located on the thoracic spine with 85% of these tumors on the ventral surface of the vertebral body and epidural space.67,68 Current treatment options include surgery, radiation, and chemotherapy. Patchell and colleagues69 found that significantly more patients treated with direct surgical decompression followed by radiation were able to walk after treatment than patients who were treated with radiotherapy alone. An anterior approach to this decompression offers the advantages of direct cord decompression with tumor resection, immediate interbody reconstruction and stabilization, and a lower wound complication rate than posterior incisions.68 Although traditional open transthoracic vertebrectomies have been used, they are associated with significant morbidity.70
Advances in endoscopic expandable interbody cages have made the excision and stabilization of these tumors possible through a thoracoscopic approach.68,71 Thoracoscopy has been shown to have substantial clinical benefits in patients with tumors, including reduced postoperative pain, shorter intensive care unit and hospital stays, faster return to activity, and reduced complication rates.68,71,72 These benefits are especially important because these patients have a mean survival of only 8 to 12 months.68 Although data on large case series are pending, thoracoscopy is a promising treatment modality for these patients.
Trauma
In principle, the treatment of thoracic spinal injuries (e.g., burst fractures, multiple compression fractures, dislocations) involves correction of malposition, decompression of the spinal canal, and stabilization. To achieve these goals, anterior column reconstruction or decompression or both are often required. Because of the morbidity associated with conventional open thoracotomy, a thoracoscopic procedure has been developed to provide a less invasive solution. Although a thoracoscopic approach requires a longer operative time, it has been shown to have good clinical results in trauma patients with burst fractures. Beisse and colleagues73 reported 371 patients who underwent thoracoscopic anterior reconstruction and fusion with instrumentation for spinal trauma. The average duration of surgery was 6 hours, and this gradually decreased to an average of 2.5 to 3 hours as the surgeon gained experience and skill. An 85% to 90% fusion rate was observed with the thoracoscopic technique, which Beisse and colleagues73 believed was comparable with the standard approach.
Thoracic Disc Herniation
Surgical treatment is required for herniated discs that cause neurologic symptoms that are refractory to conservative care. Myelopathy is the strongest indication for surgery to prevent permanent damage to the spinal cord. Approaches for treatment of thoracic disc herniation include the posterior transpedicular approach, posterolateral approach, and anterior transthoracic approach (thoracotomy and thoracoscopy). The endoscope provides a magnified view of the entire ventral surface of the spine and spinal cord. Discectomy and rib head excision provide access to the spinal canal, which must be clearly visualized to ensure the decompression is complete. Anand and Regan74 reported the outcome of 100 such cases performed endoscopically. These authors reported that thoracoscopic surgery for thoracic disc herniation has an overall long-term satisfaction rate of 84% and a clinical success rate of 70% for refractory thoracic disc disease.
Future of Thoracoscopic Spinal Surgery
Pitfalls
1 Lonner BS, Kondrachov D, Siddiqi F, et al. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis: Surgical technique. J Bone Joint Surg Am. 2007;89(Suppl 2 Pt 1):142-156.
2 Reddi V, Clarke DVJr, Arlet V. Anterior thoracoscopic instrumentation in adolescent idiopathic scoliosis: A systematic review. Spine. 2008;33:1986-1994.
3 Newton PO, Upasani VV, Lhamby J, et al. Surgical treatment of main thoracic scoliosis with thoracoscopic anterior instrumentation: A five-year follow-up study. J Bone Joint Surg Am. 2008;90:2077-2089.
4 Arlet V. Anterior thoracoscopic spine release in deformity surgery: A meta-analysis and review. Eur Spine J. 2000;9(Suppl 1):S17-S23.
1 Mack MJ, Acuff TE, Ryan WH. Implantable cardioverter defibrillator: The role of thoracoscopy. Ann Thorac Surg. 1993;56:739-740.
2 Norton RP, Patel D, Kurd MF, et al. The use of thoracoscopy in the management of adolescent idiopathic scoliosis. Spine. 2007;32:2777-2785.
3 Lenke LG. Anterior endoscopic discectomy and fusion for adolescent idiopathic scoliosis. Spine. 2003;28(15 Suppl):S36-S43.
4 Newton PO, White KK, Faro F, et al. The success of thoracoscopic anterior fusion in a consecutive series of 112 pediatric spinal deformity cases. Spine. 2005;30:392-398.
5 Luhmann SJ, Lenke LG, Kim YJ, et al. Thoracic adolescent idiopathic scoliosis curves between 70 degrees and 100 degrees: Is anterior release necessary? Spine. 2005;30:2061-2067.
6 Suk SI, Kim JH, Cho KJ, et al. Is anterior release necessary in severe scoliosis treated by posterior segmental pedicle screw fixation? Eur Spine J. 2007;16:1359-1365.
7 Dubousset J, Herring JA, Shufflebarger H. The crankshaft phenomenon. J Pediatr Orthop. 1989;9:541-550.
8 Catan H, Buluc L, Anik Y, et al. Pedicle morphology of the thoracic spine in preadolescent idiopathic scoliosis: Magnetic resonance supported analysis. Eur Spine J. 2007;16:1203-1208.
9 Sarlak AY, Atmaca H, Buluc L, et al. Juvenile idiopathic scoliosis treated with posterior arthrodesis and segmental pedicle screw instrumentation before the age of 9 years: A 5-year follow-up. Scoliosis. 2009;4:1.
10 Lapinksy AS, Richards BS. Preventing the crankshaft phenomenon by combining anterior fusion with posterior instrumentation: Does it work? Spine. 1995;20:1392-1398.
11 Gonzalez Barrios I, Fuentes Caparros S, Avila Jurado MM. Anterior thoracoscopic epiphysiodesis in the treatment of a crankshaft phenomenon. Eur Spine J. 1995;4:343-346.
12 Newton PO, Shea KG, Granlund KF. Defining the pediatric spinal thoracoscopy learning curve: Sixty-five consecutive cases. Spine. 2000;25:1028-1035.
13 Lonner BS, Kondrachov D, Siddiqi F, et al. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2006;88:1022-1034.
14 Lowe TG, Line BG. Evidence based medicine: Analysis of Scheuermann kyphosis. Spine. 2007;32(19 Suppl):S115-S119.
15 Lee SS, Lenke LG, Kuklo TR, et al. Comparison of Scheuermann kyphosis correction by posterior-only thoracic pedicle screw fixation versus combined anterior/posterior fusion. Spine. 2006;31:2316-2321.
16 Bradford DS, Ahmed KB, Moe JH, et al. The surgical management of patients with Scheuermann’s disease: A review of twenty-four cases managed by combined anterior and posterior spine fusion. J Bone Joint Surg Am. 1980;62:705-712.
17 Papagelopoulos PJ, Klassen RA, Peterson HA, et al. Surgical treatment of Scheuermann’s disease with segmental compression instrumentation. Clin Orthop Relat Res. 2001;386:139-149.
18 Sturm PF, Dobson JC, Armstrong GW. The surgical management of Scheuermann’s disease. Spine. 1993;18:685-691.
19 Lowe TG, Kasten MD. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease: A review of 32 patients. Spine. 1994;19:1680-1685.
20 Herrera-Soto JA, Parikh SN, Al-Sayyad MJ, et al. Experience with combined video-assisted thoracoscopic surgery (VATS) anterior spinal release and posterior spinal fusion in Scheuermann’s kyphosis. Spine. 2005;30:2176-2181.
21 Vitale MG, Matsumoto H, Bye MR, et al. A retrospective cohort study of pulmonary function, radiographic measures, and quality of life in children with congenital scoliosis: An evaluation of patient outcomes after early spinal fusion. Spine. 2008;33:1242-1249.
22 Kesling KL, Lonstein JE, Denis F, et al. The crankshaft phenomenon after posterior spinal arthrodesis for congenital scoliosis: A review of 54 patients. Spine. 2003;28:267-271.
23 Roaf R. The treatment of progressive scoliosis by unilateral growth-arrest. J Bone Joint Surg Br. 1963;45:637-651.
24 Samdani AF, Storm PB. Other causes of pediatric deformity. Neurosurg Clin N Am. 2007;18:317-323.
25 Winter RB. The surgical treatment of congenital spine deformity: General principles and helpful hints. Iowa Orthop J. 1995;15:79-94.
26 Early SD, Newton PO, White KK, et al. The feasibility of anterior thoracoscopic spine surgery in children under 30 kilograms. Spine. 2002;27:2368-2373.
27 King AG, Mills TE, Loe WAJr, et al. Video-assisted thoracoscopic surgery in the prone position. Spine. 2000;25:2403-2406.
28 Lieberman IH, Salo PT, Orr RD, et al. Prone position endoscopic transthoracic release with simultaneous posterior instrumentation for spinal deformity: A description of the technique. Spine. 2000;25:2251-2257.
29 Sucato DJ, Elerson E. A comparison between the prone and lateral position for performing a thoracoscopic anterior release and fusion for pediatric spinal deformity. Spine. 2003;28:2176-2180.
30 Sucato DJ, Erken YH, Davis S, et al. Prone thoracoscopic release does not adversely affect pulmonary function when added to a posterior spinal fusion for severe spine deformity. Spine. 2009;34:771-778.
31 Lischke V, Westphal K, Behne M, et al. Thoracoscopic microsurgical technique for vertebral surgery—anesthetic considerations. Acta Anaesthesiol Scand. 1998;42:1199-1204.
32 Weinzapfel B, Son-Hing JP, Armstrong DG, et al. Fusion rates after thoracoscopic release and bone graft substitutes in idiopathic scoliosis. Spine. 2008;33:1079-1083.
33 Son-Hing JP, Blakemore LC, Poe-Kochert C, et al. Video-assisted thoracoscopic surgery in idiopathic scoliosis: Evaluation of the learning curve. Spine. 2007;32:703-707.
34 Zhang H, Sucato DJ, Hedequist DJ, et al. Histomorphometric assessment of thoracoscopically assisted anterior release in a porcine model: Safety and completeness of disc discectomy with surgeon learning curve. Spine. 2007;32:188-192.
35 Connolly PJ, Ordway NR, Sacks T, et al. Video-assisted thoracic diskectomy and anterior release: A biomechanical analysis of an endoscopic technique. Orthopedics. 1999;22:923-926.
36 Wall EJ, Bylski-Austrow DI, Shelton FS, et al. Endoscopic discectomy increases thoracic spine flexibility as effectively as open discectomy: A mechanical study in a porcine model. Spine. 1998;23:9-15. discussion 15-16
37 Newton PO, Cardelia JM, Farnsworth CL, et al. A biomechanical comparison of open and thoracoscopic anterior spinal release in a goat model. Spine. 1998;23:530-535. discussion 536
38 Huntington CF, Murrell WD, Betz RR, et al. Comparison of thoracoscopic and open thoracic discectomy in a live ovine model for anterior spinal fusion. Spine. 1998;23:1699-1702.
39 Arlet V. Anterior thoracoscopic spine release in deformity surgery: A meta-analysis and review. Eur Spine J. 2000;9(Suppl 1):S17-S23.
40 Yamin S, Li L, Xing W, et al. Staged surgical treatment for severe and rigid scoliosis. J Orthop Surg. 2008;3:26.
41 Krasna MJ, Jiao X, Eslami A, et al. Thoracoscopic approach for spine deformities. J Am Coll Surg. 2003;197:777-779.
42 Levin R, Matusz D, Hasharoni A, et al. Mini-open thoracoscopically assisted thoracotomy versus video-assisted thoracoscopic surgery for anterior release in thoracic scoliosis and kyphosis: A comparison of operative and radiographic results. Spine J. 2005;5:632-638.
43 Upasani VV, Newton PO. Anterior and thoracoscopic scoliosis surgery for idiopathic scoliosis. Orthop Clin North Am. 2007;38:531-540. vi
44 Faro FD, Marks MC, Newton PO, et al. Perioperative changes in pulmonary function after anterior scoliosis instrumentation: Thoracoscopic versus open approaches. Spine. 2005;30:1058-1063.
45 Lonner BS, Auerbach JD, Estreicher M, et al. Video-assisted thoracoscopic spinal fusion compared with posterior spinal fusion with thoracic pedicle screws for thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2009;91:398-408.
46 Newton PO, Upasani VV, Lhamby J, et al. Surgical treatment of main thoracic scoliosis with thoracoscopic anterior instrumentation: A five-year follow-up study. J Bone Joint Surg Am. 2008;90:2077-2089.
47 Newton PO, Parent S, Marks M, et al. Prospective evaluation of 50 consecutive scoliosis patients surgically treated with thoracoscopic anterior instrumentation. Spine. 2005;30(17 Suppl):S100-S109.
48 Lowe TG, Alongi PR, Smith DA, et al. Anterior single rod instrumentation for thoracolumbar adolescent idiopathic scoliosis with and without the use of structural interbody support. Spine. 2003;28:2232-2241. discussion 2241-2242
49 Grewal H, Betz RR, D’Andrea LP, et al. A prospective comparison of thoracoscopic vs open anterior instrumentation and spinal fusion for idiopathic thoracic scoliosis in children. J Pediatr Surg. 2005;40:153-156. discussion 156-157
50 Deviren V, Metz LN. Anterior instrumented arthrodesis for adult idiopathic scoliosis. Neurosurg Clin N Am. 2007;18:273-280.
51 Reddi V, Clarke DVJr, Arlet V. Anterior thoracoscopic instrumentation in adolescent idiopathic scoliosis: A systematic review. Spine. 2008;33:1986-1994.
52 Lonner BS, Kondrachov D, Siddiqi F, et al. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis: Surgical technique. J Bone Joint Surg Am. 2007;89(Suppl 2 Pt 1):142-156.
53 Picetti GD, Pang D. Thoracoscopic techniques for the treatment of scoliosis. Childs Nerv Syst. 2004;20(11-12):802-810.
54 Picetti GD3rd, Pang D, Bueff HU. Thoracoscopic techniques for the treatment of scoliosis: early results in procedure development. Neurosurgery. 2002;51:978-984. discussion 984
55 Fricka KB, Mahar AT, Newton PO. Biomechanical analysis of anterior scoliosis instrumentation: Differences between single and dual rod systems with and without interbody structural support. Spine. 2002;27:702-706.
56 Lowe TG, Enguidanos ST, Smith DA, et al. Single-rod versus dual-rod anterior instrumentation for idiopathic scoliosis: A biomechanical study. Spine. 2005;30:311-317.
57 Hurford RKJr, Lenke LG, Lee SS, et al. Prospective radiographic and clinical outcomes of dual-rod instrumented anterior spinal fusion in adolescent idiopathic scoliosis: Comparison with single-rod constructs. Spine. 2006;31:2322-2328.
58 Sucato DJ, Kassab F, Dempsey M. Analysis of screw placement relative to the aorta and spinal canal following anterior instrumentation for thoracic idiopathic scoliosis. Spine. 2004;29:554-559. discussion 559
59 Qiu Y, Wang WJ, Wang B, et al. Accuracy of thoracic vertebral screw insertion in adolescent idiopathic scoliosis: A comparison between thoracoscopic and mini-open thoracotomy approaches. Spine. 2008;33:2637-2642.
60 Picetti G3rd, Blackman RG, O’Neal K, et al. Anterior endoscopic correction and fusion of scoliosis. Orthopedics. 1998;21:1285-1287.
61 Picetti GD3rd, Ertl JP, Bueff HU. Endoscopic instrumentation, correction, and fusion of idiopathic scoliosis. Spine J. 2001;1:190-197.
62 Sucato DJ. Thoracoscopic anterior instrumentation and fusion for idiopathic scoliosis. J Am Acad Orthop Surg. 2003;11:221-227.
63 Lonner BS, Scharf C, Antonacci D, et al. The learning curve associated with thoracoscopic spinal instrumentation. Spine. 2005;30:2835-2840.
64 Lonner BS, Auerbach JD, Estreicher M, et al. Video-assisted anterior thoracoscopic spinal fusion versus posterior spinal fusion: A comparative study utilizing the SRS-22 outcome instrument. Spine. 2009;34:193-198.
65 Wong HK, Hee HT, Yu Z, et al. Results of thoracoscopic instrumented fusion versus conventional posterior instrumented fusion in adolescent idiopathic scoliosis undergoing selective thoracic fusion. Spine. 2004;29:2031-2038. discussion 2039
66 Ritzman TF, Upasani VV, Pawelek JB, et al. Return of shoulder girdle function after anterior versus posterior adolescent idiopathic scoliosis surgery. Spine. 2008;33:2228-2235.
67 Byrne TN, Borges LF, Loeffler JS. Metastatic epidural spinal cord compression: Update on management. Semin Oncol. 2006;33:307-311.
68 Kan P, Schmidt MH. Minimally invasive thoracoscopic approach for anterior decompression and stabilization of metastatic spine disease. Neurosurg Focus. 2008;25:E8.
69 Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643-648.
70 Walsh GL, Gokaslan ZL, McCutcheon IE, et al. Anterior approaches to the thoracic spine in patients with cancer: Indications and results. Ann Thorac Surg. 1997;64:1611-1618.
71 Dickman CA, Rosenthal D, Karahalios DG, et al. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:279-293.
72 Kaiser LR, Shrager JB. Video-assisted thoracic surgery: The current state of the art. AJR Am J Roentgenol. 1995;165:1111-1117.
73 Beisse R, Muckley T, Schmidt MH, et al. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128-136.
74 Anand N, Regan JJ. Video-assisted thoracoscopic surgery for thoracic disc disease: Classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine. 2002;27:871-879.