CHAPTER 34 THORACIC WALL INJURIES: RIBS, STERNAL SCAPULAR FRACTURES, HEMOTHORACES, AND PNEUMOTHORACES
Although many chest injuries are potentially lethal, early man sustained and survived blunt and penetrating chest trauma. Examinations of Neanderthal skeletons have shown evidence of healed penetrating and blunt rib fractures. The Edwin Smith Papyrus, written circa 3000 BC, gave explicit instructions for the management of chest injuries including soft tissue and bony injuries.1 In fact, 8 of the 43 cases discussed concerned chest injuries, suggesting that even at that time, chest injuries accounted for 20%–25% of all trauma.
Trauma to the chest wall and the underlying lung parenchyma either in isolation or as part of multisystem trauma remains exceedingly common and are a frequent source of trauma mortality and morbidity. Hemothoraces and pneumothoraces, while technically not injuries to the thoracic wall, occur commonly in conjunction with such injuries will be considered here as well. Flail chest and its accompanying pulmonary contusion are mentioned only briefly here and are more completely discussed on pages 269–277.
INCIDENCE
Thoracic injuries remain common and are directly attributable for 20%–25% of all trauma deaths; chest injuries are further associated with another 25% of trauma deaths. Chest injuries commonly accompany other injuries and contribute to organ failure in the multiply-injured patient. Rib fractures are among the most commonly encountered injuries. In a review of over 7000 patients seen in a Level I trauma center, 10% had rib fractures; of these, 94% had associated injuries, and 12% died. Half of patients with rib fractures required operation or intensive care unit (ICU) admission, one-third developed complications, and one-third ultimately required extended care in an outpatient facility.2
Pneumothorax is found in over 20% in patients arriving to a trauma center.3 Hemothoraces are encountered with similar frequency. The incidence of both hemothoraces and pneumothoraces is underestimated by plain films, as these injuries are much better visualized by computed tomography (CT) of the chest than the traditional supine anteroposterior chest radiograph (AP CXR).
MECHANISM
Rib fractures are normally the hallmark of significant blunt chest trauma, and increasing numbers of rib fractures are related to increasing morbidity and mortality. The presence of more than three rib fractures in adults is a marker for associated solid visceral trauma and mortality, and thus has been used as a marker for trauma center transfer.4 In hemodynamically stable patients, the presence of blunt chest trauma has also been shown to double the rate of intraabdominal injuries detected by abdominal CT.5 Rib fractures are less common in children due to the resilience of their bony chest wall. Thus, children may suffer major intrathoracic injury without rib fractures and the presence of any rib fracture in a child should be considered a marker for severe injury.6 The presence of acute rib fractures in a young child whose mechanism of injury is unclear or the finding of rib fractures of varying ages should also serve as an indicator for potential child abuse.7,8 Conversely, elderly patients with brittle bones will occasionally have little in the way of intrathoracic injury despite extensive rib fractures.9
DIAGNOSIS
Physical Examination
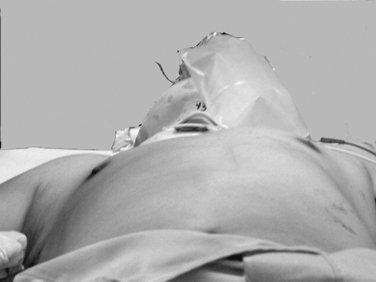
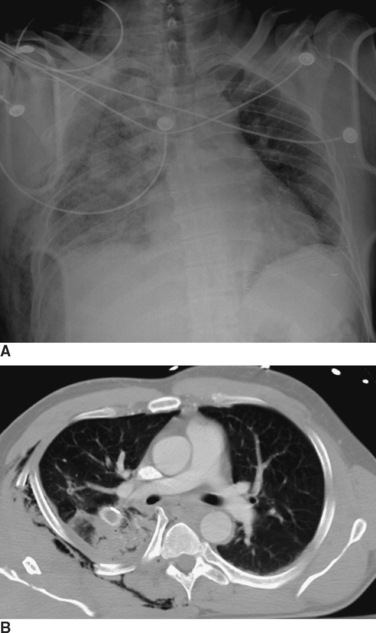
Expeditious inspection and palpation of the chest will provide much information regarding the patient’s injuries (Figure 1). Auscultation and percussion tend to be less reliable due to the high ambient noise of the trauma ED. Hypotension, tachycardia, pallor, or cyanosis suggests shock. In the presence of a known or suspected thoracic injury, shock must be assumed to be from an intrathoracic source. Inspection of the chest itself should include assessment of use of the accessory muscles suggestive of airway obstruction, the symmetry of the chest wall, number and location of wounds, presence of open chest wounds, subcutaneous emphysema, and the presence of “flail” segments. While tracheal deviation in the neck is also frequently cited as a sign of tension pneumothorax, in reality it is rarely if ever seen, even in patients with gross mediastinal deviation on chest x-ray. Palpation can reveal mobile segments of chest wall and further allows appreciation of the symmetry of chest wall motion and of crepitance. Auscultation in trauma has a high specificity but very poor sensitivity, so focus should be placed only on the presence and symmetry of air entry. Heart sounds, such as the muffled heartbeat in Beck’s triad or the “mediastinal crunch” of Hamman’s sign, are also difficult to obtain clearly in the trauma bay. The absence or asymmetry of breath sounds, however, is very suggestive of significant pathology; in the unstable patient, this is an indication for intervention on clinical grounds and a contraindication for imaging studies. Thoracic percussion, even more than auscultation, is difficult to interpret in the trauma setting and is rarely useful.
Radiographic Studies
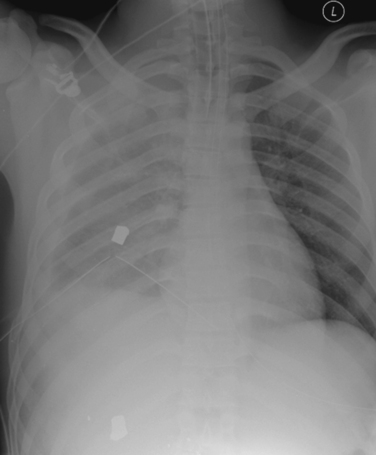
Radiographic imaging is extremely useful in the diagnosis of a hemothorax or pneumothorax. Indeed, in a hemodynamically stable patient, the diagnosis is often made on the portable AP CXR obtained for the secondary survey. In the supine position the AP CXR will reveal hemothorax only when at least 200–300 ml of blood is present in the pleural space, and is suggested by an overall opacification or haziness compared to the contralateral hemithorax as the fluid will layer posteriorly (Figure 2). False “negative”–appearing CXR may occur in the setting of bilateral hemothoraces (no difference between the two sides) or when there is a simultaneous anterior pneumothorax (decreasing the relative density more similar to the other side). In the patient with penetrating chest trauma, the CXR is best taken with the patient upright which increases the sensitivity for both hemothoraces and pneumothoraces. Chest ultrasound may help to identify the presence of pleural fluid, but its sensitivity and specificity for this purpose have not been well-defined.
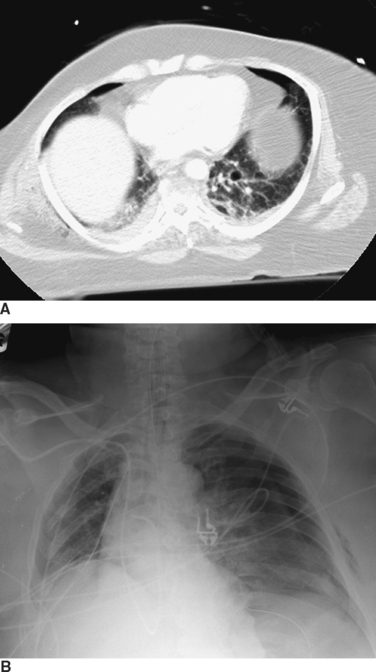
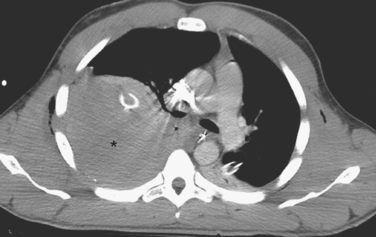
The appropriate management of the patient with “CT-only” pneumothorax is a matter of some controversy (Figure 3). The reported incidence of these pneumothoraces in blunt trauma patients is 2%–8%.10,11 The available literature suggests that 20% of these patients will require tube thoracostomy. The decision to place a chest tube, however, should be dictated by the patient’s overall status. Patients who are multiply injured, are in hemorrhagic shock, or have sustained a traumatic brain injury will not tolerate progression of even a small pneumothorax (see Figure 3). These patients would benefit from tube thoracostomy. In patients where the clinical picture appears stable, observation can be undertaken, with serial radiographs taken at 6 and 24 hours after diagnosis.
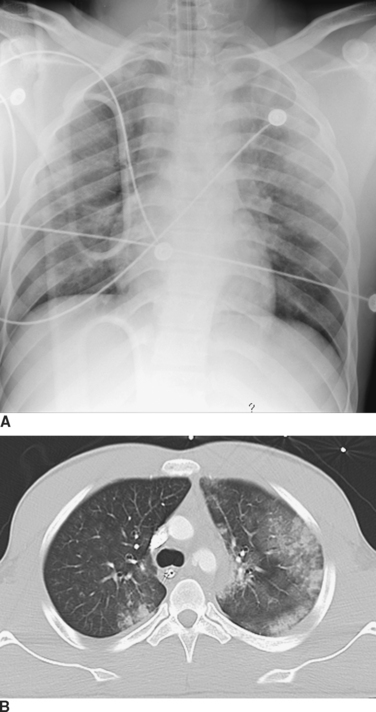
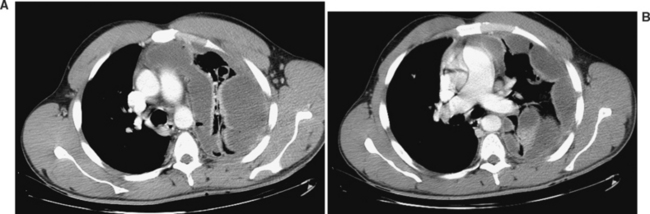
Computed tomography (CT) of the chest has become increasingly accepted in the early management of trauma. CT can reveal injuries not seen on initial CXR in about two-thirds of major trauma patients12 and can lead to therapeutic changes in 5%–30% of cases13,14 (Figure 4). In addition, CT scanning may reveal additional findings that are only suggested by an abnormal CXR (Figure 5). There are a number of specific situations in which chest CT contributes significantly to trauma management. CT of the thoracic spine is the “gold-standard” imaging modality for assessing vertebral body as well as posterior element fractures, and is also helpful in imaging the spine at the cervicothoracic junction.12,14,15 In patients with the nonspecific finding of a widened mediastinum on CXR, use of CT can help to limit the use of aortography to assess aortic injuries.16–18 As CT technology improves and as further studies become available, the role of CT in chest trauma will become better-defined.
Ultrasonography has become important in the assessment of intra-abdominal hemorrhage and pericardial fluid collections in the trauma patient. Recent reports also suggest that it might be useful to assess the pleural spaces for pneumothoraces and hemothoraces.19–21 The pleural space is interrogated by placing the ultrasound probe between the ribs and looking for the characteristic signs of pneumothorax.19,22
AAST-OIS GRADING
The American Association for the Surgery of Trauma-Organ Injury Scale (AAST-OIS) grading scales for chest wall and lung injury developed by Moore et al.23 are listed in Tables 1 and 2.
| Grade | Injury Type | Description of Injury |
|---|---|---|
| I | Contusion | Any size |
| Laceration | Skin and subcutaneous | |
| Fracture | <3 ribs, closed; nondisplaced clavicle, closed | |
| II | Laceration | Skin, subcutaneous, and muscle |
| Fracture | >3 adjacent ribs, closed | |
| Open or displaced clavicle | ||
| Nondisplaced sternum, closed | ||
| Scapular body, open or closed | ||
| III | Laceration | Full thickness, including pleural penetration |
| Fracture | Open or displaced sternum | |
| Flail sternum | ||
| Unilateral flail sternum (<3 ribs) | ||
| IV | Laceration | Avulsion of chest wall tissues with underlying rib fractures |
| Fracture | Unilateral flail chest (<3 ribs) | |
| V | Fracture | Bilateral flail chest (>3 ribs on both sides) |
Adapted from Moore EE, Cogbill TH, Malangoni MA, et al: Organ injury scaling. Surg Clin North Am 75(2):293–303, 1995.
| Grade | Injury Type | Description of Injury |
|---|---|---|
| I | Contusion | Unilateral, <1 lobe |
| II | Contusion | Unlateral, 1 lobe |
| Laceration | Simple pneumothorax | |
| Contusion | Unilateral, L1 lobe | |
| III | Laceration | Persistent (>72 hours) air leak from distal airway |
| Hematoma | Nonexpanding intraparenchymal | |
| Laceration | Major (segmental or lobar) air leak | |
| IV | Hematoma | Expanding intraparenchymal |
| Vascular | Primary branch intrapulmonary vessel disruption | |
| V | Vascular | Hilar vessel disruption |
| VI | Vascular | Total uncontained transaction of pulmonary hilum |
Adapted from Moore EE, Cogbill TH, Malangoni MA, et al: Organ injury scaling. Surg Clin North Am 75(2):293–303, 1995.
MANAGEMENT OF SPECIFIC INJURIES TO CHEST WALL
Chest Wall Defects
In large chest wall defects, the occlusive dressing is of no value. These patients must be endotracheally intubated with positive pressure ventilation. Operative management then focuses on control of hemorrhage from the chest wall and from any associated injuries. Chest wall hemorrhage in these cases may be life-threatening and may mandate emergent thoracotomy. “Damage control” with packing may be the optimal initial management of these patients. The chest defect can be temporarily closed with skin or prosthetic material. The definitive closure of these defects, which may require tissue-transfer procedures, is best deferred until the patient is fully resuscitated, physiologically sound, and able to tolerate a lengthy operation.
Rib Fractures and Flail Chest
Rib fractures can result in chest wall pain, chest wall hemorrhage, and less commonly, chest wall instability. A flail chest is said to exist when three or more adjacent ribs are segmentally fractured. The resultant chest wall instability combined with underlying pulmonary contusion is responsible for the respiratory insufficiency that develops in patients after this injury. The pathophysiology and treatment of flail chest injuries are covered in greater detail on pages 269–277.
Failure to provide sufficient analgesia in the setting of chest wall injuries has been shown to result in hypoventilation, retained secretions, increased atelectasis and lobar collapse, pneumonia, and respiratory failure.24 Persistence of pain may perpetuate the stress response to injury and may have a negative impact on post-traumatic immune function.25 Poor pain control will hamper the ability of mechanically ventilated patients to be weaned and extubated. The pharmacological approach to pain management in chest injury has consisted of the use of narcotics with or without regional anesthesia. The addition of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ketorolac have also proven effective in relieving pain from mild to moderate injuries.
Narcotics
Narcotics are the mainstay for pain control in the majority of trauma patients. Narcotic preparations can be given orally, intramuscularly, or intravenously. But whereas intramuscular administration has been the standard for decades, we strongly advise against this route of administration because it is both painful to the patient and results in unreliable absorption of the narcotic. With the exception of meperidine (Demerol) however, which narcotic chosen is far less important than ensuring adequate dosing for pain control.26 The amount of narcotic required for pain control may vary greatly depending upon previous or current narcotic use, age, and other factors that alter the patient’s perception of pain.
The use of pain scales in combination with performance on incentive spirometry can be very effective in determining the adequacy of pain control.27 Our current approach is to begin most awake patients on a patient-controlled analgesia (PCA) regimen using morphine or fentanyl. Later, patients are transitioned to long-acting narcotic preparations such as OxyContin or MS-contin with the use of oxycodone for breakthrough pain. The addition of an NSAID may reduce inflammation and augment the effect of the oral narcotics. It is also important to avoid narcotic induced constipation. This can result in severe abdominal pain, nausea, and vomiting in patients requiring long-term opioids.
Regional Anesthesia
Rib blocks can be accomplished using a mixture of 1%–2% lidocaine with 0.25% bupivacaine to decrease the pain of rib fractures. This simple technique involves the administration of 2–3 ml of the anesthetic mixture to the inferior rib margin several centimeters posterior to the site of the rib fracture. Optimal analgesia requires blocking at least one rib above and one rib below the fracture. The limitations of the technique are that the pain control is short-lived and that it can only be used for patients with mid or lower rib fractures. Last, in our experience the results vary widely and the risks of pneumothorax in patients without a chest tube are real.28
Epidural analgesia/anesthesia is the delivery mode that has been shown to have the greatest impact on pulmonary mechanics following moderate to severe chest trauma, especially in patients with bilateral injuries.29 There are various combinations of local anesthetics and narcotics which have been employed. In our institution, the most common combination is fentanyl with bupivacaine. Combination therapy can be advantageous because it works via two different mechanisms of action. Opioids modulate presynaptic and postsynaptic nerve transmission in dorsal horn neurons by effects on their specific receptors. Local anesthetics work by blocking sodium channels. Thus, the analgesic effects of the two classes of drug are synergistic. Moreover, the potential for side effects is lessened in combination therapy because the doses of each drug used can be lower than the amount used if either were administered alone.
The data supporting the use of epidural analgesia is strong. Mackersie et al.30 demonstrated that the use of epidural fentanyl was associated with significant improvements in pulmonary mechanics with 85% of the patients requiring no additional parenteral narcotics. Intravenous narcotics were associated with increases in PaCO2 and decreases in PaO2 that were not observed in the epidural group. In a randomized prospective trial, Bulger et al.31 reported epidural analgesia was associated with half the number of ventilator days compared to patients in the opioid group. They also point out that the technique was limited in trauma patients due to the presence of exclusion criteria in over 50%. Despite these limitations the use of epidural analgesia, when feasible, is of considerable benefit to patients following severe chest injury, as it has been strongly associated with a decrease in the rate of nosocomial pneumonia and a shorter duration of mechanical ventilation.
Pneumothorax and Hemothorax
Rib fractures can also cause injury to the underlying lung parenchyma, with resultant hemothoraces or pneumothoraces, although these can also occur without evidence of rib fractures. The severity of pneumothorax ranges from clinically insignificant to life-threatening. Pneumothoraces can be categorized as simple, open, and tension. A simple pneumothorax is a collection of air arising from leakage of air from an injured lung into the pleural space. An open pneumothorax arises when air enters the thoracic cavity from an open chest wound, with equalization of pressure between the thorax and the atmosphere. A tension pneumothorax occurs from a “ball-and-valve” effect in which air enters but does not exit the thoracic cavity, causing intrapleural pressure to exceed atmospheric pressure. The resultant pressure can cause the heart and great vessels to shift away from the side of injury and result in progressive circulatory compromise (see Figure 3).
The treatment goal for hemothoraces, as for pneumothoraces, is evacuation of the pleural space and re-expansion of the lung. This is accomplished initially through tube thoracostomy. Apposition of the visceral and parietal pleurae generally provides definitive control of hemorrhage, and thoracotomy is required in less than 10%–15% of all chest trauma patients. Patients may occasionally present to the trauma bay several hours after injury with a large amount of initial drainage from the chest tube. This usually represents the slow accumulation of blood rather than rapid active bleeding, particularly in the patient who remains hemodynamically stable. If the hemothorax cannot be adequately drained by one chest tube, another one may need to be placed. CXR should be obtained immediately after tube thoracostomy to demonstrate successful drainage of the pleural space and lung re-expansion. If residual blood in the pleural space is still suspected and the patient is stable, CT of the chest may help to resolve the issue. If an acute hemothorax cannot be adequately drained because the chest tube is clotted, operative drainage may be necessary as continued bleeding cannot be assessed (Figure 6). Operative treatment can be accomplished either through thoracotomy or, in selected stable patients, through video-assisted thoracoscopic surgery (VATS).
Tube Thoracostomy: Technique and Management
Once the decision is made to place a chest tube, the patient should be positioned to allow easy access to the midaxillary line in the fifth or sixth intercostal space. This location is preferred because (1) it is usually safely above the diaphragm and (2) the chest wall musculature is thinnest in this area, thus facilitating the procedure. The chest should be cleansed with an antiseptic solution and anesthetized with 10 ml of 1% lidocaine in all layers of the chest wall down to the pleura. There should be no fear of entering the pleural space when inserting a tube thoracostomy! To provide longer analgesia and increase patient comfort following insertion we suggest mixing the lidocaine with an equal volume of 0.25% bupivacaine. Conscious sedation may be administered if the patient’s hemodynamic and respiratory status permits. A 2-cm skin incision is made over the rib immediately below the interspace selected for tube insertion. Sharp dissection proceeds directly to the rib, and the pleural space is entered at its superior margin, care being taken to avoid the intercostal neurovascular bundle at the inferior border of the adjacent superior rib. While a clamp is commonly used to “pop” through the pleural space, we advocate continuing with the scalpel as it is both easier and less painful to the patient. Once the pleural space is entered, digital exploration will confirm entry into the thorax rather than the lung or abdominal cavity. The presence or absence of adhesions should be noted as well. Digital exploration is particularly important in the patient who may have a diaphragmatic rupture or who has a history of thoracic surgery or pulmonary infection. If no adhesions, diaphragmatic injury, or pulmonary pathology is encountered, the chest tube can be safely placed. The blind placement of chest tubes with trocars is illadvised.
Chest tubes, like most medical devices, have markings to aid in placement. Unfortunately, in our experience these are too often ignored by residents placing the tube in the heat of battle, which results in tubes that may be poorly positioned. One of the most common mistakes is to insert the tube too far in to ensure that it will not “fall out.” The tube will often bend at the last hole, which will prevent adequate drainage (see Figure 2). To prevent this type of malposition, the mark at the skin should be evaluated. If the tube is place in the midaxillary line at the fifth interspace, the mark at the skin level in most patients should be between 10 and 12. A tube placed deeper is likely to be kinked. In addition, once the tube is positioned it should be rotated 360 degrees before securing it in place. A tube that cannot be rotated freely is presumably kinked or otherwise malpositioned.
Chest radiographs should be obtained daily to confirm resolution of the hemopneumothorax. It is not our practice to use prophylactic antibiotics with tube thoracostomies, although opinion in the literature is divided on this issue.32,33 The chest tube can subsequently be removed when there is no air leak and when there is less than 100–150 ml of drainage over 24 hours. A prospective study has shown that a 6–8 hour trial of water seal decreases the incidence of recurrent pneumothorax when compared to chest tube removal with no water seal.34
Sternal Fractures
Sternal fractures are relatively uncommon and occur most often following blunt trauma. The usual mechanism of injury involves an unrestrained driver who strikes the sternum against the steering column of an automobile in a deceleration crash. A fall or other direct impact to the chest may also cause a sternal fracture. Most sternal fractures occur in the upper or mid-portion of the bone.35 As with scapular fractures, the presence of a sternal fracture should be regarded as a marker of potential severe multiple trauma, including rib fractures (40%), long-bone fractures (25%), and head injuries (18%).
The fracture itself often needs no treatment acutely, and more than 95% of patients are treated nonoperatively. While a baseline EKG should be obtained, particularly in patients over 40 years of age, the need for continuous cardiac monitoring is determined by the associated injuries and the patient’s cardiac status rather than by the presence of the sternal fracture itself. Most patients will be found to have a transient right ventricular dysfunction. The patient with an isolated sternal fracture and otherwise normal ED evaluation may be discharged.36–38 Similar to rib fractures, the management of sternal fractures is symptomatic and consists of analgesic administration and the avoidance of motion. Occasionally, patients who have grossly displaced fractures or who have persistent pain due to nonunion may require operative reduction and internal fixation (ORIF). Sternal repair can be accomplished though various techniques utilizing wires or small plates.
Scapular Fractures
Fractures of the scapula are uncommon, occurring with an incidence of 1%–3% in blunt trauma.39 Because of its location and structure, the scapula requires considerable direct force to fracture. As a result, associated injuries are common (80%–98%), and a patient with a scapular fracture should be considered to have sustained a severe chest trauma. Careful examination for thoracic, neurologic, vascular, and abdominal injuries, as well as other orthopedic injuries, should be undertaken. Findings on physical examination include local pain or tenderness, swelling, and crepitus. Although most scapular fractures will be seen on initial CXR, they may be obscured or overlooked. Many scapular fractures may have hitherto gone undetected, with increasing detection of nondisplaced fractures with the increasing use of modern CT technology40 (see Figure 5). CT of the shoulder may be necessary to evaluate the fracture and to look for extension into the shoulder joint.
Scapulothoracic Dissociation
Scapulothoracic dissociation results from severe blunt trauma with traction to the shoulder girdle causing the scapula, humerus, musculature of the shoulder, and neurovascular structures to be pulled away from the body. While this injury is relatively rare, it can be dramatic, involving not only the structures of the shoulder girdle, but also thoracic, craniocerebral, and spinal injuries. Damschen et al.,41 in a review of 58 cases, reported complete brachial plexus injury in 81% of patients and partial injury in 13%; subclavian or axillary artery disruption was found in 88%. Because these patients almost universally have severe neurologic deficits in the affected limb, the likelihood of a good functional outcome is low. This should be borne in mind before extensive reconstruction to salvage an essentially defunctionalized limb is undertaken.
COMPLICATIONS OF HEMOPNEUMOTHORAX
Empyema
Empyema has been reported to have an incidence of 0%–18%.33,42–44 At least some of the culture-negative “empyemas” in blunt trauma patients are probably sterile pleural collections that, being rich in inflammatory mediators, produce systemic effects clinically indistinguishable from true empyema collections.45,46 The true empyema rate in major trauma patients is probably closer to 5%, although, with the increasing survival of severely injured patients, the incidence of this complication may be increasing. Risk factors for the development of empyema include an inadequately drained pleural collection, mechanism of injury, location and number of chest tubes, presence of pulmonary contusion, and pneumonia.
The clinical presentation of empyema includes unexplained fever, elevated white count, and respiratory failure. Most patients in whom an empyema is suspected are critically ill. AP CXR in this group may be of little or no help, and liberal use of CT scanning can more easily identify pathology that is not evident on plain films (Figure 7). Early use of CT can prevent the diagnostic delay that may result in the need for more extensive surgical procedures.
Persistent Air Leaks and Bronchopleural Fistula
Air leaks are not uncommon after chest trauma, and typically resolve with tube thoracostomy. A large air leak, however, requires workup for a tracheobronchial injury. If such injury is excluded, then complete lung expansion is the cornerstone of therapy. Air leaks may persist for days or even weeks in patients requiring mechanical ventilation with high positive end-expiratory pressure (PEEP); only with weaning from the ventilator will air leaks begin to seal. Tracheostomy can, by decreasing the anatomic dead space, help to lower the peak airway pressures and thus at least slow the air leak. VATS can also help in those hemodynamically stable patients with prolonged air leaks.
Complications of Bony Injuries
Complications of bony injuries to the chest are most commonly related to pain. Sternal fractures in isolation or with only mild associated injury appear to have a relatively low incidence of post-injury sequelae; in one study, only 3% of patients at a 6-week follow-up had complaints requiring further intervention, including surgical intervention for a displaced fracture.48 Twenty-one percent of patients in that same study reported pain at 6 weeks but required no further intervention.
Scapular fractures are rarely associated with nonunion, but can cause chronic pain. Pain and disability are particularly associated with the degree of glenoid angulation and displacement if the fracture is treated nonoperatively.49
Clavicular fractures, by contrast, do carry an incidence of nonunion, though that number is somewhat in dispute. Early studies of nonoperative treatment of clavicle fractures revealed a nonunion rate as low as 0.1%.50 The nonunion rate has in more recent studies been reported to be much higher, with a rate of 15% for conservative treatment of displaced middle-third fractures.51 Even with operative fixation, some nonunion occurs. An analysis of 8 studies and 14 case series of midshaft clavicle fractures revealed an overall nonunion rate of 4.2%. Nonoperative treatment resulted in a nonunion rate of 5.9%. When results for displaced fractures were identified, a nonunion rate of 4.8% was noted with IM fixation, while displaced fractures treated nonoperatively had a nonunion rate of 15.1%.52
Scapulothoracic dissociation, as would be expected, has significant long-term sequelae. A retrospective cohort study with a mean follow-up over 12 years revealed that patients with a complete brachial plexus avulsion had significant physical and mental impairments as measured by the standard SF-36 questionnaire.53 Primary amputation of the affected limb, though a difficult decision, should be considered to avoid a defunctionalized limb where complete brachial avulsion has occurred.
1 Breasted J. The Edwin Smith Surgical Papyrus. Chicago: University of Chicago Press, 1980.
2 Ziegler DW, Agarwal NN. The morbidity and mortality of rib fractures. J Trauma. 1994;37:975.
3 Di Bartolomeo S, Sanson G, Nardi G, et al. A population-based study on pneumothorax in severely traumatized patients. J Trauma. 2001;51:677.
4 Lee RB, Bass SM, Morris JAJr, et al. Three or more rib fractures as an indicator for transfer to a Level I trauma center: a population-based study. J Trauma. 1990;30:689.
5 Livingston D, Lavery R, Passannante M, et al. Admission or observation is not necessary after a negative abdominal computed tomographic scan in patients with suspected blunt abdominal trauma: results of a prospective, multi-institutional trial. J Trauma. 1998;44:273.
6 Garcia VF, Gotschall CS, Eichelberger MR, et al. Rib fractures in children: a marker of severe trauma. J Trauma. 1990;30:695.
7 Bulloch B, Schubert CJ, Brophy PD, et al. Cause and clinical characteristics of rib fractures in infants. Pediatrics. 2000;105:E48.
8 Cadzow SP, Armstrong KL. Rib fractures in infants: red alert! The clinical features, investigations and child protection outcomes. J Paediatr Child Health. 2000;36:322.
9 Cameron P, Dziukas L, Hadj A, et al. Rib fractures in major trauma. Aust N Z J Surg. 1996;66:530.
10 Neff M, Monk JJ, Peters K, et al. Detection of occult pneumothoraces on abdominal computed tomographic scans in trauma patients. J Trauma. 2000;49:281.
11 Rhea J, Novelline R, Lawrason J, et al. The frequency and significance of thoracic injuries detected on abdominal CT scans of multiple trauma patients. J Trauma. 1989;29:502.
12 Gray L, Vandemark R, Hays M. Thoracic and lumbar spine trauma. Neu-roimaging Clin North Am. 2001;11:421.
13 Guerrero-Lopez F, Vazquez-Mata G, Alcazar-Romero PP, et al. Evaluation of the utility of computed tomography in the initial assessment of the critical care patient with chest trauma. Crit Care Med. 2000;28:1370.
14 Grieser T, Buhne KH, Hauser H, et al. Significance of findings of chest x-rays and thoracic CT routinely performed at the emergency unit: 102 patients with multiple trauma. A prospective study. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2001;173:44.
15 Jelly LM, Evans DR, Easty MJ, et al. Radiography versus spiral CT in the evaluation of cervicothoracic junction injuries in polytrauma patients who have undergone intubation. Radiographics. 2000;20:S251.
16 Parker MS, Matheson TL, Rao AV, et al. Making the transition: the role of helical CT in the evaluation of potentially acute thoracic aortic injuries. Am J Roentgenol. 2001;176:1267.
17 Downing SW, Sperling JS, Mirvis SE, et al. Experience with spiral computed tomography as the sole diagnostic method for traumatic aortic rupture. Ann Thorac Surg. 2001;72:495.
18 Pate JW, Gavant ML, Weiman DS, et al. Traumatic rupture of the aortic isthmus: program of selective management. World J Surg. 1999;23:59.
19 Dulchavsky SA, Schwarz KL, Kirkpatrick AW, et al. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma. 2001;50:201.
20 Rozycki GS, Feliciano DV, Davis TP. Ultrasound as used in thoracoabdominal trauma. Surg Clin North Am. 1998;78:295.
21 Rozycki GS, Ballard RB, Feliciano DV, et al. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228:557.
22 Lichtenstein D, Meziere G, Biderman P, et al. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440.
23 Moore EE, Cogbill TH, Malangoni MA, et al. Organ injury scaling. Surg Clin North Am. 1995;75(2):293-303.
24 Desai P. Pain management and pulmonary dysfunction. Crit Care Clin. 1999;15:151.
25 Yokoyama M, Itano Y, Mizobuchi S, et al. The effects of epidural block on the distribution of lymphocyte subsets and natural-killer cell activity in patients with and without pain. Anesth Analg. 2001;92:463.
26 Clark RF, Wei EM, Anderson PO. Meperidine: therapeutic use and toxicity. J Emerg Med. 1995;13:797.
27 Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633.
28 Shanti CM, Carlin AM, Tyburski JG. Incidence of pneumothorax from intercostal nerve block for analgesia in rib fractures. J Trauma. 2001;51:536.
29 Mackersie RC, Karagianes TG, Hoyt DB, et al. Prospective evaluation of epidural and intravenous administration of fentanyl for pain control and restoration of ventilatory function following multiple rib fractures. J Trauma. 1991;31:443.
30 Mackersie RC, Shackford SR, Hoyt DB, et al. Continuous epidural fentanyl analgesia: ventilatory function improvement with routine use in treatment of blunt chest injury. J Trauma. 1987;27:1207.
31 Bulger EM, Edwards T, Klotz P, Jurkovich GJ. Epidural analgesia improves outcome after multiple rib fractures. Surgery. 2004;136:426.
32 Wilson R, Nichols R. The EAST Practice Management Guidelines for Prophylactic Antibiotic Use in Tube Thoracostomy for Traumatic Hemopneumothorax: a commentary. J Trauma. 2000;48:758.
33 Luchette F, Barrie P, Oswanski M, et al. Practice Management Guidelines for Prophylactic Antibiotic Use in Tube Thoracostomy for Traumatic Hemopneumothorax: the EAST’ Practice Management Guidelines Work Group. Eastern Association for Trauma. J Trauma. 2000;48:753.
34 Martino K, Merrit S, Boyakye K, et al. Prospective randomized trial of thoracostomy removal algorithms. J Trauma. 1999;46:369.
35 Buckman R, Trooskin S, Flancbaum L, et al. The significance of stable patients with sternal fractures. Surg Gynecol Obstet. 1987;164:261.
36 Hills M, Delprado A, Deane S. Sternal fractures: associated injuries and management. J Trauma. 1993;35:55.
37 Jackson M, Walker W. Isolated sternal fracture: a benign injury? Injury. 1992;23:535.
38 Chiu W, D’Amelio L, Hammond J. Sternal fractures in blunt chest trauma: a practical algorithm for management. Am J Emerg Med. 1997;15:252.
39 Thompson D, Flynn T, Miller P, et al. The significance of scapular fractures. J Trauma. 1985;25:974.
40 Haapamaki W, Kiuru MJ, Koskinen SK. Multidetector CT in shoulder fractures. Emerg Radiol. 2004;11(2):89-94.
41 Damschen D, Cogbill T, Siegel M. Scapulothoracic dissociation caused by blunt trauma. J Trauma. 1997;42:537.
42 Aguilar M, Battistella F, Owings J, et al. Posttraumatic empyema. Risk factor analysis. Arch Surg. 1997;132:647.
43 Eddy AC, Luna GK, Copass M. Empyema thoracis in patients undergoing emergent closed tube thoracostomy for thoracic trauma. Am J Surg. 1989;157:494.
44 Mandal A, Thadepalli H, Mandal A, et al. Posttraumatic empyema thoracis: a 24-year experience at a major trauma center. J Trauma. 1997;44:764.
45 Watkins J, Spain D, Richardson J, et al. Empyema and restrictive pleural processes after blunt trauma: an under-recognized cause of respiratory failure. Am Surg. 2000;66:210.
46 Adams JM, Hauser CJ, Livingston DH, et al. The immunomodulatory effects of damage control abdominal packing on local and systemic neutrophil activity. J Trauma. 2001;50:792.
47 Carrillo E, Schmachl D, Gable V, et al. Thoracoscopy in the management of posttraumatic persistent pneumothorax. J Am Coll Surg. 1998;186:636.
48 Velissaris T, Tang AT M, Patel A, et al. Traumatic sternal fracture: outcome following admission to a thoracic surgical unit. Injury. 2003;34:924-927.
49 Romero J, Schai P, Imhoff AB. Scapular neck fracture—the influence of permanent malalignment of the glenoid neck on clinical outcome. Arch Orthop Trauma Surg. 2001;121(6):313-316.
50 Neer CS. Nonunion of the clavicle. JAMA. 1960;172:1006-1011.
51 Hill JM, McGuire MH, Crosby LA. Closed treatment of displaced middle-third clavicular fractures of the clavicle gives poor results. J Bone joint Surg Br. 1997;79-B(4):537-539.
52 Zlowodski M, Zelle BA, Cole PA, et al. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures. J Orthop Trauma. 2005;19:504-507.
53 Zelle BA, Pape H-C, Gerich TG, et al. Functional outcome following scapulothoracic dissociation. J Bone Joint Surg Am. 2004;86:2-8.