CHAPTER 41 THORACIC VASCULAR INJURY
One of the earliest reports of thoracic vascular injury was described by Vesalius in 1557 of a fatal, blunt traumatic rupture of the aorta in a man who was thrown from a horse.1 In 1946, DeBakey and Simeone collectively described the morbidity and the complexity of the few battlefield thoracic vascular injuries that occurred during World War II.2 It was not until 1959 that Passaro and Pace3 reported the first successful primary repair of traumatic aortic rupture performed by Klassen in 1958.
Before the development of modern trauma centers, most individuals with thoracic vascular trauma died before reaching the hospital. With the advent of rapid-response trauma systems, the incidence of thoracic vascular injury that survives to the hospital is increasing and the complexity of the injuries is becoming more challenging. Mattox et al.4 reported 1467 cardiovascular injuries in 1117 patients over a 5-year period from 1979 to 1983 in Houston, Texas.
INCIDENCE
Thoracic trauma is responsible for 50% of all trauma deaths nationally.5 The incidence of chest trauma is reported to be 12 per million per day in the United States by Beeson and Saegesser.6 In a large series of thoracic trauma patients, the aorta and great vessels were injured in 4% of cases.7 Furthermore, thoracic vascular injuries are often associated with other nonvascular injuries because of their anatomic location and mechanism of injury.
MECHANISM OF INJURY
A large number of thoracic great-vessel injuries are caused by penetrating or iatrogenic trauma.4 The mechanism of injury for penetrating thoracic vascular trauma is usually direct laceration or penetration of blood vessels. This type of injury can often present with external or internal hemorrhage, vascular thrombosis from intimal flap, or pseudoaneurysms. Because of the various types of missiles involved in penetrating vascular trauma, all thoracic vascular structures are at risk. External bleeding from skin tracts usually occurs with injuries to vessels at the thoracic inlet, whereas internal hemorrhage commonly occurs with aortic and caval injuries. Intrathoracic great-vessel injuries can present with internal bleeding into the mediastinum, pleural space, or pericardial sac. It is important to note that the presence of a normal palpable distal pulse does not rule out a proximal vascular injury. Penetrating vascular injuries can be completely contained by perivascular adventitia with blood flow preserved distally.
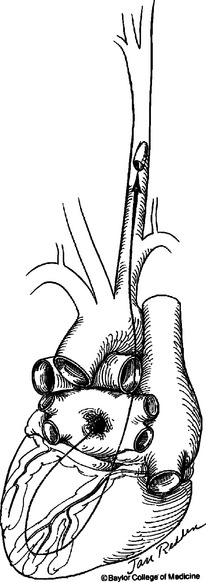
Because of the large diameter of thoracic vessels, bullets can directly enter vessels and migrate distally (Figure 1). The diagnosis of bullet embolism is often delayed because the course of the bullet may not be obvious. Bullet embolism for thoracic missiles usually lodges in the iliac and femoral vessels. The site of entry should be controlled for hemorrhage first, followed with attempts at removing the bullet emboli with endovascular intervention or separate arteriotomy.
DIAGNOSIS
Indicators of possible thoracic vascular injury are outlined in Table 1. The single most important screening tool for thoracic vascular trauma is the anteroposterior chest radiograph. There are numerous radiographic findings suggesting thoracic vascular injury as outlined in Table 2. One of the most reliable radiographic findings suggestive of blunt thoracic vascular injury is alteration of the aortic knob contour on chest radiograph.
| Mechanism of Injury |
Table 2 Radiological Findings Suggesting Great Vessel Injury
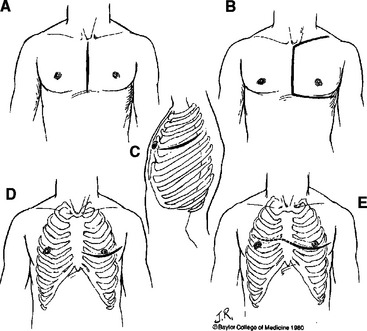
Arteriography remains the “gold standard” imaging study for evaluation of suspected thoracic vascular injury. It is important to note that preoperative angiography is never indicated in hemodynamically unstable patients with suspected thoracic vascular injury. In hemodynamically stable patients with suspected penetrating injury to the innominate, carotid, or subclavian arteries, preoperative arteriography is indicated to provide information on the type of incisions to make for proximal control of these branched aortic vessels (Figure 2). Furthermore, the proximity of missile trajectory to brachiocephalic vessels is an indication for arteriography in order to definitively rule out an injury. In blunt thoracic trauma, the need for arteriography is determined by mechanism of injury, physical examination, and screening chest radiograph. Fifty percent of patients with blunt thoracic vascular injury present without any external signs of injury.8 Seven percent of patients with blunt injury to the aorta and its branched vessels have a normal-appearing mediastinum on screening chest radiograph.9 Therefore, additional imaging studies are indicated in patients with either physical examination or chest radiograph suggesting thoracic vascular injury.
In patients with a significant mechanism of injury, but a benign physical examination and normal admission chest radiograph, it is reasonable to repeat a delayed chest radiograph to screen for radiographic evidence of thoracic vascular injury with a follow-up arteriography for a definitive diagnosis. Contrast-enhanced spiral computed tomography (CT) of the chest is being used more frequently for evaluating thoracic trauma. The sensitivity of chest CT scan for thoracic vascular injury ranges from 54% to 80%.10–16 The negative predictive value of a chest CT scan is close to 100% in evaluating thoracic vascular injury.10–16 Therefore, it is also reasonable to use chest CT scan as a screening tool in stable patients with significant mechanism of injury, normal-appearing admission chest radiograph, and benign physical examination to rule out an underlying thoracic vascular injury. However, any positive findings on CT scan should be followed by the “gold standard” arteriography for definitive diagnosis of thoracic vascular injury before operative intervention.
Transesophageal echocardiography (TEE) offers several potential advantages to arteriography in evaluating thoracic vascular injury. Avoidance of intravenous contrast, the concomitant information gained on cardiac function, and its portability are potential advantages compared with arteriography. However, published literature reports sensitivity and specificity of 85.7% and 92.0% for TEE compared with 89.0% and 100% for arteriography, respectively, in diagnosing aortic injury.17 TEE is also heavily technician and operator dependent. Furthermore, the ascending aorta, proximal aortic arch, and branch aortic vessel are extremely difficult to visualize with TEE. Therefore, its use in evaluating thoracic vascular injury is not routinely recommended.
AMERICAN ASSOCIATION FOR THE SURGERY OF TRAUMA, ORGAN INJURY SCALE
The American Association for the Surgery of Trauma (AAST) designates an organ injury scale for thoracic vascular injury from grade I to VI as outlined in Table 3, based on the size and severity of the injury.
Table 3 American Association for the Surgery of Trauma, Thoracic Vascular Injury Scale
| Gradea | Description of Injury |
|---|---|
| I | Intercostal vessels |
| Internal mammary vessels | |
| Bronchial vessels | |
| Esophageal vessels | |
| Hemiazygos vein | |
| Unnamed vessels | |
| II | Azygos vein |
| Internal jugular vein | |
| Subclavian vein | |
| Innominate vein | |
| III | Carotid artery |
| Innominate artery | |
| Subclavian artery | |
| IV | Descending thoracic aorta |
| Intrathoracic inferior vena cava | |
| Pulmonary artery/vein, primary intraparenchymal branch | |
| V | Ascending aorta |
| Aortic arch | |
| Superior vena cava | |
| Main pulmonary artery trunk | |
| Pulmonary vein, main trunk | |
| VI | Uncontained complete transaction of thoracic aorta or pulmonary hilar vessels |
a Increase one grade for multiple grade III injuries or >50% circumference involvement in grade IV injuries. Decrease one grade for grade IV injuries if <25% circumference involvement.
Modified from American Association for the Surgery of Trauma (AAST).
SURGICAL MANAGEMENT
Indications for urgent surgical intervention for thoracic vascular injuries are outlined in Table 4. The choice of incisions varies depending on the location of injury (see Figure 2). For unstable patients with presumed but undiagnosed thoracic vascular injury, the most appropriate incision is a left anterolateral thoracotomy in the supine position. This incision allows excellent exposure to the heart and aorta for resuscitative efforts. Furthermore, it can easily be converted to a clamshell incision by extending transternally to the contralateral side to provide exposure to the right lung hilum, ascending aorta, and right subclavian vessels. Median sternotomy is the incision of choice for innominate, right subclavian, right carotid, and proximal left carotid arterial injuries. Oblique cervical or transverse supraclavicular extensions can be added to provide further exposure. Left posterolateral thoracotomy in the lateral decubitus position provides the best exposure for known injuries to the descending thoracic aorta and left lung hilum.
Table 4 Indications for Operative Repair of Thoracic Great Vessel Injury
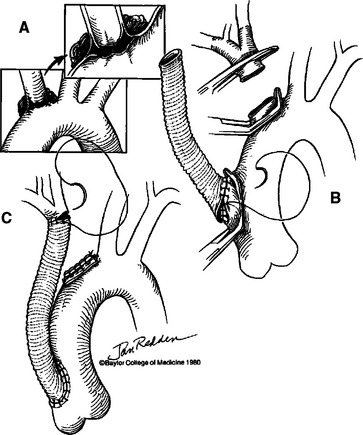
Innominate artery and proximal left common carotid artery injuries are best approached via median sternotomy incision with cervical extension if necessary. Division of the innominate vein provides excellent exposure to the transverse aortic arch for proximal control. In patients with small, partial tears of the distal innominate artery, primary repair with 4-0 polypropylene suture is often possible. In most cases, innominate artery injuries are best managed using the bypass exclusion technique18 (Figure 3). This approach allows management without the use of systemic heparinization, cardiopulmonary bypass, or hypothermic circulatory arrest. A 10-mm knitted tube graft is used to create a proximal ascending aorta to distal innominate artery bypass. After the bypass is completed, the injury at the origin of the aortic duct is oversewn. Injuries to the proximal left common carotid artery can also be managed in a similar fashion.
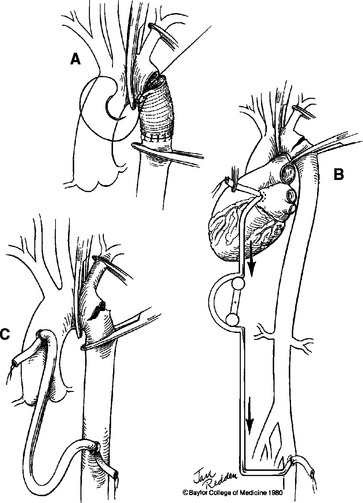
The principles of managing descending thoracic aortic injuries are proximal/distal control, addressing the injured segment, and reestablishing continuity of blood flow. Most blunt traumatic injuries in the descending thoracic aorta originate medially at the level of the ligamentum arteriosum. The most expeditious way of obtaining proximal control is to follow the left subclavian artery proximally to the aortic arch and place an umbilical tape around the aortic arch between the takeoff of the left common carotid artery and the left subclavian artery. An umbilical tape is also passed around the left subclavian artery. Care should be taken to try to avoid injury to the left recurrent laryngeal nerve as it courses posteriorly around the aortic arch near the ligamentum arteriosum. The next maneuver is to achieve vascular control distal to the anticipated injury. It is important to examine the entire length of the descending thoracic aorta in order to identify any additional tears, especially at the level of the diaphragm. There are several approaches to the basic vascular repair of the descending thoracic aorta. First is the simple clamp-and-sew technique without the use of shunts or left heart bypass. Second is the use of a passive shunt, which is less commonly used. Some surgeons advocate the use of active partial left heart bypass for repairing descending thoracic aortic injuries (Figure 4). All of these adjunct techniques should be familiar to surgeons managing this type of injury. The hematoma is entered after proximal and distal control is established. Intercostal vessels are not routinely oversewn. The extent of the injury is inspected from both external and internal aspects of the aorta. Simple partial lacerations of the aorta can be primarily closed with running sutures using 4-0 polypropylene. Complex injuries often require an interposition graft.
Heated debate continues in the literature on whether active distal perfusion decreases the dreaded morbidity of paraplegia in the management of descending thoracic aortic injuries. There are reports supporting both sides of the argument. The length of aortic cross clamp time has been argued as an independent factor contributing to increased incidence of postoperative paraplegia. Numerous studies, however, have shown that the incidence of postoperative paraplegia is multifactorial and cannot be attributed to any single cause.19–26 All distal perfusion techniques have potential complications including those from cannulation sites or from systemic heparinization. Regardless of the technique used, the overall incidence of postoperative paraplegia averages 8% according to various studies.23,27–30 To date, no prospective randomized trial has documented the superiority of any single technique.
Median sternotomy with right-sided cervical extension is the incision of choice for right subclavian artery injury. Proximal left subclavian artery injury is best repaired through a left posterolateral thoracotomy in the fourth intercostal space. Exposure of the distal left subclavian artery can be obtained through a left supraclavicular incision with proximal control via a third interspace anterolateral incision. While seldom needed, injury to multiple segments of the left subclavian artery can be managed by combining the two incisions to create a “trapdoor” incision. Injury to the phrenic nerve, lying anterior to the scalenus anticus muscle, should be avoided during exposure of the subclavian artery. The left clavicle can be divided or resected to provide better exposure if necessary. After obtaining proximal and distal control, either primary repair or interposition knitted or polytetrafluoroethylene grafts may be used depending on the extent of injury. Because of the soft nature of the subclavian artery, mobilization for end-to-end anastomosis is generally difficult. Subclavian artery injuries are often associated with concomitant brachial plexus injuries; therefore, it is helpful to note the preoperative neurological examination. Subclavian venous injuries are exposed similarly as subclavian artery injuries. Venous injuries are repaired by either primary venorrhaphy or ligation.
In a dying patient with thoracic vascular injury, damage control thoracotomy is a treatment option. A left anterolateral thoracotomy incision provides the best initial exposure. The principle of damage control thoracotomy consists of the use of simpler techniques to achieve expeditious control of hemorrhage in a single setting, or temporary measures for hemorrhage control with planned second operation for definitive repair as the patient’s physiologic status is restored to a more survivable level.31 Hilar vascular injuries can be controlled quickly by performing pneumonectomy or lobectomy with a stapling device. For vessels greater than 5 mm, synthetic grafts may be used to avoid delays in harvesting vein grafts. Temporary ligation and placement of intravascular shunts can control hemorrhage until the patient’s physiologic status is restored to a more appropriate level for definitive repair. Ligation of the subclavian artery is often well tolerated and can be used in a damage control setting. Thoracotomy incisions can be closed quickly with towel clips; however, en-mass closure using large needles encompassing all muscle layers are more hemostatic. A “Bogotá bag” or patch closure can also be used as temporary closures in patients with cardiac dysfunction in order to prevent compression of the heart.
MORTALITY
Thoracic vascular injuries have one of the highest mortality rates of any organ system because of the high incidence of other concomitant injuries in other body compartments. Patients with ascending aortic injuries rarely reach the hospital alive. The mortality rate remains as high as 50% for patients with ascending aortic injuries with stable vital signs on arrival to trauma centers.8 Injuries to the central pulmonary artery and vein are highly lethal with mortality rates in excess of 70%.8 Similarly, thoracic vena cava injuries are infrequent but extremely difficult to control and carries a mortality rate greater than 60%.8 Regardless of the surgical technique used, the mortality rate of descending thoracic aortic injuries ranges from 5% to 25%.30 The overall mortality for innominate artery injuries is reported to be 25% from 1960 to 1992.18 Subclavian artery injuries have the best prognosis with an overall mortality rate of less than 5% as reported by Graham et al.32
CONCLUSIONS
Unlike abdominal injuries where midline vertical incision is the standard exploratory incision, patients with stable thoracic vascular injuries require careful preoperative planning. Because of the rigid chest wall, ill-placed incisions and incorrect intercostal space entry significantly compromise exposure for proximal/distal control of hemorrhage from thoracic vascular injuries. Although thoracic vascular injuries have one of the highest mortality rates of any trauma, superb surgical judgment along with operative precision will translate to improved patient care and outcome.
1 Vesalius A: In Beonetus T. Sepulchretaum sive Anataomia Practica ex Cad ak Veribus Morbo Denatis. Geneva, 1700, sec. 2:290.
2 DeBakey ME, Simeone FA. Battle injuries of the arteries in WWII. Ann Surg. 1946;123:534.
3 Passaro E, Pace WG. Traumatic rupture of the aorta. Surgery. 1959;41:787.
4 Mattox KL, Feliciano DV, Burch J, Beall ACJr, Jordan GLJr, DeBakey ME. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients—epidemiological evolution 1958 to 1987. Ann Surg. 1989;209:698.
5 Trunkey DD. Thoracic trauma. In: Trunkey DD, Lewis FR, editors. Current Therapy of Trauma. St. Louis: CV Mosby; 1984:85.
6 Beeson A, Saegesser F. Color Atlas of Chest Trauma and Associated Injuries. Oradell, NJ: Medical Economics Books, 1983.
7 Bickell WH, Wall MJJr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105-1109.
8 Mattox KL. Approaches to trauma involving the major vessels of the thorax. Surg Clin North Am. 1989;69:77.
9 Woodring JH. The normal mediastinum in blunt traumatic rupture of the thoracic aorta and brachiocephalic arteries. J Emerg Med. 1990;8:467.
10 Wintermark M, Wicky S, Schnyder P. Imaging of acute traumatic injuries of the thoracic aorta. Eur Radiol. 2002;12(2):431-442.
11 Parker MS, Matheson TL, Rao AV, et al. Making the transition: the role of helical CT in the evaluation of potentially acute thoracic aortic injuries. Am J Roentgenol. 2001;176(5):1267-1272.
12 Dyer DS, Moore EE, Ilke DN, et al. Thoracic aortic injury: how predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1, 561 patients. J Trauma. 2000;48(4):673-682.
13 Dyer DS, Moore EE, Mestek MF, et al. Can chest CT be used to exclude aortic injury? Radiology. 1999;213(1):195-202.
14 Tello R, Munden RF, Hooton S, Kandarpa K, Pugatch R. Value of spiral CT in hemodynamically stable patients following blunt chest trauma. Comput Med Imaging Graph. 1998;22(6):447-452.
15 Mirvis SE, Shanmuganathan K, Buell J, Rodriguez A. Use of spiral computed tomography for the assessment of blunt trauma patients with potential aortic injury. J Trauma. 1998;45(5):922-930.
16 Durham RM, Zuckerman D, Wolverson M, et al. Computed tomography as a screening exam in patients with suspected blunt aortic injury. Ann Surg. 1994;220(5):699-704.
17 Ben-Menachem Y. Assessment of blunt aortic-brachiocephalic trauma: should angiography be supplanted by transesophageal echocardiography? J Trauma. 1997;42:969.
18 Johnston RHJr, Wall MJJr, Mattox KL. Innominate artery trauma: a thirty-year experience. J Vasc Surg. 1993;17:134.
19 Crawford ES, Rubio PA. Reappraisal of adjuncts to avoid ischemia in the treatment of aneurysm of descending thoracic aorta. J Thorac Cardiovasc Surg. 1983;85:98.
20 Culliford AT, Ayvaliotic B, Shemin R, et al. Aneurysms of the descending aorta. J Thorac Cardiovasc Surg. 1983;85:98.
21 Laschinger JC, Cunningham JNJr, Nathan IM, et al. Experimental and clinical assessment of the adequacy of partial bypass in maintenance of spinal cord flow during operations on the thoracic aorta. Ann Thorac Surg. 1983;36:417.
22 Mattox KL. Fact and fiction about management of aortic transaction. Ann Thorac Surg. 1989;48:1.
23 Mattox KL, Holtzman M, Pickard LR, et al. Cmap/repair: a safe technique for treatment of blunt injury to the descending thoracic aorta. Ann Thorac Surg. 1985;40:456.
24 Williams TE, Vasco JS, Kakos GS, et al. Treatment of acute and chronic traumatic rupture of the descending thoracic aorta. World J Surg. 1980;4:545.
25 Moore EE, Burch JM, Moore JB. Repair of the torn descending thoracic aorta using the centrifugal pump for partial left heart bypass. Ann Surg. 2004;240(1):38-43.
26 Coselli JS, LeMaire SA, Conklin LD, Adams GJ. Left heart bypass during descending thoracic aortic aneurysm repair does not reduce the incidence of paraplegia. Ann Thorac Surg. 2004;77(4):1298-1303.
27 Cowley RA, Turney SZ, Hankins JR, et al. Rupture of thoracic aorta due to blunt trauma: a 15 year experience. J Thorac Cardiovasc Surg. 1990;100:652.
28 Hilgenberg AD, Logan KL, Akins CW, et al. Blunt injuries of the thoracic aorta. Ann Thorac Surg. 1992;53:233.
29 Pate JW, Fabian TC, Walker WA. Acute traumatic rupture of the aortic isthmus: repair with cardiopulmonary bypass. Ann Thorac Surg. 1995;59:90.
30 Von Oppell UO, Dunne TT, De Groot MK, et al. Traumatic aortic rupture: twenty-year meta-analysis of mortality and risk of paraplegia. Ann Thorac Surg. 1994;58:585.
31 Wall MJJr, Soltero E. Damage control for thoracic injuries. Surg Clin North Am. 1997;77:863.
32 Graham JM, Feliciano DV, Mattox KL, et al. Management of subclavian vascular injuries. J Trauma. 1980;20:537.