207 Thoracic Trauma
 Initial Assessment
Initial Assessment
Primary Survey
The Advanced Trauma Life Support (ATLS) course of the American College of Surgeons Committee on Trauma1 provides basic tenets for the management of all injured patients. Initial treatment of seriously injured patients consists of a primary survey, resuscitation, secondary survey, diagnostic evaluation, and definitive care. Although the concepts are presented in a sequential fashion, in reality, they often proceed simultaneously. The process begins with the primary survey, designed to identify and treat conditions that constitute an immediate threat to life. The primary survey includes a stepwise evaluation of the “ABCs”: Airway, with cervical spine protection; Breathing; and Circulation.
Resuscitative Thoracotomy
Some trauma victims who arrive in extremis may be candidates for resuscitative thoracotomy in the ED (EDT). The primary objectives of EDT are to (1) release pericardial tamponade, (2) control intrathoracic hemorrhage, (3) control bronchovenous air embolism or bronchopleural fistula, (4) perform open cardiac massage, and (5) temporarily occlude the descending thoracic aorta to redistribute limited blood flow to the brain and myocardium and attenuate subdiaphragmatic hemorrhage. The critical determinants of survival following this procedure are the mechanism of injury and the patient’s condition at the time of thoracotomy. The best outcomes are seen in adult patients with isolated penetrating cardiac injuries who present to the ED with detectable blood pressure; survival averages 35% in large series. For penetrating noncardiac injuries, the salvage rate is 15% for patients who present with vital signs and less than 10% if only signs of life (i.e., pupillary activity, spontaneous respirations, narrow complex cardiac activity) are present. Resuscitative thoracotomy is least beneficial in the treatment of blunt injury or in the absence of signs of life, with only 1% to 2% of patients surviving.2
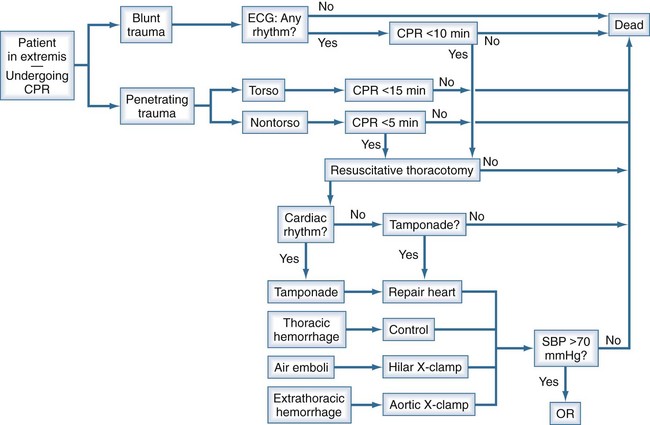
The value of thoracotomy in the resuscitation of a patient in profound shock but not yet dead is unquestioned. Its indiscriminate use, however, renders it a low-yield, high-cost procedure, including risks to the health care team. A recent Western Trauma Association (WTA) multicenter study attempted to determine the limits of EDT to enable the development of rational guidelines to withhold or terminate resuscitative efforts.3 The WTA multicenter experience suggests EDT is unlikely to yield productive survival when patients: (1) sustain blunt trauma and require more than 10 minutes of prehospital cardiopulmonary resuscitation (CPR) without response, (2) have penetrating wounds and undergo more than 15 minutes of prehospital CPR without response, or (3) manifest asystole without pericardial tamponade. There are likely to be exceptions, and the clinician must individualize care in each case. Based on our experience and that reflected in the current literature, we have formulated a decision algorithm for resuscitation of moribund trauma patients (Figure 207-1). Patients arriving in extremis following blunt injury undergo thoracotomy only if they have a rhythm on electrocardiography (ECG) and have had fewer than 10 minutes of CPR. Penetrating trauma victims in extremis undergo thoracotomy if they have had fewer than 15 minutes (for torso injuries) or 5 minutes (for non-torso injuries) of CPR. If, upon opening the chest, there is no organized cardiac activity and no blood in the pericardium, the patient is declared dead. All other patients are treated according to the injury. Pericardial tamponade is decompressed, and bleeding from cardiac wounds is controlled. Suspected air embolism is treated by application of a pulmonary hilar cross-clamp, vigorous cardiac massage, and aortic root and left ventricular aspiration for air. Intrathoracic hemorrhage is controlled. Cardiovascular collapse from suspected intraabdominal hemorrhage is temporized by occluding the descending thoracic aorta. Those patients who respond to treatment and have a systolic blood pressure above 70 mm Hg are rapidly transported to the operating room for definitive treatment of their injuries.
 Pleural Space
Pleural Space
Pneumothorax
Pneumothorax is a common sequela of thoracic trauma. Visceral pleural disruption due to penetrating trauma, blunt shearing, or lacerations from fractured bones allows egress of air into the pleural space as negative intrapleural pressure is created during inspiration. Physical findings include decreased breath sounds, hyperresonance to percussion, and decreased expansion of the chest wall on the affected side. If not relieved, a simple pneumothorax may progress to a tension pneumothorax, especially if the patient is receiving positive-pressure ventilation. In this setting, the mediastinal structures are shifted away from the affected side. In addition to the mechanical impediment to gas exchange, venous return to the heart is impaired secondary to vena caval distortion, and shock ensues. Immediate decompression is mandatory and can be lifesaving (see Tube Thoracostomy).
With the growing use of thoracoabdominal computed tomography (CT) in the evaluation of trauma patients, small pneumothoraces that are not seen on plain radiographs are often discovered. The treatment of these so-called occult pneumothoraces is not as well defined as the treatment of the usual pneumothorax. Generally they do not require treatment but should be monitored for progression. The notion of “prophylactic” tube thoracostomy in the setting of positive-pressure ventilation has been challenged, but vigilance is important to detect progression to tension pneumothorax in approximately 10% of patients.4
Tube Thoracostomy
In the setting of tension pneumothorax, if tube thoracostomy is not immediately available, the chest can be decompressed with a large-bore needle as a temporizing measure. Although many authors promote decompression via the second intercostal space in the midclavicular line, injuries to the great vessels and heart have been described as a result of this procedure. Further, catheters may be misdirected or kinked in the pectoralis major muscle or breast tissue, rendering them ineffective, often unbeknownst to the clinician. The author’s preference is to insert the needle through the fifth intercostal space in the midaxillary line. This site allows rapid, reliable entry into the pleural space, and the risk of great vessel injury is essentially nil.5
The major morbidity related to tube thoracostomy is infectious (pneumonia, empyema), reported in up to 20% of patients. Some investigators have proposed routine prophylactic antibiotics to prevent such morbidity, but this has been controversial. A multicenter prospective randomized clinical trial comparing prophylactic antibiotics versus placebo found that antibiotics did not reduce the incidence of empyema or pneumonia. Moreover, the use of antibiotics was associated with a definite pattern of resistance in subsequent hospital-acquired infections.6
Pneumothoraces and air leaks should be resolved before removal of the tube, and ideally, drainage should be less than 2 mL/kg/d. After 12 to 24 hours without an air leak, the tube may be removed while on suction. However, a 6- to 12-hour trial of waterseal drainage is generally warranted to observe for an occult air leak.7 It has been recommended that tubes be removed at maximal deep inspiration with a Valsalva maneuver, but recurrent pneumothorax may occur in 6% to 8% of patients regardless of respiratory phase.8 More than 20% of patients require longer than 3 days to resolve an air leak; their hospital course may be expedited by the use of thoracoscopy.9
 Chest Wall Injury
Chest Wall Injury
Rib Fracture
Rib fractures are estimated to occur in 10% of patients presenting for evaluation by trauma services. Ziegler and Agarwal reported that more than 90% of patients with rib fractures had associated injuries, and half of these patients required intensive care unit (ICU) care.10 In their series, the overall mortality of patients presenting with rib fractures was 12%. Multiple rib fractures, fractures of the first or second rib, and scapular fractures signify higher-energy injuries and should prompt a search for associated intraabdominal injury or thoracic vascular injury.
Single rib fractures in young patients are generally of little consequence; however, rib fractures in elderly patients can lead to diminished pulmonary function with potentially disastrous infectious complications. Patients over the age of 65 have two- to fivefold increases in morbidity and mortality compared with younger patients with similar injuries.11,12 Bulger et al. found that for each additional rib fracture in the elderly, the risk of pneumonia increases by 27%, and mortality increases by 19%.11 A key factor in the management of these patients is pain control to facilitate coughing and clearance of secretions. Epidural catheters have proved to be efficacious and superior to patient-controlled analgesia in this regard and may also modify the immune response.13,14 Rib blocks may provide immediate relief in the ED or ICU while awaiting epidural catheter placement. Bupivacaine or a lidocaine-bupivacaine mixture may be injected into the intercostal bundle (with care taken not to inject intravascularly) of the fractured ribs and those above and below them. An intercostal catheter provides another alternative in the event an epidural catheter is unavailable or contraindicated.15
Flail Chest
Two or more ribs fractured in two or more places produce a flail segment of the chest wall. This segment moves paradoxically—inward during inspiration, outward during expiration—because it is detached from the chest wall and thus susceptible to the forces of intrapleural pressure. The mechanical effects on respiration are related to the size of the flail segment. However, a more important cause of respiratory compromise following flail chest injury is the pulmonary contusion that invariably accompanies it. Treatment is supportive, including supplemental oxygen, analgesia, and pulmonary toilet. Endotracheal intubation with positive-pressure ventilation is sometimes necessary. Surgical stabilization of the flail segment, and rib fracture repair in general, has been performed for decades. At this time, there is a need for multicenter randomized trials with long-term follow-up to identify appropriate patients and optimal techniques.16
Sternal Fracture
Early series of sternal fractures described the “steering wheel syndrome” (rapid deceleration, with impact of the sternum on the steering wheel) as the most common cause of sternal fracture. In these series, associated blunt cardiac injury (see later) was common, so sternal fractures were thought to be harbingers of significant occult thoracic injury. More recently, however, sternal fractures have been reported more commonly with the “seatbelt syndrome” (in conjunction with three-point, or bandolier, seat belts). Because the elements of deceleration and steering wheel impact are no longer prominent, associated injuries are relatively infrequent.17 Stable patients without dyspnea, ECG abnormalities, or significantly displaced fractures can be safely discharged from the ED. Rest and analgesia are adequate treatment.
 Lung Injury
Lung Injury
Pulmonary Contusion
Pulmonary contusion is a common problem, occurring in one-quarter of patients with injury severity scores (ISS) over 15 and in a majority of patients sustaining major chest trauma. The injury may result from a direct blow, shearing or bursting at gas/liquid or high-density/low-density interfaces, or the transmission of a shock wave. The pathophysiologic changes fundamentally include hemorrhage with surrounding edema, with a broad range of severity up to “hepatization” of the lung. The clinical result is hypoxia and increased work of breathing due to ventilation/perfusion mismatching and decreased pulmonary compliance. Pulmonary contusions may not appear on initial chest radiograph, although they are usually seen by 6 hours after the injury; chest CT is more sensitive at diagnosing early pulmonary contusions. Treatment is supportive, including supplemental oxygen, pain control, pulmonary toilet, and judicious fluid management. There is no role for either routine antibiotics or steroid therapy.18 Intubation and mechanical ventilation are employed only as necessary. The degree of pulmonary dysfunction usually peaks at 72 hours and generally resolves within 7 days in the absence of associated nosocomial pneumonia. Mortality related to pulmonary contusion has improved greatly with advances in critical care.
Posttraumatic pulmonary pseudocysts are cavitary lesions that occur in approximately 3% of lung parenchymal injuries.19 They may be asymptomatic or associated with mild nonspecific symptoms and are often noted incidentally on the chest radiograph. Most resolve spontaneously within 2 to 4 months. However, surgical intervention is indicated for infection, bleeding, and rupture. The lesion can be distinguished from an abscess by CT-guided aspiration. If infected, catheter drainage may be required for definitive management.
Pulmonary Laceration
Penetrating trauma, blunt shearing, or the ends of fractured bones can cause pulmonary laceration and parenchymal disruption. The typical clinical presentation is a hemopneumothorax. Bleeding is usually self-limited, and the vast majority of these injuries are definitively managed by tube thoracostomy alone. Of the 10% of patients requiring thoracotomy, approximately 20% need lung resection. Historically, this group has experienced high morbidity and mortality, with mortality following pneumonectomy approaching 100%. In 1994, Wall and colleagues introduced the concept of pulmonary tractotomy as a nonresectional means of managing penetrating lung injuries.20 It is indicated for deep through-and-through injuries that do not involve central hilar vessels or airways. The wound tract is exposed by passing clamps (as originally described) or a stapling device (our preference) through the wound and dividing the bridge of lung tissue. Air leaks and bleeding points are sutured, and the wound tract is left open. The literature contains mixed reports of the success of this approach, but the morbidity and mortality compare favorably with those associated with anatomic resections.21
 Pneumomediastinum
Pneumomediastinum
Pneumomediastinum has classically been considered a sign of aerodigestive injury. This was particularly true of pneumomediastinum seen on plain radiography; however, with expanding use of chest CT, pneumomediastinum is being seen with increasing frequency. Recent analyses have found that pneumomediastinum is present on approximately 5% of chest CT scans following trauma, but that only 10% of these patients actually have aerodigestive injuries.22 In the absence of signs or symptoms or additional suspicious findings on CT scan, further investigation is not necessary.22,23
 Tracheobronchial Injury
Tracheobronchial Injury
Tracheobronchial injuries are uncommon but should be excluded in the presence of cervical subcutaneous emphysema, pneumomediastinum (see earlier), or pneumothorax with a persistent air leak. Although CT may reveal the injury, the preferred definitive diagnostic test is bronchoscopy. Most penetrating injuries occur in the cervical area and are approached via cervical incisions, with partial or complete sternotomy as needed. Blunt injuries more commonly occur in the distal trachea or right mainstem bronchus and are approached via sternotomy or thoracotomy. Tracheal injuries can usually be repaired primarily or by resection and reanastomosis without tracheostomy; late stenosis is uncommon. On the other hand, laryngotracheal injuries often require tracheostomy as an adjunct to repair, and tracheal stenosis is a common late complication. Absorbable monofilament sutures are preferred. Bronchial injuries may be repaired, but severe disruptions or associated vascular injuries may necessitate pneumonectomy or lobectomy. Positive end-expiratory pressure is avoided postoperatively.24
 Esophageal Injury
Esophageal Injury
Esophageal perforation from blunt-force trauma is a rare event caused by a sudden rise in intraluminal pressure or by the upper esophagus being crushed between the trachea and a vertebral body. More commonly, esophageal injury is the result of penetrating trauma. Early signs and symptoms of injury can be subtle, so a high index of suspicion is important. Pneumomediastinum should prompt consideration of this injury (see earlier). Barium esophagography is considered the diagnostic study of choice and can be readily obtained in a stable, awake patient.25 However, videoendoscopy can be done at the bedside virtually anywhere in the hospital and has excellent accuracy, particularly in the pharyngeal area. Thus, it is preferred in critically ill or unstable patients in the ICU or operating room.26
Evaluation should be expeditious because delays in definitive care are associated with increased morbidity and mortality. If the injury is identified within 24 hours, it can usually be treated with débridement, primary repair, and drainage. Injuries identified after 24 hours are better treated with débridement and drainage, cervical esophagostomy, and feeding tube placement.25
 Blunt Cardiac Injury
Blunt Cardiac Injury
Cardiac Rupture
Cardiac rupture is the most severe form of BCI; 80% to 90% of ruptures are lethal within minutes. Cardiac rupture may result from direct-impact force to the heart or pressure transmitted via venous channels; deceleration with lacerations at junctions between fixed and mobile structures (e.g., atriocaval disruptions); myocardial contusion, with subsequent necrosis and rupture; and broken ribs or sternum penetrating the heart. The most common chambers ruptured are the right atrium and ventricle, followed by the left atrium and then the left ventricle.27,28 A coexistent pericardial laceration allows free hemorrhage into the pleural or peritoneal cavity. Those who reach the hospital alive typically have a pericardial effusion and may develop pericardial tamponade. A characteristic mill-wheel murmur, the bruit de moulin, may be heard.
Pericardial Injury
Pericardial tears may result from direct thoracic impact or from an acute increase in intraabdominal pressure. The tears most commonly occur on the left (64%), paralleling the phrenic nerve; the diaphragmatic surface (18%), right pleuropericardium (9%), and mediastinum (9%) are the next most frequent sites.27 Herniation of the heart through a large tear may be associated with significant cardiac dysfunction. A pericardial rub may be detected on physical examination. The chest radiograph may demonstrate pneumopericardium, displacement of the heart, or bowel gas in the chest. Echocardiography or CT may be required to confirm the injury. In a stable patient, a subxiphoid pericardial window should be performed, followed by sternotomy in the presence of hemopericardium or a visible pericardial tear. An unstable patient may require EDT. Pericardial lacerations should be repaired, but large holes that cannot be closed primarily should be left widely open to prevent future cardiac herniation. A late complication is the postpericardiotomy syndrome, manifested by fever, chest pain, pericardial effusion, a pericardial rub, and ECG abnormalities; this is adequately treated with antiinflammatory agents.
Valvular Injury
Lethal cardiac trauma involves the valves in approximately 5% of patients. The most commonly injured valve is the aortic, followed by the mitral, tricuspid, and pulmonary. The aortic cusps may be lacerated or avulsed when a sudden increase in intrathoracic pressure leads to a concomitant increase in aortic pressure. The result is often acute severe cardiac failure, but a mild injury may present with syncope or anginal symptoms.29 Violent compression of the heart in early systole during isovolumetric contraction may tear mitral valve leaflets but more commonly ruptures the papillary muscles or chordae tendineae. Acute heart failure may ensue, and a holosystolic murmur of mitral regurgitation is heard.30 Tricuspid valve injuries are rare; they usually occur in the subvalvular area following compression in late diastole. They are generally of less hemodynamic consequence than aortic or mitral valve injuries, but endocarditis and hepatic dysfunction from chronic venous congestion have been reported. Cardiac catheterization and echocardiography are used to confirm the diagnosis. Most valve injuries are amenable to supportive care until other injuries have been stabilized. Valve repair is generally preferred over valve replacement when feasible.31
Septal Injury
Small septal defects may heal primarily, allowing expectant management with periodic follow-up. Surgical repair—either primary or with a patch graft—is indicated if the patient is hemodynamically compromised or has a left-to-right shunt with a shunt ratio of 2 : 1 or greater. Repair of the defect is delayed for several weeks if possible.32
Coronary Artery Injury
Direct injuries to coronary arteries are rare. The left anterior descending artery is most susceptible (76% of cases), followed by the right coronary artery (12%) and the circumflex coronary artery (6%). The sequela of coronary artery dissection or thrombosis is myocardial infarction, with ischemic consequences dependent on the vessel and level of injury. Cardiac catheterization is indicated, and therapeutic angioplasty or stenting may be performed occasionally; however, the usual treatment is medical. Recanalization of arteries is frequently reported, but surgical revascularization or repair of delayed complications related to infarction, such as ventricular pseudoaneurysms, may be indicated.33
Diagnosis, Monitoring, and Treatment
The frequency of the diagnosis of BCI depends on the diagnostic criteria, which may include specific ECG abnormalities (e.g., ventricular dysrhythmias, atrial fibrillation, sinus bradycardia, bundle branch block), cardiac enzyme elevation, or evidence of cardiac dysfunction on echocardiography or nuclear medicine studies. Unfortunately, none of these tests is predictive of the uncommon but life-threatening complications of ventricular dysrhythmias and cardiac pump failure.34 The pivotal issue is to identify patients at risk and have them in a setting where the complication can be identified and treated.
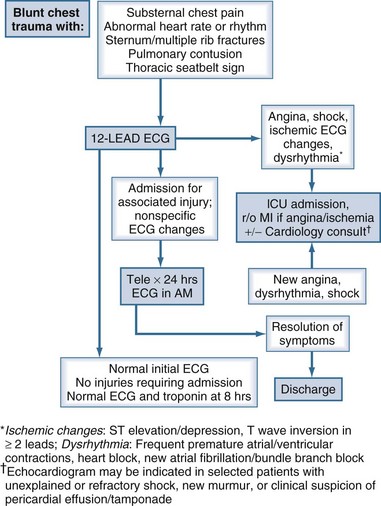
Our practice guidelines for monitoring patients with suspected BCI are depicted in Figure 207-2. BCI should be suspected in all individuals who sustain major chest trauma. The initial evaluation should include an ECG as part of the secondary survey. Patients with shock from any cause, ischemic changes on the ECG, or significant dysrhythmias are admitted to the ICU. If angina or ischemic ECG changes are noted, a standard “rule out myocardial infarction” protocol is followed. Nonspecific ECG findings are rarely associated with significant BCI, and patients may be discharged after 24 hours of cardiac monitoring if no new symptoms occur. Patients with significant blunt chest trauma who are being admitted for associated injuries should have cardiac monitoring for 24 hours. A subset of patients may not require admission for other injuries. These patients can be safely discharged from the ED if ECG at presentation and at 8 hours is normal, and if a troponin-I level at 8 hours is less than 1.5 ng/mL.35
Commotio cordis is a distinct entity in which “virtually instantaneous cardiac arrest is produced by nonpenetrating chest blows in the absence of heart disease or identifiable morphologic injury to the chest wall or heart.”36 In a series of 70 cases, Maron and colleagues reported a 90% mortality rate in a young (mean age 12 years) population of patients.36 An experimental model demonstrated that ventricular fibrillation is reproducibly triggered by a precisely timed blow during a narrow window within the repolarization phase of the cardiac cycle (15-30 msec before the peak of the T wave). Heart block may be produced by a blow during the QRS complex.37
 Penetrating Cardiac Injury
Penetrating Cardiac Injury
Cardiac penetration is rapidly lethal in 90% of gunshot wounds and up to 50% of stab wounds. The most important factors for survival are rapid transport to the trauma center, early diagnosis, and immediate treatment. Patients arriving in extremis after penetrating chest trauma should undergo EDT. All patients in shock with penetrating chest injuries between the right midclavicular line and left anterior axillary line should be considered to have a cardiac injury until proven otherwise.38 The right ventricle, with its maximal anterior exposure, is at greatest risk, followed by the left ventricle, right atrium, and left atrium. Multiple cardiac structures are involved in a third of patients. Stab wounds are more commonly associated with tamponade, while gunshot wounds generally exsanguinate through a large pericardial defect.
Repair of cardiac injuries can be accomplished through either a median sternotomy or a thoracotomy incision. In a hemodynamically compromised patient, left anterior thoracotomy with transsternal extension is used for definitive repair. Otherwise, in a hemodynamically stable patient, sternotomy is generally preferred. A limitation of sternotomy is access to posterior injuries or associated aortic or esophageal injuries. In any case, control of hemorrhage is the first priority. Satinsky clamps are useful in isolating atrial or caval injuries, whereas small ventricular lacerations are controlled digitally. Larger wounds may be stapled. Insertion of a Foley catheter with temporary balloon occlusion of the wound may facilitate repair, but one must be careful to not extend the injury.39 Wounds that are too large for balloon occlusion are occasionally salvageable using temporary caval inflow occlusion.40
Pericardial Tamponade
Potential pericardial tamponade should be suspected in all patients sustaining penetrating injuries to the anterior chest wall. Pericardial tamponade can be a two-edged sword: although it may limit initial blood loss, it can prove fatal by restricting diastolic filling of the heart.41 As blood leaks out of the injured heart, it accumulates in the pericardial sac. Because the pericardium is not acutely distensible, the pressure in the pericardial sac rises to match that of the injured chamber. When this pressure approaches that of the right atrium, right atrial filling is impaired, and right ventricular preload is reduced; ultimately, this leads to decreased right ventricular output. Increased intrapericardial pressure also impedes myocardial blood flow, which leads to subendocardial and later subepicardial ischemia, with a further reduction of cardiac output. This vicious cycle may progress insidiously with injury to low-pressure conduits, or it may occur precipitously with a ventricular wound. Acute tamponade of as little as 100 mL of blood within the pericardial sac can produce life-threatening hemodynamic compromise.
In the setting of suspected pericardial tamponade, ultrasonography using subxiphoid and parasternal views (or formal echocardiography if immediately available in the ED) is extremely helpful if the findings are positive, although a negative ultrasonographic examination may be misleading if there is a pericardial laceration.42 If pericardial fluid is demonstrated, the patient should be transported immediately to the operating room for sternotomy. However, if ultrasonography is equivocal, a central venous pressure line should be inserted promptly. Persistently elevated central venous pressure in a patient with thoracic trauma should prompt consideration of ultrasound-guided pericardiocentesis or subxiphoid pericardial window. If the pericardial ultrasonography is positive and there will be any delay in getting to the operating room, pericardiocentesis should be done even if the patient appears hemodynamically stable, because subclinical myocardial ischemia can lead to sudden lethal dysrhythmias. The pericardial tap should be performed with a pigtail catheter to allow repeated aspiration during preparation for thoracotomy. In the setting of shock, evacuation of as little as 15 mL of blood may dramatically improve the patient’s hemodynamic profile. Pericardiocentesis is successful in decompressing tamponade in approximately 80% of cases; most failures are due to clotted blood within the pericardium. Although a subxiphoid pericardial window can be created under local anesthesia in the ED, hemorrhage may be difficult to control if an injury is found. If pericardiocentesis is unsuccessful and the patient remains severely hypotensive (systolic blood pressure <70 mm Hg), EDT should be performed.
 Transmediastinal Penetrating Trauma
Transmediastinal Penetrating Trauma
Transmediastinal trajectory of a bullet should be considered in the setting of (1) entry and exit wounds on opposite sides of the thorax, (2) a single entry wound with the bullet ending up on the opposite side of the thoracic cavity or in close proximity to the mediastinum, or (3) multiple gunshot wounds to the thorax. Significant injury, especially to the heart or great vessels, often results in prehospital death or hemodynamic instability. There is little controversy regarding the management of unstable patients: they should have emergent thoracotomy. However, stable patients may harbor occult injuries to critical mediastinal structures (heart, great vessels, trachea, esophagus). Consequently, patients have routinely been submitted to a battery of invasive diagnostic tests: echocardiography or subxiphoid pericardial window, arch aortography, bronchoscopy, esophagoscopy, and esophagography.43 The last two have been employed together to improve on the sensitivity of each test individually. This array of tests can be expensive and time consuming. Further, only a small percentage of hemodynamically stable, asymptomatic patients have clinically significant injuries.44
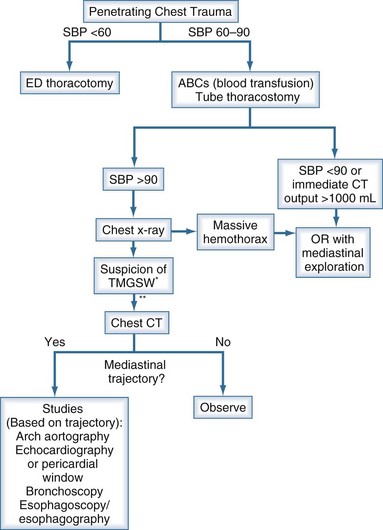
Helical CT of the chest has proved useful in demonstrating the trajectory of missiles in the thorax.45,46 In the setting of a potential transmediastinal gunshot wound, a CT scan may confirm a trajectory remote from the mediastinum, obviating further testing. A proven transmediastinal trajectory mandates further evaluation. However, rather than performing all the aforementioned tests, the investigation can be tailored to the specific clinical scenario. For example, trajectory near the pericardium warrants echocardiography or pericardial window. If CT suggests great vessel injury, arteriography should follow (see later). Bronchoscopy is indicated for pneumomediastinum, respiratory distress, or bronchopleural fistula or massive air leak. The esophagus is evaluated as outlined earlier. Our current approach to evaluating these patients is outlined in Figure 207-3.
 Thoracic Great Vessel Injury
Thoracic Great Vessel Injury
Patients with penetrating injuries to extrapericardial thoracic great vessels usually succumb in the field; however, an occasional patient arrives with a contained hematoma. Early chest radiography is critical to identify hemothorax, as well as a widened mediastinum or apical capping. Patients who are hemodynamically unstable should be taken directly to the operating room; those in extremis should undergo EDT. A reasonable approach can be inferred from the chest radiograph and the location of the wounds. If the patient has a left hemothorax, a left anterolateral thoracotomy in the third or fourth interspace should be performed. Patients with a right hemothorax should likewise be approached via a right anterolateral thoracotomy. Unstable patients with injuries near the sternal notch may have large mediastinal hematomas or may have lost blood externally. These patients should be explored via a median sternotomy with cervical extension, similar to a penetrating zone I neck wound. Hemorrhage should be controlled digitally until the vascular injury is delineated. In a hemodynamically stable patient, angiography can facilitate a more directed approach. Recent series suggest that clinical assessment may be adequate to detect injuries, obviating arteriography in cases in which the suspicion is based on periclavicular trajectory alone.47,48 However, it must be remembered that collateral flow around the shoulder girdle can result in palpable pulses, even in the presence of a significant subclavian artery injury.
The great vessels are rather fragile and can be easily torn during dissection or crushed with a clamp. For this reason, injuries adjacent to the aortic arch are oversewn, and a graft is inserted onto a new location on the arch. The graft is then sewn (without tension) to the distal artery. Nonoperative management of nonocclusive peripheral arterial injuries has proved successful, and there are limited data supporting similar management within the thorax for certain patients. Similarly, those lesions associated with severe neurologic injuries are usually managed nonoperatively. Experience with intravascular stenting is growing, although long-term outcomes have not been reported.49 Clearly unstable patients require operative control and repair; however, it appears that stent graft treatment of subclavian artery injuries is preferred in stable patients.50
Blunt Thoracic Aortic Injury
Perhaps the most feared occult injury in trauma surgery is a blunt thoracic aortic injury (BTAI). The mechanism of aortic tears is believed to be primarily a shearing force. The tear usually occurs just distal to the left subclavian artery where the aorta is tethered by the ligamentum arteriosum. In 5% of cases, the tear occurs in the ascending aorta, in the transverse arch, or at the diaphragm. An estimated 85% of thoracic aortic injuries are fatal at the injury scene. A multicenter report from the American Association for the Surgery of Trauma (AAST) analyzed 274 accident-scene survivors of BTAI.51 Motor vehicle crashes accounted for 81% of the injuries, with frontal impact in 72%, lateral impact in 24%, and rear impact in 4%. Two additional series also documented substantial numbers of BTAI following lateral-impact crashes: 57 of 165 (35%) autopsy cases reported by Burkhart et al.,52 and 48 of 97 (50%) cases reviewed by Katyal et al.53 Thus the surgeon should suspect this injury whenever there is significant energy transfer, regardless of directionality.
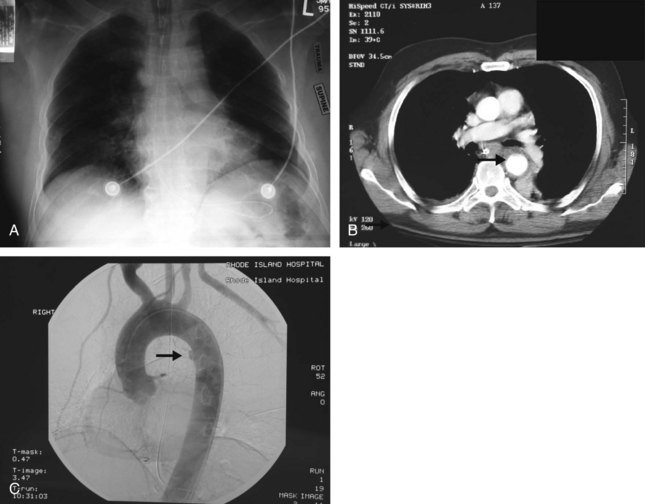
Chest radiograph is considered the initial screening tool for determining whether further investigation is needed for BTAI. Commonly associated radiographic findings include mediastinal widening, obscured aortic knob, deviation of the left mainstem bronchus (downward) or nasogastric tube (rightward), and opacification of the aortopulmonary window (Figure 207-4, A). In the AAST multicenter study,51 widening of the mediastinum on the anteroposterior chest radiograph was present in 85% of cases. However, 7% of patients with torn aortas had normal chest radiographs. Dyer and colleagues reported normal initial radiographs in 13% of patients.54 Thus, additional investigations are warranted in the setting of significant energy transfer. Thoracic aortography was previously considered the gold standard for diagnosis (see Figure 207-4, B). However, helical CT scan is now well accepted as an excellent screening test (see Figure 207-4, C).54–56 When hematoma adjacent to the thoracic aorta is considered a positive finding, the sensitivity of CT for aortic injury is 100%. Most authors advocate omitting the aortogram and operating on the basis of CT alone, but this is up to the individual surgeon. Transesophageal echocardiography is portable and fairly sensitive and specific; however, it is highly operator dependent and is not reliable for visualizing the ascending or transverse aorta or its branches. It has been supplanted by CT, and its primary role may be in following small intimal injuries that are managed nonoperatively. Intravascular ultrasonography is another tool with a poorly defined role.
There are currently a number of areas of controversy in the management of BTAI: immediate versus delayed repair, management of minimal aortic injuries (MAI), and open versus endovascular repair.57
Immediate Versus Delayed Repair
Until the 1990s, BTAI was thought to require emergent repair to avoid early rupture. Recognizing significant morbidity and mortality in patients with severe associated injuries and comorbid medical conditions, the concept of immediate repair was challenged. The administration of beta-blockade to decrease systolic blood pressure (<100 mm Hg) and heart rate (<100 bpm), and therefore reduce aortic shear pressure, allowed optimization of associated injuries stabilization of targeting a systolic blood pressure and heart rate.55 Numerous studies have established the safety of this approach. In fact, a recent AAST prospective multicenter trial found that delayed repair is associated with significant survival benefit.58 Although patients with major associated injuries are most likely to benefit, the study supported delayed repair in all patients, irrespective of risk factors.
Management of Minimal Aortic Injury
With increasing sensitivity of CT scans (as discussed with regard to pneumomediastinum), more MAIs are being diagnosed. These are defined as small (<1 cm) intimal lesions with minimal to no periaortic hematoma.59 Fabian and colleagues51 identified MAI in 10% of BTAI and found that half of these lesions were missed on arteriography. Although the name suggests benign behavior, the Memphis group reported that 50% of MAIs had progressed to pseudoaneurysm formation by 8 weeks post injury.59 MAIs are generally treated with beta-blockade and CT surveillance.
Open Versus Endovascular Repair
Over the past several years, open repair has been largely supplanted by thoracic endovascular aortic repair (TEVAR).60 A number of studies have reported lower mortality and paraplegia, as well as fewer blood transfusions and strokes, associated with TEVAR.49,57,60 However, there are still issues with device-related complications and the need for reinterventions. These issues will likely be improved with developing technology, but long-term studies are needed. In the meantime, TEVAR will no doubt continue to increase.
In those patients who require open repair, a primary concern has been the occurrence of paraplegia from ischemic injury of the spinal cord. Conceptually, two techniques have been advocated. The simpler technique, often referred to as “clamp and sew,” is accomplished with application of vascular clamps proximal and distal to the aortic injury. Razzouk et al.61 have successfully employed this technique in the majority of their patients over a 25-year period. However, this method results in transient hypoperfusion of the spinal cord distal to the clamps, as well as of abdominal organs. In the AAST study,51 the paraplegia incidence was 1.6% in patients with cross-clamp times less than 30 minutes, but 12% if the time was greater than 30 minutes. A 20-year meta-analysis found a 19% incidence of paraplegia associated with this method and noted that average cross-clamp times were over 40 minutes.62 The alternative approach is to provide some method for maintaining spinal perfusion during cross-clamping. Two techniques have been used to accomplish this goal, one passive and one active. Passive shunting uses a temporary extra-anatomic route around the clamps. A heparin-impregnated tube, the Gott shunt, was specifically designed for this purpose. However, blood flow to the distal aorta is inadequate; consequently, this technique is no longer used. With the availability of centrifugal pumps that do not require systemic anticoagulation, the current preferred method is to use either active partial left heart bypass (siphoning blood from the left heart and pumping it to the distal aorta) or full bypass such as femoral-femoral bypass. The former can be a significant benefit in a patient with multiple injuries, particularly in those with intracranial hemorrhage. However, occasional small cerebral infarcts have occurred, so heparin is administered unless contraindicated. The injury may be primarily repaired, or a graft may be inserted. A large multicenter trial suggested that polytetrafluoroethylene is the preferred graft material for aortic replacement, given its long-term patency and apparent resistance to infection.63
Key Points
Cothren CC, Moore EE. Emergency department thoracotomy. In Feliciano DV, Mattox KL, Moore EE, editors: Trauma, 6th ed, New York: McGraw-Hill, 2008.
Dyer DS, Moore EE, Ilke DN, et al. Thoracic aortic injury: how predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1,561 patients. J Trauma. 2000;48:673-683.
Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374-380.
Nirula R, Diaz JJ, Trunkey DD, et al. Rib fracture repair: indications, technical issues, and future directions. World J Surg. 2009;33:14-22.
This review provides a comprehensive overview of techniques and devices for rib fracture repair.
Wall MJ, Hirshberg A, Mattox KL. Pulmonary tractotomy with selective vascular ligation for penetrating injuries to the lung. Am J Surg. 1994;168:665-669.
The original description of pulmonary tractotomy.
Wu JT, Mattox KL, Wall MJ. Esophageal perforations: new perspectives and treatment paradigms. J Trauma. 2007;63:1173-1184.
1 American College of Surgeons Committee on Trauma. Advanced Trauma Life Support for Doctors, 8th ed. Chicago: American College of Surgeons; 2008.
2 Cothren CC, Moore EE. Emergency department thoracotomy. In Feliciano DV, Mattox KL, Moore EE, editors: Trauma, 6th ed, New York: McGraw-Hill, 2008.
3 Moore EE, Knudson MM, Burlew CC, et al and the WTA Study Group. Defining the limits of resuscitative emergency department thoracotomy: A contemporary Western Trauma Association perspective. J Trauma (in press)
4 Ball CG, Kirkpatrick AW, Feliciano DV. The occult pneumothorax: What have we learned? Can J Surg. 2009:52-E173.
5 Biffl WL. Needle thoracostomy: A cautionary note. Acad Emerg Med. 2004;11:795-796.
6 Maxwell RA, Campbell DJ, Fabian TC, et al. Use of presumptive antibiotics following tube thoracostomy for traumatic hemopneumothorax in the prevention of empyema and pneumonia–a multi-center trial. J Trauma. 2004;57:742-749.
7 Martino K, Merrit S, Boyakye K, et al. Prospective randomized trial of thoracostomy removal algorithms. J Trauma. 1999;46:369-374.
8 Bell RL, Ovadia P, Abdullah F, et al. Chest tube removal: End-inspiration or end-expiration. J Trauma. 2001;50:674-677.
9 Schermer CR, Matteson BD, Demarest GB, et al. A prospective evaluation of video-assisted thoracic surgery for persistent air leak due to trauma. Am J Surg. 1999;177:480-484.
10 Ziegler DW, Agarwal NN. The morbidity and mortality of rib fractures. J Trauma. 1994;37:975-979.
11 Bulger EM, Arneson MA, Mock CN, et al. Rib fractures in the elderly. J Trauma. 2000;48:1040-1046.
12 Bergeron E, Lavoie A, Clas D, et al. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma. 2003;54:478-485.
13 Moon MR, Luchette FA, Gibson SW, et al. Prospective, randomized comparison of epidural versus parenteral opioid analgesia in thoracic trauma. Ann Surg. 1999;229:684-692.
14 Wu CL, Jani ND, Perkins FM, et al. Thoracic epidural analgesia versus intravenous patient controlled analgesia for the treatment of rib fracture pain after motor vehicle crash. J Trauma. 1999;47:564-568.
15 Haenel JB, Moore FA, Moore EE, et al. Extrapleural bupivacaine for amelioration of multiple rib fracture pain. J Trauma. 1995;38:22-26.
16 Nirula R, Diaz JJ, Trunkey DD, et al. Rib fracture repair: Indications, technical issues, and future directions. World J Surg. 2009;33:14-22.
17 Athanassiadi K, Gerazounis M, Mustardas, et al. Sternal fractures: Retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246.
18 Cohn SM, DuBose JJ. Pulmonary contusion: An update on recent advances in clinical management. World J Surg. 2010;34:1959-1970.
19 Crestanello JA, Samuels LE, Kaufman MS, et al. Posttraumatic pulmonary pseudocyst. J Trauma. 1998;44:401-403.
20 Wall MJ, Hirshberg A, Mattox KL. Pulmonary tractotomy with selective vascular ligation for penetrating injuries to the lung. Am J Surg. 1994;168:665-669.
21 Cothren C, Moore EE, Biffl WL, et al. Lung-sparing techniques are associated with improved outcome compared with anatomic resection for severe lung injuries. J Trauma. 2002;53:483-487.
22 Macleod JBA, Tibbs BM, Freiberger DJ, et al. Pneumomediastinum in the injured patient: Inconsequential or predictive? Am Surg. 2009;75:375-377.
23 Dissanaike S, Shalhub S, Jurkovich GJ. The evaluation of pneumomediastinum in blunt trauma patients. J Trauma. 2008;65:1340-1345.
24 Karmy-Jones R, Wood DE. Traumatic injury to the trachea and bronchus. Thorac Surg Clin. 2007;17:35-46.
25 Wu JT, Mattox KL, Wall MJ. Esophageal perforations: New perspectives and treatment paradigms. J Trauma. 2007;63:1173-1184.
26 Ahmed N, Massier C, Tassie J, et al. Diagnosis of penetrating injuries of the pharynx and esophagus in the severely injured patient. J Trauma. 2009;67:152-154.
27 Fulda G, Brathwaite CEM, Rodriguez A, et al. Blunt traumatic rupture of the heart and pericardium: A ten-year experience (1979-1989). J Trauma. 1991;31:167-173.
28 Perchinsky MJ, Long WB, Hill JG. Blunt cardiac rupture: The Emanuel Trauma Center experience. Arch Surg. 1995;130:852-857.
29 Pretre R, Faidutti B. Surgical management of aortic valve injury after nonpenetrating trauma. Ann Thorac Surg. 1993;56:1426-1431.
30 Lin JC, Ott RA. Acute traumatic mitral valve insufficiency. J Trauma. 1999;47:165-168.
31 Halstead J, Hosseinpour AR, Wells FC. Conservative surgical treatment of valvular injury after blunt chest trauma. Ann Thorac Surg. 2000;69:766-768.
32 Wu JJK, Yu TJ, Wang JJ, et al. Early repair of traumatic ventricular septal defect and mitral valve regurgitation. J Trauma. 1995;39:1191-1193.
33 Ginzburg E, Dygert J, Parra-Davila E, et al. Coronary artery stenting for occlusive dissection after blunt chest trauma. J Trauma. 1998;45:157-161.
34 Biffl WL, Moore FA, Moore EE, et al. Cardiac enzymes are irrelevant in the patient with suspected myocardial contusion. Am J Surg. 1994;169:523-528.
35 Velmahos GC, Karaiskakis M, Salim A, et al. Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J Trauma. 2003;54:45-51.
36 Maron BJ, Link MS, Wang PJ, et al. Clinical profile of commotio cordis: An underappreciated cause of sudden death in the young during sports and other activities. J Cardiovasc Electrophysiol. 1999;10:114-120.
37 Link MS, Wang PJ, Pandian NG, et al. An experimental model of sudden death due to low-energy chest-wall impact (commotio cordis). N Engl J Med. 1998;338:1805-1811.
38 Nagy KK, Lohmann C, Kim DO, et al. Role of echocardiography in the diagnosis of occult penetrating cardiac injury. J Trauma. 1995;38:859-862.
39 Feliciano DV, Burch JM, Mattox KL, et al. Balloon catheter tamponade in cardiovascular wounds. Am J Surg. 1990;160:583-587.
40 Asensio JA, Stewart BM, Murray J, et al. Penetrating cardiac injuries. Surg Clin North Am. 1996;76:685-724.
41 Moreno C, Moore EE, Majure JA, et al. Pericardial tamponade: A critical determinant for survival following penetrating cardiac wounds. J Trauma. 1986;26:821-825.
42 Ball CG, Williams BH, Wyrzykowski AD, et al. A caveat to the performance of pericardial ultrasound in patients with penetrating cardiac wounds. J Trauma. 2009;67:1123-1124.
43 Richardson JD, Flint LM, Snow NJ, et al. Management of transmediastinal gunshot wounds. Surgery. 1981;90:671-676.
44 Renz BM, Cava RA, Feliciano DV, et al. Transmediastinal gunshot wounds: A prospective study. J Trauma. 2000;48:416-422.
45 Grossman MD, May AK, Schwab CW, et al. Determining anatomic injury with computed tomography in selected torso gunshot wounds. J Trauma. 1998;45:446-456.
46 Stassen NA, Lukan JK, Spain DA, et al. Reevaluation of diagnostic procedures for transmediastinal gunshot wounds. J Trauma. 2002;53:635-638.
47 Gasparri MG, Lorelli DR, Kralovich KA, et al. Physical examination plus chest radiography in penetrating periclavicular trauma: The appropriate trigger for angiography. J Trauma. 2000;49:1029-1033.
48 Gonzalez WA, Falimirski ME. The role of angiography in periclavicular penetrating trauma. Am Surg. 1999;65:711-714.
49 Hershberger RC, Aulivola B, Murphy M, et al. Endovascular grafts for treatment of traumatic injury to the aortic arch and great vessels. J Trauma. 2009;67:660-671.
50 du Toit DF, Lambrechts AV, Stark H, et al. Long-term results of stent-graft treatment of subclavian artery injuries: Management of choice for stable patients? J Vasc Surg. 2008;47:739-743.
51 Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: Multicenter trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42:374-380.
52 Burkhart HM, Gomez GA, Jacobson LE, et al. Fatal blunt aortic injuries: A review of 242 autopsy cases. J Trauma. 2001;50:113-115.
53 Katyal D, McLellan BA, Brenneman FD, et al. Lateral impact motor vehicle collisions: Significant cause of blunt traumatic rupture of the thoracic aorta. J Trauma. 1997;42:769-772.
54 Dyer DS, Moore EE, Ilke DN, et al. Thoracic aortic injury: How predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1,561 patients. J Trauma. 2000;48:673-683.
55 Fabian TC, Davis KA, Gavant ML, et al. Prospective study of blunt aortic injury: Helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg. 1998;227:666-676.
56 Mirvis SE, Shanmuganathan K, Buell J, et al. Use of spiral computed tomography for the assessment of blunt trauma patients with potential aortic injury. J Trauma. 1998;45:922-930.
57 Atkins MD, Marrocco CJ, Bohannon WT, et al. Stent-graft repair for blunt traumatic aortic injury as the new standard of care: Is there evidence? J Endovasc Ther. 2009;16(Suppl I):153-162.
58 Demetriades D, Velmahos GC, Scalea TM, et al. Blunt traumatic thoracic aortic injuries: Early or delayed repair- Results of an American Association for the Surgery of Trauma prospective study. J Trauma. 2009;66:967-973.
59 Malhotra AK, Fabian TC, Croce MA, et al. Minimal aortic injury: A lesion associated with advancing diagnostic techniques. J Trauma. 2001;51:1042-1048.
60 Demetriades D, Velmahos GC, Scalea TM, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: Results of an American Association for the Surgery of Trauma multicenter study. J Trauma. 2008;64:561-571.
61 Razzouk AJ, Gundry SR, Wang N, et al. Repair of traumatic aortic rupture: A 25-year experience. Arch Surg. 2000;135:913-918.
62 von Oppell UO, Dunne TT, De Groot MK, et al. Traumatic aortic rupture: Twenty-year meta-analysis of mortality and risk of paraplegia. Ann Thorac Surg. 1994;58:585-593.
63 Prager M, Polterauer P, Bohmig HJ, et al. Collagen versus gelatin-coated Dacron versus stretch polytetrafluoroethylene in abdominal aortic bifurcation graft surgery: Results of a seven-year prospective, randomized multicenter trial. Surgery. 2001;130:408-414.