Chapter 30 Thoracic Metastatic Disease
Introduction
Metastatic disease is the most common chest malignancy and the chest acquires more metastases than any system.1 In autopsy series, pulmonary metastases are present in 20% to 54% of patients with a primary malignancy.2,3 The most common extrathoracic malignancies to metastasize to the chest include breast cancer, gastrointestinal (GI) malignancies (colon, pancreatic, and gastric cancer), melanoma, head and neck tumors, and renal cell cancer.1 Rarely, metastatic disease to the thorax can be the initial presentation of a malignancy. Tumor spread to the chest can occur via hematogenous, lymphatic, or endobronchial routes. Endobronchial spread most commonly occurs in primary lung adenocarcinoma and from tumors of the upper airway and paranasal sinuses. Chest radiographs and computed tomography (CT) are the main modalities used to assess thoracic metastatic disease. Other modalities currently serve complementary roles and include magnetic resonance imaging (MRI), ultrasound (US) and positron emission tomography (PET)/CT. Technique and typical and atypical CT imaging manifestations of pulmonary metastases, pleural metastases, and cardiac metastases are addressed.
Technique
Imaging is indispensable in the evaluation of patients with a primary tumor. In these patients, the principal functions of imaging are to stage patients and to detect or exclude the presence of metastatic disease. Achieving this goal with the appropriate imaging technique is essential. Chest radiographs, posteroanterior and lateral views, generally serve as the initial evaluation of patients with possible metastatic disease. In patients whose radiographs demonstrate obvious signs of metastatic disease, additional imaging modalities may not be necessary and chest radiographic follow-up may be all that is needed.4 However, chest radiographs lack sensitivity and CT scanning has been shown to be superior to chest radiographs in the detection of pulmonary nodules. CT scanning is now accepted as the state-of-the-art modality in the search for metastatic disease and in characterizing lesions in the thorax.5,6 The evolution of CT as the standard began with improvements in CT technology that include the ability to obtain thinner collimation and development of spiral and subsequently multidetector row computed tomography (MDCT) scanners. Thinner collimation brought on the development of the science of high-resolution computed tomography (HRCT) to assess diffuse lung disease, improved anatomic assessment and characterization of small lesions. Current MDCT scanners obtain a continuous volume acquisition and, thus, decrease misregistration artifacts and improve nodule detection by eliminating interslice gaps. This aids assessment of diffuse lung diseases, increases our sensitivity for small lung nodules, and improves multiplanar image reconstructions. In addition to the increased sensitivity provided by CT scanning, other benefits include its ability to quantify disease in the case of possible metastectomy, assess response to therapy on sequential studies, and provide a detailed roadmap to guide biopsy.
Our present CT chest protocol for assessing patients with possible metastatic disease include MDCT scan parameters that allow image review in both standard and lung algorithms at a 2.5-mm image thickness. The CT parameters also permit 1.25-mm-thick image reconstructions to be performed retrospectively, as needed. Images (2.5-mm image thickness) are also reconstructed in both the coronal and the sagittal planes. Images are reviewed at workstations that have cine mode capabilities.
1. Contrast enhancement permits a more exact delineation of the mediastinum and hilar regions and is also useful to detect subtle pleural metastatic disease.
2. Patients with primary malignancy are at increased risk for developing pulmonary emboli. In retrospective reviews of CT scans of oncologic patients, between 3.3% and 4.0% of patients had incidentally discovered pulmonary emboli with higher risks associated with inpatients, advanced disease, gynecologic malignancy, and melanoma.7,8
3. Patients undergoing chemotherapy often have indwelling catheters that may lead to thrombotic occlusion of vessels and visualization of collateral vessels.
The advances in MDCT, changes in image review from hard-copy film to workstations, and the use of the cine mode have dramatically improved our sensitivity to small solid lesions.9 In addition, sensitivity in detection of nonsolid and semisolid lesions has increased. It is widely accepted that CT scanning is a sensitive but nonspecific modality in assessing metastatic disease. False-positive nodules are often caused by intraparenchymal lymph nodes and noncalcified granulomas. Image processing can also improve image interpretation. It has been shown that the addition of maximum intensity projection images, by demonstrating vascular structures as clearly tubular branching structures and enhanced anatomic orientation, increases reader sensitivity to small lung nodules.10
Other modalities such as US, MRI, and PET serve complementary roles in evaluating metastatic disease of the thorax. Electrocardiogram (ECG)-gated cardiac magnetic resonance imaging (CMRI) is useful in evaluating the heart and surrounding cardiac structures for questionable findings on staging CT and equivocal findings on echocardiography that may suggest metastatic disease. CMRI is a cardiac-gated study that eliminates cardiac motion and has excellent temporal resolution and excellent soft tissue contrast. In a similar vein, ECG-gated CT has emerged as an alternative to CMRI in the evaluation of cardiac tumors in patients who are unable to undergo CMRI, for example, those patients who have an implanted ferromagnetic device or discomfort lying on the MRI table for prolonged periods of time.11
Patterns of Metastatic Disease
Simple Nodules
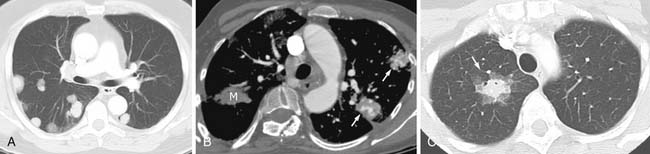
Most metastases to the chest are spread hematogenously. The characteristic appearance of these metastatic lesions include multiple bilateral peripheral spherical or ovoid sharply marginated nodules of variable sizes predominantly affecting the periphery of the lungs and the lower lobes (where the majority of the blood flow is directed)1,3,12 (Figure 30-1A). The typical sharply marginated edges of metastatic lesions help differentiate these tumors from primary lung cancer that normally show ill-defined, spiculated margins that extend into the adjacent lung parenchyma. Growth of metastatic lesion is extremely variable and volume doubling times, an increase in the diameter of a nodule by 26%, have been reported to range from 1 to 2 weeks for rapidly growing lesions such as sarcomas, melanomas, and germ cell tumors to months for thyroid malignancy.13 Hematogenous spread of metastases can also simulate a miliary pattern, typical of medullary thyroid malignancy.1 Occasionally, imaging metastatic nodules with contrast-infused CT imaging demonstrates areas of enhancement with dilated, tortuous tubular enhancing vessels. This feature can be seen in sarcomas, particularly alveolar soft part sarcoma or leiomyosarcoma6,14 (see Figure 30-1B).
Benign tumors rarely metastasize, but occasionally, metastases are found in the lung. Benign tumors that have been shown to metastasize to the lung include leiomyoma of the uterus, hydatidiform mole, giant cell tumor of bone, chondroblastoma, pleomorphic adenoma of the salivary gland, and meningioma. Slow growth of these solid benign nodules is typical but these are indistinguishable from other malignant metastatic nodules.6
Occasionally, metastatic nodules followed on sequential scans may not change in appearance and these nodules may, in fact, represent sterilized metastatic disease. These residual nodules are indistinguishable from viable tumor on CT scans. Biopsied materials show areas of necrosis and/or areas of fibrosis. Testicular cancer, breast cancer, and choriocarcinoma are common tumors that present in this manner.15,16 In some cases, following serum markers such as beta-human chorionic gonadotropin, alphafetoprotein, or PET scanning can be helpful in following and assessing viability. In addition, growth of metastatic nonseminomatous germ cell lesions with negative serum markers frequently represents a conversion to a benign mature teratoma.17
Nodules with a Ground Glass “Halo”
Hemorrhage around a metastatic lesion can create the appearance of a solid nodule surrounded by a ground glass rim, known as the CT halo sign. Fragility of the neovascular bed is a proposed mechanism. Metastatic tumors that can be associated with this appearance include choriocarcinoma and angiosarcoma among other lesions18,19 (see Figure 30-1C). The appearance is not pathognomonic for metastatic disease and can be seen in other lesions that bleed including infections such as invasive aspergillosis (the most common condition showing the CT halo sign in immunocompromised patients) and inflammatory processes such as Wegener’s granulomatosis. In addition, primary malignancy can simulate this appearance; examples include minimally invasive adenocarcinoma (the most common condition showing the CT halo sign in immunocompetent patients) and lymphoma.6,19
Cavitary Nodules
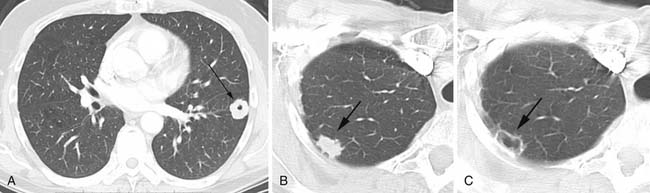
Cavitation in metastases is less frequent than in primary lung cancers. Metastatic squamous cell lesions, from any source, were thought to have the highest rate of cavitation, approximately 10%. However, a similar rate of cavitation was demonstrated in a series of metastatic adenocarcinoma.6 Typical cavitary metastases have thick irregular walls (Figure 30-2A). Less frequently, cavitary metastases can have thin walls and this feature is particularly noted in patients with metastatic adenocarcinoma or sarcoma. Chemotherapy can induce cavitation in lesions, and this is more common in sarcomatous metastatic lesions than in other cancers1,6,20 (see Figure 30-2B and C).
Calcified Nodules
Calcification is often considered a sign of benignity, representing either a granuloma or a hamartoma; however, infrequently calcification can occur in a variety of metastatic lesions through different mechanisms. Most frequently, calcification in metastases is associated with matrix-forming primary tumors such as osteosarcoma and chondrosarcoma12,21 (Figure 30-3A). Mucoid-producing tumors can lead to calcified metastases and include tumors of the GI tract and breast cancer (see Figure 30-3B). In addition, dystrophic calcification can be seen in metastatic thyroid cancer, synovial sarcoma, and giant cell tumors.21–24 Dystrophic calcification can also occur in areas of necrosis after therapy.13 Aside from the four characteristic patterns of benign calcification (diffuse, central, popcorn and laminar), CT scanning cannot reliably differentiate benign and malignant lesions based on other patterns of calcifications.6,13
Airspace Nodules, Airspace Disease
An airspace pattern of disease in the lung parenchyma, mimicking pneumonia, can be seen in metastatic disease. These lesions can be ground glass or consolidative in density or a mixture of these densities and preserve the lung architecture with air bronchograms. Adenocarcinomas of the GI tract and the breast can spread to the lung and then grow along the lung scaffolding, known as lepidic growth, to create this radiographic appearance.25–27 In one study of metastatic GI tumor, an airspace pattern of metastatic disease was identified in approximately 10% of patients.27 This pattern of disease spread is also similar in appearance to primary well-differentiated adenocarcinoma of the lung.
Key Points Nodular pattern
• Nodules are the most common form of metastatic disease.
• Calcification in metastatic disease is possible but overlaps with forms of calcification in benign lesions.
• Metastatic nodules can mimic nonmalignant forms of disease and include solid nodules, (granuloma), airspace nodules (pneumonia), and nodules with a halo (including invasive aspergillosis).
Branching Metastatic Disease
Reticular and Nodular Opacities (Lymphangitic Carcinomatosis)
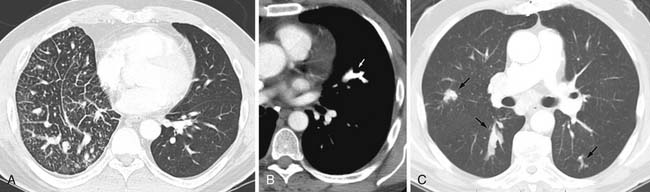
Reticular and nodular opacities in the lung related to metastatic disease can be caused by lymphangitic carcinomatosis. Lymphangitic carcinomatosis is usually a result of hematogenous spread of malignancy and secondary invasion of the lymphatic system. Less frequently, lymphangitic carcinomatosis can be a result of direct spread from mediastinal and hilar lymph nodes. Only 50% of cases of lymphangitic tumor have hilar adenopathy supporting a hematogenous mechanism as the primary mode of lymphangitic tumor spread. Lymphangitic carcinomatosis most often results from spread of carcinomas of the lung; breast; upper GI malignancies including pancreas and stomach; prostate, uterus, cervical, or thyroid cancer, and adenocarcinomas of unknown primary site.1 The process can be a focal or a bilateral diffuse process and is often associated with pleural effusions 60% of the time. Chest radiograph findings in lymphangitic carcinomatosis include reticulonodular opacities and may also demonstrate thickening of the interlobular septa. Optimal evaluation includes thin images that result in decreased summation artifacts, and therefore, HRCT shows advantages over routine CT scanning. Typical CT features include thickening of the bronchovascular bundles and peripheral interlobular septa that may be beaded or smooth. Smooth peripheral interlobular septal thickening needs to be differentiated from the Kerley-B lines of pulmonary edema. There is often prominence of the centrilobular artery, thickening of core structures in the secondary pulmonary artery creating a central “dot.” Importantly, to aid in the differentiation from other reticular processes such as fibrosis, in lymphangitic carcinomatosis, the architecture of the secondary pulmonary lobule is preserved28,29 (Figure 30-4A).
Key Points Branching pattern
• Branching metastatic disease includes lymphangitic carcinomatosis and endobronchial and endovascular metastases.
• Lymphangitic carcinomatosis can be smooth or beaded and spares the architecture of the secondary pulmonary lobule.
• Examination of sequential thin-cut images helps establish the origin of the branching pattern.
Endovascular Metastases
Two other forms of branching nodular metastatic disease can occur and include lesions to the pulmonary artery known as tumor emboli and lesions to the airway or endobronchial metastases. Tumor emboli are similar to hematogenous metastatic lesions in mode of spread but tumor emboli differ in that tumor emboli proliferate only in the vasculature. At autopsy, microscopic tumor emboli were seen in up to 26% of patients with solid malignancy.30 CT findings include multifocal dilatation and areas of beading and generally affect mid to small pulmonary arteries (see Figure 30-4B). Infrequently, central vessels are involved.31 Peripheral airspace disease can be seen secondary to infarction.31–33 Tumors most frequently associated with tumor emboli include hepatoma, breast cancer, renal cancer, prostate cancer, gastric cancer, and choriocarcinoma.34
Endobronchial Metastases
Endobronchial metastases are infrequent, occurring in less than 2% to 4% of patients at autopsy.35,36 Routes of metastatic disease spread to bronchi include (1) direct deposition, either aspiration of tumor cells or lymphatic or hematogenous spread, and (2) direct invasion from tumor in the adjacent lung or lymph nodes. Endobronchial metastases are only rarely visible on radiographs, most often manifesting as distal lung parenchyma volume loss. CT can detect these lesions as small as soft tissue filling defects in the airways, areas of narrowing, or obstruction35 (see Figure 30-4C). Endobronchial lesions noted on CT scans need to be differentiated from primary lung cancers. Clinically, the patients can present with dyspnea, cough, and hemoptysis.37 Generally, endobronchial metastases are a late manifestation of disease with a median survival after diagnosis of 9 to 18 months.37,38 Renal, breast, and colorectal cancers are most frequently associated with endobronchial metastatic disease.37,38 Occasionally, it is difficult to differentiate the branching patterns of endobronchial and endovascular metastases. Differentiation is facilitated by sequential thin-cut image review often in the cine mode and allows separation of the normal pulmonary artery or bronchus from the involved structure.
Adenopathy
Metastatic Intrathoracic Adenopathy
Metastatic intrathoracic adenopathy can be seen in all nodal groups. Generally, metastatic adenopathy occurs with parenchymal lung metastases, but occasionally, metastatic intrathoracic adenopathy can develop in the absence of parenchymal nodules. Metastatic intrathoracic adenopathy from tumors other than lung cancer is most frequently associated with cancers of the genitourinary system, head and neck tumors, breast cancer, and melanoma.13
Enlarged nodes in the mediastinum and hilar regions can develop in patients with an extrathoracic primary without harboring metastatic cells and, on radiologic studies, appears identical to metastatic adenopathy. This pattern is known as a sarcoid-like reaction. This pattern of disease has to be distinguished from a metastatic adenopathy. Biopsy results of these enlarged nodes show noncaseous necrosis, without evidence of malignancy.39
Pleural Manifestations of Metastatic Disease
Pleural Metastases
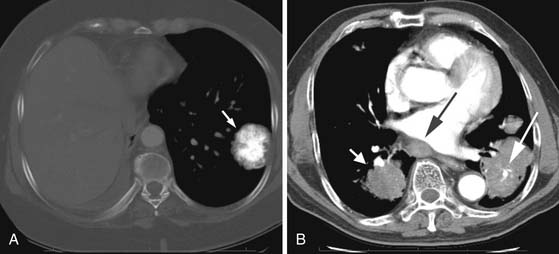
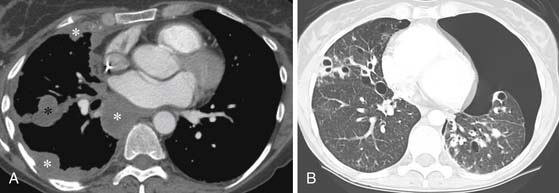
Pleural metastases are generally associated with metastatic adenocarcinoma and are frequently associated with tumors of the lung, breast, pancreas, and stomach.1 Pleural metastases most frequently are a result of hematogenous spread of disease but can also be secondary to lymphangitic spread or related to hepatic metastases.1 Chest radiographs and CT scans of pleural metastases can present as an effusion or smooth or nodular pleural thickening (Figure 30-5A). In the absence of irregular or nodular pleural thickening, it is difficult to distinguish a benign from a malignant pleural effusion. Occasionally, diffuse thickening can mimic mesothelioma, and this pattern is typically associated with metastatic lesions from renal cell carcinoma and lymphoma.1
Pneumothorax Associated with Metastatic Disease
A pneumothorax can result from a metastatic lesion. Proposed mechanisms include cavitation of a metastatic nodule adjacent to the pleura or an aggressive tumor that creates a bronchopleural fistula. A pneumothorax associated with metastatic disease is frequently related to osteosarcoma but can occur with other sarcomas or aggressive tumors12,40 (see Figure 30-5B). The incidence of a pneumothorax in a patient with osteosarcoma has been estimated to be 5% to 7%. When a pneumothorax occurs secondary to metastatic disease, patients most frequently have other known metastatic disease; however, occasionally, a pneumothorax can be the initial manifestation of a metastatic lesion.41 In people who present with a pneumothorax, fewer than 1% will have an underlying malignancy.42
Cardiac Metastases
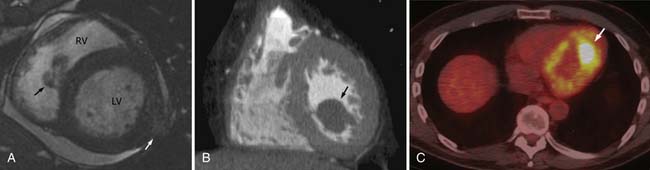
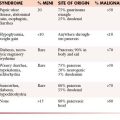
Metastatic disease to the heart and pericardium is 22 times more frequent than primary tumors.43 In a retrospective autopsy review of patients with a primary malignancy, 10.7% of patients were found to have cardiac metastases with most metastases arising from lung cancer (36.4%).44 Nonsolid primary tumors (lymphoma, leukemia, and Kaposi’s sarcoma) are also relatively common.43,44 By cell type, adenocarcinoma accounted for the most cardiac metastases; however, melanoma had the highest propensity to involve the heart, approximately 50% of cases.44 The majority of the lesions are located in the pericardium and epicardium followed by myocardium and endocardium with approximately one third of epicardial lesions having an associated pericardial effusion. The majority of cardiac metastases are clinically silent.
The typical imaging pattern for cardiac metastases includes multiple nodules or masses, but they can be diffusely infiltrative (Figure 30-6). In many cases, the appearance of cardiac metastases is relatively nonspecific. CMRI is the preferred method to evaluate these lesions and most lesions show a low T1 signal and high T2 signal. Occasionally, in patients with metastatic melanoma, CMRI characteristics are helpful in distinguishing metastases from other lesions in which the T1 signal may be high. Enhancement post contrast is generally heterogeneous. CT imaging is also nonspecific, demonstrating soft tissue lesions.11
Differential Diagnosis
Generally, when multiple nodules in the chest are detected in patients with a known primary malignancy, no further diagnostic tests are necessary and nodules can be assumed to be metastatic. The appearance of multiple nodules in patients with more than one known primary is problematic and can necessitate the need for tissue sampling in order to make a more specific diagnosis and institute the best therapy. Also problematic is the situation in which a solitary pulmonary nodule is detected in a patient with an extrathoracic primary malignancy. In these cases, differentiation between a primary and a secondary malignancy in the chest is important. In a published series on patients with breast cancer, the incidence of a second primary lung cancer was higher than a metastatic lesion.45 The propensity for a second primary to develop is highest in cases in which the initial primary originated from a head and neck cancer but is also common when there is a primary in the lung, breast, prostate, or stomach, especially in those people who smoke.1 Statistically, conversely, primary cancers from melanoma and sarcomas will more likely develop solitary metastatic lesions in the lung rather than a secondary primary lung neoplasm.1
A difficult issue that frequently arises in the evaluation of possible metastatic disease is small nodules that are detected on CT scanning in the workup of a patient with a primary malignancy. It should be stressed that most of the small solid noncalcified nodules, less than 4 mm in diameter, discovered at CT scanning, even in patients with a known primary extrathoracic malignancy, will turn out to be benign, most often sequela of granulomatous disease. Despite this fact, the current recommendation is to follow these nodules to exclude metastatic disease. In our experience, the vast majority of metastatic nodules will show changes on the initial 3-month follow-up study.
Key Points Cardiac
• Metastatic disease to the heart is 22 times more common than a primary tumor.
• More than one third of cardiac metastases are from lung cancer.
• Melanoma is the tumor with the highest association of cardiac metastases.
• Epicardium is the most frequently involved layer, followed by the myocardium and endocardium.
1. Herold C.J., Bankier A.A., Fleischmann D. Lung metastases. Eur Radiol. 1996;6:596-606.
2. Crow J., Slavin G., Kreel L. Pulmonary metastasis: a pathologic and radiologic study. Cancer. 1981;47:2595-2602.
3. Davis S.D. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology. 1991;180:1-12.
4. Davis S.D., Westcott J., Fleishon H., et al. Screening for pulmonary metastases. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215(Suppl):655-662.
5. Peuchot M., Libshitz H.I. Pulmonary metastatic disease: radiologic-surgical correlation. Radiology. 1987;164:719-722.
6. Seo J.B., Im J.G., Goo J.M., et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics. 2001;21:403-417.
7. Gladish G.W., Choe D.H., Marom E.M., et al. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology. 2006;240:246-255.
8. Cronin C.G., Lohan D.G., Keane M., et al. Prevalence and significance of asymptomatic venous thromboembolic disease found on oncologic staging CT. AJR Am J Roentgenol. 2007;189:162-170.
9. Tillich M., Kammerhuber F., Reittner P., et al. Detection of pulmonary nodules with helical CT: comparison of cine and film-based viewing. AJR Am J Roentgenol. 1997;169:1611-1614.
10. Gruden J.F., Ouanounou S., Tigges S., et al. Incremental benefit of maximum-intensity-projection images on observer detection of small pulmonary nodules revealed by multidetector CT. AJR Am J Roentgenol. 2002;179:149-157.
11. Hoey E.T., Mankad K., Puppala S., et al. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64:1214-1230.
12. Libshitz H.I., North L.B. Pulmonary metastases. Radiol Clin North Am.. 1982;20:437-451.
13. McLoud T.C. Thoracic Radiology: The Requisites. St. Louis: CV Mosby; 1998. :336-337
14. Choi J.I., Goo J.M., Seo J.B., et al. Pulmonary metastases of alveolar soft-part sarcoma: CT findings in three patients. Korean J Radiol. 2000;1:56-59.
15. Libshitz H.I., Jing B.S., Wallace S., et al. Sterilized metastases: a diagnostic and therapeutic dilemma. AJR Am J Roentgenol. 1983;140:15-19.
16. Swett H.A., Westcott J.L. Residual nonmalignant pulmonary nodules in choriocarcinoma. Chest. 1974;65:560-562.
17. Panicek D.M., Toner G.C., Heelan R.T., et al. Nonseminomatous germ cell tumors: enlarging masses despite chemotherapy. Radiology. 1990;175:499-502.
18. Tateishi U., Hasegawa T., Kusumoto M., et al. Metastatic angiosarcoma of the lung: spectrum of CT findings. AJR Am J Roentgenol. 2003;180:1671-1674.
19. Lee Y.R., Choi Y.W., Lee K.J., et al. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol. 2005;78:862-865.
20. Thalinger A.R., Rosenthal S.N., Borg S., et al. Cavitation of pulmonary metastases as a response to chemotherapy. Cancer. 1980;46:1329-1332.
21. Maile C.W., Rodan B.A., Godwin J.D., et al. Calcification in pulmonary metastases. Br J Radiol. 1982;55:108-113.
22. deSantos L.A., Lindell M.M.Jr., Goldman A.M., et al. Calcification within metastatic pulmonary nodules from synovial sarcoma. Orthopedics. 1978;1:141-144.
23. Maxwell J.R., Yao L., Eckardt J.J., et al. Case report 878: densely calcifying synovial sarcoma of the hip metastatic to the lungs. Skeletal Radiol. 1994;23:673-675.
24. Hall F.M., Frank H.A., Cohen R.B., et al. Ossified pulmonary metastases from giant cell tumor of bone. AJR Am J Roentgenol. 1976;127:1046-1047.
25. Ohnishi H., Haruta Y., Yokoyama A., et al. Metastatic breast cancer presenting as air-space consolidation on chest computed tomography. Intern Med. 2009;48:727-731.
26. Jung J.I., Kim H.H., Park S.H., et al. Thoracic manifestations of breast cancer and its therapy. Radiographics. 2004;24:1269-1285.
27. Gaeta M., Volta S., Scribano E., et al. Air-space pattern in lung metastasis from adenocarcinoma of the GI tract. J Comput Assist Tomogr. 1996;20:300-304.
28. McLoud T.C. Thoracic Radiology. St. Louis: CV Mosby; 1998. :216-218
29. Webb W.R., Müller N.L., Naidich D.P. High-resolution CT of the Lung, 2nd ed., Philadelphia: Lippincott-Raven, 1996. :149-150
30. Winterbauer R.H., Elfenbein I.B., Ball W.C.Jr. Incidence and clinical significance of tumor embolization to the lungs. Am J Med. 1968;45:271-290.
31. Shepard J.A., Moore E.H., Templeton P.A., et al. Pulmonary intravascular tumor emboli: dilated and beaded peripheral pulmonary arteries at CT. Radiology. 1993;187:797-801.
32. Kang C.H., Choi J.A., Kim H.R., et al. Lung metastases manifesting as pulmonary infarction by mucin and tumor embolization: radiographic, high-resolution CT, and pathologic findings. J Comput Assist Tomogr. 1999;23:644-646.
33. Kim A.E., Haramati L.B., Janus D., et al. Pulmonary tumor embolism presenting as infarcts on computed tomography. J Thorac Imaging. 1999;14:135-137.
34. Chan C.K., Hutcheon M.A., Hyland R.H., et al. Pulmonary tumor embolism: a critical review of clinical, imaging, and hemodynamic features. J Thorac Imaging. 1987;2:4-14.
35. Ikezoe J., Johkoh T., Takeuchi N., et al. CT findings of endobronchial metastasis. Acta Radiol. 1991;32:455-460.
36. Braman S.S., Whitcomb M.E. Endobronchial metastasis. Arch Intern Med. 1975;135:543-547.
37. Akoglu S., Ucan E.S., Celik G., et al. Endobronchial metastases from extrathoracic malignancies. Clin Exp Metastasis. 2005;22:587-591.
38. Katsimbri P.P., Bamias A.T., Froudarakis M.E., et al. Endobronchial metastases secondary to solid tumors: report of eight cases and review of the literature. Lung Cancer. 2000;28:163-170.
39. Hunsaker A.R., Munden R.F., Pugatch R.D., et al. Sarcoidlike reaction in patients with malignancy. Radiology. 1996;200:255-261.
40. Dines D.E. Pneumothorax and metastatic sarcomas. Chest. 1978;73:681-682.
41. Furrer M., Althaus U., Ris H.B. Spontaneous pneumothorax from radiographically occult metastatic sarcoma. Eur J Cardiothorac Surg. 1997;11:1171-1173.
42. Smevik B., Klepp O. The risk of spontaneous pneumothorax in patients with osteogenic sarcoma and testicular cancer. Cancer. 1982;49:1734-1737.
43. Lam K.Y., Dickens P., Chan A.C. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027-1031.
44. Klatt E.C., Heitz D.R. Cardiac metastases. Cancer. 1990;65:1456-1459.
45. Cahan W.G., Castro E.B. Significance of a solitary lung shadow in patients with breast cancer. Ann Surg. 1975;181:137-143.