CHAPTER 10 Thoracic Metastases, Mimics, and Treatment Effects
Most breast cancer patients with intrathoracic metastases are asymptomatic, the exception being those with significant pleural effusions. Therefore, unless pleural, parenchymal, or nodal metastases are demonstrated at initial staging, thoracic metastases are most commonly found at the time of follow-up imaging. A significant number are found on chest x-rays done for other reasons. Isolated involvement of the lung or pleural space occurs in 15% to 25% of women with metastatic breast cancer.1
Pulmonary metastases are typically peripheral,2 and, if large enough, may be discovered on preoperative chest radiographs. Because of its low cost, chest radiography may be a reasonable choice as a baseline study or in follow-up; however, the extremely low yield, false-positive results, inferior sensitivity compared with CT, and lack of data supporting an outcome benefit limit routine use in follow-up.
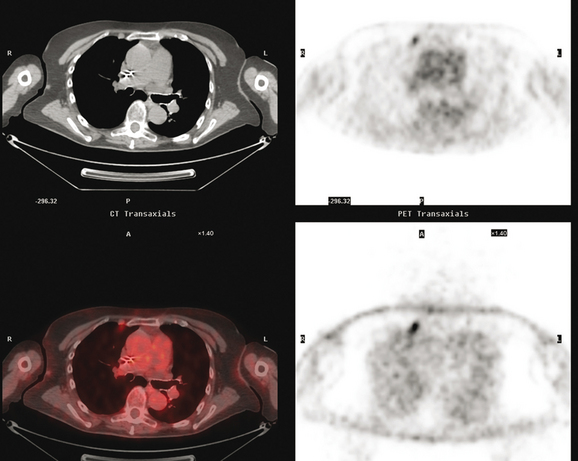
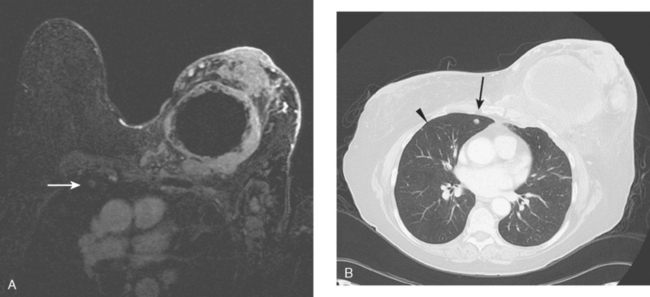
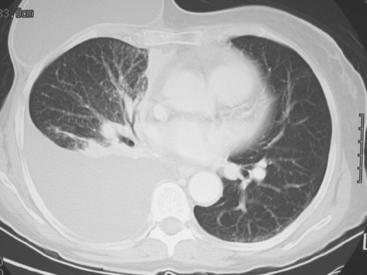
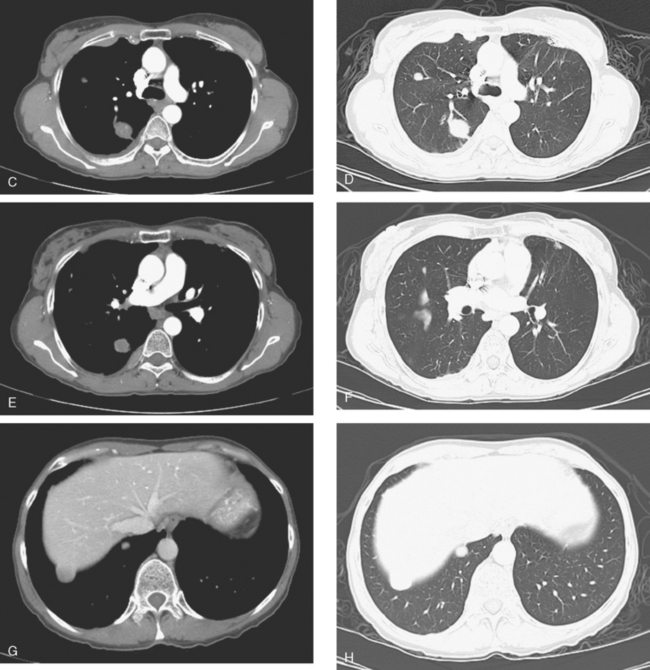
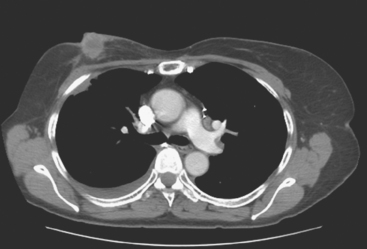
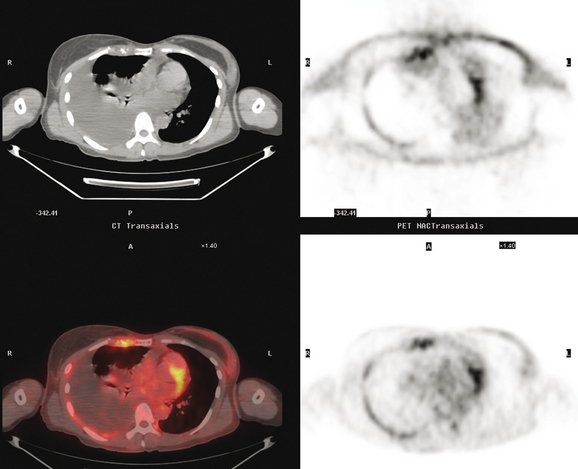
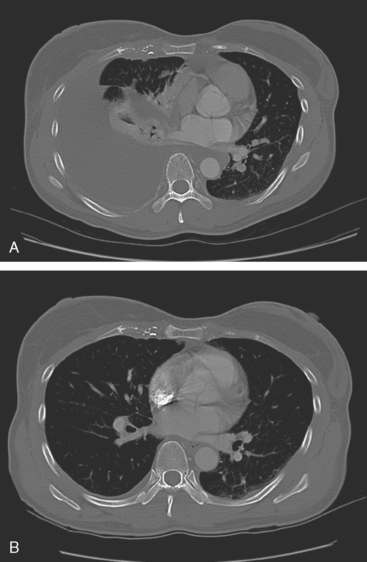
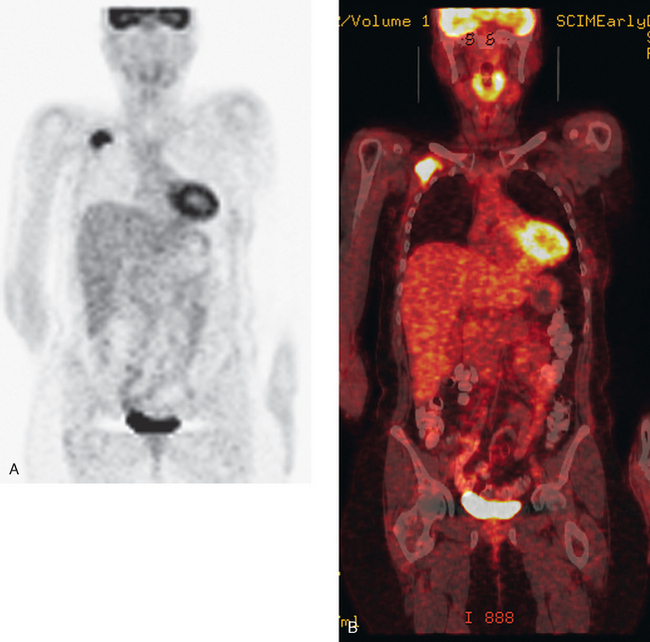
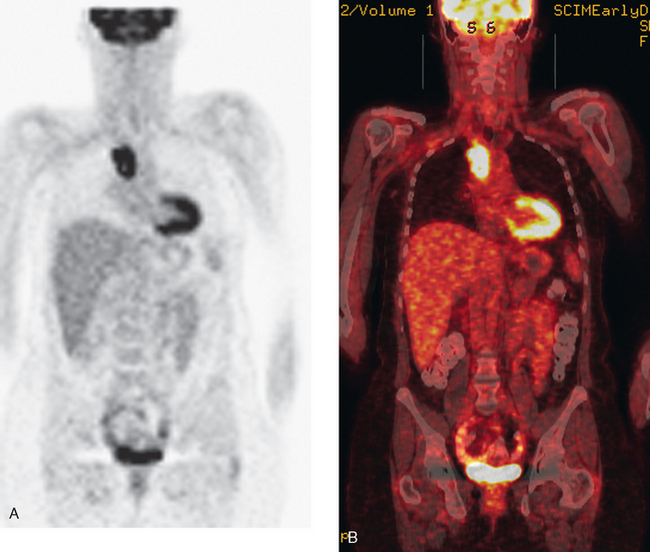
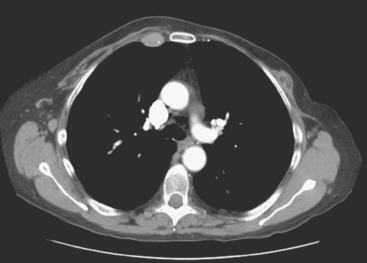
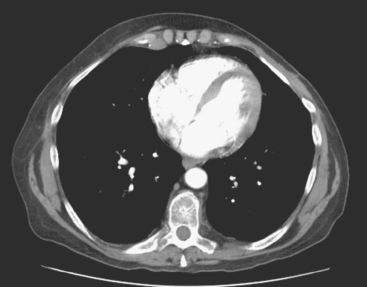
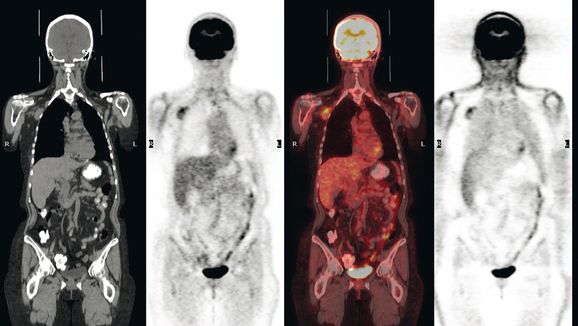
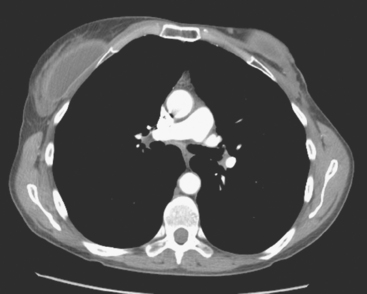
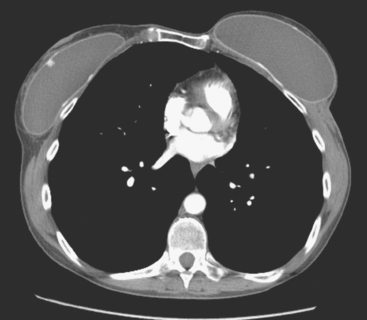
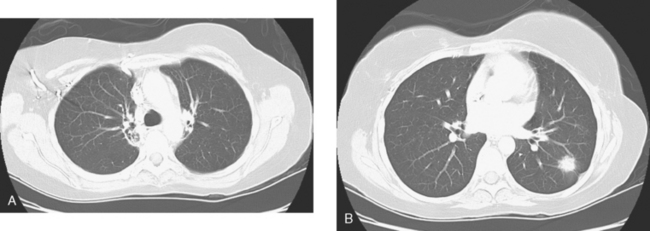
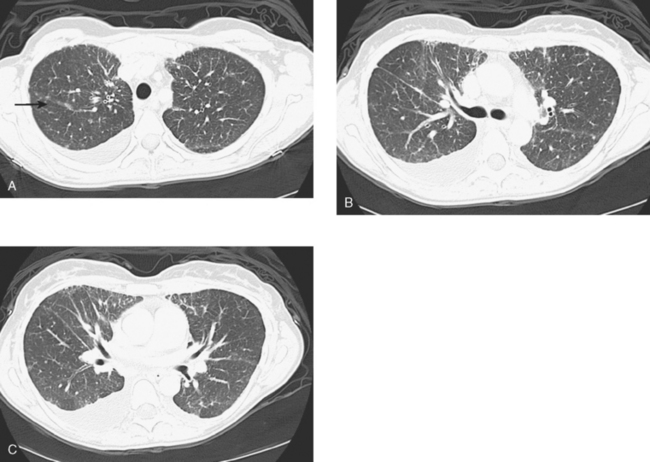
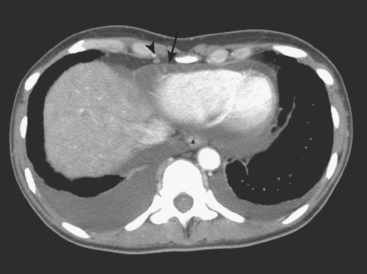
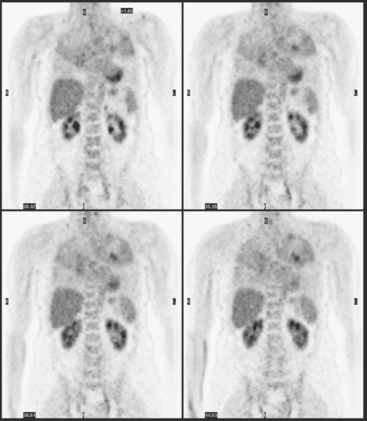
CT is the preferred modality for investigation of thoracic metastases. CT has proven efficacy for detecting local, regional, and distant disease. Normal-sized internal mammary nodes are not visualized on CT, so a patient in whom CT identifies a node or nodes larger than 6 mm is at risk for malignant involvement.3 Because of the recognized problem of relying on size only to diagnose disease, positron emission tomography (PET)/CT is presently considered the modality of choice for assessing internal mammary nodes (Figure 1). In women who die from disseminated breast cancer, intrathoracic nodal metastases may occur in more than 70% of patients.4 As with internal mammary nodes, using size as the major criterion for disease identification reduces both sensitivity and specificity. Magnetic resonance imaging (MRI) can detect most lesions larger than 5 mm, does not expose the patient to ionizing radiation, and has acceptable imaging times; however, it is generally viewed as complementary to CT, rather than as a replacement for CT (Figure 2).5–9 Cardiac and respiratory motion, reduced sensitivity for lesions smaller than 5 mm (compared with CT), calcified metastases, and suboptimal ability to assess lymphangitic spread are recognized limitations of MRI in the thorax.10
In one series of 73 patients who underwent FDG PET and CT for recurrent or metastatic disease, PET proved more sensitive (85% versus 50%) and specific (90% versus 83%) than CT. Also, PET uptake in mediastinal and internal mammary nodes was 2 times more prevalent than enlarged nodes on CT.11 FDG PET has been shown to be significantly more accurate in staging the mediastinum in patients with non–small cell lung cancer, using histology as the gold standard.12–13 Extrapolation of these data to patients with breast cancer may be problematic. It has been recognized that certain subtypes of breast cancer (e.g., invasive lobular, tubular, carcinoma in situ) show relatively low FDG uptake, resulting in reduced sensitivity compared with infiltrating ductal carcinoma (IDC) primary tumors.14–15 Theoretically, patients whose initial staging FDG PET is negative in these tumor subtypes (especially if the primary has not been excised) may demonstrate reduced sensitivity for detection of metastases, including within the chest, on restaging studies.
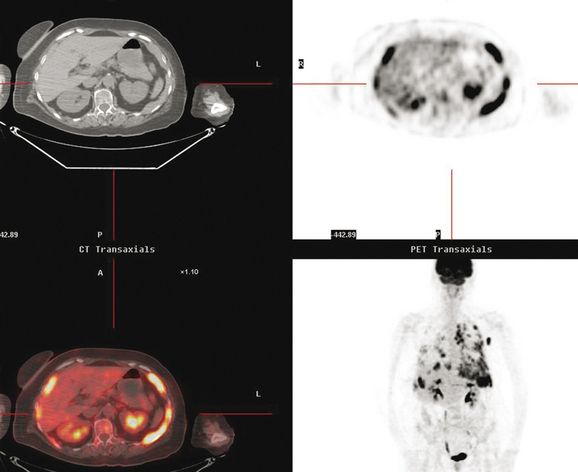
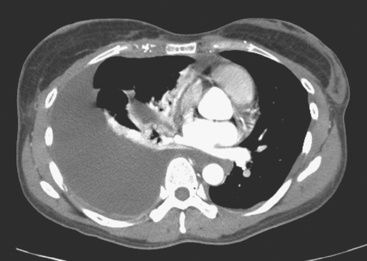
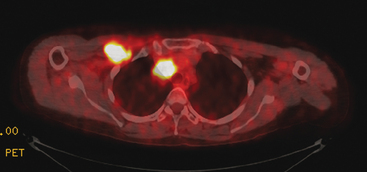
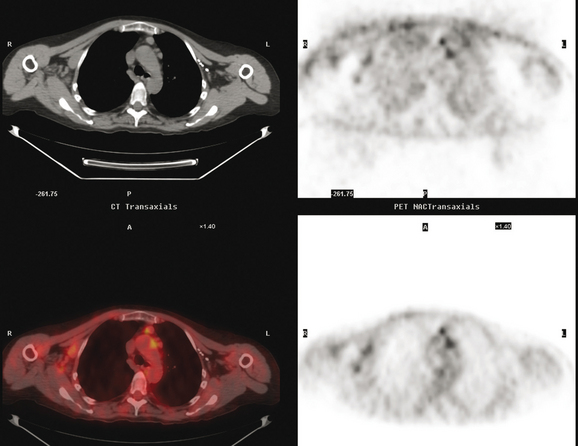
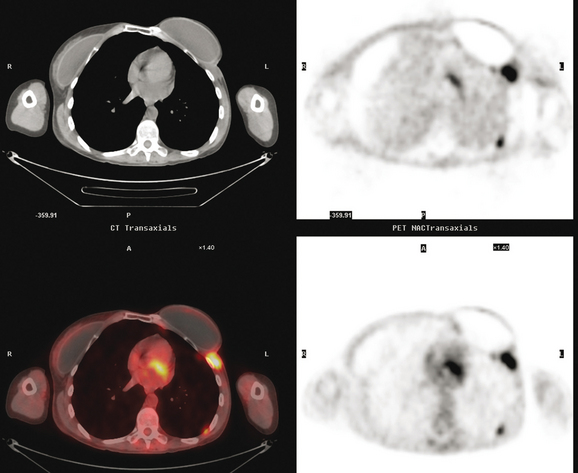
Pleural involvement may be suspected based on clinical signs on physical examination or symptoms (e.g., cough, shortness of breath, pleuritic-type chest pain, orthopnea), the discovery of a pleural effusion on plain radiography or CT, or the identification of pleural abnormalities on CT or FDG PET (Figure 3). Pleural effusion is often unilateral and on the same side as the breast primary.16 The effusion can be confirmed by chest radiography, CT, or even ultrasound. Thoracentesis may be performed for both diagnostic and therapeutic purposes. If the result is nondiagnostic, or negative with a strong clinical suspicion of malignancy, consideration may be given to FDG PET imaging (preferably, PET/CT).
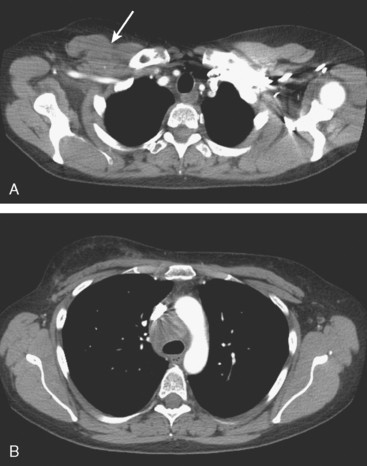
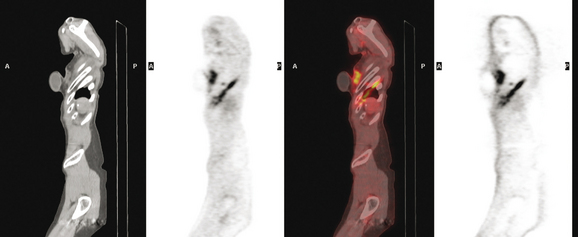
Many special circumstances exist with respect to the thoracic manifestations of breast cancer, reviewed in depth by Jung and associates.17 One important consideration is radiation therapy–related complications. Radiation pneumonitis typically occurs within 3 months of radiation therapy and progresses from diffuse haziness to coalescence of areas of consolidation to fibrous changes on chest x-ray or CT. Findings in the irradiated field may persist for months to years (Figure 4). Although PET/CT has demonstrated efficacy in separating scar from active tumor in many malignancies, its use following radiation therapy in the irradiated field may be problematic for months to 1 year or more. The heightened metabolic activity of activated monocytes at sites of radiation pneumonitis can last for a variable period of time, especially in the lung and with head and neck tumors. A negative FDG PET study at a site of postirradiation change on chest x-ray or CT is reassuring; however, a positive study must be interpreted with caution and reassessed or further investigated before initiation of additional tumor-targeted therapy.
The development of a solitary pulmonary nodule in patients who have been treated for breast cancer should not be assumed to be a breast recurrence without histologic proof. About half of these patients will have lung cancer, and a small percentage of cases will be benign.18 These patients are excellent candidates for PET/CT. Hypermetabolic uptake in the nodule directs the patient to tissue sampling (sometimes obviated by the finding of widespread metastases). Absent FDG uptake, as well as an otherwise negative study, may allow watchful waiting with short-term CT follow-up (e.g., 3 to 6 months). Recall the limitations of PET with respect to histology and lesion size.
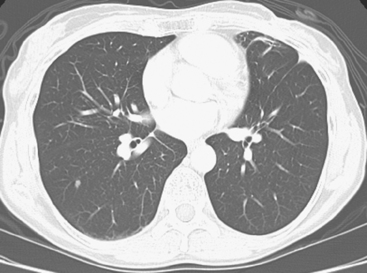
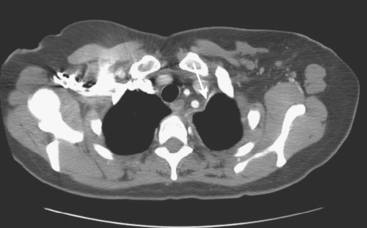
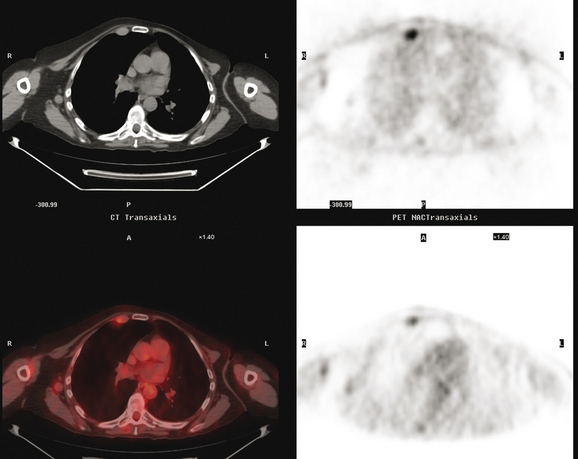
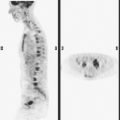
Lymphangitic metastases are extremely common in women who die from breast cancer.19 CT is the imaging modality of choice for the identification of this process, which is most often bilateral (Figure 5). Findings include irregular thickening of the interlobar septa and peribronchovascular sheaths as well as thickening of the structures in the central regions of the secondary pulmonary lobules.20
1 Patanaphan V, Salazar OM, Ricco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1998;81(9):1109-1112.
2 Shaw JP, Glassman LR. Thoracic metastases from breast cancer. In: Breast Cancer. Philadelphia: Elsevier Churchill Livingston; 2005:661-666.
3 Meyer JE, Munzenrider JE. Computed tomographic demonstration of internal mammary lymph-node metastases in patients with locally recurrent breast carcinoma. Radiology. 1981;139:661-663. (A)
4 Thomas JM, Redding WH, Sloane JP. The spread of breast cancer: importance of intrathoracic lymphatic route and its relevance to treatment. Br J Cancer. 1979;40:540-547.
5 Feirerstein IM, Jicha DL, Pass HL, et al. Pulmonary metastases: MR imaging with surgical correlation—a prospective study. Radiology. 1992;181:123-129.
6 Müller NL, Gamsu G, Webb WR. Pulmonary nodules: detection using magnetic resonance and computed tomography. Radiology. 1985;155:687-690.
7 Panicek DM. MR imaging for pulmonary metastases? Radiology. 1992;182:10-11.
8 Webb WR, Sustman HD. MR imaging of thoracic disease: clinical uses. Radiology. 1992;182:621-630.
9 Ohno Y, Sugimura K, Hatabu H. MR imaging of lung cancer. Eur J Radiol. 2002;44(3):172-181.
10 Vogt FM, Herborn CU, Hunold P, et al. HASTE MRI versus chest radiography in the detection of pulmonary nodules: comparison with MDCT. AJR Am J Roentgenol. 2004;183:71-78.
11 Eubank WB, Mankoff DA, Takasugi J, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19:3516-3523.
12 Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16(6):2142-2149.
13 Scott WJ, Gobar LS, Terry JD, et al. Mediastinal lymph node staging of non-small-cell lung cancer: a prospective comparison of computer tomography and positron emission tomography. J Thorac Cardiovasc Surg. 1996;111(3):642-648.
14 Crippa F, Seregni E, Agresti R, et al. Association between [18F]-fluorodeoxyglucose uptake and postoperative histology, hormone receptor status, thymidine labeling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25:1429-1434.
15 Avril N, Menzel M, Dose J, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9-16.
16 Connolly JEJr, Erasmus JJ, Patz EFJr. Thoracic manifestations of breast carcinoma: metastatic disease and complications of treatment. Clin Radiol. 1999;54:487-494.
17 Jung JI, Kim HH, Park SH, et al. Thoracic manifestations of breast cancer and its therapy. RadioGraphics. 2004;24:1269-1285.
18 Casey JJ, Stempel BG, Scanlon EF, et al. The solitary pulmonary nodule in the patient with breast cancer. Surgery. 1984;96:801-805.
19 Kreisman H, Wolkove N, Finkelstein HS, et al. Breast cancer and thoracic metastases: review of 119 patients. Thorax. 1983;38:175-179.
20 Webb WR, Müller NL, Naidich NP. High-Resolution CT of the Lung, 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 2001.
CASE 1 Solitary pulmonary nodule in the breast cancer patient: Primary lung cancer versus breast cancer metastasis
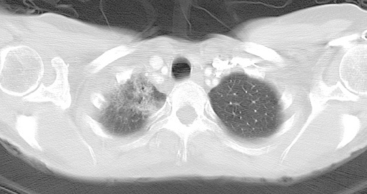
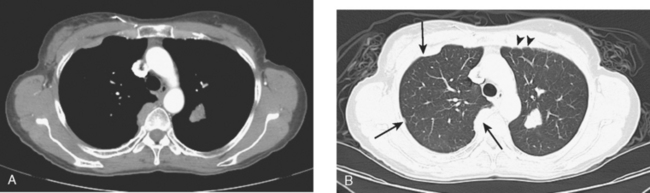
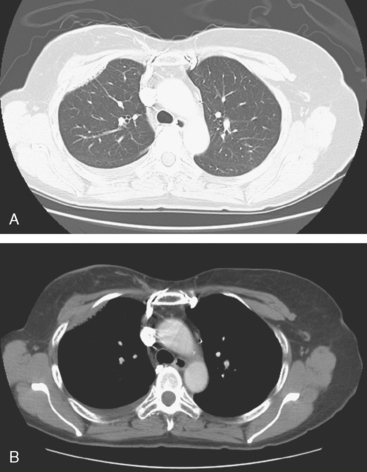
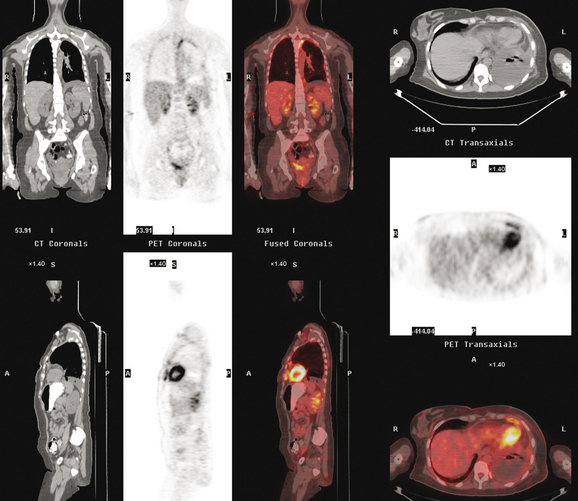
An 80-year-old woman with emphysema was found to have a left upper lobe (LUL) mass on chest x-ray. This was confirmed on chest CT, which also showed a similar-appearing, suspicious right lower lobe (RLL) lesion (Figure 1). Fine-needle aspiration (FNA) of the LUL lesion was consistent with a non–small cell lung carcinoma. The patient’s past medical history was significant for prior left mastectomy for breast cancer nearly 20 years earlier. Lymph nodes were reportedly negative, and the patient did not undergo additional therapy at that time. She also had a significant past smoking history, on the order of 1.5 packs per day.
The PET scan showed both the LUL and RLL lesions to be hypermetabolic (Figure 2). Based on the information available, this result seemed most consistent with synchronous lung cancers. However, subsequent immunocytochemical stains performed on the material obtained from FNA suggested the cause to be metastatic breast carcinoma.
CASE 2 Lung metastases, progression to pleural metastases
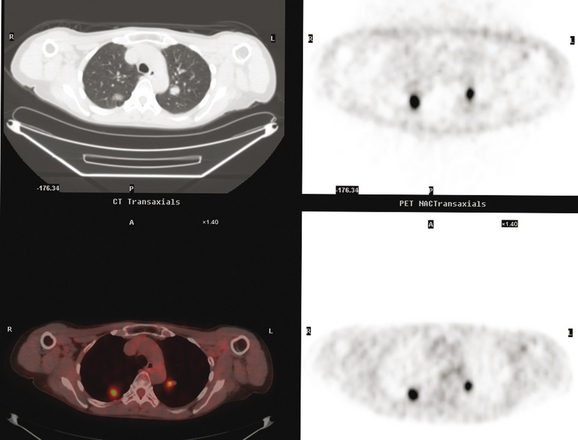
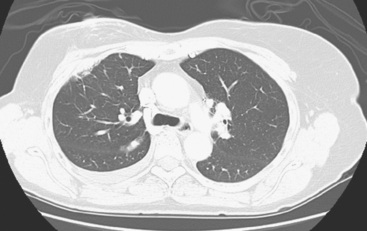
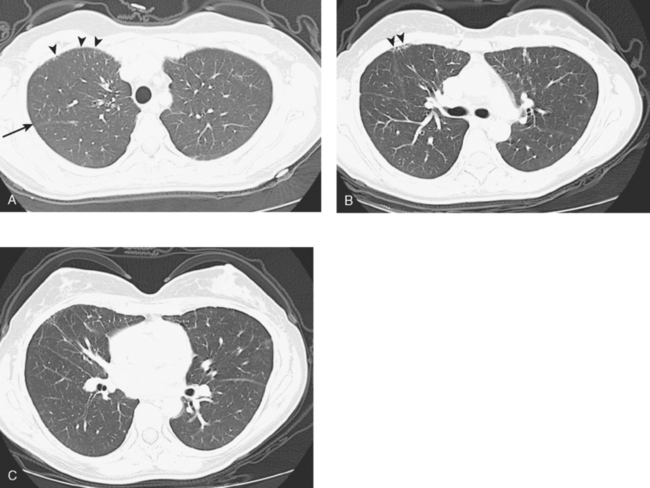
At the time of restaging, the patient was essentially asymptomatic. Chest CT and positron emission tomography (PET) showed four lung nodules on CT, three of which were hypermetabolic on PET (a 3 × 7-mm nodule was probably too small) (Figures 1, 2, 3, 4, and 5).
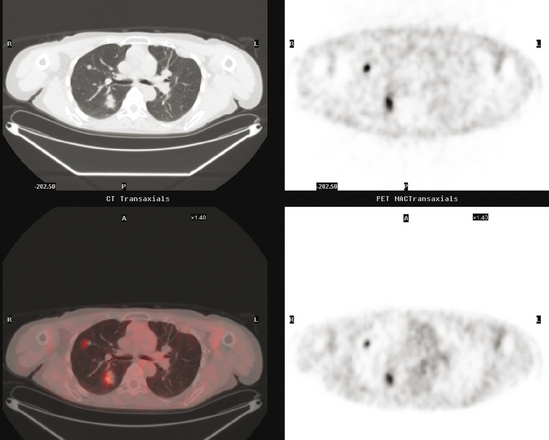
FIGURE 4 A more inferior PET/CT image shows hypermetabolism in the two right lung lesions depicted in Figure 1.
Seven months later, follow-up CT scans showed new right pleural and mediastinal disease, confirmed as fluorodeoxyglucose avid on PET (Figures 6 and 7). The patient was not particularly symptomatic, and was reluctant to have chemotherapy. She began a trial of androgenic hormone therapy (Halotestin).
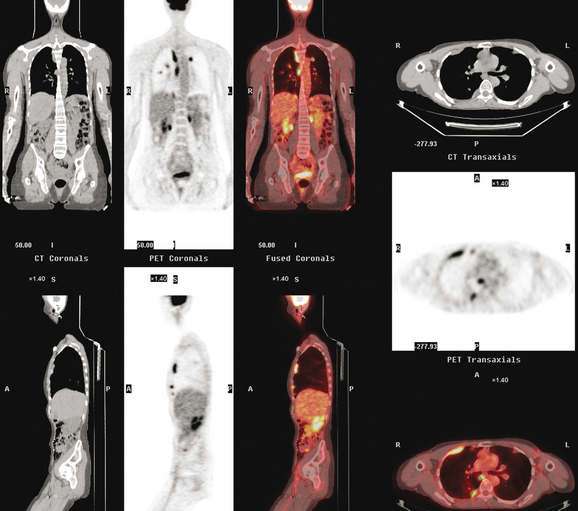
FIGURE 7 Concurrent PET/CT, 7 months after PET scan in Figures 3 to 5, shows new peripheral linear and nodular right hemithoracic hypermetabolism, which corresponds to the new pleural masses on CT and represents pleural metastases.
When she was re-evaluated 3 months later, she noted right posterior chest pain and dry cough. Imaging showed further progression. She was restaged in conjunction with the entrance requirements of a chemotherapy clinical trial. These studies showed progression of right pleural metastases and new liver metastases (Figure 8).
CASE 3 Chest wall, pleural, and thoracic nodal recurrence
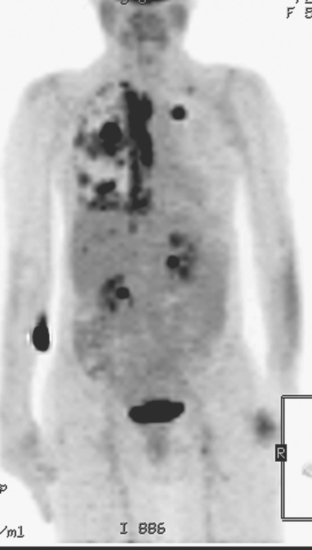
Initial evaluations suggested local recurrence only, and mastectomy was planned. However, her CA-125 was noted to be elevated, leading to restaging with positron emission tomography (PET)/CT, diagnostic enhanced body CT scans, and bone scan. PET and CT findings suggested thoracic recurrence (Figures 1, 2, 3, 4, and 5). CT scans showed a moderate-sized right pleural effusion with modest associated metabolic activity on PET. A 1-cm nodule in the right major fissure was metabolically active, as was a left hilar lymph node. The known right breast local recurrence was intensely hypermetabolic and could be faintly visualized as soft tissue activity on bone scan (Figure 6).
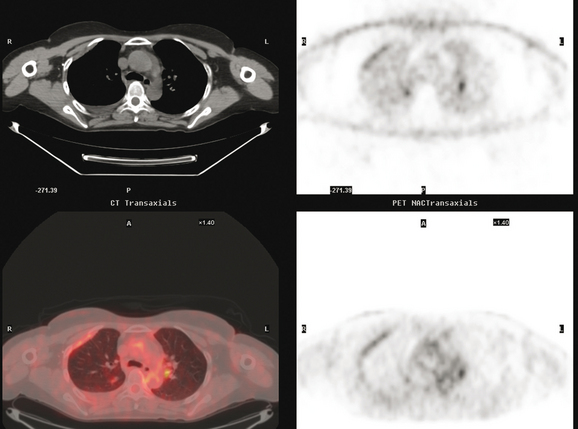
FIGURE 4 Axial PET/CT images, corresponding in level to Figure 2, show the right pleural nodules to be metabolically active. The associated activity is better seen on the NAC image (upper right) than on the AC image (lower right). A linear band of right anterolateral subpleural activity corresponds with the radiation fibrosis changes on CT, and was 8 years old. Activity corresponding with the left hilar lymph node is also seen. Note also subtle increased dependent activity in the right hemothorax compared with the left, seen on fused PET/CT (lower left) and AC PET (lower right), correlating with the pleural effusion. This, coupled with the pleural nodularity and activity, suggests a malignant etiology of the effusion, which was subsequently confirmed histologically.
Based on the PET and CT findings, CT-guided thoracentesis and FNA of the right pleural nodule were performed. Both confirmed metastatic breast cancer by morphology and immunohistochemistry. Thyroid transcription factor-1 was noted to be negative, largely eliminating lung cancer from the differential.
TEACHING POINTS
Pleural metastases are commonly manifested by the development of pleural fluid, which most often occurs on the same side as the primary breast carcinoma. The route of spread is thought to be lymphatic rather than hematogenous, which would be expected to be bilateral. This propensity for same-side laterality of pleural metastases is demonstrated in this case. The findings can range from effusion alone to fluid with variable degrees of pleural thickening and enhancement. This can be fairly smooth, as in this case, or frankly nodular and masslike (see Case 5). Activity of pleural effusions on bone scans (see Case 4) and PET scans suggests a malignant etiology. In this case, both the pleural fluid and the nodules in the major fissure showed metabolic activity on PET, a constellation of findings strongly suggesting a malignant etiology, which was subsequently confirmed histologically.
CASE 4 Pleural recurrence; bone scan and CT findings
Diagnostic thoracentesis showed malignant cells consistent with breast primary, which were estrogen receptor and progesterone receptor positive. The patient was placed on anastrozole (Arimidex) after restaging studies showed no evidence of other disease (Figures 1 and 2).
TEACHING POINTS
Causes of asymmetrical nuclear medicine activity of the hemithoraces in breast cancer patients include prior surgery or radiation, presence of implants, and chest wall and pleural metastases. Knowledge of the prior clinical history is essential to assess the significance of asymmetry. Patients who have undergone mastectomy without reconstruction have less breast soft tissue to attenuate bone scan activity and consequently may show better visualization of rib activity on anterior views on the mastectomy side. Patients who have been treated with mastectomy and implant reconstruction may show decreased rib activity due to attenuation on the treated side. Radiation to the breast or chest wall may result in discernible photopenia of ribs on the treated side. In general, these treatment-induced changes are better visualized on anterior views. Asymmetrical soft tissue activity such as seen here, visualized both anteriorly and posteriorly, suggests the possibility of tumor-related soft tissue activity. Most commonly, findings such as these are seen in the setting of a malignant pleural effusion, although such findings could be produced by extensive chest wall soft tissue recurrence.
CT findings of pleural recurrences range from effusion alone, to variable degrees of pleural enhancement and thickening (smooth or otherwise), to frank nodularity (see Case 5). The presence of pleural fluid serves as a natural contrast agent to allow interpreters to better visualize the pleura itself, which normally is thin to nearly imperceptible. Ready visualization of a thickened pleura in the presence of pleural fluid, in a patient with a cancer history, should heighten suspicion for a possible malignant etiology.
CASE 5 Pleural and chest wall recurrence
A 44-year-old woman with a history of breast cancer developed shortness of breath, and a chest radiograph showed a large right pleural effusion (Figure 1). The patient was 2 years post–right mastectomy and flap reconstruction for a node-negative, estrogen receptor–positive, progesterone receptor–negative, 1.5-cm infiltrating ductal carcinoma. The patient had declined chemotherapy and hormonal therapy.
Cytology of the serosanguineous thoracentesis aspirate confirmed carcinoma, consistent with breast primary. Workup with positron emission tomography (PET)/CT (Figures 2, 3 and 5) and enhanced body CT scans (Figures 4 and 6) showed findings of a malignant right pleural effusion, with abnormal right hemithoracic pleural thickening, nodularity, and enhancement, with a corresponding pleural rind of hypermetabolism on PET. Right anterior chest wall involvement was demonstrated, with hypermetabolism in asymmetrical soft tissue masses (Figures 2–6).
The patient was begun on chemotherapy with doxorubicin (Adriamycin) and docetaxel (Taxotere), as well as zoledronic acid (Zometa). Two months later, after three chemotherapy cycles, the chest CT was repeated to assess the response (Figures 7 and 8). In addition to resolution of the right pleural effusion, reduced pleural tumor burden was present, with tiny residual nodules where there previously had been bulky disease. In addition, small occasional sclerotic bone foci were now identified, thought to represent healing, formerly lytic bone metastases, previously unrecognized. These findings were stable and unchanged at repeat CT imaging 1 month later, after completion of chemotherapy, at which time the PET scan had normalized. The patient was then begun on tamoxifen.
TEACHING POINTS
As this case illustrates, some patients respond well to chemotherapy. As in all of imaging, knowledge of the clinical history and familiarity with the prior imaging studies is critical to the accurate interpretation of follow-up examinations. Correlation of the follow-up CT with the prior study enables the correct conclusions to be drawn, namely that the patient is responding well to chemotherapy, with the pleural effusion resolved and the bulky pleural nodules reduced to tiny pleural-based residuals. This excellent response was subsequently confirmed by a repeat PET scan, which showed complete resolution of the hypermetabolic pleural rind. Interpreting the follow-up study without considering the prior studies could lead to serious misinterpretation, such as that the nodules are new parenchymal disease, which could suggest the patient is not responding and mislead the oncologist into considering an alternative chemotherapeutic course. Critical observations on the way to the correct interpretation of the follow-up study are that all of the tiny nodules are along pleural surfaces. Of course, comparison with the preceding study showing bulky pleural nodular disease, aids in the correct assessment.
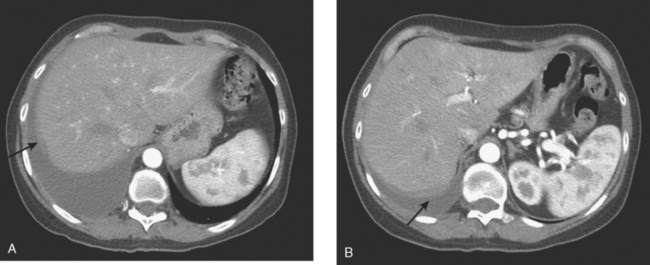
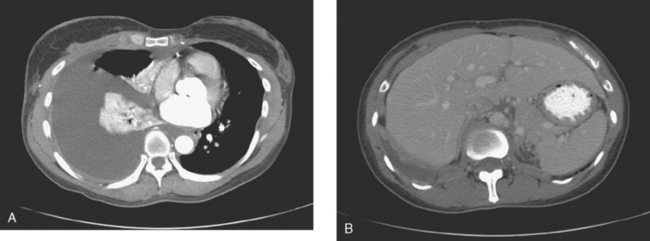
This case also serves to remind us of important anatomic relationships. Disease overlying the surface of the liver may be below the diaphragm and may represent serosal implants on the liver surface or peritoneal implants. This disease overlying the liver (see coronal projection image from the PET, Figure 2, far right; and abdominal CT image, Figure 4B) is above the diaphragm, in the pleural space, which PET reminds us extends far inferiorly to overlap the liver.
Finally, this case demonstrates an interesting manifestation of clinically unrecognized and asymptomatic bone metastases. The follow-up study, after initiation of chemotherapy, showed several tiny new osseous sclerotic foci in the spine. No corresponding abnormality could be seen, even in retrospect, at some of these levels on the prior study. This response is illustrated in the right side of the sternum (see Figure 8). Lytic change can be seen on the initial study in the right sternum, where sclerosis develops on the follow-up study after chemotherapy. Presumably, these lytic, small bone metastases (too small to be seen on PET and mostly subradiographic on pretreatment CT) developed sclerosis as a healing response to chemotherapy.
CASE 6 CT and PET findings of talc pleurodesis for malignant pleural effusion
A left-sided pleural effusion was initially identified when the patient was evaluated for a cough 4 years after surgical treatment for breast cancer. An abnormality at T10 was noted during the same period, and biopsy confirmed metastatic breast cancer. The patient underwent thoracenteses twice of 1.5 liters of fluid, with negative cytologies. Ultimately, for management of the recurrent effusion, the patient underwent left hemithoracic thoracoscopy and thoracotomy, with drainage of the left pleural effusion, visceral and parietal pleurectomy, decortication of the left lung, and mechanical and talc pleurodesis. A pericardial effusion was also drained and a pericardial window created. Cytologies of both the pericardial and pleural fluid were positive for carcinoma, consistent with breast primary. Biopsies of the pleura, diaphragm, and pericardium confirmed metastatic carcinoma, consistent with metastatic lobular carcinoma. Evaluation of the visceral pleural specimens noted carcinoma extending into the subpleural alveolar septa.
The patient was begun on chemotherapy. Positron emission tomography (PET)/CT scans 1 and 3 months after the left pleurodesis showed similar findings, with left linear pleural space hypermetabolism (Figure 1), corresponding to the distribution of hyperdense talc on CT (Figures 2 and 3).
Asad S, Aquino SL, Piyavisetpat N, et al. False-positive FDG positron emission tomography uptake in nonmalignant chest abnormalities. AJR Am J Roentgenol. 2004;182(4):983-989.
Kwek BH, Aquino SL, Fischman AJ. Fluorodeoxyglucose positron emission tomography and CT after talc pleurodesis. Chest. 2004;125:2356-2360.
Murray JG, Erasmus JJ, Bahtiarian EA, et al. Talc pleurodesis simulating pleural metastases on F18 FDG PET imaging. AJR Am J Roentgenol. 1997;168:359-360.
CASE 7 Infraclavicular and mediastinal nodal recurrence presenting with brachial plexopathy symptoms
A 38-year-old woman elected to undergo prophylactic left mastectomy and bilateral transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction 10 months after finishing radiation therapy for a right stage II, T2N1, 3-cm infiltrating ductal carcinoma, estrogen receptor and progesterone receptor negative and HER-2/neu negative, with 3 of 22 involved axillary lymph nodes. She had been treated with right mastectomy with clear margins, chemotherapy with four cycles each of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) and paclitaxel (Taxol), and right chest wall, supraclavicular, and posterior axillary boost radiation therapy (see Case 18 in Chapter 3 for the presenting imaging features and staging of this patient).
The patient’s postoperative course was complicated by development of abdominal wound infection, requiring débridement twice of infected, necrotic tissue and intravenous antibiotic therapy. She also required anticoagulation for pulmonary embolism. (See Case 3 in Chapter 6 for imaging features of a complicated TRAM flap donor site.)
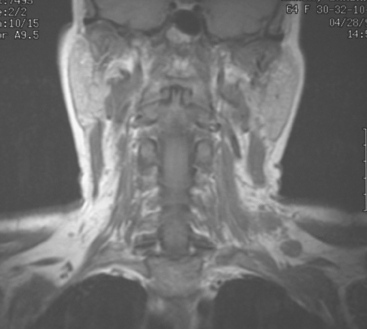
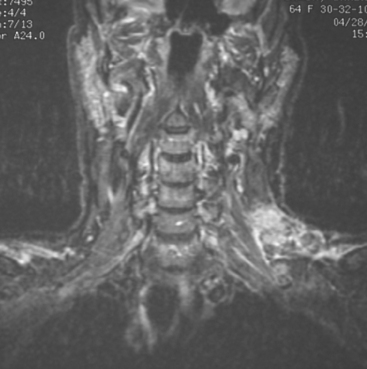
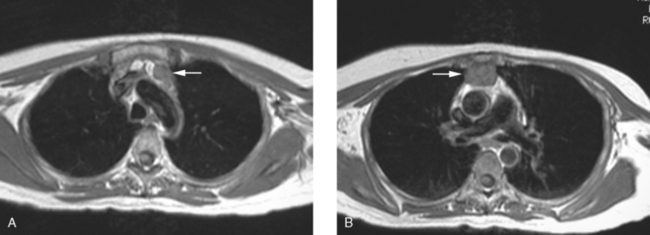
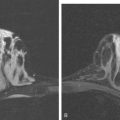
During the patient’s recovery from these procedures, she began to complain of right upper arm and shoulder pain. A lump developed under her right clavicle. She was imaged with enhanced body CT and PET/CT, which showed hypermetabolic nodal masses in the right paratracheal mediastinum and in the right infraclavicular region (Figures 1, 2, 3, and 4). These findings represented changes from prior studies, indicative of recurrent disease. Ultrasound of the right infraclavicular region identified a corresponding nodal mass (Figure 5), which was sampled with fine-needle aspiration technique, and confirmed recurrent adenocarcinoma, consistent with breast primary. To better define the extent of disease in light of the patient’s symptoms of right shoulder pain, a brachial plexus MRI was obtained (Figures 6, 7, 8, 9, 10, and 11).
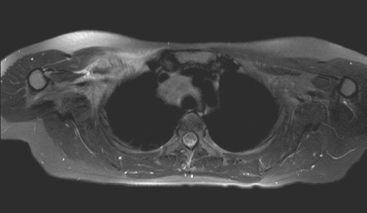
FIGURE 9 Fat-saturated, T2-weighted image, at a comparable level to Figure 7, shows hyperintensity of both the right paratracheal nodal mass and the interpectoral (Rotter’s) nodal mass. The medial aspects of both the right pectoralis major and minor muscles are also increased in signal intensity.
TEACHING POINTS
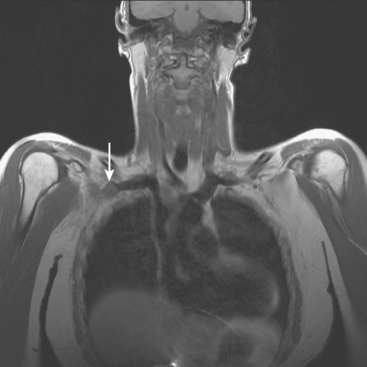
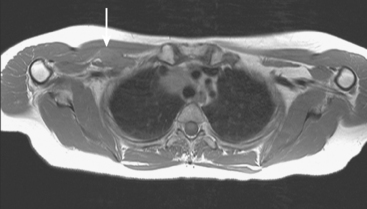
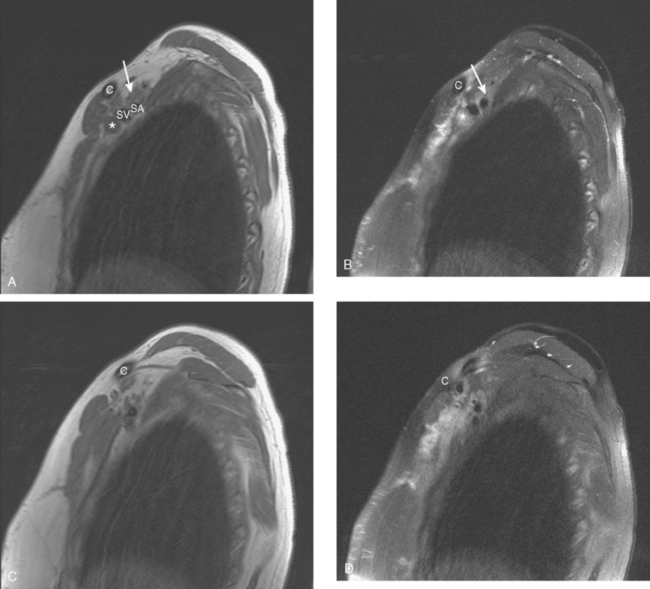
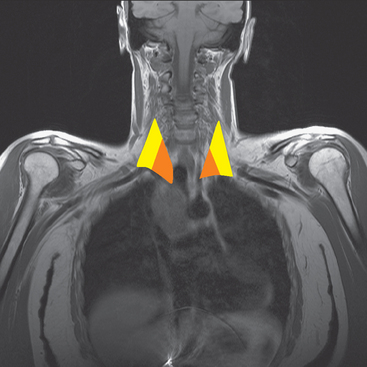
The brachial plexus is formed from the anterior rami of C5 through T1 in most people. The anterior rami reside just beyond the neural foramen. They form the trunks, which course between the anterior and middle scalene muscles (Figure 12), above the subclavian artery. The interscalene fat pad is an important anatomic landmark to evaluate for symmetry or effacement on coronal sequences because tumors or other processes at this level will impinge on brachial plexus trunks (Figure 13). These trunks ramify to form divisions posterior to the clavicle, which run in the supraclavicular triangle above the subclavian artery. These divisions ramify again to form cords inferior to the clavicle. These cords are the infraclavicular component of the brachial plexus with which we are most concerned in breast cancer patients. (Remember: Cords are the brachial plexus component behind the clavicle.) Although breast cancer metastases can arise anywhere along the course of the brachial plexus, the critical area is in the retroclavicular region around the cords of the brachial plexus. At this level, the cords are in close proximity to the axillary artery and vein (as the subclavian artery and vein are known lateral to the first rib).
Patients like this, with axillary nodal disease, are at a known 10% to 20% risk for recurrence at the apical axillary nodal level (axillary level III). Lymph node dissections target lymph nodes at axillary level I (lateral to pectoralis minor muscle) and level II (under the pectoralis minor muscle) and generally do not extend to encompass apical axillary nodes (level III), which lie in an infraclavicular site superior to the medial border of the pectoralis muscle. These apical axillary nodes are in continuity with supraclavicular nodes and are clustered around the axillary vessels in proximity to the cords of the brachial plexus.
Castillo M. Imaging the anatomy of the brachial plexus: review and self-assessment module. AJR Am J Roentgenol. 2005;185:S196-S204.
Hathaway PB, Mankoff DA, Maravilla KR, et al. Value of combined FDG PET and MR imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology. 1999;210:807-814.
Qayyum A, MacVicar AD, Padhani AR, et al. Symptomatic brachial plexopathy following treatment for breast cancer: utility of MR imaging with surface-coil techniques. Radiology. 2000;214:837-842.
CASE 8 Brachial plexus involvement
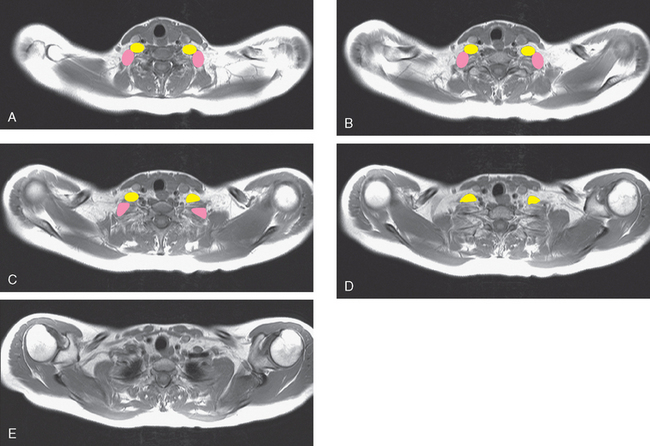
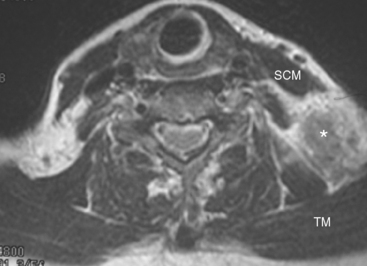
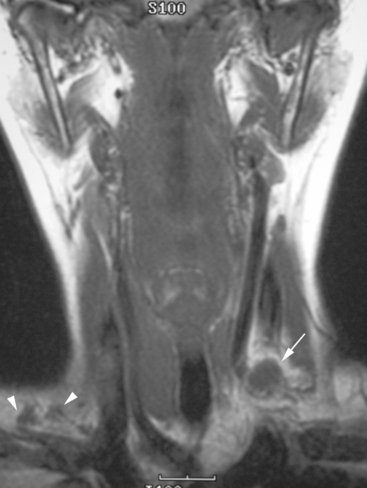
At the time of evaluation of the mild brachial plexopathy symptoms, a palpable left supraclavicular nodal mass was noted on physical examination. Fine-needle aspiration confirmed metastatic adenocarcinoma. CT and MRI of the brachial plexus confirmed stippled, nodular infiltration of the left supraclavicular triangle (Figures 1, 2, 3, 4, and 5). After progression on a trial of an experimental chemotherapeutic agent, the supraclavicular region was radiated. The supraclavicular mass decreased in size and became more mobile, and the patient’s shoulder paresthesias improved.
TEACHING POINTS
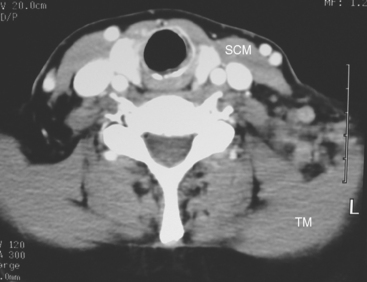
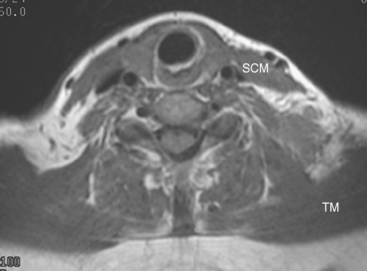
This patient had mild symptoms of an early left brachial plexopathy and developed a palpable supraclavicular node to suggest the etiology and location of the causative disease. From an anatomic perspective, MRI is the imaging modality with the best combination of soft tissue contrast and resolution for delineation of the location, size, and distribution of disease recurrence affecting the brachial plexus. If the findings were more subtle, positron emission tomography (PET) could be a valuable adjunct to image the region functionally, especially if the area had been previously surgically altered or radiated, which would make anatomic evaluation more difficult. The bulk of this recurrent disease is in the supraclavicular triangle, delineated by the sternocleidomastoid (SCM) muscle anteriorly and the trapezius muscle (TM) posteriorly. The brachial plexus divisions pass through the supraclavicular triangle above the subclavian artery, before ramifying behind the clavicle to form cords, which constitute the infraclavicular components of the brachial plexus, en route to the axilla. For a case of infraclavicular brachial plexus recurrence, see Case 7 in this chapter.
Castagno AA, Shuman WP. MR imaging in clinically suspected brachial plexus tumor. AJR Am J Roentgenol. 1987;149:1219-1222.
Castillo M. Imaging the anatomy of the brachial plexus: review and self-assessment module. AJR Am J Roentgenol. 2005;185:S196-S204.
Hathaway PB, Mankoff DA, Maravilla KR, et al. Value of combined FDG PET and MR imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology. 1999;210:807-814.
Qayyum A, MacVicar AD, Padhani AR, et al. Symptomatic brachial plexopathy following treatment for breast cancer: utility of MR imaging with surface-coil techniques. Radiology. 2000;214:837-842.
Wittenberg KH, Adkins MC. MR imaging of nontraumatic brachial plexopathies: frequency and spectrum of findings. RadioGraphics. 2000;20:1023-1032.
CASE 9 Nodal recurrence to mediastinum and supraclavicular regions
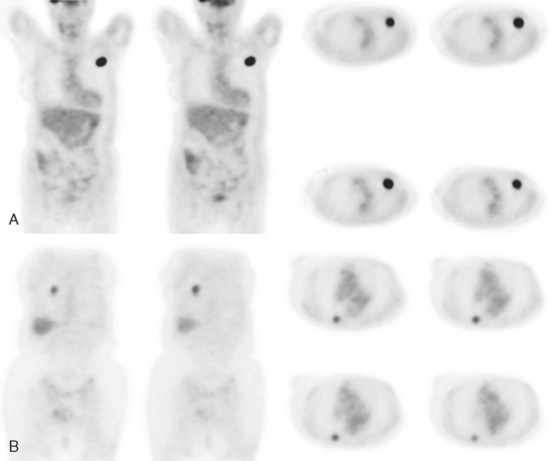
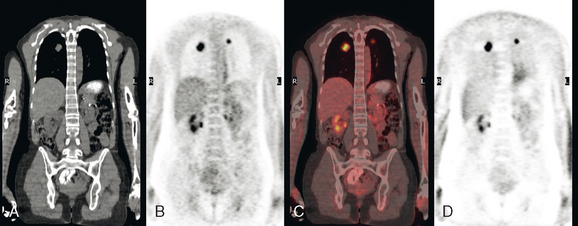
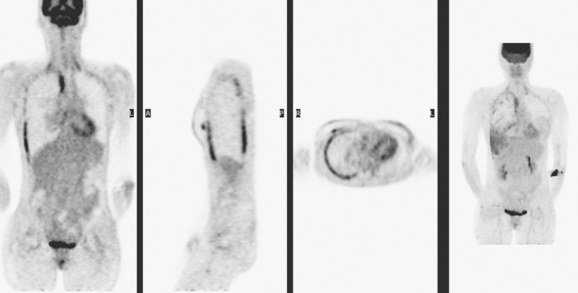
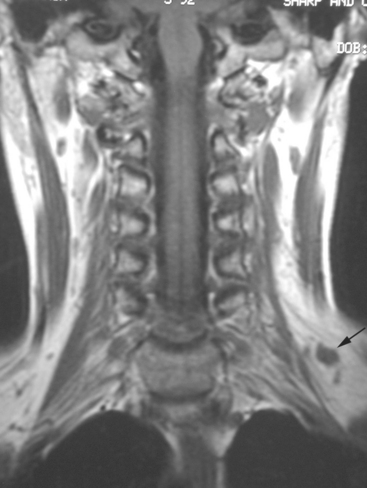
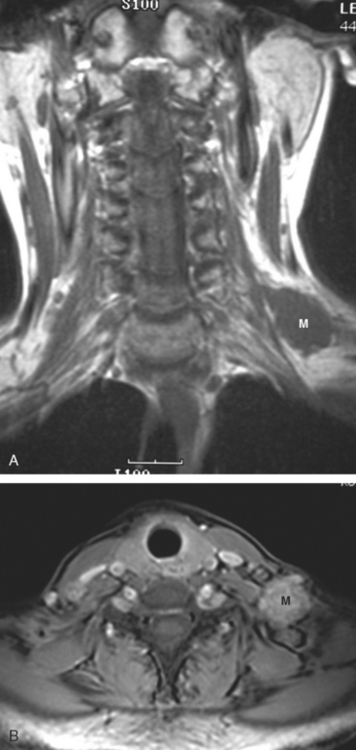
The patient was first diagnosed with a 1-cm left breast infiltrating ductal carcinoma at age 32 and treated with mastectomy. Fifteen lymph nodes were negative. Two and a half years before PET scanning, left neck nodal recurrence was found and treated with 6 months of chemotherapy. Six months later, she again developed a recurrent left neck lump and was treated with an additional eight cycles of chemotherapy, which finished 1 year before. At the time of PET evaluation, she had a persistent hard, fixed, palpable left supraclavicular nodule. MRI showed only a normal-sized (1 cm) left supraclavicular lymph node at that level (Figure 1).
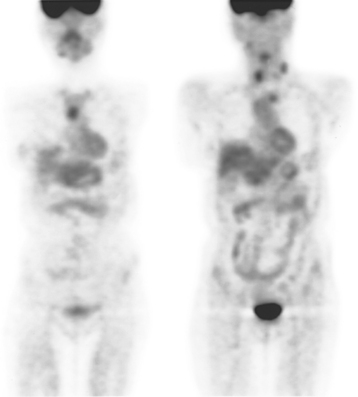
PET scan confirmed a small left supraclavicular hypermetabolic nodule at the level in question (Figure 2). A trial of hormonal therapy yielded no clinical response, so 3 months later, the node was surgically excised and proved to be metastatic breast cancer. She was restarted on chemotherapy and had completed four cycles when she was re-evaluated with neck and chest MRI and PET scan. Unfortunately, these studies showed interval progression, with new prevascular mediastinal and bilateral neck and supraclavicular foci of activity on PET, which correlated with MRI masses (Figures 3, 4, 5, and 6).
CASE 10 PET-positive thoracic nodal recurrence mimic due to silicone implant leak
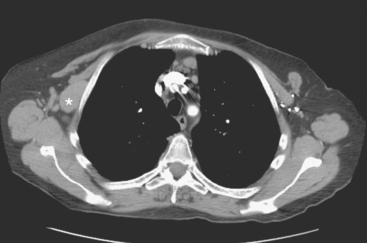
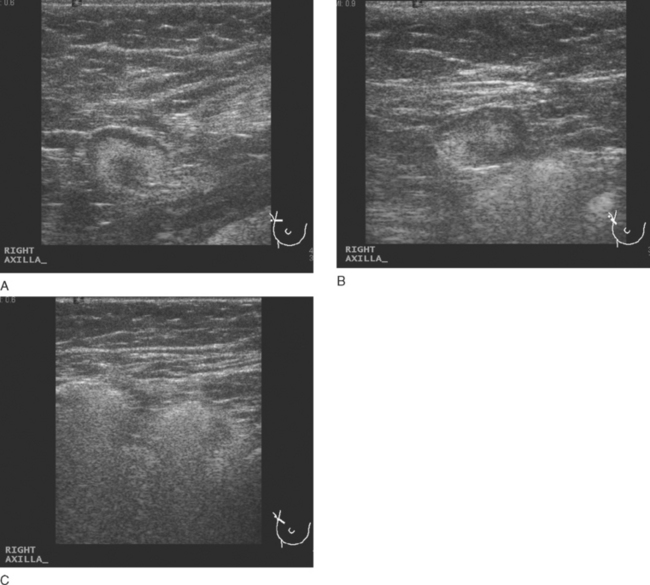
Physical examination identified a 2-cm right axillary lymph node. Positron emission tomography (PET)/CT and contrast-enhanced CT scans showed multiple abnormally enlarged and metabolically active lymph nodes in the right axilla, prevascular mediastinum, and right internal mammary regions (Figures 1, 2, 3, 4, 5, and 6). Correlation with right axillary sonography showed characteristic findings suggesting silicone as the etiology, rather than nodal recurrent disease (Figure 7).
TEACHING POINTS
Not all increased metabolic activity in regional lymphatics of treated breast cancer patients represents recurrent tumor. Like Case 16 in Chapter 6, iatrogenic etiologies of lymphadenopathy can simulate recurrent disease and should be considered when there is a prior history of reconstruction, implant rupture, or other viable alternative explanation. As in this case, it may take another modality or even tissue sampling to completely characterize such abnormalities.
Unlike metastatic tumor cells, which lodge preferentially in the subcapsular and cortical sinusoids of lymph nodes, foreign material such as silicone tends to accumulate in medullary sinusoids. There is a range of appearances on ultrasound of lymph nodes containing silicone, depending on the amount of material accumulated. Hardest to recognize are lesser degrees of involvement, with ill-defined shadowing arising from the mediastinum (illustrated to variable degrees by Figures 7A and 7B). With progressive accumulation of silicone in the medullary sinuses, the hilus becomes increasingly hyperechoic and may shadow, as in Figure 7A. Most characteristic is the snowstorm appearance of lymph nodes so silicone laden that even the cortex is hyperechoic and not readily differentiated from the medulla, with dirty shadowing emanating from the whole of the extensively involved lymph node, as in Figure 7C.
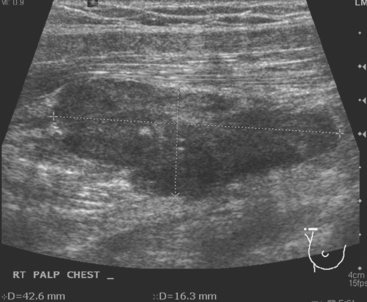
CASE 11 Unusual pattern of chest wall recurrence (intercostal muscle infiltration)
Seven years later, the patient presented with small palpable subcutaneous nodules and chronic left chest wall pain. Ultrasound showed hypoechoic subcutaneous nodules (Figure 1). An ultrasound-guided biopsy confirmed recurrent infiltrating lobular carcinoma (ILC), estrogen receptor positive, progesterone receptor negative, infiltrating skeletal muscle and adipose tissue.
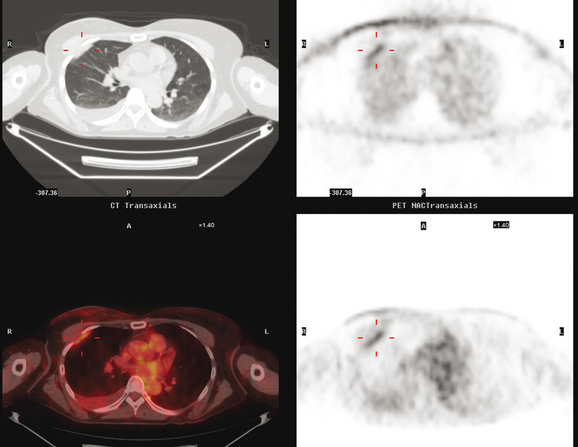
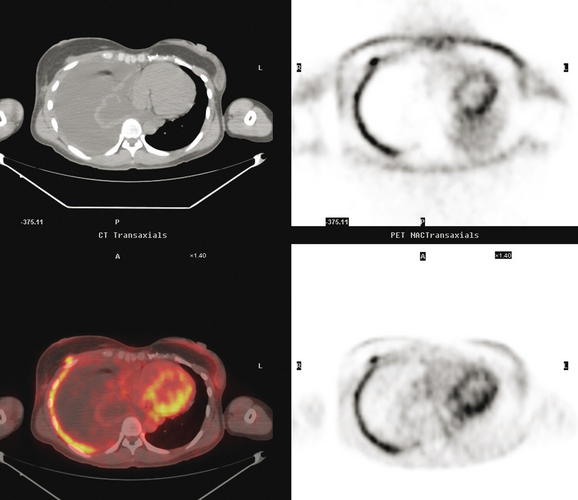
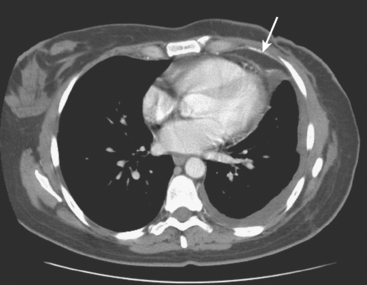
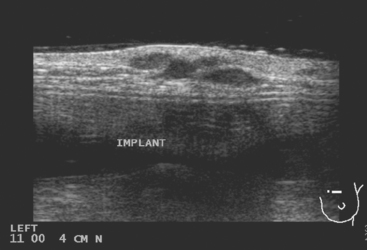
Restaging with positron emission tomography (PET) and CT showed multiple small hypermetabolic subcutaneous nodules as well as hypermetabolism lateral to the implant in the region of dense fixation and along an intercostal space in the left chest wall (Figures 2, 3, 4, 5, 6, and 7).
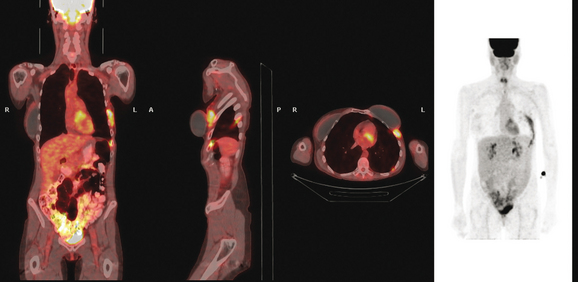
FIGURE 5 Three plane fused PET/CT images show multiple foci of intense hypermetabolism in the left chest wall. The coronal projection volumetric image (right) displays a curvilinear shape, following the oblique orientation of a rib. Intense hypermetabolism lateral to the implant (best seen on the axial section) corresponds with the asymmetrical soft tissue lateral to the left breast implant shown in Figure 4.
TEACHING POINTS
An unusual pattern of chest wall recurrence is illustrated by this case. The curvilinear, obliquely oriented activity was confounding to sort out. Based on its appearance on the PET volumetric image and sagittal projections, the course of the activity suggests it is in an involved rib. In fact, it had previously been suggested clinically that perhaps the patient’s chronic chest wall pain was due to radionecrosis of a rib. However, this was never substantiated. A bone scan 8 months before was negative, review of the CT bone windows showed no rib abnormality, and the activity seemed to localize to an intercostal space and not to a rib. Although there was some asymmetry in the CT appearance of the chest wall musculature, there did not appear to be any focal correlate in the intercostal space implicated on PET. Because respiratory motion can significantly affect localization of PET abnormalities, this was carefully scrutinized. The registration between the CT and PET data appeared perfect. After much discussion among the consultants (the patient was being considered for surgery, with chest wall reconstruction), it was elected to pursue the intercostal abnormality despite the lack of a focal CT correlate. Using landmarks, the interspace was identified, and biopsy confirmed recurrent breast carcinoma.
With the second positive biopsy confirming the PET impression of much more extensive chest wall involvement than suspected previously, it became clear that surgery was unlikely to render this patient disease free. She was started on fulvestrant (Faslodex), a direct estrogen receptor blocker.
CASE 12 Drug reaction (pulmonary toxicity) due to chemotherapy
A 42-year-old woman was found to have lung and nodal breast cancer metastases after evaluation with positron emission tomography (PET) and CT for rising tumor markers. Her diagnosis of breast cancer was 4 years before, and was stage IIB, T2N1. She underwent bilateral mastectomy and received adjuvant CMF chemotherapy and tamoxifen. Her prior medical history was notable for treatment of Hodgkin’s lymphoma at age 17 with radiation and chemotherapy.
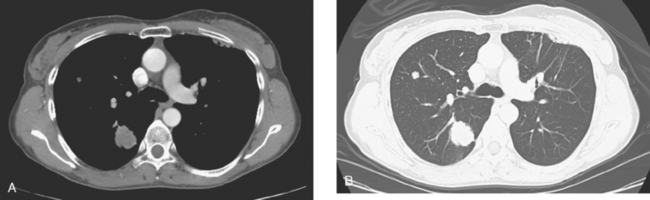
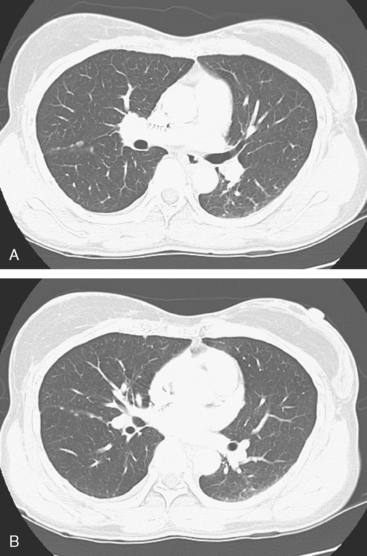
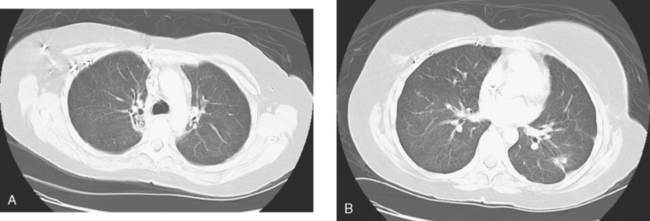
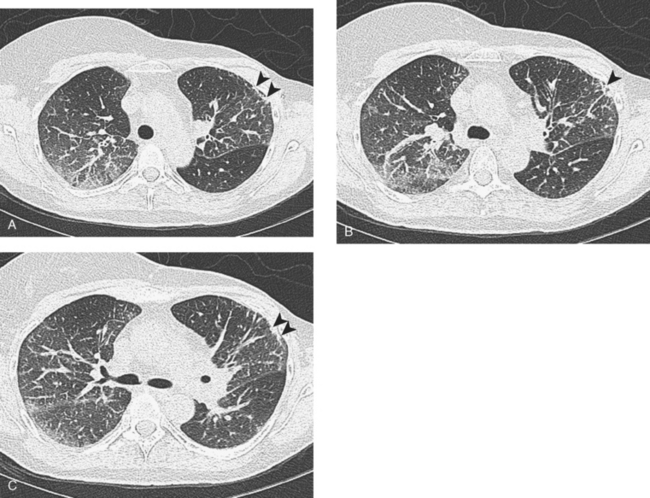
A chest CT obtained just before cycle seven of chemotherapy showed that the index lung metastases were responding (Figures 1 and 2). However, new, vague, bilateral ground-glass lung opacities were noted. A drug reaction was suggested as the etiology. The patient complained of fatigue but was otherwise asymptomatic. Her tumor markers had normalized.
Four months later, the patient noted increasing dyspnea with exertion. Her chemotherapy was interrupted and consultation with chest medicine obtained. Additional symptoms of intermittent cough and low-grade fever were noted by this time. Pulmonary function tests were abnormal, with evidence of moderate restriction and low diffusing capacity, suggesting interstitial lung disease. Bronchoscopy was performed to exclude an infectious etiology. All cultures were negative. On a chemotherapy holiday, the patient’s symptoms improved dramatically, and the ground-glass infiltrates seen on chest CT resolved (Figure 3). Repeat pulmonary function tests 3 months later showed improvement.
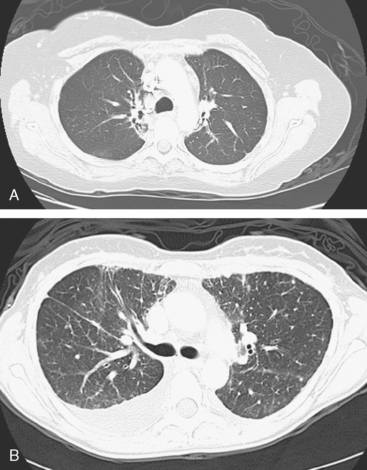
FIGURE 3 A and B, Six months since the CT in Figure 1, and after chemotherapy was discontinued 3 months, CT scan shows that the diffuse lung changes have resolved. The index LLL lung metastasis has grown.
Akira M, Ishikawa H, Yamamoto S. Drug-induced pneumonitis: thin-section CT findings in 60 patients. Radiology. 2002;224:852-860.
Camus PH, Foucher P, Bonniaud PH, Ask K. Drug-induced infiltrative lung disease. Eur Respir J. 2001;18:93S-100.
Padley SP, Adler B, Hansell DM, et al. High-resolution computed tomography of drug-induced lung disease. Clin Radiol. 1992;46(4):232-236.
CASE 13 Lymphangitic tumor
A 37-year-old woman with extensive bony metastatic breast cancer known for 1 year developed increased aching, weight loss, depressed appetite, and rising tumor markers while on goserelin acetate (Zoladex), zoledronic acid (Zometa), and exemestane (Aromasin). Positron emission tomography (PET)/CT and enhanced body CT scans showed new liver metastases (see Case 2 in Chapter 9). Chemotherapy was recommended but declined. She was started on fulvestrant (Faslodex) and restaged after 2 months, at which time fatigue, shortness of breath, and rising tumor markers were noted. Repeat PET/CT and enhanced body CT scans showed progression of liver metastases, new pleural and pericardial effusions as well as pulmonary metastases, and evidence of lymphangitic spread (Figures 1, 2, 3, 4, 5). One month later, the patient was admitted with confusion, thought to be hepatic encephalopathy due to the extensive liver metastases. She died soon thereafter.
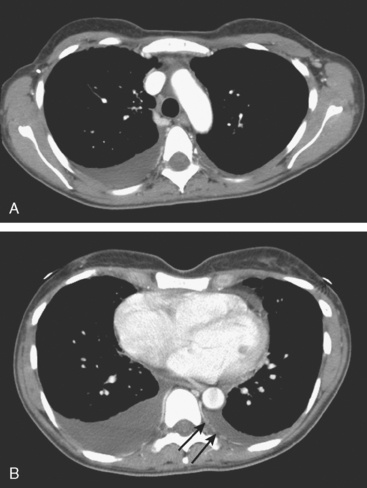
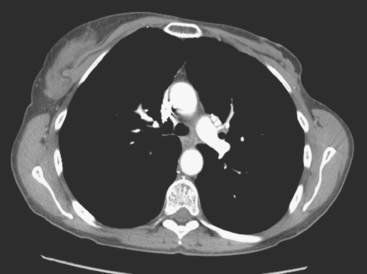
FIGURE 4 Contrast-enhanced chest CT images (A and B, soft tissue window) from the final CT (same study as Figure 3) show bilateral pleural effusions, right greater than left. The pleura is enhancing, smoothly in some places and nodularly in others (as seen here best on the left, arrows), suggesting malignant effusions. A small new pericardial effusion is also partially visualized here.
Her original tumor had been treated 5 years earlier and was an estrogen receptor– and progesterone receptor–positive, T1cN1b(4) infiltrating ductal carcinoma. She was treated with breast conservation and axillary dissection, with 4 of 20 lymph nodes positive and showing microscopic extracapsular extension. Additional treatment was with chemotherapy (four cycles of doxorubicin [Adriamycin] and cyclophosphamide [Cytoxan] [AC] and four of paclitaxel [Taxol]); right breast, supraclavicular, and axillary radiation therapy; and tamoxifen. Four years after the original breast cancer diagnosis and 1 year before detection of liver metastases, diffuse bone metastases were found. See Case 3 in Chapter 8 for discussion of the imaging manifestations of this patient’s bone metastases.
TEACHING POINTS
The diagnosis is suggested by high-resolution CT showing reticular or reticulonodular interstitial markings, irregular and nodular thickening of interlobular septa (Kerley B lines), and uneven thickening of bronchovascular bundles, especially with a beaded or nodular appearance. CT underestimates the extent of subpleural tumor compared with pathology.
Connolly JEJr, Erasmus JJ, Patz EFJr, et al. Thoracic manifestations of breast carcinoma: metastatic disease and complications of treatment. Clin Radiol. 1999;54:487-494.
Thomas JM, Redding WH, Sloane JP. The spread of breast cancer: importance of the intrathoracic lymphatic route and its relevance to treatment. Br J Cancer. 1979;40:540-547.
CASE 14 Lymphangitic tumor
A 55-year-old woman with stage III breast cancer diagnosed 3 years before was noted to have rising tumor markers (CA 27.29), while finishing a delayed year of trastuzumab (Herceptin) therapy. She had been treated at another facility for a 4.5-cm, estrogen receptor– and progesterone receptor–negative, HER-2/neu positive, infiltrating ductal carcinoma, with mastectomy, chemotherapy (four cycles of doxorubicin [Adriamycin] plus cyclophosphamide [Cytoxan] [AC] and four cycles of docetaxel [Taxotere]), and radiation. Ten of 12 lymph nodes were involved. The patient presented for medical oncology second opinion 9 months after finishing primary treatment and began a year’s therapy with trastuzumab (Herceptin).
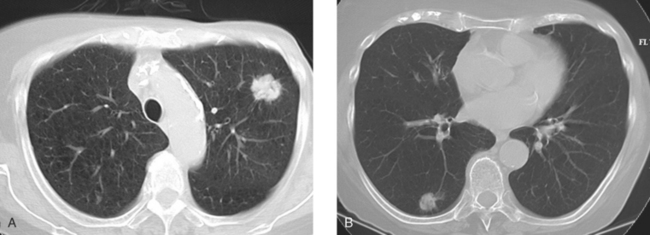
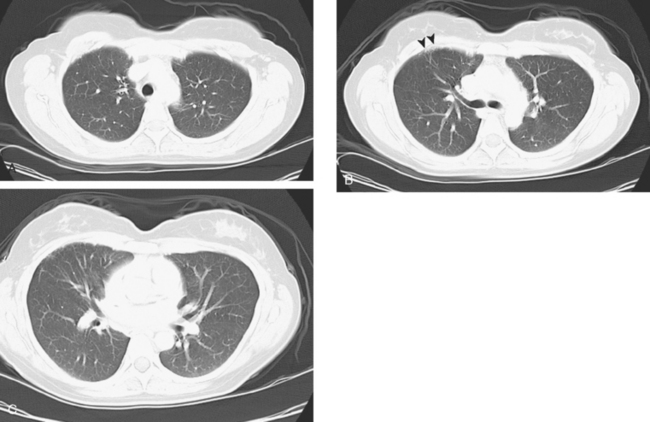
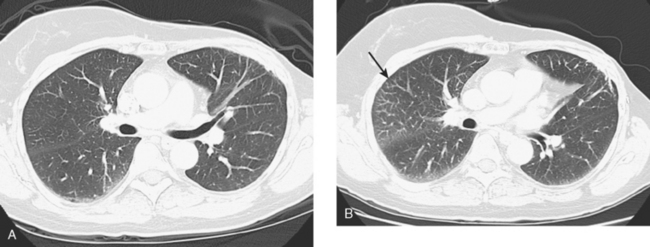
A repeat chest CT 4 months later showed increased interstitial lung changes, and the specter of lymphangitic tumor was raised (Figures 1 and 2). Repeat PET scan showed new abnormalities, with bilateral suprahilar hypermetabolic uptake, as well as new mild, generalized, increased upper lobe activity, corresponding to the new parenchymal findings (Figure 3).
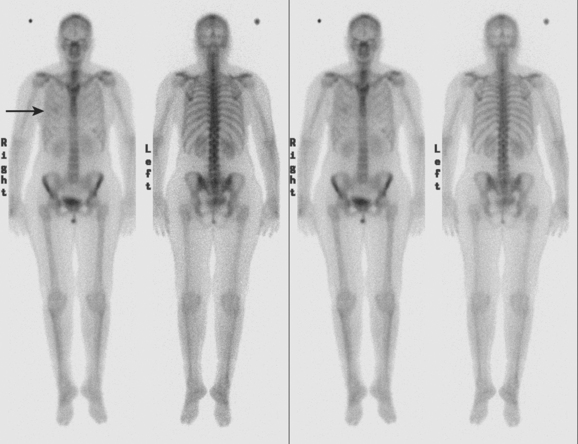
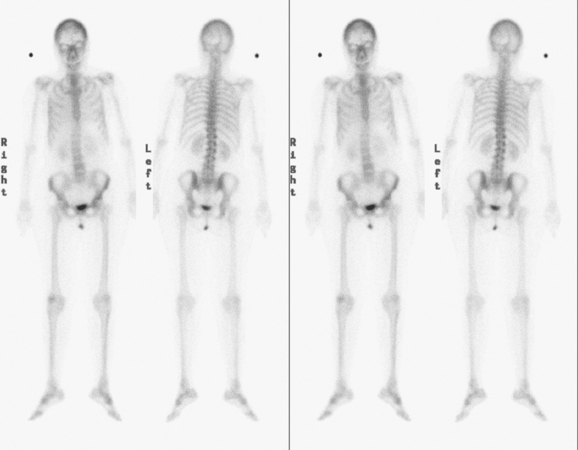
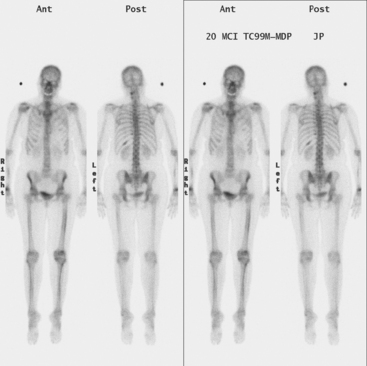
Bronchoscopy showed a normal tracheobronchial tree. RUL lavage and transbronchial biopsy confirmed metastatic breast cancer, estrogen receptor and progesterone receptor negative. Bone scan, obtained to evaluate complaints of left knee pain, showed findings suggesting hypertrophic osteoarthropathy as a possible etiology for the pain (Figure 4).
TEACHING POINTS
This patient’s tumor markers began rising during the last few months of a year of Herceptin therapy. Workup showed only minimal RUL peripheral micronodularity (Figure 2B) and PET was negative. Repeat chest CT and PET, 4 months later and 2 months after completing Herceptin, show dramatic interval change, suggesting lymphangitic tumor spread. Metastatic breast cancer was confirmed by bronchoscopic biopsy, directed to the RUL, where the most extensive findings were seen.
Hypertrophic osteoarthropathy may cause bone pain and arthralgias due to periostitis. This is manifested scintigraphically by increased long bone cortical activity, most often recognized in the lower extremities, which can be seen before clinical symptoms develop. It is seen with a variety of thoracic conditions, both malignant and benign. Lung cancer is the most common association, but thoracic metastases are one of the less often seen causes.