CHAPTER 78 Thoracic and Lumbar Spinal Injuries
Incidence
The thoracolumbar junction is the most common injury site for thoracic and lumbar trauma. Most patients are young males involved in high-energy accidents. More than half of fractures occur between T11 and L1, and another 30% occur between L2 and L5.1–3 More than 50% of injuries are sustained in motor vehicle accidents, and another 25% are sustained in a fall from greater than 6 feet. Complete neurologic injuries occur in about 20%, and incomplete neurologic injuries occur in about 15% of patients. Associated injuries including fractures, head trauma, pulmonary injuries, and intra-abdominal injuries occur more than 50% of the time. Noncontiguous spine injuries remote from the site of the primary injury occur in 5% of patients.1–3
Anatomic Considerations
Considerable anatomic differences exist throughout the thoracolumbar spine, contributing to the differences in spinal injury patterns in the thoracic spine, thoracolumbar junction (T11 to L2), and lower lumbar spine. The thoracic spine is kyphotic and has the greatest amount of intrinsic stability because of the rib cage, but it also has a relatively narrow canal that gives little reserve for neural element protection after spine trauma.4 Axial rotation is greater in the thoracic spine than the lumbar spine because of the coronal alignment of the facets. The lower lumbar spine is lordotic and has the greatest amount of flexion and extension capabilities due to the sagittal facet joint orientation.5,6 The human body’s center of gravity is anterior to the thoracic and thoracolumbar spine, which places compressive forces on the vertebral bodies and tensile forces on the posterior ligamentous structures in the upright position. The center of gravity is located more posteriorly in the lower lumbar spine, in part due to the greater degree of regional lordosis, and the posterior elements, in particular the osseous components, experience significant compressive rather than tensile forces in the upright position.7–10 The posterior osteoligamentous complex consists of the lamina, facets, facet joint capsules, and interspinous ligaments. These structures are commonly referred to as the posterior ligamentous complex, reflecting the key role of the ligaments in maintaining spinal stability when there is injury to the vertebral body and intervertebral discs. The lower lumbar spine is inherently stable and somewhat protected from injury due to its lordotic configuration, distribution of compressive forces across the vertebral bodies and posterior elements, and relatively large vertebral bodies. The thoracolumbar junction is the transition zone from the rigid, stable kyphotic thoracic spine to the mobile, lordotic, relatively stable lower lumbar spine and is therefore particularly susceptible to injury.
Mechanisms of Injury
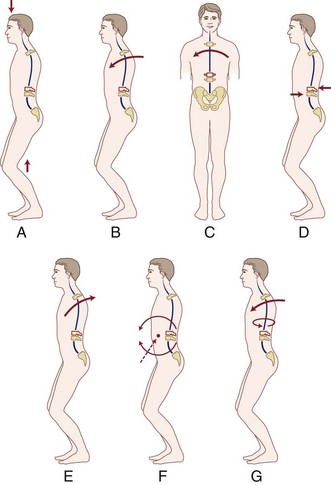
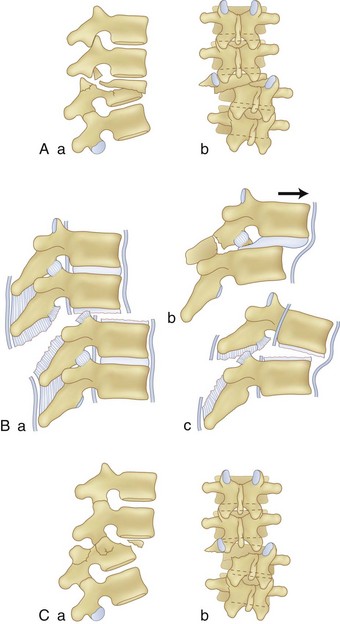
Thoracic and lumbar spinal injury patterns can usually be explained by the application of one or two force vectors. These forces cause relatively consistent injury types that serve as the basis for the main classification schemes, which are described later. The most common primary forces are axial compression, lateral compression, flexion, extension, distraction, shear, and rotation (Fig. 78–1A-E). The most common force combinations are flexion-rotation and flexion-distraction (Fig. 78–1F-G).
Initial Evaluation and Management
Associated injuries are common including chest and abdominal injuries, orthopedic injuries including pelvis and extremity fractures, head trauma, as well as contiguous and noncontiguous spine injuries.11,12 The potential for these injuries must be kept in mind and reassessed periodically, especially in the early stages and in obtunded or severely injured patients who cannot direct attention to their injuries. The initial assessment and subsequent surveys should be systematic and thorough from head to toe, knowing that even in the highest volume and most experienced trauma centers, injuries in the spine and elsewhere sometimes go undetected and can have significant functional consequences.3,13–15
The use of pharmacologic agents in spinal cord injury as part of the initial resuscitative protocol has been extensively studied and debated. The rationale is to minimize the harmful secondary cascade of events such as the inflammatory response and oxidative cell injury that occurs after mechanical injury to the spinal cord. The National Acute Spinal Cord Injury Studies (NASCIS II and III) examined methylprednisolone, naloxone, and tirilazad. If administered within 8 hours of injury, there may be improvement in long-term neurologic function with high-dose methylprednisolone. The study dose was a bolus of 30 mg/kg, followed by a 5.4 mg/kg/hour infusion for 23 hours if therapy is initiated within 3 hours of injury or 48 hours if initiated at 3 to 8 hours after injury. There are significant potential complications such as pneumonia, postoperative wound infection, gastrointestinal bleeding, and sepsis with the use of steroids.16–18 Steroids have not been shown to be effective in purely lower-motor neuron lesions. GM-1 ganglioside has been studied as well.19 The use of steroids has not been universally accepted and remains controversial but can be considered as a treatment option, as can GM-1 ganglioside.20 There is interest in systemic hypothermia after spinal cord injury, but clinical data are limited.21
Initial Neurologic Evaluation
Neurologic injury may have a profound effect on a patient’s ultimate function, and the presence or absence of a neurologic deficit often guides subsequent injury management including operative versus nonoperative treatment. A detailed neurologic examination is therefore critical and consists of testing of motor and sensory function and reflexes in a sequential and systematic manner. Findings should be carefully documented to enable accurate assessment of neurologic deterioration or recovery. Motor function is assessed in the main muscle groups in the upper and lower extremities, which can sometimes be difficult in an injured extremity, but every attempt should be made under these circumstances. Motor function should be graded on a 0 to 5 scale. A score of 5 is normal with full range of motion against gravity and resistance, 4 is full range of motion against gravity and slight resistance, 3 is full range of motion against gravity only, 2 is full range of motion only with gravity eliminated, 1 is palpable or visible contraction of a muscle without joint motion, and 0 is a muscle without any function. Rectal sphincter tone and volitional control should be tested as well. Sensory function should be assessed in a dermatomal distribution. This should include testing of the perineal region because sparing of the sacral dermatomes may provide an indicator for functional recovery in patients sustaining a spinal cord injury. Temperature, pain, and light touch are spinothalamic tract functions and can be assessed with a sterile needle and an alcohol swab. Vibration and position sense are posterior column functions and can be assessed with a tuning fork and limb or digit positioning. Reflexes include superficial abdominal (stroking of the abdominal skin causes the umbilicus to be drawn toward the stimulated area, above the umbilicus T7-T10, below the umbilicus T11-L1); cremasteric (in males, stroking of the inner thigh causes the scrotum to be drawn upwards, T12-L1); patellar tendon (quadriceps reflex or knee jerk, L3-L4); Achilles tendon (ankle jerk, S1); anal wink (anal sphincter contraction with stimulation of the perineal skin, S2-S4); and bulbocavernosus reflex (described later, S2-S4). Pathologic reflexes indicating an upper motor neuron lesion include clonus and an abnormal Babinski test. The Babinski test is normal when a stroke along the lateral plantar aspect of the foot results in down-going toes and is abnormal with up-going toes. The neurologic examination is covered in greater detail in Chapter 11.
Neurologic Injury Classification
The central cord syndrome usually involves an injury to the cervical spine in older patients and is characterized by upper extremity weakness with relative sparing of the lower extremities and sacral nerves. Although this injury pattern usually applies to cervical spine injuries because of the upper extremity involvement, Bohlman described a central cord injury pattern in thoracic spine trauma with a greater degree of muscle weakness in the trunk and proximal muscle groups with relative sparing of the distal muscle groups and preserved bowel and bladder function.22 Although the central cord syndrome is the most common of the incomplete patterns when considering all spinal cord injuries, it is rare when cervical injuries are excluded and thoracic injuries alone are considered. The anterior cord syndrome is the most common incomplete pattern in thoracic spine injury, characterized by loss of all motor and most sensory function below the level of the lesion, with sparing of the vibratory and position sense via the posterior columns.22 This has a poor prognosis for recovery and is a debilitating injury pattern. The posterior cord syndrome is rare and involves loss of vibratory and position sense. Patients cannot rely on tactile lower extremity feedback for spatial orientation and must use visual cues when ambulatory. They have preserved bowel and bladder function. The Brown-Séquard syndrome is usually a result of cord hemisection by penetrating trauma and is characterized by ipsilateral loss of motor function and contralateral loss of pain, temperature, and light touch sensation. Patients typically have preserved bowel and bladder function, are ambulatory but may require assistive devices, and have the best prognosis for recovery of the incomplete spinal cord injury syndromes.
The conus medullaris marks the anatomic transition from the upper motor neurons of the spinal cord to the lower motor neurons of the cauda equina and is usually found at the T12-L1 levels. The conus medullaris injury pattern is characterized by flaccid paralysis, loss of reflexes, and sensory loss of the lower extremities with bowel, bladder, and sexual dysfunction. Injury to the cauda equina below the conus medullaris is a purely lower motor neuron injury and presents with the same types of findings as with conus medullaris injury, except that there is a higher incidence of asymmetric involvement and can occur at any level below the conus medullaris. These injuries may be complete or incomplete.23,24
Radiologic Evaluation
Anteroposterior (AP) and lateral conventional radiographs of the thoracic and lumbar spine are the most basic imaging modalities available. A swimmer’s view of the upper thoracic spine may be necessary to visualize the vertebral bodies through the shoulder region. The lateral radiograph is used to examine the height, width, and alignment of the vertebral bodies, pedicles, spinal canal, neural foramina, facets, and spinous processes. The cortical margins of the vertebral bodies should be smooth and contiguous and, when viewed in conjunction with adjacent vertebral bodies, should form a gently arcing line both at the anterior margin (anterior vertebral body line) and posterior margin (posterior vertebral body line). The AP radiograph allows assessment of coronal alignment, interpedicular distance, and alignment of the spinous processes and best visualizes the ribs and transverse processes. Anterior wedging of the vertebral body with a break in the anterior cortical margin signifies a compression fracture. Loss of posterior vertebral body height, a break in the posterior vertebral body cortical margin, spinal canal narrowing, and widening of the interpedicular distance signifies a burst fracture.25 The posterior vertebral body angle can also be used to distinguish subtle burst fractures from compression fractures.26 Facet widening; spinous process splaying or malalignment; fracture lines in the pedicle, lamina, facets, and spinous processes; and vertebral body translation signifies injury to the posterior elements including possible ligamentous injury and are radiographic markers of potential instability.27 Conventional radiography of the thoracolumbar spine has technical, logistical, and diagnostic limitations and therefore CT has become a key modality in evaluating thoracolumbar trauma.
Many trauma centers now use screening spiral CT of the chest, abdomen, and pelvis as part of the standard trauma evaluation. These images include collateral thoracic and lumbar spine information, and as a result, some centers are adopting CT protocols as the initial screening radiologic modality for spine trauma.28–30 Studies have shown that CT scans have a higher sensitivity than conventional radiographs in the detection of thoracic and lumbar spine fractures, although this applies mainly to stable rather than unstable fractures.31–33 CT is better than conventional radiography at discerning compression fractures from burst fractures, and misdiagnosis may occur in up to 25% of injuries when relying on conventional radiography alone.34–36 Screening CT scans may decrease the incidence of missed, delayed, or incorrect diagnoses of thoracic and lumbar trauma, especially in obtunded and multiply injured patients, but the clinical significance of this has not been determined and it is not standard in all trauma centers to obtain screening CT scans. The typical protocol for a trauma-screening CT scan consists of sequential 5-mm axial images with or without reconstructions, whereas a dedicated spine CT protocol consists of 2- to 3-mm axial images with sagittal and coronal reconstructions. It may be necessary to obtain a dedicated spine protocol CT scan if it seems that some important detail may not have been imaged during the screening examination. If the thoracic or lumbar spine injury is initially diagnosed on conventional radiographs alone and a high-energy mechanism is known or suspected, a dedicated spine protocol CT scan should be considered. The main limitation of CT is the limited ability to visualize the soft tissues, for which a MRI scan may be useful.
MRI allows for high-resolution imaging of soft tissues and is particularly important in the evaluation of spinal cord injury because the neural elements can be visualized. This helps in delineating edema, hemorrhage, hematoma, compression, and transection of the neural elements. MRI findings may contribute to the understanding of injury severity including the potential for spinal instability and the need for surgical intervention, and they may be useful for predicting the potential for neurologic recovery.37 Hemorrhage within the spinal cord and edema extending over multiple cord levels are correlated with poor prognosis for neurologic recovery.38–40 The posterior ligamentous structures can be reliably evaluated with MRI.41–44 Unless contraindicated, MRI should be considered in all cases with neurologic injury and in cases where further evaluation of the posterior ligamentous complex is necessary in deciding between operative versus nonoperative treatment. The main limitations for MRI are logistics, cost, and the inferior visualization of the bony elements, so MRI is usually complementary to CT rather than a substitute.
Concept of Spinal Stability and Its Role in Fracture Classification Systems
Spinal mechanical stability refers to the structural integrity of the spinal column and in its simplest form is the ability to resist physiologic loads without progressive deformity or damage to the neural elements.45 Spinal neurologic stability refers to the presence or absence of a neurologic deficit. The concept of mechanical stability serves as the basis for the main recognized fracture classifications and, more recently, attempts have been made to incorporate neurologic stability into the classification schema. Classification systems are potentially useful for communication, research, determining prognosis, and guiding treatment. With respect to thoracic and lumbar spine injuries, certain fracture patterns and associated injury mechanisms have been recognized and terminology has evolved to form a core group of specific injuries with relatively consistent nomenclature, even though there is not a universally accepted classification system. The majority of fractures can be grouped into these main well-recognized categories, but some injuries are of a mixed pattern and are not readily classifiable. The underlying theme of modern classification systems is to determine whether or not an injury is mechanically stable, and the lack of universal acceptance of one classification system reflects the great difficulty in assessing stability in some cases. As the injury severity increases, so does the degree of instability, but this occurs along a continuum and it is not always possible to determine the point where the transition from a stable injury to an unstable injury occurs. Despite intense work in this area, assessment of mechanical stability remains a judgment call at times, and this mainly explains the disparity in treatment approach for certain injuries (especially burst fractures) from one surgeon to the next.
Fracture Classification Systems
Classifications have evolved since their introduction in the mid-20th century. Descriptions of thoracic and lumbar injuries first appeared in print publications in the mid-1900s.46–48 Nicoll49 credited these early authors and classified injuries as anterior wedge fractures, lateral wedge fractures, fracture-dislocations, and isolated neural arch fractures in 1949. Holdsworth expanded on this classification and detailed the traumatic forces causing distinct fracture patterns, described as flexion, flexion and rotation, extension, and compression. He conceptualized the two-column theory of spinal stability, with the anterior column resisting compressive loads and the posterior ligamentous complex resisting tensile forces. Wedge compression and compression burst fractures were considered stable due to preservation of the posterior ligamentous complex integrity, whereas dislocations, extension fractures and dislocations, and rotational fracture-dislocations were considered unstable.50 Kelly and Whitesides51 built on Holdsworth’s work and formally presented the two-column theory as a conceptualization of spinal stability as it relates to spine trauma. These classification systems did not incorporate the extent of neurologic injury, and other authors focused on this aspect of the injury.52,53
The application of CT to spine imaging marked a major change in the understanding of the various fracture patterns, and the modern classification systems evolved. Denis analyzed 412 thoracic and lumbar spine injuries with CT scans and classified major injuries as compression fractures, burst fractures, flexion-distraction injuries, and fracture-dislocations (Figs. 78-2 to 78-5). He proposed a three-column theory of spinal stability and delineated the concepts of mechanical instability and neurologic instability. In this scheme the anterior column is composed of the anterior longitudinal ligament (ALL), anterior annulus, and the anterior portion of the vertebral body. The middle column is composed of the posterior vertebral body, posterior annulus, and posterior longitudinal ligament. The posterior column is composed of the ligamentum flavum, neural arch, facet joints and facet capsules, and the posterior ligamentous complex. Mechanical instability begins to occur with failure of two of the three columns.54,55 The three-column theory was later validated with biomechanical cadaver studies.56 Injuries with a neurologic deficit were deemed by Denis to have neurologic instability. Neurologic instability was felt to be an important concept because injuries severe enough to cause neurologic injury are usually also mechanically unstable. Subsequent classification systems and current treatment algorithms are based on the perceived mechanical stability of the injured spine and the presence or absence of neurologic injury. Mechanical stability is a continuum, and great controversy exists in determining at what point a “stable” injury becomes “unstable.” This controversy is most evident with burst fractures.
McAfee described a classification based on analysis of the CT scan appearance of 100 fractures. Six basic injury patterns were proposed: wedge-compression, stable burst, unstable burst, Chance, flexion-distraction, and translational.57,58 Ferguson and Allen59,60 combined the three-column theory with the forces causing the injury and proposed a mechanistic classification. Fracture types included compressive flexion, distractive flexion, lateral flexion, translational, torsional flexion, vertical compression, distractive extension, and isolated transverse process fractures. The Arbeitsgemeinschaft für Osteosynthesefragen (AO) system was an attempt at comprehensively classifying spine injuries. Three main injury types were recognized: vertebral body compression, anterior and posterior element injury with distraction, and anterior and posterior element injury with rotation. Multiple subtypes exist within each group.61,62 Most recently, the Spine Trauma Study Group has integrated key concepts of these various classification systems to create a more simple and reproducible scheme. Neurologic status is included in the classification, with the goal of generating a more standardized thoracic and lumbar spine injury treatment approach. The Thoracolumbar Injury Classification and Severity Score (TLICS) is based on three categories: injury morphology, integrity of the posterior ligamentous complex, and neurologic status. Injuries are analyzed and point values assigned to subgroups within each category. More severe injuries receive higher injury scores, and therefore treatment theoretically may be guided by calculating the injury score.63–67
Description and Diagnosis of Specific Fracture Types
Sprains
Injuries to the spinal column involving the ligaments and musculotendinous units that do not cause facet joint subluxation, fracture, or listhesis are considered sprains and are stable.68 This tends to be a diagnosis of exclusion based on the history, physical examination, and imaging studies. With a reliable history, mild physical examination findings, and otherwise normal spine anatomy, plain radiographs may be sufficient to rule out spinal instability and diagnose a sprain. If there is a high-energy mechanism, unreliable history, significant physical examination findings such as obvious swelling or neurologic injury, or abnormal anatomy such as with ankylosing spondylitis or congenital anomaly, advanced imaging studies are warranted. CT and then MRI are often necessary under these circumstances to diagnose a sprain rather than a more significant injury.
Disc Herniations
Traumatic disc herniations caused by high-energy mechanisms are relatively rare as an isolated injury. Fractures are often associated with disc disruption, and the displaced disc material may contribute to neurologic injury, but the category of injury described here refers to an otherwise intact spinal column in association with a disc herniation that appears to be traumatic on the basis of the patient’s history, symptoms, and physical examination combined with correlating imaging studies. Disc herniations are commonly associated with minor trauma such as lifting an object, but the disk herniations considered here are due to high-energy trauma such as motor vehicle accidents that also caused or had the potential to cause other significant injuries. Diagnosis is made with MRI. There may be other imaging or clinical evidence of injury to the spinal column such as a soft tissue injury that would otherwise be called a sprain, but in order to be considered an isolated disc injury, there must not be any facet subluxation or traumatic listhesis. The classification scheme is anatomically descriptive and identical to that used in atraumatic disc herniations: bulging, protruded, extruded, or sequestered.69,70 It may be difficult to definitively establish a disc herniation as traumatic rather than preexisting because disc herniations are common in the asymptomatic population and it is a rare luxury to have available preinjury magnetic resonance images. From a clinical perspective it is not so important to establish a disc herniation as traumatic or preexisting because management of the disc herniation depends primarily on the presence or absence of neurologic symptoms (radicular pain), the physical examination findings (sensory and motor deficits), and the apparent temporal sequence in which these signs and symptoms developed. The spinal column itself is usually stable with isolated disc injuries.
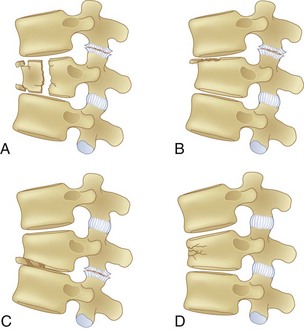
Compression Fractures
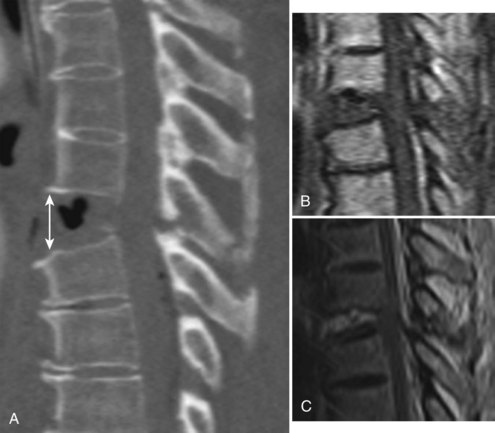
Compression fractures result from axial compression through the vertebral body, with failure through the anterior column. The fracture may involve the superior or inferior endplate alone, both endplates, or buckling of the anterior cortex with endplate preservation. The posterior column is usually not involved, but occasionally is. When this is the case, what appears to be a compression fracture may be a more significant flexion-distraction injury, described later as a “Chance” fracture (Fig. 78–6). The main distinguishing feature of the compression fracture is predominant failure of the anterior column in compression, whereas the flexion-distraction injury is characterized by predominant failure of the posterior column in tension.
Plain radiographs are usually diagnostic, but CT scans may be necessary to confirm the diagnosis because extension into the middle column may be subtle and burst fractures may be misdiagnosed as compression fractures 25% of the time on the basis of plain radiographs alone.36
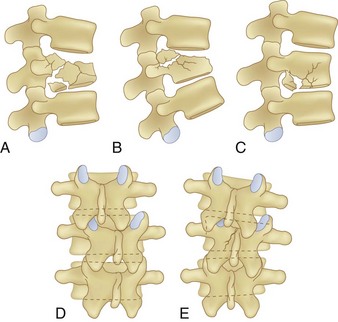
Burst Fractures
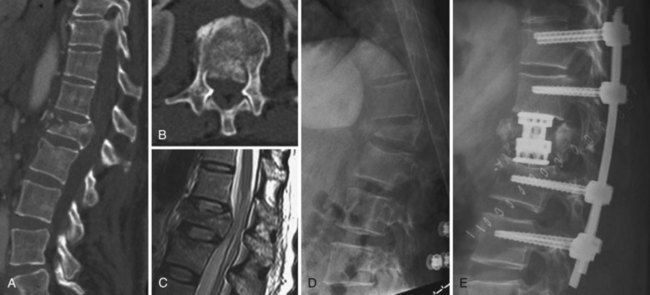
Burst fractures, like compression fractures, are caused by axial compression through the vertebral body. The fracture may involve the superior or inferior endplate alone, both endplates, or rarely with preservation of the endplates. Lateral compression forces may cause an injury variant that is asymmetrical in the coronal plane. The posterior column may or may not be involved, though more frequently it is involved when compared with compression fractures. In more severe injuries, the distinction between a burst fracture and a compression fracture is clear, with retropulsion of the fractured middle column fragments into the spinal canal being the hallmark of a burst fracture. But in less severe injuries, the distinction between compression and burst fractures is less clear and practically speaking may be in nomenclature alone, such as when the middle column fracture is minimally displaced and barely extends into the posterior cortex and there is minimal retropulsion of the middle column fragments (Fig. 78–7). The generally accepted differentiation between compression and burst fractures occurs at the middle column, which is spared in compression fractures and involved with burst fractures. Practically speaking, the transition from compression fracture to burst fracture represents a continuum of injury rather than distinct injury patterns because the same force vector is responsible.
Plain radiographs are often diagnostic, but CT scans are usually necessary to distinguish compression fractures from subtle burst fractures.36 For classification and descriptive purposes, if on CT scan the fracture extends into the posterior cortex of the vertebral body, regardless of the degree of displacement, it is referred to as a burst fracture.
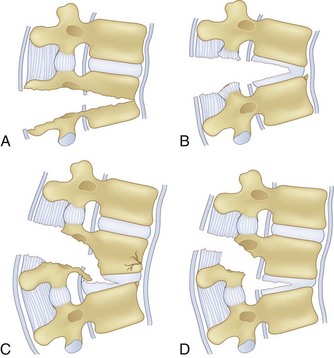
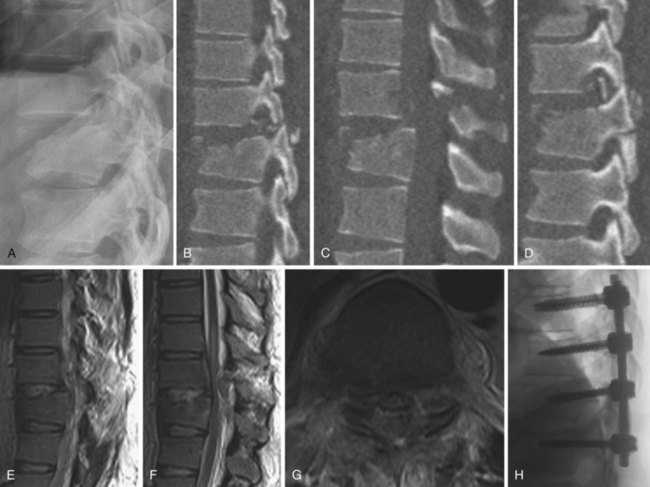
Flexion-Distraction Injuries (Chance Fractures and Chance Variants)
Flexion-distraction injuries are characterized by primary distractive forces on the spine, rather than crushing forces as seen with compression and burst fractures. These distractive forces cause tension failure of the posterior ligamentous complex and associated injuries to the anterior and middle column, either involving the disc or vertebral body. The axis of rotation is within or just in front of the anterior column, and at times the height of the anterior column can be relatively well maintained. The injury may be entirely osseous or entirely discoligamentous in nature or a combination of osseous and ligamentous (Fig. 78–8). Chance described the purely osseous lesion as a horizontal fracture extending through the spinous process, through the lamina and pedicles, and into the vertebral body.57 These injuries are commonly referred to as Chance fractures. Variants of this injury involve disc and ligamentous disruption with or without fracture. Anterior column disruption occurs through the disc space, not the vertebral body, and posterior column disruption is manifested as facet dislocation and ligamentous rupture, rather than fracture through the pedicle. These discoligamentous injuries are commonly referred to as Chance variants. If the axis of rotation is anterior to the spine, the vertebral body may be completely intact without fracture; if such an injury is severe, all the spine ligaments including the ALL may be disrupted, resulting in a severely unstable injury. If the axis of rotation is within the anterior column, there may be a vertebral body fracture with radiographic findings consistent with a compression or burst fracture, and if attention is not paid to the posterior elements and the distractive nature of the injury is not appreciated, underdiagnosis as a compression or burst fracture may occur.
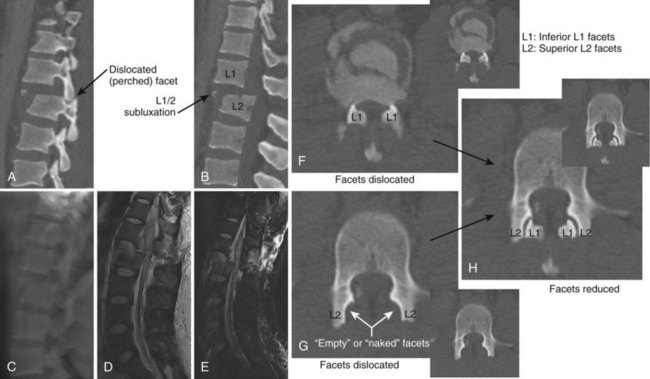
The diagnosis of a flexion-distraction injury may be made with plain radiographs, but CT is helpful in defining the precise anatomy of the osseous injury and identifies minimally displaced and nondisplaced injuries. Facet dislocations have a characteristic appearance on axial CT images, known as the “naked facet” or “empty facet” sign.71 This was first described in Chance variant injuries but is probably seen more commonly with fracture-dislocations.72 MRI is particularly good at detailing the extent of ligament and disc involvement with Chance fractures and Chance variants.
Fracture-Dislocations
Diagnosis can often be made on plain radiographs. The AP view may show lateral translation, whereas the lateral view may show anterior or posterior translation. CT scans will better quantify the bony injury and often demonstrate the “naked facet” or “empty facet” sign (Fig. 78–9).71,72 MRI should also be performed to assess the degree of disk and ligament injury and to visualize the neural elements.
Extension and Extension-Distraction Injuries
Injuries caused by an extension mechanism are relatively rare and are most commonly seen in patients with an ankylosed spine such as ankylosing spondylitis (AS) or diffuse idiopathic skeletal hyperostosis (DISH). The ankylosed spine is nonmobile and often demonstrates a kyphotic deformity. The discs and ligaments are ossified, so the spine acts as a single contiguous segment. Fractures do not follow typical patterns and come to resemble long-bone fractures more than vertebral fractures (Fig. 78–10). These fractures are usually unstable. Extension injuries can also occur in the otherwise normal spine.
In those circumstances where an extension injury is evident radiographically but AS or DISH is not present and the spine is otherwise normal, MRI is helpful to characterize the disc and ligamentous injury. These injuries are typically due to an extension-distraction mechanism and are rare, identified by gapping in the anterior and middle spinal columns rather than the more typical gapping in the posterior column seen in flexion and axial-load type injuries.73 The facets may be dislocated with the axial CT images, demonstrating the “naked facet” sign.72
Penetrating Injuries
Gunshot injuries to the spine are the most common form of penetrating injury. These injuries are almost always inherently stable. Radiographs and CT scans are usually sufficient to characterize the injury. The use of MRI when the bullet is in the spinal canal is controversial because of the potential risk of neurologic deterioration if the bullet migrates due to the pull of the MRI magnet. Bullet migration has been reported but appears to be quite rare.74–76
General Treatment Principles: Operative and Nonoperative
A nonoperative treatment option that is less commonly considered in the era of modern spinal fixation systems is prolonged bed rest.52,77,78 This is a consideration in the setting of a spinal injury that is too mechanically unstable to treat with a brace, but for some reason surgery is contraindicated or refused by the patient. This approach is most likely to be successful when the injury is primarily bony in nature and is not associated with significant deformity. However, prolonged bed rest is often associated with complications such as deep venous thrombosis, pulmonary embolism, pneumonia, and decubitus ulcers. Because this treatment approach is so rarely employed and is usually a result of necessity rather than surgeon preference, the discussion hereafter centers on bracing principles and surgical principles.
Surgical Principles
Surgical Principle #1: Achieving and Maintaining Anatomic Reduction and Stability—Surgical Approach and Instrumentation Choice
In order to achieve and maintain anatomic reduction, the forces that caused the injury must be counteracted by the instrumentation construct, which should be robust enough to withstand physiologic loads until the injury heals. Posterior pedicle screw and rod constructs are more rigid than anterior constructs and have become the mainstay of spinal instrumentation in thoracic and lumbar trauma.79 The posterior approach is quite versatile and allows for powerful reduction techniques. However, the anterior spine performs the majority of the axial load bearing and this must be taken into consideration. Anterior approaches are primarily used for neural element decompression and structural restoration of the anterior column. The integrity of the anterior column may be restored by performing a corpectomy of a highly comminuted vertebral body fracture followed by reconstruction with structural bone grafting or a structural device such as a titanium cage.80 Anterior internal fixation devices may provide additional stability and may obviate the need for additional posterior instrumentation. This requires further exposure to place the internal fixation device in the intact vertebral bodies above and below the fractured level, which may be difficult and risky anteriorly, especially in the lower lumbar spine and at the thoracolumbar junction.81–84 In cases where an anterior approach is performed as the primary component of the fracture treatment, consideration may be given to supplementing the anterior construct with posterior pedicle screw instrumentation. This is especially true if the posterior ligamentous complex is disrupted because the combined construct is stronger than either construct alone and more reliably handles the load demands of early mobilization.85
Surgical Principle #2: Decompression
Neural element decompression is generally reserved for patients with neurologic deficits, irrespective of the degree of spinal canal compromise. Neurologically intact patients with significant canal compromise of 50% or more do not benefit from decompression. It has been shown that resorption of retropulsed bone occurs naturally, and late spinal stenosis has not been shown to be a problem provided there is maintenance of spinal alignment.86–89 Patients with neurologic deficit may benefit from decompression, either indirectly or directly. Indirect decompression is best achieved within the first 48 hours after injury and relies on ligamentotaxis to reduce retropulsed fragments as the fracture is reduced and spinal alignment restored.90–93 Direct decompression may be performed anteriorly or posteriorly. Posterior decompression via laminectomy is useful when a piece of fractured lamina or infolded ligamentum flavum is protruding into the canal or a single nerve root requires decompression. Retropulsed vertebral body fragments in the spinal canal cause most neurologic deficits, and these require a direct decompression. Because the compression is anterior to the thecal sac, an anterior approach is the most direct method to effect the decompression. In some instances the decompression may be achieved via a posterolateral transpedicular approach, especially in the lumbar spine at the nerve root level, where the dural tube may be retracted more safely than at cord level.94 The offending bone fragments are identified and either removed or tamped anteriorly. Posterior pedicle screw instrumentation is then performed to achieve stability.
Surgical Principle #3: Minimization of Construct Length
Minimization of construct length is more important in the mobile lumbar spine than the thoracic spine. Classically, “long-segment” constructs consisted of instrumentation 2 or 3 levels above and below the injured level.95,96 With the advent of modern pedicle screw systems, constructs now typically include one or two levels above and below the injured level, provided the bone quality is normal. The addition of sublaminar hooks at the same level as the terminal screws may improve the biomechanical performance of the construct while minimizing the construct length.97–99 “Short-segment” fixation is the term used to describe a construct limited to one level above and below the injury. Initial reports with this technique were disappointing due to relatively high rates of instrumentation failure and loss of reduction.100–103 As techniques, implants, and indications have been refined, short-segment fixation may be used with a similar success rate as long-segment fixation in certain injury patterns, which maintains as much native spinal motion as possible.104–106 Placement of screws in the injured level when technically possible may provide enough additional rigidity to allow for short-segment fixation in cases that would have been traditionally treated with long-segment fixation.107 The surgeon may choose to support the instrumentation construct with an orthosis depending on individual patient factors such as screw purchase and patient compliance.
Surgical Principle #4: Appropriate Surgical Timing
The optimal timing for surgical decompression and stabilization in patients with neurologic injury is not well established.108–110 The potential benefit of early decompression must be weighed against other factors such as medical optimization, management of coexisting injuries, and operating room conditions such as availability of neurophysiological monitoring and the appropriate anesthesia team and operating room personnel. Those patients with a progressive neurologic deficit should certainly be taken to surgery as quickly as possible.
Surgical Principle #5: Avoidance of Complications
The main complications associated with surgery include dural tear, iatrogenic neural injury, pseudoarthrosis, failure of fixation, iatrogenic flat back, infection, and medical complications. Burst fractures with associated lamina fractures have a high incidence of dural tear, especially in the setting of a neurologic deficit.111 The surgeon must be aware of the possibility of nerve roots entrapped in the lamina fracture and be prepared to repair dural tears or place a cerebrospinal fluid drain if needed. Iatrogenic neural injuries may occur during prone patient positioning or from direct injury during surgery. Great care must be taken when positioning patients with unstable injuries. Consideration should be given to performing awake positioning in patients with severe spinal instability and intact neural function or incomplete neural injuries. The patient may be able to self-protect his or her spine to some degree with muscle tone, and the surgeon can directly and continuously monitor the patient’s neurologic function.
Treatment of Specific Injuries
Sprains and Minor Fractures
Sprains and isolated minor fractures are not associated with neurologic deficits or spinal instability and can usually be treated symptomatically, with or without bracing.112,113 If a brace is chosen for comfort, it need not be rigid. The main concern with these injuries is misdiagnosis and undertreatment of a more serious spinal injury, such as with disruption of the posterior ligamentous complex, or sacral fracture associated with a L5 transverse process fracture. Consideration must be given to the mechanism of injury and clinical examination, and if there is doubt, advanced imaging techniques should be used to assess for more significant occult injury. It should also be kept in mind that patients with minor spine fractures may have more significant intra-abdominal injuries.114 Patients with isolated sprains and minor fractures should be able to mobilize readily, and full recovery is expected over a 6-week to 6-month period depending on the extent of the injury, with most patients returning to unrestricted activities within 6 to 12 weeks.
Traumatic Disc Herniations
Isolated disc herniations secondary to high-energy trauma are sufficiently rare that it is not possible to comment on the rate of neurologic injury, appropriate treatment, or long-term outcomes. Terhaag115 found only four traumatic disc herniations in his series of 1771 patients with disc lesions. The literature is scarce on this subject.115–118 Treatment protocols are similar to that of nontraumatic disc herniations, with an initial trial of nonoperative management and early mobilization. Bracing is not necessary. Preference may be given to early surgical intervention if there is severe radiculopathy or a dense nerve root motor deficit caused by the disc herniation. Emergency surgery would be indicated if the patient presented with or developed a cauda equina syndrome.
Compression Fractures
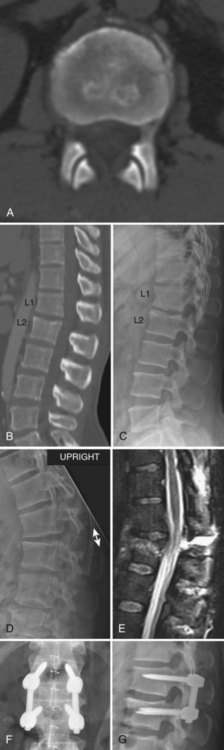
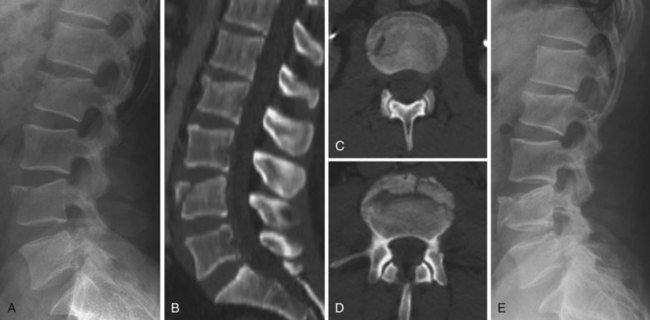
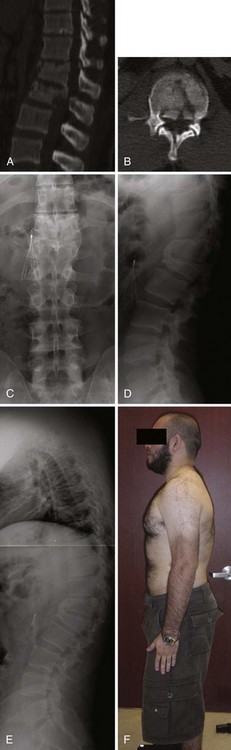
Compression fractures are usually intrinsically stable and rarely cause neurologic deficits. They occur most frequently due to a low-energy mechanism in elderly osteopenic patients but also occur due to higher-energy mechanisms in patients with normal bone. This discussion is directed toward fractures in patients with normal bone because osteoporotic compression fracture treatment is discussed in more detail in Chapter 88. Compression fractures were classically treated with postural reduction and plaster cast immobilization.119,120 The use of plaster casts gave way to less cumbersome brace treatment, and these fractures can be reliably treated with bracing the vast majority of the time, using either a three-point hyperextension type or a custom-molded orthosis (Fig. 78–11).121,122 The prognosis is generally favorable, though some patients will complain of persistent back pain long after the fracture has healed. Several clinical reviews did not find a correlation between the initial injury severity and the degree of residual pain.49,123,124 Other studies, however, have shown a correlation between the severity of injury and back pain.125–130 It is from these studies that the degree of vertebral body height loss and traumatic kyphosis became a criteria for surgical treatment in a small proportion of these fractures.
Surgical stabilization may be considered when there is significant traumatic kyphosis and/or vertebral body height loss, especially in the setting of posterior ligamentous complex compromise. It is difficult to say with certainty at what point the degree of deformity is deemed significant and the transition should be made from brace to surgical treatment, but commonly cited numbers are 30 degrees of traumatic kyphosis and 50% vertebral body height loss, with these fractures thus deemed unstable.127 Individual patient factors and overall spinal alignment need to be considered as well. Posterior pedicle-screw instrumentation with fusion is the usual mode of surgical treatment, either short-segment or long-segment depending on the circumstances. Provided the fracture is treated surgically within the first 7 to 10 days of injury, significant reduction can usually be achieved posturally and additional correction may be achieved with distraction through the pedicle screws with the intact anterior longitudinal ligament preventing overdistraction. An anterior approach is not necessary for decompression because there is no retropulsion into the canal, but it may be considered when there is a residual bone void anteriorly after the fracture is reduced or in delayed fracture treatment when an anterior release is necessary to achieve a reduction. Fractures treated surgically generally have a good prognosis, similar to stable compression fractures treated in a brace. There are no meaningful randomized trials comparing surgery with brace treatment in these “unstable” high-energy compression fractures. Osteoporotic compression fractures are generally stable and may be treated in a brace, although persistent pain may be an indication for vertebroplasty or kyphoplasty. The efficacy of vertebroplasty is a source of controversy, and this is discussed more thoroughly in Chapter 88.131,132
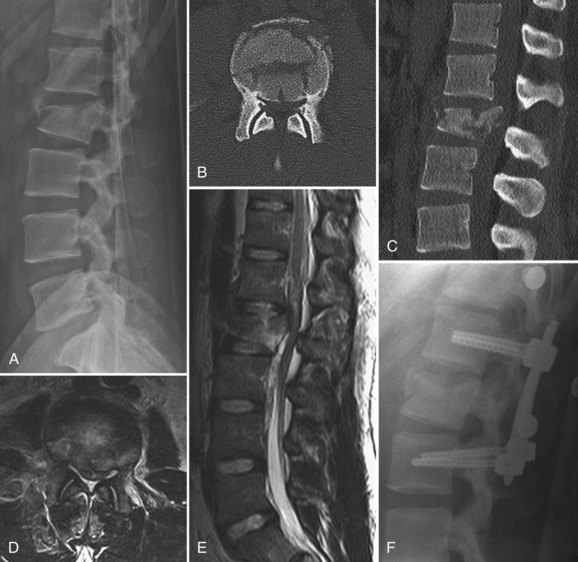
Burst Fractures
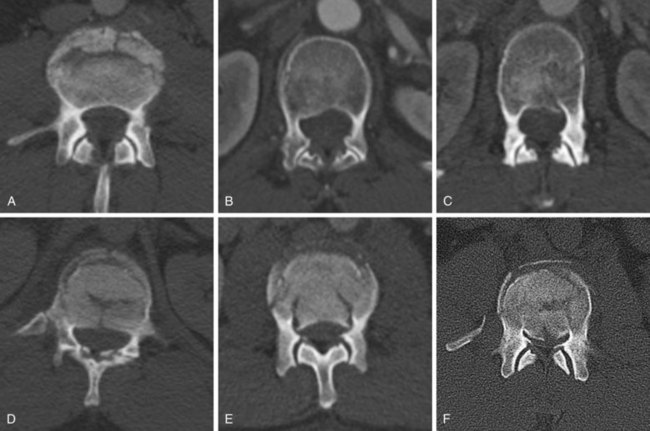
Brace treatment has been established as appropriate for neurologically intact patients with less than 30 degrees of traumatic kyphosis, less than 50% vertebral body height loss, and no or minimal injury to the posterior ligamentous complex.133–136 A few authors have even advocated brace treatment for burst fractures with greater than 50% height loss and in some instances when kyphosis exceeds 30 degrees, provided the injury is otherwise stable.137 It is important to consider individual patient factors and overall sagittal alignment in deciding on operative versus nonoperative management, rather than relying on strict radiographic criteria.
Burst fractures with a neurologic deficit are defined as unstable because of the neurologic injury, regardless of the extent of deformity or the integrity of the posterior elements, and are best treated surgically. This gives the best chance of neurologic recovery and prevents neurologic deterioration in incomplete injuries by stabilizing the fracture, and it allows early mobilization of the patient with paralysis. Exceptions can be made for mild isolated nerve root injuries in an otherwise mechanically stable fracture pattern, with a high rate of neurologic recovery under these circumstances.138 The dorsally displaced fragments from the vertebral body usually cause the neural element injury, and direct decompression is typically necessary. Laminectomy alone is contraindicated because it tends to be destabilizing and does not effectively decompress the neural elements.22,122 Direct decompression is most reliably accomplished via an anterior approach, but it can also be performed via a posterior transpedicular approach.94,139–142
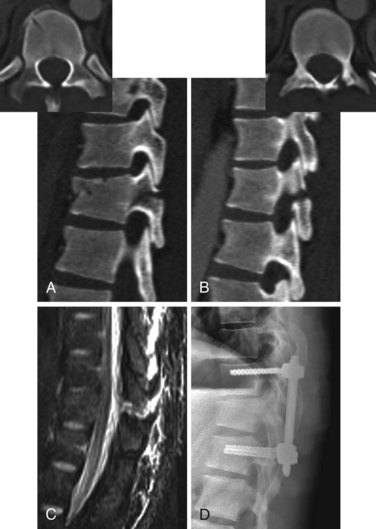
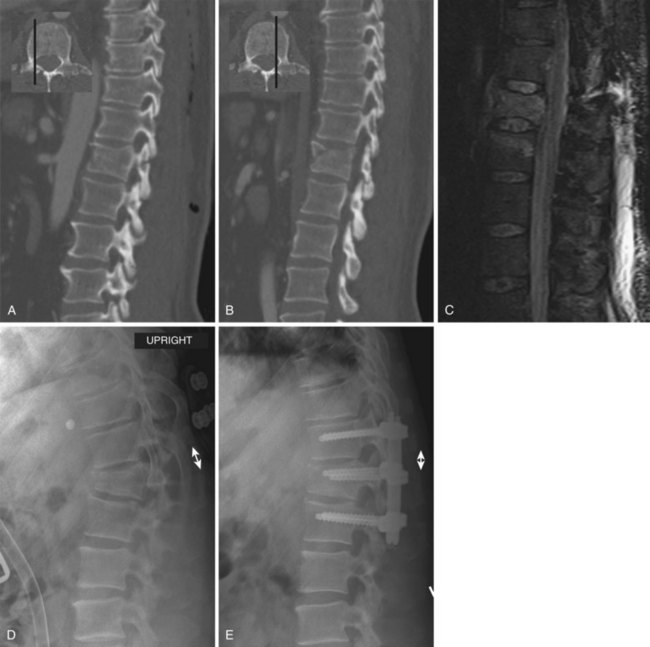
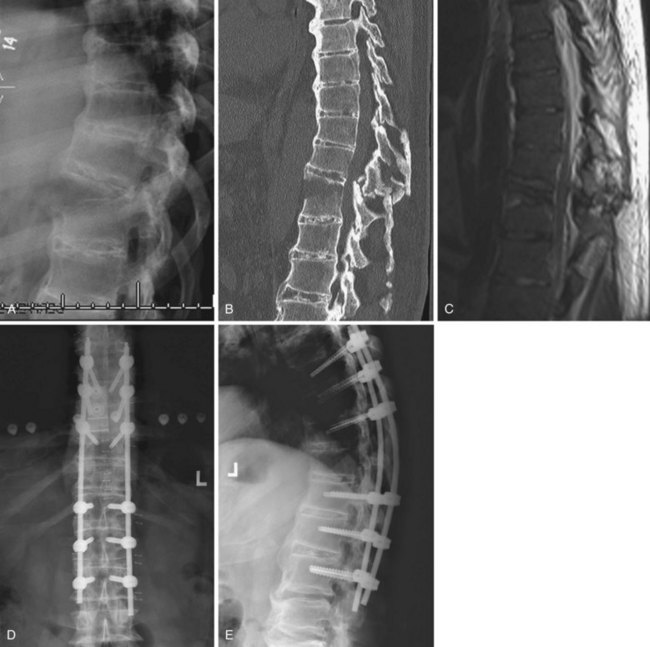
Burst fractures with posterior ligamentous complex disruption are mechanically unstable and are best treated with surgery. Posterior pedicle screw instrumentation and fusion restores the posterior ligament tension-band effect and is the treatment of choice in neurologically intact patients (Fig. 78–12).143,144 In cases with a highly comminuted vertebral body and/or loss of greater than 50% of the vertebral body height, consideration may be given to performing anterior surgery to restore the integrity of the anterior column (Fig. 78–13). Exceptions to surgical management of burst fractures with posterior column injury should be made when the posterior injury consists of isolated sagittal lamina fractures, minimally displaced facet or spinous process fractures, and minimal facet opening. Under these circumstances the posterior ligamentous complex may retain its integrity, and these injuries are therefore potentially stable. Consideration should be given to nonoperative treatment. True disruption of the posterior ligamentous complex means the tension-band stabilizing effect of the posterior elements is lost, as may occur with facet subluxation, dislocation, and fracture-dislocation. This leads to progressive instability, and surgery is therefore indicated for stabilization purposes. In those fractures with posterior element injury, the difficulty lies in discerning the extent and significance of this component of the injury and its implications on stability. When the significance of a posterior element injury is borderline, it is usually safe to carefully mobilize a neurologically intact patient in a brace and assess for progressive instability with serial upright radiographs.
Long-term outcomes are mainly related to the extent of neurologic injury. For patients who are neurologically intact, the prognosis is generally favorable.133–136 Rates of residual pain and disability and the ability to return to vigorous work are similar in surgically and nonsurgically treated patients, though there are few prospective randomized studies and these show conflicting results.145–147 If a kyphotic deformity reduction is performed surgically or with bracing, some loss of correction can be expected on long-term follow-up.148,149 Radiographic parameters including residual kyphosis, vertebral body height loss, and canal compromise have not been definitively shown to be associated with long-term outcomes (Fig. 78–14).135,136,145,147,149 Of these three measurements, kyphosis greater than 30 degrees is the least desirable and is probably most commonly believed to be a predictor of long-term back pain.1
Flexion-Distraction Injuries (Chance Fractures and Chance Variants)
The incidence of neurologic deficit in flexion-distraction injuries is relatively low, perhaps 10% to 15%.150 The injury classically occurs with lap-belt car restraints and is associated with a high incidence of intra-abdominal injury.151 The deforming forces occur in the sagittal plane, with displacement manifesting as focal kyphosis due to gapping of the posterior elements. Injuries through bone may be acutely unstable but tend to heal reliably via primary osteosynthesis. Injuries through the posterior ligamentous complex do not heal as well and carry a more guarded prognosis. Brace treatment therefore is more likely to be successful in stable bony injuries. Minimally angulated bony injuries may be treated in a brace. Minimally angulated ligamentous injuries may also be considered for brace treatment but need to be followed closely to make sure healing occurs. Ligamentous injuries are more likely to require surgery than a purely osseous injury. Caution should be used when declaring these injures minimally angulated because the kyphotic deformity may reduce with gravity when the patient is imaged in the typical supine position. Swelling and tenderness over the spinous processes is potentially indicative of such a situation. MRI will show the extent of posterior injury in questionable cases.
Unstable injuries of both the bony and ligamentous types are best treated surgically.
The main difficulty with this type of injury is determining stability. Although a minimally angulated fracture may be obviously stable, and a large focal kyphotic deformity may be obviously unstable, it is the cases that fall between these extremes that pose a dilemma. Legay’s series of 18 patients showed an association of initial kyphotic deformity greater than 17 degrees with a poor prognosis.152 Glassman’s series of 12 cases in children showed brace treatment failed when the initial kyphotic deformity was greater than 20 degrees.153 These numbers are consistent with biomechanical studies that show substantial structural injury with potential instability occurring somewhere between 12 and 17 degrees of traumatic focal kyphosis, and definite instability beyond 19 to 22 degrees of focal kyphosis.154–157 These numbers are helpful in guiding treatment in borderline cases. It is also reasonable under borderline circumstances to brace and cautiously mobilize the neurologically intact patient and obtain serial radiographs to see if instability becomes apparent.
Surgery typically consists of posterior pedicle screw instrumentation and fusion across the injured level (Fig. 78–15). This serves as a tension-band and effectively counteracts the distractive forces across the posterior elements. Instrumentation may cross two or three levels depending on the anatomy of the injury. Consideration may be given to performing percutaneous pedicle screw instrumentation without fusion in a primarily bony injury, allowing the fractures to heal, then removing the instrumentation 6 to 9 months after injury, although this technique has not been proven. Whatever technique is chosen, the goal is to achieve anatomic reduction and stabilization, which under most circumstances is readily achieved because this injury occurs as a result of tensile forces and so tends to be less destructive than those injuries that occur secondary to compressive forces. Careful intraoperative patient positioning on a standard spine frame with support under the hips and chest usually results in excellent reduction of the injury, and the native anatomy is restored. Long-term prognosis therefore tends to be favorable in neurologically intact patients.152,153,158,159
Fracture-Dislocations
Fracture-dislocations are highly unstable injuries and result in neurologic injury in 75% or more of patients.1,54 There is no role for bracing. Surgical treatment should proceed as soon as possible. If surgery is delayed for whatever reason, the patient should be carefully transferred to a roto-rest bed. Awake positioning is usually well tolerated with proper patient coaching before surgery and may be considered in patients with a normal neurologic examination or incomplete neurologic injury. This allows the patient to maintain muscle tone and self-protect against further spinal subluxation, and it also allows for real-time monitoring of the neurologic status. Posterior pedicle screw instrumentation and fusion can usually be limited to two levels above and below the injury, though longer constructs may be required (Fig. 78–16). Anterior procedures are usually not necessary. Prognosis depends primarily on neurologic status.
Extension and Extension-Distraction Injuries
Extension injuries are rare, so it is difficult to comment on the incidence of neurologic deficit, but it is high, perhaps 75%.73,160 These injuries tend to be unstable, especially in the AS and DISH populations, but also when there is posterior element disruption in an otherwise normal spine. Brace treatment may be considered when the injury is minimally displaced in an otherwise normal spine, but this injury pattern is unusual. Otherwise, surgery is indicated. The spine should be assumed to be highly unstable and consideration given to awake positioning. In the ankylosed spine, multiple points of fixation are necessary because of poor bone quality, usually three levels above and below the injury. Percutaneous pedicle screw instrumentation may be appropriate because the spine is nonmobile and fusion is not necessary (Fig. 78–17). Healing reliably occurs via primary osteosynthesis. Prognosis depends primarily on neurologic status.
Penetrating Injuries: Gunshot Wounds
Penetrating injuries are usually secondary to gunshot wounds and are rarely mechanically unstable, even in the setting of a neurologic deficit. Neurologic injury is common, with gunshot wounds being the third leading cause of spinal cord injury after motor vehicle accidents and falls.161 Injuries involving a single spinal column do not require bracing, but those involving two or three columns should be braced for 6 to 12 weeks. Instability is seen in those rare cases with significant comminution of the vertebral body with extension of the injury through the posterior elements. This is the rare type of gunshot wound to the spine that may require surgical stabilization. Low-velocity (handgun) wounds are the most common type of injury in the civilian population, and local wound care can usually be performed in the emergency department. High-velocity (rifle) gunshot wounds are less common in the civilian population, and débridement should be performed in the operating room.
Surgery may be indicated to remove a bullet from the spinal canal if it is causing compression of the neural elements and there is a neurologic deficit. Below the level of the conus, significant motor improvement may be seen, although this does not seem to be the case above T12.161 With a stable neurologic deficit, surgery should be performed at 7 to 10 days after injury to allow the dura and surrounding soft tissues to heal, thus simplifying the operation.162 Cerebrospinal fluid-cutaneous fistulas occur 6% of the time after laminectomy to remove the bullet, but delaying surgery 7 to 10 days after injury may decrease the incidence.162,163 With a progressive neurologic deficit, surgery should be performed as soon as it is safe to proceed. Instrumentation is usually not required unless instability is created during decompression to remove the bullet. Surgery may also be considered to remove a bullet from the disc space if mechanical symptoms occur and to avoid potential lead poisoning. Lead toxicity may result from a bullet bathed in synovial fluid, and this may also be true with a bullet in the disc space.164
Spine infections following gunshot wounds are well recognized, most commonly following injury to a hollow viscus such as the colon. For gunshots not involving visceral injury, prophylactic parenteral antibiotics should be administered for 72 hours, with a low incidence of subsequent spine infection. For gunshots involving contaminated visceral injury, broad-spectrum parenteral antibiotics should be administered for 7 to 10 days. Infection rates are 5% to 10% with this regimen.162,165
It is common for patients with spinal cord injuries secondary to a gunshot wound to experience local and referred pain radiating into the paralyzed extremities. Surgical decompression does not seem to help.161,163 Pain usually slowly resolves with time, and medications such as nonsteroidal anti-inflammatory drugs, amitriptyline, carbamazepine, gabapentin (Neurontin), or pregabalin may be of benefit.
Summary
Pearls
Pitfalls
Key Points
1 Bohlmann HH, Kirkpatrick JS, Delamarter RB. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Rel Res. 1994;300:24-29.
2 Cantor JB, Lebwohl NH, Garvey T, Eismont FJ. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine. 1993;19:1731-1740.
3 Denis F. The three-column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
4 Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990 May 17;322(20):1405-1411.
5 Wood K, Buttermann G, Mehbod A, Garvey T, Jhanjee R, Sechriest V, Butterman G. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003 May;85-A(5):773-781.
1 Gertzbein SD. Scoliosis Research Society. Multicenter spine fracture study. Spine. 1992;17:528-540.
2 Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;17:126-133.
3 Calenoff L, Chessare JW, Rogers LF, et al. Multiple level spinal injuries: importance of early recognition. AJR Am J Roentgenol. 1978;130:665-669.
4 Stagnara P, De Mauroy JC, Dran G, et al. Reciprocal angulation of vertebral bodies in a sagittal plane: approach to references for the evaluation of kyphosis and lordosis. Spine. 1982;7:335-342.
5 White AA3rd, Panjabi MM. The basic kinematics of the human spine. A review of past and current knowledge. Spine. 1978;3:12-20.
6 Vialle R, Levassor N, Rillardon L, et al. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87:260-267.
7 Panjabi MM, Brand RAJr, White AA3rd. Three-dimensional flexibility and stiffness properties of the human thoracic spine. J Biomech. 1976;9:185-192.
8 Roussouly P, Gollogly S, Noseda O, et al. The vertical projection of the sum of the ground reactive forces of a standing patient is not the same as the C7 plumb line: a radiographic study of the sagittal alignment of 153 asymptomatic volunteers. Spine. 2006;31:E320-E325.
9 Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30:346-353.
10 Kuntz C4th, Levin LS, Ondra SL, et al. Neutral upright sagittal spinal alignment from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104-112.
11 Saboe LA, Reid DC, Davis LA, et al. Spine trauma and associated injuries. J Trauma. 1991;31:43-48.
12 Henderson RL, Reid DC, Saboe LA. Multiple noncontiguous spine fractures. Spine. 1991;16:128-131.
13 Reid DC, Henderson R, Saboe L, Miller JD. Etiology and clinical course of missed spine fractures. J Trauma. 1987;27:980-986.
14 Bohlman HH. Acute fractures and dislocations of the cervical spine. An analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119-1142.
15 Anderson S, Biros MH, Reardon RF. Delayed diagnosis of thoracolumbar fractures in multiple-trauma patients. Acad Emerg Med. 1996;3:832-839.
16 Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405-1411.
17 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
18 Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
19 Geisler FH, Coleman WP, Grieco G, et al. The Sygen multicenter acute spinal cord injury study. Spine. 2001;26(24 Suppl):S87-S98.
20 Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery. 2002;50(Suppl):S63-S71.
21 Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26:407-415.
22 Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
23 Kingwell SP, Curt A, Dvorak MF. Factors affecting neurological outcome in traumatic conus medullaris and cauda equina injuries. Neurosurg Focus. 2008;25:E7.
24 Harrop JS, Hunt GEJr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus. 2004;16:E4.
25 Harris JHJr. Radiographic evaluation of spinal trauma. Orthop Clin North Am. 1986;17:75-86.
26 McGrory BJ, VanderWilde RS, Currier BL, Eismont FJ. Diagnosis of subtle thoracolumbar burst fractures. A new radiographic sign. Spine. 1993;18:2282-2285.
27 Atlas SW, Regenbogen V, Rogers LF, Kim KS. The radiographic characterization of burst fractures of the spine. AJR Am J Roentgenol. 1986;147:575-582.
28 Berry GE, Adams S, Harris MB, et al. Are plain radiographs of the spine necessary during evaluation after blunt trauma? Accuracy of screening torso computed tomography in thoracic/lumbar spine fracture diagnosis. J Trauma. 2005;59:1410-1413.
29 Epstein O, Ludwig S, Gelb D, et al. Comparison of computed tomography and plain radiography in assessing traumatic spinal deformity. J Spinal Disord Tech. 2009;22:197-201.
30 Diaz JJJr, Cullinane DC, Altman DT, et al. Practice management guidelines for the screening of thoracolumbar spine fracture. J Trauma. 2007;63:709-718.
31 Hauser CJ, Visvikis G, Hinrichs C, et al. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003;55:228-234.
32 Sheridan R, Peralta R, Rhea J, et al. Reformatted visceral protocol helical computed tomographic scanning allows conventional radiographs of the thoracic and lumbar spine to be eliminated in the evaluation of blunt trauma patients. J Trauma. 2003;55:665-669.
33 Inaba K, Munera F, McKenney M, et al. Visceral torso computed tomography for clearance of the thoracolumbar spine in trauma: a review of the literature. J Trauma. 2006;60:915-920.
34 Campbell SE, Phillips CD, Dubovsky E, et al. The value of CT in determining potential instability of simple wedge-compression fractures of the lumbar spine. AJNR Am J Neuroradiol. 1995;16:1385-1392.
35 Bagley LJ. Imaging of spinal trauma. Radiol Clin North Am. 2006;44:1-12.
36 Ballock RT, Mackersie R, Abitbol JJ, et al. Can burst fractures be predicted from plain radiographs? J Bone Joint Surg Br. 1992;74:147-150.
37 Lammertse D, Dungan D, Dreisbach J, et al. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2007;30:205-214.
38 Flanders AE, Spettell CM, Tartaglino LM, et al. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649-655.
39 Miyanji F, Furlan JC, Aarabi B, et al. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome – prospective study with 100 consecutive patients. Radiology. 2007;243:820-827.
40 Flanders AE, Spettell CM, Friedman DP, et al. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol. 1999;20:926-934.
41 Oner FC, van Gils AP, Dhert WJ, Verbout AJ. MRI findings of thoracolumbar spine fractures: a categorisation based on MRI examinations of 100 fractures. Skeletal Radiol. 1999;28:433-443.
42 Lee HM, Kim HS, Kim DJ, et al. Reliability of magnetic resonance imaging in detecting posterior ligament complex injury in thoracolumbar spinal fractures. Spine. 2000;25:2079-2084.
43 Lee JY, Vaccaro AR, Schweitzer KMJr, et al. Assessment of injury to the thoracolumbar posterior ligamentous complex in the setting of normal-appearing plain radiography. Spine J. 2007;7:422-427.
44 Vaccaro AR, Lee JY, Schweitzer KMJr, et al. Assessment of injury to the posterior ligamentous complex in thoracolumbar spine trauma. Spine J. 2006;6:524-528.
45 White AA, Panjabi MM. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1978.
46 Bohler L. The Treatment of Fractures, ed 4. Baltimore: W. Wood; 1935.
47 Watson-Jones R. Fractures and Joint Injuries, 3rd ed. Edinburgh: E & S Livingstone; 1943.
48 Key JA, Conwell HE. The Management of Fractures, Dislocations, and Sprains, 4th ed. St Louis: CV Mosby & Co; 1946.
49 Nicoll EA. Fractures of the dorso-lumbar spine. J Bone Joint Surg Am. 1949;31:376-394.
50 Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534-1551.
51 Kelly RP, Whitesides TEJr. Treatment of lumbodorsal fracture-dislocations. Ann Surg. 1968;167:705-717.
52 Bedbrook GM. Treatment of thoracolumbar dislocation and fractures with paraplegia. Clin Orthop Relat Res. 1975;112:27-43.
53 Lucas JT, Ducker TB. Motor classification of spinal cord injuries with mobility, morbidity and recovery indices. Am Surg. 1979;45:151-158.
54 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
55 Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984 Oct;189:65-76.
56 Panjabi MM, Oxland TR, Kifune M, et al. Validity of the three-column theory of thoracolumbar fractures. A biomechanic investigation. Spine. 1995;20:1122-1127.
57 Chance GQ. Note on a type of flexion fracture of the spine. Br J Radiol. 1948;21:452.
58 McAfee PC, Yuan HA, Fredrickson BE, Lubicky JP. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461-473.
59 Ferguson RL, Allen BLJr. A mechanistic classification of thoracolumbar spine fractures. Clin Orthop Relat Res. 1984;189:77-88.
60 Ferguson RL, Allen BLJr. An algorithm for the treatment of unstable thoracolumbar fractures. Orthop Clin North Am. 1986;17:105-112.
61 Gertzbein SD. Classification of thoracic and lumbar fractures. In: Gertzbein SD, editor. Fractures of the Thoracic and Lumbar Spine. Baltimore: Williams and Wilkins, 1992.
62 Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184-201.
63 Vaccaro AR, Lehman RAJr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325-2333.
64 Patel AA, Dailey A, Brodke DS, Daubs M, et al. Thoracolumbar spine trauma classification: the Thoracolumbar Injury Classification and Severity Score system and case examples. J Neurosurg Spine. 2009;10:201-206.
65 Rihn JA, Anderson DT, Harris E, et al. A review of the TLICS system: a novel, user-friendly thoracolumbar trauma classification system. Acta Orthop. 2008;79:461-466.
66 Whang PG, Vaccaro AR, Poelstra KA, et al. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine. 2007;32:791-795.
67 Patel AA, Vaccaro AR, Albert TJ, et al. The adoption of a new classification system: time-dependent variation in interobserver reliability of the thoracolumbar injury severity score classification system. Spine. 2007;32:E105-E110.
68 Fast A, Sosner J, Begeman P, et al. Lumbar spinal strains associated with whiplash injury: a cadaveric study. Am J Phys Med Rehabil. 2002;81:645-650.
69 Costello RF, Beall DP. Nomenclature and standard reporting terminology of intervertebral disk herniation. Magn Reson Imaging Clin N Am. 2007;15:167-174.
70 Milette PC. Classification, diagnostic imaging, and imaging characterization of a lumbar herniated disk. Radiol Clin North Am. 2000;38:1267-1292.
71 Gellad FE, Levine AM, Joslyn JN, et al. Pure thoracolumbar facet dislocation: clinical features and CT appearance. Radiology. 1986;161:505-508.
72 O’Callaghan JP, Ullrich CG, Yuan HA, Kieffer SA. CT of facet distraction in flexion injuries of the thoracolumbar spine: the “naked” facet. AJR Am J Roentgenol. 1980;134:563-568.
73 Denis F, Burkus JK. Shear fracture-dislocations of the thoracic and lumbar spine associated with forceful hyperextension (lumberjack paraplegia). Spine. 1992;17:156-161.
74 Bono CM, Heary RF. Gunshot wounds to the spine. Spine J. 2004;4:230-240.
75 Moon E, Kondrashov D, Hannibal M, et al. Gunshot wounds to the spine: literature review and report on a migratory intrathecal bullet. Am J Orthop. 2008;37:E47-E51.
76 Finitsis SN, Falcone S, Green BA. MR of the spine in the presence of metallic bullet fragments: is the benefit worth the risk? AJNR Am J Neuroradiol. 1999;20:354-356.
77 Jacobs RR, Asher MA, Snider RK. Thoracolumbar spinal injuries. A comparative study of recumbent and operative treatment in 100 patients. Spine. 1980;5:463-477.
78 Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179-192.
79 Kallemeier PM, Beaubien BP, Buttermann GR, et al. In vitro analysis of anterior and posterior fixation in an experimental unstable burst fracture model. J Spinal Disord Tech. 2008;21:216-224.
80 Kaneda K, Abumi K, Fujiya M. Burst fractures with neurologic deficits of the thoracolumbar-lumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9:788-795.
81 An HS, Lim TH, You JW, et al. Biomechanical evaluation of anterior thoracolumbar spinal instrumentation. Spine. 1995;20:1979-1983.
82 Sasso RC, Renkens K, Hanson D, et al. Unstable thoracolumbar burst fractures: anterior-only versus short-segment posterior fixation. J Spinal Disord Tech. 2006;19:242-248.
83 Oskouian RJJr, Johnson JP. Vascular complications in anterior thoracolumbar spinal reconstruction. J Neurosurg. 2002;96(1 Suppl):1-5.
84 Fantini GA, Pappou IP, Girardi FP, et al. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine. 2007;32:2751-2758.
85 Gurwitz GS, Dawson JM, McNamara MJ, et al. Biomechanical analysis of three surgical approaches for lumbar burst fractures using short-segment instrumentation. Spine. 1993;18:977-982.
86 Chakera TM, Bedbrook G, Bradley CM. Spontaneous resolution of spinal canal deformity after burst-dispersion fracture. AJNR Am J Neuroradiol. 1988;9:779-785.
87 Dai LY. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res. 2001;382:119-123.
88 de Klerk LW, Fontijne WP, Stijnen T, et al. Spontaneous remodeling of the spinal canal after conservative management of thoracolumbar burst fractures. Spine. 1998;23:1057-1060.
89 Sjöström L, Jacobsson O, Karlström G, et al. Spinal canal remodelling after stabilization of thoracolumbar burst fractures. Eur Spine J. 1994;3:312-317.
90 Crutcher JPJr, Anderson PA, King HA, Montesano PX. Indirect spinal canal decompression in patients with thoracolumbar burst fractures treated by posterior distraction rods. J Spinal Disord. 1991;4:39-48.
91 Doerr TE, Montesano PX, Burkus JK, Benson DR. Spinal canal decompression in traumatic thoracolumbar burst fractures: posterior distraction rods versus transpedicular screw fixation. J Orthop Trauma. 1991;5:403-411.
92 Harrington RM, Budorick T, Hoyt J, et al. Biomechanics of indirect reduction of bone retropulsed into the spinal canal in vertebral fracture. Spine. 1993;18:692-699.
93 Edwards CC, Levine AM. Early rod-sleeve stabilization of the injured thoracic and lumbar spine. Orthop Clin North Am. 1986;17:121-145.
94 Garfin SR, Mowery CA, Guerra JJr, Marshall LF. Confirmation of the posterolateral technique to decompress and fuse thoracolumbar spine burst fractures. Spine. 1985;10:218-223.
95 Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine. A comparative study of three fixation devices in 70 patients. Spine. 1993;18:450-460.
96 Tasdemiroglu E, Tibbs PA. Long-term follow-up results of thoracolumbar fractures after posterior instrumentation. Spine. 1995;20:1704-1708.
97 de Peretti F, Hovorka I, Cambas PM, et al. Short device fixation and early mobilization for burst fractures of the thoracolumbar junction. Eur Spine J. 1996;5:112-120.
98 Chiba M, McLain RF, Yerby SA, et al. Short-segment pedicle instrumentation. Biomechanical analysis of supplemental hook fixation. Spine. 1996;21:288-294.
99 Farcy JP, Weidenbaum M, Glassman SD. Sagittal index in management of thoracolumbar burst fractures. Spine. 1990;15:958-965.
100 Kramer DL, Rodgers WB, Mansfield FL. Transpedicular instrumentation and short-segment fusion of thoracolumbar fractures: a prospective study using a single instrumentation system. J Orthop Trauma. 1995;9:499-506.
101 Tezeren G, Kuru I. Posterior fixation of thoracolumbar burst fracture: short-segment pedicle fixation versus long-segment instrumentation. J Spinal Disord Tech. 2005;18:485-488.
102 McLain RF, Burkus JK, Benson DR. Segmental instrumentation for thoracic and thoracolumbar fractures: prospective analysis of construct survival and five-year follow-up. Spine J. 2001;1:310-323.
103 Scholl BM, Theiss SM, Kirkpatrick JS. Short segment fixation of thoracolumbar burst fractures. Orthopedics. 2006;29:703-708.
104 Parker JW, Lane JR, Karaikovic EE, Gaines RW. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 4 1/2-year series. Spine. 2000;25:1157-1170.
105 Dai LY, Jiang LS, Jiang SD. Posterior short-segment fixation with or without fusion for thoracolumbar burst fractures. a five to seven-year prospective randomized study. J Bone Joint Surg Am. 2009;91:1033-1041.
106 McLain RF. The biomechanics of long versus short fixation for thoracolumbar spine fractures. Spine. 2006;31(11 Suppl):S70-S79.
107 Mahar A, Kim C, Wedemeyer M, et al. Short-segment fixation of lumbar burst fractures using pedicle fixation at the level of the fracture. Spine. 2007;32:1503-1507.
108 Vaccaro AR, Daugherty RJ, Sheehan TP, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22:2609-2613.
109 Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13-B26.
110 La Rosa G, Conti A, Cardali S, et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503-512.
111 Cammisa FPJr, Eismont FJ, Green BA. Dural laceration occurring with burst fractures and associated laminar fractures. J Bone Joint Surg Am. 1989;71:1044-1052.
112 Homnick A, Lavery R, Nicastro O, et al. Isolated thoracolumbar transverse process fractures: call physical therapy, not spine. J Trauma. 2007;63:1292-1295.
113 Bradley LH, Paullus WC, Howe J, Litofsky NS. Isolated transverse process fractures: spine service management not needed. J Trauma. 2008;65:832-836.
114 Miller CD, Blyth P, Civil ID. Lumbar transverse process fractures—a sentinel marker of abdominal organ injuries. Injury. 2000;31:773-776.
115 Terhaag D, Frowein RA. Traumatic disc prolapses. Neurosurg Rev. 1989;12(Suppl 1):588-594.
116 Vazquez D, Solano I, Pages E, et al. Thoracic disc herniation, cord compression, and paraplegia caused by electrical injury: case report and review of the literature. J Trauma. 1994;37:328-332.
117 Ando T, Mimatsu K. Traumatic lumbar disc herniation. A case report. Spine. 1993;18:2355-2357.
118 Martin G. The role of trauma in disc protrusion. N Z Med J. 1978;87:208-211.
119 Birch-Jensen A. Fracture of the spine; conservative treatment with plaster jacket without preceding reduction; a follow-up examination of 42 patients with isolated fractures of the vertebral body. Acta Chir Scand. 1955;109:377-383.
120 Davis AG. Fractures of the Spine. J Bone Joint Surg. 1929;11:133-156.
121 Jewett EL. Fracture of the spine; new treatment without plaster casts. J Int Coll Surg. 1950;13:407-414.
122 Bohlman HH. Treatment of fractures and dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1985;67:165-169.
123 Hazel WAJr, Jones RA, Morrey BF, Stauffer RN. Vertebral fractures without neurological deficit. A long-term follow-up study. J Bone Joint Surg Am. 1988;70:1319-1321.
124 Young MH. Long-term consequences of stable fractures of the thoracic and lumbar vertebral bodies. J Bone Joint Surg Br. 1973;55:295-300.
125 Westerborn A, Olsson O. Mechanics, treatment and prognosis of fractures of the dorso-lumbar spine. Acta Chir Scand. 1951;102:59-83.
126 Baab OD, Howorth MB. Fractures on the dorsal and lumbar vertebrae. J Am Med Assoc. 1951;146:97-100.
127 Day B, Kokan P. Compression fractures of the thoracic and lumbar spine from compensable injuries. Clin Orthop Relat Res. 1977;124:173-176.
128 Härkönen M, Kataja M, Lepistö P, et al. Fractures of the thoracic spine. Clinical and radiological results in 98 patients. Arch Orthop Trauma Surg. 1979;94:179-184.
129 Härkönen M, Kataja M, Keski-Nisula L, et al. Fractures of the lumbar spine. Clinical and radiological results in 94 patients. Arch Orthop Trauma Surg. 1979;94:43-48.
130 Härkönen M, Kataja M, Keski-Nisula L, et al. Injuries of the thoracolumbar junction. Clinical and radiological results in 149 patients. Arch Orthop Trauma Surg. 1979;94:35-41.
131 Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569-579.
132 Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568.
133 Cantor JB, Lebwohl NH, Garvey T, Eismont FJ. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine. 1993;18:971-976.
134 Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine. 1993;18:955-970.
135 Moller A, Hasserius R, Redlund-Johnell I, et al. Nonoperatively treated burst fractures of the thoracic and lumbar spine in adults: a 23- to 41-year follow-up. Spine J. 2007;7:701-707.
136 Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated conservatively: a long-term follow-up. Spine. 1988;13:33-38.
137 Chow GH, Nelson BJ, Gebhard JS, et al. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and early mobilization. Spine. 1996;21:2170-2175.
138 Dai LY, Jiang LS, Jiang SD. Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine. 2008;33:2536-2544.
139 Kaneda K, Taneichi H, Abumi K, et al. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997;79:69-83.
140 McAfee PC, Bohlman HH, Yuan HA. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67:89-104.
141 McNamara MJ, Stephens GC, Spengler DM. Transpedicular short-segment fusions for treatment of lumbar burst fractures. J Spinal Disord. 1992;5:183-187.
142 Kaya RA, Aydin Y. Modified transpedicular approach for the surgical treatment of severe thoracolumbar or lumbar burst fractures. Spine J. 2004;4:208-217.
143 McLain RF, Burkus JK, Benson DR. Segmental instrumentation for thoracic and thoracolumbar fractures: prospective analysis of construct survival and five-year follow-up. Spine J. 2001;1:310-323.
144 Danisa OA, Shaffrey CI, Jane JA, et al. Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. J Neurosurg. 1995;83:977-983.
145 Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85:773-781.
146 Seybold EA, Sweeney CA, Fredrickson BE, et al. Functional outcome of low lumbar burst fractures. A multicenter review of operative and nonoperative treatment of L3-L5. Spine. 1999;24:2154-2161.
147 Siebenga J, Leferink VJ, Segers MJ, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881-2890.
148 Tropiano P, Huang RC, Louis CA, et al. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine. 2003;28:2459-2465.
149 Willén J, Anderson J, Toomoka K, Singer K. The natural history of burst fractures at the thoracolumbar junction. J Spinal Disord. 1990;3:39-46.
150 Eismont FJ. Flexion-distraction injuries of the thoracic and lumbar spine. In: Levine AM, Eismont FJ, Garfin SR, et al, editors. Spine Trauma. Philadelphia: WB Saunders; 1998:404-405.
151 Gertzbein SD, Court-Brown CM. Flexion-distraction injuries of the lumbar spine. Mechanisms of injury and classification. Clin Orthop Relat Res. 1988;227:52-60.
152 LeGay DA, Petrie DP, Alexander DI. Flexion-distraction injuries of the lumbar spine and associated abdominal trauma. J Trauma. 1990;30:436-444.
153 Glassman SD, Johnson JR, Holt RT. Seatbelt injuries in children. J Trauma. 1992;33:882-886.
154 Neumann P, Nordwall A, Osvalder AL. Traumatic instability of the lumbar spine. A dynamic in vitro study of flexion-distraction injury. Spine. 1995;20:1111-1121.
155 Neumann P, Osvalder AL, Nordwall A, et al. The mechanism of initial flexion-distraction injury in the lumbar spine. Spine. 1992;17:1083-1090.
156 Osvalder AL, Neumann P, Lövsund P, Nordwall A. A method for studying the biomechanical load response of the (in vitro) lumbar spine under dynamic flexion-shear loads. J Biomech. 1993;26:1227-1236.
157 Nagel DA, Koogle TA, Piziali RL, Perkash I. Stability of the upper lumbar spine following progressive disruptions and the application of individual internal and external fixation devices. J Bone Joint Surg Am. 1981;63:62-70.
158 Gumley G, Taylor TK, Ryan MD. Distraction fractures of the lumbar spine. J Bone Joint Surg Br. 1982;64:520-525.
159 Levine AM, Bosse M, Edwards CC. Bilateral facet dislocations in the thoracolumbar spine. Spine. 1988;13:630-640.
160 Graham B, Van Peteghem PK. Fractures of the spine in ankylosing spondylitis. Diagnosis, treatment, and Complications. Spine. 1989;14:803-807.
161 Waters RL, Adkins RH. The effects of removal of bullet fragments retained in the spinal canal. Spine. 1991;16:934-939.
162 Eismont FJ. Gunshot wounds of the wpine. In: Levine AM, Eismont FJ, Garfin SR, et al, editors. Spine Trauma. Philadelphia: WB Saunders; 1998:525-543.
163 Stauffer ES, Wood RW, Kelly EG. Gunshot wounds of the spine: the effects of laminectomy. J Bone Joint Surg Am. 1979;61:389-392.
164 Grogan DP, Bucholz RW. Acute lead intoxication from a bullet in an intervertebral disk space. J Bone Joint Surg Am. 1981;63:1180-1182.
165 Roffi RP, Waters RL, Adkins RH. Gunshot wounds to the spine associated with a perforated viscus. Spine. 1989;14:808-811.