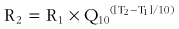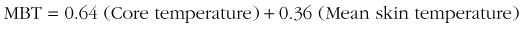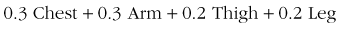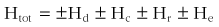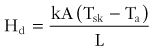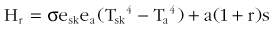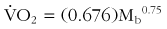Chapter 4 Thermoregulation
For online-only figures, please go to www.expertconsult.com ![]()
Conceptualizing the Thermoregulatory System
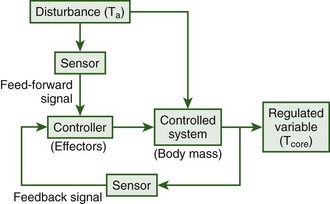
Humans are homeotherms and as such are capable of maintaining a relatively constant body temperature across a wide range of ambient temperatures. Such constancy is attained through the use of behavioral processes that involve maintaining or searching for a preferable environment and autonomic processes such as vasodilation of the skin blood vessels and sweating in the heat and shivering in the cold. These processes are controlled via a negative feedback system with a primary feed-forward input from skin sensors that monitor ambient temperature (Figure 4-1).89 The feed-forward input from the skin allows for the elicitation of thermoregulatory responses without a change in core body temperature. Thus, the primary regulated variable, core temperature, can remain relatively constant under widely varying environmental conditions. Without the feed-forward element, if a hot or cold stress were encountered, large amounts of heat would be gained or lost before deep body sensors were sufficiently affected to elicit restorative responses. In addition, when greater thermal stresses were encountered, greater deviations in core temperature would be necessary to elicit sufficient restorative responses.
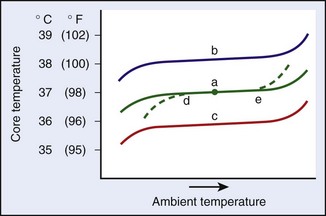
Figure 4-2 illustrates some of the concepts that relate to the thermoregulatory system and that will be discussed in this chapter. Under normal conditions, body temperature is relatively constant under a range of ambient temperatures, as is depicted by trace “a.” The breadth of this range of ambient temperature is called the range of normothermia. The midpoint of this range can be conveniently called the regulated temperature, which is shown by the dot in trace “a.” Toward the upper and lower ends of trace “a,” the core temperature inflects up and down. These inflections represent ambient temperature extremes at which the regulation begins to fail. Altered core temperatures can also accrue when there are alterations in the regulated temperature. Such alterations could be caused by the presence of bacterial toxins (e.g., fever) or starvation, which would cause increases (trace “b”) or decreases (trace “c”) in the regulated temperature. In addition, under various conditions, the effector responses can become compromised, which leads to decreases in the ability to defend against low temperatures (dashed line at “d”), which could indicate a problem with metabolic stores, or high temperatures (dashed line at “e”), which may indicate dehydration. Of course, various combinations of regulatory changes and altered effector responsiveness occur in conjunction with many threatening situations.
Basics of Core Temperature
Typical measurements of core temperature provide a good estimate of the temperature of critical internal organs and are quite stable across individuals. In a study that involved 700 observations of 148 healthy individuals,113 90% of the early-morning oral core-temperature measurements were between 36.0° C (96.9° F) and 37.1° C (98.9° F). Core temperature is vigorously defended by the body. At low temperatures, regional heterothermy that results from peripheral vasoconstriction forms an important aspect of this defense. The lowered skin temperature decreases the thermal gradient from the skin to the environment and thus decreases heat loss. At cooler temperatures, there can be a large amount of peripheral tissue that is well below core temperature, which leads to a major decrease in the overall heat content of the body. A nude human resting at 35° C (95° F) or 20° C (68° F) exhibits similar temperatures at various locations within the core. However, because of decreased temperatures in the outer shell, a person resting at 20° C will have a total heat content that is about 200 kcal lower than when resting at 35° C.168 If the peripheral vessels were suddenly dilated, an immediate drop in core temperature of about 3.5° C (6.3° F) would result. In a hypothermic individual, the discrepancy between core and shell temperatures could be considerably greater and could result in a dangerous postdilation drop in core temperature. A method for making a rough estimate of the potential drop in core temperature after peripheral vessel dilation is given in Estimating Mean Body Temperature, later.
A different type of heterothermy may be present in hyperthermic humans. As the brain temperature reaches high levels, blood flow that normally moves outward from the intracranium to the face via the ophthalmic vein is redirected and flows from the face inward.75 This results in the brain being cooled by blood that has passed through areas that have been cooled by facial sweating and leads to a brain temperature that is lower than that of the remainder of the core. Although brain cooling is clearly documented and accepted in many mammals,5 there has been some controversy about its importance in humans.15,24 Nevertheless, there is unanimous agreement that the head is an extremely important area for heat loss.15,25 Thus, for hyperthermic patients, it is important to optimize the heat loss from that region and, when necessary, to augment cranial heat dissipation by fanning and moistening.
Consequences of Altered Core Temperature
When tissue temperatures change, there are immediate and important effects on metabolism as well as on other physiologic mechanisms. With a 10° C (18° F) increase in temperature, the metabolism of typical human tissue increases by a factor (Q10) of about 2.7. The metabolic rate of the entire organism—apart from thermoregulatory responses—responds similarly. For temperature differences other than 10° C (18° F), these effects can be calculated with the following equation:168
where R2 and R1 are the two rates of physiologic response; T2 and T1 are the two temperatures; and Q10 is the increase in rate caused by a 10° C (18° F) increase in temperature.
A core temperature of 34° to 36° C (93.2° to 96.9° F) disrupts many important physiologic functions, which, taken together, may significantly affect patient outcome. Such mild hypothermia impairs recovery from surgical procedures as a result of such things as impaired peripheral blood flow and oxygen availability, increased possibility of cardiovascular complications, decreased antibody and cellular immune defenses, impaired coagulation, and increased metabolic expenditure for heat production.47,57,103,107 In most situations, it is very important to maintain the patient at normothermic levels.
Traumatic brain injury can be present in wilderness accidents, and it may be accompanied by hyperthermia. Heightened temperatures can exacerbate cerebral inflammation and lead to increased neuronal damage.185 There is current interest in invoking mild hypothermia to minimize damage to the central nervous system after neurologic injury.139 However, when this approach is used, care must be taken to deal with the side effects mentioned previously.148
Monitoring the Temperature of the Core and Other Sites
Monitoring the Core Temperature
A history of clinical thermometry is available111 as are good overviews of the assessment of core temperature.10,32,201 Sites for taking the temperature, in order of increasing invasiveness, include the forehead, axilla, oral cavity, tympanum, rectum, esophagus, bladder, and pulmonary artery. There is no clear-cut choice regarding the best site to monitor; particular situations demand different techniques. Thermometers that have been employed clinically include mercury-in-glass thermometers (which are now obsolete), electronic thermometers, tympanic radiation thermometers, and liquid crystal thermometers. Whatever instrument is used should have an accuracy of ±0.1° C (0.2° F). The handheld electronic thermometer is a good choice for field emergencies.
Measuring Instruments
Tympanic infrared radiometers are often used in hospital settings. However, even in this relatively predictable environment, some controversy exists regarding their ability to accurately assess core temperature. These instruments monitor the electromagnetic radiation that emanates from the ear canal; various manufacturers make use of different and complicated electronic circuitry to produce a temperature display. An advantage is that the reading takes only a few seconds,32 but questions remain regarding the overall accuracy of the measurement displayed. In a laboratory situation in which the auditory canal is plugged with a sponge and the probe measures only radiation that emanates from the tympanum, infrared tympanic thermometry provides an excellent estimate of the core temperature.174 In clinical settings, the results are less consistent. In one study, infrared tympanic thermometry produced core temperatures that were much more variable than rectal temperatures. Even after correcting for the higher rectal values (0.5° C [0.9° F]), tympanic measurements still inaccurately displayed one-third of the temperatures that were more than 37.7° C (99° F). An extended training program did not significantly alter the accuracy of the readings.147
In one instance, a child who arrived at an emergency department presented with tachycardia and skin vasoconstriction. Separate tympanic infrared thermometers gave core temperatures of 36.4° and 37.6° C (97.5° and 99.7° F); the rectal temperature was determined to be 42.2° C (108° F).158 Alternatively, in a hospital setting with a trained operator and immobile patients, two brands of infrared tympanic thermometers produced readings that were closer to pulmonary artery readings than those obtained from the axilla or the rectum.155
The potential benefits of a continuous and easily applied core temperature monitor have led to repeated attempts to validate liquid-crystal thermometers, which are typically placed on the head or neck surface. Unfortunately, the temperature readings produced by this method are not reliable.10,111 Because these measurements are compromised by the thermoregulatory vascular changes associated with heat conservation and heat dissipation and by changes in ambient temperature,80 they are particularly unsuited for field emergency measurements.
Measurement Sites
Although the deep internal temperatures of normothermic humans are reasonably similar, no specific anatomic site represents the “official” core temperature. The temperature at each location is a consequence of a combination of the local metabolic rate, local perfusion rate, proximity to the outer shell, and proximity to other locations that have differing rates of metabolism and perfusion. Nevertheless, because of the generally high overall rates of tissue perfusion in mammals, deep core temperatures rarely differ by more than 0.5° C (0.9° F). The temperature of the pulmonary artery is a good reference temperature for the overall status of the thermal core. At steady state, accepted sites for assessing core temperature differ with regard to varying amounts from this temperature. Esophageal and tympanic temperatures are essentially the same as the temperature of the pulmonary artery,155,166 whereas rectal temperature averages about 0.4° C (0.7° F) higher, and axillary and oral temperatures are about 0.2° C (0.4° F) and 0.4° C (0.7° F) lower, respectively.10,110,155
Tympanic temperature as an estimation of core temperature has long been controversial. Because the tympanic membrane is highly vascular and supplied by branches of the external and internal carotid arteries, it should be an ideal site. Nevertheless, over the years many studies have indicated that tympanic temperature is affected by ambient temperature and local facial cooling.166 Conditions under which these complications can be avoided have now been clarified: (1) if the ear canal is insulated; (2) if the thermocouple is made of fine wire and insulated except at the active junction; and (3) if the thermocouple is in direct contact with the tympanum (which causes the patient to “hear” a continuous and low-pitched sound), then alterations in core temperature are detected more rapidly than by an esophageal probe, are not affected by facial skin temperature, and are otherwise identical to esophageal temperature.166 In fact, if these conditions are adhered to during facial fanning, tympanic temperature provides a better estimate of brain temperature than do measurements made in the esophagus or the rectum.115 Accurate results were also obtained when an insulated probe was used in conjunction with an optical sensor to detect infrared radiation from the tympanum.174 Whether the conditions met in these carefully controlled studies can be duplicated in the field is unknown.
In steady-state conditions, rectal temperature is a good index of core temperature, and it can also be used to estimate brain temperature.204 However, when the heat content of the body or of the internal thermal compartments is in flux, rectal temperature changes more slowly than temperatures measured in other commonly used sites.155 There is a thermal gradient along the rectum, so all measurements should be made at a standard depth; 4 cm is recommended.10 The higher temperatures recorded in this region may be caused by a combination of low perfusion rates, digestive reactions, and bacterial activity, but there is not clear evidence of this.111 For assessing core temperature during outdoor exercise in the heat, the National Athletic Trainers’ Association recommends that only rectal temperature be used.29
Oral temperature is an excellent index of core temperature, provided that the mouth is kept closed. The sublingual pocket is well perfused by blood flow, and responds quite rapidly to alterations in core temperature. Mastication, smoking, fluid intake, and mouth breathing can affect sublingual temperature; these should be avoided during the period that immediately precedes the measurement.10,110,111 The use of an electronic thermometer with a rapidly responding sensor makes this measurement considerably more accurate and rapid than when it is performed with a mercury-in-glass thermometer.
Although axillary temperature does reflect core temperature, it has a number of negative characteristics and should be used only as a last resort. The axillary temperature is affected by local blood flow as well as by thermal and nonthermal sweating.10 Changes in core temperature are slow to affect the axillary temperature, and there is high interpatient variability.155 However, this measurement has proved to be particularly useful for assessing core temperature in infants.10,111
Estimating Mean Body Temperature
Mean body temperature (MBT) provides a mass-weighted average of body tissue temperature and thus can be related to the heat content of the entire body. For a severely hypothermic patient, MBT provides a way to gauge the potential fall in core temperature (afterdrop) after vessel dilation caused by rapid surface warming. Traditionally, estimates of MBT have been made with the use of a formula that combines mean skin temperature and core temperature. Recently, the validity of such estimates was evaluated for patients undergoing various procedures, including cardiac surgery during extracorporeal circulation; these studies included core temperatures as low as 18.5° C (65.3° F).106 “Peripheral compartment temperatures were estimated using fourth-order regression and integration over volume from 18 intramuscular needle thermocouples, 9 skin temperatures, and ‘deep’ hand and foot temperatures.”106 The authors concluded that the estimation of MBT from Burton’s original formula23 “is generally accurate and precise.”106 That formula is as follows:
Ramanathan151 found that a rough but reasonably accurate estimate of mean skin temperature could be provided by the temperature of the medial thigh, and that a very accurate estimate of mean skin temperature could be made by measuring and weighting the temperature of four skin sites as follows:
Physical Factors That Govern Heat Exchange: The Heat Balance Equation
The physical laws that govern heat transfer determine the net energy flux into or out of the body.* The heat balance equation is a convenient method for partitioning and quantifying the flow of energy between the environment and the body. A high rate of metabolic heat production is critical for maintaining a constant body temperature in mammals. This is represented by total heat production (Htot) on the left side of the following equation. For a person whose body is at thermal equilibrium, the equation is balanced and given as follows:
where Htot is the total metabolic heat production; Hd is the conductive heat exchange; Hc is the convective heat exchange; Hr is the radiative heat exchange; and He is the evaporative heat exchange.
Conductive Heat Exchange
Heat transfer between objects that are in direct contact is called conduction (Hd). The direction of heat flow is always from the higher to the lower temperature. Because conduction involves a direct interaction (i.e., contact) between molecules, this type of heat transfer is minimal except under certain circumstances, such as when sitting on a cold rock with a little insulation. Under such conditions, the heat lost to the rock would be similar to that lost from the remainder of the body surface by radiation and convection. Adequate insulation should be placed under patients who are in contact with hot or cold substrates.125 The equation that governs heat exchange by conduction is
where k is thermal conductivity; A is the area of contact; Tsk is the skin temperature; Ta is the ambient temperature; and L is the distance between the two surfaces.
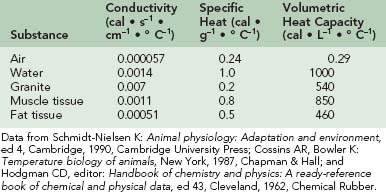
The thermal conductivity of a number of substances is given in Table 4-1. Note that water has 25 times the conductivity of air but only one fifth that of granite. Muscle tissue has about twice the conductivity of fat tissue. The conduction of heat through a tissue is called thermal diffusivity. This expression is obtained by dividing the thermal conductivity by the product of the density and the specific heat. The specific heats of various substances are also given in Table 4-1. Water and muscle tissue, which consists mostly of water, have particularly high values. However, specific heats can be misleading, so the volumetric heat capacities are also listed in Table 4-1. Although the specific heat of water is four times that of air, it takes about 3500 times as much heat to raise the temperature of a given volume of water by 1° C (1.8° F) as it does to accomplish the same feat with a similar volume of air. For someone in the water, the consequence of these properties is that skin temperature is within 1° C (1.8° F) of water temperature, and heat transfer to or from the environment is greatly facilitated. In cool water during rest, skin blood flow is minimized as a result of peripheral vasoconstriction. Heat loss is importantly determined by the subcutaneous fat layer; an average-size fat person with 36% body fat by weight begins shivering at a water temperature of about 27° C (81° F), whereas a lean person with less than 10% body fat starts shivering at about 33° C (91° F).135
Convective Heat Exchange
Convection (Hc) can be seen as the facilitation of conduction caused by the movement of molecules in a gas or liquid. This movement decreases the functional value of L, which is the denominator in the conduction equation. Convection can be either forced or natural (free). Forced convection results from gas or liquid movement caused by the application of an external force, such as the movement of a fan or the pumping of a heart. Natural convection results from density changes that are produced by heating or cooling molecules adjacent to the body. These density changes cause the molecules to move with respect to the body surface. For humans, natural convection predominates at air speeds of less than 0.2 m/sec (0.7 ft/sec), whereas forced convection is more important at greater air speeds.124
Brengelmann and Brown16 have noted that, under relatively neutral conditions (Ta = 29° C [84.2° F], wind velocity = 0.9 m/sec [3 ft/sec]), about 40% of heat loss from a nude human is mediated by convection. Increases in air or fluid velocity greatly increase convective heat transfer. Fanning a minimally clothed patient will greatly augment heat loss in a cool environment.
Radiative Heat Exchange
where Hr is the radiative heat exchange; s is the Stefan-Boltzmann proportionality constant; esk is the emissivity of the skin; ea is the emissivity of the environment; Tsk is the skin temperature (given in K); Ta is the ambient temperature (given in K); a is the absorptance; r is the reflectance; and s is the solar radiation.
For temperatures in the physiologic range and where (Tsk − Ta) is less than 20° C (68.0° F), several authors have noted that infrared radiation heat exchange is roughly proportional to Tsk − Ta.14,168 Also of note is that the spectrum of emitted radiation depends on the temperature of the object. At physiologic temperatures, the predominant wavelengths of emitted radiation are longer (infrared), whereas, at higher temperatures (e.g., like that of the sun’s surface), the predominant wavelengths are shorter (visible radiation) and can be detected by the human eye. This difference leads to some important consequences. The middle infrared radiation that is emitted by mammals is maximal, regardless of skin pigmentation or the color of clothing. However, solar radiation peaks in the visible portion of the spectrum and is differentially absorbed. In other words, dark clothes absorb more heat from solar radiation than do light clothes, but both types emit similar amounts of radiation energy.
Incident radiation can vary drastically under different environmental conditions and may severely tax the body’s ability to respond. Heat input from solar radiation on a cloudless day may exceed by several times the heat produced by basal metabolism; on a cloudless night, there is a significant net loss of radiation to the sky. Under the relatively thermoneutral conditions noted earlier by Brengelmann and Brown,16 radiant heat loss accounts for about 45% of the total.
Evaporative Heat Exchange
When water changes state, a large amount of energy is either absorbed or given off. Evaporation of 1 g (0.035 oz) of water at 35° C (95.0° F), which is the usual skin temperature of a person who is sweating,168 requires the input of 0.58 kcal of thermal energy. In the field, the preferred cooling measure is to splash water on the patient, and this is coupled with air fanning.66 Heat absorbed by the evaporation of 100 cc of water will lower body temperature by about 1° C (1.8° F). In a neutral thermal environment, sweating does not occur, and evaporation accounts for only 15% of the total heat loss. Of this, slightly more than one-half is the result of evaporation from the respiratory tract, with the remainder coming from water that passively diffuses through the skin and evaporates.16
Thermoregulatory Network
Peripheral Thermal Sensors
The entire outer surface of the body is well supplied with sensitive thermoreceptive structures. Because one destination of the output of these transducers is the sensory cortex, many properties of the receptors can be gleaned from direct experience. Afferent thermal information produces both hot and cold sensations, and it is particularly rate sensitive. In addition to cortical input that arrives via the medial lemniscus and the ventrobasal thalamus, the brain receives a large amount of thermal information from pathways that synapse in the reticular area.20 Although cortical thermal input is part of the sensory information that is used to reconstruct the external thermal environment, reticular inputs are more important to the behavioral and autonomic regulation of body temperature.43 This distinction was pointed out by Cabanac,26 who found that internal body temperature determined whether a particular surface temperature was perceived as pleasant or unpleasant. However, altered body temperature did not affect the discriminative (cortically mediated) aspects of the thermal stimulus; subjects had no problem correctly identifying the actual peripheral temperature. This study also confirms the intimate relationship between the thermoregulatory network and the pleasure–pain system.142
The structure, location, and properties of peripheral thermoreceptors are well documented. Thermal sensors are free nerve endings, and they are categorized as either warm or cold. Cold receptors are found immediately beneath the epidermis, whereas warm receptors are located slightly deeper in the dermis. The hallmark of both types of receptors is their extremely high rate sensitivity (Figure 4-3, online). Although the static firing rate of cold receptors is usually less than 10 impulses per second, under conditions of rapid temperature change, firing rates are often higher by an order of magnitude. Cold receptors are excited by cooling and inhibited by warming, and they have static maxima at about 25° C (77.0° F); these receptors are active from about 10° C (50.0° F) to 40° C (104.0° F). Warm receptors are excited by warming and inhibited by cooling, and they have static maxima at more than 40° C; they are active from about 30° C (86.0° F) to 45° C (113.0° F). At both ends of the spectrum, more extreme temperatures activate neuronal responses that are phenomenologically reported as “cold pain” and “warm pain.”72
Recent work with the use of cloning and ion-channel characterization has elucidated peripheral temperature transduction mechanisms. A family of related temperature-activated transient receptor potential (TRP) ion channels is highly sensitive to temperature. The cloned receptors TRPV3 and TRPV4 respond over a range similar to that of the warm receptors just described, whereas TRPM8 responds similarly to the cool receptors. TRPM8 also responds to menthol, eucalyptol, and icillin.117,146 TRPV1, TRPV2, and TRPA1 respond similarly to the heat-pain–sensitive and cold-pain–sensitive neurons. The heat-pain channels (vanilloid receptor 1 [VR1]) also respond to low pH, ethanol, and capsaicin, which is the active ingredient in chili peppers.144 Alternatively, TRPM2, TRPM4, and TRPM5 respond to warm temperatures and involve insulin secretion187 and taste.183 However, they are not regarded as warm receptors for thermal sensation, because sensory neurons have none of those receptors. In addition to TRP channels, TREK-1 and TRAAK channels may also be related to control of warm and cold perception.137
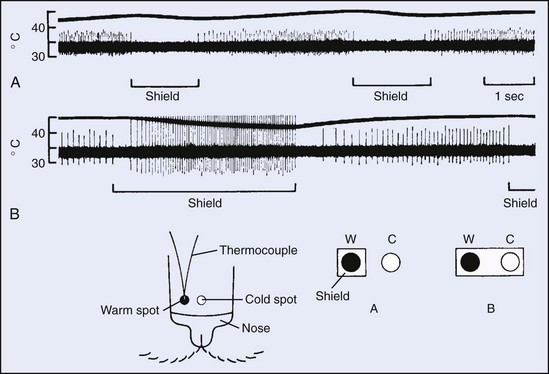
Psychophysical and physiologic studies indicate that thermal receptors are not uniformly distributed across the body surface and that there are far more cold receptors.72 Cold receptors are abundant in the face and trunk areas, especially in the lips; however, they are less numerous in the feet and lower legs. The face and fingers have a greater number of warm spots.153,181 Threshold temperature for the perception of thermal sensation follows the anatomic distribution and is not uniform across the body. The face, particularly near the mouth, is exquisitely sensitive, whereas the extremities, by comparison, have poor sensitivity. Other regions of the body are intermediate in sensitivity.179
Because peripheral thermal input is intimately involved in regulation of body temperature, heating and cooling different body sites can differentially affect the magnitude of the restorative physiologic response produced. In one study, cooling the forehead was found to be more than three times as effective (per unit area) for decreasing ongoing sweating as was cooling the lower leg.41 A separate study evaluated regional trunk and appendage sensitivity to cooling by assessing the magnitude of the gasping response that occurs at the onset of immersion. In this case, exposing various parts of the body to water at 15° C (59.0° F) indicated that the upper torso had the greatest cold receptor density or sensitivity (or both). The lower torso was somewhat less sensitive, with the arms and legs exhibiting similar but considerably lower sensitivity.22
Central Thermal Sensors
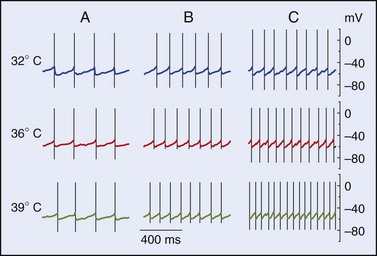
Many sites within the body are capable of eliciting generalized thermoregulatory responses. Such areas include the abdominal viscera, spinal cord, hypothalamus, and lower portions of the brainstem.12,69 The genesis of input to the regulator that results from heating or cooling these areas is poorly understood. Some of the effects may result from modulation of synaptic connections rather than from stimulation of specific thermodetectors per se. Input from central detectors is not rate sensitive; rather, it is a direct reflection of the absolute temperature. The area with the highest thermal sensitivity and that has received the greatest amount of experimental attention is the preoptic area/anterior hypothalamus (POAH). Heating or cooling this portion of the brainstem elicits the entire array of autonomic and behavioral heat loss and heat gain responses, respectively.68 Neurons in this portion of the brain exhibit both warm sensitivity and cold sensitivity.12 Recent work on hypothalamic slice preparations with the use of synaptic blockers has indicated that warm sensitivity may be an inherent property of some of the POAH neurons, whereas cold sensitivity in this area of the brain requires synaptic input.12,45 Figure 4-4, online illustrates the effects of temperature on the firing rates of three representative types of hypothalamic neurons.
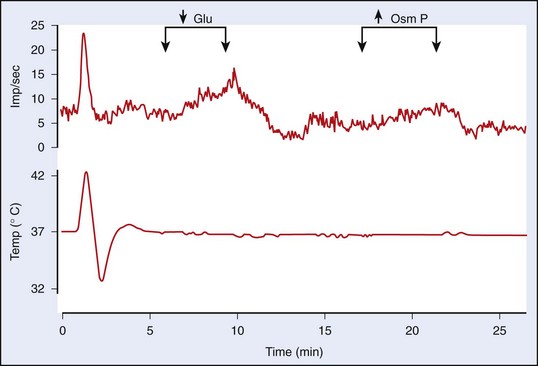
The high level of temperature sensitivity shown by one of the cells (labeled “C”) results from the temperature-dependent characteristic of the prepotential. Voltage-clamp experiments indicate that the altered rate of depolarization is most likely the result of an effect on hyperpolarizing (K+) conductances.64 Work involving the use of hypothalamic slices has also established that about one-half of the thermosensitive neurons also respond to nonthermal stimuli such as osmotic pressure, glucose concentration, and steroid hormone concentration. Such neurons could form the basis for the interactions between the homeostatic systems that are described later in this chapter. Figure 4-5, online illustrates the response of a warm-sensitive POAH neuron in a slice preparation. This cell is excited by increased temperature, low glucose, or increased osmotic pressure.13 TRPV protein expression has been detected in the POAH; thus, it was proposed that the TRPV channels may underlie the thermosensitivity found in POAH neurons144 and that both TRPV1 and TRPV2 channels may be active within the physiologic range of temperature.93,149 However, a large body of evidence is at odds with the proposal of TRPV1 as a thermosensor.157 Furthermore, TRPV channels respond to warming with persistent inward cationic currents, and this would produce a change in the resting membrane potential. In the POAH, both warm-sensitive neurons and temperature-insensitive neurons show identical membrane potential responses to temperature change.203 In addition, several in vivo studies have shown that TRPV1, TRPV3, and TRPV4—all of which are expressed in the hypothalamus—are not likely to be involved in thermoregulation in either the heat or the cold.31,107,118 It is more likely that POAH warm sensitivity is caused by brief ionic currents that determine the rate of change in depolarizing the prepotentials that occur between two successive action potentials.64
Regulator
As mentioned previously, the brain is capable of accurately regulating body temperature under a wide range of conditions. Although almost all portions of the central nervous system can potentially be involved, the most critical neuroanatomic structures for thermoregulation include the spinal cord, brainstem, hypothalamus, and septum. The preoptic area and anterior hypothalamus are particularly important for both integration and sensing of internal temperature.68,70 Recent advances in neurophysiologic and neuroanatomic techniques have been used to extend our understanding of the systems involved in regulation of body temperature; a number of excellent sources document this progress.11,122,200 Notable advances include demonstration of the relative independence of populations of neurons that control separate effector systems91,202 and elucidation of a pathway that conveys cold-sensitive afferent (feed-forward) information to the hypothalamus.131
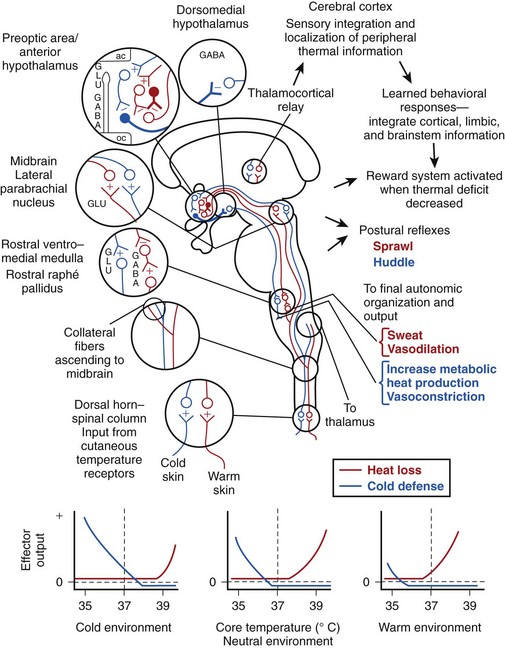
A schematic model for regulation of body temperature is presented in Figure 4-6. Credit for much of the information and organization in the figure comes from the authors listed in the figure legend as well as from many others in the field. For ease of understanding, many aspects of the system’s complexity have been simplified or modified. Among the many inputs to this system that are not shown include those mentioned in the section about central thermoreceptors (e.g., glucose concentration, osmotic pressure) as well as factors that are covered later in this chapter (e.g., time of day, hormone levels, pyrogen titer, oxygen concentration of the blood). In addition, central thermoreceptors from many locations in the body provide input to this system.
The key sensing elements of Figure 4-6 involve the peripheral warm and cold receptors and the central thermodetectors, the latter of which are depicted by solid cell bodies and bold axons. Peripheral input originates in the skin and enters the central nervous system via the spinal or trigeminal dorsal horns; this information ascends to both the midbrain and the thalamus. The thalamic neurons that receive thermal information project to the sensory cortex and subserve sensory integration and localization of peripheral thermal information. They are also likely involved to some degree in learned behavioral thermoregulatory responses.
Thermal afferent pathways reaching the midbrain are involved in the feed-forward aspect of regulation.131 Axons of the cell bodies in the midbrain synapse on cells in the midline subregion of the preoptic area. These preoptic cells receive excitatory input from peripheral warm and cold sensors, but they inhibit the cells upon which they synapse. The systems that subserve heat loss and cold defense act as if they reciprocally inhibit each other and receive output from a unitary integrating system. However, as mentioned previously, the systems are actually functionally separate to a large degree. In the schema of Figure 4-6, both systems depend on inherently warm-sensitive cells that have a high spontaneous firing rate at normal body temperatures. These cells inhibit the succeeding neurons, but the connections are such that, in a cold environment or when the body temperature is below normal, cold defense responses are disinhibited and heat loss responses are further suppressed. The opposite would occur in a warm environment or when the body becomes excessively hot.
The output of these systems under different conditions is illustrated in the panels below the neuroanatomic diagram of Figure 4-6. The middle panel illustrates the situation for a person with a body temperature of 37° C (98.6° F) in a thermoneutral environment. At 37° C (98.6° F), both systems are inhibited, and there is no effector response. If the body cools or becomes warmer, the inherently temperature-sensitive neurons disinhibit either the cold defense or the heat-loss system. The panel on the left illustrates the action of the feed-forward cold neurons in a cold environment. Although the inherently thermosensitive neurons do not change their firing rate as a result of a local temperature that stays constant, input from the peripheral cold-sensitive pathway disinhibits the cold defense system. A similar situation—but in reverse—occurs when a person encounters a warm environment; that situation is depicted in the right panel. The error signal or output driving force is shown as the difference between the horizontal dashed line and the effector output at a given body temperature. The system is extremely accurate, and the error signal created by peripheral feed-forward input is usually just sufficient to counteract the sensed disturbance and to maintain body temperature at a constant level.
In addition to autonomic responses that are organized and transmitted from the brainstem (e.g., sweating, shivering), various complex whole-animal responses are also activated by the thermoregulatory system. Postural reflexes (e.g., huddling, sprawling) and complex learned behavioral thermoregulatory responses are initiated by cold and warm error signals. Although the mechanism is not understood, appropriate behavior reduces the error signal and activates the reward system, which likely culminates in the release of dopamine in the nucleus accumbens of the septum via cell bodies located in the ventral midbrain.81
Effector Responses
Vascular Adjustments
Excellent overviews of the role of the vasculature in coping with thermal stresses are available.16,33 One function of the circulatory system is to maintain a relatively homogeneous internal body temperature. Heat from metabolically active organs is convectively distributed to portions of the body where less heat is produced. More commonly appreciated are alterations of blood flow patterns that increase or decrease the overall thermal conductivity of the body during exposure to hot or cold environments, respectively. Some of these alterations in conductivity result from the preferential shunting of peripheral blood flow superficial or deep relative to the subcutaneous fat layer. Indeed, fat has about one-half the tissue conductivity of muscle. Nevertheless, shunting blood away from major portions of the body is at least as important for determining overall conductivity as is the conductive property of the tissue itself. For example, during immersion in cold water, muscle accounts for about 90% of the total tissue insulation of the forearm.48 Thus directing blood away from poorly insulated (and more highly conductive) regions reduces heat loss and preserves core temperature.
In addition to capillaries, microcirculatory units contain arterioles, metarterioles, and arteriovenous anastomoses. Flow through all of these vessels is under the control of smooth muscle. The smooth muscle of precapillary sphincters is largely influenced by local factors such as the partial pressures of oxygen and carbon dioxide, whereas the other vessels are well supplied with receptors that respond to both neuronal and endocrine inputs. Glabrous skin (i.e., the palms, lips, and soles) contains numerous arteriovenous anastomoses and is innervated only by sympathetic vasoconstrictor fibers. Nonglabrous (i.e., hairy) skin is innervated by sympathetic vasoconstrictor and vasodilator fibers and contains few arteriovenous anastomoses. The vasoconstrictor system acts primarily through α-1 and α-2 adrenoceptors. By contrast, the active vasodilator system makes use of acetylcholine. This was verified by experiments that involved intradermal injection of botulinum toxin, which blocks the release of acetylcholine.96 However, in the same experiments, involvement of an unidentified cotransmitter was also suggested. Vasoactive intestinal polypeptide is a likely candidate, because nitric oxide is an important end product for inducing active vasodilation.6 About 90% of the elevation in skin blood flow during heat stress is a result of input from the active vasodilator system, and, of this, about 30% is the result of nitric oxide.6 Thermoregulatory skin blood flow can reach up to 8 L/min and involve 60% of the cardiac output.33
Operation of the vasomotor effector system is affected by excessive exposure to ultraviolet B radiation. Moderate sunburn impairs the vasoconstrictor response to cold; an associated uncontrolled increase in thermal conduction is still present 1 week after exposure, although the original erythema will have disappeared.140
Central Signal
Vascular changes are bioenergetically the least costly thermoregulatory autonomic effector response. Because of the high sensitivity of the vasomotor system, ambient temperatures between the thresholds for sweating and shivering are often referred to as being in the zone of vasomotor regulation. If a particular vascular bed is kept at a relatively constant temperature, output from the central nervous regulator can be assessed. Under these conditions in dogs, manipulations of hypothalamic temperature confirm a high level of vasomotor activity between the thresholds for the activation of panting and shivering.71 In humans, forearm blood flow increases rapidly as core temperature rises; a sixfold increase in blood flow can occur with a core temperature that has risen to only 38° C (100° F).191 Within the vasomotor zone (i.e., skin temperatures of 33° to 35° C [91.5° to 95.1° F]), core and skin temperatures linearly combine to control skin blood flow. Skin blood flow responds accurately and rapidly to changes in skin temperature, which leads to a very stable core temperature.17
Although most peripheral arterioles are well supplied with adrenergic receptors, output from the thermoregulatory centers is not homogeneously distributed. Extensive nervous inputs from the thermoregulator occur only in the lips, ears, and distal extremities; thus immersing the feet in cold water leads to marked vasoconstriction in the hands and forearms but not in the abdomen or upper arms.63
Local Modulation
Local temperature has a great effect on the vasomotor status of the peripheral vessels, and, in some cases, may be largely responsible for the observed thermal conductivities. Local heating produces a biphasic response. Initial dilation occurs in a few minutes and depends on release of various factors from the nerve ending. The VR1 receptors mentioned previously are involved in this response. The longer-lasting second phase of dilation is not dependent on local nerve endings, and involves nitric oxide. Local cooling can decrease superficial cutaneous blood flow to almost zero.33 Although vascular beds on the skin surface constrict in response to cooling, other vascular beds dilate when cooled.51 The specific response to cold shown by cutaneous vessels follows from the observed distribution and properties of the α-adrenergic vascular receptors. Although in most of the vasculature α1-receptors predominate, in the superficial cutaneous areas, α2-receptors constitute a clear majority. The usual predominance of α1-receptors is found in the deeper blood vessels. Local temperature affects the α2– and α1-receptors in a reciprocal manner. Although cooling augments the response of the α2-receptors, it either inhibits or does not affect the response of the α1-receptors. Thus cooling the skin not only constricts the superficial vessels but also concomitantly dilates many underlying vessels. The ensuing flow pattern increases tissue insulation and augments countercurrent exchange between incoming cool blood and outgoing warm blood.51,53 Although initial work was done on canine vessels, subsequent studies that involved α-adrenergic agonists and antagonists have demonstrated that a similar mechanism exists in human fingers.49,52
The responsiveness of cutaneous blood vessels is diminished in people with type 2 diabetes mellitus. The increased incidence of this disorder among the general population presages a greater number of patients exhibiting severe hyperthermia.33
Evaporative Responses
At high workloads and at environmental temperatures approaching 37° C (98.6° F), the only way to maintain thermal balance is to augment evaporative cooling by mobilizing the eccrine sweat glands. This sympathetic and cholinergically innervated organ system is spread over the entire body surface, but it is more profuse in some areas than in others. A person who is acclimatized to heat can produce several liters of sweat per hour.76 High rates of sweating occur on the forehead, neck, anterior and posterior portions of the trunk, and dorsal surfaces of the hands and forearms. Low rates occur on the medial femoral regions, lateral trunk areas, and palms and soles.133 Sweat is secreted in these latter two areas in response to emotional rather than thermal inputs.16 Sweating is cholinergically mediated, and can be completely abolished by atropine. Sweat gland activity interacts with the regional vasculature; metabolic products of active sweat glands increase blood flow in areas of active sweating. In well-hydrated individuals, the degree of anhidrosis is correlated with the severity of generalized autonomic failure.63,109 Several reviews discuss the many aspects of sweating disorders.34,109
Central Signal
By controlling the local milieu at different skin sites, it has been possible to separate the central thermoregulatory drive to sweat glands from local effects on the glands themselves. The central thermoregulatory system provides a proportional output that is influenced by both internal and whole-body skin temperatures. Per degree increase above thermoneutral values, internal temperature is about 10 times as important as is mean skin temperature for eliciting an output to the sweat glands.126,128
Local Modulation
Local effects are also important for determining the output of sweat glands. Temperature exerts a multiplicative effect on sweat secretion; the Q10 for this augmentation is about 3.70. In addition, skin wetness has an important local effect on sweat glands. The wetter the skin, the greater the suppression of sweating.127
Moderate sunburn disrupts evaporative cooling. This effect is locally mediated and involves decreases in both responsiveness and capacity of the sweat glands.141
Metabolic Responses
Heat is an inevitable by-product of the inefficiencies of the body’s metabolic reactions. When oxidizing foodstuffs to carbon dioxide and water during adenosine triphosphate (ATP) production and transferring the ATP produced to the functional systems of the cells, about 75% of the original chemical potential energy appears as heat. With the exception of excreted or that used to perform physical work, the remaining 25% of the original energy is also converted to heat when ATP is used in the numerous metabolic reactions of the body.169 Mammals—as compared with poikilotherms such as reptiles or fish—use much more ATP to maintain ionic and electrochemical balances of the cells178 and for many other functions. This leads to greatly increased metabolic heat production, which forms the basis for homeothermy. It also creates the need to maintain a substantial thermal gradient between the body and the environment to dissipate the high levels of heat that are produced.
An increased rate of metabolism above basal levels is critical for maintenance of body temperature in cold environments. The elevated heat production is derived from shivering and nonshivering responses. Shivering consists of simultaneous rhythmic excitation of agonistic and antagonistic skeletal muscles. Under normal circumstances, carbohydrate oxidation provides the major substrate for shivering. In glycogen-depleted individuals, shivering levels are maintained by the greatly increased oxidation of lipid and protein reserves.67 Nonshivering heat production is associated with the presence of uncoupling protein 1 in brown adipose tissue. Uncoupling protein 1 is a transmembrane protein that is located in the mitochondrial inner membrane, which, on activation by the sympathetic nervous system, allows for protons to reenter the mitochondrial matrix without passing through ATP synthase. Because the energy released during substrate oxidation is not conserved as ATP, heat is generated. Although brown adipose tissue was believed to be of little importance in adult humans, recent research has indicated the presence of potentially significant amounts of active brown adipose tissue in adults.44,162
There is evidence that both epinephrine and thyroid hormones are released in humans after cold exposure.56 Because these hormones augment overall tissue metabolism, they are components of the response to cold environments. In the absence of thyroid hormone, metabolic heat production is reduced by 30% or more. Thyroid hormone acts by both accelerating ATP turnover and reducing efficiency of ATP synthesis.175
Basal metabolic rate, when calculated on a weight-specific basis, decreases with body size. This relationship holds within as well as across species. Because drug potency as determined by the rate of inactivation is most closely related to metabolic rate, when drug dosing is extrapolated to persons of different sizes, it should not be done on a per-kilogram or per-pound basis unless the weights are similar. Basal metabolic rate relates to size according to the following equation:168
where 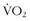 is oxygen consumption in L/hr and Mb is body mass in kg.
is oxygen consumption in L/hr and Mb is body mass in kg.
Central Signal
Of the various thermoregulatory outputs, metabolism is the easiest to evaluate quantitatively; the most complete documentation is available for this response, and most models of the thermoregulatory system are based on this information. Experiments on medium-size mammals have allowed for separate thermal manipulation of various parts of the brain, body core, and skin. This work has made it clear that the thermoregulatory centers act as a proportional controller and that skin temperature provides a feed-forward input to the system.68,84 Thus greater decreases in either core or skin temperature or both below neutral values elicit proportionally larger compensatory increases in metabolism. In addition to an impaired ability to generate metabolic heat, hypothyroidism is also associated with a decrease in regulated core temperature of about 1° C (1.8° F).196
Evidence indicates that humans have a control system similar to that of medium-sized mammals. In a summary of their data and of that collected previously, Hong and Nadel77 noted that central output for shivering is augmented by increased rate of skin cooling. They also concluded that a given decrease in core temperature elicits 10 to 20 times the metabolic response of an equivalent decrease in mean skin temperature. Exercise is not incompatible with shivering, but increased levels of exercise exert increasing degrees of suppression on the shivering response, possibly as a consequence of an increased arousal response.77
Local Modulation
Although the central and local effects of decreased core temperature on shivering have not been directly partitioned, both inputs are important. Slight decreases in core temperature create large compensatory responses, as delineated previously. However, even moderate hypothermia decreases the metabolic response to cold, and, at about 30° C (86.0° F), the shivering response is lost.16 This decrement must involve nervous system malfunction, because the muscles themselves are quite responsive below this temperature. For example, limb muscles and diaphragm muscles develop peak tensions that are not greatly affected by temperatures down to 25° C (77.1° F), and fatigue resistance is considerably increased at 25° C (77.1° F).150,170 Likewise, altering the skin and superficial muscle temperature of the anterior thigh through a range of temperatures between 12° and 40° C (between 53.6° and 104.0° F) for 30 minutes had little effect on subsequent isometric peak torque production during isometric knee extensions to exhaustion. Time to fatigue was longer at the coolest temperature.186
Behavioral Responses
In most wilderness situations, a variety of ambient temperatures is available, and external insulation is easily adjusted. Under these conditions, the choice of thermal microenvironment and clothing provides a far higher gain than any of the autonomic effector systems discussed previously. Whole-body adjustments are achieved by all motile animals, and are particularly well developed in vertebrates.40 In addition to moving the body, the somatic effectors are important for optimizing autonomic responses to thermal stress. Thus spreading out the arms and legs during heat stress increases the surface area available for the autonomic augmentation of conductive, convective, evaporative, and radiative heat losses.
Cabanac26 found that internal body temperature determined whether a particular surface temperature was perceived as pleasant or unpleasant. When subjects were hypothermic, a warm stimulus applied to the hand was experienced as pleasant and a cold stimulus as unpleasant. The opposite responses were observed in hyperthermic subjects. An overall sensation of thermal pleasantness is obtained when environmental conditions are appropriate for maintaining a normal body temperature with no fluid or energy expenditure. However, altered body temperature did not affect the discriminative (cortically mediated) aspects of the thermal stimuli; subjects had no problem correctly identifying the actual peripheral temperature. This study also confirmed the intimate relationship between the thermoregulatory network and the pleasure–pain system.142
Central Signal
As compared with the knowledge about autonomic thermoregulation, we know little about the neural mechanisms that underlie behavioral thermoregulation. As noted in previous sections, the preoptic area plays a key role in autonomic thermoregulation. Animals with lesions of the POAH are severely compromised with regard to their ability to use autonomic thermoregulatory effectors; however, their ability to behaviorally thermoregulate is relatively intact.28,164 These results indicate that the preoptic area is not as crucial for behavioral thermoregulatory processes as it is for autonomic processes.27,163 Alternatively, lesions of the lateral hypothalamus, which is involved in various reward systems, result in the loss of behavioral thermoregulation.165
Recent studies involving the use of positron emission tomography and functional magnetic resonance imaging have demonstrated that temperature signals from the body surface reach the insular cortex.38,79,138 However, insular activation correlates with the discrimination between hot and cold rather than with thermal pleasure, so this system is likely minimally involved with behavioral thermoregulatory processes. Alternatively, thermal pleasantness is clearly important for behavioral thermoregulation. In studies involving functional magnetic resonance imaging, it has been shown that activation of the amygdala, mid-orbitofrontal and pregenual cingulate cortices, and striatum is correlated with thermally related pleasant and unpleasant feelings.91,156
Regional differences in the contribution of the body surface to thermal comfort are present.132 Facial cooling produces the most pleasant experiences during mild heat exposure, whereas during cold exposure, local warming of the chest and abdomen leads to the most pleasant sensations. This would induce us to preferentially cool the head in the heat and to huddle or add clothing to warm the torso in the cold.
Important Modifications of Thermoregulatory Responses
Normal Variations in the Regulated Temperature and in the Ability to Maintain Body Temperature
Level of Activity
Activity normally leads to increases in body temperature. However, the level of activity does not appear to provide direct input to the regulator of body temperature, unlike the feed-forward input that is received from the peripheral temperature. Thus, the magnitude of the error signal for increased heat dissipation is determined simply by the increase in body temperature.180 Someone who is exercising heavily (or who has just exercised) in a neutral environment will have an unusually high body temperature, whereas someone who is sleeping or resting quietly will have a relatively low body temperature. At a given level of exercise, core temperature will plateau 30 to 40 minutes after the exercise is initiated; higher levels of exercise result in higher plateau levels of the core temperature. For someone who is working at 50% of their maximal aerobic capacity, the increase in core temperature is about 1° C (1.8° F).135
Circadian Changes
Body temperature shows cyclic changes throughout the day. Some of this variation is the result of the daily cycle of activity, as described previously. However, there also exists a circadian rhythm for the regulated body temperature. This sinusoidal rhythm accounts for much of the observed variations in body temperature. In a study that involved 700 observations of 148 healthy individuals, the daily mean oral reading was 36.8° C (98.2° F). However, this was only a midpoint; the mean early-morning low was 36.4° C (97.6° F), and the mean late-afternoon high was 36.9° C (98.4° F).135 These diurnal changes definitely reflect alterations in the controller, because the body temperature thresholds for eliciting sweating and peripheral vasodilation are significantly lower in the early morning than in the afternoon or evening, whereas the sensitivities and maximal response levels remain unchanged.2,3 The body’s biologic clock may directly modulate the body temperature rhythm. The suprachiasmatic nucleus in the hypothalamus is the main pacemaker for the circadian system of the body. The core molecular mechanisms of the circadian clock, which consist of autoregulatory transcription and translation loops by the clock genes, show a periodicity of approximately 24 hours. Removing the circadian clock abolishes the body temperature rhythm as well as the circadian influences on thermoregulatory responses.130,188
Thermoregulatory responses to exercise are affected by the circadian time. At the daily low (5:00 AM), the perceived exertion for a standardized task is the greatest, and thermoregulation is less effective than at the time of maximum temperature (5:00 PM). At the time when the body temperature is rising at the fastest rate (11:00 AM), heat loss mechanisms are less responsive; alternatively, at 11:00 PM, when the body temperature is falling at the fastest rate, heat loss mechanisms are much more responsive.190 An excellent overview of body temperature cycles is available.154
Interindividual Differences
Most oral temperature measurements are between 36.0° C (96.9° F) and 37.1° C (98.9° F) in the early morning. Corresponding values for the late afternoon are 36.3° C (97.4° F) and 37.4° C (99.4° F). On the basis of interindividual differences and diurnal changes, it has been suggested that the upper limit for a normal oral temperature should be 37.2° C (98.9° F) in early morning, which then increases gradually to 37.8° C (99.9° F) by early afternoon and remains at that level until early evening.110 These values delineate the 99th percentile for body temperature observed during the respective time periods.
Age
The circadian rhythm of body temperature develops soon after birth. Although newborns display small-amplitude rhythms, the patterns are not circadian. Circadian rhythmicity begins to develop during the second and third weeks of life, and, after a progressive increase in amplitude, the typical adult temperature rhythms are reached by the age of 2 years.154 Under thermoneutral conditions, rectal temperatures of older adults are similar to those of younger people, whereas oral and axillary temperatures are slightly lower.88
Of the major regulatory systems, temperature regulation is unique in the extent to which the effector organs are “borrowed” from other systems. This makes developmental assessments difficult, because functional changes may be secondary to changes in primary systems such as the skeletal muscles or the blood vessels. Other difficulties, which have been detailed by Cooper,36 include inconsistencies between chronologic age and physiologic viability and the increased incidence of interfering disease states and cerebral microinfarcts as aging progresses.
Thermoregulatory capacities of the young show a progressive increase, but they are not fully developed until after puberty. Effectors that are more important to infants than to adults include certain behavioral responses (e.g., calling for help) and the ability to activate thermogenic brown adipose tissue. Shivering is not present in infants; it develops fully only after several years of nervous system maturation. Metabolism in infants is increased to some degree by the increase in motor activity that accompanies cold stress.97
Sweating is present and effective in children, but the typical high capacity for evaporative heat loss that is present in adults is attained only during the changes that occur with puberty.50 Factors that affect loss of body heat during cold stress throughout the adult years have been investigated with a multiple regression analysis to evaluate fitness, fatness, and age from the 20s to the early 50s. Fitness has no effect, but fatness retards heat loss. Aging during this period is correlated with progressive weakening of the vasoconstrictor response to cold.21
Individuals in their late 60s and beyond have a definite decrease in thermoregulatory capacity. Sweating is lessened in response to passive heating,82 vascular responses to heating and cooling are significantly reduced,94,97 and a distinct shivering tremor is rarely observed.97 In older adults, thermoregulatory sympathetic nerve impulses to the skin are reduced by 60%,62 and there is lower resting metabolic rate.192
Young children and older adults are, without a doubt, particularly vulnerable to thermal extremes and should be given treatment priority when possible. Both groups are susceptible to climatic heat injury, and, when their core temperatures exceed 40° C (104° F) and are accompanied by altered mental status, treatment should be immediate, even at the risk of misdiagnosing a febrile condition. As for all such cases, most clothing should be removed; ice, if it is available, should be placed around the groin, in the axillae, and around the neck. Cool water should be sprayed on the skin, and then the individual should be fanned. Blood gas and electrolyte status should be determined and any appropriate treatment measures taken.25,30
Gender
Women have a number of physiologic and morphologic characteristics that produce subtle differences in the regulation of body temperature. Such attributes include smaller blood volume, lower hemoglobin concentration, smaller heart, smaller lean body mass, greater percentage of subcutaneous and total body fat, greater surface area–to–mass ratio, higher threshold core temperature for cutaneous vasodilation and sweating, greater resting vasoconstriction in the hands and feet, geometrically thinner extremities, and cyclic changes in sex hormone levels.120
Some reports have shown that when age, thermal acclimation, body size, maximal aerobic capacity, cardiovascular responses during exercise, and relative workload are matched, thermoregulatory gender differences are relatively negligible.58 Nevertheless, in situations such as those encountered in the U.S. Navy, men and women do not tend to be matched for such nonthermoregulatory factors, and different thermal exposure standards may be required for different genders.65
The menstrual cycle, menopause, and pregnancy are all associated with important effects on the thermoregulatory system. Relative to the early follicular phase of the menstrual cycle, core temperature is typically 0.3° C (0.5° F) higher during the late follicular phase and 0.7° C (1.3° F) higher during the luteal phase.102 Postmenopausal hot flashes are experienced by most women and involve increases in sweating, vasodilation, and heart rate. Heat and exercise may be particularly stressful during and after these episodes. The proximate mechanism appears to involve estrogen activating the central noradrenergic system53 as well as circulating serotonin levels.116 In addition, hot flashes may be associated with activation of the insular cortex.54 Some information about hormonal effects has been gained by studying postmenopausal women who are undergoing hormone replacement therapy. Administered estrogen acts to lower the core temperature at which heat loss effector mechanisms are activated and results in lowered core temperature. The addition of exogenous progestins blocks these effects.18 By contrast, animal studies have shown that estrogen acts to augment heat production78 and to maintain core temperature in the cold.189 Epidemiologic studies have indicated that lower testosterone concentration may be associated with the sensation of cold.60
Pregnancy is of special concern to the physician in the wilderness. This concern does not apply to well-hydrated women who are exercising at submaximal levels, because the thermoregulatory system makes adaptive adjustments as pregnancy proceeds. In the course of a pregnancy, basal body temperature shows a continuous decline, and heat-loss responses are elicited at progressively lower levels so that, near term, the steady-state temperature during exercise is about 1° C (1.8° F) lower than it was before conception. This reduces thermal stress on the fetus, which is typically 0.5° C (0.9° F) warmer than the mother.35,177
However, concern is warranted if hyperthermia develops in pregnant women. Animal experiments and epidemiologic analysis both indicate that, during pregnancy, it is dangerous for body temperature to exceed 39° C (102.2° F). During the first half of pregnancy, excessive body temperature is likely to produce birth defects; during the latter half, birth weight is more likely to be affected. The fifth week after conception, which is the period of neural tube closure, is a particularly vulnerable time for the fetus.108 Because women in the early stages of pregnancy may be unaware of their condition, it is critical that heat stress be promptly treated in women of childbearing age. When exercising, it is important that pregnant or potentially pregnant women acclimatize gradually to extreme thermal environments, remain well hydrated, wear loose-fitting clothing, exercise at a comfortable pace, and avoid swimming in warm water or immersing themselves in hot tubs.177 A program of water aerobics for pregnant women was reported to decrease requests for analgesia when these women gave birth, and was not found to be detrimental to the health of the mother or the child.4
Induced Alterations of the Regulated Temperature
Fever
Increased body temperatures have been associated with illnesses for thousands of years. On the basis of the population data presented previously, febrile body temperatures for resting young adults include morning temperatures that are equal to or that exceed 37.3° C (99.2° F), which increase gradually to 37.8° C (100.0° F) for early afternoon and evening. Such elevated temperature needs to reflect a regulated increase to be considered a true fever. Pathogens and cancers are the usual causes of such increases in the regulated temperature, but they are not directly responsible for the increased body temperature; rather they interact with components of the immune system such as macrophages, T cells, monocytes, and Kupffer cells as well as with glial, epithelial, and many other types of cells. This interaction stimulates the cells to produce pyrogenic cyotokines,113 including interleukin-1, interleukin-6, and macrophage inflammatory protein-1. Cytokines act on cells in the vicinity of the anterior hypothalamus and induce them to release prostaglandin E2, which leads to an increase in the regulated temperature.100 Important avenues by which cytokine-mediated pyrogenic signals reach the brain include peripheral inputs from the abdominal vagus nerve160 and central inputs via the subfornical organs.182 Aspirin and related drugs block fever by inhibiting prostaglandin synthesis.100 Interleukin-1 and other cytokines have many effects in addition to causing fever; these include decreased appetite, hypoferremia, activation of B and T lymphocytes, and increased slow-wave sleep.99
The increase in body temperature during fever helps with many immune functions; neutrophil migration, release of reactive oxygen intermediates and nitric oxide by neutrophils, and interferon production are all augmented. It has been suggested that the most important aspect of fever is to greatly increase the temperatures of the peripheral tissues via selection of a warmer microclimate, addition of insulation, and postural changes. As peripheral temperatures increase from typical levels (i.e., 29° to 33° C [84.2° to 91.4° F]) to those that approximate core temperature, activation, proliferation, and effector production in peripheral cells involved in cell-mediated and humoral immunity are greatly increased and show temperature coefficients (Q10) of 100 to 1000. By contrast, the Q10 for the effectiveness of the newly created effectors themselves, as well as for antigen-nonspecific defense systems, are much lower at about 1.5 to 5.36.100,101,161
The presence and beneficial effects of fever have been documented in a variety of cold- and warm-blooded vertebrates and even in some invertebrates. Under most conditions, it is probably not advisable to alleviate a fever. Exceptions include malignant hyperthermia and particularly high fevers during pregnancy.100,101 In addition, for patients with limited fluid, oxygen transport, or cardiopulmonary reserves, a febrile response should be treated with antipyretics.94 Consequences of the decreased immune response can be treated after the emergency.
Alcohol, Anesthetics, and Toxins
Increases in the blood concentrations of ethanol, anesthetics, and a number of toxic substances lead to substantial decreases in body temperature.42,172 In many cases, this fall is caused by a decrease in the regulated temperature. In the case of high concentrations of alcohol and certain toxins, the reduction appears to be an adaptive adjustment that promotes survival. These chemicals disrupt protein structures within the cell membrane, and this effect is counteracted by a lower temperature.42 Indeed, mouse studies have shown that lowered body temperature counteracts ethanol toxicity.114 In humans as well, decrease in the regulated temperature is caused by increases in blood ethanol concentration. After ingestion of ethanol (3.0 mL/kg body weight) at 33° C (91.4° F), sweat rate increased and body temperature fell. Although skin temperature did not increase, subjects reported a warm sensation that paralleled the increase in sweat rate.199 At 18° C (64.4° F), body temperature decreased continuously before and after the drinking of alcohol, with no facilitation of metabolic heat production. During this period, the thermal discomfort sensation became more intense, although the discrimination of cold per se was impaired after the ingestion of ethanol.197,198 However, lower blood ethanol levels associated with moderate consumption in humans have minimal and inconsistent effects on thermal balance of the whole body.46,86
Excellent overviews of the effects of general anesthetics on perioperative thermoregulation are available.171,173 Many of these substances (e.g., halothane, fentanyl and nitrous oxide, enflurane, isoflurane) in anesthetic doses act in a similar manner. Heat-loss thresholds are increased by about 1° C (1.8° F), and heat-maintenance thresholds are lowered by approximately 2.5° C (4.5° F). Interestingly, in the typical clinical dose range, the gain (sensitivity) of the effector responses is nearly normal. In the conditions under which general anesthetics are normally administered, body temperature decreases significantly. An initial rapid drop is caused by redistribution of heat; cool blood from the periphery lowers central core temperature. A second and slower decrease results from a fall in body heat content. Finally, a plateau is reached, either because heat production and heat loss are passively balanced or because heat maintenance thresholds are reached. During postanesthetic recovery, there is vigorous shivering. Anesthetic-induced hypothermia is reduced in patients with higher preoperative systolic blood pressures. This difference is associated with higher preoperative plasma norepinephrine levels, which may intensify the vasoconstriction response.92 Cutaneous warming before and during anesthesia prevents the development of hypothermia and decreases the incidence of infectious complications.103,171
Severe Hypoxia and Endotoxin Shock
When inspired oxygen concentration falls to 10% to 12%, a substantial decrease in the regulated temperature occurs. This reaction has been documented with the use of behavioral responses in fish, amphibians, reptiles, and mammals.59,193 For humans who are exercising in 28° C (82.4° F) water under eucapnic conditions, decreasing inspired oxygen to 12% lowers the core temperature thresholds for vasoconstriction and shivering and increases the rate of core cooling by 33%.87 The value of the resultant lowered body temperature is clear: the affinity of hemoglobin for oxygen is increased, and overall metabolic rate is decreased. The mechanism underlying the change in the regulated temperature may involve differential sensitivities of central neurons; hypoxia specifically increases activity of warm-sensitive neurons in the POAH.184
A somewhat similar regulated hypothermic response occurs when an animal is exposed to very high levels of pyrogens; the same response occurs under less extreme conditions in weak or malnourished animals. The lowered body temperature may serve to decrease the energy costs of maintaining a high body temperature for a severely compromised animal.159
Thermal Acclimation
Thermoregulation is affected by chronic exposure to very cold or hot environments as well as by chronic exercise in cool or warm ambient temperatures. Such exercise in a cool environment greatly increases responsiveness of the sweat glands; if exercise is in the heat, the central temperature at which sweating is initiated is also lowered. The net consequence of these adjustments is that a heat- and exercise-acclimated individual can workout at a given level with far less increase in core temperature.129 Exercise in humid heat appears to decrease resting core temperature in acclimated individuals.145 Increases in both core and skin temperatures contribute to various changes involved in heat acclimation.152 Physical training also increases skin blood flow at any given increase in core temperature.85 Although acclimation to warm conditions produces many changes in the cardiovascular system, the basic baroreflex responses are not altered.195
Heat acclimation may result in protective cellular adaptations. Intracellular heat shock protein 72 is likely involved in the maintenance of cellular protein conformation and homeostasis during hyperthermia, inflammation, and injury.123 A 10-day heat- and exercise-acclimation test increased heat shock protein 72 levels in peripheral blood mononuclear cells.194
Chronic cold exposure alters many thermoregulatory systems and involves long-term evolutionary changes and thermal acclimation. In many cases, resting metabolic rate is increased after repeated cold exposure;20,204 however, repeated exposure to very cold environments may produce the opposite effect. For example, eighty 30-minute sessions at 5° C (41° F) decreased the metabolic response to a standard cold-air test and often led to lower internal temperatures in these cold-acclimated individuals.74
When the extremities are initially exposed to cold, strong vasoconstriction is seen. At some point, however, cold-induced removal of the superficial α-receptor inhibition leads to vasodilation of the fingers, which warms the extremities and protects against frostbite. This response has a genetic component; for individuals who are not cold acclimated, this response is very strong among Inuit Eskimos, moderate among whites, and minimal among Chinese from Hong Kong. Individual differences in the vasodilation response to cold determine the relative likelihood of the development of frostbite. An environmental influence in this response is also likely; fishermen in northeastern Canada did not exhibit vasoconstriction of the fingers when exposed to cold.63
Competition with Other Homeostatic Systems
In addition to a constant core temperature, the body has many other requirements. When fluid balance or energy requirements are not met, thermoregulatory responses can be compromised. For heat production and heat conservation, an adequate energy supply, patent nervous system, and functional effector organs are critical. Thus, hypoglycemia decreases the core temperature at which shivering is initiated while leaving the thresholds for sweating and vasodilation unaffected.143
During exercise, heat is generated by activity of the muscles. About 80% of the energy consumption is converted into heat, with only about 20% going to the actual work produced by contraction of the muscles. High muscle temperature increases efficiency of muscle contraction.7,8 However, an excessive rise in body temperature impairs endurance performance. It is well appreciated that 40° C (104° F) is very near the upper limit of core body temperature when trying to maintain prolonged exercise. The time to exhaustion during heavy exercise is affected by the initial body temperature.61,134,136 In a heat-acclimation study, subjects exercised until exhaustion at 60% of their maximum 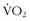 for 9 to 12 consecutive days at 40° C (104° F).136 The time to exhaustion became progressively longer with acclimation, but core temperature at the time of exhaustion remained at about 40° C (104° F). When body temperature was altered by water immersion before bicycle ergometer exercise at 40° C (104° F), time to exhaustion became progressively longer as initial body temperature was progressively lowered. Again, body temperature at exhaustion remained at about 40° C (104° F).61
for 9 to 12 consecutive days at 40° C (104° F).136 The time to exhaustion became progressively longer with acclimation, but core temperature at the time of exhaustion remained at about 40° C (104° F). When body temperature was altered by water immersion before bicycle ergometer exercise at 40° C (104° F), time to exhaustion became progressively longer as initial body temperature was progressively lowered. Again, body temperature at exhaustion remained at about 40° C (104° F).61
For the maintenance of exercise performance and body temperature in a warm environment, body water status is critical.121,167 It is common for a person who is working in the heat to lose 1 L of water per hour. Even when fluids are readily available, maintaining a euhydrated state may be difficult. For hypohydration during continued activity, each percent decrease in body weight leads to a core temperature increase of about 0.15° C (0.27° F). This decreased heat dissipation is mediated by two mechanisms. At a given core temperature, hypertonicity decreases the sweating response, and hypovolemia reduces skin blood flow.167 Hyperhydration has no effect on thermoregulation during compensable exercise heat stress.104 During uncompensable exercise heat stress, hyperhydration slightly increases the time to exhaustion but only by delaying hypohydration; thermoregulation is not affected.105 It is important to be alert to the possibility of dehydration in many atypical situations. For example, swimmers training in an outdoor pool with a water temperature of 26° C (78.8° F) lost sufficient fluid (i.e., 2.5% of their body weight) in 3 hours to compromise thermoregulatory responses.176
Hypoxia can undermine thermoregulatory vascular responses because of demands that compete or operate at cross-purposes. Thus, in a hot environment, hypoxia reduces skin blood flow at a given sweat rate. In the cold, hypoxia enhances core cooling by delaying the onset of vasoconstriction and cooling.119
Alcohol, Drugs, Anesthetics, and Toxins
Although moderate doses of anesthetics and toxins may elicit adaptive changes in a regulated body temperature, elevated doses of these substances impair or abolish both autonomic and behavioral aspects of thermoregulation. Body temperature then changes passively, depending on the thermal environment. This can be particularly dangerous when elevated levels of alcohol or similar substances are combined with heat stress. Impaired ability to dissipate heat is then combined with the enhanced toxicity of increased tissue temperature. Drugs that increase metabolic heat production (e.g., amphetamines, ecstasy [3,4-methylenedioxymethamphetamine], cocaine) are also particularly dangerous to use in the heat. In addition, cocaine interferes with heat dissipation mechanisms and with the perception of warmth.19,39
1 Almeida MC, Steiner AA, Branco LG, et al. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One. 2006;1:e1.
2 Aoki K, Kondo N, Shibasaki M, et al. Circadian variation in skin blood flow responses to passive heat stress. Physiol Behav. 1998;63:1.
3 Aoki K, Kondo N, Shibasaki M, et al. Circadian variation of sweating responses to passive heat stress. Acta Physiol Scand. 1997;161:397.
4 Baciuk EP, Pereira RI, Cecatti JG, et al. Water aerobics in pregnancy: Cardiovascular response, labor and neonatal outcomes. Reprod Health. 2008;5:10.
5 Baker MA. Brain cooling in endotherms in heat and exercise. Annu Rev Physiol. 1982;44:85.
6 Bennett LA, Johnson JM, Stephens DP, et al. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol. 2003;552:223.
7 Bishop D. Warm up I: Potential mechanisms and the effects of passive warm up on exercise performance. Sports Med. 2003;33:439.
8 Bishop D. Warm up II: Performance changes following active warm up and how to structure the warm up. Sports Med. 2003;33:483.
9 Blatteis CM. Functional anatomy of the hypothalamus from the point of view of temperature. In: Szelényi Z, Székely M, editors. Contributions to thermal physiology. Budapest: Akademiai Kiado, 1980.
10 Blatteis CM. Methods of body temperature measurement. In: Blatteis CM, editor. Physiology and pathophysiology of temperature regulation. Singapore: World Scientific, 1998.
11 Boulant JA. Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol. 2006;100:1347.
12 Boulant JA, Curras MC, Dean JB. Neurophysiological aspects of thermoregulation. In: Wang LCH, editor. Advances in comparative and environmental physiology, vol 4. Berlin: Springer-Verlag; 1989.
13 Boulant JA, Silva NL. Neuronal sensitivities in preoptic tissue slices: interactions among homeostatic systems. Brain Res Bull. 1988;20:871.
14 Brengelmann GL. Body temperature regulation, ed 21. Patton HD, Fuchs AF, Hille B, et al, editors. Textbook of physiology: circulation, respiration, body fluids, metabolism, and endocrinology, vol 2. WB Saunders, Philadelphia, 1989..
15 Brenglemann GL. Specialized brain cooling in humans? FASEB J. 1993;7:1148.
16 Brengelmann G, Brown AC. Temperature regulation. In Ruch TC, Patton HD, editors: Physiology and biophysics, ed 19, Philadelphia: WB Saunders, 1965.
17 Brengelmann GL, Savage MV. Temperature regulation in the neutral zone. Ann N Y Acad Sci. 1997;813:39.
18 Brooks EM, Morgan AL, Pierzga JM, et al. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function is postmenopausal women. J Appl Physiol. 1997;83:477.
19 Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (“ecstasy”): modulation by environmental conditions. Eur J Neurosci. 2004;20:51.
20 Brück K, Hinckel P. Thermoafferent networks and their adaptive modifications. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
21 Budd GM, Brotherhood JR, Hendrie AL, et al. Effects of fitness, fatness, and age on men’s responses to whole body cooling in air. J Appl Physiol. 1991;71:2387.
22 Burke WEA, Mekjavic IB. Estimation of regional cutaneous cold sensitivity by analysis of the gasping response. J Appl Physiol. 1991;71:1933.
23 Burton AC. Human calorimetry: the average temperature of the tissues of the body. J Nutr. 1935;9:261.
24 Bytomski JR, Squire DL. Heat illness in children. Curr Sports Med Rep. 2003;2:320.
25 Cabanac M. Human selective brain cooling. Heidelberg: Springer-Verlag; 1995.
26 Cabanac M. Physiological role of pleasure. Science. 1971;173:1103.
27 Carlisle HJ. Behavioural significance of hypothalamic temperature-sensitive cells. Nature. 1966;209:1324.
28 Carlisle HJ. Effect of preoptic and anterior hypothalamic lesions on behavioral thermoregulation in the cold. J Comp Physiol Psychol. 1969;69:391.
29 Casa DJ, Becker SM, Ganio MS, et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42:333.
30 Caspani ML, Savioli M, Crotti S, et al. Heat stress: Characteristics, pathophysiology and avoidable mistakes. Minerva Anestesiol. 2004;70:617.
31 Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306.
32 Cetas TC. Thermometers. In Mackowiak PA, editor: Fever: basic mechanisms and management, ed 2, Philadelphia: Lippincott-Raven, 1997.
33 Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603.
34 Cheshire WP, Freeman MB. Disorders of sweating. Semin Neurol. 2003;23:399.
35 Clapp JFIII. The changing thermal response to endurance exercise during pregnancy. Am J Obstet Gynecol. 1991;165:1684.
36 Cooper KE. Thermoregulation in the elderly. In: Bender BS, Scarpace PJ, editors. Physiology and pathophysiology of temperature regulation. Singapore: World Scientific, 1998.
37 Cossins AR, Bowler K. Temperature biology of animals. New York: Chapman & Hall; 1987.
38 Craig AD, Chen K, Bandy D, et al. Thermosensory activation of insular cortex. Nature Neurosci. 2000;3:184.
39 Crandall CG, Vongpatanasin W, Victor RG. Mechanism of cocaine-induced hyperthermia in humans. Ann Intern Med. 2002;136:785.
40 Crawshaw LI. Temperature regulation in vertebrates. Annu Rev Physiol. 1980;42:473.
41 Crawshaw LI, Nadel ER, Stolwijk JA, et al. Effect of local cooling on sweating rate and cold sensation. Pflügers Arch. 1975;354:19.
42 Crawshaw LI, Wallace H, Crabbe J. Ethanol, body temperature and thermoregulation. Clin Exp Pharmacol Physiol. 1998;25:150.
43 Crawshaw LI, Wollmuth LP, O’Connor CS, et al. Body temperature regulation in vertebrates: comparative aspects and neuronal elements. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
44 Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509.
45 Dean JB, Boulant JA. Effects of synaptic blockade on thermosensitive neurons in rat diencephalon in vitro. Am J Physiol. 1989;257:R65.
46 Desruelle AV, Boisvert P, Candas V. Alcohol and its variable effect on human thermoregulatory response to exercise in a warm environment. Eur J Appl Physiol. 1996;74:572.
47 Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17:535.
48 Ducharme MB, Tikuisis P. In vivo thermal conductivity of the human forearm tissues. J Appl Physiol. 1991;70:2682.
49 Ekenvall L, Lindblad LE, Norbeck O, et al. Alpha-adrenoceptors and cold-induced vasoconstriction in human finger skin. Am J Physiol. 1988;255:H1000.
50 Falk B, Bar-Or O, Calvert R, et al. Sweat gland response to exercise in the heat among pre-, mid-, and late-pubertal boys. Med Sci Sports Exerc. 1992;24:313.
51 Flavahan NA. The role of vascular a2-adrenoreceptors as cutaneous thermosensors. New Physiol Sci. 1991;6:251.
52 Flavahan NA, Lindblad LE, Verbeuren TJ, et al. Cooling and α1- and α2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol. 1985;249:H950.
53 Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332.
54 Freedman RR, Benton MD, Genik RJII, et al. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674.
55 Freedman RR, Sabharwal SC, Moten M, et al. Local temperature modulates α1- and α2-adrenergic vasoconstriction in men. Am J Physiol. 1992;32:H1197.
56 Fregly M. Activity of the hypothalamic-pituitary-thyroid axis during exposure to cold. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
57 Fröhlich D, Wittmann S, Rothe G, et al. Mild hyperthermia down-regulates receptor-dependent neutrophil function. Anesth Analg. 2004;99:284.
58 Fu Q, Levine BD. Cardiovascular response to exercise in women. Med Sci Sports Exerc. 2005;37:1433.
59 Gautier H, Bonora M, M’Barek SB, et al. Effects of hypoxia and cold acclimation on thermoregulation in the rat. J Appl Physiol. 1991;71:1355.
60 Goetmar A, Hammar M, Fredrikson M, et al. Symptoms in peri- and postmenopausal women in relation to testosterone concentrations: data from the Women’s Health In the Lund Area (WHILA) study. Climacteric. 2008;11:304.
61 Gonzalez-Alonso J, Taller C, Andersen S, et al. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86:1032.
62 Grassi G, Saravalle G, Turri C, et al. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729.
63 Grayson J. Responses of the microcirculation to hot and cold environments. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
64 Griffin JD, Kaple ML, Chow AR, et al. Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J Physiol. 1996;492:231.
65 Grucza R. Thermoregulatory responses to heat loads in men and women. In: Johannsen BN, Nielsen R, editors. Thermal physiology. Copenhagen: August Krogh Institute, 1997.
66 Hadad E, Rav-Acha M, Heled Y, et al. Heat stroke: a review of cooling methods. Sports Med. 2004;34:501.
67 Haman F, Péronnet F, Kenny GP, et al. Effects of carbohydrate availability on sustained shivering: I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol. 2004;96:32.
68 Hammel HT. Regulation of internal body temperature. Annu Rev Physiol. 1968;30:641.
69 Hardy JD. Body temperature regulation, ed 2. Mountcastle VB, editor. Medical physiology, vol 2. Mosby, St. Louis, 1980..
70 Hardy JD. Physiology of temperature regulation. Physiol Rev. 1961;41:521.
71 Hellstrom B, Hammel HT. Some characteristics of temperature regulation in the unanesthetized dog. Am J Physiol. 1967;213:547.
72 Hensel H. Cutaneous thermoreceptors. In: Iggo A, editor. Handbook of sensory physiology, vol 2: Somatosensory system. Berlin: Springer-Verlag, 1973.
73 Hensel H, Kenshalo DR. Warm receptors in the nasal region of cats. J Physiol (Lond). 1969;204:99.
74 Hesslink RLII, D’Alesandro MM, Armstrong DWIII, et al. Human cold air habituation is independent of thyroxin and thyrotropin. J Appl Physiol. 1992;72:2134.
75 Hirashita M, Shido O, Tanabe M. Blood flow through the ophthalmic veins during exercise in humans. Eur J Appl Physiol. 1992;64:92.
76 Hölzle E. Pathophysiology of sweating. Curr Probl Dermatol. 2002;30:10.
77 Hong S, Nadel ER. Thermogenic control during exercise in a cold environment. J Appl Physiol. 1979;47:1084.
78 Hosono T, Chen XM, Zhang YH, et al. Effects of estrogen on thermoregulatory responses in freely moving female rats. Ann N Y Acad Sci. 1997;813:207.
79 Hua le H, Strigo IA, Baxter LC, et al. Anteroposterior somatotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol. 2005;289:319.
80 Ikeda T, Sessler DI, Marder D, et al. Influence of thermoregulatory vasomotion and ambient temperature variation on the accuracy of core-temperature estimates by cutaneous liquid-crystal thermometers. Anesthesiology. 1997;86:603.
81 Ikemoto S. Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27.
82 Inoue Y, Nakao M, Araki T, et al. Regional differences in the sweating responses of older and younger men. J Appl Physiol. 1991;71:2453.
83 Jessen C. Thermal afferents in the control of body temperature. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
84 Johnson JM. Physical training and the control of skin blood flow. Med Sci Sports Exerc. 1998;30:382.
85 Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly ML, Blatteis CM, editors. Handbook of physiology, sect 4: Environmental physiology, vol 1. Bethesda, Md.: Oxford University Press; 1996.
86 Johnston CE, Bristow GK, Elias DA, et al. Alcohol lowers the vasoconstriction threshold in humans without affecting core cooling rate during mild cold exposure. Eur J Appl Physiol. 1996;74:293.
87 Johnston CE, White MD, Wu M, et al. Eucapnic hypoxia lowers human cold thermoregulatory response thresholds and accelerates core cooling. J Appl Physiol. 1996;80:422.
88 Jones SR. Fever in the elderly. In Mackowiak PA, editor: Fever: basic mechanisms and management, ed 2, Philadelphia: Lippincott-Raven, 1997.
89 Kanosue K, Crawshaw LI, Nagashima K, et al. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur J Appl Physiol. 2010;109:5.
90 Kanosue K, Hosono T, Zhang YH, et al. Neuronal networks controlling thermoregulatory effectors. Prog Brain Res. 1998;115:49.
91 Kanosue K, Sadato N, Okada T, et al. Brain activation during whole body cooling in humans studied with functional magnetic resonance imaging. Neurosci Lett. 2002;329:157.
92 Kasai T, Hirose M, Matsukawa T, et al. Preoperative blood pressure and catecholamine related to hypothermia during general anesthesia. Acta Anaesthesiol Scand. 2003;47:208.
93 Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci. 2009;32:215.
94 Kayman H. Management of fever: making evidence-based decisions. Clin Pediatr. 2003;42:383.
95 Kellogg DL, Pergola PE, Piest KL, et al. Cutaneous active vasodiliation in humans is mediated by cholinergic nerve cotransmission. Circulation Res. 1995;77:1222.
96 Kenney GP, Périard J, Journeay WS, et al. Effect of exercise intensity on the post exercise sweating threshold. J Appl Physiol. 2003;95:2355.
97 Khan F, Spence VA, Belch JJF. Cutaneous vascular responses and thermoregulation in relation to age. Clin Sci. 1992;82:521.
98 Kleinebeckel D, Klussman FW. Shivering. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
99 Kluger MJ. Is fever beneficial? Yale J Biol Med. 1986;59:89.
100 Kluger MJ, Kozak W, Leon LR, et al. Fever and antipyresis. In: Sharma HS, Westman J, editors. Brain function in hot environment. New York: Elsevier Science, 1998.
101 Kluger MJ, Wieslaw K, Conn CA, et al. The adaptive value of fever. In Mackowiak PA, editor: Fever: basic mechanisms and management, ed 2, Philadelphia: Lippincott-Raven, 1997.
102 Kolka MA, Stephenson LA. Resetting the thermoregulatory set-point by endogenous estradiol or progesterone in women. Ann N Y Acad Sci. 1997;813:204.
103 Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209.
104 Latzka WA, Sawka MN, Montain SJ, et al. Hyperhydration: thermoregulatory effects during compensable exercise-heat stress. J Appl Physiol. 1997;83:860.
105 Latzka WA, Sawka MN, Montain SJ, et al. Hyperhydration: tolerance and cardiovascular effects during uncompensable exercise-heat stress. J Appl Physiol. 1998;84:1858.
106 Lenhardt R, Sessler DI. Estimation of mean body temperature from mean skin and core temperature. Anesthesiol. 2006;105:1117.
107 Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17:485.
108 Lindqvist PG, Marsal K, Merlo J, et al. Thermal response to submaximal exercise before, during and after pregnancy: a longitudinal study. J Matern Fetal Neonatal Med. 2003;13:152.
109 Low PA. Evaluation of sudomotor function. Clin Neurophysiol. 2004;115:1506.
110 MacKenzie MA. Poikilothermia. In: Man: pathophysiological aspects and clinical implications [thesis]. Nijmegen, The Netherlands: University of Nijmegen; 1996.
111 Mackowiak PA. Clinical thermometric measurements. In Mackowiak PA, editor: Mechanisms and management, ed 2, Philadelphia: Lippincott-Raven, 1997.
112 Mackowiak PA. History of clinical thermometry. In Mackowiak PA, editor: Fever: Basic mechanisms and management, ed 2, Philadelphia: Lippincott-Raven, 1997.
113 Mackowiak PA, Bartlett JG, Borden EC, et al. Concepts of fever: recent advances and lingering dogma. Clin Infect Dis. 1997;25:119.
114 Malcolm RD, Alkana RL. Temperature dependence of ethanol depression in mice. J Pharmacol Exp Ther. 1983;35:306.
115 Mariak Z, White MD, Lyson T, et al. Tympanic temperature reflects intracranial temperature changes in humans. Pflügers Arch Eur J Physiol. 2003;446:279.
116 Maswood N, Cosmi S, Alfinito PD, et al. The role of the selective serotonin reuptake inhibitor fluoxetine in temperature regulation in ovariectomized rat models. Neuroendocrinology. 2006;84:330.
117 McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52.
118 Mezey E, Toth ZE, Cortright DN, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655.
119 Minson CT. Hypoxic regulation of blood flow in humans. In: Roach RC, Wagner PD, Hackett PH, editors. Hypoxia: through the lifecycle. New York: Kluwer Academic/Plenum, 2003.
120 Mitchell JH, Tate C, Raven P. Acute response and chronic adaptation to exercise in women. Med Sci Sports Exerc. 1992;24:S258.
121 Morimoto T, Itoh T, Takamata A. Thermoregulation and body fluid in hot environment. In: Sharma HS, Westman J, editors. Progress in brain research, vol 115. New York: Elsevier; 1998.
122 Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773.
123 Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413.
124 Mount LE. Adaptation to thermal environment: man and his productive animals. Baltimore: University Park Press; 1979.
125 Mount LE. The climatic physiology of the pig. London: Edward Arnold; 1968.
126 Nadel ER, Bullard RW, Stolwijk JAJ. Importance of skin temperature in the regulation of sweating. J Appl Physiol. 1971;31:80.
127 Nadel ER, Mitchell JW, Saltin B, et al. Peripheral modifications to the central drive for sweating. J Appl Physiol. 1971;31:828.
128 Nadel ER, Pandolf KB, Roberts MF, et al. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515.
129 Nadel ER, Stolwijk JAJ. Effect of skin wettedness on sweat gland response. J Appl Physiol. 1973;35:689.
130 Nagashima K, Matsue K, Konishi M, et al. The involvement of Cry1 and Cry2 genes in the regulation of the circadian body temperature rhythm in mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R329.
131 Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62.
132 Nakamura M, Yoda T, Crawshaw LI, et al. Regional differences in temperature sensation and thermal comfort in humans. J Appl Physiol. 2008;105:1897.
133 Newburgh LH. Physiology of heat regulation and the science of clothing. Philadelphia: WB Saunders; 1949.
134 Nielsen B, Hales J, Strange S, et al. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467.
135 Nielsen B, Kaciuba-Uscilko H. Temperature regulation in exercise. In: Blatteis CM, editor. Physiology and pathophysiology of temperature regulation. Singapore: World Scientific, 1998.
136 Nielsen B, Strange S, Christensen N, et al. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflügers Arch. 1997;434:49.
137 Noel J, Zimmermann K, Busserolles J, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308.
138 Olausson H, Charron J, Marchand S, et al. Feelings of warmth correlate with neural activity in right anterior insular cortex. Neurosci Lett. 2005;389:1.
139 Olsen TS, Weber UJ, Kammersgaard LP. Therapeutic hypothermia for acute stroke. Lancet Neurol. 2003;2:410.
140 Pandolf KB, Gange RW, Latzka WA, et al. Human thermoregulatory responses during cold water immersion after artificially induced sunburn. Am J Physiol. 1992;262:R617.
141 Pandolf KB, Gange RW, Latzka WA, et al. Human thermoregulatory responses during heat exposure after artificially induced sunburn. Am J Physiol. 1992;262:R610.
142 Panksepp J. Hypothalamic integration of behavior: rewards, punishments, and related psycho-biological processes. In: Morgane PJ, Panksepp J, editors. Handbook of the hypothalamus, vol 3. New York: Marcel Dekker; 1981. part B
143 Passias TC, Meneilly GS, Mekjavíc IB. Effect of hypoglycemia on thermoregulatory responses. J Appl Physiol. 1996;80:1021.
144 Patapoutian A, Peier AM, Story GM. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev. 2003;4:529.
145 Patterson MJ, Stocks JM, Taylor NAS. Humid heat acclimation does not elicit a preferential sweat redistribution toward the limbs. Am J Physiol Regul Integr Comp Physiol. 2003;286:R512.
146 Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705.
147 Petersen MH, Hauge HN. Can training improve the results with infrared tympanic thermometers? Acta Anaesthesiol Scand. 1997;41:1066.
148 Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Intensive Care Med. 2004;30:757.
149 Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985.
150 Prezant DJ, Richner B, Valentine DE, et al. Temperature dependence of rat diaphragm muscle contractility and fatigue. J Appl Physiol. 1990;69:1740.
151 Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531.
152 Regan JM, Macfarlane DJ, Taylor NAS. An evaluation of the role of skin temperature during heat adaptation. Acta Physiol Scand. 1996;158:365.
153 Rein H. Über die topographie der warmempfindung. Beziehungen zwischen innervation und rezeptorischen endorganen. Z Biol. 1925;82:513.
154 Reinberg A, Smolensky M. Chronobiology and thermoregulation. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon Press, 1990.
155 Robinson J, Charlton J, Seal R, et al. Oesophageal, rectal, axillary, tympanic and pulmonary artery temperatures during cardiac surgery. Can J Anaesth. 1998;45:317.
156 Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. Neuroimage. 2008;41:1504.
157 Romanovsky AA, Almeida MC, Garami A, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228.
158 Romanovsky AA, Quint PA, Benikova Y, et al. A difference of 5 degrees C between ear and rectal temperatures in a febrile patient. Am J Emerg Med. 1997;15:383.
159 Romanovsky AA, Shido O, Sakurada S, et al. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol. 1996;39:R693.
160 Romanovsky AA, Simons CT, Szekely M, et al. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:R407.
161 Rosenspire AJ, Kindzelskii AL, Petty HR. Cutting edge: fever-associated temperatures enhance neutrophil responses to lipopolysaccharide: a potential mechanism involving cell metabolism. J Immunol. 2002;169:5396.
162 Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526.
163 Satinoff E. Behavioral thermoregulation in response to local cooling of the rat brain. Am J Physiol. 1964;206:1389.
164 Satinoff E, Rutstein J. Behavioral thermoregulation in rats with anterior hypothalamic lesions. J Comp Physiol Psychol. 1970;71:77.
165 Satinoff E, Shan S. Loss of behavioral thermoregulation after lateral hypothalamic lesions in rats. J Comp Physiol Psychol. 1971;77:302.
166 Sato KT, Kane NL, Soos G, et al. Reexamination of tympanic membrane temperature as a core temperature. J Appl Physiol. 1996;80:1233.
167 Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc. 1992;24:657.
168 Schmidt-Nielsen K. Animal physiology: adaptation and environment, ed 5. Cambridge: Cambridge University Press; 1997.
169 Schönbaum E, Lomax P. Temperature regulation and drugs: an introduction. In: Schönbaum E, Lomax P, editors. Thermoregulation: physiology and biochemistry. New York: Pergamon, 1990.
170 Segal SS, Faulkner JA, White TP. Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol. 1986;61:660.
171 Sessler DI. Perianesthetic thermoregulation and heat balance in humans. FASEB J. 1993;7:638.
172 Sessler DI. Perioperative hypothermia. N Engl J Med. 1997;336:1730.
173 Sessler DI. Perioperative thermoregulation and heat balance. Ann NY Acad Sci. 1997;813:757.
174 Shibasaki M, Kondo N, Tominaga H, et al. Continuous measurement of tympanic temperature with a new infrared method using an optical fiber. J Appl Physiol. 1998;85:921.
175 Silva JE. The thermogenic effect of thyroid hormone and it clinical implications. Ann Intern Med. 2003;139:205.
176 Soler R, Echegaray M, Rivera MA. Thermal responses and body fluid balance of competitive male swimmers during a training session. J Strength Cond Res. 2003;17:362.
177 Soultanakis-Aligianni HN. Thermoregulation during exercise in pregnancy. Clin Obstet Gynecol. 2003;46:442.
178 Stevens ED. The evolution of endothermy. J Theor Biol. 1973;38:597.
179 Stevens JC, Choo KK. Temperature sensitivity of the body surface over the life span. Somatosens Mot Res. 1998;15:13.
180 Stolwijk JAJ, Nadel ER. Thermoregulation during positive and negative work exercise. Fed Proc. 1973;32:1607.
181 Strughold H, Porz R. Die dichte der kaltpunkte auf der haut des menschlichen körpers. Z Biol. 1931;91:563.
182 Takahashi Y, Smith P, Ferguson A, et al. Circumventricular organs and fever. Am J Physiol. 1997;273:R1690.
183 Talavera K, Yasumatsu K, Voets T, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022.
184 Tamaki Y, Nakayama T. Effects of air constituents on thermosensitivities of preoptic neurons: hypoxia versus hypercapnia. Pflügers Arch. 1987;409:1.
185 Thompson HJ, Tkacs NC, Saatman KE, et al. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12:163.
186 Thornley LJ, Maxwell NS, Cheung SS. Local tissue temperature effects on peak torque and muscular endurance during isometric knee extension. Eur J Appl Physiol. 2003;90:588.
187 Togashi K, Hara Y, Tominaga T, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804.
188 Tokizawa K, Uchida Y, Nagashima K. Thermoregulation in the cold changes depending on the time of day and feeding condition: physiological and anatomical analyses of involved circadian mechanisms. Neuroscience. 2009;164:1377.
189 Uchida Y, Kano M, Yasuhara S, et al. Estrogen modulates central and peripheral responses to cold in female rats. J Physiol Sci. 2010;60:151.
190 Waterhouse J, Edwards B, Bedford P, et al. Thermoregulation during mild exercise at different circadian times. Chronobiol Int. 2004;21:253.
191 Wenger CB, Roberts MF, Stolwijk JA, et al. Forearm blood flow during body temperature transients produced by leg exercise. J Appl Physiol. 1975;38:58.
192 Wilson MG, Morley JE. Aging and energy balance [invited review]. J Appl Physiol. 2003;95:1728.
193 Wood SC. Interactions between hypoxia and hypothermia. Annu Rev Physiol. 1991;53:71.
194 Yamada PM, Amorim FT, Moseley P, et al. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol. 2007;103:1196.
195 Yamazaki F, Hamasaki K. Heat acclimation increases skin vasodilation and sweating but not cardiac baroreflex responses in heat-stressed humans. J Appl Physiol. 2003;95:1567.
196 Yang Y, Gordon CJ. Regulated hypothermia in the hypothyroid rat induced by administration of propylthiouracil. Am J Physiol. 1997;272:R1390.
197 Yoda T, Crawshaw LI, Nakamura M, et al. Effects of alcohol on thermoregulation during mild heat exposure in humans. Alcohol. 2005;36:195.
198 Yoda T, Crawshaw LI, Saito K, et al. Effects of alcohol on autonomic responses and thermal sensation during cold exposure in humans. Alcohol. 2008;42:207.
199 Yoda T, Crawshaw LI, Saito K, et al: Effects of alcohol on thermoregulation in humans. Paper presented at the International Union of Physiological Sciences satellite conference on thermoregulation, 2004.
200 Yoshida K, Xiaodong L, Cano G, et al. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954.
201 Young CC, Sladen RN. Temperature monitoring. Int Anesthesiol Clin. 1996;34:149.
202 Zhang YH, Yanase-Fujiwara M, Hosono M, et al. Warm and cold signals from the preoptic area: which contribute more to the control of shivering? J Physiol. 1995;485:195.
203 Zhao Y, Boulant JA. Temperature effects on neuronal membrane potentials and inward currents in rat hypothalamic tissue slices. J Physiol. 2005;564:245.
204 Zweifler RM, Voorhees ME, Mahmood MA, et al. Rectal temperature reflects tympanic temperature during mild induced hypothermia in nonintubated subjects. J Neurosurg Anesthesiol. 2004;16:232.