Chapter 55 Therapeutic photomedicine
1. What is phototherapy?
3. How does traditional phototherapy work?
Immunomodulation locally, and possibly systemically, is the primary mechanism of action of phototherapy. Effects on keratinocyte proliferation and differentiation are likely secondary to the immunomodulatory effects. Immunomodulatory effects include: T-cell apoptosis (CD8+ in epidermis, CD4+ in dermis, CD25+ in both epidermis and dermis), inhibition and depletion of antigen-presenting cells (Langerhans cells in epidermis, dermal dendritic cells in dermis), release of immunosuppressive cytokines (interleukin [IL]-10 and IL-4, reduced expression of tumor necrosis factor–α [TNF-α]), interferon (IFN)-γ, and IL-12 locally, photoisomerization of trans-urocanic acid to cis-uracanic acid (which suppresses cellular immune responses, such as antigen presentation by Langerhans cells), and upregulation of CD95L (Fas ligand that binds to the Fas receptor on T cells inducing apoptosis).
Walters IB, Ozawa M, Cardinale I, et al: Narrowband (312-nm) UV-B suppresses interferon gamma and interleukin (IL) 12 and increases IL-4 transcripts: differential regulation of cytokines at the single-cell level: Arch Dermatol 139:155–161, 2003.
4. How is phototherapy administered?
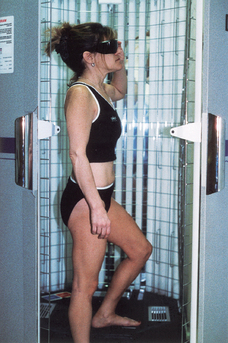
It is usually administered in a physician’s office or treatment center. Less commonly, patients purchase UVB phototherapy units and treat themselves at home. Patients receive a controlled dose of ultraviolet light while standing in a booth lined with high-output ultraviolet light bulbs (Fig. 55-2). The initial ultraviolet light exposure is determined by assessing the patient’s skin type and history of burning or by establishing the minimal erythema dose (MED).
5. Compare the induction phase, maintenance phase, and tapering phase for various forms of phototherapy.
• BBUVB: Daily treatments, increasing approximately 10% each treatment until pink or therapeutic level reached
• NBUVB: 3 times weekly, increasing approximately 10% each treatment until pink or therapeutic level reached
• UVA1: Daily or 3 times weekly, starting at 5 to 10 J/cm2, usually set at a standard dose depending on condition being treated. In general, high-dose regimens (130 J/cm2), medium-dose regimens (30 to 50 J/cm2) and low-dose regimens (20 J/cm2) have been used.
6. What advantages does narrowband UVB have over broadband UVB?
Narrowband UVB utilizes a TL-01 light source emitting UV light almost exclusively at 311 nm, which is near the most effective action spectrum for induction of T-cell apoptosis in inflammatory skin diseases such as psoriasis. The absence of other wavelengths of UV light decreases side effects (erythema, carcinogenesis, photoaging) without sacrificing efficacy. Unlike broadband UVB, suberythemogenic doses are effective.
7. What advantages does narrowband UVB have over PUVA?
Narrowband UVB does not have the risks, side effects, and inconvenience of taking oral psoralens, including nausea, vomiting, dizziness, headache, insomnia, depression and anxiety, hepatotoxicity, drug interactions, allergic reactions, photoallergic reactions, PUVA keratoses, PUVA lentigines, PUVA pain, transient nail pigmentation, photoonycholysis, facial hypertrichosis, licheoid eruptions, photosensitivity for 24 hours (even through windows), risk of cataracts, increased risk of melanoma and other skin cancers including genital skin cancers, narrow window of treatment after taking medication (1 to 2 hours), variability of tissue response depending on medication absorption and distribution (which is time sensitive), and need to wear sunglasses and avoid natural sunlight after taking medication for 24 hours. Narrowband UVB has not demonstrated increased carcinogenicity, thus far in humans, and the efficacy is similar. However, increased carcinogenicity has been noted in rats, and long-term follow-up is not yet available.
8. What is targeted laser phototherapy?
An excimer (XeCl) laser, which emits a wavelength of 308 nm (near the narrowband UVB wavelength of 311 nm) is effective in treating individual psoriatic plaques. Supererythemogenic doses, from 6 to 8 MEDs, are used on individual plaques for 8 to 12 treatments and may induce months of remission. This localized form of UVB phototherapy is also an effective treatment for patches of vitiligo. It is advantageous in that it avoids irradiating healthy, nonlesional skin. It more effectively induces T-cell apoptosis, as well. In psoriasis, it is able to target individual plaques with higher fluences and reaches deeper T cells within the dermis. The pulsed dye laser at 585 to 595 nm has also been shown to be efficacious in treating individual psoriatic plaques, but appears less effective than the 308 nm excimer laser.
9. What is UVA1 phototherapy and why is it used?
UVA1 is 340 to 400 nm UV light used mainly to treat atopic dermatitis and scleroderma/morphea, but it is less effective than UVB in treating psoriasis, mycosis fungoides, and other inflammatory skin disorders. It has different mechanism of action: It induces singlet oxygen–induced T-cell apoptosis, reduces Langerhans cell ability to migrate out of the epidermis, decreases the number of IgE-bearing Langerhans cells and dendritic cells in the epidermis and dermis, decreases collagen synthesis, and increases collagenase I and matrix metalloproteinases in sclerosing disorders of the skin. It penetrates deeper than UVB and appears more effective in cutaneous mastocytosis compared to UVB in depleting mast cells from the skin. UVA1 does not increase viral load in HIV-positive patients, unlike UVB, which has shown a 6- to 10-fold increase of cutaneous viral load.
11. What is balneophototherapy?
Salt water bathing in combination with UV phototherapy. Potential mechanisms of action include the following: Magnesium salts may decrease antigen presentation in the skin, water-soaked skin allows more UV light to penetrate the skin due to decreased reflectance, and perhaps there is a neuropsychiatric influence (relaxation, vacation, decreased stress).
12. What do MED and MPD mean and why are they important?
13. What is Goeckerman therapy?
Goeckerman therapy, named in honor of Dr. William H. Goeckerman, who developed it in 1925, is UVB phototherapy used in combination with topical coal tar to treat psoriasis. The therapy is administered by having patients apply crude coal tar or tar derivatives to the skin and removing the excess tar before exposure to UVB. After treatment, the patient takes a bath or shower to remove any remaining tar or scale. With each visit, the dose of UVB administered is gradually increased, with the treatment being administered three or more times per week for 3 to 4 weeks or longer. This extremely safe treatment can be supplemented with topical corticosteroid preparations or descaling agents. Once remission is achieved, patients may stay in remission for 12 to 18 months or longer. Long remission rates and relative safety have made this the therapy of choice in many psoriasis treatment centers. Disadvantages include its inconvenience, messiness of the tar, and need for numerous office visits.
14. What are the most common acute UVB phototherapy side effects?
The most common acute side effect is a sunburn-like effect within 24 hours of treatment manifested by erythema and tenderness of the skin. When this occurs, therapy is usually withheld until the erythema fades, and the amount of UVB administered is usually reduced at the next treatment session. Patients who fail to wear eye protection that blocks UVB may develop corneal burns. Occasionally, patients with psoriasis may experience temporary pustular flares of psoriasis during treatment. Topical preparations used in conjunction with UVB phototherapy, such as emollients and tar, may produce a folliculitis. This complication can be prevented by instructing the patient to apply topical preparations in a downward fashion to prevent follicular irritation. A severe blistering burn is rare when UVB is properly administered, and may be associated with Koebner’s isomorphic phenomenon resulting in psoriatic plaques in the burned area of skin.
15. What are the most common long-term UVB phototherapy side effects?
Long-term side effects include skin cancer and skin aging (dermatoheliosis). The exact risk of skin cancer has not been determined, but it is greater in patients with fair skin, with a family history of skin cancer, or who use other therapies associated with a risk of producing skin cancer (i.e., PUVA). Patients with prolonged UVB therapy and other risk factors should have periodic skin examinations to detect early skin cancers.
16. What is PUVA phototherapy?
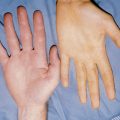
PUVA is an acronym for psoralen and ultraviolet light, type A. It involves the combined use of a prescription psoralen (methoxsalen or trioxsalen) and long-wave ultraviolet light (UVA). PUVA therapy for psoriasis was approved by the Food and Drug Administration in 1982 and has since become one of the treatments of choice for many adult patients with extensive patch and plaque-type psoriasis and mycosis fungoides. The psoralen usually is administered orally (sometimes topically) and followed by exposure to UVA light. While UVA is most commonly delivered in booths, special portable units are also available to treat the hands, feet, and scalp (Fig. 55-3).
17. What are psoralens?
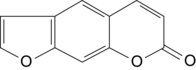
Psoralens are a subclass of drugs that belong to a group of compounds called furocoumarins, which are derived from the fusion of a furan with coumarin (Fig. 55-4). Psoralens are natural constituents in a large variety of medicinal plants (e.g., limes, lemons, figs, parsnips). The two psoralens that are used therapeutically in the United States are methoxsalen and trioxsalen. Methoxsalen (8-methoxypsoraIen, 8-MOP) is a naturally occurring photoactive plant substance found in the seeds of Amnii majus, a plant that grows wild along the Nile delta. Methoxsalen is absorbed from the upper gastrointestinal tract and metabolized by the intestine and liver. Ninety percent is excreted within 24 hours, with the major portion being excreted in the urine. Trioxsalen is a synthetic psoralen usually reserved for the treatment of vitiligo.
18. How do the psoralens work?
Psoralen compounds by themselves do not affect the skin in the absence of UVA, but in the presence of UVA (320 to 400 nm), they are potent photosensitizers. Ninety minutes after oral ingestion, absorption of UVA photons photochemically links the DNA by forming cycloadditive products between the intercalated psoralen and the pyrimidine bases of cellular DNA. These psoralen-DNA cross-links cause a decrease in the rate of epidermal DNA synthesis, which some authorities believe to be the primary mechanism of action of these agents. Irradiation of psoralens also induces the formation of reactive oxygen species that can damage both cell membranes and organelles, as well as activate arachidonic acid metabolism. There is considerable evidence that PUVA therapy has a direct effect on the cutaneous immune system, and some authorities feel that this may be more important in terms of the therapeutic effect. The induction of T-cell apoptosis, decreased function of antigen-presenting cells, and altered cytokine profile is likely the most important component of PUVA therapy in most modern views.
19. Are there contraindications to using PUVA?
Contraindications include a history of psoralen hypersensitivity reactions; photosensitive diseases including lupus erythematosus, porphyria, xeroderma pigmentosum, and albinism; malignant melanoma; pregnancy; and aphakia (absence of a lens may produce retinal damage). PUVA photochemotherapy should be used with caution in patients with fair skin, a history of previous ionizing radiation, history of multiple skin cancers, cataracts, immunosuppression, uremia, or renal failure. Patients with severe myocardial or other diseases that may disallow standing for prolonged periods in the treatment cabinet may not be able to receive photochemotherapy.
20. What is bath PUVA?
The patient is immersed for 15 minutes in a bathtub containing five 10-mg capsules of methoxsalen dissolved in the water. Topical methoxsalen gradually loses its photoactivity, and the patient should ideally be treated with the standard UVA light treatments within 15 minutes after immersion. Bath PUVA therapy using trimethylpsoralen (TMP), which is more hydrophobic than 8-methoxypsoralen (8-MOP), has been used for more than 30 years in Sweden and Finland, with no observed increase in the number of skin cancers. It is also useful for patients who are not able to tolerate oral methoxsalen because of nausea, and for children under 15 years of age. This technique also may be adapted for use on the palms and soles for hand and foot dermatoses.
Key Points: Therapeutic Photomedicine
1. Phototherapy is used to treat many inflammatory skin diseases, is safe and effective, has relatively low cost, and provides another tool for the dermatologist to treat patients.
2. Narrowband UVB is now the phototherapy treatment of choice for psoriasis, vitiligo, and possibly mycosis fungoides (patch stage) and atopic dermatitis (in some experts’ opinions) given its superior efficacy to broadband UVB and decreased side effects compared to oral PUVA.
3. Phototherapy exerts its effects primarily through immunomodulation (T-cell apoptosis within the epidermis and dermis, and suppression/depletion of Langerhans cells within the epidermis).
4. Photodynamic therapy utilizes aminolevulinic acid, which is converted to protoporphyrin IX in keratinocytes, and when illuminated with blue or red light is activated, producing phototoxic products and free radicals that induce cellular apoptosis.
21. What is RePUVA?
RePUVA treatment uses an oral retinoid (usually acitretin) and PUVA in combination. Studies suggest that this therapeutic combination offers a practical way to clear psoriasis (with an overall response rate of 73%) with less cumulative UVA exposure than PUVA alone. Additionally, RePUVA can be successfully applied in patients resistant to standard PUVA. Patients receiving acitretin plus PUVA clear 40% faster than those treated with PUVA alone, even though the UVA dose is reduced by 50%. Most commonly, the oral acitretin is begun 7 to 10 days before the first PUVA treatment, and the two therapies are given concurrently until 100% clearing occurs. The acitretin is usually discontinued, and the patient is maintained on PUVA for about 2 months. Retinoids may also be used in combination with BBUVB and NBUVB.
22. What is photopheresis?
Photopheresis, also known as extracorporeal photochemotherapy (ECP), is a form of apheresis therapy. First introduced for the treatment of cutaneous T-cell lymphoma (CTCL), it has since been evaluated in studies and randomized trials as a potential treatment for autoimmune diseases, solid organ transplant rejection, and graft-versus-host disease. ECP is a relatively new procedure that involves discontinuous leukapheresis by centrifugation, followed by exposure of the buffy coat lymphocytes to UVA light in a special unit about 2 hours after the administration of methoxsalen. Following exposure of the lymphocytes to UVA, the photoirradiated cells are reinfused into the patient. The procedure is done on two consecutive days at 4-week intervals. The adverse side effects are minimal; patients may experience nausea and about 10% develop a transient fever after reinfusion.
23. Which diseases have been treated with extracorporeal photopheresis?
Photopheresis is approved for the treatment of cutaneous T-cell lymphoma (mycosis fungoides). It is most effective in the erythrodermic variants (Sézary syndrome) that involve >25% of the body surface. The response rate in this group of patients is about 64%. Photopheresis, as a form of immunotherapy, has also been tried on other T cell–mediated diseases, such as pemphigus vulgaris, chronic Lyme arthritis, psoriatic arthritis, and progressive systemic sclerosis.
24. How is photodynamic therapy used in dermatology?
Photodynamic therapy is a two-step process. A photosensitizing agent is topically applied to the patient’s skin, followed by illumination with a light source of the proper wavelength. Aminolevulinic acid (ALA) is used most often in the United States and is converted to protoporphyrin IX by keratinocytes. Keratinocytes lack the final enzyme in the heme synthesis pathway, and the process ends with protoporphyrin IX, which is photosensitizing, particularly in the Soret band (400 to 410 nm). Porphyrins used in combination with blue (415 to 425 nm) or red light (600 to 700 nm) are the most common forms of photodynamic therapy. Smaller absorption peaks are also noted around 585 to 595 nm, which correlates with the pulsed dye laser absorption spectrum. Cancer cells and precancerous cells derived from keratinocytes (actinic keratoses, squamous cell carcinomas, basal cell carcinomas) accumulate porphyrins at higher concentrations than normal keratinocytes and are more susceptible to the phototoxic effects of the treatment. In acne, P. acnes is susceptible to both blue light alone and to porphyrin-induced injury. The sebaceous glands in the skin accumulate significant amounts of porphyrin and are very susceptible to injury by photodynamic therapy (PDT). When used in combination with the pulsed dye laser at 585 to 595 nm, deeper penetration into the sebaceous gland is achieved and a better therapeutic outcome is noted when treating acne. After 3 monthly treatments, acne may stay in remission for up to a year.
25. What is blue-light phototherapy?
Some degree of jaundice is common in newborns and is seen in up to 50% of infants between the second and fourth day of life. Before birth, bilirubin is conjugated and excreted chiefly by the placenta; however, after birth, this function shifts to the neonatal liver. Transient neonatal hyperbilirubinemia is the result of insufficient activity of the conjugative hepatic enzyme glucuronyl transferase. Phototherapy with blue light (460 nm) is an effective means of preventing hyperbilirubinemia by producing a photoproduct called photobilirubin, which is nontoxic. In the treatment of hyperbilirubinemia, phototherapy should be considered at serum bilirubin levels of 222 to 260 mmol/L, taking into account other clinical factors. Blue light phototherapy alone is also used to treat acne vulgaris. P. acnes is susceptible to blue light (415 to 425 nm) and is significantly reduced in number within the pilosebaceous unit. Improvement in acne is well documented.