CHAPTER 116 Therapeutic Injections and Radiofrequency Denervation
INTRODUCTION
For more than 70 years, the sacroiliac joint complex has been implicated as a significant and discreet structural source of low back pain. However, of the three major groups of spinal structures identified as contributing to chronic low back pain, the sacroiliac joint complex (SIJC) stands apart from the zygapophyseal joints and intervertebral discs as the least understood from a neuroanatomic, diagnostic, and therapeutic standpoint. The prevalence of low back pain arising from the SIJC has been estimated at 15–30%,1,2 and is likely higher in the older population. The SIJC possesses a unique and complex three-dimensional anatomy with multisegmental sensory innervation. Unlike the zygapophyseal joints and intervertebral discs, the sensory innervation of the SIJC has been described in a detailed fashion only recently, and the definitive gold standard for successful treatment of SIJC pain has not yet been rigorously defined.
Numerous studies have demonstrated a lack of pathognomonic historical, symptom, physical examination, or imaging findings specific for the individual diagnosis of zygapophyseal joint, disc, or SIJC pain.1,3–7 The identification of specific structural sources of low back pain must currently be made through validated interventional diagnostic means. A structure-specific diagnosis is ultimately essential for the eventual consideration of appropriate evidence-supported treatment options. A specific structural diagnosis also leads to a definable prognosis, as the long-term outcomes following treatment for zygapophyseal joint pain differ from disc pain, and from SIJC pain as well.8–14
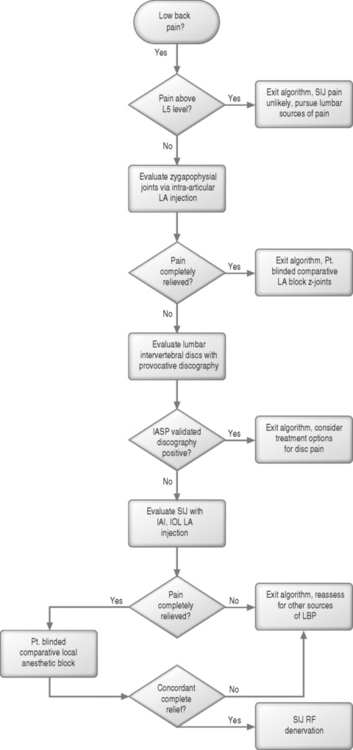
A rational, evidence-based diagnostic algorithm for the identification of the structural source of low back pain is thus invaluable (Fig. 116.1). The area of the SIJC is frequently involved with referred pain patterns associated with other lumbar structures,2,3,15–17 and pain of SIJC origin frequently involves somatic referred pain to various areas of the low back, pelvis, abdomen, and lower extremities,18 with nearly one in seven patients experiencing pain radiating to the foot in a pseudoradicular pattern. Successful implementation of such a diagnostic algorithm is therefore ultimately dependent not only on the physician’s understanding of the technical aspects of diagnostic interventions, but also the overlapping distributions of referred somatic pain arising from different lumbar structures, and the defined sensitivities and specificities of each test considered.
The current gold standard for the diagnosis of SIJC pain involves controlled intra-articular anesthetic injections (IAI).1,2,6,7,19 Pain may also arise from the deep interosseous ligament (DIOL), which is exquisitely innervated. The nociceptive potential of the DIOL is supported by anatomic and histologic findings. Although a technique for injection of the DIOL has been described, the clinical overlap in diagnostic sensitivity and specificity between controlled SIJC IAI and DIOL anesthetic injections has yet to be determined,10,20–22 and represents an ongoing area of clinical and anatomical research. Once the diagnosis of SIJC pain has been established and the presence of other sources of lumbar spine pain excluded, definitive SIJC therapeutic intervention may be considered.
A definitive, highly successful, and durable intervention for the treatment of SIJC pain may be just around the corner. Limited reports of the efficacy of surgical fusion23,24 and radiofrequency denervation10,25,26 are available; however, long-term prospective, controlled or comparative studies are currently lacking or are under investigation. This chapter will focus on current evidence supporting therapeutic injection and radiofrequency SIJC denervation.
RELEVANT ANATOMY
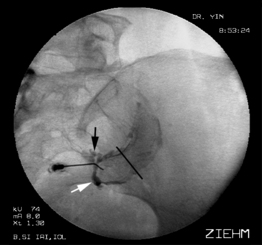
The synovial component of the SIJC is composed of two divergent joint planes, a larger lateral (or anterior) pole and the smaller medial (or posterior) pole. Percutaneous access to the medial pole traverses a minimum of ligamentous tissue, whereas access to the lateral pole must traverse the DIOL (Fig. 116.2). The total volume of the synovial component of the SIJC (1.5 mL) is small compared to its surface area.15,27
The ‘capsule’ of the sacroiliac joint is frequently incompetent, even in asymptomatic patients. In one series, 61% of intra-articular injections demonstrated extracapsular extravasation,22 and 25% of asymptomatic volunteers undergoing intra-articular injection demonstrated ventral capsular insufficiencies,27 with contrast extravasating in the region of the traversing lumbosacral plexus. Detailed dissection of the ventral joint capsule in cadavers suggests that these ventral capsular ‘defects’ often appear as small foramina rather than traumatic capsular rents.28
Neuroanatomy of the sacroiliac joint complex
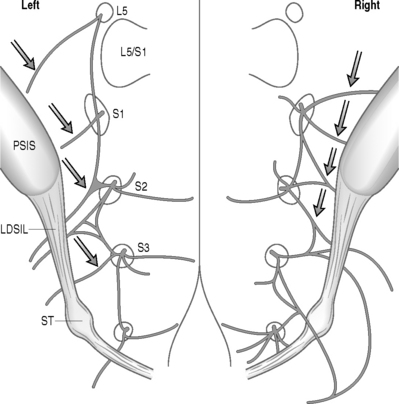
Early studies of the sacroiliac joint suggested a combination of ventral and dorsal innervation,29,30 but recent investigation has demonstrated a predominant dorsal innervation in humans21,22 arising from the lateral branches of the L5 dorsal ramus and S1–4 dorsal rami, and composed of a wide range of sensory fiber types.21,30 The lateral branch nerves arise from a lateral dorsal sacral plexus, and divide into multiple smaller branches, some of which enter the DIOL whereas others do not. The segmental location and number of lateral branch nerves innervating the SIJC is extremely variable. On the posterior aspect of the sacrum, these branches are often closely related (Fig. 116.3).20 Unlike the lumbar medial branch nerves of the dorsal rami supplying innervation to the zygapophyseal joints, the complex three-dimensional anatomy of the lateral branch nerves innervating the SIJC combined with their close proximity to the dorsal sacral foramina renders consideration of selective anesthetic blockade futile (Fig. 116.4).27
DIAGNOSTIC INJECTION TECHNIQUES
Once other structures of low back pain have been excluded, consideration of SIJC-specific diagnostic interventions may be entertained. The current gold standard involves blinded, controlled intra-articular local anesthetic injections of the SIJC, or saline placebo-controlled blocks. Because the synovial component of the SIJC is rarely accessed successfully without image guidance, injection of the joint must be performed under fluoroscopy, or possibly computed tomography (CT) guidance.31,32 However, it must be remembered that the sacroiliac joint capsule is frequently incompetent, and extravasation of local anesthetic into surrounding structures, especially from the ventral joint capsule in the region of the traversing lumbosacral plexus, will render such an injection non-specific, and therefore nondiagnostic. Additionally, since the false-positive rate of single anesthetic injections may approach 40%,33–36 no therapeutic decisions regarding ablation or surgery should be made based on a single response to an isolated analgesic test. Patient responses to controlled, comparative or placebo-controlled anesthetic testing must be rigorously assessed and documented. As with any diagnostic procedure, analgesic tests generate negative, indeterminate, and positive results, and have associated false-negative and false-positive rates. Understanding what constitutes a negative, indeterminate, and positive result is essential. The methodology of comparative and placebo-controlled analgesic testing has been extensively validated elsewhere, and should be considered mandatory reading for all interventional spine diagnosticians.34,35,37–39 It is further recommended that any analgesic test be performed without sedation. Patient responses in the immediate postprocedural period should be rigorously documented, preferably with a q15 minute or q30 minute pain log. Sedation is an inadequate excuse for poor injection technique.
Access techniques for the medial40–44 and lateral poles10,45 of the synovial component of the SIJC have been described, as has a technique for isolated injection of the DIOL.10,45 Any image-guided injection of the SIJC must include the use of radiopaque contrast to obviate vascular uptake, and verify contained distribution of injected local anesthetic. Fluoroscopy offers several intrinsic advantages over CT, including the capability for real-time imaging (and detection of vascular uptake of contrast), although the superior contrast and spatial resolution of CT may offer potential advantages in documenting extracapsular or extraligamentous extravasation patterns. In patients with spinal or pelvic hardware, advanced fluoroscopic imaging technologies, such as digital subtraction algorithm (DSA) imaging permits imaging free of the metallic imaging artifacts common to CT.
THERAPEUTIC INTERVENTIONS
Injections of the SIJC have not been demonstrated to have therapeutic value except in the isolated instance of sacroiliitis associated with seronegative spondyloarthropathies.46–50 No structure-specific diagnostic information can be gleaned from steroid injection; if a patient experiences relief beyond the duration of action of local anesthetic, all that can be ascertained is that a steroid response may be present. A prolonged steroid response implies that a component of inflammation may be present, but is not structure-specific; local anesthetic injection in and of itself may also provide prolonged analgesia, well beyond the expected duration of conduction block.37 Once the diagnosis of SIJC pain has been established, therapeutic intervention may be considered.
Several denervation procedures for SIJC pain have described targeting the lateral branch nerves of the dorsal rami at various points between the dorsal sacral foramina and the posterior SIJ capsule. Among the first techniques is that described by Kline, involving the generation of a series of bipolar radiofrequency (RF) lesions along the posterior joint line (Fig. 116.5).51 Recognizing the multisegmental innervation of the SIJC, Kline reasoned that the lateral branch nerves must converge along the posterior joint capsule, and serial lesions along the posterior joint line should effectively denervate the joint. However, a study by Ferrante et al., utilizing uncontrolled single anesthetic diagnostic blocks, found that only 36% of patients experienced more than 6 months of >60% relief, and no patients experienced complete relief.26 A more rigorous evaluation with controlled anesthetic blocks and rigorous postblock assessment may have yielded better results, as initial false-positive block responders would have been eliminated from consideration.
Another approach toward denervation of lateral branch nerves at L5 and S1 has been described under CT guidance by Gevargez et al.25 Like the Ferrante study, a single anesthetic block was used for patient selection. Thirty-five of 38 patients were followed for 3 months, with 34.2% reporting complete relief, and an additional 32% reporting ‘substantial relief.’
Based on a detailed neuroanatomic study of the dorsal sacral plexus and lateral branch topography, Yin et al. published results of a pilot study utilizing a sensory stimulation-guided approach to select pain-conducting from non pain-conducting lateral branch nerves for subsequent RF denervation.10 Dual analgesic injections of the SIJC DIOL were utilized to identify patients with probable SIJC pain after no relief was reported following anesthetic blocks of the lumbar zygapophyseal joints. In this study, provocative discography was not performed to rule out disc pain. Sensory stimulation-guided localization of the lateral branch nerves was performed to identify painful versus nonpainful nerves. The frequency of painful L5 and S1 lateral branch nerves identified in this group of patients was 100%, with 78% of patients demonstrating a painful branch arising from S2 and 42% with painful S3 branches. Although follow-up was limited to 6 months, preliminary results were encouraging, with 64% of patients experiencing longer than 6 months of >60% relief, and among responders, more than half (36% of total) experiencing 100% relief. No complications were noted. A prospective, long-term follow-up study is currently in progress. Ultimately, prospective, controlled studies will be required to identify a gold standard for the treatment of SIJC pain.
DESCRIPTION OF SENSORY STIMULATION-GUIDED LATERAL BRANCH RADIOFREQUENCY NEUROTOMY TECHNIQUE
Background
Detailed neuroanatomic examination of the dorsal sacral plexus and associated lateral branch nerves innervating the SIJC demonstrates that these nerves follow a complex and variable course to the DIOL and posterior joint capsule. Penetrating numerous ligaments along their course, the segmental lateral branch nerves are inconsistent with regard to number and location in three dimensions soon after exiting the dorsal sacral foramina (Fig. 116.6).10,28 Because consistent lateral branch contributions to the SIJC have been demonstrated to arise from the S2 and S3 dorsal rami, procedures that only target the lateral branch nerves at L5 and S1 may miss pain-bearing conducting from these lower dorsal rami. A description of the sensory stimulation-guided radiofrequency neurotomy targeting the L5 through S3 lateral branch nerves follows.
Procedural details
Once the L5 dorsal ramus has been eliminated as a potential source of pain, the lateral branch is localized. The lateral branch of L5 typically lies 5–10 mm lateral to the lateral aspect of the superior articular process of S1 (Fig. 116.7A). The electrode is repositioned along the dorsal sacral ala as described, and stimulation repeated. The electrode is manipulated along the dorsal sacral ala until the lateral branch is identified. The lateral branch of the dorsal ramus of L5 does not have a known motor component, and localization depends solely on sensory findings.
Prior to lesion generation, 0.5–1.0 mL of local anesthetic (e.g. 0.5–0.75% bupivacaine) is injected. As the lateral branch nerves are closely invested within a labyrinthine network of ligaments surrounding the dorsal sacral plexus, injected local anesthetic may not be directed in the area desired; the electrode may have to be rotated in order to achieve diffusion of sufficient local analgesia sufficient to permit painless coagulation. Care should be taken to return the electrode to the exact position where stimulation resulted in pain reproduction prior to lesion generation. The author creates three overlapping 90° C lesions, each of 60 seconds duration, on each symptomatic branch identified. Lesion parameters, including initial temperature and impedance, volts, watts, and milliamperes, should be recorded. Any complaints of pain, especially that which radiates down the lower extremity, should result in the immediate cessation of lesion generation and confirmation of appropriate electrode placement well dorsal to the segmental spinal nerves.
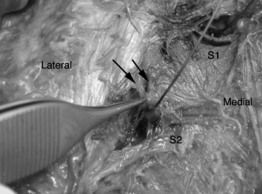
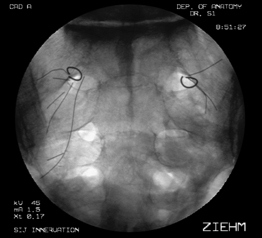
Once the L5 lateral branch has been located and mapped, attention is directed towards mapping of the lateral branch nerves arising from the dorsal ramus of S1. As the S1 dorsal ramus supplies the most lateral branch nerves going to the SIJC, care should be taken to map this level carefully. More than one symptomatic branch may be encountered. Because the lateral branch nerves arising from the dorsal sacral plexus are invariably located lateral the cephalocaudad meridian of the foramen, mapping efforts are systematically dedicated to this area (i.e. if the dorsal foramina was viewed as a clock face, between the 12 o’clock and 6 o’clock position) (see Fig. 116.7B). The lateral margin of the sacral foramina is frequently difficult to visualize on fluoroscopy, but can be easily identified by feel as the electrode tip ‘slips over’ the edge of the foramen. Stimulation of the lateral branch nerves or communicating arcade can result in referral of pain to the lower extremity, groin, or abdomen. It is imperative that such sensation be differentiated from stimulation of the sacral spinal nerves themselves, which may occur if the electrode is inadvertently advanced through the dorsal foramina. If the depth of electrode placement is a concern, nonionic radiopaque contrast may be injected through the electrode and oblique and lateral fluoroscopic imaging used to verify placement of the electrode along the dorsal sacral plate. Any symptomatic branches identified at S1 are lesioned as previously described for the dorsal ramus of L5.
In a sequential fashion, the lateral branch nerves of S2 and S3 are mapped. Symptomatic branches are coagulated as described (see Fig. 116.7C,D). At the conclusion of the procedure, the electrodes and introducer catheter are removed.
Postoperative care
Prior to discharge, the patient is instructed about expected postprocedural side effects, and counseled on signs and symptoms of infection, acute neurological changes, or other issues that may herald a serious postoperative complication. Typically, the postoperative course is benign, with postoperative discomfort lasting between 1 and 3 weeks. The author has not found it necessary to prescribe opioid analgesics or to escalate existing opioid regimens. If required, one of many nonopioid analgesic agents may be prescribed instead. Occasionally, patients will experience hypoesthesia, focal anesthesia, or dysesthesia affecting a small (i.e. 7.5 × 7.5 cm) region of the buttocks. In the author’s experience, dysesthesiae are self-limited, and resolve 3–6 weeks after surgery. In all of the author’s cases save one, hypoesthesiae and anesthesia have also resolved spontaneously within 3–6 weeks. Return to work may occur as early as the first postoperative day. A return to normal activities is encouraged as quickly as possible. No specific postoperative rehabilitation exercises or regimens are typically required. Routine outpatient follow-up 4–6 weeks after operation is scheduled. Maximum benefit from the procedure may be masked by postoperative discomfort, and may not be obvious for up to 4–6 weeks. If no improvement is seen by 6 weeks after operation, the possibility of a false-positive diagnosis or procedural failure is considered.
REPRESENTATIVE CASE
Provocative rate, volume, and manometrically controlled lumbar discography is then performed at the L2–3 through L5–S1 levels. A posterior transdural midline approach is necessary at L5–S1 due to previous posterolateral fusion. Although significant degenerative changes are present at all levels, including frank annular incompetence at L4–5 and L5–S1, provocative discography is painless at all studied levels.
1 Maigne J, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889-1892.
2 Schwarzer A, Aprill C, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
3 Schwarzer AC, et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20(17):1878-1883.
4 Schwarzer AC, et al. Prevalence and clinical features of lumbar zygapophyseal joint pain: a study in an Australian population with chronic low back pain. Ann Rheum Dis. 1995;54(2):100-106.
5 Schwarzer AC, et al. Clinical features of patients with pain stemming from the lumbar zygapophyseal joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19(10):1132-1137.
6 Dreyfuss P, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21(22):2594-2602.
7 Slipman CW, et al. The predictive value of provocative sacroiliac joint stress maneuvers in the diagnosis of sacroiliac joint syndrome. Arch Phys Med Rehabil. 1998;79(3):288-292.
8 van Kleef M, et al. Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine. 1999;24(18):1937-1942.
9 Dreyfuss P, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophyseal joint pain. Spine. 2000;25(10):1270-1277.
10 Yin W, et al. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: technique based on neuroanatomy of the dorsal sacral plexus. Spine. 2003;28(20):2419-2425.
11 Karasek M, Bogduk N. Twelve-month follow-up of a controlled trial of intradiscal thermal annuloplasty for back pain due to internal disc disruption. Spine. 2000;25(20):2601-2607.
12 Karasek M, Karasek D, Bogduk N. A controlled trial of the efficacy of intra-discal electrothermal treatment for internal disc disruption. North American Spine Society, 14th Annual Meeting. 1999; Chicago, IL.
13 Derby R, et al. The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes. Spine. 1999;24(4):364-371. discussion 371–372
14 Madan SS, Harley JM, Boeree NR. Circumferential and posterolateral fusion for lumbar disc disease. Clin Orthop. 2003;409:114-123.
15 Fortin J, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: Asymptomatic volunteers. Spine. 1994;19(13):1475-1482.
16 Carragee E, Paragioudakis S, Khurana S. Lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25(23):2987-2992.
17 Carragee E, et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25(11):1373-1381.
18 Slipman CW, et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000;81(3):334-338.
19 Fortin J, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part II: Clinical evaluation. Spine. 1994;19(13):1483-1489.
20 Willard F, Carreiro J, Manko W. The long posterior interosseous ligament and the sacrococcygeal plexus. Third Interdisciplinary World Congress on Low Back and Pelvic Pain. 1998.
21 Grob K, Neuhuber W, Kissling R. [Innervation of the sacroiliac joint of the human]. Z Rheumatol. 1995;54(2):117-122.
22 Fortin J, et al. Sacroiliac joint innervation and pain. Am J Orthop. 1999;28(12):687-690.
23 Giannikas KA, et al. Sacroiliac joint fusion for chronic pain: a simple technique avoiding the use of metalwork. Eur Spine J. 2004;13(3):253-256.
24 Guner G, et al. Anterior sacroiliac fusion: a new video-assisted endoscopic technique. Surg Laparosc Endosc. 1998;8(3):233-236.
25 Gevargez A, et al. CT-guided percutaneous radiofrequency denervation of the sacroiliac joint. Eur Radiol. 2002;12(6):1360-1365.
26 Ferrante FM, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137-142.
27 Dreyfuss P, Park K, Bogduk N. Do L5 dorsal ramus and S1–4 lateral branch blocks protect the sacro-iliac joint from an experimental pain stimulus? A randomized double-blinded controlled trial. ISIS 8th Annual Scientific Meeting. San Francisco, CA, USA: International Spinal Injection Society, 2000.
28 Willard F, Carreiro J, Manko W. The long posterior interosseous ligament and the sacrococcygeal plexus. Third Interdisciplinary World Congress on Low Back and Pelvic Pain, 1998.
29 Ikeda R. Innervation of the sacroiliac joint. Macroscopic and histological studies. J Nippon Med School. 1991;58:587-596.
30 Solonon K. The sacroiliac joint in light of anatomical, roentgenological, and clinical studies. Acta Orthop Scand [Suppl]. 1957;27:1-127.
31 Rosenberg JM, Quint TJ, de Rosayro AM. Computerized tomographic localization of clinically-guided sacroiliac joint injections. Clin J Pain. 2000;16(1):18-21.
32 Hansen H. Is fluoroscopy necessary for sacroiliac joint injections. Pain Phys. 2003;6(2):155-158.
33 Dreyfuss P, et al. Specificity of lumbar medial branch and L5 dorsal ramus blocks. A computed tomography study. Spine. 1997;22(8):895-902.
34 Barnsley L, et al. False-positive rates of cervical zygapophyseal joint blocks. Clin J Pain. 1993;9(2):124-130.
35 Lord SM, Barnsley L, Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophyseal joint pain. Clin J Pain. 1995;11(3):208-213.
36 Schwarzer AC, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophyseal joints. Pain. 1994;58(2):195-200.
37 Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophyseal joint pain. Pain. 1993;55(1):99-106.
38 Lord S, et al. Chronic cervical zygapophyseal joint pain after whiplash: A placebo-controlled prevalence study. Spine. 1996;22:1737-1744.
39 Bogduk N, McGuirk B. Medical management of acute and chronic low back pain. An evidence-based approach. Pain research and clinical management, Vol. 13 . Elsevier, Amsterdam, 2002;169-185.
40 Haldeman K, Soto-Hall R. The diagnosis and treatment of the sacroiliac condition by the injection of procaine. J Bone Joint Surg. 1938;20:675-685.
41 Miskew DB, Block RA, Witt PF. Aspiration of infected sarco-iliac joints. J Bone Joint Surg [Am]. 1979;61(7):1071-1072.
42 Aprill C. The role of anatomically specific injections into the sacroiliac joint. First Interdisciplinary World Congress on Low Back Pain and its Relation to the Sacroiliac Joint. San Diego, CA: ECO, Rotterdam, 1992.
43 Hendrix RW, Lin PJ, Kane WJ. Simplified aspiration or injection technique for the sacro-iliac joint. J Bone Joint Surg [Am]. 1982;64(8):1249-1252.
44 Dussault RG, Kaplan PA, Anderson MW. Fluoroscopy-guided sacroiliac joint injections. Radiology. 2000;214(1):273-277.
45 Yin W. Neuroanatomy of the dorsal sacral plexus: implications for a functional stereotactic approach towards sacro-iliac joint complex radiofrequency neurotomy. 8th Annual Scientific Meeting of the International Spinal Injection Society. San Fransisco, CA: International Spinal Injection Society, 2000.
46 Bollow M, et al. CT-guided intra-articular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: indication and follow-up with contrast-enhanced MRI. J Comput Assist Tomogr. 1996;20(4):512-521.
47 Braun J, et al. Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: clinical outcome and follow-up by dynamic magnetic resonance imaging. J Rheumatol. 1996;23(4):659-664.
48 Luukkainen R, et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondyloarthropathy. Clin Exp Rheumatol. 1999;17(1):88-90.
49 Maugars Y, et al. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondyloarthropathy. Arthritis Rheum. 1992;35(5):564-568.
50 Maugars Y, et al. Assessment of the efficacy of sacroiliac corticosteroid injections in spondyloarthropathies: a double-blind study. Br J Rheumatol. 1996;35(8):767-770.
51 Kline MT. Stereotactic radiofrequency lesions as part of the management of chronic pain. Orlando, FL: Paul M Deutsch, 1992;86.