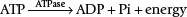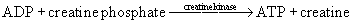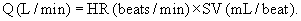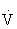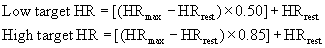Chapter 18 Therapeutic Exercise
General Principles
Regular physical activity is an important component of a healthy lifestyle. Increases in physical activity and cardiorespiratory fitness have been shown to reduce the risk for death from coronary heart disease as well as from all causes. The primary focus on achieving these health-related goals in the past has been on prescribing exercise to improve cardiorespiratory fitness, body composition, and strength. More recently the Centers for Disease Control and Prevention (CDC) and the American College of Sports Medicine (ACSM) suggested that the focus be broadened to address the needs of more sedentary individuals, especially those who cannot or will not engage in structured exercise programs. There is increasing evidence showing that regular participation in moderate-intensity physical activity is associated with health benefits, even when aerobic fitness remains unchanged. To reflect this evidence, the CDC and ACSM are now recommending that every adult in the United States accumulate 30 minutes or more of moderate-intensity physical activity on most, and preferably all, days of the week. Those who follow these recommendations can experience many of the health-related benefits of physical activity, and if they are interested are ready to achieve higher levels of fitness.44,45,108,121
Energy Systems
A 70-kg human has an energy expenditure at rest of about 1.2 kcal/min, with less than 20% of the resting energy expenditure attributed to skeletal muscle. During intense exercise, however, total energy expenditure can increase 15 to 25 times above resting values, resulting in a caloric expenditure between 18 and 30 kcal/min. Most of this increase is used to provide energy to the exercising muscles that can increase energy requirements by a factor of 200.26,103
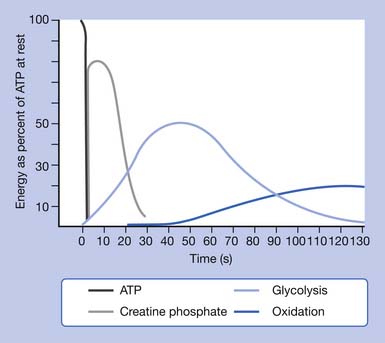
The limited stores of ATP in skeletal muscles can fuel approximately 5 to 10 seconds of high-intensity work (Figure 18-1). ATP must be continuously resynthesized from adenosine diphosphate (ADP) to allow exercise to continue.70,114 Muscle fibers contain three metabolic pathways for producing ATP: the creatine phosphate system, rapid glycolysis, and aerobic oxidation.26,103,108
Creatine Phosphate System
There is enough creatine phosphate stored in skeletal muscle for approximately 25 seconds of high-intensity work (see Figure 18-1). The ATP–creatine phosphate system lasts for about 30 seconds (5 seconds for the stored ATP and 25 seconds for creatine phosphate). This provides energy for activities such as sprinting and weightlifting. The creatine phosphate system is considered an anaerobic system, because oxygen is not required.26,103,108
Rapid Glycolysis (Lactic Acid System)
Anaerobic oxidation starts as soon as high-intensity exercise begins and dominates for approximately 1½ to 2 minutes (see Figure 18-1). It would fuel activities such as middle-distance sprints (400-, 600-, and 800-m runs) or events requiring sudden bursts of energy such as weightlifting.
Although glycolysis is considered an anaerobic pathway, it can readily participate in the aerobic metabolism when oxygen is available and is considered the first step in the aerobic metabolism of carbohydrates.26,103,108
Aerobic Oxidation System
The final metabolic pathway for ATP production combines two complex metabolic processes: the Krebs cycle and the electron transport chain. The aerobic oxydation system resides in the mitochondria. It is capable of using carbohydrates, fat, and small amounts of protein to produce energy (ATP) during exercise, through a process called oxidative phosphorylation. During exercise, this pathway uses oxygen to completely metabolize the carbohydrates to produce energy (ATP), leaving only carbon dioxide and water as byproducts. The aerobic oxidation system is complex and requires 2 to 3 minutes to adjust to a change in exercise intensity (see Figure 18-1). It has an almost unlimited ability to regenerate ATP, however, limited only by the amount of fuel and oxygen that is available to the cell. Maximal oxygen consumption, also known as 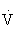 O2max, is a measure of the power of the aerobic energy system, and is generally regarded as the best indicator of aerobic fitness.26,103,108
O2max, is a measure of the power of the aerobic energy system, and is generally regarded as the best indicator of aerobic fitness.26,103,108
All the energy-producing pathways are active during most types of exercise, but different exercise types place greater demands on different pathways. The contribution of the anaerobic pathways (creatine phosphate system and glycolysis) to exercise energy metabolism is inversely related to the duration and intensity of the activity. The shorter and more intense the activity, the greater the contribution of anaerobic energy production, whereas the longer the activity and the lower the intensity, the greater the contribution of aerobic energy production. In general, carbohydrates are used as the primary fuel at the onset of exercise and during high-intensity work. But during prolonged exercise of low to moderate intensity (longer than 30 minutes), a gradual shift from carbohydrate toward an increasing reliance on fat as a substrate occurs. The greatest amount of fat use occurs at about 60% of maximal aerobic capacity (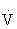 O2max).26,103,108
O2max).26,103,108
Cardiovascular Exercise
Cardiorespiratory Physiology
The cardiorespiratory system consists of the heart, lungs, and blood vessels. The purpose of this system is the delivery of oxygen and nutrients to the cells, as well as the removal of metabolic waste products to maintain the internal equilibrium.70,103,108
Cardiac Function
Heart Rate
Normal resting heart rate (HR) is approximately 60 to 80 beats/min. HR increases in a linear fashion with the work rate and oxygen uptake during exercise. The magnitude of HR response is related to age, body position, fitness, type of activity, the presence of heart disease, medications, blood volume, and environmental factors such as temperature and humidity. HR during maximal exercise can exceed 200 beats/min, depending on the person’s age and training state. With the onset of dynamic exercise, HR increases in proportion to the relative workload. The maximal HR (HRmax) decreases with age, and can be estimated in healthy men and women by using the following formula: HRmax = 220 – age. There is considerable variability in this estimation for any fixed age, with a standard deviation of ±10 beats/min.70,103,108
Stroke Volume
SV is also affected by body position, with SV being greater in the supine or prone position and lower in the upright position. Static exercise (weight training) can also cause a slight decrease in SV because of increased intrathoracic pressure.70,103,108
Cardiac Output
Resting cardiac output in both trained and sedentary individuals is approximately 4 to 5 L/min, but during exercise the maximal cardiac output can reach 20 L/min. Maximal cardiac output in an individual depends on many factors, including age, posture, body size, presence of cardiac disease, and physical conditioning. During dynamic exercise, cardiac output initially increases with increasing exercise intensity by increases in SV and HR. Increases in cardiac output initially beyond 40% to 50% of 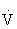 O2max, however, are accounted for only by increases in HR.70,103,108
O2max, however, are accounted for only by increases in HR.70,103,108
Blood Flow
At rest, 15% to 20% of the cardiac output is distributed to the skeletal muscles, with the remainder going to visceral organs, the brain, and the heart. During exercise, 85% to 90% of the cardiac output is selectively delivered to working muscles and shunted away from the skin and splanchnic vasculature. Myocardial blood flow can increase four to five times with exercise, whereas blood supply to the brain is maintained at resting levels. The difference between the oxygen content of arterial blood and the oxygen content of venous blood is termed the arteriovenous oxygen difference. It reflects the oxygen extracted from arterial blood by the tissues. The oxygen extraction at rest is approximately 25%, but at maximal exercise the oxygen extraction can reach 75%.70,103,108
Venous Return
Venous return is maintained or increased during exercise by the following mechanisms70,103,108:
Blood Pressure
Blood pressure is the driving force behind blood flow. Systolic blood pressure (SBP) is the maximal force of the blood against the walls of the arteries when cardiac muscle is contracting (systole). Normal resting SBP is less than 130 mm Hg. Diastolic blood pressure (DBP) is the force of the blood against the walls of the arteries when the heart is relaxing (diastole). Normal resting DBP is less than 85 mm Hg.103
SBP increases linearly with increasing work intensity, at 8 to 12 mm Hg per metabolic equivalent (MET) (where 1 MET = 3.5 mL of O2 per kilogram per minute). Maximal values typically reach 190 to 220 mm Hg. Maximal SBP should not be greater than 260 mm Hg. DBP remains unchanged or only slightly increases with exercise.70,103
Because blood pressure is directly related to cardiac output and peripheral vascular resistance, it provides a noninvasive way to monitor the inotropic performance (pumping capacity) of the heart. Failure of SBP to rise, decreased SBP with increasing work rates, or a significant increase in DBP are all abnormal responses to exercise, and indicate either severe exercise intolerance or underlying cardiovascular disease.70,103,108
Postural Considerations
In the supine position, gravity has less effect on return of blood to the heart, so the SBP is lower. When the body is upright, gravity works against the return of blood to the heart, so SBP increases. DBP does not change significantly with body position in healthy individuals.48,101,108
Effects of Arm Versus Leg Exercise
At similar oxygen consumptions, HR, SBP, and DBP are higher during arm work than during leg work. This is primarily because the total muscle mass in the arms is smaller, and consequently a greater percentage of the available mass is recruited to perform the work. In addition, arm work is less mechanically efficient than leg work.70,103,108
Pulmonary Ventilation
Pulmonary ventilation (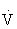 e) is the volume of air exchanged per minute, and generally is approximately 6 L/min at rest in an average sedentary adult man. However, at maximal exercise,
e) is the volume of air exchanged per minute, and generally is approximately 6 L/min at rest in an average sedentary adult man. However, at maximal exercise, 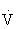 e increases 15- to 25-fold over resting values. During mild to moderate exercise,
e increases 15- to 25-fold over resting values. During mild to moderate exercise, 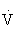 e increases primarily by increasing tidal volume, but during vigorous activity it increases by increasing the respiratory rate.48,101
e increases primarily by increasing tidal volume, but during vigorous activity it increases by increasing the respiratory rate.48,101
Increases in 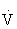 e are generally directly proportional to an increase in oxygen consumption (
e are generally directly proportional to an increase in oxygen consumption (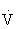 O2) and carbon dioxide that is produced (
O2) and carbon dioxide that is produced (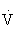 CO2). At a critical exercise intensity (usually 47% to 64% of the
CO2). At a critical exercise intensity (usually 47% to 64% of the 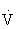 O2max in healthy untrained individuals and 70% to 90%
O2max in healthy untrained individuals and 70% to 90% 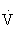 O2max in highly trained individuals), however,
O2max in highly trained individuals), however, 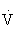 e increases disproportionately relative to the
e increases disproportionately relative to the 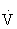 O2 (paralleling an abrupt increase in serum lactate and
O2 (paralleling an abrupt increase in serum lactate and 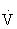 CO2). This is called the anaerobic (ventilatory) threshold.48,101,108
CO2). This is called the anaerobic (ventilatory) threshold.48,101,108
The Anaerobic Threshold
The anaerobic threshold signifies the onset of metabolic acidosis during exercise, and traditionally has been determined by serial measurements of blood lactate. It can be noninvasively determined by assessment of expired gases during exercise testing, specifically 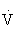 e and carbon dioxide production (
e and carbon dioxide production (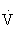 CO2). The anaerobic threshold signifies the peak work rate or oxygen consumption at which the energy demands exceed the circulatory ability to sustain aerobic metabolism.48,101,108
CO2). The anaerobic threshold signifies the peak work rate or oxygen consumption at which the energy demands exceed the circulatory ability to sustain aerobic metabolism.48,101,108
Maximal Oxygen Consumption
The resting oxygen consumption of an individual (250 mL/min) divided by body weight (70 kg) gives the resting energy requirement, 1 MET (about 3.5 mL/kg per minute). Multiples of this value are used to quantify levels of energy expenditure. For example, running a 6-mph pace requires 10 times the resting energy expenditure, giving an aerobic cost of 10 METs, or 35 mL/kg per minute. Because there is little variation in HRmax and maximal systemic arteriovenous oxygen difference with physical training, 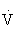 O2max virtually defines the pumping capacity of the heart. When expressed as milliliters of oxygen per kilogram of body weight per minute (mL/kg per minute) or in METs, it is considered the best index of physical work capacity or cardiorespiratory fitness.48,101,108
O2max virtually defines the pumping capacity of the heart. When expressed as milliliters of oxygen per kilogram of body weight per minute (mL/kg per minute) or in METs, it is considered the best index of physical work capacity or cardiorespiratory fitness.48,101,108
Oxygen Pulse
The oxygen pulse (mL/beat) is the ratio of 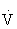 O2 (mL/min) to HR (beats/min), when both measures are obtained simultaneously. Oxygen pulse increases with increasing work effort. A low value during exercise indicates an excessive HR for workload and can be an indicator of heart disease.29
O2 (mL/min) to HR (beats/min), when both measures are obtained simultaneously. Oxygen pulse increases with increasing work effort. A low value during exercise indicates an excessive HR for workload and can be an indicator of heart disease.29
Respiratory Quotient and Respiratory Exchange Ratio
The respiratory exchange ratio (RER) reflects pulmonary exchange of CO2 and O2 at rest and during exercise. The RER also ranges between 0.7 and 1.0 during rest, and can also reflect substrate preference. During strenuous exercise, however, the RER can exceed 1.0 because of increasing metabolic activity not matched by 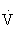 O2 and additional CO2 derived from bicarbonate buffering of lactic acid. The terms RQ and RER are often used interchangeably, but their distinction is important.29
O2 and additional CO2 derived from bicarbonate buffering of lactic acid. The terms RQ and RER are often used interchangeably, but their distinction is important.29
Effects of Exercise Training
Cardiovascular System
The effects of regular exercise on cardiovascular activity can be grouped into changes that occur at rest, during submaximal exercise, and during maximal work (Box 18-1).103,108 Regular exercise can also affect a number of physiologic parameters (Box 18-2).
BOX 18-1 Effects of Regular Exercise on Cardiovascular Activity
Changes at Rest
Changes at Submaximal Work∗
BOX 18-2 Physiologic Changes After a Regular Exercise Program
Energy System Changes
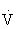 O2max. It increases muscle glycogen and triglyceride stores, as well as the rate at which carbohydrates and fat are metabolized.103,108
O2max. It increases muscle glycogen and triglyceride stores, as well as the rate at which carbohydrates and fat are metabolized.103,108ATP, Adenosine triphosphate; 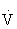 O2max, maximal oxygen consumption.
O2max, maximal oxygen consumption.
Detraining
The changes induced by regular exercise training generally are lost after 4 to 8 weeks of detraining. If training is re-established, the rate at which the training effects occur does not appear to be faster.103,108
Overtraining
Overtraining fatigue syndrome presents as a prolonged decreased sport-specific performance, usually lasting greater than 2 weeks. It is characterized by premature fatigability, emotional and mood changes, lack of motivation, infections, and overuse injuries. Recovery is markedly longer and variable among affected athletes, sometimes taking months before the athlete returns to baseline performance (Box 18-3).103,108
Exercise Prescriptions
Exercise prescriptions are designed to enhance physical fitness, promote health by reducing risk factors for chronic disease, and ensure safety during exercise participation. The fundamental objective of the prescription is to bring about a change in personal health behavior to include habitual physical activity. The optimal exercise prescription for an individual is determined from an objective evaluation of that individual’s response to exercise, including observations of HR, blood pressure, rating of perceived exertion (RPE) to exercise, electrocardiogram when appropriate, and 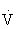 O2max measured directly or estimated during a graded exercise test. The exercise prescription should be developed with careful consideration of the individual’s health status, medications, risk factor profile, behavioral characteristics, personal goals, and exercise preferences.45,108,121
O2max measured directly or estimated during a graded exercise test. The exercise prescription should be developed with careful consideration of the individual’s health status, medications, risk factor profile, behavioral characteristics, personal goals, and exercise preferences.45,108,121
Components of an Exercise Prescription
Exercise Prescription for Cardiorespiratory Endurance
Cardiorespiratory endurance is the ability to take in, deliver, and use oxygen. It is dependent on the function of the cardiorespiratory system (heart and lungs) and the cellular metabolic capacities. The degree of improvement that can be expected in cardiorespiratory fitness is directly related to the frequency, intensity, duration, and mode of exercise. 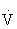 O2max can increase between 5% and 30% with training. It has become apparent recently, however, that the level of physical activity necessary to achieve the majority of health benefits is less than that needed to attain a high level of cardiorespiratory fitness.45,108,121
O2max can increase between 5% and 30% with training. It has become apparent recently, however, that the level of physical activity necessary to achieve the majority of health benefits is less than that needed to attain a high level of cardiorespiratory fitness.45,108,121
ACSM Recommendations for Cardiorespiratory Endurance Training
Mode
Intensity
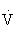 O2max that noticeably increases HR and breathing).
O2max that noticeably increases HR and breathing).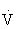 O2max that results in substantial increases in HR and breathing) is ideal for the attainment of improvements in health and fitness in most adults.45,108,121
O2max that results in substantial increases in HR and breathing) is ideal for the attainment of improvements in health and fitness in most adults.45,108,121Calculating Intensity
Because of limitations in using 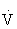 O2 calculations for prescribing intensity, the most common methods of setting the intensity of exercise to improve or maintain cardiorespiratory fitness use HR and RPE.45,100,108
O2 calculations for prescribing intensity, the most common methods of setting the intensity of exercise to improve or maintain cardiorespiratory fitness use HR and RPE.45,100,108
Heart Rate Methods
Heart rate is used as a guide to set exercise intensity, because of the relatively linear relationship between HR and percentage of 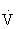 O2max. It is best to measure HRmax during a progressive exercise test whenever possible, because HRmax declines with age. HRmax can be estimated by using the following equation: HRmax = 220 – age. This estimation has significant variance, with a standard deviation of 10 beats/min.45,100,108,121
O2max. It is best to measure HRmax during a progressive exercise test whenever possible, because HRmax declines with age. HRmax can be estimated by using the following equation: HRmax = 220 – age. This estimation has significant variance, with a standard deviation of 10 beats/min.45,100,108,121
Maximal heart rate method
One of the oldest methods of setting the target HR range uses a straight percentage of the HRmax. Using 70% to 85% of an individual’s HRmax approximates 55% to 75% of 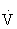 O2max and provides the stimulus needed to improve or maintain cardiorespiratory fitness.45,100,108,121 For example, if the HRmax is 180 beats/min, then the target HR (70% to 85% of HRmax) would range from 126 to 153 beats/min. (See also Chapter 33.)
O2max and provides the stimulus needed to improve or maintain cardiorespiratory fitness.45,100,108,121 For example, if the HRmax is 180 beats/min, then the target HR (70% to 85% of HRmax) would range from 126 to 153 beats/min. (See also Chapter 33.)
Heart rate reserve method
The small but systematic differences between the two HR methods occur because the percentage HRmax is 55% to 75% of 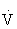 O2max, whereas the percentage HRRmax is 60% to 80% of
O2max, whereas the percentage HRRmax is 60% to 80% of 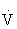 O2max. Either method can be used to approximate the range of exercise intensities known to increase or maintain cardiorespiratory fitness or
O2max. Either method can be used to approximate the range of exercise intensities known to increase or maintain cardiorespiratory fitness or 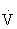 O2max.45,108,121
O2max.45,108,121
Rating of Perceived Exertion
The RPE is a subjective grading of how hard individuals feel they are exercising. Use of RPE is considered an adjunct to monitoring HR. It has proven to be a valuable aid in prescribing exercise for individuals who have difficulty with HR palpation, and in cases where the HR response to exercise may have been altered because of a change in medication. The most commonly used scale of perceived exertion is the Borg Scale (see Table 18-1). The average RPE range associated with physiologic adaptation to exercise is 13 to 16 (“somewhat hard” to “hard”) on the Borg Scale category. One should suit the RPE to the individual on a specific mode of exercise, and not expect an exact matching of the RPE to a percentage HRmax or percentage HRR. It should be used only as a guideline in setting the exercise intensity.45,94,100,108,121
The appropriate exercise intensity is one that is safe and compatible with a long-term active lifestyle for that individual and achieves the desired outcome given the time constraints of the exercise session. The ACSM recommends an intensity that will elicit an RPE within a range of 12 to 16 on the original 6 to 20 Borg Scale (Table 18-1).
| Level | Perceived Exertion |
|---|---|
| 6 | — |
| 7 | Very, very light |
| 8 | |
| 9 | Fairly light |
| 10 | |
| 11 | |
| 12 | |
| 13 | Somewhat hard |
| 14 | |
| 15 | Hard |
| 16 | |
| 17 | Very hard |
| 18 | |
| 19 | Very, very hard |
| 20 |
Duration
Frequency
Medical Clearance
Exercise training might not be appropriate for everyone. Patients whose adaptive reserves are severely limited by disease processes might not be able to adapt to or benefit from exercise. In this small subpopulation of people with severe or unstable cardiac, respiratory, metabolic, systemic, or musculoskeletal disease, exercise programming can be fatal, injurious, or simply not beneficial, depending on the clinical status and condition of the individual.46,121
The recommended level of screening before beginning or increasing an exercise program depends on the risk for the individual and the intensity of the planned physical activity. For individuals planning to engage in low- to moderate-intensity activities, the Physical Activities Readiness Questionnaire (PAR-Q) (Box 18-4) should be considered the minimal level of screening. The PAR-Q was designed to identify the small number of adults for whom physical activity might be inappropriate or those who should receive medical advice concerning the most suitable type of activity.46,108,121
BOX 18-4 Physical Activity Readiness Questionnaire∗
Preexercise Evaluation
Identification of Those Who Need an Exercise Stress Test
Indications for an exercise stress test according to the American College of Cardiology and American Heart Association are as follows114:
The ACSM guidelines are summarized in Box 18-5 and Tables 18-2 and 18-3.78,121 Contraindications to exercise testing are listed in Box 18-6.
BOX 18-5 Major Symptoms or Signs Suggestive of Cardiopulmonary Disease78, 121
From Kenny W, Humphrey R, Bryant C: ACSM’s guidelines for exercise testing and prescription, ed 5, Philadelphia, 1995, Williams & Wilkins, 1995, with permission of Williams & Wilkins.
Table 18-2 American College of Sports Medicine Recommendations for Medical Examination and Exercise Testing Before Participation and for Physician Supervision of Exercise Tests
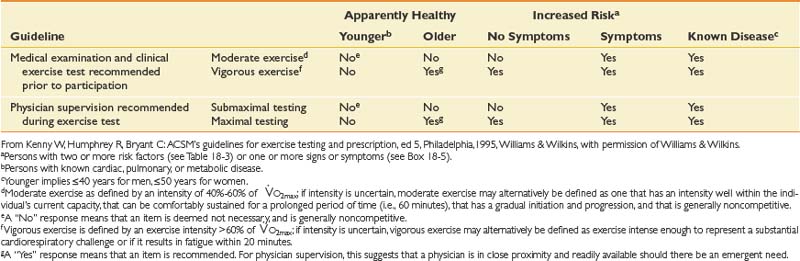
Table 18.3 Coronary Artery Disease Risk Factor Thresholds for Use With ACSM Risk Stratification
| Risk Factors | Defining Criteria |
|---|---|
| Positive | |
| Family history | Myocardial infarction, coronary revascularization, or sudden death before 55 years of age in father or other male first-degree relative (i.e., brother or son) or before 65 years of age in mother or other female first-degree relative(i.e., sister or daughter) |
| Cigarette smoking | Current cigarette smoker or those who quit within the previous 6 months |
| Hypertension | Systolic blood pressure of ≥140 mm Hg or diastolic ≥90 mm Hg, confirmed by measurements on at least two separate occasions, or on antihypertensive medication |
| Hypercholesterolemia | Total serum cholesterol of >200 mg dL (5.2 mmoL/L) or high-density lipoprotein cholesterol of <35 mg/dL (0.9 mmoL/L) or on lipid-lowering medication. If low-density lipoprotein cholesterol is available, use >130 mg/dL (3.4 mmoL/L) rather than total cholesterol of >200 mg/dL |
| Impaired fasting glucose | Fasting blood glucose of ≥110 mg/dL (6.1 mmoL/L) confirmed by measurements on at least two separate occasions |
| Obesity∗ | Body mass index of ≥30 kg · m–2, or waist girth of >100cm |
| Sedentary lifestyle | Persons not participating in a regular exercise program or meeting the minimal physical activity recommendations in the U.S. Suregeon General’s report |
| Negative | |
| High serum HDL cholesterol† | >60 mg/dL (1.6 mmoL/L) |
∗ Professional opinions vary regarding the most appropriate markers and thresholds for obesity; therefore exercise professionals should use clinical judgment when evaluating this risk factor.
† Accumulating 30 minutes or more of moderate physical activity on most days of the week. It is common to sum risk factors in making clinical judgments. If high-density lipoprotein (HDL) cholesterol is high, subtract one risk factor from the sum of positive risk factors because high HDL decreases coronary artery disease (CAD) risk.3
Modified from Glass SC: Health appraisal and fitness testing in Bibi KW, Niederproein MG (eds) ACSM’s Certification review, 3rd ed, Philadelphia, 2010, Lippincott Williams & Wilkins.
BOX 18-6 Contraindications to Exercise Testing47, 121
Absolute
Muscle Physiology
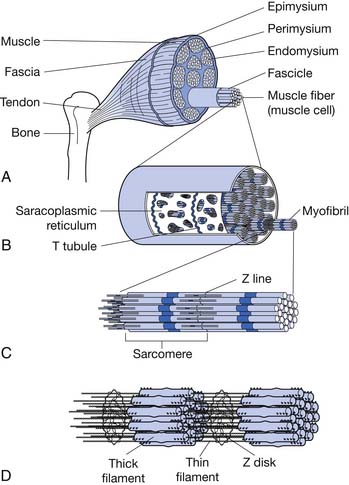
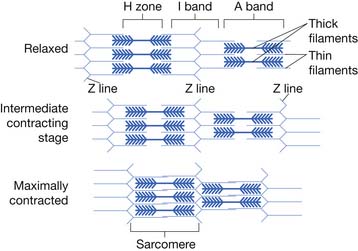
Each skeletal muscle is made of many muscle fibers, which range in diameter between 10 and 80 μm. Each muscle fiber, in turn, contains hundreds to thousands of myofibrils. Each myofibril comprises about 1500 myosin (thick) filaments and 3000 actin (thin) filaments, which are responsible for muscle contraction (Figure 18-2).61,120
Myosin and actin filaments partially interdigitate, causing myofibrils to have alternate light and dark bands. The light bands contain only actin filaments and are called I bands (because they are isotropic to polarized light). Dark bands contain myosin as well as the ends of the actin filaments where they overlap the myosin, and are called A bands (because they are anisotropic to polarized light). Small projections, called cross-bridges, protrude from the surface of myosin filaments along their entire length, except in the very center. The interaction between the myosin cross-bridge and the actin filaments results in contraction.61,120
The ends of actin filaments are attached to Z disks. From the Z disk, actin filaments extend in either direction, interdigitating with the myosin filaments. The Z disk passes from myofibril to myofibril, attaching the myofibrils across the muscle fiber. Thus the entire muscle fiber has light and dark bands, as do individual myofibrils, and thus the striated appearance of the muscle fiber.61,120
The portion of a myofibril or the whole muscle fiber between two Z disks is called a sarcomere. The myofibrils within the muscle fibers are suspended in a matrix called sarcoplasm. The sarcoplasm contains potassium, magnesium, phosphate, enzymes, and mitochondria. The sarcoplasm also contains the sarcoplasmic reticulum, an extensive endoplasmic reticulum important in the control of muscle contraction.57,120
Physiology of Muscle Contraction
Sliding Filament Mechanism
Muscle contraction occurs by a sliding filament mechanism. In the relaxed state the ends of actin filaments derived from two successive Z disks barely overlap each other, while at the same time completely overlapping the myosin filaments. In the contracted state the actin filaments overlap each other to a great extent, and the Z disks are pulled up to the end of the myosin filaments (Figure 18-3).
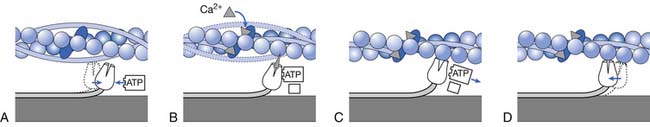
The muscle contraction is initiated by the release of acetylcholine from the motor nerve. Acetylcholine opens protein channels in the muscle fiber membrane, allowing sodium to flow into the muscle fiber membrane and initiating a muscle action potential. The action potential depolarizes the muscle fiber membrane, causing the sarcoplasmic reticulum to release calcium. Calcium, in turn, generates attraction between actin and myosin cross-bridges, causing them to slide together.61,71,120
Molecular Characteristics of the Contractile Filaments
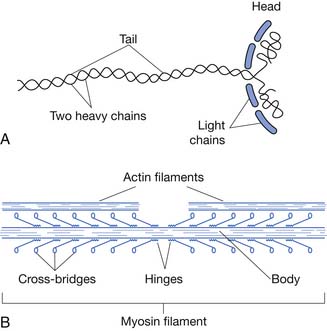
Each myosin filament is composed of 200 or more myosin molecules. Each myosin molecule is composed of six polypeptide chains: two heavy chains and four light chains. The two heavy chains are wrapped around each other to form a double helix, the tail and arm of the myosin molecule. One end of each of the chains is folded into a globular mass called the myosin head. Therefore two myosin heads are lying side by side. The four light chains are also parts of the myosin heads, two to each head (Figure 18-4).
The tails of myosin molecules are bonded together, forming the body of the myosin filament. Protruding from the body, the arm and heads of the myosin molecules are called cross-bridges, which are flexible at two points called hinges. In addition to serving as a component of the cross-bridge, the myosin head also functions as adenosine triphosphatase (ATPase), allowing the head to cleave ATP and energize contraction (Figure 18-5).
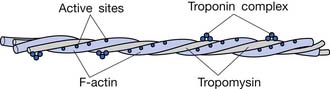
Actin filaments are composed of three protein components: actin, tropomyosin, and troponin (Figure 18-6). Several G-actin molecules form strands of F-actin. Two F-actin strands are then wound in a double helix. One molecule of ADP is attached to each G-actin molecule. These ADP molecules represent the active sites of the actin filaments with which myosin cross-bridges interact to cause muscle contraction.
In the resting state the troponin-tropomyosin complex is thought to cover the active sites of actin, inhibiting contraction. In the presence of calcium, this inhibitory effect is removed, allowing contraction to proceed.61,120
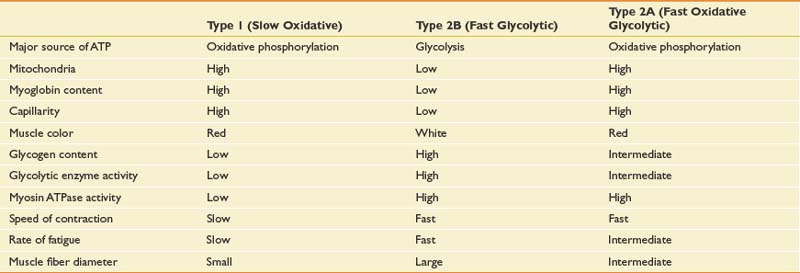
Muscle Fiber Types
Muscle fibers can be characterized based on their speed of contraction or twitch. Type 1 fibers (slow oxidative) are best suited for endurance activities requiring aerobic metabolism. Type 2 fibers (fast twitch) are most active during activities requiring strength and speed. Type 2 fibers are further categorized into type 2A (fast, oxidative glycolytic) and type 2B (fast glycolytic). Type 2A represents a type of hybrid that retains some oxidative capacity. Features of each muscle type are summarized in Table 18-4.
During isometric contractions, type 2 fibers are generally recruited when force exceeds 20% of the maximal voluntary contraction. If sustained for long periods, however, type 2 units can be recruited at thresholds below 20% of maximal voluntary contraction.24,57,120
Types of Muscle Contraction
Isometric contractions are contractions in which there is no change in the length of the muscle. No joint or limb motion occurs. Isotonic contractions occur when the muscle changes length, producing limb motion. Concentric contractions occur when the muscle shortens. Eccentric contractions occur when the muscle lengthens. More fast-twitch fibers are recruited during eccentric contractions. Isokinetic contractions occur when muscle contraction is performed at a constant velocity. This can be done only with the assistance of a preset rate-limiting device. This type of exercise does not exist in nature.
Factors Affecting Muscle Strength and Performance
Determinants of Strength
A muscle’s ability to produce force is directly proportional to its cross-sectional area. For parallel muscles, this corresponds to the cross-section at the bulkiest part of the muscle. For pennate muscles, multiple cross-sections are taken at right angles to each of the muscle fibers. Pennate muscles are particularly adapted to force production because many more muscle fibers are contained in pennate muscles and these fibers are shorter.78
Length-Tension Relationship
Efficiency (percentage of energy that is converted into work instead of heat) occurs at a velocity of contraction of about 30% of maximum.23,24
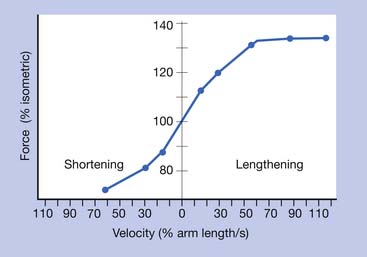
Torque-Velocity Relationship
The greatest amount of force is generated by a muscle during fast eccentric (lengthening) contractions.23,24 The least amount of force is produced during fast concentric (shortening) contractions. The amount of force developed in the order of most force to least force can be summarized as follows: fast eccentric, isometric, slow concentric, and fast concentric.(Figure 18-7).
Effects of Exercise Training
Neural Adaptations
Observed strength gains within the first few weeks of a weightlifting program are mostly because of neuromuscular adaptations. The nervous system recruits larger motor units with higher frequencies of stimulation to provide the force necessary to overcome the imposed resistance. Early strength gains and increased muscle tension production from training therefore result from a more efficient neural recruitment process. This means that most of the improvement in strength-related functional activities gained on inpatient rehabilitation units are due to neural recruitment rather than muscle hypertrophy, due to the relatively short length of stays.82
Muscle Hypertrophy
Muscle hypertrophy represents enlargement of total muscle mass and cross-sectional area. Muscle hypertrophy is more common in fast-twitch than in slow-twitch muscles. Type 2A fibers exhibit the greatest growth, more so than type 2B and type 1 fibers. Muscle hypertrophy is typically experienced after 6 to 7 weeks of resistance training.28,65 Conversely, muscle atrophy resulting from disuse occurs primarily in type 2 fibers.
Virtually all muscle hypertrophy occurs from hypertrophy of the individual muscle fibers. During muscle hypertrophy the rate of muscle contractile protein synthesis is greater than decay, leading to greater numbers of actin and myosin filaments in the myofibrils. The myofibrils within each muscle fiber split, resulting in more myofibrils in each muscle fiber. Only under very rare conditions of extreme muscle force generation do the numbers of muscle fibers increase (fiber hyperplasia), and even then by only a few percent.24,61
Another type of muscle hypertrophy occurs when muscles are stretched to a greater-than-normal length, causing new sarcomeres to be added at the ends of muscle fibers where they attach to the tendons. Conversely, when a muscle remains shortened at less than its resting length, sarcomeres at the end of the muscle fibers disappear.22,24
Exercise Prescription
Progressive Resistance Exercise
DeLorme
In the DeLorme method, three sets are performed for each exercise. Ten repetitions are performed in each set. The weight for the first set is 50% of the 10 RM; the second set, 75% of the 10 RM; and the third set, 100% of the 10 RM.25 This is usually referred to clinically as progressive resistive exercise.
Daily Adjusted Progressive Resistance Exercise
The DAPRE method of strength training guides the athlete through four sets of exercise per muscle group. The first set in DAPRE involves 10 repetitions at 50% of the athlete’s predetermined 6 RM. The second set consists of six repetitions at 75% of the athlete’s 6 RM. The third set consists of as many repetitions as can be performed at the athlete’s 6 RM. The number of repetitions performed in the third set determines the resistance for the fourth set. If five to seven repetitions were performed in the third set, resistance stays the same. If fewer than five repetitions were performed in the third set, the weight is lowered by 5 lb. If more than seven repetitions are performed in the third set, the weight is raised by 5 lb. The fourth set consists of as many repetitions as can be performed to fatigue. The working weight for the next day is established based on the athlete’s performance during the fourth set, using the same formula for determining resistance change from the third to the fourth set.65,81
Increasing Number of Repetitions or Rate
Hellebrandt and Houtz67 advocate increasing the rate of contraction (controlled by a metronome) each session while lifting the same number of repetitions (10 to 20).24
Effects of Aging
Although it was previously believed that strength training in the elderly was due only to learning or neural factors,92 reports have also demonstrated that the muscles of older persons can demonstrate hypertrophy after strength training.49,78
Exercise for Fat Reduction
The threshold for change in body weight appears to be 30 minutes of exercise per day, with more marked losses noted with 60 min/day. Land-based exercise such as cycling and walking appear effective choices. Swimming in the absence of caloric restriction did not appear to result in weight loss. In fact, subjects gained weight, although this appeared to be all muscle weight, thus increasing lean body mass. It stands to reason, however, that swimming can be effective in weight loss if combined with caloric restriction.24,62
ACSM Guidelines for Prescription of Strength-Training Exercise
Recommended guidelines for strength training are listed in Table 18-5.
| Component | Details |
|---|---|
| Mode | Perform a minimum of 8-10 exercises that train the major muscle groups. |
| Intensity | One set of 8-12 repetitions resulting in volitional fatigue for each exercise∗ |
| Duration | The entire program should last no more that 1 hour. Programs lasting greater than 1 hour are associated with a higher dropout rate. |
| Frequency | At least 2 days per week∗ |
∗ Although more frequent training with additional sets or repetitions might elicit additional strength gains, the additional improvement is considered relatively small.75
Flexibility
Flexibility generally describes the range of motion commonly present in a joint or group of joints that allows normal and unimpaired function.17 More specifically, flexibility has been defined as “the total achievable excursion (within limits of pain) of a body part through its range of motion.”104 With regard to flexibility, the following generalizations can be made. Flexibility is an individually variable, joint-specific, inherited characteristic that decreases with age; varies by gender and ethnic group; bears little relationship with body proportion or limb length; and most importantly for the purposes of this chapter, can be acquired through training.17,52,58–60,126
From a developmental standpoint, flexibility is greatest during infancy and early childhood. Flexibility reaches minimal levels between 10 and 12 years of age. It then improves again toward early adulthood, but not sufficiently to allow ranges of motion seen in childhood.33 The early adolescent growth spurt results in short-term tightness of the joints, probably as a result of increased tension in the connective tissue. Girls are generally more flexible than boys,80,97,102 and this advantage probably persists into adulthood.
Achieving a maximal functional range of motion is an important goal of many therapeutic exercise regimens. Most typically, increased range of motion is achieved via the process of stretching. The term stretching defines an activity that applies a deforming force along the rotational or translational planes of motion of a joint.104 Stretching should respect the lines of geometry of the joint as well as its planes of stability. In addition to stretching, mobilization is used to maintain flexibility. Mobilization moves a joint through its range of motion without applying a deforming force.
Importance of Flexibility
Flexibility has only recently been identified as an important component of therapeutic exercise. Cureton21 emphasized flexibility as an important component of physical fitness after working with swimmers during the 1932 Olympic Games. Later, Kraus84 stressed the importance of flexibility in preventing low back pain. His work inspired much of the subsequent research on flexibility. It was not until 1964 that Fleishman42 proved flexibility to be an independent factor in physical fitness that was unrelated to other factors, including strength, power, endurance, and coordination. During the same decade and in another landmark work, DeVries and Housh32 proved the value of passive stretching in improving flexibility and range of motion. Subsequent study has demonstrated the value of flexibility training for patients in the industrial and athletic settings, for patients with back pain, and for patients who are status post orthopedic surgical procedures.
Subsequent to Kraus’s work, biomechanical studies have shown that lower limb flexibility is needed for the prevention of lumbar spine injuries.40 An increased frequency of spondylolysis and spondylolisthesis in subjects with severe hamstring inflexibility has been reported.96 Cady et al.12 demonstrated an inverse relationship between flexibility and the incidence of back injuries and workers’ compensation costs in a cohort study of firefighters placed on a fitness program.
The work of Salter and associates79,106,127 has emphasized the importance of maintaining motion and flexibility postoperatively in patients who have undergone orthopedic procedures. The benefits of the mobilization of postoperative joints to the surrounding ligamentous and musculotendinous structures have been well established.
In the realm of athletic training, flexibility has been both extensively applied and extensively studied. The proposed benefits of flexibility to athletes include injury prevention, reduced muscle soreness, skill enhancement, and muscle relaxation.∗ With regard to injury prevention, muscles possessing greater extensibility are less likely to be overstretched during athletic activity, lessening the likelihood of injury. A 1987 review of all studies of soccer injuries suggested an important role for flexibility in the prevention of injury, and attributed up to 11% of all injuries to poor flexibility.77 A prospective study of a flexibility program in soccer players demonstrated a correlation between improvement in range of motion and reduction in incidence of muscle tears.38 There is some evidence that delayed muscular soreness can be prevented and treated by static stretching.30,31
Flexibility has generally been hypothesized to improve athletic performance through skill enhancement. For example, mastery of the serve in tennis requires sufficient shoulder flexibility. Similarly, proficient golf skills require flexibility throughout the hips, trunk, and shoulders.16 On the biomechanical level, prestretching a muscle has been shown in several studies to enhance the force of muscle contraction.8,9,13,14
There is, however, considerable uncertainty regarding two of the most important proposed benefits of flexibility training for athletes: prevention of injury and improvement of performance. Although currently held teaching states that stretching is a preventive measure for athletic injury, it has been pointed out that little conclusive epidemiologic evidence supports this idea.63,66 In fact, it has been proposed that a certain degree of tightness might protect against injury by allowing load sharing when joints are stressed.66 Hypermobility or excessive stretching could theoretically result in increased stress on the ligaments, bone, and cartilage at the joint, resulting in injury or arthritis.58,93 In support of this is the fact that there is general agreement that the major predictive factor for joint injury is a previous joint injury or indeed the presence of excessive joint laxity, rather than inadequate flexibility.∗ Presently the role of preexercise stretching in the prevention of sports-related injury is unclear. A systematic review performed by Thacker et al.119 for the ACSM failed to find “sufficient evidence to endorse or discontinue routine stretching before or after exercise to prevent injury among competitive or recreational athletes.”
With regard to athletic performance, several laboratories have shown that among runners, less flexible individuals have a lower rate of oxygen consumption while covering the same distance at the same speed as their more flexible cohorts.18 In addition, the aforementioned improvement in contraction strength resulting from prestretching has not been consistently observed in the world of athletics. In fact, it has been shown repeatedly that passive stretching can result in an acute loss of strength.† Along the same lines, a recent study of elite female soccer players demonstrated that static stretching before sprinting resulted in worsened performance.107 Prior stretching does appear to have one reproducible benefit with regard to performance, however: maintenance of strength with the muscle in a lengthened position during and after eccentric exercise.91 This benefit might be important in resisting injurious muscle elongation during continued sport performance.91
Determinants of Flexibility
The determinants of joint mobility can be subdivided into static and dynamic factors. Static factors include the types of tissues involved, the types and state of collagen subunits in the tissue, the presence or absence of inflammation, and the temperature of the tissue. Dynamic factors include neuromuscular variables such as voluntary muscle control and the length-tension “thermostat” of the musculotendinous unit, as well as external factors such as pain associated with injury.104
Static Factors
The most important tissue with regard to flexibility is the muscle-tendon unit, which is the primary target of flexibility training.104 This structure includes the full length of the muscle and its supporting tissue, the musculotendinous junction, and the full length of the tendon to the tendon-bone junction. Within the muscle-tendon unit, it is the muscle that has the largest capacity for percent lengthening72,115,116 of the tissues involved in a stretch. A ratio of 95% to 5% for the muscle-to-tendon length change has been demonstrated.116
From a mechanical standpoint, muscle is composed of contractile and elastic elements arranged in parallel.61 Muscle can respond to an applied force or stretch with permanent elongation. Animal studies have shown that this results from an increase in the number of sarcomeres, which translates to increased peak tension of a muscle at longer resting lengths. By contrast, muscle at rest has a tendency to shorten because of its contractile element. This shortening can be permanent and is associated with a reduction in sarcomeres.56,58,128 Tendon has a much more limited capacity for lengthening than muscle, probably because of its proteoglycan content and collagen cross-links (2% to 3% of its length, compared with 20% for muscle).115,116,130 Of the external static factors, temperature has been studied the most. Warmer tissues are generally more distensible than cold ones.36,124,126
Dynamic Factors
An additional complicating factor is that receptors in the musculotendinous unit called the Golgi tendon organs act to inhibit muscle contraction at the point of critical stresses to the structure. The Golgi tendon organs allow lengthening and facilitate relaxation. When acting in conjunction, these dynamic mechanisms facilitate a response to a stretch in the following way. As the muscle spindle is initially stretched, it sends impulses to the spinal cord that result in reflex muscle contraction. If the stretch is maintained longer than 6 seconds, the Golgi tendon organ fires, causing relaxation.104
The relative contribution of static muscle factors and dynamic neural factors to flexibility remains somewhat controversial. It seems clear that the changes in flexibility noted immediately after the institution of a stretching program occur too rapidly to be attributable solely to structural alteration of the muscle and connective tissue. The consensus view is that neural factors probably play the major role in this early flexibility. After prolonged periods of training, changes in sarcomere number can play a role in the establishment of a new elongated muscle length.104
Assessment of Flexibility
Flexibility is generally assessed in terms of joint range of motion. Joint range of motion in turn is generally assessed with a goniometer or similar device. A goniometer consists of a 180-degree protractor designed for easy application to joints. The methods used when using a goniometer, as well as the normal ranges of motion encountered with these methods, are well standardized.37,99 Interobserver and intraobserver reliability are good.35 Limitations of the standard goniometer include application to only single joints at a given time, static measurements only, and difficulty of application to certain joints (e.g., costoclavicular).
The Leighton Flexometer contains a rotating circular dial marked in degrees and a pointer counterbalanced to remain vertical. It can be strapped to a body segment, and range of motion is determined with respect to the perpendicular. Its reliability is good but is not quite equivalent to that of the standard goniometer.64
With regard to measuring trunk flexibility, goniometric devices are generally considered inadequate. The Schober test, originally designed to measure spinal flexion and extension in patients with ankylosing spondylitis, is commonly used, as modified by Moll and Wright. Two marks are made along the proximal and distal ends of the lumbar spine, and tape measurements are made between them with the spine in flexion, neutral, and extension. This test has been shown to be more reliable than other methods, including fingertip to floor measurements and the Loebl inclinometer technique. “Eyeball” measurements show marked variability. These tests of trunk flexibility are all nonspecific, and each is limited to a gross measurement of compound motion of the entire thoracolumbar spine. None of these methods can assess articular mobility in the translational and rotational planes.104 The optimal measurement of trunk flexibility is probably that obtained with plain films, but these have the obvious disadvantages of cost and radiation exposure.
Methods of Stretching
It is important to take several factors into consideration when using a stretching program. Prevention of injury and treatment of specific joint injury, as well as the presence and effects of pain or muscle spasm, require modification of the program. Stretching can be dangerous, and might result in significant injury if performed incorrectly.105,109,110 As with any form of therapeutic exercise, flexibility training must be approached within a program aimed at addressing the specific functional needs of the individual.
Ballistic
Ballistic stretching uses the repetitive rapid application of force in a bouncing or jerking maneuver. Momentum carries the body part through the range of motion until the muscles are stretched to the limits. This method is less efficient than other methods, because muscle will contract under these stresses to protect itself from overstretching. Additionally, the rapid increase in force can cause injury.109,118 An example would be the 10-count bouncing toe touches popularized in the 1970s, but since abandoned because of lack of efficacy and risk for injury.
Neuromuscular Facilitation
The efficacy of stretching afforded by neuromuscular facilitation techniques has been documented in several studies.105,117 These methods typically require a trained therapist, aide, or trainer. The specific activities most frequently used include hold-relax and contract-relax techniques, characterized by an isometric or concentric contraction of the musculotendinous unit followed by a passive or static stretch. The prestretch contraction is thought to facilitate relaxation and flexibility via the muscle length–tension thermostat discussed previously in this chapter.
ACSM Guidelines for Prescription of Exercise for Musculoskeletal Flexibility
Optimal musculoskeletal function requires that adequate range of motion be maintained in all joints. Particularly important is maintenance of flexibility in the low back and posterior thigh muscles. Poor flexibility in these regions can predispose to low back pain.78,121
Some common stretching exercises might not be appropriate for all people, particularly those with a prior injury, joint insufficiency, or other conditions that could place them at risk for injury. Furthermore, exercises requiring substantial flexibility or skill are not recommended for older, less flexible, or less experienced persons.75
Recommended guidelines for flexibility training are listed in Table 18-6.78,121
Table 18-6 Recommended Guidelines for Flexibility Training121
| Component | Details |
|---|---|
| Mode | Static, dynamic, and PNF stretching of major muscle groups including the low back and posterior thigh |
| Intensity | To a mild degree of tightness without discomfort |
| Duration | Static stretches are held 15-60 seconds. A 6-second contraction followed by 10-30 seconds assisted stretch for PNF. |
| Frequency | At least 2-3 days per week |
| Repetitions | Four or more per muscle group |
PNF, Proprioceptive neuromuscular facilitation.
Plyometrics
Plyometrics allows the body to store elastic energy briefly in the muscle during the eccentric phase. This stored energy, combined with activation of the myotatic stretch reflex, results in a more powerful concentric contraction than is otherwise possible. This type of relatively complex action relies more heavily on the interplay between central nervous system and muscular system than do many other forms of exercise. Feedback from the central nervous system to the muscles influences the length of each muscle at any point during the movement, as well as the tension required for maintaining postural stability and initiating or stopping movement.15 With training, according to proponents of plyometrics, this neuromuscular interplay can be finely tuned. The widespread use of plyometric training in the athletic community suggests general acceptance of these methods by trainers, therapists, and athletes. However, many techniques in use have not been adequately studied. Results of research so far have generally been promising.
Hewett et al.69 have reported that plyometric jump training improved lower body strength in high school–age girls. Specifically, hamstring isokinetic strength and vertical jump height were improved after a 6-week program. A 22% decrease in peak ground reaction forces and a 50% decrease in the abduction-adduction moments at the knee during landing were also observed. In a later study using the same plyometric program, Hewett et al.68 prospectively analyzed the effect of this neuromuscular training on the incidence of serious knee injuries in female athletes. The authors reported a statistically significant decrease in the number of knee injuries sustained by the trained group versus matched control subjects.
Plyometric exercises vary in intensity, from simple, two-footed, in-place jumps, to hopping and bounding for maximum distance, to depth jumps from boxes of varying height. Plyometrics has been shown to result in ground reaction forces of four to seven times the body weight.7,129 Clearly these exercises should be approached with caution and begun at an elementary level. Progression to more advanced exercises should be based on the patient’s proficiency with the basic movements, taking into account baseline levels of strength, stability, and coordination.
Proprioception
Proprioception denotes the process by which information about the position and movement of body parts is related to the central nervous system. Proprioceptive organs, including muscle (particularly intrafusal spindle fibers), skin, ligaments, and joint capsules, generate afferent information that is crucial to the effective and safe performance of motor tasks. The process of proprioception is unfortunately subject to impairment from injury and disease. For example, knee and ankle ligament injuries have been shown to reduce proprioception. The same is true for both osteoarthritis and rheumatoid arthritis.6,41 Neuropathies, most notably diabetic neuropathy, can also cause significant loss of proprioception.111 Proprioception has also been shown to decrease with age.112
The importance of proprioception to injury prevention and rehabilitation from injury is generally accepted. Impaired proprioception has been associated with an increased risk for joint damage, athletic injury, and falls. Decreased joint proprioception is thought to influence the progressive joint deterioration associated with osteoarthritis, rheumatoid arthritis, and Charcot disease.5,6 In a study of soccer players, a significantly greater incidence of ankle injury was observed among players with abnormal proprioceptive testing results as compared with those who tested within normal parameters.123 Some findings also suggest that return to sport after knee injury might be more dependent on proprioception than on ligament tension.6 It has also been demonstrated in several studies that the risk for falling in the elderly population correlates with postural sway, a variable that is determined in large part by proprioception.85,87,88,122
Proprioceptive exercise regimens, by definition, seek to improve joint and limb position sense. These exercises are typically used after an injury has occurred to a joint that has resulted in a deficit in proprioception. For example, the tilt or wobble board is commonly used after ankle ligamentous injuries. Classically, the unidirectional boards are used first, with a progression to multidirectional boards. This type of training has led to measurably improved position sense in athletes.51 Other proprioceptive exercises include carioca (sideways running) and backward walking or running. It has also been shown that elastic bandaging improves position sense in subjects with previously impaired proprioception,6 perhaps through stimulation of proprioceptors in the skin.
Neurofacilitation Techniques
Proprioceptive Neuromuscular Facilitation
This form of therapy uses resistance to indirectly facilitate movement. The therapist provides maximal resistance to the stronger motor components of specific spiral and diagonal movement patterns, thereby facilitating the weaker components of the patterns. Proprioceptive neuromuscular facilitation techniques are best applied to patients with hypotonia associated with supraspinal lesions to promote normalization of tone. In patients with spasticity, these techniques can actually further increase tone in a potentially detrimental fashion.74,75
Brunnstrom
These techniques use resistance and primitive postural reactions to facilitate gross synergistic movement patterns and increase muscle tone during early recovery from central nervous system injury.10 During later stages, Brunnstrom techniques emphasize development of isolated movement and control. Like proprioceptive neuromuscular facilitation, this approach is thought to be effective in normalizing tone in a hypotonic or flaccid hemiplegic patient.
Bobath
The neurodevelopmental techniques developed by the Bobaths differ significantly from proprioceptive neuromuscular facilitation and Brunnstrom methods. Bobath techniques use reflex inhibitory movement patterns to inhibit increased tone. These inhibitory patterns, which are generally antagonistic to the primitive synergistic patterns, are performed without resistance. Neurodevelopmental techniques also incorporate advanced postural reactions to stimulate recovery. Advocates of these techniques claim reduction of hypertonicity and facilitation of motor recovery as their primary benefits.50
The techniques described above are all in common clinical use, with most therapists using an eclectic approach, borrowing some from each. There is no convincing evidence, however, that any of these methods actually alter the natural history of recovery from neurologic insult. These approaches to therapy seem to be most useful in providing compensatory techniques during the course of recovery. Using these methods, patients are able to improve performance in and gain independence with such tasks as making transfers, stretching, bed mobility, and safe ambulation.50
Exercise for Special Populations
Pregnancy
Special considerations exist during pregnancy because of the possible competition between exercising maternal muscle and the fetus for blood flow, oxygen delivery, glucose availability, and heat dissipation. Metabolic and cardiorespiratory adaptations to pregnancy can alter the responses from exercise training. The acute physiologic responses to exercise are generally increased during pregnancy compared with prepregnancy levels. There are no data in humans to indicate that pregnant women should or should not limit exercise intensity and lower target HRs because of potential adverse effects.78,121 Healthy, pregnant women without exercise contraindications are encouraged to exercise throughout the pregnancy. Regular exercise during pregnancy provides health and fitness benefits to the mother and child.27,121 Exercise might also reduce the risk for developing conditions associated with pregnancy, such as pregnancy-induced hypertension and gestational diabetes mellitus.27,98 For women who do not have any additional risk factors for adverse maternal or perinatal outcomes, the American College of Obstetricians and Gynecologists (ACOG) has established guidelines for the safe prescription of exercise.1,2 The Canadian Society for Exercise Physiology Physical Activity Readiness Medical Examination, termed the PARmed-X for Pregnancy, should be used for the health screening of pregnant women before their participation in exercise programs.121 Participation in a wide range of recreational activities appears to be safe during pregnancy. The safety of each sport is determined largely by the specific movements required by that sport. Participation in recreational sports with a high potential for contact, such as ice hockey, soccer, and basketball, could result in trauma to both the woman and the fetus. Recreational activities with an increased risk for falling, such as gymnastics, horseback riding, downhill skiing, and vigorous racquet sports, have an inherently high risk for trauma in pregnant and nonpregnant women. Those activities with a high risk for falling or for abdominal trauma should be avoided during pregnancy. Scuba diving should be avoided throughout pregnancy, because during this activity the fetus is at increased risk for decompression sickness secondary to the inability of the fetal pulmonary circulation to filter bubble formation. Exertion at altitudes of up to 6000 feet appears to be safe, but engaging in physical activities at higher altitudes carries various risks.
The ACOG recommends that women who currently participate in a regular exercise program can continue their training during pregnancy, following the above recommendations. Studies have demonstrated that women naturally decrease their exercise duration and intensity as their pregnancy advances. Those who begin an exercise program after becoming pregnant are advised to receive physician authorization and begin exercising with low-intensity, low-impact (or nonimpact) activities, such as walking and swimming.1,2 Contraindications for exercise during pregnancy have also been established by the ACOG (Box 18-7).2,121
BOX 18-7 Contraindications to Aerobic Exercise During Pregnancy121
Many of the physiologic and morphologic changes of pregnancy persist 4 to 6 weeks postpartum. Therefore the ACSM recommends in general to resume exercise 4 to 6 weeks after delivery. This will vary from one individual to another, with some women able to resume an exercise routine within days of delivery. There are no published studies to indicate that, in the absence of medical complications, rapid resumption of activities will result in adverse affects. Having undergone detraining, resumption of activities should be gradual. No known maternal complications are associated with resumption of training.121
The Elderly
The elderly can demonstrate improvements in aerobic capacity and muscle strength when given a sufficient training stimulus. Resistance training can enable elderly individuals to perform activities of daily living with greater ease, and counteract muscle loss and frailty in “older elderly” persons. The same general principles of exercise prescription apply to individuals of all ages. The wide range of health and fitness levels observed among older adults, however, make generic exercise prescription more problematic.78,121 Care must be taken in establishing the type, intensity, duration, and frequency of exercise. Specific recommendations for the elderly are outlined in Table 18-7.78,121
Table 18-7 Guidelines for Aerobic Exercise Prescription for the Elderly121
| Component | Details |
|---|---|
| Mode | The exercise modality should be one that does not impose significant orthopedic stress. |
| The activity should be accessible, convenient, and enjoyable to the participant—all factors directly related to exercise adherence. | |
| Consider walking, stationary cycling, water exercise, swimming, or machine-based stair climbing. | |
| Intensity | Intensity must be sufficient to stress (overload) the cardiovascular, pulmonary, and musculoskeletal systems without overtaxing them. |
| High variability exists for maximal heart rates in persons older than 65 years. It is always better to use a measured HRmax rather than age-predicted HRmax whenever possible. | |
| For similar reasons the HR reserve method is recommended for establishing a training HR in older individuals, rather than a straight percentage of HRmax. | |
| The recommended intensity for older adults is 50%-70% of HR reserve. | |
| Because many older persons have a variety of medical conditions, a conservative approach to prescribing aerobic exercise is warranted. | |
| Duration | During the initial stages of an exercise program, some older adults can have difficulty sustaining aerobic exercise for 20 minutes. One viable option can be to perform the exercise in several 10-minute bouts throughout the day. |
| To avoid injury and ensure safety, older individuals should initially increase exercise duration rather than intensity. | |
| Frequency | Alternate between days that involve primarily weight-bearing and non–weight-bearing exercise. |
HRmax, Maximal heart rate.
Individualization of resistance training prescriptions is also essential and should be based on the health and fitness status and specific goals of the participant. Some guidelines follow, with reference to the intensity, frequency, and duration of exercise (Table 18-8).78,121
Table 18-8 Guidelines for Resistance Exercise Prescription for the Elderly121
| Component | Details |
|---|---|
| Intensity | Perform one set of 8-10 exercises that train all the major muscle groups (e.g., gluteals, quadriceps, hamstrings, pectorals, latissimus dorsi, deltoids, and abdominals). Each set should involve 8-12 repetitions that elicit a perceived exertion rating of 12-13 (somewhat hard). |
| Frequency | Resistance training should be performed at least twice a week, with at least 48 hours of rest between sessions. |
| Duration | Sessions lasting longer than 60 minutes can have a detrimental effect on exercise adherence. Following the above guidelines should permit individuals to complete total body resistance training sessions within 20-30 minutes. |
Regardless of which specific protocol is adopted, several common-sense guidelines pertaining to resistance training for older adults should be followed.78,121
Children
Children tend to be more active than adults, and accordingly tend to maintain adequate levels of physical fitness. Healthy children should be encouraged, nonetheless, to engage in physical activity on a regular basis. However, because children are anatomically, physiologically, and psychologically immature, special precautions should be applied when designing exercise programs. Children can experience a higher incidence of overuse injuries, or damage the epiphyseal growth plates if endurance exercise is excessive. The risk for injury can be significantly decreased by ensuring appropriate matching of competition in terms of size, maturation or skill level, the use of properly fitted protective equipment, liberal adaptation of rules toward safety, proper conditioning, and appropriate skill development. Children have less efficient thermoregulation than that of adults, and are more prone to hyperthermia and hypothermia.78,121
The current rise in childhood obesity underscores the importance of regular exercise. In the United States 32% of children are overweight or obese.86,95 This increase in obesity has been linked to increases in comorbidities including glucose intolerance, type-2 diabetes, hypertension, and hyperlipidemia.86 Studies have demonstrated that monitored programs of moderate to vigorous exercise can result in a decrease in percent body fat and improvement of insulin resistance.90 Consensus guidelines for 2005 recommend that schools provide for 30 to 34 minutes of daily vigorous activity.113 The Endocrine Society recommends 60 minutes of daily vigorous activity.4
Specific considerations for children include the following121:
ACSM guidelines for exercise prescription in children are detailed in Table 18-9.121
| Component | Details |
|---|---|
| Frequency | At least 3-4 days/wk and preferably daily |
| Intensity | Moderate (physical activity that noticeably increases breathing, sweating, and HR) to vigorous (physical activity that substantially increases breathing, sweating, and HR) intensity |
| Time | 30 min/day of moderate and 30 min/day of vigorous intensity to total 60 min/day of accumulated physical activity |
| Type | A variety of activities that are enjoyable and developmentally appropriate for the child or adolescent |
HR, Heart rate.
Hypertension
The ACSM makes the following recommendations regarding exercise testing and training of persons with hypertension78,121:
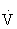 O2max) appears to lower blood pressure as much, or more, than exercise at higher intensities. This can be especially important in specific hypertensive populations, such as the elderly.
O2max) appears to lower blood pressure as much, or more, than exercise at higher intensities. This can be especially important in specific hypertensive populations, such as the elderly.Specific guidelines for exercise in patients with hypertension are as listed in Table 18-10.78,121
Table 18-10 Guidelines for Exercise Prescription in Patients With Hypertension121
| Component | Details |
|---|---|
| Frequency | Aerobic exercise on most (preferably all days of the week; resistance exercise 2-3 days/wk) |
| Intensity | Moderate-intensity aerobic exercise (i.e., 40% to <60% 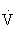 O2R) supplemented by resistance training at 60%-80% 1-RM O2R) supplemented by resistance training at 60%-80% 1-RM |
| Time | 30-60 min/day of continuous or intermittent aerobic exercise; if intermittent, use a minimum of 10-minute bouts. |
| Type | Emphasis should be placed on aerobic activities. |
RM, Repetition maximum; 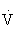 O2R, oxygen uptake reserve.
O2R, oxygen uptake reserve.
Peripheral Vascular Disease
Severe peripheral vascular disease is treated initially with exercise and medications that decrease blood viscosity. Treatment with angioplasty or bypass grafting might also be indicated. Weight-bearing exercise is preferred to facilitate greater functional changes, but might not be well tolerated initially. Prescription of non–weight-bearing exercise (which can permit a greater intensity or longer duration) is a suitable alternative.78,121 Specific guidelines for exercise in patients with peripheral vascular disease are listed in Table 18-11.78,121
Table 18-11 Guidelines for Exercise Prescription in Patients With Peripheral Vascular Disease121
| Component | Details |
|---|---|
| Frequency | Weight-bearing aerobic exercise 3-5 days/wk; resistance exercise at least 2 days/wk |
| Intensity | Moderate intensity (i.e., 40% to <60% 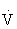 O2R) that allows patients to walk until they reaches a pain score of 3 (i.e., intense pain) on the 4-point pain scale.122 Between bouts of activity, individuals should be given time to allow ischemic pain to subside before resuming exercise.55,122 O2R) that allows patients to walk until they reaches a pain score of 3 (i.e., intense pain) on the 4-point pain scale.122 Between bouts of activity, individuals should be given time to allow ischemic pain to subside before resuming exercise.55,122 |
| Time | 30-60 min/day, but initially some patients may need to start with 10-minute bouts |
| Type | Weight-bearing aerobic exercise, such as walking, and non–weight-bearing activity, such as arm ergometry. Cycling may be used as a warmup, but should not be the primary type of activity. Resistance training is recommended to enhance and maintain muscular strength and endurance. |
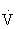 O2R, Oxygen uptake reserve.
O2R, Oxygen uptake reserve.
Diabetes
Hypoglycemia can occur not only during the exercise but for up to 4 to 6 hours after an exercise bout.78,121 The risk for hypoglycemic events can be minimized by taking the following precautions:
Other precautions that should be taken include the following78,121:
Specific guidelines for exercise in patients with diabetes are listed in Table 18-12.78,121
Table 18-12 Guidelines for Exercise Prescription in Patients With Diabetes121
| Component | Details |
|---|---|
| Frequency | 3-7 days/wk |
| Intensity | 50%-80% 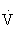 O2R or HRR corresponding to an RPE of 12-16 on a scale from 6 to 2024 O2R or HRR corresponding to an RPE of 12-16 on a scale from 6 to 2024 |
| Time | 20-60 min/day continuous or accumulated in bouts of at least 10 minutes to total 150 min/wk of moderate physical activity, with additional benefits of increasing to 300 minutes or more of moderate-intensity physical activity |
| Type | Emphasize activities that use large muscle groups. |
| Resistance training should be encouraged for people with diabetes mellitus in the absence of contraindications, retinopathy, and recent laser treatments. | |
| Frequency | 2-3 days/wk with at least 48 hours separating the exercise sessions |
| Intensity | 2-3 sets of 8-12 repetitions at 60%-80% 1-RM |
| Time | 8-10 multijoint exercises of all major muscle groups in the same session (whole body) or sessions split into selected muscle groups |
HRR, Heart rate reserve; RM, repetition maximum; RPE, rating of perceived exertion; 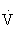 O2R, oxygen uptake reserve.
O2R, oxygen uptake reserve.
1. American College of Obstetricians and Gynecologists. Exercise during pregnancy and the post partum period. Washington, DC: American College of Obstetricians and Gynecologists; 1994.
2. American College of Obstetricians and Gynecologists. Exercise during pregnancy and the post partum period. Obstet Gynecol. 2002;99:171-173.
3. [Anonymous]. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA. 1993;269(23):3015-3023.
4. August G.P., Caprio S., Fennoy I., et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2009;93(12):4576-4599.
5. Barrack R.L., Skinner H., Cook S., et al. Effect of articular disease and total knee arthroplasty on knee joint-position sense. J Neurophysiol. 1983;50(3):684-687.
6. Barrett D.S. Proprioception and function after anterior cruciate reconstruction. J Bone Joint Surg Br. 1991;73(5):833-837.
7. Bobbert M., Mackey M., Schenkelshoek D. Biomechanical analysis of drop and countermovement jumps. Eur J Appl Physiol. 1986;54:566-573.
8. Bosco C., Komi P.V. Potentiation of the mechanical behavior of the human skeletal muscle through prestretching. Acta Physiol Scand. 1979;106(4):467-472.
9. Bosco C., Tihani J., Komi P. Store and recoil of elastic energy in slow and fast types of human skeletal muscles. Acta Physiol Scand. 1982;116:343-349.
10. Brunnstrom S. Movement therapy in hemiplegia. New York: Harper & Row; 1971.
11. Bryant S. Flexibility and stretching. Physician Sports Med. 1984;12:171.
12. Cady L.D.Jr., Thomas P.C., Karwasky R.J. Program for increasing health and physical fitness of fire fighters. J Occup Med. 1985;27(2):110-114.
13. Cavagna G., Dusman B., Margaria R. Positive work done by a previously stretched muscle. J Appl Physiol. 1968;24:21-32.
14. Cavagna G., Saibene F., Margaria R. Effects of negative work on the amount of positive work performed by an isolated muscle. J Appl Physiol. 1965;20:157-158.
15. Chu D. Jumping into plyometrics. Champaign: Human Kinetics; 1998.
16. Ciullo J.V. Biomechanics of the musculotendinous unit. Clin Sports Med. 1983;2:71.
17. Corbin C. Flexibility. Clin Sports Med. 1984;3:101-117.
18. Craib M.W., Mitchell V.A., Fields K.B., et al. The association between flexibility and running economy in sub-elite male distance runners. Med Sci Sports Exerc. 1996;28:737-743.
19. Cramer J.T., Housh T.J., Johnson G.O., et al. Acute effects of static stretching on peak torque in women. J Strength Cond Res. 2004;18:236-241.
20. Cramer J.T., Housh T.J., Weir J.P., et al. The acute effects of static stretching on peak torque, mean power output, electromyography and mechanomyography. Eur J Appl Physiol. 2005;93:530-539.
21. Cureton T. Flexibility as an aspect of physical fitness. Res Q. 1951;12:381-390.
22. D’Ambrosia R., Drez D. Prevention and treatment of running injuries. Thorofare: Charles Slack; 1982.
23. DeLateur B.J. Therapeutic exercise to develop strength and endurance. In: Kottke F.J., Stillwell G.K., Lehmann J.F., editors. Krusen’s handbook of physical medicine and rehabilitation. Philadelphia: Saunders, 1982.
24. DeLateur B.J. Therapeutic exercise. In: Braddom R.L., editor. Physical medicine and rehabilitation. Philadelphia: Saunders, 1996.
25. DeLateur B.J., Lehman J.F., Fordyce W.E. A test of the DeLorme axiom. Arch Phys Med Rehabil. 1968;49:245-248.
26. Demaree S.R., Powers S.K., Lawler J.M. Fundamentals of exercise metabolism. In: Roitman J.L., Haver E.J., Herridge M., et al, editors. ACSM resource manual for guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins, 2001.
27. Dempsey F.C., Butler F.L., Williams F.A. No need for a pregnant pause: physical activity may reduce the occurrence of gestational diabetes mellitus and preeclampsia. Exerc Sport Sci Rev. 2005;33:141-149.
28. Deschenes M., Kraemer W. Performance and physiologic adaptations to resistance exercise. Am J Phys Med Rehabil. 2002;81(suppl 11):S3-S16.
29. Deuster P., Keyser D. Basics in exercise physiology. In: O’Connor F., Sallis R., Wilder R., et al, editors. Sports medicine: just the facts. New York: McGraw Hill, 2005.
30. DeVries H. Electromyographic observations of effects of static stretching upon muscular distress. Res Q. 1960;32:468-479.
31. DeVries H., Housh T. Prevention of muscular distress after exercise. Res Q. 1960;32:177-185.
32. DeVries H., Housh T.J. Evaluation of static stretching procedures for improvement of flexibility. Res Q. 1962;33:222-229.
33. DeVries H., Housh T.J. Physiology of exercise for physical education, athletics and exercise science, ed 5. Dubuque: William C. Brown; 1994.
34. DeVries H.A., Whistle R.A., Bulbulian R. Tranquilizer effect of exercise. Acute effects of moderate aerobic exercise on spinal reflex activation level. Am J Phys Med. 1981;60:57-66.
35. Doucette S., Globe E. The effect of exercise on patellar tracking in lateral patellar compression syndrome. Am J Phys Med. 1992;20:434-440.
36. East J., Smith F., Burry L. Evaluation of warm-up for improvement in flexibility. Am J Phys Med. 1986;14:316-319.
37. Ekstrand J., Gillquist J. The frequencies of muscle tightness and injuries in soccer players. Am J Phys Med. 1982;10:75-78.
38. Ekstrand J., Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
39. Evetovich T.K., Nauman N.J., Conley D.S., et al. Effect of static stretching of the biceps brachii on torque, electromyography and mechanomyography during concentric isokinetic muscle actions. J Strength Cond Res. 2003;17:484-488.
40. Farfan H., Gracovetsky S. The mechanism of the lumbar spine. Spine. 1989;6:249-262.
41. Ferrell W.R., Crighton A., Sturrock R.D. Position sense at the proximal interphalangeal joint is distorted in patients with rheumatoid arthritis of finger joints. Exp Physiol. 1992;77(5):675-680.
42. Fleishman E. The structure and measurement of physical fitness. Englewood Cliffs: Prentice Hall; 1964.
43. Fowles J.R., Sale D.G., MacDougall J.D. Reduced strength after passive stretch of the human plantar flexors. J Appl Physiol. 2000;89:1179-1188.
44. Franklin B., Whaley M., Howley E. Benefits and risks associated with exercise. ACSM’s guidelines for exercise testing and prescription, ed 6. Philadelphia: Lippincott Williams & Wilkins, 2000.
45. Franklin B., Whaley M., Howley E. General principles of exercise prescription. ACSM’s guidelines for exercise testing and prescription, ed 6. Philadelphia: Lippincott Williams & Wilkins, 2000.
46. Franklin B., Whaley M., Howley E. Health screening and risk stratification. ASCM’s guidelines for exercise testing and prescription, ed 6. Philadelphia: Lippincott Williams & Wilkins, 2000.
47. Franklin B., Whaley M., Howley E. Physical fitness testing and interpretation. ACSM’s guidelines for exercise testing and prescription, ed 6. Philadelphia: Lippincott Williams & Wilkins, 2000.
48 Franklin B.A. Normal cardiorespiratory response to acute aerobic exercise. In: Roitman J.L., Haver E.J., Herridge M., editors. ACSM resource manual for guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins, 2001.
49. Frontera W., Meredith C.N., O’Reilly K.P. Strength conditioning in older men: skeletal muscle hypertrophy and improved functioning. J Appl Physiol. 1988;64:1038-1044.
50. Frontera W.R., Moldover J.R., Borg-Stein J., et al. Exercise. In Gonzalez E.G., Myers S.J., editors: Downey and Darling’s physiological basis of rehabilitation medicine, ed 3, Boston: Butterworth-Heinemann, 2001.
51. Gauffin H., Tropp H., Odenrick P. Effect of ankle disk training on postural control in patients with functional instability of the ankle joint. Int J Sports Med. 1988;9(2):141-144.
52. Gleim G.W., McHugh M.P. Flexibility and its effects on sports injury and performance. Sports Med. 1997;24(5):289-299.
53. Glick J. Muscle strains: prevention and treatment. Physician Sports Med. 1980;6:73-77.
54. Godshall R.W. The predictability of athletic injuries: an eight-year study. J Sports Med. 1975;3(1):50-54.
55. Goldspink D. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977;264:267-282.
56. Goldspink G., Williams P. The nature of the increased passive resistance in muscle following immobilization of the mouse soleus muscle. J Physiol. 1979;289:55P.
57. Golnick P., Karlsson J., Peihl K. Selective glycogen depletion in skeletal muscle fibers of man following sustained contractions. J Physiol. 1974;241:59-67.
58. Grahame R. Joint hypermobility: clinical aspects. Proc R Soc Med. 1971;64(6):692-694.
59. Grahame R., Jenkins J.M. Joint hypermobility: asset or liability? A study of joint mobility in ballet dancers. Ann Rheum Dis. 1972;31(2):109-111.
60. Grana W.A., Moretz J.A. Ligamentous laxity in secondary school athletes. JAMA. 1978;240(18):1975-1976.
61. Guyton A. Textbook of medical physiology. Philadelphia: Saunders; 1996.
62. Gwinup G. Weight loss without dietary restriction: efficacy of different forms of aerobic exercise. Am J Sports Med. 1987;15:275-279.
63. Halpern B., Thompson N., Curl W. High school football injuries: identifying the risk factors. Am J Sports Med. 1987;15:316-320.
64. Flexibility Harris M. Phys Ther. 1968;49:591-601.
65. Hart J., Ingersoll C. Weightlifting. In: O’Connor F., Sallis R., Wilder R., et al, editors. Sports medicine: just the facts. New York: McGraw-Hill, 2005.
66. Hattori K., Ohta S. Ankle joint flexibility in college soccer players. J Hum Ergol. 1986;15:85-89.
67. Hellebrandt F.A., Houtz S.J. Methods of muscle training: the influence of pacing. Phys Ther Rev. 1958;38:319-322.
68. Hewett T., Lindenfield T., Riccobene J. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Phys Med. 1999;24:765-773.
69. Hewett T.E., Stroupe A.L., Nance T.A., et al. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765-773.
70. Holly R.G., Shaffrath J.D. Cardiorespiratory endurance. In: Roitman J.L., Haver E.J., Herridge M., et al, editors. ACSM resource manual for guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins, 2001.
71. Huxley A.F., Simmons R.M. Mechanical properties of the cross-bridges of frog striated muscle. J Physiol. 1971;218(1):59P-60P.
72. Johns R., Wright V. Relative importance of various tissues in joint stiffness. J Appl Physiol. 1962;17:814-828.
73. Johnson J.E., Sim F.H., Scott S.G. Musculoskeletal injuries in competitive swimmers. Mayo Clin Proc. 1987;62(4):289-304.
74. Kabat H. Studies on neuromuscular dysfunction, XI. New principles of neuromuscular reeducation. Perm Found Med Bull. 1947;5:111.
75. Kabat H. Proprioceptive facilitation in therapeutic exercise. In Licht S., editor: Therapeutic exercise, ed 2, New Haven: E Licht, 1961.
76. Kalenak A., Morehouse C.A. Knee stability and knee ligament injuries. JAMA. 1975;234(11):1143-1145.
77. Keller C., Noyes F., Buchner R. Sports traumatology series: the medical aspects of soccer injury epidemiology. Am J Phys Med. 1987;15:230-237.
78. Kenny W., Humphrey R., Bryant C. ACSM’s guidelines for exercise testing and prescription, ed 5. Philadelphia: Williams & Wilkins; 1995.
79. Kim H., Kerr R., Cruz T., et al. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen arthritis. J Rheumatol. 1995;22:1714-1721.
80. Kirchner G., Glines D. Comparative analysis of Eugene, Oregon elementary school children using the Kraus–Weber test of minimum muscular fitness. Res Q. 1957;28:16-25.
81. Knight K.L. Knee rehabilitation by the daily adjustable progressive resistive exercise technique. Am J Sports Med. 1979;7:336-337.
82. Knuttgen H.G. Development of muscular strength and endurance. In: Knuttgen H.G., editor. Neuromuscular mechanisms for therapeutic and conditioning exercises. Baltimore: University Park Press, 1976.
83. Kokkonen J., Nelson A.G., Cornwell A. Acute muscle stretching inhibits maximal strength performance. Res Q Exerc Sport. 1998;69:411-415.
84. Kraus H. Backache, stress, and tension. Their cause, prevention and treatment. New York: Simon & Schuster; 1965.
85. Lichtenstein M.J., Shields S.L., Shiavi R.G., et al. Clinical determinants of biomechanics platform measures of balance in aged women. J Am Geriatr Soc. 1988;36(11):996-1002.
86. Lieb D.C., Snow R.E., DeBoer M.D. Socioeconomic factors in the development of childhood obesity and diabetes. Clin Sports Med. 2009;28:349-378.
87. Lord S.R., Sambrook P.N., Gilbert C., et al. Postural stability, falls and fractures in the elderly: results from the Dubbo Osteoporosis Epidemiology Study. Med J Aust. 1994;160(11):684-685.
88. Maki B.E., Holliday P.J., Fernie G.R. Aging and postural control: a comparison of spontaneous and induced-sway balance tests. J Am Geriatr Soc. 1990;38(1):1-9.
89. Marek S.M., Cramer J.F., Fincher A.L., et al. Acute effects of static and proprioceptive neuromuscular facilitation stretching on muscle strength and power output. J Athl Train. 2005;40:94-103.
90. McCall A., Raj R. Exercise for prevention of obesity and diabetes in children and adolescents. Clin Sports Med. 2009;28:393-421.
91. McHugh M.P., Neese M. Effect of stretching on strength loss and pain after eccentric exercise. Med Sci Sports Exerc. 2008;40(3):566-573.
92. Moritani T., de Vries H. Potential for gross muscle hypertrophy in older men. J Gerontol. 1980;35:672-682.
93. Nicholas J.A. Injuries to knee ligaments. Relationship to looseness and tightness in football players. JAMA. 1970;212(13):2236-2239.
94. Noble B., Borg G., Jacobs I. A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc. 1983;15:523-528.
95. Ogden C.L., Carroll M.D., Flegal K.M. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299(20):2401-2405.
96. Phalen G., Dickson J. Spondylolisthesis and tight hamstrings. J Bone Joint Surg Am. 1961;43:505-512.
97. Phillips M. Analysis of results from the Kraus–Weber test of minimum fitness in children. Res Q. 1955;26:314-323.
98. Pivarnik J.M., Chambliss H.O., Clapp J.F., et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38:989-1006.
99. Polley H., Hunder G. Physical examination of the joints, ed 2. Philadelphia: Saunders; 1978.
100. Pollock M., Gaesser G., Butcher J. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. American College of Sports Medicine position stand. Med Sci Sports Exerc. 30(6), 1998.
101. Roitman J.L., Haver E.J., Herridge M., editors. ACSM’s resource manual for guidelines to exercise testing and prescriptions. Philadelphia: Lippincott Williams & Wilkins, 2010.
102. Ross J., Gilbert G: National Children, Fitness Youth. Study: a summary of findings. J Phys Educ Recreation Dance. 1985;56(1):45-50.
103. Rupp J.C. Exercise physiology. In: Roitman J.L., Bibi K.W., Thompson W.R., editors. ACSM health fitness certification review. Philadelphia: Lippincott Williams & Wilkins, 2001.
104. Saal J. Flexibility training. In: Kibler W., editor. Functional rehabilitation of sports and musculoskeletal injuries. Gaithersburg: Aspen, 1998.
105. Sady S.P., Wortman M., Blanke D. Flexibility training: ballistic, static or proprioceptive neuromuscular facilitation? Arch Phys Med Rehabil. 1982;63(6):261-263.
106. Salter R. History of rest and motion and the scientific basis for early continuous passive motion. Hand Clin. 1996;12:1-11.
107. Al Sayers, Farley R.S., Fuller D.K., et al. The effect of static stretching on phases of sprint performance in elite soccer players. J Strength Cond Res. 2008;22(5):1416-1421.
108. Seto C. Basic principles of exercise training. In: O’Connor F., Sallis R., Wilder R., et al, editors. Sports medicine: just the facts. New York: McGraw-Hill, 2005.
109. Shellock F., Prentice W. Warming up and stretching for improved physical performance and prevention of sports-related injuries. Sports Med. 1985;2:267-278.
110. Shyne K., Dominquez R. To stretch or not to stretch? Physician Sports Med. 1982;10:137-140.
111. Simoneau G.G., Derr J.A., Ulbrecht J.S., et al. Diabetic sensory neuropathy effect on ankle joint movement perception. Arch Phys Med Rehabil. 1996;77(5):453-460.
112. Skinner H.B., Barrack R.L., Cook S.D. Age-related decline in proprioception. Clin Orthop Relat Res. 1984;184:208-211.
113. Speiser P.W., Rudolf M.C., Anhalt H., et al. Childhood obesity [review]. J Clin Endocrinol Metab. 2005;90(3):1871-1887. Epub Dec 14, 2004
114. Stephens M.B., O’Connor F., Deuster P. Exercise and nutrition. American Academy of Family Physician’s home study self-assessment program, monograph 283. Leawood KS. 2002. American Academy of Family Physicians
115. Stolov W.C., Weilepp T.G.Jr. Passive length–tension relationship of intact muscle, epimysium, and tendon in normal and denervated gastrocnemius of the rat. Arch Phys Med Rehabil. 1966;47(9):612-620.
116. Stolov W.C., Weilepp T.G.Jr., Riddell W.M. Passive length–tension relationship and hydroxyproline content of chronically denervated skeletal muscle. Arch Phys Med Rehabil. 1970;51(9):517-525.
117. Surburg P. Neuromuscular facilitation techniques in sports medicine. Physician Sports Med. 1981;9:115-127.
118. Surburg P. Flexibility exercises re-examined. J Athl Train. 1983;18:370-374.
119. Thacker S.B., Gilchrist J., Stroup D.F., et al. The impact of stretching on sports injury risk: a systematic review of the literature. Med Sci Sports Exerc. 2004;36:371-378.
120. Thibodeau G.A., Patton K.T. Anatomy and physiology. St Louis: Mosby; 1999.
121. Thompson W.R., editor. ACSM’s guidelines for exercise testing and prescription, ed 8, Philadelphia: Lippincott Williams & Wilkins, 2010.
122. Topper A.K., Maki B.E., Holliday P.J. Are activity-based assessments of balance and gait in the elderly predictive of risk of falling and/or type of fall? J Am Geriatr Soc. 1993;41(5):479-487.
123. Tropp H., Ekstrand J., Gillquist J. Stabilometry in functional instability of the ankle and its value in predicting injury. Med Sci Sports Exerc. 1984;16(1):64-66.
124. Warren C., Lehmann J., Koblanski J. Heat and stretch procedures: an evaluation using rat tail tendon. Arch Phys Med Rehabil. 1976;57:122-126.
125. Whaley M.H., Kaminsky L.A. Epidemiology of physical activity, physical fitness and selected chronic diseases. In: Roitman J.L., Haver E.J., Herridge M., et al, editors. ACSM resource manual for guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins, 2001.
126. Wiktorsson-Moller M., Oberg B., Ekstrand J., et al. Effects of warming up, massage, and stretching on range of motion and muscle strength in the lower extremity. Am J Sports Med. 1983;11(4):249-252.
127. Williams J., Moran M., Thonar E., et al. Continuous passive motion stimulates repair of rabbit knee articular cartilage after matrix proteoglycan loss. Clin Orthop. 1994;304:252-262.
128. Williams P., Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat. 1978;127(3):459-468.
129. Witzke K.A., Snow C.M. Effects of plyometric jump training on bone mass in adolescent girls. Med Sci Sports Exerc. 2000;32(6):1051-1057.
130. Zarins B. Soft tissue repair: biomechanical aspects. Int J Sports Med. 1982;3:9-11.