Chapter 1 Theoretical Considerations
Introduction
Ophthalmic ultrasonography is the main diagnostic imaging modality of the eye. It is a safe, non-invasive diagnostic tool providing instant feedback for the evaluation of a variety of ophthalmic disorders. Diagnostic ophthalmic ultrasonography is most useful in the presence of opaque ocular media due to corneal opacities, anterior chamber opacities, cataracts, vitreous hemorrhage or inflammatory opacities. Ophthalmic ultrasonography is also valuable in the presence of clear media, for evaluation of the iris, lens, ciliary body and orbital structures. Intraocular tumors are routinely documented, measured, and differentiated by ultrasonographic techniques (Chapter 11). This chapter provides a brief overview of the basic physics of ultrasound, instrumentation, and special examination techniques used in ophthalmic ultrasonography.
Basic physics
Laws of acoustic energy
The use of ultrasound in medicine is dependent on the physical laws of acoustic energy: reflection, refraction, and absorption.1 The angle of incidence is an important factor in the strength of the returning echoes. To accurately access structures based on the intensity of the returning echoes, the sound beam needs to be directed perpendicular to the desired structure. Sound beams directed at an oblique angle towards an interface result in the reflection of some of the sound beams away from the probe causing a weaker signal. Variations in the shape and size of the acoustic interface can also result in scattering of some of the sound beams. Ultrasound directed at a coarse, irregular surface results in significant loss of echo strength due to diversion of reflected echoes.
Frequency and resolution
Frequencies currently used in ophthalmic ultrasound machines range from 8–80 MHz, compared with 2–6 MHz typically used in other fields of diagnostic ultrasound. The use of higher frequencies allows for increased resolution, which is essential in the evaluation of small ophthalmic structures. The superficial location of the eye and the low absorptive properties of its primarily aqueous based structures, make the use of high frequencies practical.2 The high frequencies are achieved with mechanical scanning by single-element focused transducers. Electronically scanned arrays are not usually found in ophthalmic imaging devices because it is difficult to assemble array elements with the necessary half-wavelength spacing.3 The unique anatomy of the ocular structures allows the sound beam in ophthalmic devices to reach all areas of the eye in a close to optimal perpendicular orientation by movement of the eye and positioning of the transducer.
Instrumentation
In 1956 Mundt and Hughes published the first report of in vivo A-scan ultrasonography of intraocular tumors.4 Other clinical applications were published soon after.5 Techniques for B-scan ophthalmic examination and ultrasonographic features of specific ocular diseases and tumors were described within 2 years of the initial publication.6 Since then, many investigators have aided in the design and improvement of ophthalmic ultrasound instrumentation as well as expanded upon the diagnostic techniques. The most frequently utilized ophthalmic ultrasound instrumentation includes A-scan, B-scan (Chapters 2 and 3), and ultrasound biomicroscopy (Chapters 4 and 6). Color Doppler ultrasonography (Chapter 5) and 3-D ultrasonography (Chapter 19) has limited ophthalmic applications.
A-scan
A-scan is a one-dimensional display of echo strength over time. Vertical spikes correspond to echo intensity and are shown on the horizontal axis as a function of time. There are two primary types of A-scan used in ophthalmic ultrasonography; biometric A-scan and standardized diagnostic A-scan.7 Each has slightly different operating frequencies and amplification algorithms.
Biometric A-scan
Biometric A-scan is optimized for axial eye length measurements (Chapter 7). It utilizes a probe with an operating frequency of 10–12 MHz and a linear amplification curve.8 The sound velocity in ocular structures along the visual axis at physiological temperatures are well established resulting in highly accurate measurements.9–11 The primary function of biometric A-scan in ophthalmology is to determine the axial eye lengths (AEL) for patients undergoing cataract surgery so that the dioptric power of the intraocular lens (IOL) to be implanted can be accurately determined.
Standardized A-scan
Standardized A-scan is a special diagnostic instrument developed by Ossoinig.12,13 It utilizes a probe with an operating frequency of 8 MHz and an S-shaped amplification curve. The S-shaped curve provides the benefit of the wide range of logarithmic amplification and the high sensitivity of linear amplification. The primary feature of standardized A-scan is the tissue sensitivity, or standardized decibel setting used for the detection and differentiation of abnormal intraocular tissues. Standardized A-scan is designed to display an echo spike for retina that is 100% on the echo intensity scale when the sound beam is directed perpendicular to the retina (Chapter 3). Highly dense ocular structures including choroid and sclera will also produce 100% echo spikes. All intraocular structures that have a density lower than retina including vitreous opacities and membranes will produce echoes of less than 100% intensity. The reflectivity of the A-scan spike also allows intraocular and orbital tumor cell structure to be evaluated and differentiated. In combination with B-scan, diagnostic A-scan is essential in the differentiation of vitreoretinal membranes (Chapter 10).
B-scan
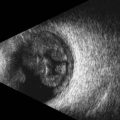
Contact B-scan is a two-dimensional display of echoes using both the horizontal and vertical orientations to show shape, location and extent. Dots on the screen represent echoes and the strength of the echo is determined by the brightness of the dot. Most ophthalmic ultrasound machines utilize logarithmic or S-shaped amplification and a frequency in the range of 10 MHz.7 The term contact refers to the direct application of the probe to the surface of the eye with methylcellulose as a coupling agent in the absence of a water bath (Chapter 3).
B-scan images are highly accurate representations of ocular structures and provide the foundation for diagnostic ultrasound in ophthalmology.14,15 Evaluation and differentiation of intraocular lesions is one of the primary indications for ophthalmic ultrasonography (Chapter 11). Contact B-scan is most informative regarding topographic features including the location, shape, and extension of the lesion. It is important to note that the evaluation of static B-scan images in isolation can lead to misdiagnosis.16 B-scan evaluation is a dynamic process requiring specific attention to the mobility of the displayed echoes.
Standardized echography, the combined use of contact B-scan and standardized A-scan, provides a reliable method to evaluate ocular lesions based on the topographic, quantitative and kinetic properties of the echo amplitudes and patterns.13,17–19 These methods are well established, most extensively for choroidal melanoma, and used in clinical trials for the documentation of tumor differentiation and growth (Chapter 11).20–23 Three basic B-scan probe orientations are used in ophthalmic ultrasonography and referred to in the chapters that follow: axial, transverse and longitudinal (Chapter 3).
Special techniques
Ultrasound biomicroscopy (UBM)
UBM is an ultrasound instrument introduced by Pavlin that utilizes frequencies from 35 to 80 MHz for the acoustic evaluation of anterior segment of the eye.24 Details of this instrument and technique are described in Chapter 4.
Immersion B-scan
Immersion B-scan refers to the use of balanced salt solution (BSS) between the probe and the surface of the eye. Immersion B-scan is not routinely used for evaluation of posterior segment structures. The vessel holding the BSS, usually a bottomless cup that is fitted for the eye, is placed in a fixed position. The mobility of the probe is significantly limited, which prohibits the sound beams from reaching posterior structures in the desired perpendicular manner. However, immersion B-scan is valuable in the evaluation of pathology located near the ora serrata (anterior limit of the retina), an area that is too anterior to image with contact B-scan and too posterior to image with UBM (Chapter 3).
Color Doppler ultrasonography
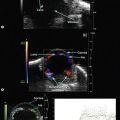
Color Doppler imaging (CDI) simultaneously allows for two-dimensional B-scan imaging of structure and evaluation of blood flow. Conventional duplex scanning of ocular and orbital structures produces a waveform graph of Doppler information on one screen and a B-scan image on a separate screen. The small vessel diameters found in intraocular and orbital vasculature are too small to be imaged with B-scan and Doppler spectra are obtained without knowing the exact location of the vessels.25 CDI allows real-time blood flow information to be color encoded and superimposed on the gray-scale B-scan image.26 Doppler shifts are usually displayed at the red end of the spectrum when flow is moving towards the transducer and at the blue end of the spectrum when flow is moving away. CDI has been proven to be effective in the display of a variety of pathological ocular conditions including the detection of ocular and orbital tumor vasculature,27,28 carotid disease,26 central retinal artery and vein occlusions25,29 and non-arteritic ischemic optic neuropathy (Chapter 5).30
3D ultrasonography
In 3D ultrasonography multiple consecutive two-dimensional B-scan images are utilized to create a 3D block (Chapter 19). The probe is held in fixed, transscleral orientation and serial images are rapidly obtained as the transducer rotates 200 degrees.31 Software transforms the data into a 3D image that can be sectioned in longitudinal, transverse, oblique and coronal views. Three-dimensional ultrasound has been shown to be useful in clinical settings including estimating the volume of intraocular lesions32,33 and for evaluation of retrobulbar optic nerve.34,35
1 Lizzi FL, Feleppa EJ. Practical physics and electronics of ultrasound. Int Ophthalmol Clin. 1979;19(4):35-63.
2 Fledelius HC. Ultrasound in ophthalmology. Ultrasound Med Biol. 1997;23(3):365-375.
3 Lizzi FL, Coleman DJ. History of ophthalmic ultrasound. J Ultrasound Med. 2004;23(10):1255-1266.
4 Mundt G, Hughes W. Ultrasonics in ocular diagnosis. Am J Ophthalmol. 1956;41:488-498.
5 Oksala A, Lehtinen A. Diagnostic value of ultrasonics in ophthalmology. Ophthalmologica. 1957;134(6):387-395.
6 Baum G, Greenwood I. The application of ultrasonics locating techniques to ophthalmology. Am J Ophthalmol. 1958;46(5 Part 2):319-329.
7 Byrne S, Green R. Ultrasound of the Eye and Orbit, 2nd ed. St Louis: Mosby; 2002.
8 Byrne S. A-scan Axial Eye Length Measurements – A Handbook for IOL Calculations. Mars Hill, NC: Grove Park Publishers; 1995.
9 Oksala A, Lehtinen A. Measurement of the velocity of sound in some parts of the eye. Acta Ophthalmol. 1958;36(4):633-639.
10 Jansson F, Sundmark E. Determination of the velocity of ultrasound in ocular tissues at different temperatures. Acta Ophthalmol. 1961;39:899-910.
11 Coleman DJ. Ophthalmic biometry using ultrasound. Int Ophthalmol Clin. 1969;9(3):667-683.
12 Ossoinig KC. Quantitative echography – the basis of tissue differentiation. J Clin Ultrasound. 1974;2(1):33-46.
13 Ossoinig KC. Standardized echography: basic principles, clinical applications, and results. Int Ophthalmol Clin. 1979;19(4):127-210.
14 Bronson NR. Development of a simple B-scan ultrasonoscope. Trans Am Ophthalmol Soc. 1972;70:365-408.
15 Feibel RM. Diagnostic ultrasonography. Int Ophthalmol Clin. 1978;18(1):167-178.
16 Fisher YL. Contact B-scan ultrasonography: a practical approach. Int Ophthalmol Clin. 1979;19(4):103-125.
17 Ossoinig KC. Ruling out posterior segment lesions with echography. Int Ophthalmol Clin. 1978;18(2):117-120.
18 Byrne SF. Standardized echography. Part I: A-Scan examination procedures. Int Ophthalmol Clin. 1979;19(4):267-281.
19 Byrne SF. Standardized echography in the differentiation of orbital lesions. Surv Ophthalmol. 1984;29(3):226-228.
20 Char DH, Ljung BM, Miller T, et al. Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology. 1988;95(5):625-630.
21 Echography (ultrasound) procedures for the Collaborative Ocular Melanoma Study (COMS), Report no. 12, Part II. J Ophthal Nursing Technol. 1999;18(5):219-232.
22 Echography (ultrasound) procedures for the Collaborative Ocular Melanoma Study (COMS), Report no. 12, Part I. J Ophthal Nursing Technol. 1999;18(4):143-149.
23 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19.[Erratum appears in Ophthalmology 2004;111(8):1514]. Ophthalmology. 2002;109(12):2197-2206.
24 Pavlin CJ, Harasiewicz K, Sherar MD, et al. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98(3):287-295.
25 Lieb WE, Cohen SM, Merton DA, et al. Color Doppler imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Ophthalmol. 1991;109(4):527-531.
26 Erickson S, Hendrix L, Massaro B, et al. Color Doppler flow imaging of the normal and abnormal orbit. Radiology. 1989;173:511-516.
27 Lieb WE, Shields JA, Cohen SM, et al. Color Doppler imaging in the management of intraocular tumors. Ophthalmology. 1990;97(12):1660-1664.
28 Guthoff R, Berger R, Winker P. Doppler ultrasonography of malignant melanoma of the uvea. Arch Ophthalmol. 1991;109:537.
29 Baxter GM, Williamson TH. Color Doppler flow imaging in central retinal vein occlusion: a new diagnostic technique? Radiology. 1993;187(3):847-850.
30 Williamson TH, Harris A. Color Doppler ultrasound imaging of the eye and orbit. Surv Ophthalmol. 1996;30(4):316-317.
31 Fisher Y, Hanutsaha P, Tong S, et al. Three-dimensional ophthalmic contact B-scan ultrasonography of the posterior segment. Retina. 1998;18:251-256.
32 Finger PT, Khoobehi A, Ponce-Contreras MR, et al. Three dimensional ultrasound of retinoblastoma: initial experience. Br J Ophthalmol. Oct 2002;86(10):1136-1138.
33 Romero JM, Finger PT, Rosen RB, et al. Three-dimensional ultrasound for the measurement of choroidal melanomas. Arch Ophthalmol. 2001;119(9):1275-1282.
34 Garcia JPSJr, Garcia PT, Rosen RB, et al. A 3-dimensional ultrasound C-scan imaging technique for optic nerve measurements. Ophthalmology. 2004;111(6):1238-1243.
35 Garcia JPSJr, Garcia PMT, Rosen RB, et al. Optic nerve measurements by 3D ultrasound-based coronal “C-scan” imaging. Ophthal Surg Lasers Imaging. 2005;36(2):142-146.