Chapter 22
The Vestibular System
Peripheral Vestibular Labyrinth
Morphologic Polarization of Hair Cells
Semicircular Canals and Otolith Organs
Function of Semicircular Canals
Rotational Vestibuloocular Reflex
Vestibulothalamocortical Network
Humans have the ability to control posture and movements of the body and eyes relative to the external environment. The vestibular system mediates these motor activities through a network of receptors and neural elements. This system integrates peripheral sensory information from vestibular, somatosensory, visceromotor, and visual receptors as well as motor information from the cerebellum and cerebral cortex. Central processing of these inputs occurs rapidly, with the output of the vestibular system providing an appropriate signal to coordinate relevant movement reflexes. Although the vestibular system is considered to be a special sense, most vestibular activity is conducted at a subconscious level. However, in situations producing unusual or novel vestibular stimulation, such as rough air in a plane flight or wave motion on ships, vestibular perception becomes acute, with dizziness, vertigo, or nausea often resulting.
OVERVIEW
The vestibular system is an essential component in the production of motor responses that are crucial for daily function and survival. Throughout evolution, the highly conserved nature of the vestibular system is revealed through striking similarities in the anatomic organization of receptors and neuronal connections in fish, reptiles, birds, and mammals.
For the present discussion, the vestibular system can be divided into five components:
1. The peripheral receptor apparatus resides in the inner ear and is responsible for transducing head motion and position into neural information.
4. The vestibulospinal network coordinates head movements, axial musculature, and postural reflexes.
PERIPHERAL VESTIBULAR LABYRINTH
The vestibular labyrinth contains specialized sensory receptors and is located lateral and posterior to the cochlea in the inner ear (Fig. 22-1). The vestibular labyrinth consists of five separate receptor structures, three semicircular canals and two otolith organs, which are contained in the petrous portion of the temporal bone. The labyrinth is actually composed of two distinct components. The bony labyrinth is a surrounding shell that contains and protects the sensitive underlying vestibular sensory structures (Fig. 22-1). In humans, the bony labyrinth can be visualized only on excision of the mastoid process. Inside the bony labyrinth is a closed, fluid-filled system, the membranous labyrinth, which consists of connecting tubes and prominences (Fig. 22-2). Vestibular receptors are located in specialized regions of the membranous labyrinth.
Figure 22-1. A cross section of the outer, middle, and inner ear.
Between the membranous labyrinth and bony labyrinth is a space containing fluid called perilymph, which is similar to cerebrospinal fluid. Perilymph has a high sodium content (150 mM) and a low potassium content (7 mM), and it bathes the vestibular portion of the eighth cranial nerve.
The membranous labyrinth is filled with a different type of fluid, called endolymph, which covers the specialized sensory receptors of both the vestibular and the auditory systems. Endolymph has a high concentration of potassium (150 mM) and a low concentration of sodium (16 mM). It is important to note the differences in these two fluids because both are involved in the normal functioning of the vestibular system. Disturbances in the distribution or ionic content of endolymph often lead to vestibular disease.
Vestibular Receptor Organs
The five vestibular receptor organs in the inner ear complement each other in function. The semicircular canals (horizontal, anterior, and posterior) transduce rotational head movements (angular accelerations). The otolith organs (utricle and saccule) respond to translational head movements (linear accelerations) or to the orientation of the head relative to gravity. Each semicircular canal and otolith organ is spatially aligned to be most sensitive to movements in specific planes in three-dimensional space.
In humans, the horizontal semicircular canal and the utricle both lie in a plane that is slightly tilted anterodorsally relative to the nasooccipital plane (Fig. 22-3). When a person walks or runs, the head is normally declined (pitched downward) by approximately 30 degrees, so that the line of sight is directed a few meters in front of the feet. This orientation causes the plane of the horizontal canal and utricle to be parallel with the earth and perpendicular to gravity. The anterior and posterior semicircular canals and the saccule are arranged vertically in the head, orthogonal to the horizontal semicircular canal and utricle (Fig. 22-3). The two vertical canals in each ear are positioned orthogonal to each other, whereas the plane of the anterior canal on one side of the head is coplanar with the plane of the contralateral posterior canal (Fig. 22-3).
The receptor cells in each vestibular organ are innervated by primary afferent fibers that join with those from the cochlea to comprise the vestibulocochlear (eighth) cranial nerve. The cell bodies of these bipolar vestibular afferent neurons are in the vestibular ganglion (Scarpa ganglion), which lies in the internal acoustic meatus (Fig. 22-4). The central processes of these bipolar cells enter the brainstem and terminate in the ipsilateral vestibular nuclei and cerebellum.
The blood supply to the labyrinth is primarily via the labyrinthine artery, usually a branch of the anterior inferior cerebellar artery. This vessel enters the temporal bone through the internal auditory meatus. Although it is not as important as the labyrinthine artery, the stylomastoid artery also provides branches to the labyrinth, mainly to the semicircular canals. An interruption of blood supply to the labyrinth will compromise vestibular (and cochlear) function, resulting in labyrinth-associated symptoms, such as vertigo or oscillopsia, and clinical signs, such as nystagmus or unstable gait.
Membranous Labyrinth
The membranous labyrinth is supported inside the bony labyrinth by connective tissue. The three ducts of the semicircular canals connect to the utricle, and each duct ends with a single prominent enlargement, the ampulla (Fig. 22-2). Sensory receptors for the semicircular canals reside in a neuroepithelium at the base of each ampulla. The receptors in the utricle are oriented longitudinally along its base, and in the saccule they are oriented vertically along the medial wall (Fig. 22-2). Endolymph in the labyrinth is drained into the endolymphatic sinus via small ducts. In turn, this sinus communicates through the endolymphatic duct with the endolymphatic sac, which is located adjacent to the dura mater (Fig. 22-2). The saccule is also connected to the cochlea by the ductus reuniens.
Meniere Disease
The balance between the ionic contents of endolymph and perilymph is maintained by specialized secretory cells in the membranous labyrinth and the endolymphatic sac. In cases of advanced Meniere disease, there is disruption of normal endolymph volume, resulting in endolymphatic hydrops (an abnormal distention of the membranous labyrinth). Symptoms of Meniere disease include severe vertigo (a sense of spinning in space), positional nystagmus, and nausea. Affected persons often have unpredictable attacks of auditory and vestibular symptoms, including vomiting, tinnitus (ringing in the ears), and a complete inability to make head movements or even to stand passively. For patients with frequent debilitating attacks, the first course of treatment is often administration of a diuretic (e.g., hydrochlorothiazide) and a salt-restricted diet to reduce the hydrops. If persistent symptoms of Meniere disease continue, second treatment options include either the implantation of a small shunt into the abnormally swollen endolymphatic sac or the delivery of a vestibulotoxic agent such as gentamicin into the perilymph.
Semicircular Canal Dehiscence
On occasion, a condition may develop in which a portion of the temporal bone overlying either the anterior or the posterior semicircular canal thins so much that an opening (dehiscence) is created next to the dura (Fig. 22-5). In affected patients, the canal dehiscence exposes the normally closed bony labyrinth to the extradural space. Symptoms can include vertigo and oscillopsia (a sense that objects are moving to and fro, oscillating, in the visual fields) in response to loud sounds (the Tullio phenomenon) or in response to maneuvers that change middle ear or intracranial pressure. The eye movements evoked by these stimuli (nystagmus) align with the plane of the dehiscent superior canal. Surgical closure of the defect by bone replacement is often performed.
VESTIBULAR SENSORY RECEPTORS
Hair Cell Morphology
The sensory receptor cells in the vestibular system, like those in the auditory system, are called hair cells because of the stereocilia that project from the apical surface of the cell (Fig. 22-6A). Each hair cell contains 60 to 100 hexagonally arranged stereocilia and a single longer kinocilium. The stereocilia are oriented in rows of ascending height, with the tallest lying next to the lone kinocilium. The stereocilia arise from a region of dense actin, the cuticular plate, located at the apical end of the hair cell. The cuticular plate acts as an elastic spring to return the stereocilia to the normal upright position after bending. Each stereocilium is connected to its neighbor by small filaments.
There are two types of hair cells, and they differ in their pattern of innervation by fibers of the eighth cranial nerve (Fig. 22-6A). Type I hair cells are chalice shaped and typically are surrounded by an afferent terminal that forms a nerve calyx. Type II hair cells are cylindric and are innervated by simple synaptic boutons. Excitatory amino acids such as aspartate and glutamate are the neurotransmitters at the receptor cell–afferent fiber synapses. Both types of hair cells, or their afferents, receive synapses from vestibular efferent fibers that control the sensitivity of the receptor. These efferent fibers contain acetylcholine and calcitonin gene–related peptide as neurotransmitters. Efferent cell bodies are located in the brainstem just rostral to the vestibular nuclei and lateral to the abducens nucleus. They are activated by behaviorally arousing stimuli or by trigeminal stimulation.
Within each ampulla, the hair cells and their supporting cells lie embedded in a saddle-shaped neuroepithelial ridge, the crista, which extends across the base of the ampulla (Fig. 22-6B). Type I hair cells are concentrated in central regions of the crista, and type II hair cells are more numerous in peripheral areas. Arising from the crista and completely enveloping the stereocilia of the hair cells is a gelatinous structure, the cupula. The cupula attaches to the roof and walls of the ampulla, forming a fluid-tight partition that has the same specific density as that of endolymph. Rotational head movements produce angular accelerations that cause the endolymph in the membranous ducts to be displaced so that the cupula is pushed to one side or the other like the skin of a drum. These cupular movements displace the stereocilia (and kinocilium) of the hair cells in the same direction.
For the otolith organs, a structure analogous to the crista, the macula, contains the receptor hair cells (Fig. 22-6C). The hair cell stereocilia of otolith organs extend into a gelatinous coating called the otolith membrane, which is covered by calcium carbonate crystals called otoconia (from the Greek, meaning “ear stones”). Otoconia are about three times as dense as the surrounding endolymph, and they are not displaced by normal endolymph movements. Instead, changes in head position relative to gravity or linear accelerations (forward-backward, upward-downward) produce displacements of the otoconia, resulting in bending of the underlying hair cell stereocilia.
Hair Cell Transduction
The response of hair cells to deflection of their stereocilia is highly polarized (Figs. 22-7 and 22-8A). Movements of the stereocilia toward the kinocilium cause the hair cell membranes to depolarize, which results in an increased rate of firing in the vestibular afferent fibers. If the stereocilia are deflected away from the kinocilium, however, the hair cell is hyperpolarized and the afferent firing rate decreases.
Figure 22-7. Physiologic responses of vestibular hair cells and their vestibular afferent fibers. Asp, aspartate; Glu, glutamate.
The mechanisms underlying the depolarization and hyperpolarization of vestibular hair cells depend, respectively, on the potassium-rich character of endolymph and the potassium-poor character of the perilymph that bathes the basal and lateral portions of the hair cells. Deflection of the stereocilia toward the kinocilium causes potassium channels in the apical portions of the stereocilia to open. Potassium flows into the cell from the endolymph, depolarizing the cell membrane (Fig. 22-7). This depolarization in turn causes voltage-gated calcium channels at the base of the hair cells to open, allowing calcium to enter the cell. The influx of calcium causes synaptic vesicles to release their transmitter (aspartate or glutamate) into the synaptic clefts, and the afferent fibers respond by undergoing depolarization and increasing their rate of firing. When the stimulus subsides, the stereocilia and kinocilium return to their resting position, allowing most calcium channels to close and voltage-gated potassium channels at the base of the cell to open. Potassium efflux returns the hair cell membrane to its resting potential (Fig. 22-7).
Deflection of the stereocilia away from the kinocilium causes potassium channels in the basolateral portions of the hair cell to open, allowing potassium to flow out from the cell into the interstitial space. The resulting hyperpolarization of the cell membrane decreases the rate at which the neurotransmitter is released by the hair cells and consequently decreases the firing rate of afferent fibers.
Almost all vestibular primary afferent fibers have a moderate spontaneous firing rate at rest (approximately 90 spikes per second). Therefore it is likely that some hair cell calcium channels are open at all times, causing a slow, constant release of neurotransmitter. The ototoxic effects of some aminoglycoside antibiotics (e.g., streptomycin, gentamicin) may be due to direct reduction of the transduction currents of hair cells.
Morphologic Polarization of Hair Cells
Given that deflections of the stereocilia toward and away from the kinocilium cause opposing physiologic responses, it is clear that the directional orientation of the hair cells in the vestibular organs will play an essential role in signaling the direction of movement. On the cristae of the horizontal semicircular canal, the hair cells are all arranged with their kinocilium on the side closer to the utricle (Fig. 22-8B). Thus movement of endolymph toward the ampulla in the horizontal canal causes the stereocilia to be deflected toward the kinocilium, resulting in depolarization of the hair cell. In the vertical semicircular canals, the hair cells are arranged with their kinocilium on the side farther from the utricle (closest to the endolymphatic duct). Thus the hair cells of the vertical canals are hyperpolarized by movement of endolymph toward the ampulla (ampullipetal movement) and are depolarized by movement away from the ampulla (ampullifugal movement).
In both the utricle and the saccule, the otolith membrane overlying the hair cells contains a small, curving depression, the striola, that roughly bisects the underlying macula (Fig. 22-8C). Hair cells on the utricular macula are polarized so that the kinocilium is always on the side toward the striola (Figs. 22-6C and 22-8C), which effectively splits the receptors into two morphologically opposed groups. In contrast, the kinocilia of saccular hair cells are oriented on the side away from the striola. Because the striola curves through the macula, otolith hair cells are polarized in many different directions (Fig. 22-8C). In this way, utricular and saccular hair cells are directionally sensitive to a wide variety of head positions and linear movements.
SEMICIRCULAR CANALS AND OTOLITH ORGANS
As stated previously, the vestibular receptors transduce movement and position stimuli into neural signals that are sent to the brain. The semicircular canals are responsive to rotational acceleration resulting from turns of the head or body. The otolith organs are responsive to linear accelerations. The most prominent linear acceleration on earth is the constant force of gravity. Linear motion, such as experienced during swinging on a swing or flying in an airplane through turbulence, couples with gravity to change the direction and amplitude of the resultant gravitoinertial acceleration (GIA). The GIA is sensed by the otolith organs and can be greatly reduced during space flight. Linear accelerations also occur in situations such as up-and-down motion during running and acceleration of an automobile. The otolith organs are also responsive to tilting of the head relative to gravity (pitch and roll movements). Forward and backward tilting is called pitch; side-to-side tilting is called roll.
Function of Semicircular Canals
The membranous semicircular duct can be thought of as a fluid-filled tube with a partition (the cupula) in the middle (Fig. 22-6B). Because the utricles are located medially, compared with the horizontal canals, on each side of the head, the hair cells of the complementary left and right semicircular canals are oppositely polarized. An example is seen in rotational head movements made in the horizontal plane (Fig. 22-9). When the head is stationary (no angular acceleration), the endolymph and the cupula remain still and the afferents from the two horizontal semicircular canals fire at the same (resting) rate (Fig. 22-9A). When the head turns to the right or left, however, the horizontal semicircular ducts turn with it, but the endolymph lags owing to inertial forces and the viscous drag between the fluid and the duct wall. The lagging endolymph deflects the cupula, which in turn deflects the stereocilia of the hair cells. As Figure 22-9B shows, a leftward turn of the head causes the stereocilia in the left horizontal canal ampulla to be deflected toward their kinocilia, resulting in an increase in the discharge rate of the eighth nerve afferents on the left side. Simultaneously, the hair cells in the right horizontal canal ampulla are hyperpolarized, so their afferents show a decreased rate of firing. A rightward head turn produces the opposite pair of responses (Fig. 22-9C).
The left and right semicircular canals of each functional pair (such as the left and right horizontal canals) always respond oppositely to any head movement that affects them. This fact leads to the “push-pull” concept of vestibular function, which states that directional sensitivity to head movement is coded by opposing receptor signals. Because of commissural connections, neurons in the vestibular nuclei receive information from receptors on both sides of the head. These neurons act as comparator units that interpret head rotation on the basis of the relative discharge rates of left and right canal afferents. This pattern of connections also increases the sensitivity of the system, so that even small differences in the discharge rates of afferents from corresponding canal pairs (such as in slow head movements) can be perceived. During a leftward head turn, the comparator units receive impulses at a higher frequency from the left horizontal canal than from the right horizontal canal; the difference is interpreted as a left head turn. Similar conditions exist when the head is pitched or rolled so that the vertical semicircular canals are stimulated by rotational accelerations in their respective planes. However, in the case of the vertical canals, the opposing push-pull responses occur between the anterior semicircular canal in one ear and the posterior semicircular canal of the opposite ear (Fig. 22-3).
Head trauma or disease can change the normal resting activity in eighth nerve afferent fibers. This change may be interpreted by the brain as turning, even though the head is stationary. For example, a lesion of the eighth nerve, such as that produced by a glomus tumor or vestibular schwannoma (Fig. 22-10), may reduce the frequency of impulses in the ipsilateral afferent fibers or block their impulse transmission entirely. The comparator units of the vestibular nuclei will then consistently receive a higher impulse frequency from the intact side, which will be interpreted as a head turn away from the side of the lesion.
Function of Otolith Organs
The receptor hair cells in the maculae do not respond to head rotation but are sensitive to linear acceleration and tilt of the head (Fig. 22-11). When the head is moved with respect to gravity (rolled or pitched), the otoconia crystals are displaced because of their density with respect to the surrounding endolymph. This displacement shifts the underlying gelatinous coating on the maculae and produces stereocilia deflection in the hair cells. Like the responses of semicircular canal hair cells, otolith organ hair cells are either depolarized or hyperpolarized with stereocilia deflection toward or away from the kinocilium, respectively. However, hair cells on the maculae are oriented according to their position relative to the striola (Fig. 22-8). Hair cells on one side of the striola will be depolarized, and hair cells on the other side of the striola will be hyperpolarized (Fig. 22-11). Because the striola is curved, only certain groups of cells will be affected by a specific direction of head tilt or linear acceleration. Thus movement is encoded by a macular map of directional space. The eighth nerve fibers maintain the directional signal because each afferent innervates only hair cells from a small region on the macular neuroepithelium.
VESTIBULAR NUCLEI
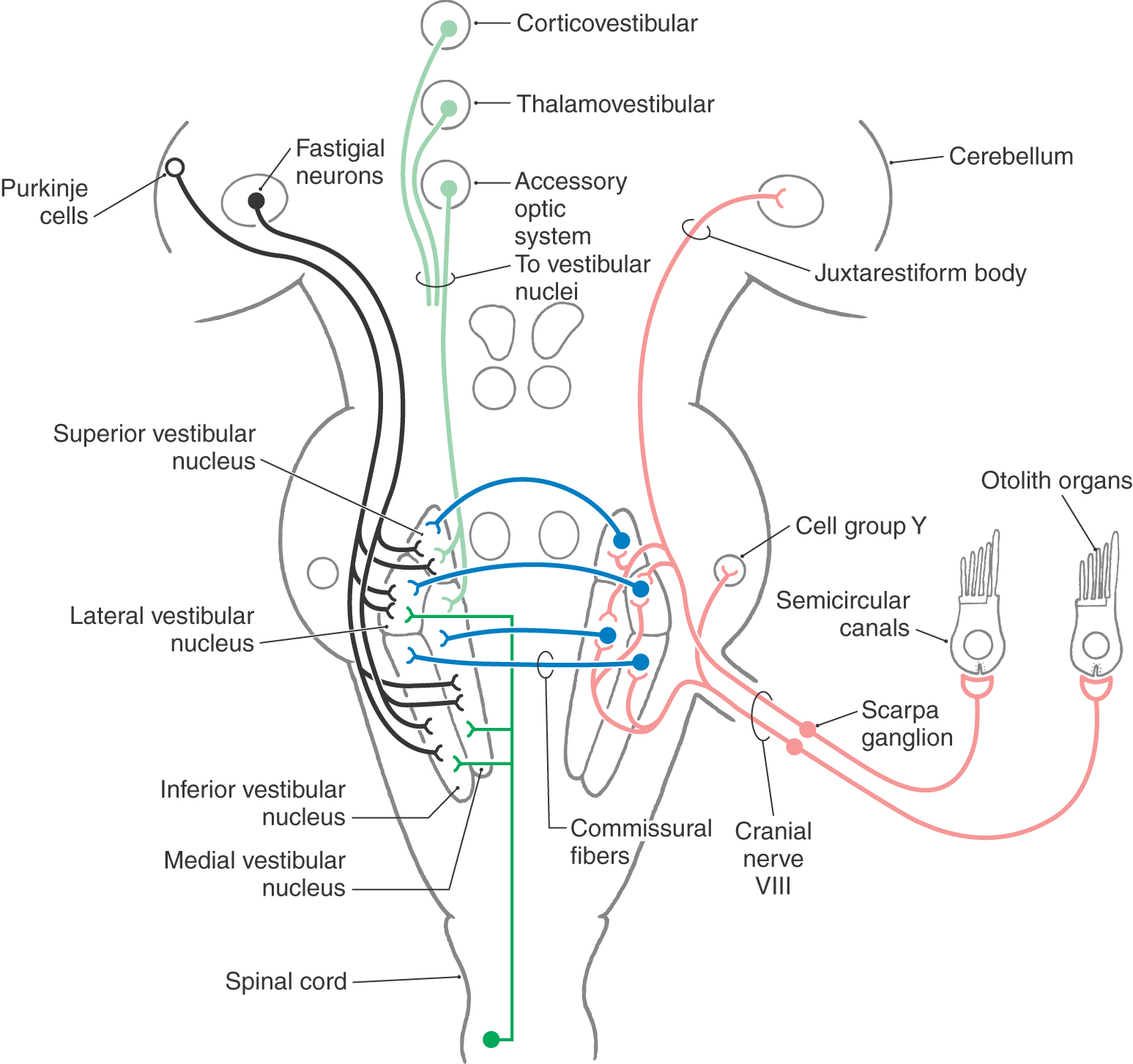
Neural information carried on vestibular afferent fibers is transmitted to the four vestibular nuclei, which lie in the rostral medulla and caudal pons (Fig. 22-12). The superior vestibular nucleus lies superolaterally in the central pons and is bordered by the restiform body and the fourth ventricle (Fig. 22-12B). The medial vestibular nucleus lies in the lateral floor of the fourth ventricle throughout most of its rostrocaudal extent (Fig. 22-12B-E). The lateral vestibular nucleus lies lateral to the medial vestibular nucleus (Fig. 22-12B, C) and contains some large neurons known as Deiters cells. Located lateral to the medial vestibular nucleus, the inferior (or descending) vestibular nucleus extends through much of the medulla (Fig. 22-12D-F).
The processing of positional and movement information for control of visual and postural reflexes largely takes place in the vestibular nuclei. Consequently, the major targets for efferents of the vestibular nuclei include the oculomotor nuclei, the vestibulocerebellum, the contralateral vestibular nuclei, the spinal cord, the reticular formation, and the thalamus. Each vestibular nucleus differs in its cytoarchitecture and its afferent and efferent connections.
Vestibular Afferent Inputs
Vestibular primary afferent fibers enter the brainstem at the pontomedullary junction. These fibers traverse the restiform body and then bifurcate into ascending and descending branches. Afferent fibers from the semicircular canals project primarily to the superior and medial vestibular nuclei, although lesser inputs also reach the lateral and inferior vestibular nuclei (Fig. 22-13). The otolith organs project primarily to the lateral, medial, and inferior vestibular nuclei. Saccular afferents also project to cell group Y, which in turn excites neurons in the contralateral oculomotor nucleus and influences vertical eye movements.
Figure 22-13. Afferents to the vestibular nuclei. Open cell bodies represent inhibitory projections.
The termination of vestibular afferent fibers on neurons of the vestibular nuclei is highly ordered. Individual central neurons in the superior and medial vestibular nuclei appear to receive information from otolith receptors and from one semicircular canal pair (either horizontal or vertical). Vestibular neurons in the lateral and inferior nuclei mostly receive information from several canal pairs and otolith receptors. As a result of their input, neurons in the vestibular nuclei show directional selectivity for particular head movements and can encode both the angular and linear components of head movements. These cells distribute information about both the direction and the speed of the head movement, as well as the position of the head with respect to gravity, to many different regions of the brain.
Cerebellar Connections
The vestibular labyrinth is the only sensory organ in the body that sends direct primary afferent projections to the cerebellar cortex and nuclei (Fig. 22-13). These primary vestibulocerebellar fibers course through the juxtarestiform body, the smaller medial part of the inferior cerebellar peduncle. Primary vestibulocerebellar fibers send collaterals to the dentate nucleus and terminate as mossy fibers in the nodulus, the uvula, and perhaps the flocculus. Neurons in all four vestibular nuclei also send axons to the cerebellum as secondary vestibulocerebellar projections. These axons end in the flocculonodular lobe, the uvula, the immediately adjacent portions of the paraflocculus, and the fastigial and dentate nuclei of the cerebellum.
The cerebellum forms reciprocal connections with the vestibular nuclei. This cerebellovestibular projection includes Purkinje cell axons (cerebellar corticovestibular fibers) from the nodulus, uvula, flocculus, and other areas of the cerebellar vermis. In addition, projections from the fastigial nucleus (fastigiovestibular fibers) also innervate the vestibular nuclei. Purkinje cells are GABAergic (γ-aminobutyric acid) and therefore inhibitory, whereas the fastigiovestibular fibers use glutamate or aspartate and are excitatory. These vestibulocerebellar and cerebellovestibular fibers all pass through the juxtarestiform body. The reciprocal connections between the cerebellum and the vestibular nuclei constitute important regulatory mechanisms for the control of eye movements, head movements, and posture.
Commissural Connections
Commissural vestibulovestibular fibers arise from all vestibular nuclei, but they appear to be most prominent from the superior and medial nuclei. Many of these fibers form reciprocal connections with the analogous contralateral nucleus. Most vestibulovestibular cells contain the inhibitory neurotransmitter GABA or glycine, although some may use the excitatory amino acids. These commissural fibers provide the pathways by which information from pairs of corresponding semicircular canals and otolith organs can be compared. Commissural fibers also play a major role in vestibular compensation, a process by which reflexes and postural control that are impaired as a result of unilateral loss of vestibular receptor function (through trauma or disease) are restored gradually by means of central adjustment.
Other Afferent Connections
Spinovestibular fibers arise from all levels of the spinal cord and provide proprioceptive input primarily to the medial and lateral vestibular nuclei. Information concerning movement of the head through the visual world also reaches vestibular nuclei neurons through the accessory optic system (see Chapter 28). Vestibular nuclei neurons also receive input from the reticular formation, primarily from cells relaying information about proprioception but also from serotonergic cells in raphe nuclei that exert modulatory or arousal effects. Finally, both the thalamus and several cortical regions provide direct descending connections to the vestibular nuclei.
Other Efferent Connections
Vestibular neurons send efferent projections to the hair cells and afferent terminals in the vestibular labyrinth, although their function remains enigmatic. Other vestibular efferent projections include the reticular formation, the posterior (dorsal) pontine nuclei, and the nucleus of the solitary tract (NTS). Cells in the medial and inferior vestibular nuclei project to the NTS and ventrolateral medullary reticular nuclei, where they mediate changes in respiration and circulation that occur with changes in posture. These compensatory vestibular autonomic responses serve to stabilize respiration, heart rate, and blood pressure during normal body position changes relative to gravity and during locomotion. Acute vestibular disorders often result in dysfunction of the vestibular autonomic responses that can lead to orthostatic hypotension, postural tachycardia, and falls. Other efferents may also be important for induction of motion sickness and emesis.
VESTIBULOOCULAR NETWORK
It is often necessary to keep one’s gaze fixed on an object of interest while the head is moving—as in reading a sign on a building while walking down the street. The vestibular system provides this capability by eliciting compensatory eye movements through a network of neural connections. These stabilizing eye movements, collectively known as the vestibuloocular reflex, are said to be compensatory because they are equal in magnitude and opposite in direction to the head movement perceived by the vestibular system. The vestibuloocular reflex occurs for any direction or speed of head movement, whether the movement is rotational, linear, or a combination of both. The reflex can also be suppressed at will if, for example, one wishes to focus on a moving target while turning the head in the same direction (as when watching an airplane or baseball move across the sky).
Rotational Vestibuloocular Reflex
There are three types of rotationally induced eye movements: horizontal, vertical, and torsional. Each of the six pairs of eye muscles (see Chapter 28) must be controlled in unison to produce the appropriate response. Thus the vertical semicircular canals and the saccule are responsible for controlling vertical eye movements, whereas the horizontal canals and the utricle control horizontal eye movements. Torsional eye movements are controlled by the vertical semicircular canals and the utricle.
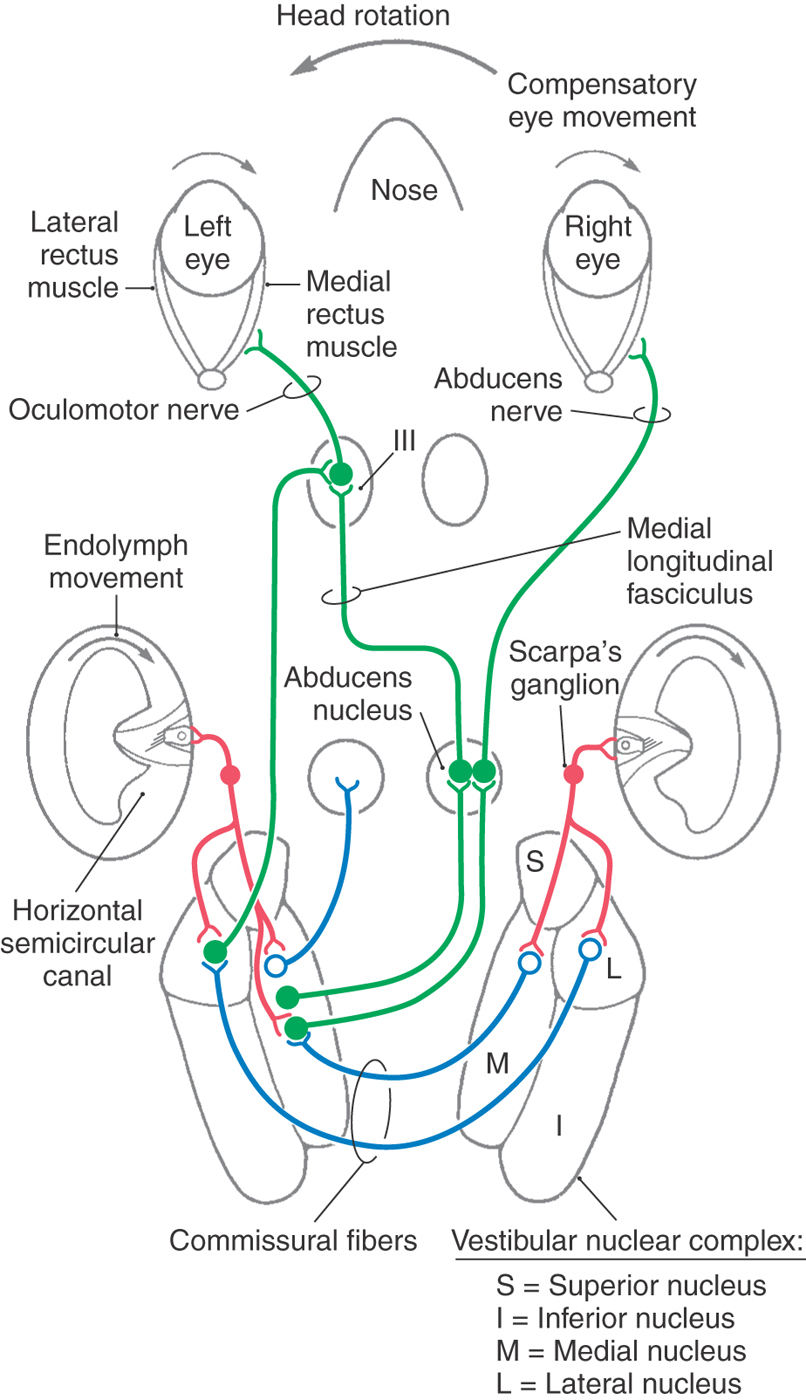
For purposes of example, only the horizontal vestibuloocular reflex is described here (Fig. 22-14). Primary afferents from the horizontal semicircular canals project to specific neurons in the medial and lateral vestibular nuclei. Most of these cells send excitatory signals through the medial longitudinal fasciculus to the contralateral abducens nucleus. Abducens motor neurons send impulses via the sixth cranial nerve to excite the ipsilateral lateral rectus muscle. At the same time, abducens interneurons send excitatory signals to motor neurons in the contralateral oculomotor nucleus, which innervates the medial rectus muscle. A second population of vestibular neurons sends excitatory signals to the medial rectus subdivision of the ipsilateral oculomotor nucleus. A third group of vestibular neurons carry inhibitory signals to the ipsilateral abducens nucleus.
During a leftward head turn, excitatory signals from the left horizontal semicircular canal afferents increase the firing rate of neurons in the left vestibular nuclei (Fig. 22-14). At the same time, inhibitory signals from the right vestibular nuclei are decreased via commissural neurons. Neurons in the left vestibular nuclei then excite both the contralateral abducens motor neurons and interneurons, which in turn produce contraction in the right lateral rectus and the left medial rectus muscles (Fig. 22-14). The resulting rightward eye movement keeps the object of interest on the fovea. Through matching bilateral connections, the left lateral rectus and right medial rectus eye muscles are inhibited.
A similar pattern of connections links the vertical semicircular canals with the motor neurons in the trochlear and oculomotor nuclei to control vertical and torsional responses (see also Chapter 28). The vertical vestibuloocular reflex originates primarily from neurons in the superior vestibular nucleus, although some medial vestibular nucleus neurons also participate.
Linear Vestibuloocular Reflex
During linear movements that do not involve head rotation, an appropriate vestibuloocular reflex also occurs. These reflexes depend on input from the otolith organ receptors and involve connections to the extraocular motor neuron pools that are similar to those described previously for the rotational vestibuloocular reflex. For example, side-to-side head movements result in a horizontal eye movement in a direction opposite to the head movement. Vertical displacements of the body, such as occur during walking or running, elicit oppositely directed vertical eye movements to stabilize gaze. During roll tilts of the head, the compensatory eye movement is termed a counterroll and is actually a torsional eye movement (Fig. 22-11).
Nystagmus
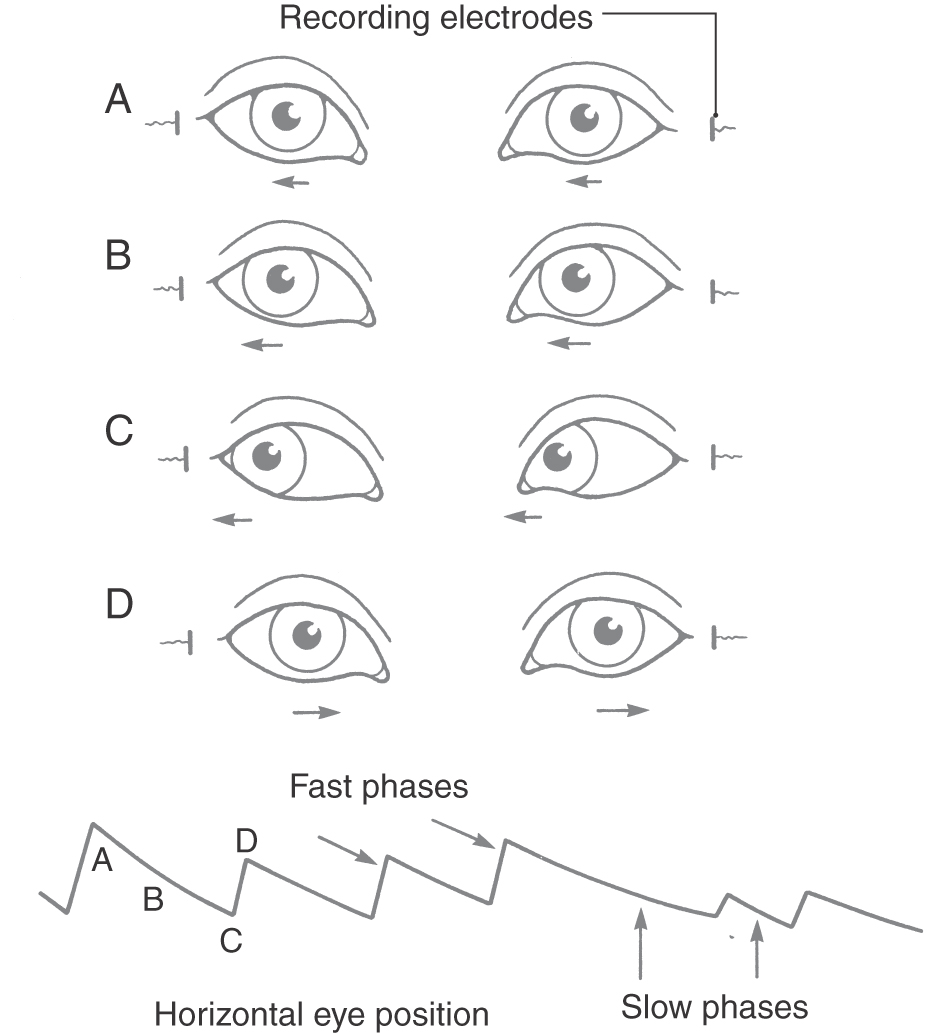
With large head rotations, such as with a 360-degree body turn, compensatory eye movements take another form (Fig. 22-15). Initially, the vestibuloocular reflex directs the eyes slowly in the direction opposite to the head motion. This movement is called the slow phase. When the eye reaches the limit of how far it can turn in the orbit, it springs back rapidly to a central position, moving in the same direction as that of the head, also known as the fast phase. Another slow phase then begins. This combination of slow compensatory phases punctuated by fast return phases is called nystagmus. Nystagmus movements are named for the direction of the fast return phase, for example, as leftward-beating nystagmus or downward-beating nystagmus. Nystagmus takes many forms and is often observed clinically (see also Chapter 28). In cases of head injury with acute temporal bone fracture, the semicircular canals can be affected, producing rapid spontaneous nystagmus that can persist for hours or days.
Figure 22-15. Vestibular nystagmus in the horizontal plane showing slow-phase (A to C) and fast-phase (D) eye movements.
Nystagmus can be used as a diagnostic indicator of vestibular system integrity. Typically, in patients complaining of dizziness or vertigo, the function of the vestibular labyrinth is assessed by administration of a caloric test. Either warm (40° C) or cold (30° C) water is introduced into the external auditory canal. In normal persons, warm water induces nystagmus that beats toward the ear into which the water has been introduced, whereas cold water induces nystagmus that beats away from the ear into which the water has been introduced. (This relationship is encapsulated in the mnemonic COWS: Cold water produces nystagmus beating to the Opposite side; Warm water produces nystagmus beating to the Same side.) In normal persons, the two ears give equal responses. If there is a unilateral lesion in the vestibular pathway, however, nystagmus will be reduced or absent on the side of the lesion.
VESTIBULOSPINAL NETWORK
The vestibular system influences muscle tone and produces reflexive postural adjustments of the head and body through two major descending pathways to the spinal cord, the lateral vestibulospinal tract and the medial vestibulospinal tract (Fig. 22-16). There is also a reticulospinal pathway that receives input from the vestibular system.
 Figure 22-16. Pathways making up the vestibulospinal system.
Figure 22-16. Pathways making up the vestibulospinal system.
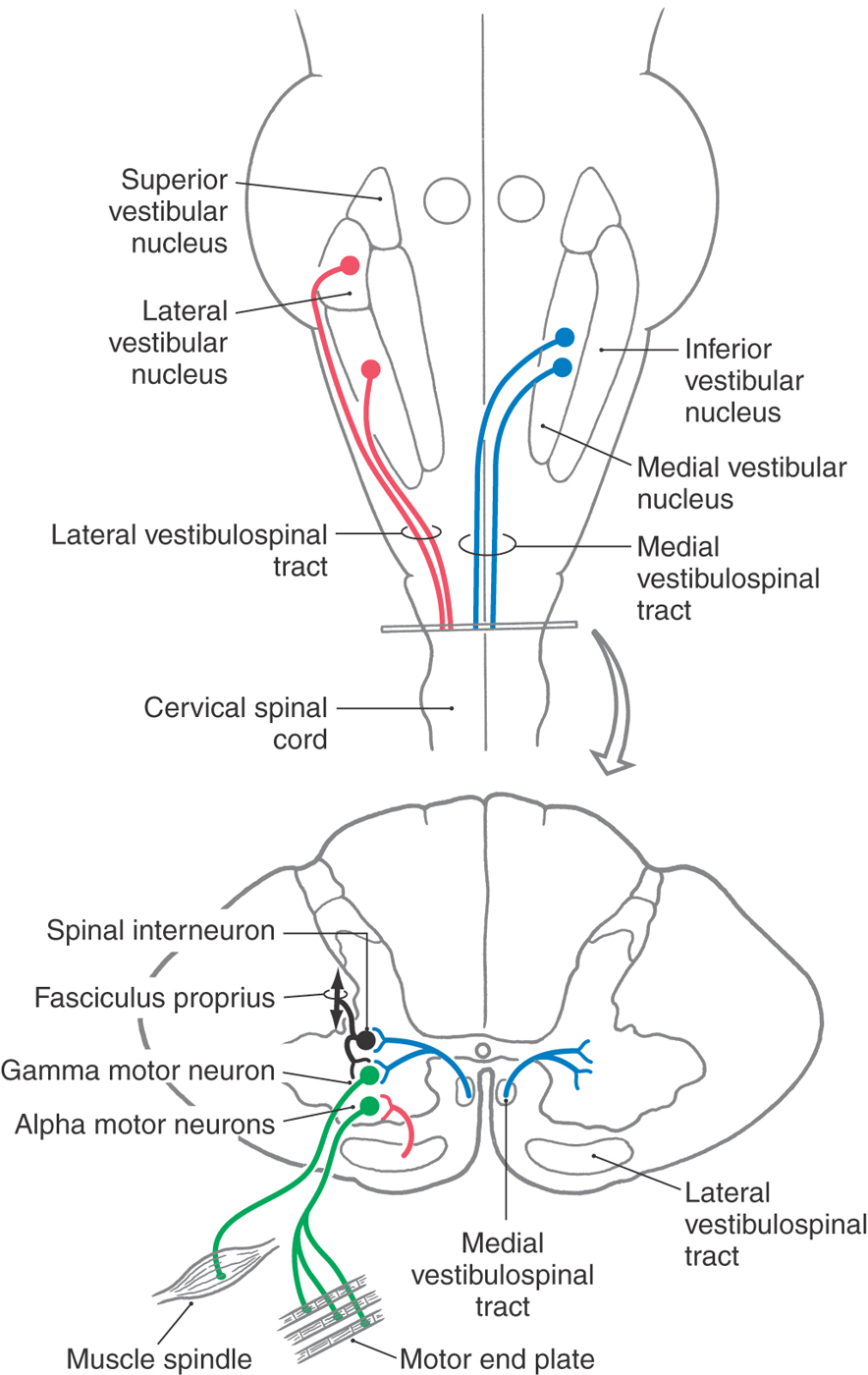
Lateral Vestibulospinal Tract
The lateral vestibulospinal tract (LVST) arises primarily from neurons in the lateral and inferior vestibular nuclei and projects to all levels of the ipsilateral spinal cord. This projection is topographically organized. Cells in anterorostral areas of the lateral nucleus project to the cervical cord, whereas cells in posterocaudal regions project to the lumbosacral cord. These vestibulospinal neurons receive substantial input from orthogonal semicircular canal pairs, the otolith organs, the vestibulocerebellum, and the fastigial nucleus as well as proprioceptive inputs from the spinal cord.
Fibers of the LVST course through the lateral medulla dorsal to the inferior olivary complex and then through the anterior funiculus of the cord (Fig. 22-16) to terminate directly on alpha and gamma motor neurons and on interneurons in laminae VII to IX. Axons of many LVST neurons give off collaterals in different segments of the cord, thus ensuring that different muscle groups will be coordinated during postural control. The LVST neurons contain either acetylcholine or glutamate as a neurotransmitter and exert an excitatory influence on extensor muscle motor neurons. The coordinated actions of neurons that make up the LVST and provide postural stabilization are not completely understood. However, if a person begins tilting to the right, ipsilateral LVST fibers elicit extension of the left axial and limb musculature. Concurrently, right extensor muscles are inhibited. These actions stabilize the body’s center of gravity and preserve upright posture.
Medial Vestibulospinal Tract
The action of vestibular stimulation on neck muscles arises primarily through neurons in the medial vestibulospinal tract (MVST). These fibers originate primarily from the medial vestibular nucleus, although lesser projections arise from the inferior and lateral vestibular nuclei. Similar to LVST neurons, cells of the MVST receive input from vestibular receptors and the cerebellum as well as somatosensory information from the spinal cord. Fibers of the MVST descend bilaterally through the medial longitudinal fasciculus to terminate in laminae VII to IX of the cervical spinal cord (Fig. 22-16). These MVST fibers carry both excitatory and inhibitory signals, and they terminate on neck flexor and extensor motor neurons as well as on propriospinal neurons.
The effects of vestibular function–induced responses can be seen in the vestibulocolic reflex, which is actually a series of responses that stabilize the head in space. If, for example, a person falls forward, MVST neurons will receive signals on downward linear acceleration from the saccule, signals on the changing head position relative to gravity from both the utricle and the saccule, and signals on forward rotational acceleration from the vertical semicircular canals. The MVST neurons process this information and transmit excitatory signals to the dorsal neck flexor muscles (splenius, biventer cervicis, and complexus muscles). At the same time, inhibitory signals are sent to the anterior neck extensor muscles. The result is a neck movement upward, opposite to the falling motion, to protect the head from impact.
VESTIBULOTHALAMOCORTICAL NETWORK
Vestibular Thalamus
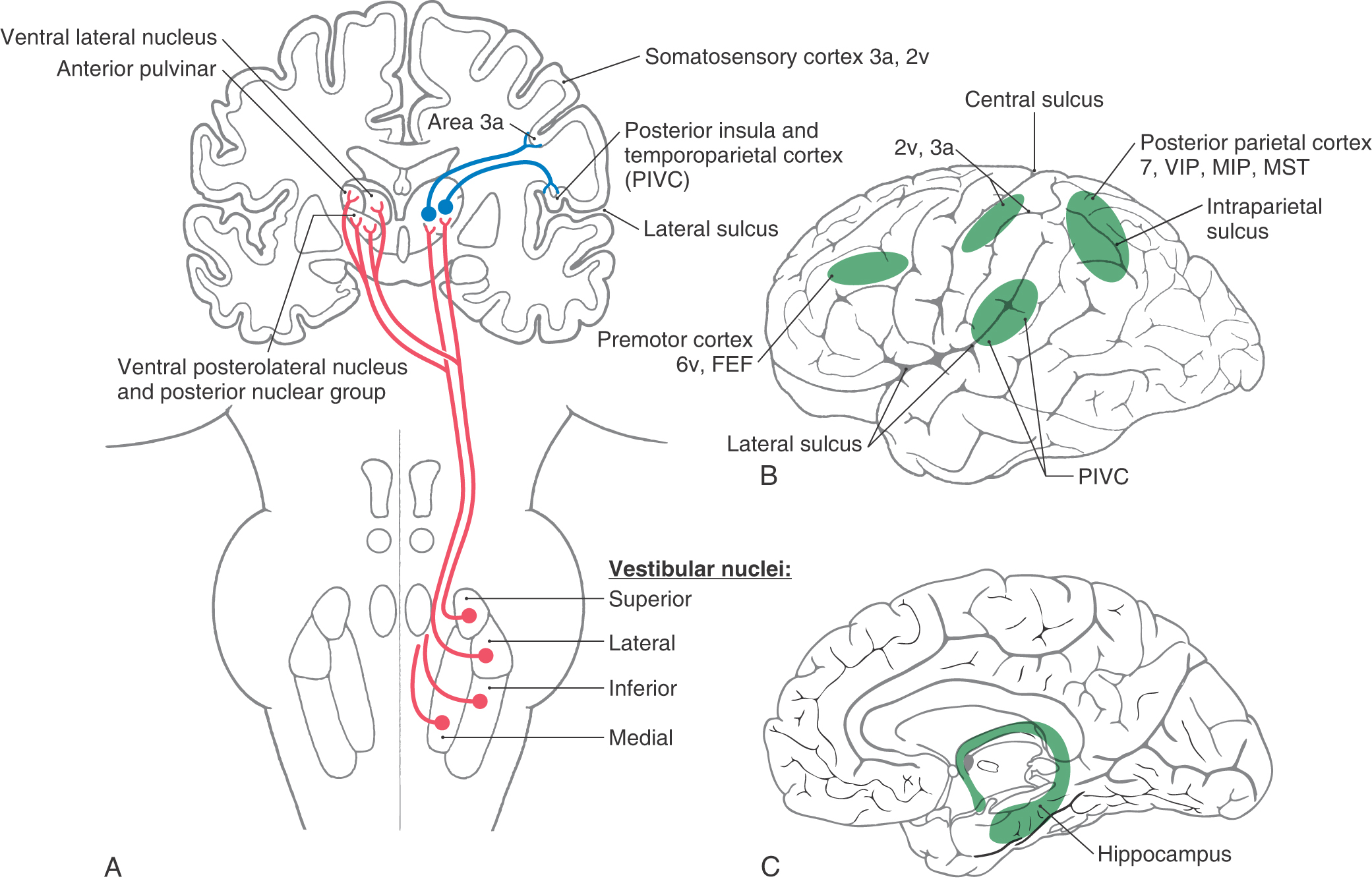
The cognitive perceptions of motion and spatial orientation arise through convergent information from the vestibular, visual, and somatosensory systems at the thalamocortical level. Neurons in all four vestibular nuclei project bilaterally to the thalamus, but most fibers terminate in the contralateral thalamic nuclei (Fig. 22-17). Several major thalamic regions have been identified as responding to vestibular stimulation.
One is located in the ventral posterolateral nucleus (VPL), with some adjacent cells located in the ventral posteroinferior nucleus (VPI). These nuclei receive somatosensory input and project to the primary somatosensory cortex (area 3a, 2v), the secondary somatosensory cortex, the posterior parietal cortex (areas 5 and 7), and the insula of the temporal cortex. In fact, many of these thalamic cells are multimodal and respond to both vestibular and somatosensory stimulation; others appear to be purely vestibular.
Another area receiving vestibular information is the posterior nuclear group (PO), which is located adjacent to the medial geniculate body. The PO receives additional auditory afferents from the inferior colliculus and inputs from the superior colliculus and spinal cord, indicating an integration of multiple sensory signals.
The anterior pulvinar, located dorsal to the VPL, which projects to cortical area 3a as well as to the posterior insula and temporoparietal cortex (PIVC), is also a target of vestibular input. Thalamic regions represent separate parallel pathways of vestibular information from the brainstem to the cortex for processing of motion and body orientation information. In humans, electrical stimulation of the thalamic areas produces sensations of movement and sometimes dizziness. Recently, patients with VPL lesions were found to have misperceptions of visual vertical and postural instability.
Vestibular Cortex
Although vestibular signals are widely distributed to a number of cortical regions, all are multimodal and none seems to represent a primary “vestibular” cortex, similar to other modalities such as vision and audition. There are four main cortical areas that respond to vestibular stimulation (Fig. 22-17).
First are areas 2v and 3a. In the primary somatosensory cortex, area 2v lies at the base of the intraparietal sulcus just posterior to the hand and mouth areas of the postcentral gyrus. Electrical stimulation of area 2v in humans produces sensations of whole body motion. Area 3a lies at the base of the central sulcus, adjacent to the motor cortex, and receives input from VPL and VPI thalamic neurons. Because these cells project to area 4 of the motor cortex, it is believed that one of their functions is to integrate motor control of the head and body.
Second is area 7 of the parietal cortex. Vestibular neurons have also been observed in the posterior parietal cortex: in area 7, the ventral intraparietal area (VIP), the medial intraparietal area (MIP), and the medial superior temporal area (MST). VIP contains multimodal neurons involved in spatial coding. MIP and MST neurons respond to both visual (optic flow) and vestibular motion signals to integrate cues about body motion through space. Lesions of the parietal cortical areas can result in confusion in spatial awareness.
A third cortical region responding to vestibular motion signals lies in the retroinsular and granular insula areas of the lateral sulcus, together termed the parietoinsular vestibular cortex (PIVC). Many PIVC cells are multisensory, responding to body motion, somatosensory, proprioceptive, and visual motion stimuli. Patients with PIVC lesions report episodes of vertigo, unsteadiness, and a loss of perception for visual vertical.
Fourth, in the prefrontal cortex, area 6 and the superior frontal gyrus receive vestibular signals and are related to the frontal eye field (FEF). The FEF is involved in the control of saccades and smooth pursuit eye movements (see Chapter 28). There is an extensive network of connections between vestibular cortical regions, primarily centered through the PIVC. In addition to cortical interconnections, there are direct corticofugal projections to the vestibular nuclei, the parabrachial nuclei, and the prepositus hypoglossi. How these different regions give rise to our perception of motion and spatial orientation is still not understood.
Navigation
The ability to maintain a sense of direction and to navigate through spatial locations is an essential cognitive function. Studies suggest that an internal model for the spatial representation of our environment (cognitive map) exists and is used for navigation. Certain cells in the limbic system and the hippocampus are involved in navigation tasks; damage to these areas impairs a variety of spatial and directional abilities. Three cell types contributing to spatial orientation have been identified, including place cells, grid cells, and head direction cells. Place cells are primarily located in the hippocampus and discharge in relation to the animal’s location in the environment. Grid cells in the entorhinal cortex respond in a tessellated pattern to spatial location. Head direction cells in the anterior dorsal thalamus discharge as a function of the animal’s heading direction, independent of location or behavior.
These regions have interconnections that are thought to function together to provide for spatial orientation, spatial memory, and our ability to navigate to learned locations, such as driving to the store or walking through our house in the dark. Both place cells and head direction cells depend on a functioning vestibular system to maintain their tuning responsiveness. The pathway by which vestibular signals reach the navigation network is not well understood. However, patients with disease or trauma to the vestibular system, hippocampus, and dorsal thalamus regions may have severe deficits in their ability to orient in familiar environments, to navigate from place to place, or even to find their way home.
DIZZINESS AND VERTIGO
Dizziness is a nonspecific term that generally means a spatial disorientation that may or may not involve feelings of movement. Dizziness may be accompanied by nausea or postural instability. A large number of factors may produce a dizzy sensation, and many are not exclusively vestibular in origin.
Vertigo is a specific perception of body motion, often spinning or turning, experienced when no real motion is taking place. Vertigo may be perceived as subjective vertigo or as objective vertigo. In subjective vertigo, the patient experiences the sensation of spinning while things in the environment are not moving; in objective vertigo, the sensation is one of objects spinning while the patient is not moving. As children, we all learn to produce vertigo by whirling in place as fast as possible and then abruptly stopping. For a few moments, the world seems to be spinning in the opposite direction. Examination of the eyes during this phase will reveal a nystagmus that beats in the direction opposite to the original direction of rotation. Vertigo can also be elicited optokinetically if the visual surroundings are revolved while the body remains stationary. Many modern amusement games take advantage of this phenomenon to produce the sensation of motion.
Benign Paroxysmal Positional Vertigo
One of the most common vestibular disorders observed clinically is benign paroxysmal positional vertigo. This condition is characterized by brief episodes of vertigo that coincide with particular changes in body position. Typically, episodes may be triggered by turning over in bed, getting up in the morning, bending over, or rising from a bent position. The pathophysiologic mechanism of benign positional vertigo is not clearly understood, but posterior canal abnormalities are implicated. One possible explanation is that otoconial crystals from the utricle separate from the otolith membrane and become lodged in the cupula of the posterior canal (a condition called cupulolithiasis). The resulting increased density of the cupula produces abnormal cupula deflections when the head changes position relative to gravity.
Vestibular Neuritis
Patients often present with severe vertigo, nausea, and vomiting yet have no accompanying hearing loss or other central nervous system abnormalities. In many of these cases, vestibular neuritis is diagnosed and is thought to involve edema of the vestibular nerve (or ganglion). The edema is most commonly believed to be produced through an acute viral infection, such as herpes simplex virus. In fact, some patients report a recent history of upper respiratory tract infection, cold, or influenza. Treatment options include antiemetics, vestibular suppressants, corticosteroids to reduce inflammation, and antiviral agents.
Sources and Additional Readings
Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564.
Baloh RW, Halmagyi GM. Disorders of the Vestibular System. New York: Oxford University Press; 1996.
Beitz AJ, Anderson JH. Neurochemistry of the Vestibular System. New York: CRC Press; 2000.
Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404.
Wilson VJ, McIvill Jones G. Mammalian Vestibular Physiology. New York: Plenum Press; 1979.
Yates BJ, Miller AD. Vestibular Autonomic Regulation. New York: CRC Press; 1996.

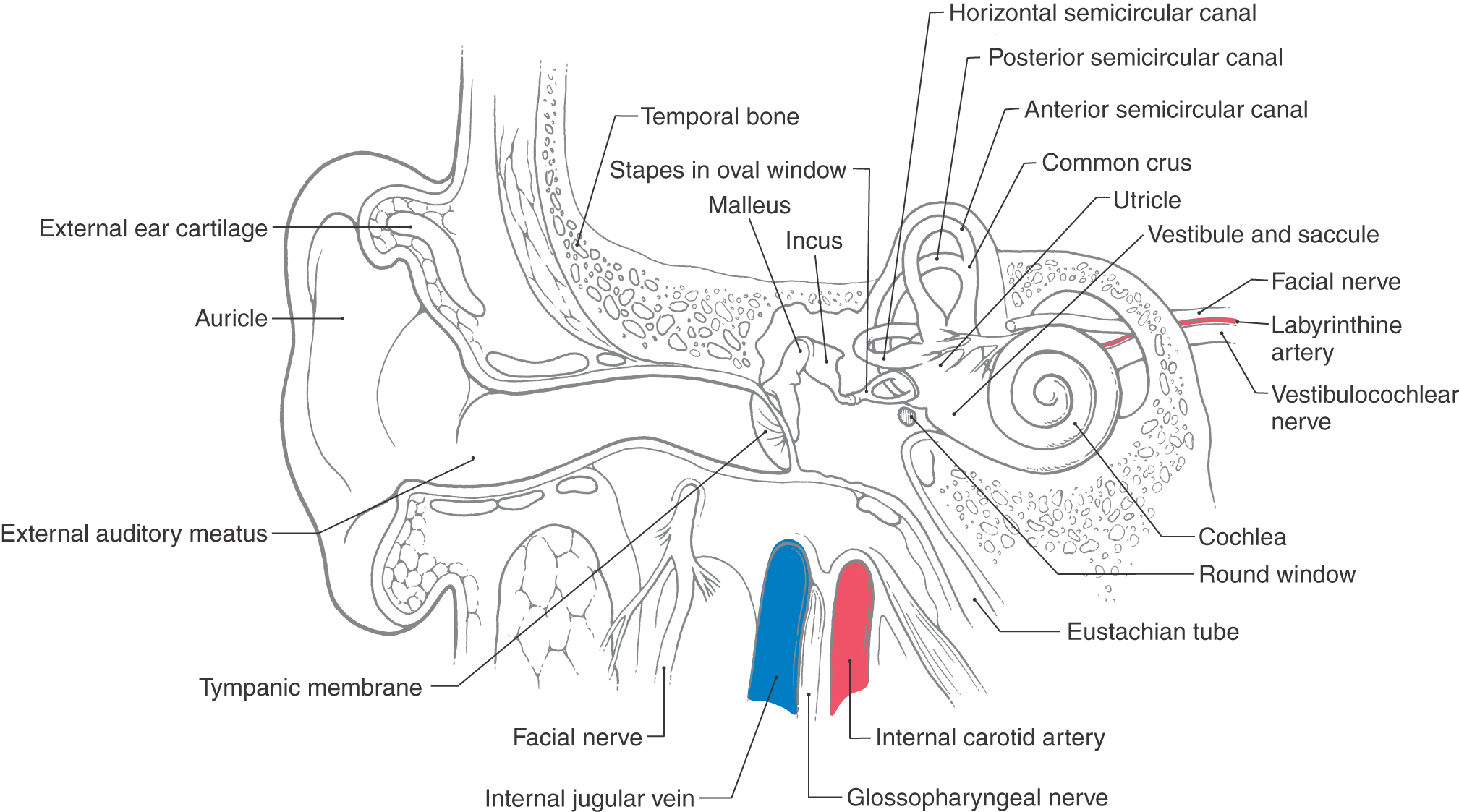
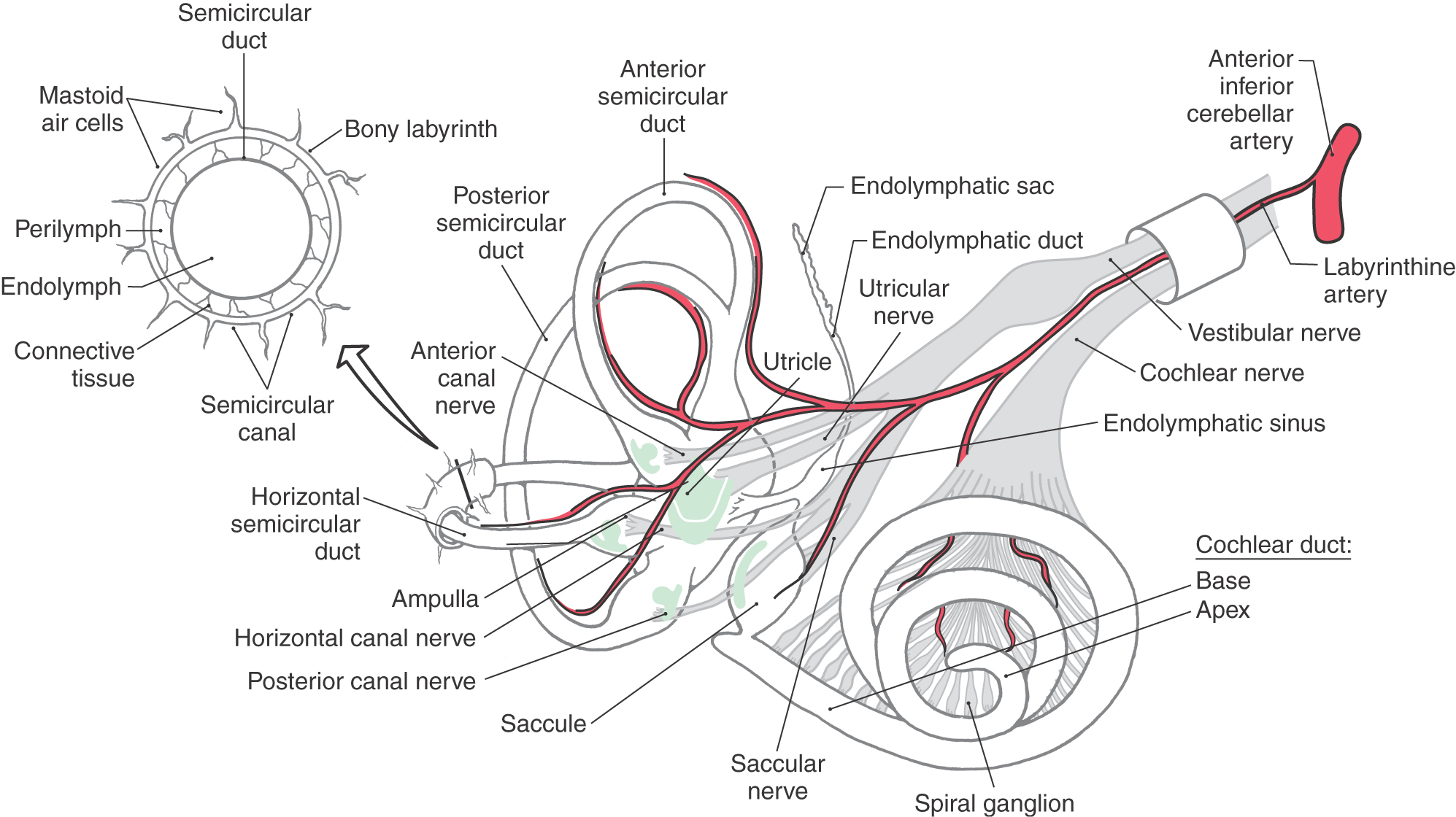
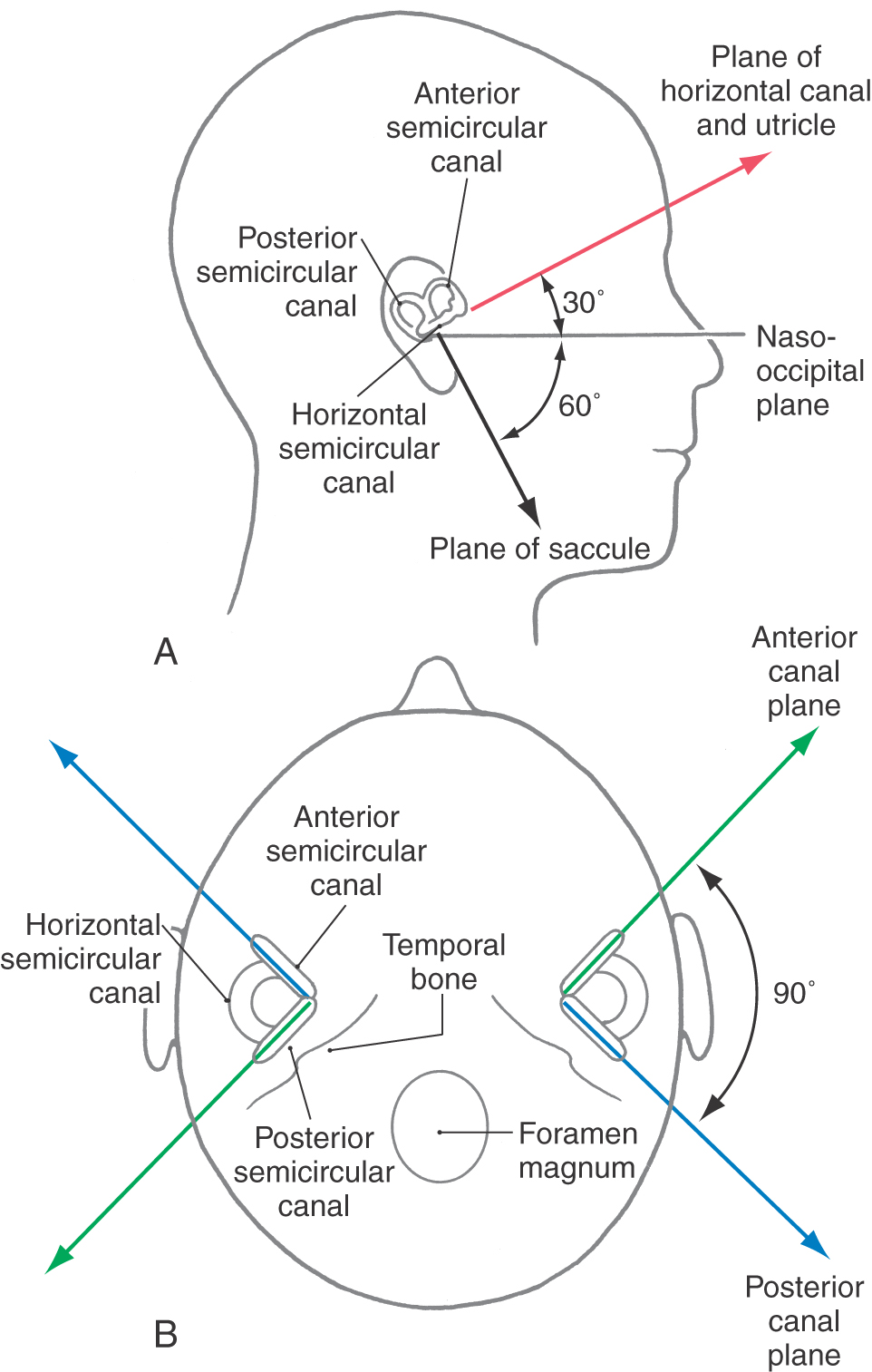
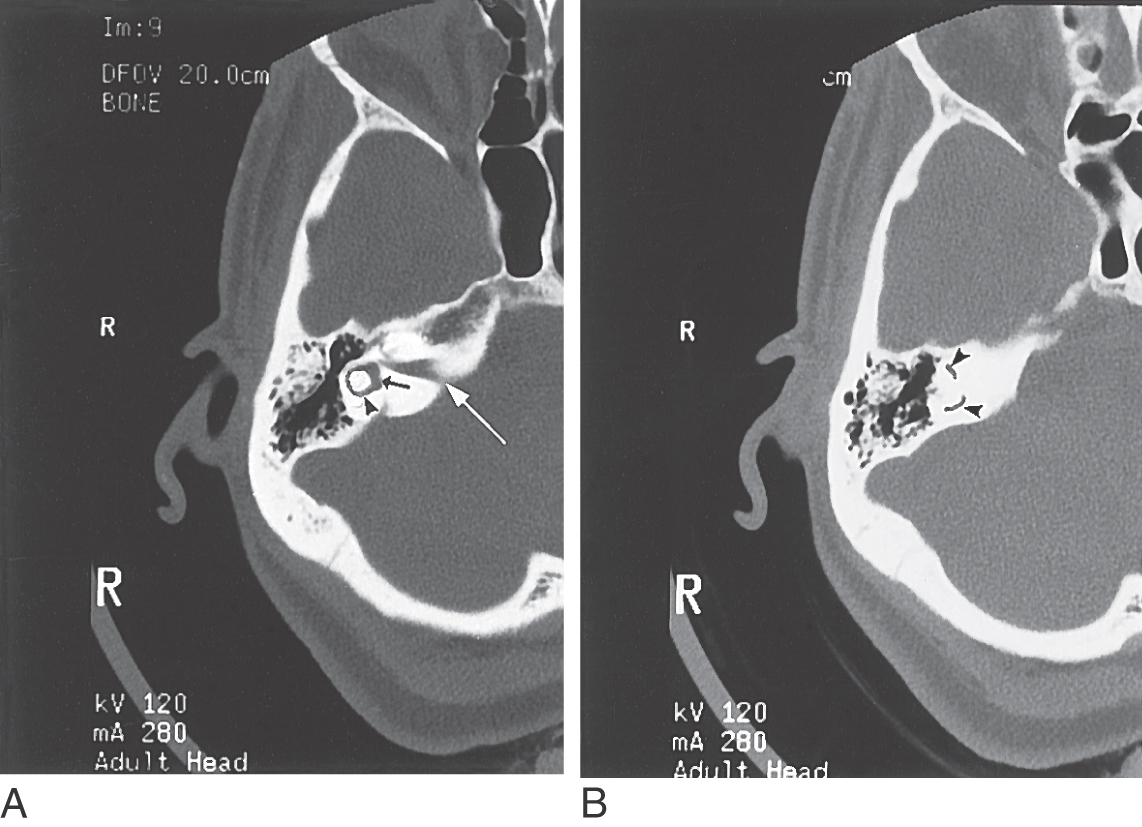
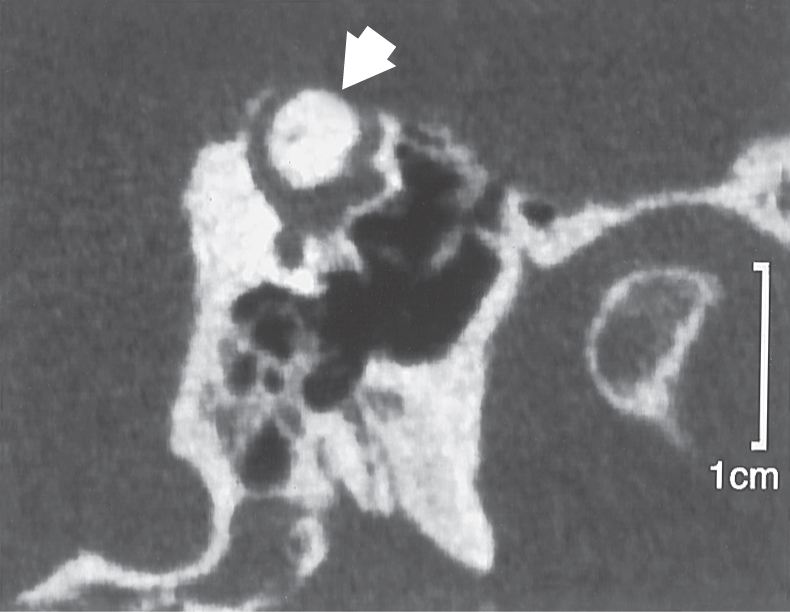
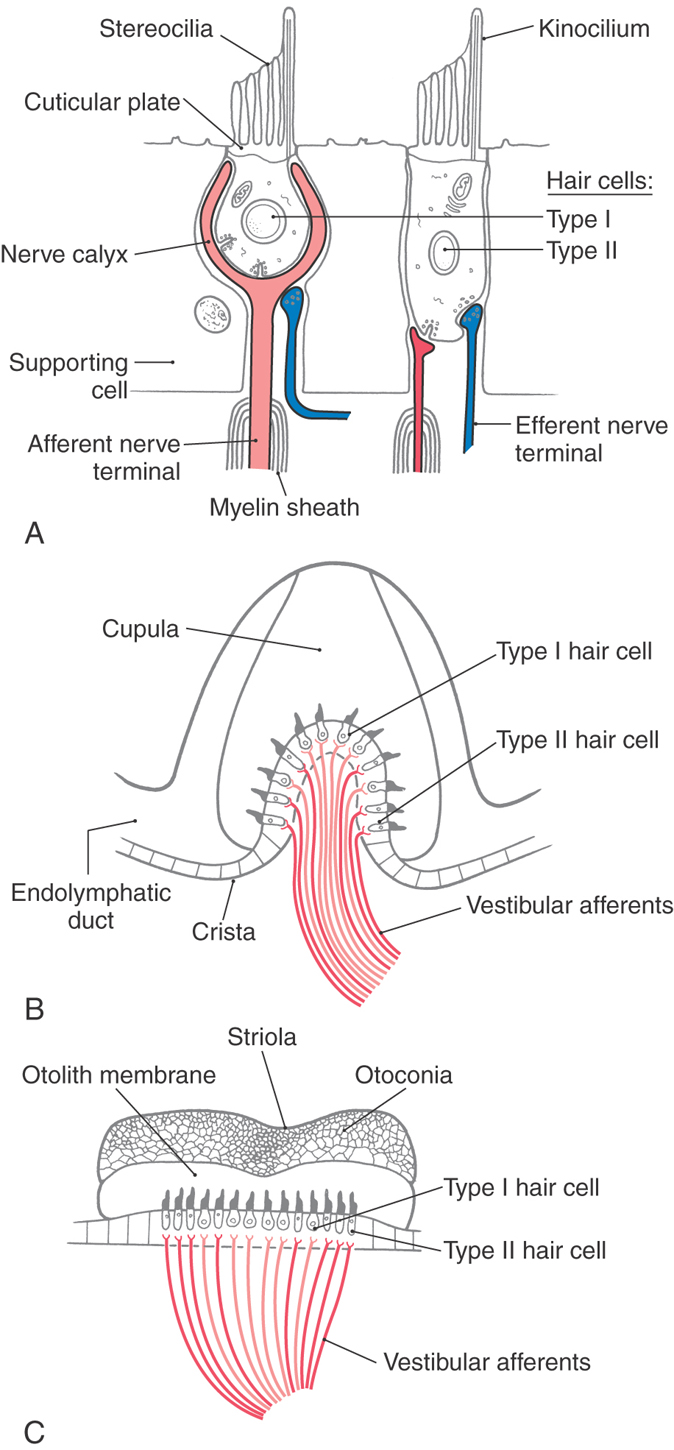
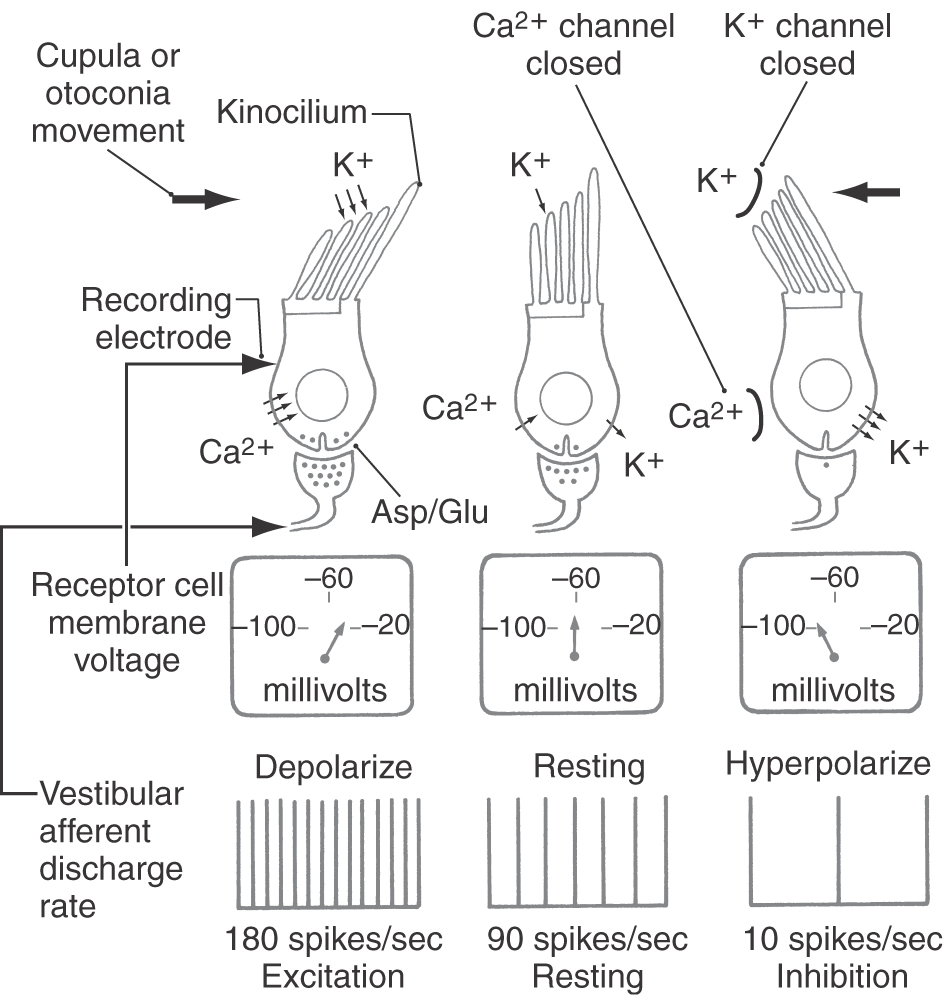
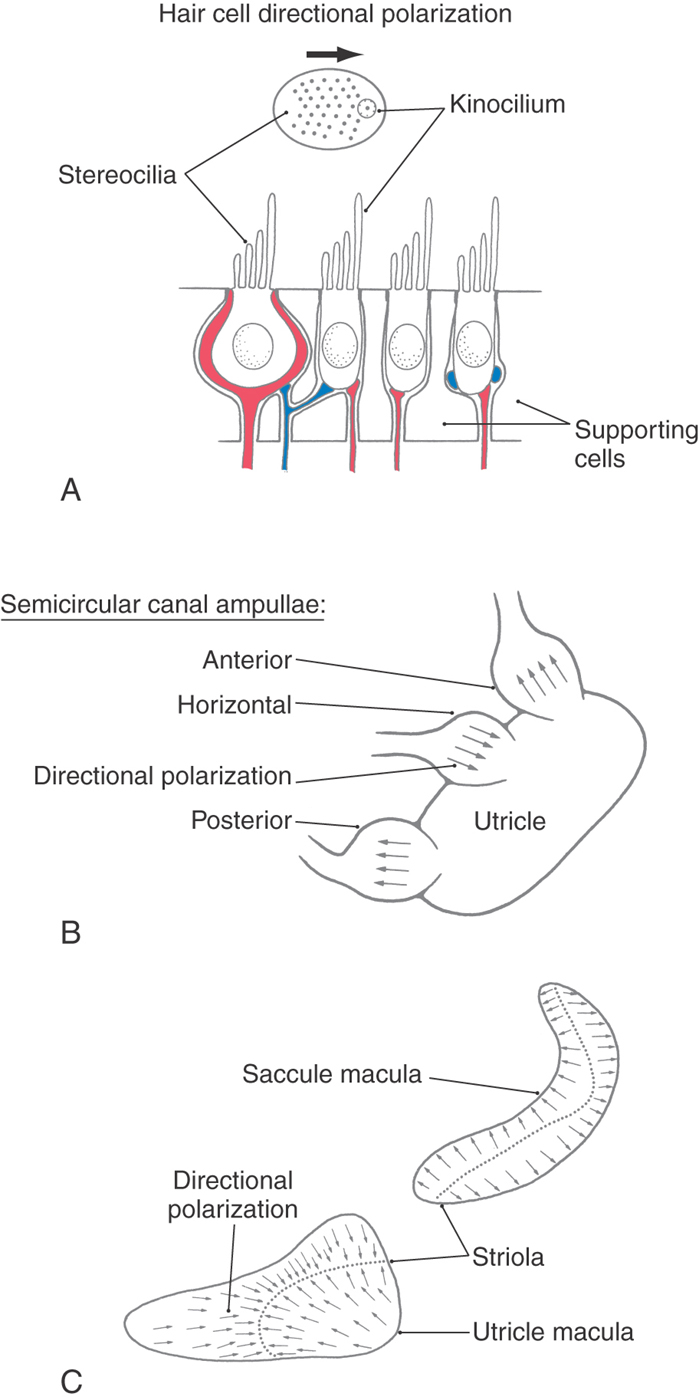
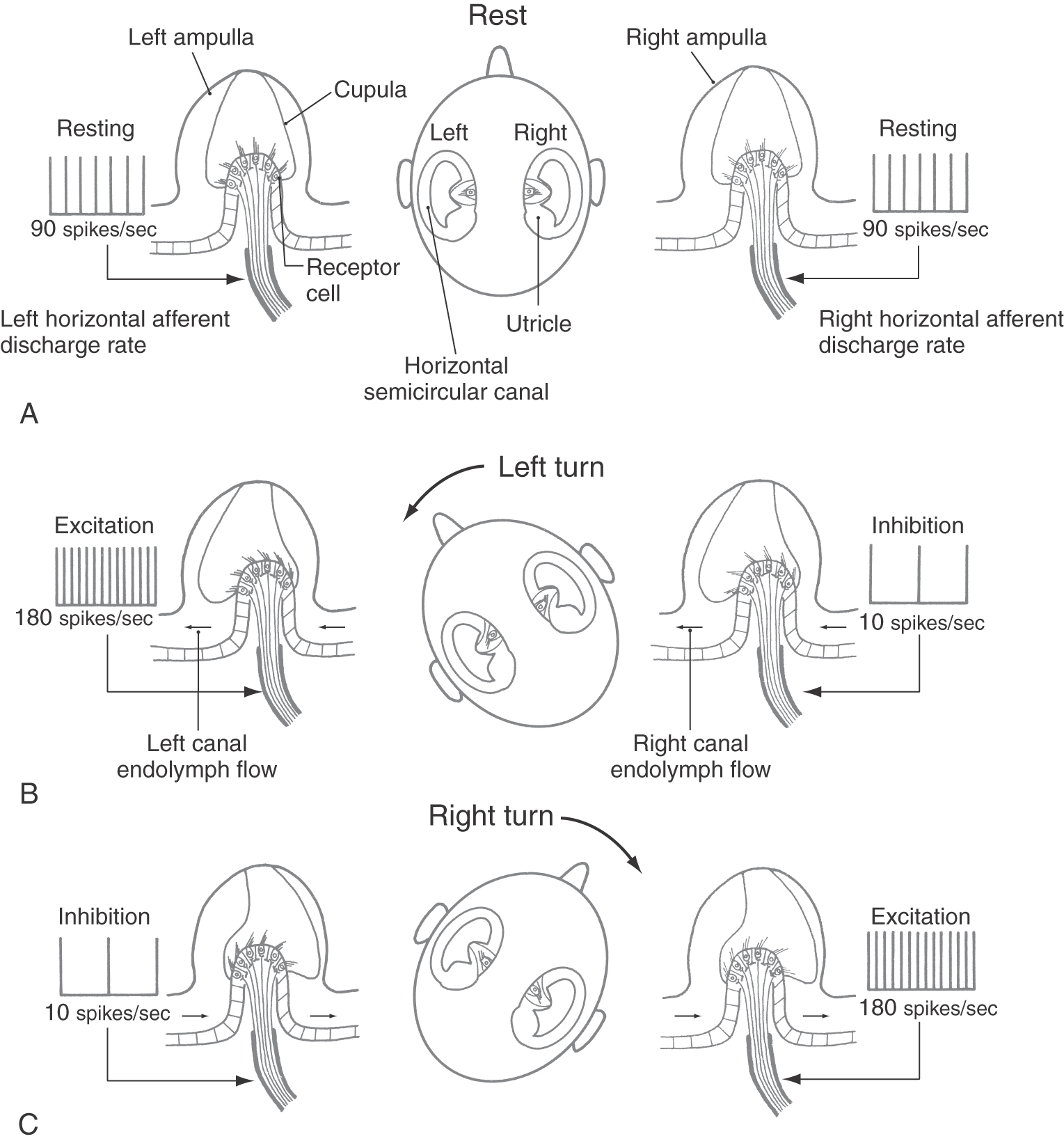
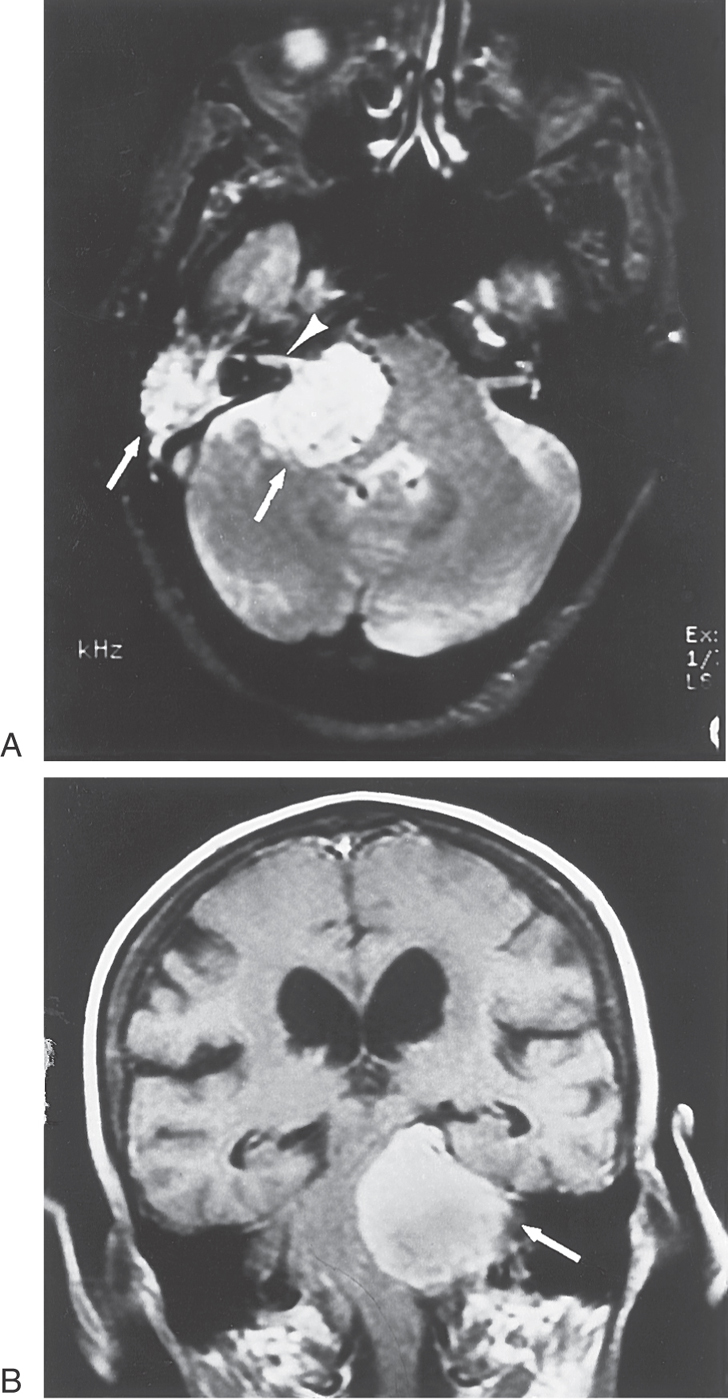
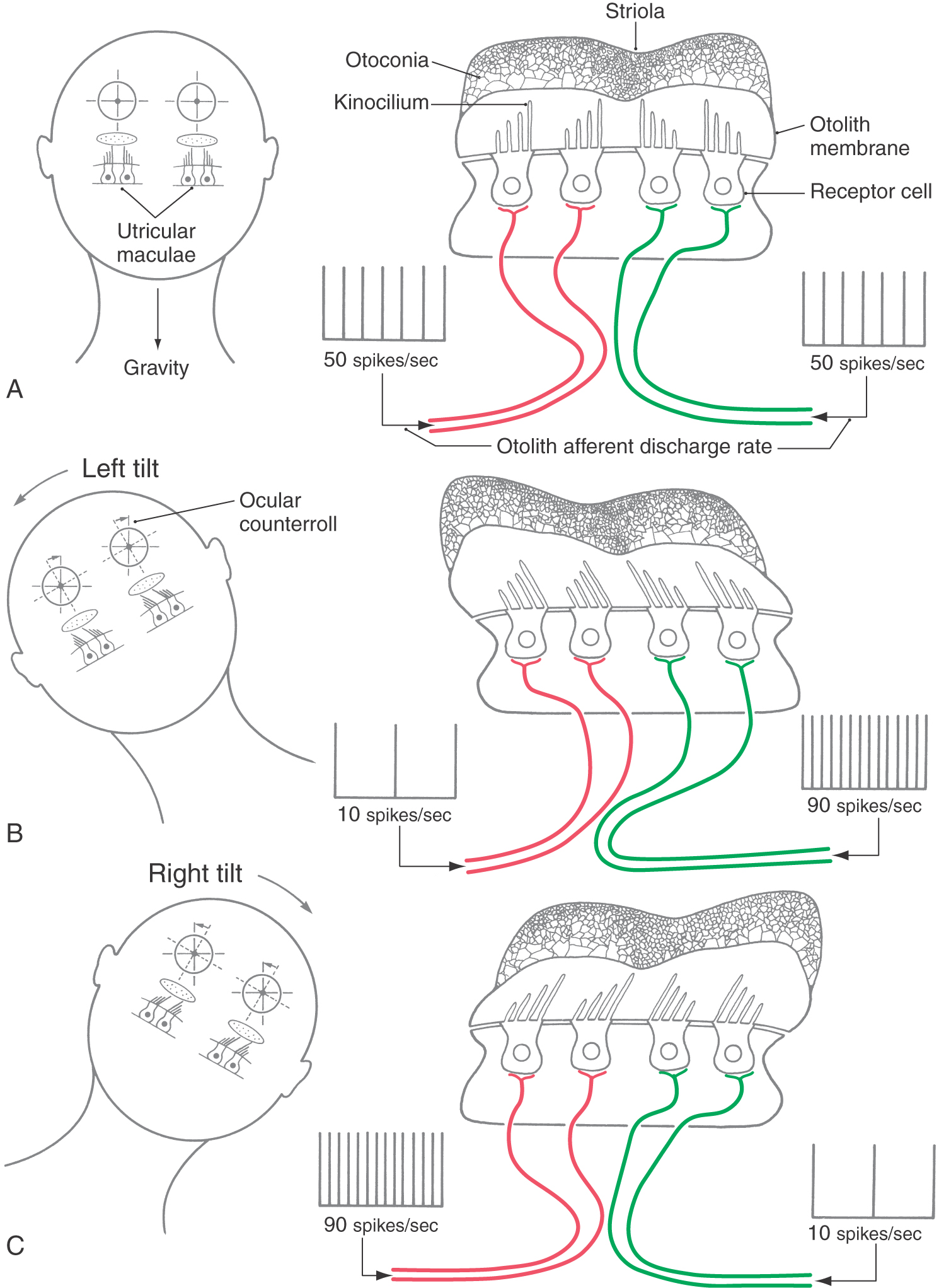
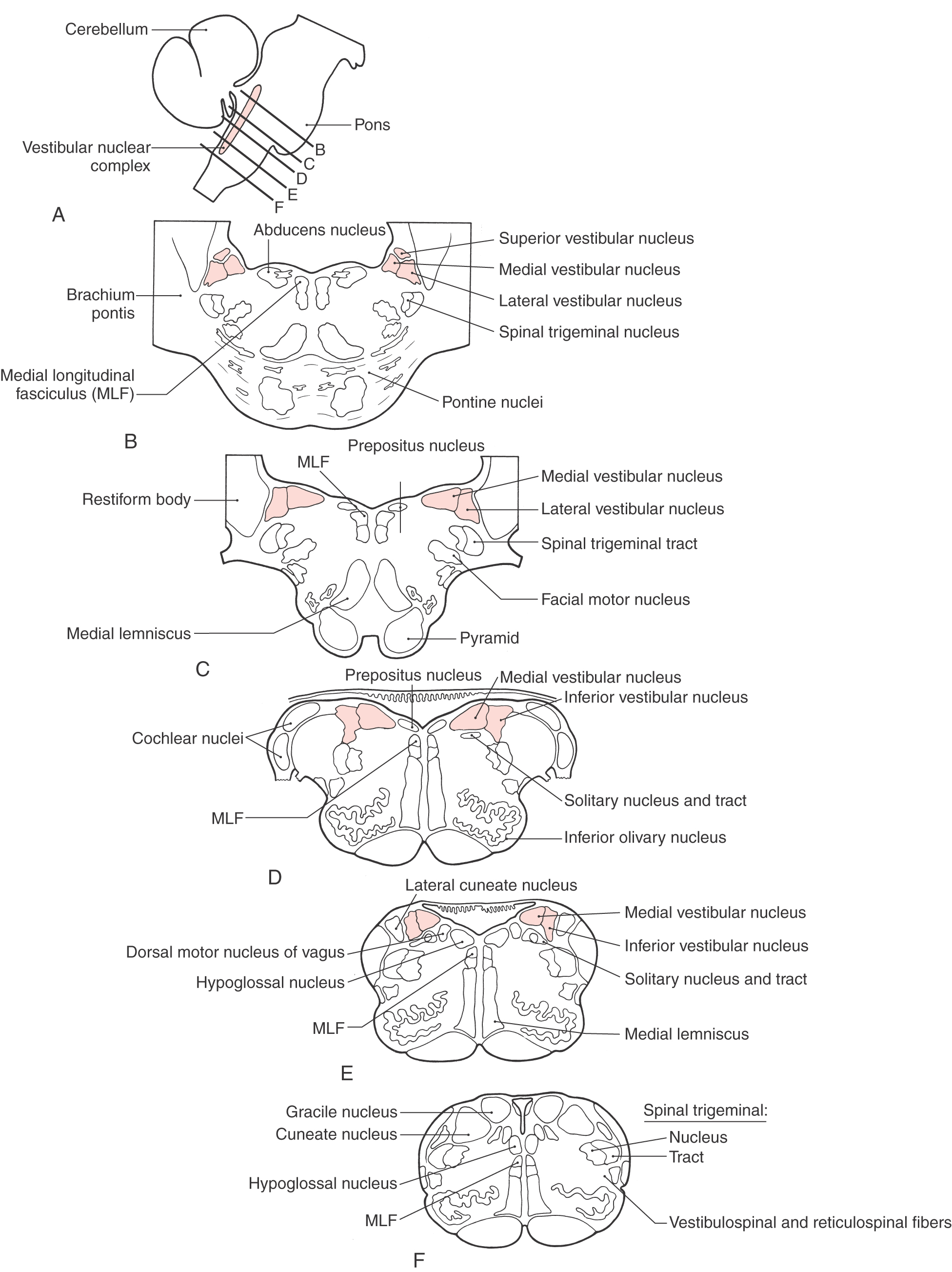
 Figure 22-12.
Figure 22-12.