Chapter 6
The Ventricles, Choroid Plexus, and Cerebrospinal Fluid
Foramina of the Fourth Ventricle
Formation of the Choroid Plexus
Hemorrhage into the Ventricles
Ependyma, Choroid Plexus, and Cerebrospinal Fluid
Cerebrospinal Fluid in Health and Disease
Cerebrospinal Fluid Production and Circulation
Hydrocephalus and Related Conditions
The ventricular spaces of the brain are the adult elaborations of the neural canal of early developmental stages. These spaces, the choroid plexuses in them, and the cerebrospinal fluid (CSF) produced by the choroid plexus are essential elements in the normal function of the brain.
OVERVIEW
By about the third week of development, the nervous system consists of a tube closed at both ends and somewhat hook shaped rostrally (Fig. 6-1). The cavity of this tube, the neural canal, eventually gives rise to the ventricles of the adult brain and the central canal of the spinal cord. The ventricles become elaborate as the various parts of the brain differentiate, whereas the central canal becomes progressively smaller as the spinal cord differentiates into its adult pattern.
The choroid plexus, which secretes the CSF that fills the ventricles and the subarachnoid space, arises from tufts of cells that appear in the wall of each ventricle during the first trimester. These cells are specialized for a secretory function. The production of CSF is an active process that requires an expenditure of energy by the choroidal cells.
Any condition that causes CSF to accumulate, such as overproduction or an obstruction of its movement through the ventricle system, produces serious neurologic deficits. Perhaps the most widely recognized example is hydrocephalus as seen in a fetus or in a newborn. This condition is usually caused by an obstruction of CSF flow with resultant enlargement of the ventricular spaces upstream to the blockage. The bones of the developing skull move apart, and the head may enlarge significantly. In most of these cases, some type of surgical diversion of CSF flow (a shunting procedure) is necessary.
DEVELOPMENT
The anterior (rostral) and posterior (caudal) neuropores close at about 24 and 26 days, respectively. At this point, the neural tube is lined by the differentiating neuroepithelial cells of the ventricular zone, which are undergoing waves of cell division. Some of these precursor cells give rise to the ependymal cells that line the developing (and mature) ventricular system and the central canal.
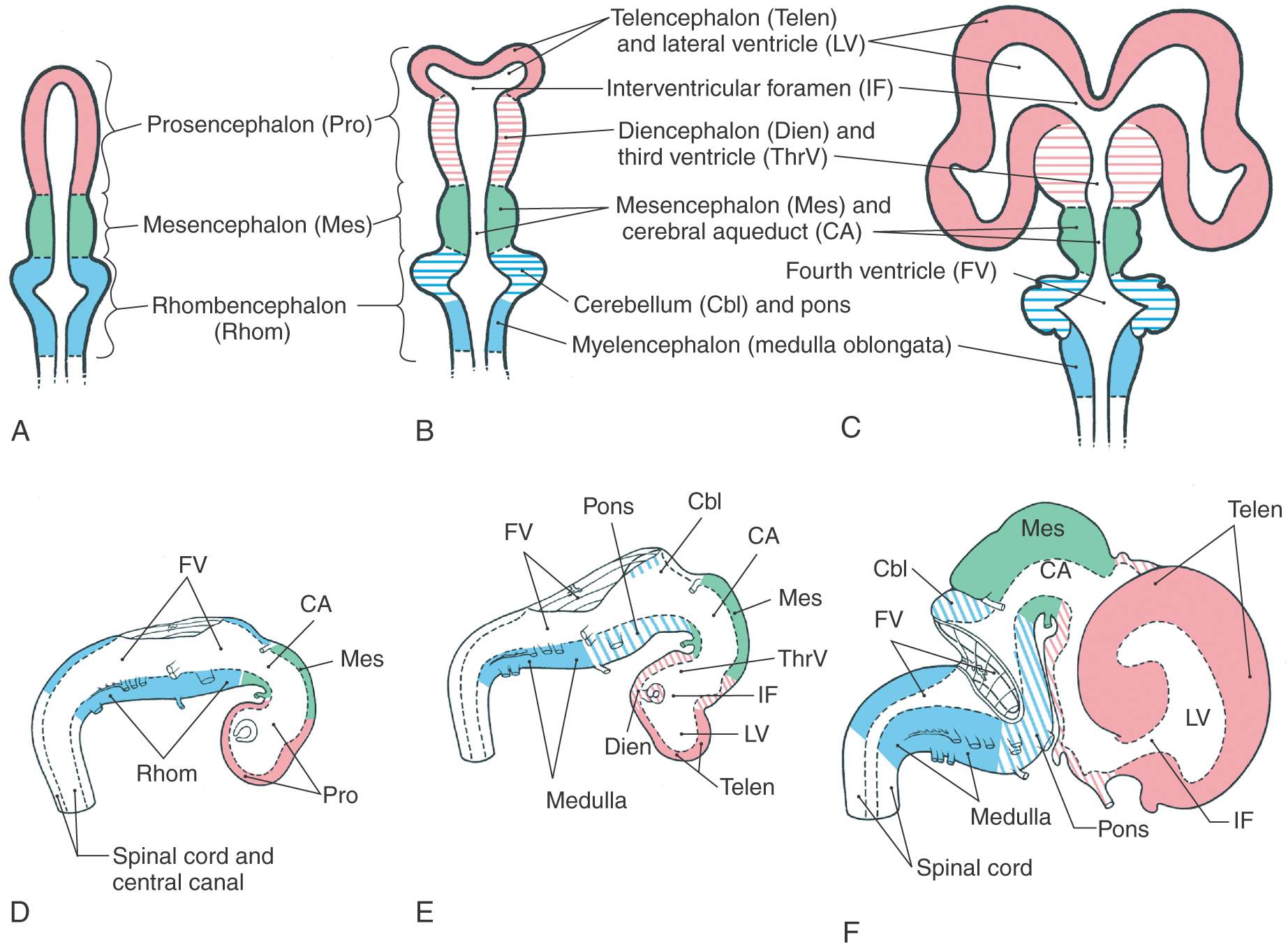
The brain is initially composed of three primary brain vesicles—rhombencephalon, mesencephalon, and prosencephalon—each containing a portion of the cavity of the neural tube (Fig. 6-1A, D). The appearance of the pontine flexure in the rhombencephalon and a progressively deepening groove that separates the diencephalon from the telencephalon (the diencephalic-telencephalic sulcus, shortened here to telencephalic flexure) divide these three vesicles into the five brain vesicles (myelencephalon, metencephalon, mesencephalon, diencephalon, telencephalon) characteristic of the adult brain (Fig. 6-1B, E). With subsequent development, the telencephalon enlarges significantly (Fig. 6-1C, F). As the brain enlarges from three to five vesicles, each part pulls along a portion of the cavity of the primitive neural tube. These spaces in each brain vesicle form the ventricle of that part of the brain in the adult. Consequently, the shape of the ventricular system conforms, in general, to the changes in configuration of the surrounding parts of the brain.
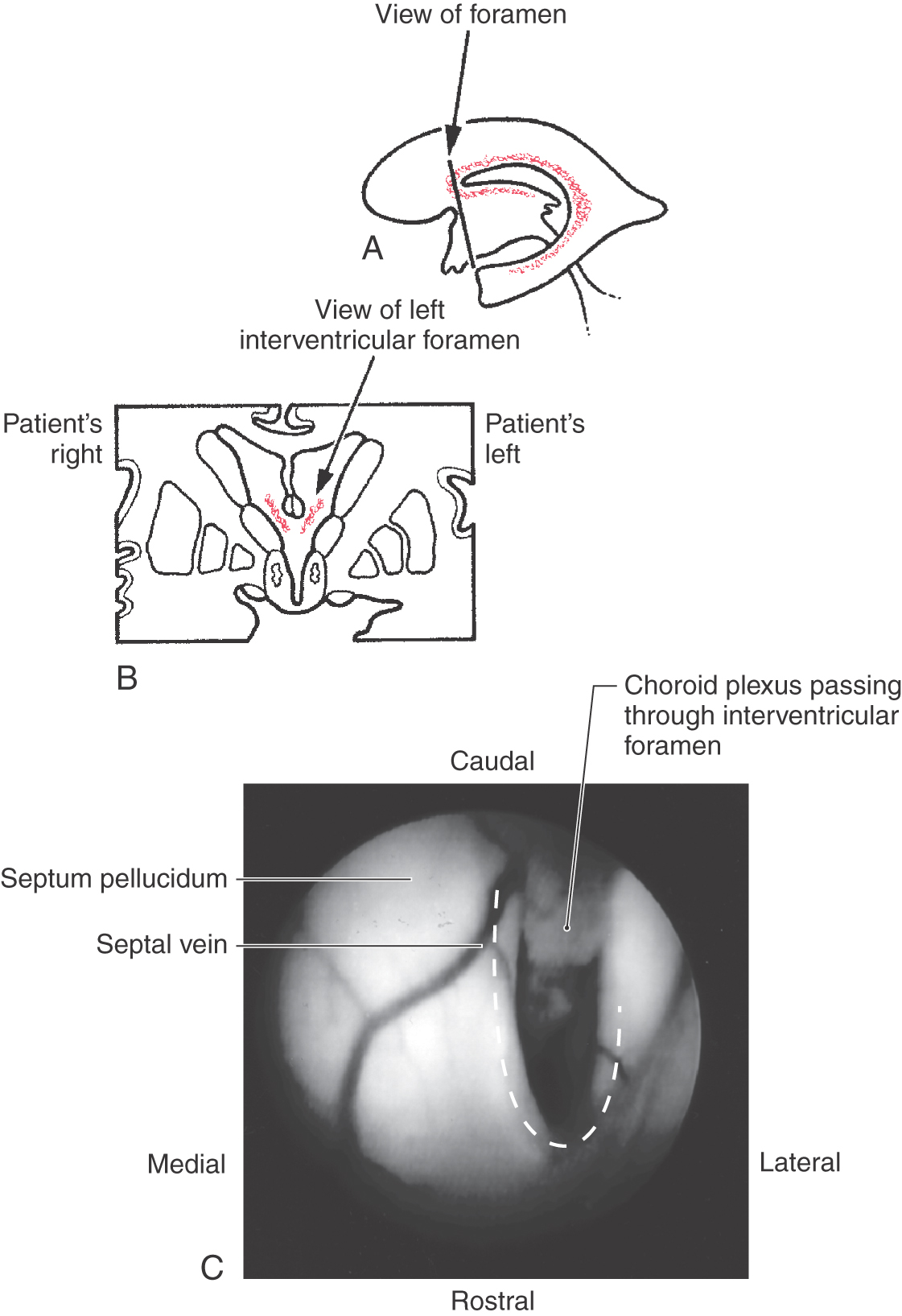
The lateral ventricles follow the enlarging cerebral hemispheres, and the third ventricle remains a single midline space (Fig. 6-1A-C). The communications between the lateral ventricles and the third ventricle, the interventricular foramina (of Monro), are initially large but become small, in proportion to the enlarging brain, as development progresses (Fig. 6-1C, F). A colloid cyst is a type of glioma that, although comprising only about 1% of all intracranial tumors, has a predilection for being found at the position of the interventricular foramen. In this location, it may block the egress of CSF from the lateral ventricles and result in enlarged ventricles and thinning of the corpus callosum. These patients usually present with headache, nausea, and vomiting (features of increased intracranial pressure) and with mental changes and gait disturbances. Proliferation of the neural elements of the mesencephalon results in a reduction in the size of the cavity of this vesicle to form the cerebral aqueduct of the adult brain (Fig. 6-1C, F). This creates a constricted region in the ventricular system and thus a point at which the flow of CSF may be easily blocked. Occlusion of the cerebral aqueduct during development may be the result of glial scarring (gliosis) due to infection or a consequence of developmental defects of the forebrain, a rupture of the amnionic sac in utero, or forking of the aqueduct. The last entity is a genetic sex-linked condition in which the aqueduct is reduced to two or more very small channels that do not properly meet. In addition, the cerebral aqueduct may be reduced to such a small channel that CSF flow is reduced or essentially blocked. Whatever the cause, occlusion of the cerebral aqueduct results in a lack of communication between the third and fourth ventricles and blocks the egress of CSF from the third ventricle. Caudally, the cerebral aqueduct flares open into the fourth ventricle (Fig. 6-1B, C, E, F).
Foramina of the Fourth Ventricle
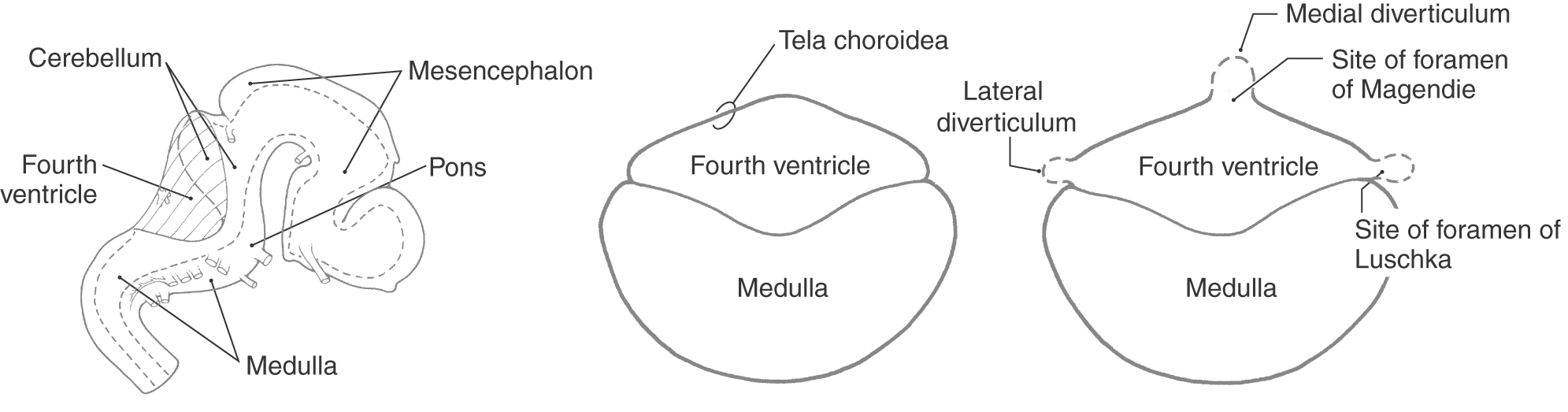
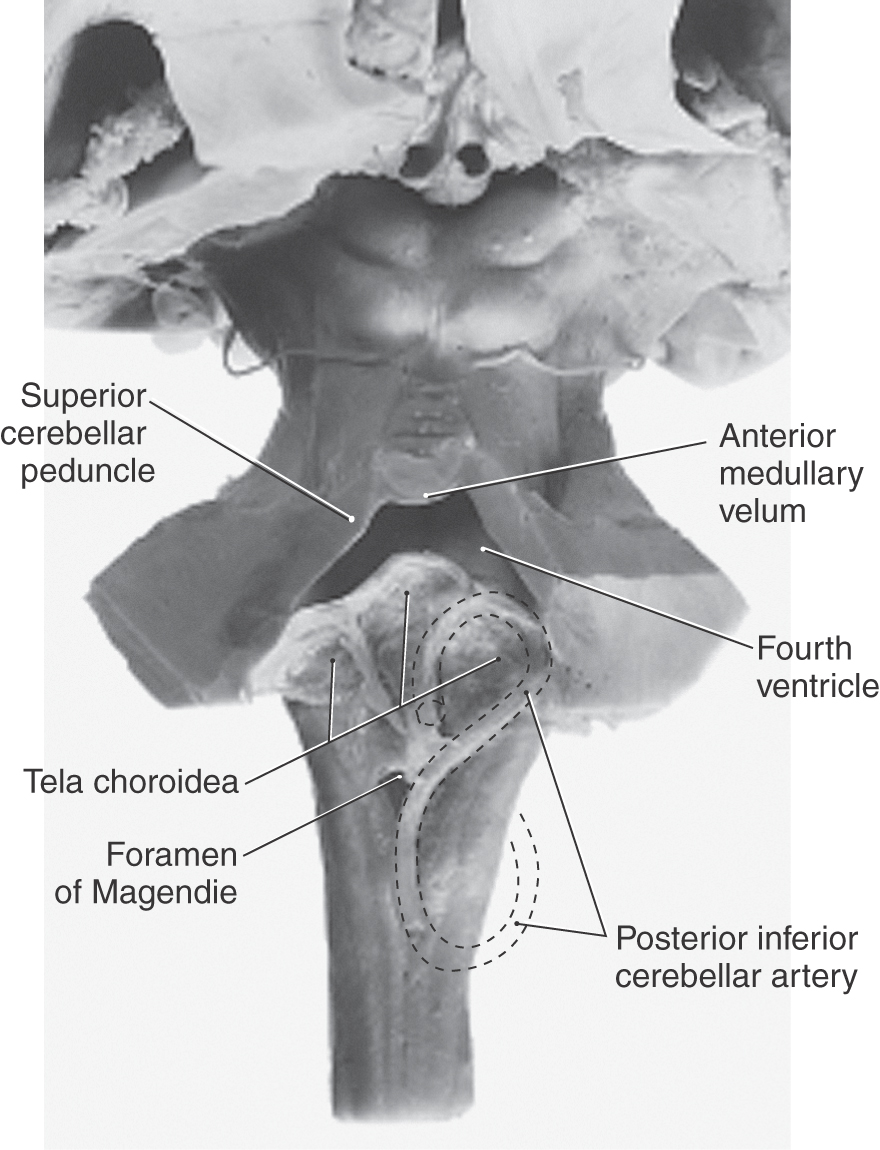
The ventricles and central canal of the spinal cord initially form a closed system. However, in the second and third months of development, three openings form in the roof of the fourth ventricle, rendering the ventricular system continuous with the subarachnoid space surrounding the brain and spinal cord. The caudal part of the roof of the fourth ventricle consists of a layer of ependymal cells internally and a delicate layer of connective tissue externally (Fig. 6-2). The future apertures first appear in the form of small bulges in the roof of the fourth ventricle. The membrane forming the roof at these points becomes thinned and breaks down. The resultant openings are the medial foramen of Magendie and the lateral foramina of Luschka (Fig. 6-2).
Figure 6-2. Development of the foramina of Luschka and Magendie in the fourth ventricle.
Formation of the Choroid Plexus
The caudal roof of the fourth ventricle is composed of ependymal cells on the luminal surface and a delicate layer of connective tissue, the pia mater, on its external surface. These collectively form the tela choroidea (Fig. 6-3). Developing arteries in the immediate vicinity invaginate the roof of the ventricle to form a narrow groove, the choroid fissure, in the tela choroidea. These small developing arteries are branches of what will become the posterior inferior cerebellar artery in the adult. The involuted ependymal cells, along with vessels and a small amount of connective tissue, represent the primordial choroid plexus inside the ventricular space. As development progresses, the choroid plexus enlarges, forms many small elevations called villi, and begins to secrete CSF (Fig. 6-3; see also Fig. 6-18). By about the end of the first trimester, the choroid plexus is functional, the openings in the fourth ventricle are patent, and there is circulation of CSF through the ventricular system and into the subarachnoid space.
Figure 6-3. Development of the choroid plexus.
The choroid plexuses of the third and lateral ventricles develop much in the same manner (Fig. 6-4). A choroid fissure appears in the roof of the third ventricle and in the medial wall of the lateral ventricle. The choroid plexus develops along these lines, bulges into the respective space, and is continuous from lateral to third ventricles through the interventricular foramen. The small arteries serving the choroid plexus of the third ventricle are branches of the medial posterior choroidal artery, and those serving the choroid plexus of the lateral ventricles are branches of the lateral posterior choroidal artery and the anterior choroidal artery (see Chapter 8).
In the adult, the choroid plexus is found in both lateral ventricles and in the third and fourth ventricles (Fig. 6-4). The development of this structure is essentially the same in all of these spaces and is described here for the fourth ventricle.
VENTRICLES
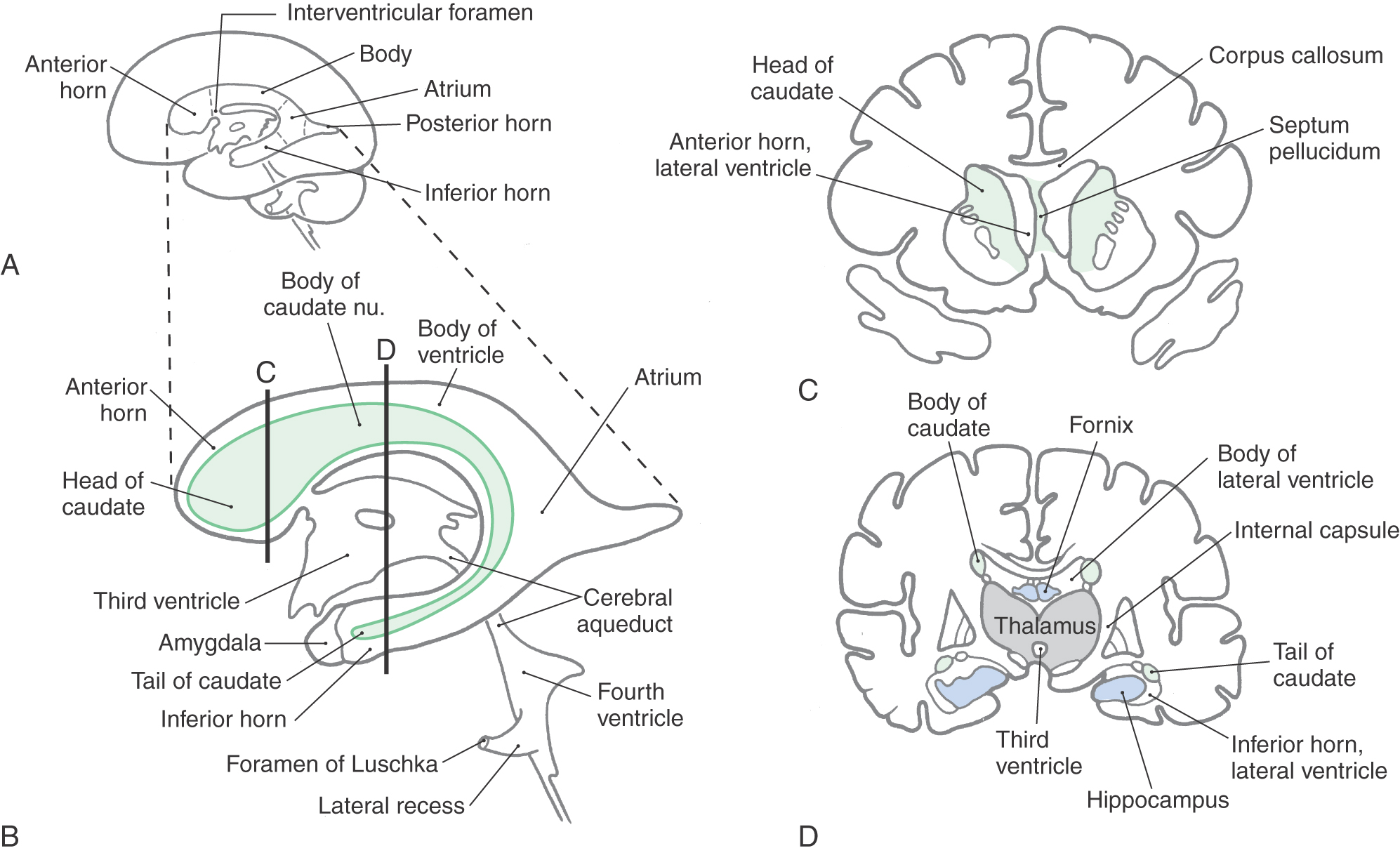
Lateral Ventricles
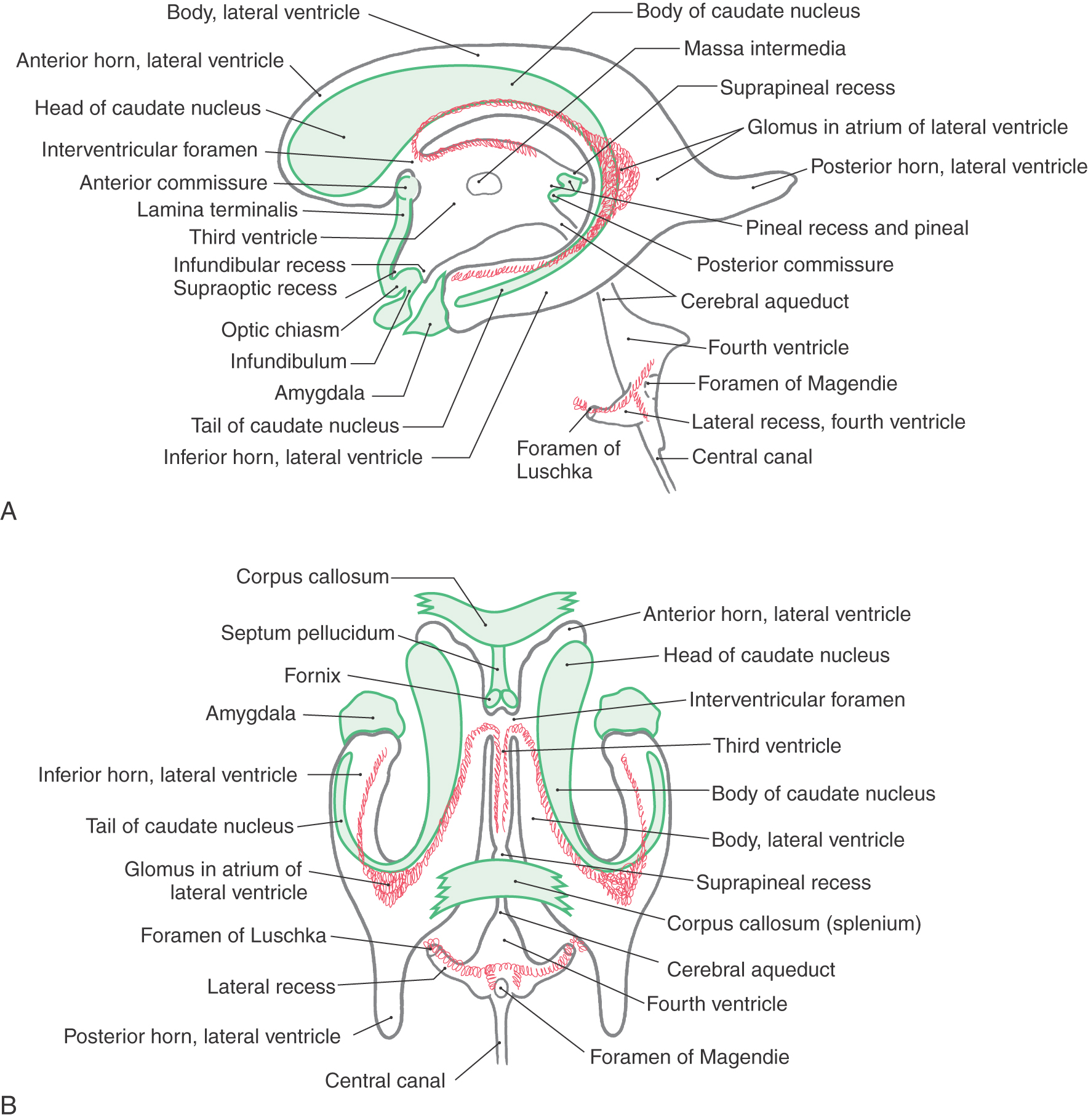
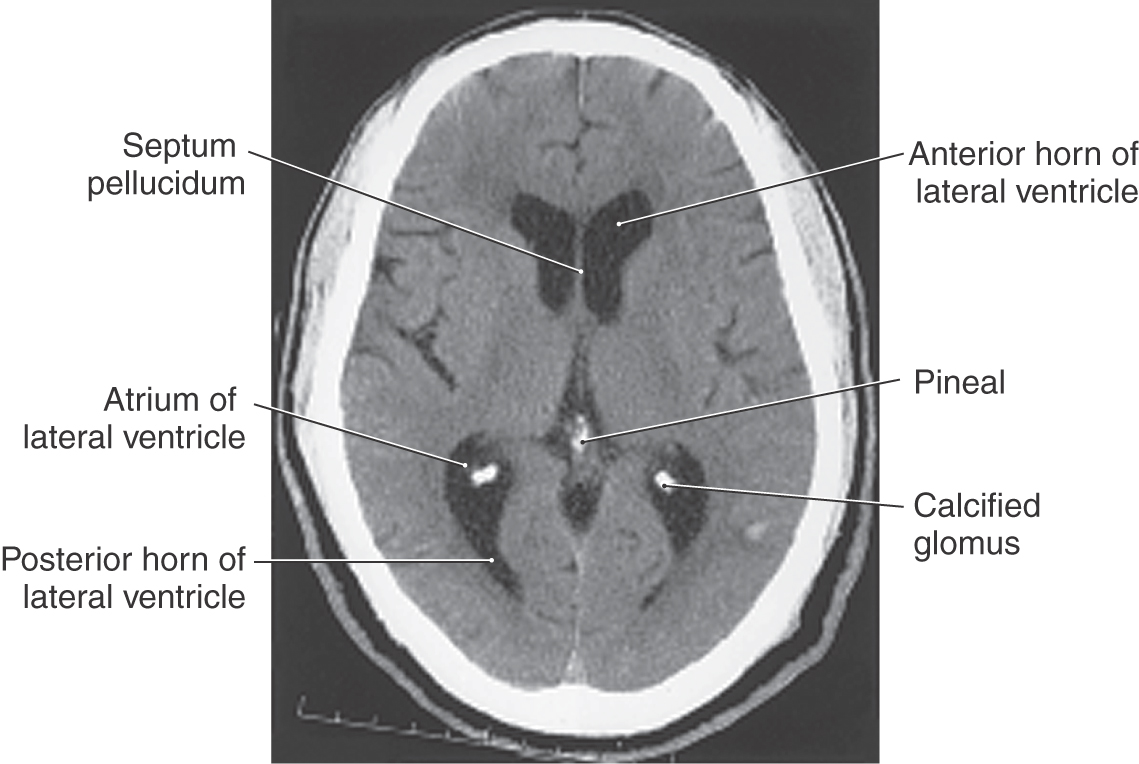
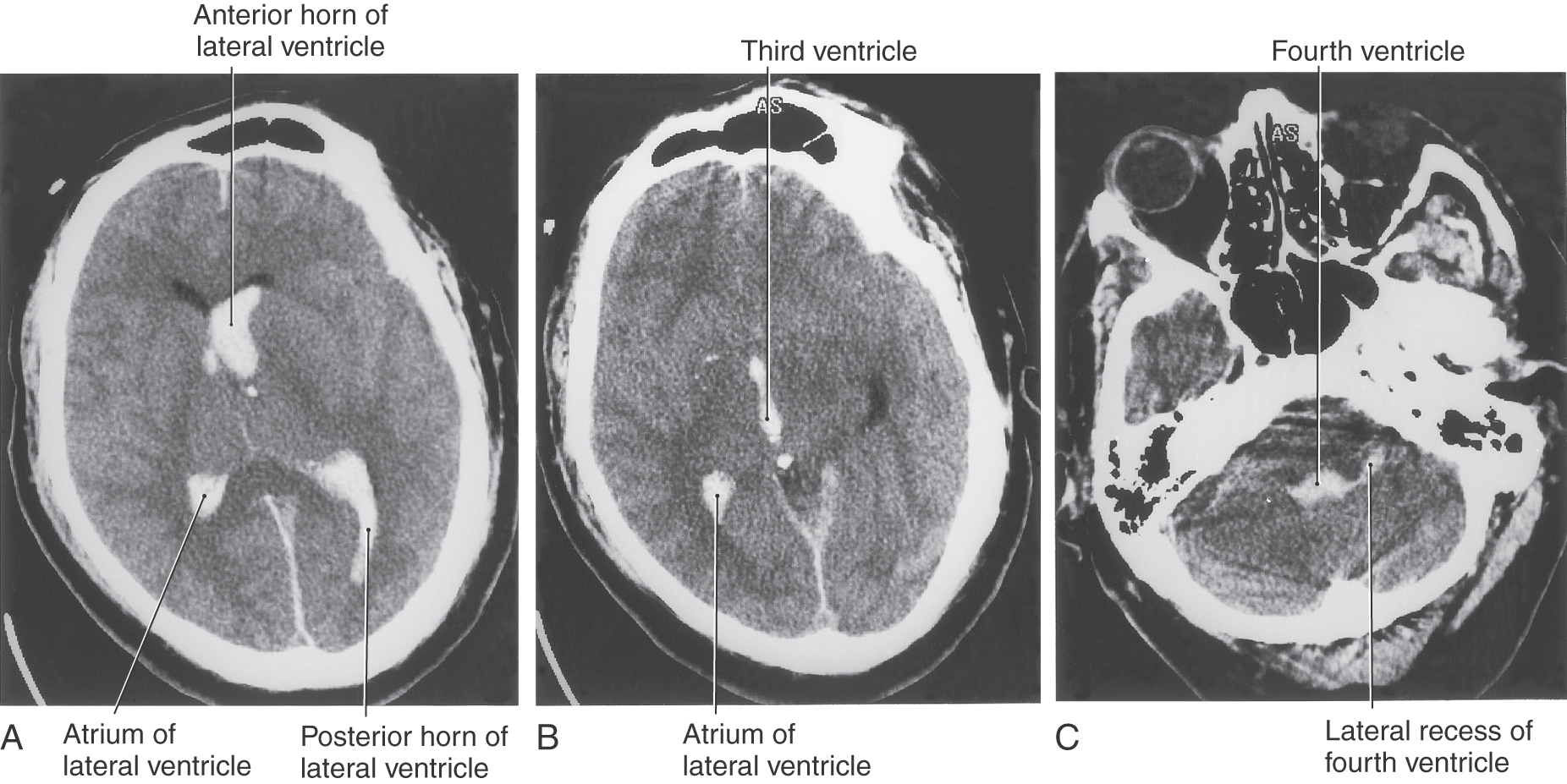
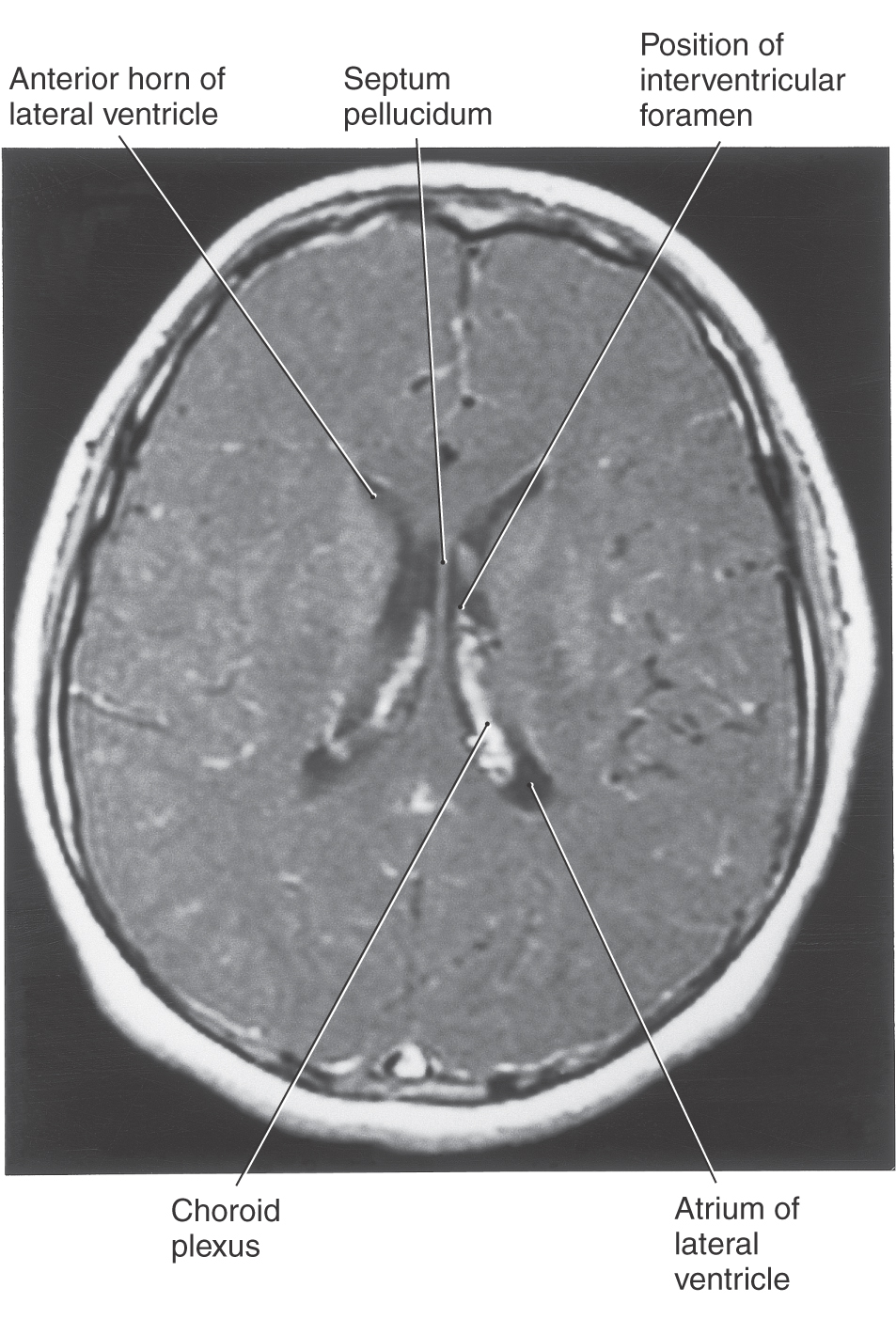
The cavities of the telencephalon are the lateral ventricles, of which there is one in each hemisphere (Fig. 6-4). As the development of the hemispheres creates the frontal, temporal, and occipital lobes, the lateral ventricles are pulled along and thus acquire their definitive adult shape of a flattened C with a short tail (Fig. 6-4). This shape is present by birth. The lateral ventricle consists of an anterior horn, a body, and posterior and inferior horns (Fig. 6-4). The junction of the body with the posterior and inferior horns constitutes the atrium of the lateral ventricle. An especially large clump of choroid plexus, the glomus (or glomus choroideum), is found in the atrium (Fig. 6-4). In adults and especially in elderly persons, the glomus may contain calcifications that are visible (as white spots) on radiographs or computed tomography (CT) scans (Fig. 6-5). Shifts in the position of the glomus, usually accompanied by alterations in the volume or shape of the surrounding ventricle, may indicate some type of ongoing pathologic process or space-occupying lesion.
The elaborate shape of the lateral ventricle means that different structures border on different parts of this space. The anterior horn and body of the lateral ventricle are bordered medially by the septum pellucidum (at rostral levels) and by a bundle of fibers called the fornix (at caudal levels) and posteriorly (superiorly) by the corpus callosum (Figs. 6-4 and 6-6). The floor of the body of the lateral ventricle is made up of the thalamus, and the caudate nucleus is characteristically found in the lateral wall of the lateral ventricle throughout its extent (Figs. 6-4 and 6-6). In the temporal lobe, the inferior horn of the lateral ventricle contains the tail of the caudate nucleus in its lateral wall, the hippocampal formation in its medial wall, and a large group of cells (the amygdaloid complex) in its rostral end (Figs. 6-4 and 6-6).
The openings between the lateral and third ventricles, the interventricular foramina, are located between the column of the fornix and the rostral and medial ends of the thalamus. There are two interventricular foramina, one opening from each lateral ventricle into the single midline third ventricle (Fig. 6-4B).
Third Ventricle
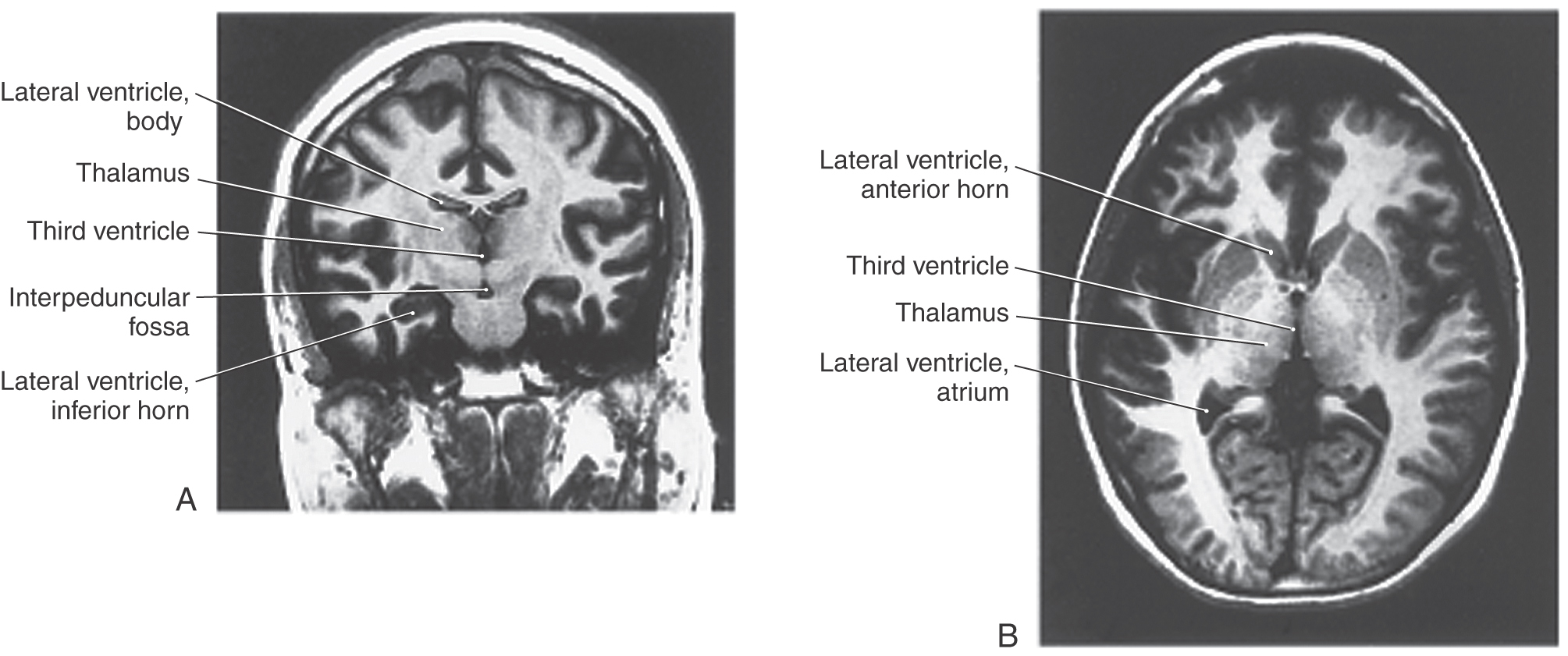
The third ventricle, the cavity of the diencephalon, is a narrow, vertically oriented midline space that communicates rostrally with the lateral ventricles and caudally with the cerebral aqueduct (Figs. 6-4 and 6-8). The third ventricle has an elaborate profile on a sagittal view (Fig. 6-4A), but it is narrow in the coronal and axial planes (Fig. 6-7).
Figure 6-7. Magnetic resonance images of the third ventricle in coronal (A) and axial (B) views.
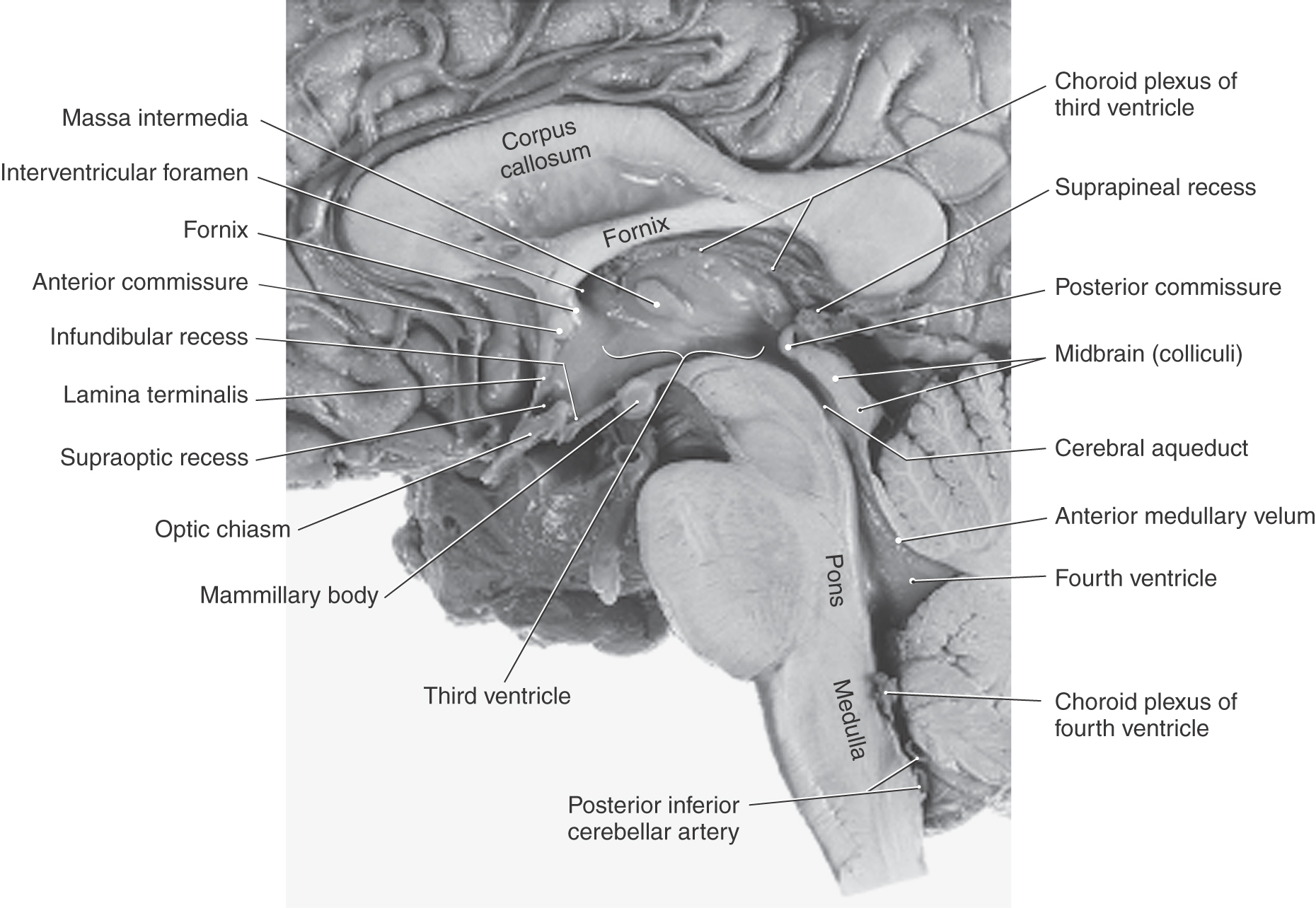
The boundaries of the third ventricle are formed by a variety of structures, the most important being the dorsal thalamus and hypothalamus, and by structures that form small outpocketings called recesses (Figs. 6-4A and 6-8). These are the supraoptic recess (above the optic chiasm), the infundibular recess (in the infundibulum, the stalk of the pituitary), the pineal recess (in the stalk of the pineal), and the suprapineal recess (above the pineal). The rostral wall of the third ventricle is formed by a short segment of the anterior commissure and a thin membrane, the lamina terminalis, that extends from the anterior commissure anteriorly (ventrally) to the rostral edge of the optic chiasm (Figs. 6-4A and 6-8). The floor of the third ventricle is formed by the optic chiasm and infundibulum and their corresponding recesses plus a line extending caudally along the rostral aspect of the midbrain to the cerebral aqueduct. The caudal wall is formed by the posterior commissure and the recesses related to the pineal, whereas the roof is the tela choroidea, from which the choroid plexus is suspended (Figs. 6-4A and 6-8).
Cerebral Aqueduct
The cerebral aqueduct, the extension of the ventricle through the mesencephalon, communicates rostrally with the third ventricle and caudally with the fourth ventricle (Figs. 6-4A and 6-8). This midline channel is about 1.5 mm in diameter in adults and contains no choroid plexus. Its narrow diameter makes it especially susceptible to occlusion. For example, cellular debris in the ventricular system (from infections or hemorrhage) may clog the aqueduct. Tumors in the area of the midbrain (such as pinealoma) may compress the midbrain and occlude the aqueduct. The result is a blockage of CSF flow and enlargement of the third and lateral ventricles at the expense of the surrounding brain tissue. The cerebral aqueduct is surrounded on all sides by a sleeve of gray matter that contains primarily small neurons; this is the periaqueductal gray or central gray.
Fourth Ventricle
The fourth ventricle is a roughly pyramid-shaped space that forms the cavity of the metencephalon and myelencephalon (Figs. 6-4 and 6-8). The apex of this ventricle extends into the base of the cerebellum, and caudally it tapers to a narrow channel that continues into the cervical spinal cord as the central canal. Laterally the fourth ventricle extends over the surface of the medulla as the lateral recesses, eventually to open into the area of the pons-medulla-cerebellum junction, the cerebellopontine angle, through the foramina of Luschka (Figs. 6-4 and 6-9). The irregularly shaped foramen of Magendie is located in the caudal sloping roof of the ventricle (Figs. 6-4 and 6-10). Although the roof of the caudal part of the fourth ventricle and the lateral recesses is composed of tela choroidea, the rostral boundaries of this space are formed by brain structures. These include the cerebellum (covering about the middle third of the ventricle) and the superior cerebellar peduncles and anterior medullary velum (covering the rostral third of the ventricle). The floor of the fourth ventricle, the rhomboid fossa (see Fig. 10-3), is formed by the pons and medulla (Fig. 6-8). The only openings between the ventricles of the brain and the subarachnoid space surrounding the brain are the foramina of Luschka and Magendie in the fourth ventricle.
Hemorrhage into the Ventricles
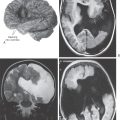
A variety of events may result in the accumulation of blood in the ventricular spaces in the brain (Fig. 6-11). These include hemorrhage into the substance of the brain (such as cerebral hemorrhage) that subsequently ruptures into the ventricular space, rupture of an intracranial aneurysm (especially those located immediately adjacent to the third or fourth ventricles), and severe head trauma. In this last case, there may also be blood in the subarachnoid space or in the substance of the brain, depending on the degree of injury. Additional but less frequent causes are rupture or bleeding from an intraventricular arteriovenous malformation and bleeding from a tumor located in or invading the ventricular space. Whatever the cause, blood in the ventricles, especially acute blood, is clearly seen on CT (Fig. 6-11). The white (hyperdense) appearance of the blood characteristically outlines the ventricular spaces and is clearly distinguishable from blood at other intracranial locations. In fact, blood in the ventricular spaces can create an in vivo cast showing details of the ventricular spaces and their relationships (Fig. 6-11). Alterations of size, shape, or position of a ventricle containing blood may be indicative of further neurologic complications.
EPENDYMA, CHOROID PLEXUS, AND CEREBROSPINAL FLUID
Ependyma
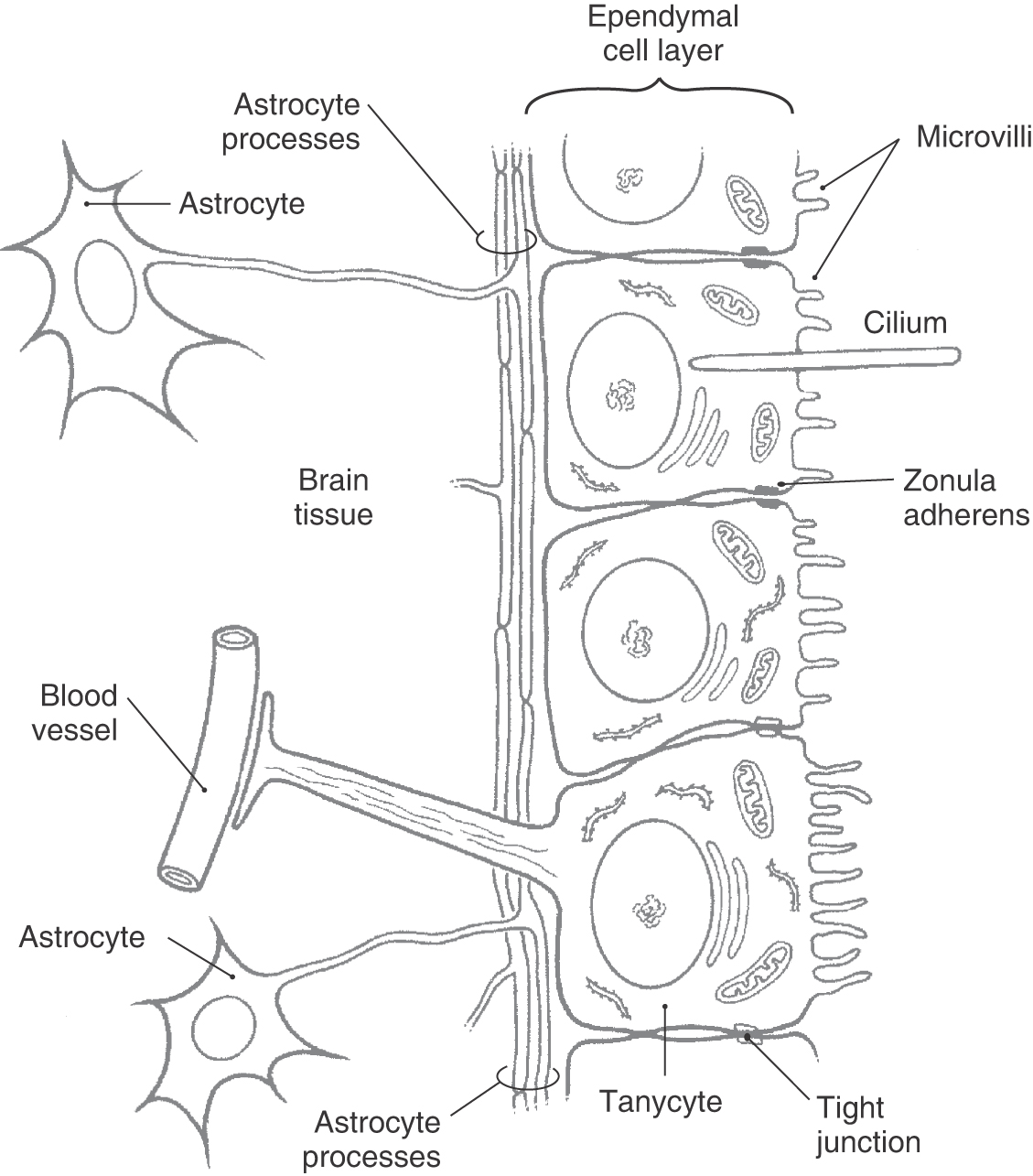
The ventricles of the brain and the central canal of the spinal cord are lined by a simple cuboidal epithelium, the ependyma. Ependymal cells contain abundant mitochondria and are metabolically active. Their luminal surfaces are ciliated and have microvilli, and the bases contact the subependymal layer of astrocytic processes. There is not a continuous basal lamina between ependymal cells and the subjacent glial cell processes (Fig. 6-12). Ependymal cells are attached to each other by zonulae adherens (desmosomes).
In some regions, particularly the third ventricle, there are patches of specialized ependymal cells called tanycytes (Fig. 6-12). Tanycytes have basal processes that extend through the layer of astrocytic processes to form end-feet on blood vessels and in the neuropil. They may function to transport substances between the ventricles and the blood. In contrast to ependymal cells, tanycytes are attached to each other and to immediately adjacent ependymal cells by tight junctions. Desmosomes are also present between tanycytes.
Ependymomas
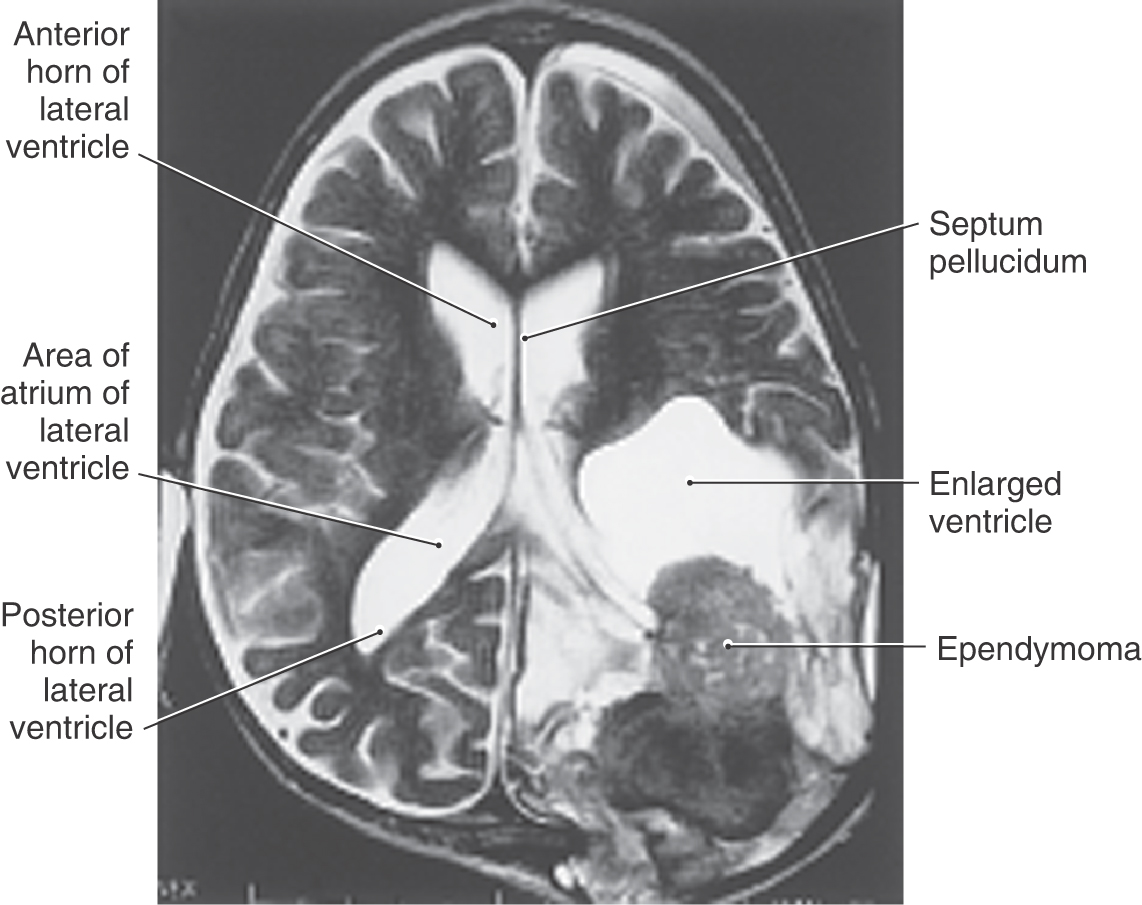
This tumor, which constitutes 5% to 6% of all glial cell neoplasms, originates from the ependymal cells lining the ventricles (Fig. 6-13). Although these tumors may appear in any ventricle, the majority (60% to 75%) are located in the spaces of the posterior fossa. These neoplasms are seen most frequently in children younger than 5 years, and their location may determine the symptoms experienced by the patient. Supratentorial lesions (Fig. 6-13) may result in hydrocephalus in the case of blocked CSF flow or seizure activity. Lesions in infratentorial locations frequently cause nausea and vomiting, headache, other signs and symptoms related to increased intracranial pressure, and cranial nerve signs and symptoms indicative of compression of or tumor infiltration into the brainstem.
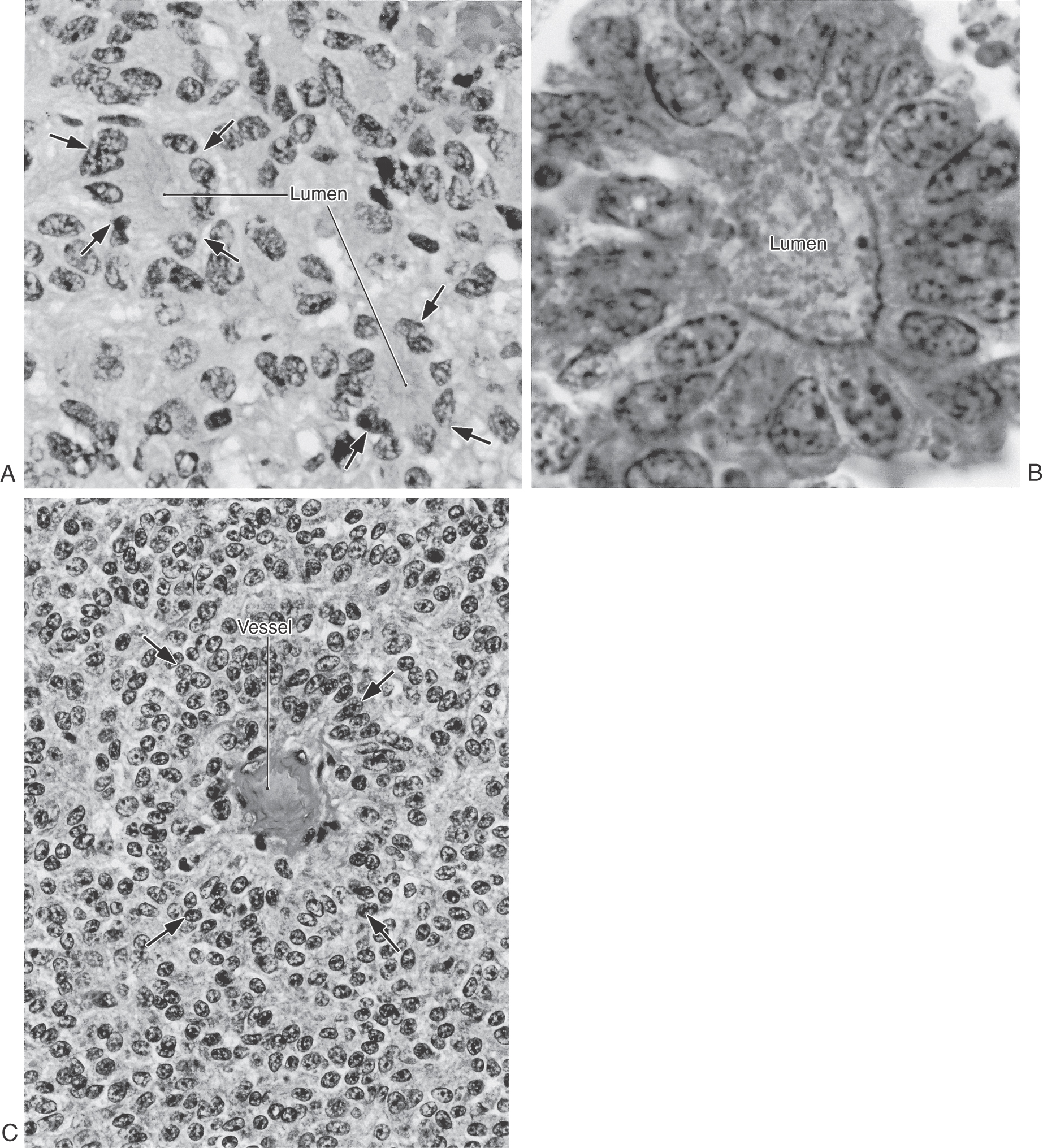
The histologic appearance of ependymomas may vary. In general, these tumors are characterized by clusters of various sizes that are composed of polygonal or columnar cells arranged in a circle facing a lumen (true rosettes) (Fig. 6-14A, B) or a small blood vessel (perivascular rosettes or pseudorosettes) (Fig. 6-14C). These configurations are made up of long cell processes impinging on a vessel with cell bodies and nuclei located somewhat distal to these processes. Less commonly seen are ependymal rosettes consisting of cell clusters surrounding lumina of varying sizes. The cell apexes in these clusters may contain a basal body (blepharoplast) associated with the cilium of the ependymal cell. The blepharoplast is visualized with stains for glial fibrillary proteins. The presence of this basal body in a stained section is one feature that differentiates this tumor from a choroid papilloma as described later.
Treatment of patients with ependymoma is primarily with surgical removal followed by focal irradiation. Incomplete removal, for example, in cases with tumor infiltration into the brainstem, reduces survival rates even with radiation therapy and chemotherapy.
Choroid Plexus
The choroid plexus extends from the inferior horn of the lateral ventricle into the atrium (where the glomus choroideum is located) along the floor of the body of the lateral ventricle, continues through the interventricular foramen, and attaches to the roof of the third ventricle (Figs. 6-4, 6-15, and 6-16). A tumor of the choroid plexus at the point where it is continuous through the interventricular foramen (Fig. 6-16) may result in an enlargement of the lateral ventricle on that side with signs and symptoms of increased intracranial pressure (vomiting, lethargy, headache, possible papilledema). Such lesions are candidates for surgical removal. The choroid plexus of the fourth ventricle is attached to the caudal roof and extends laterally into the foramen of Luschka (Figs. 6-4 and 6-9).
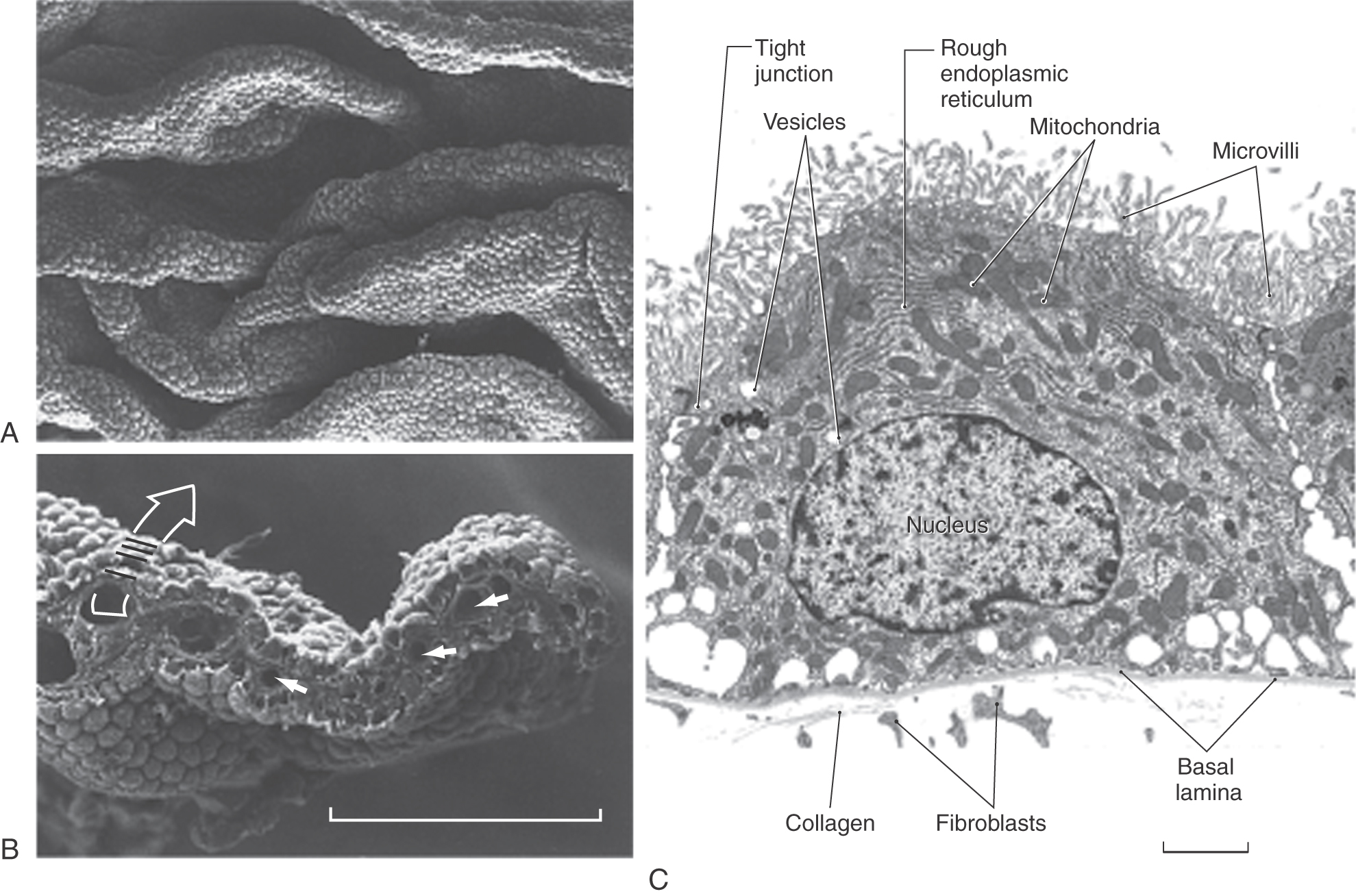
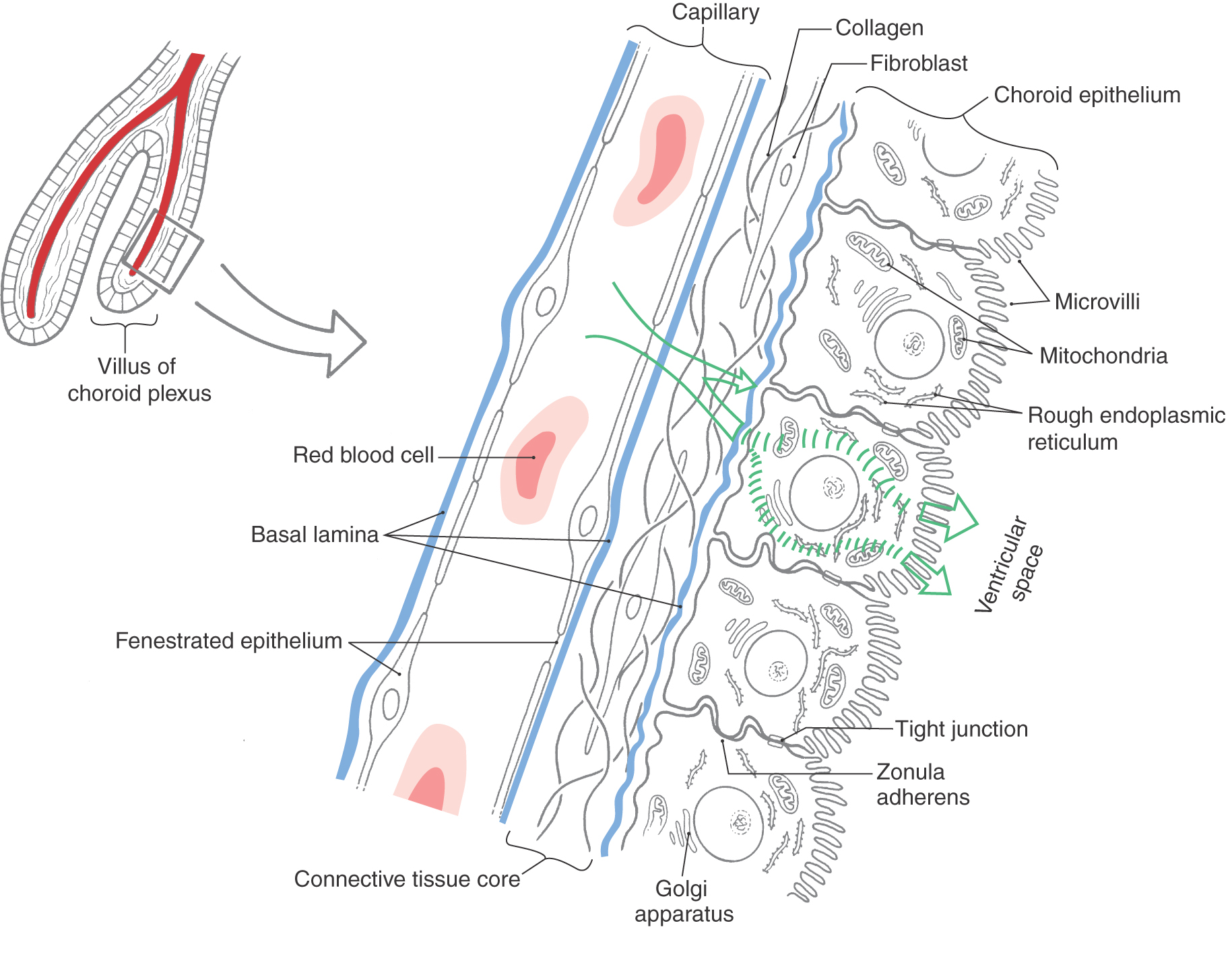
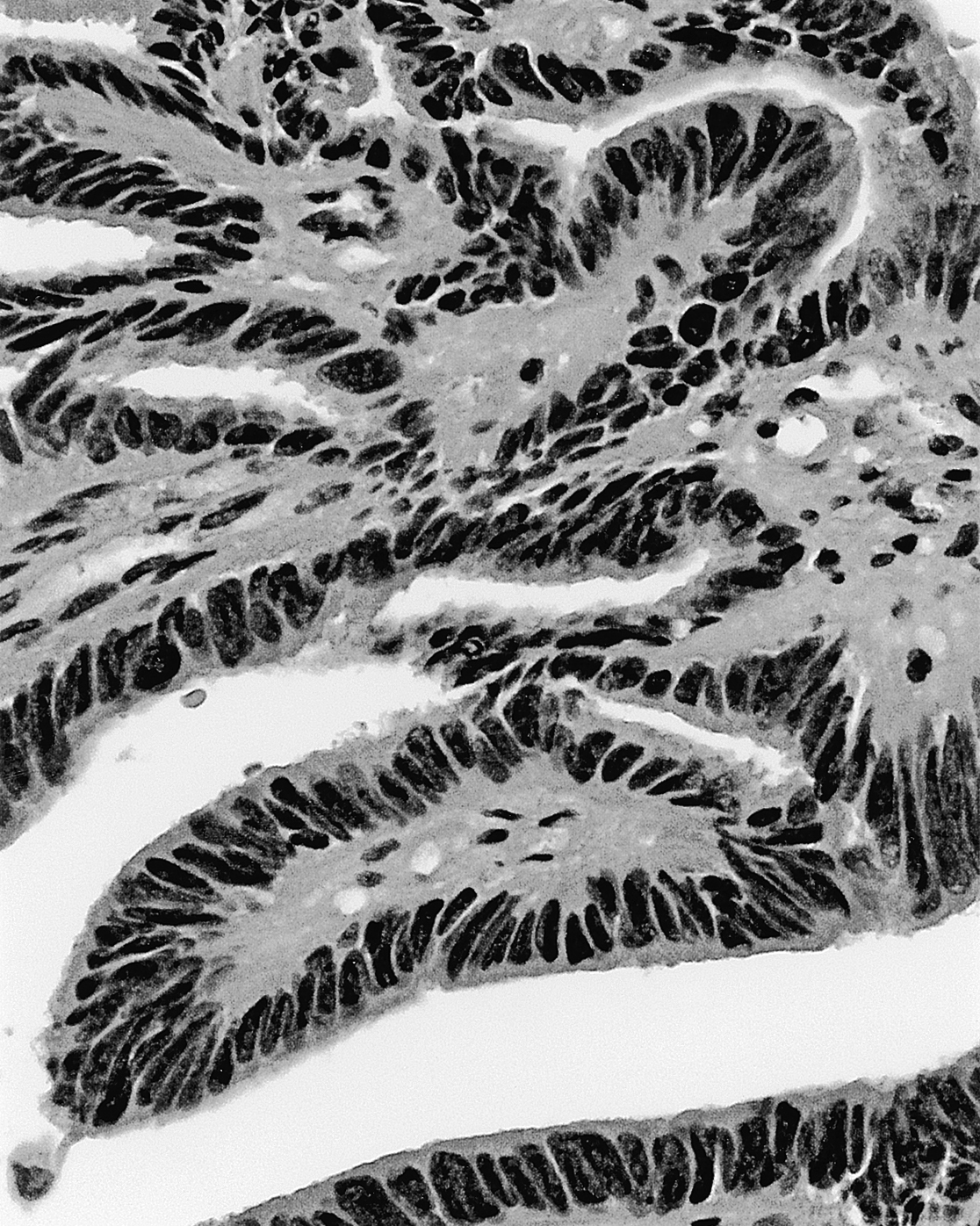
The choroid plexus in each ventricle is thrown into a series of folds called villi (singular, villus). These are covered on their ventricular (luminal) surfaces by a continuum of dome-shaped structures, each with numerous microvilli (Fig. 6-17A, B). Each dome represents the luminal surface of one choroid epithelial cell, and the shallow grooves between domes are the points of contact between adjacent choroid cells (Fig. 6-17B, C). Each villus consists of a core of highly vascularized connective tissue derived from the pia mater and a simple cuboidal covering (the choroid epithelial cell layer), which is derived from ependymal cells (Figs. 6-3 and 6-17B, C). The abundant capillaries in the connective tissue core of each villus are surrounded by a basal lamina. The endothelial cells of these capillaries have numerous fenestrations, which allow a free exchange of molecules between blood plasma and the extracellular fluid in the connective tissue core (Fig. 6-18). The connective tissue core itself consists of fibroblasts and collagen fibrils. Another basal lamina is formed at the interface between the connective tissue core and the choroid epithelial cells that form the surface of each villus (Figs. 6-17C and 6-18). These choroid cells have microvilli on their apical (ventricular) surface, interdigitating cell membranes on their sides, and irregular bases. Each is attached to its neighbor by continuous tight junctions (zonulae occludentes) that seal off the subjacent extracellular space from the ventricular space (Figs. 6-17C and 6-18). This represents the blood-CSF barrier. Choroid epithelial cells contain a nucleus, numerous mitochondria, rough endoplasmic reticulum, and a small Golgi apparatus (Figs. 6-17C and 6-18). Thus they are specialized to control the flow of ions and metabolites into the CSF.
Although choroid epithelial cells are joined by tight junctions, ependymal cells are not (Fig. 6-18). Therefore, fluid exchange occurs freely between CSF and the extracellular fluid of the brain parenchyma. The composition of CSF can thus sometimes reflect disease processes occurring in brain tissue.
In humans the blood supply to the choroid plexuses is via the choroidal arteries and the posterior cerebellar arteries. Choroid plexus in the inferior horn, atrium of the lateral ventricle, and body of the lateral ventricle is served by the anterior choroidal artery (a branch of the internal carotid) and the lateral posterior choroidal artery (a branch of P2). The medial posterior choroidal artery (also a branch of P2) serves the choroid plexus of the third ventricle. The choroid plexus located inside the fourth ventricle is served by branches of the posterior inferior cerebellar artery (Figs. 6-8 and 6-10), and the tuft that extends out of the foramen of Luschka into the subarachnoid space (Fig. 6-9) is served by the anterior inferior cerebellar artery.
Tumors of the Choroid Plexus
Tumors of the choroid plexus are relatively rare, comprising somewhat less than 1% of all intracranial tumors. In general, these lesions are classified as choroid plexus papillomas (Fig. 6-19). These are benign and occur more frequently than choroid plexus carcinomas, which are malignant and rarely seen. Although these tumors may be seen in patients of any age, they are more common between birth and 10 years. They more often occur in the fourth ventricle (50% to 60%) but may also be found in the lateral and third ventricles. These patients present with signs and symptoms of increased intracranial pressure (headache, nausea, vomiting, lethargy), hydrocephalus (excessive production of CSF), or deficits of eye movement due to pressure on the roots of III, IV, or VI. The treatment of choice for the more commonly seen tumor, a choroid plexus papilloma, is surgical removal. The more rarely seen choroid plexus carcinoma is treated first with chemotherapy, followed by surgery, then with a combination of chemotherapy and radiation therapy.
On histologic examination, these tumors are characterized by clusters of cuboidal or columnar cells that are strikingly similar to normal choroid plexus epithelium (Fig. 6-19). These cell clusters are insinuated between comparatively thin areas containing small vessels and loose connective tissue. This is one important difference between this tumor and an ependymoma, which has thick intervening areas that are composed of glial cell processes (compare Figs. 6-14 and 6-19). Mitotic figures are infrequently seen but when present may indicate that the tumor is malignant.
Cerebrospinal Fluid in Health and Disease
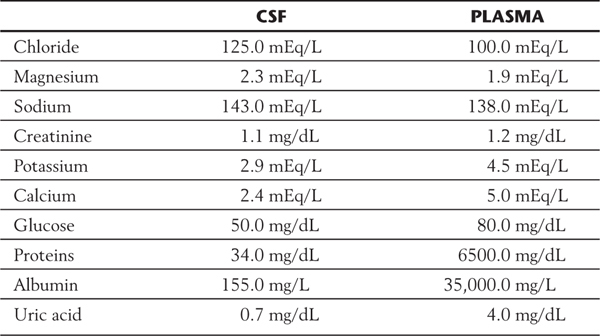
Choroid epithelial cells secrete CSF by selective transport of materials from the connective tissue extracellular space (Fig. 6-18). Sodium chloride is actively transported into the ventricles, and water passively follows the concentration gradient thus established. Other materials, including large molecules, are transported in pinocytotic vesicles from the basal to the apical surface of the epithelium and exocytosed into the CSF. Compared with blood plasma, CSF has higher concentrations of chloride, magnesium, and sodium; similar concentrations of creatinine; and lower concentrations of potassium, calcium, glucose, proteins, albumin, and uric acid (Table 6-1). Deviation from these normal values is indicative of a pathologic state or ongoing pathologic process.
Table 6-1 A Comparison of the Constituents of Cerebrospinal Fluid (CSF) with Blood Plasma*
*These are general averages that approximate the center of a range.
Normal CSF is clear and colorless and contains very little protein (15 to 45 mg/dL), little immunoglobulin, and only one to five cells (leukocytes) per milliliter. Changes from these normal values are useful in the diagnosis of a variety of disease processes (Table 6-1).
Lumbar puncture is used to collect a sample of CSF for analysis and to measure CSF pressure. A needle is inserted between the third and fourth (or fourth and fifth) lumbar vertebrae into the dural sac (lumbar cistern), the spinal fluid pressure may be measured (range of 100 to 250 mm H2O opening pressure), and a few milliliters of fluid is withdrawn. Because the average volume of CSF in the adult is about 120 to 140 mL, and the rate of production is 15 to 20 mL/hr (and about 400 to 500 mL/day), the sample removed is quickly replaced. When there is evidence of blood in the retrieved sample of CSF, it is important to establish whether this observation is due to subarachnoid hemorrhage or due to damage to a vessel during the procedure: a traumatic tap. What is commonly called the three-tube test provides the answer. Three successive tubes of CSF are drawn. If the first tube contains blood, the second little or none, and the third none, it was most likely a traumatic tap. If all three tubes contain the same amount of bloody CSF that is also xanthochromic (yellow tinted), it most likely means that there is bleeding into the subarachnoid space.
The numbers and types of cells found in CSF vary according to the type of disease. In bacterial meningitis or brain abscesses, neutrophils predominate and may reach concentrations of 1000 to 20,000/mL, and the CSF is cloudy. In syphilitic meningitis, by contrast, 200 to 300 cells/mL would be typical, and most of these would be lymphocytes. Lymphocytes are also the predominant cell type found in viral meningitis and encephalitis. In active multiple sclerosis (MS), there are usually fewer than 50 cells/mL of CSF. The diagnosis of MS also rests on changes in the immunoglobulin G content of CSF and a slight increase in the number of mononuclear cells; immunoglobulin G is both derived from the blood and produced by lymphocytes in the CSF, where it is released during an MS attack.
In marked contrast to the elevated numbers of white blood cells seen in central nervous system (CNS) infections, numerous red blood cells are present in the CSF of patients who have bleeding into the subarachnoid space (subarachnoid hemorrhage). For example, this condition may result from rupture of an intracranial aneurysm or arteriovenous malformation. Patients with subarachnoid hemorrhage or those with primary CNS tumors usually have elevated protein levels in their CSF. Elevated CSF protein is also seen in patients with syphilis or meningitis and in cancer patients in whom the tumor has spread (metastasized) into the CSF. The CSF of cancer patients may contain malignant cells characteristic of their primary lesions.
Cerebrospinal Fluid Production and Circulation
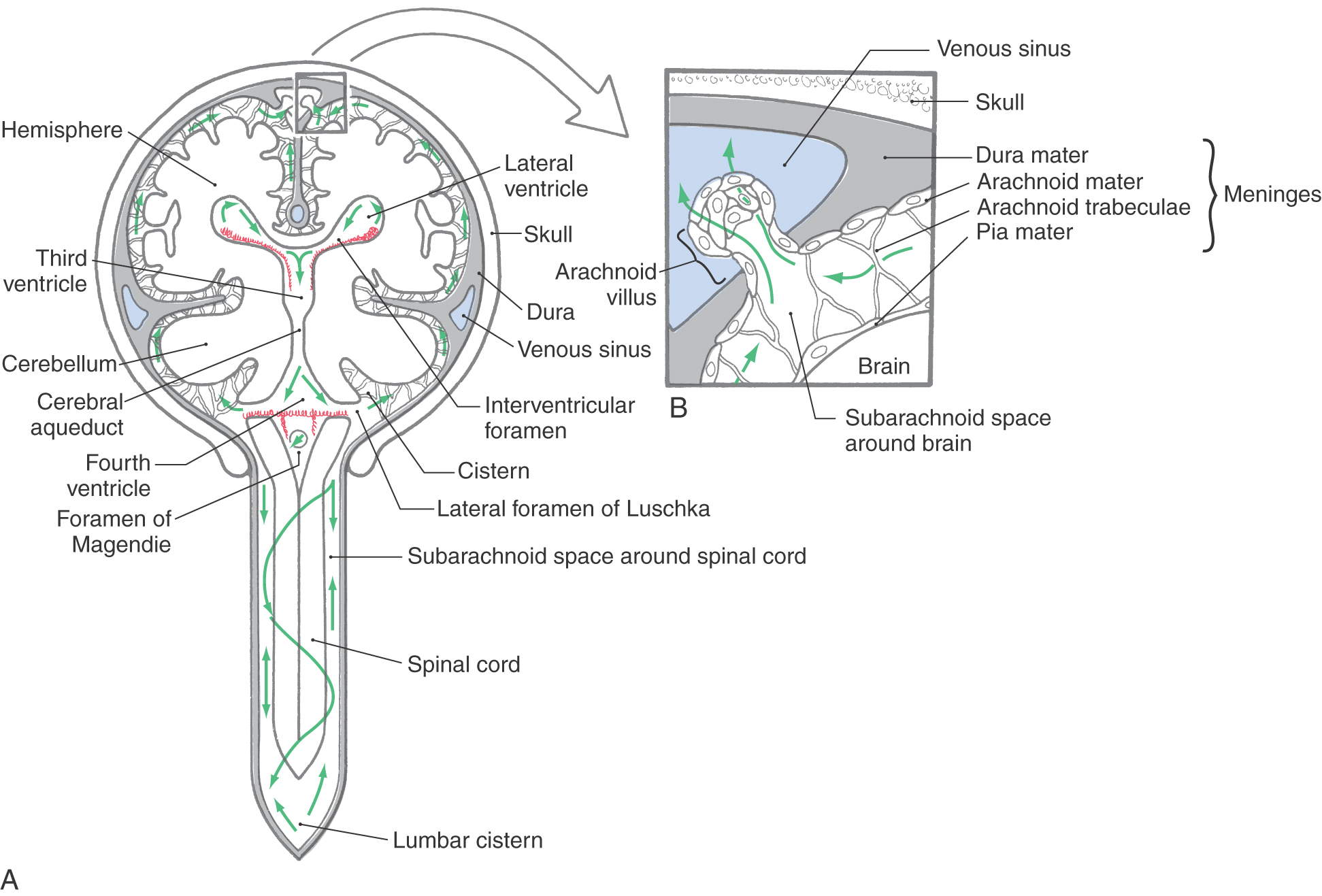
The CSF produced by the choroid plexuses passes through the ventricular system to exit the fourth ventricle through the foramina of Luschka and Magendie (Fig. 6-20). At this point, the CSF enters the subarachnoid space, which is continuous around the brain and spinal cord. The CSF in the subarachnoid space provides the buoyancy necessary to prevent the weight of the brain from crushing nerve roots and blood vessels against the internal surface of the skull. The weight of the brain, about 1400 g in air, is reduced to about 45 g when it is suspended in CSF. Consequently, the tethers formed by delicate connective tissue strands traversing the subarachnoid space, the arachnoid trabeculae (Fig. 6-20), are adequate to maintain the brain in a stable position within its CSF envelope.
The movement of CSF through the ventricular system and the subarachnoid space is influenced by several factors. First, the ciliary movement of intact ependymal cells moves CSF along from the lateral and third ventricles, through the cerebral aqueduct, into the fourth ventricle, and into the subarachnoid space. Second, there is a subtle pressure gradient between points of CSF production (choroid plexus) and points of CSF transfer into the venous system (arachnoid villi). Because CSF is not compressible, it tends to move along this gradient. Third, pulsations of arteries in the subarachnoid space, arterioles in the Virchow-Robin spaces, the passive movement of CSF through ependyma and brain tissue (the “sink action” of the brain), and subtle movements of the brain and spinal cord during normal activity also contribute to CSF circulation.
After passing through the subarachnoid space, the CSF reaches the arachnoid villi that extend into the superior sagittal sinus and into the venous lakes (lateral lacunae or lateral lacunae of the superior sagittal sinus) of the superior sagittal sinus (Fig. 6-20). The subarachnoid space and the CSF it contains extend into the core of each villus. At this point, CSF enters the venous circulation through two routes. A limited amount passes between the cells making up the arachnoid villus, whereas most is transported through these cells in membrane-bound vesicles (see also Chapter 7). About 330 to 380 mL of CSF enters the venous circulation per day, and about 120 to 140 mL is present in ventricles and subarachnoid space at any given time.
HYDROCEPHALUS AND RELATED CONDITIONS
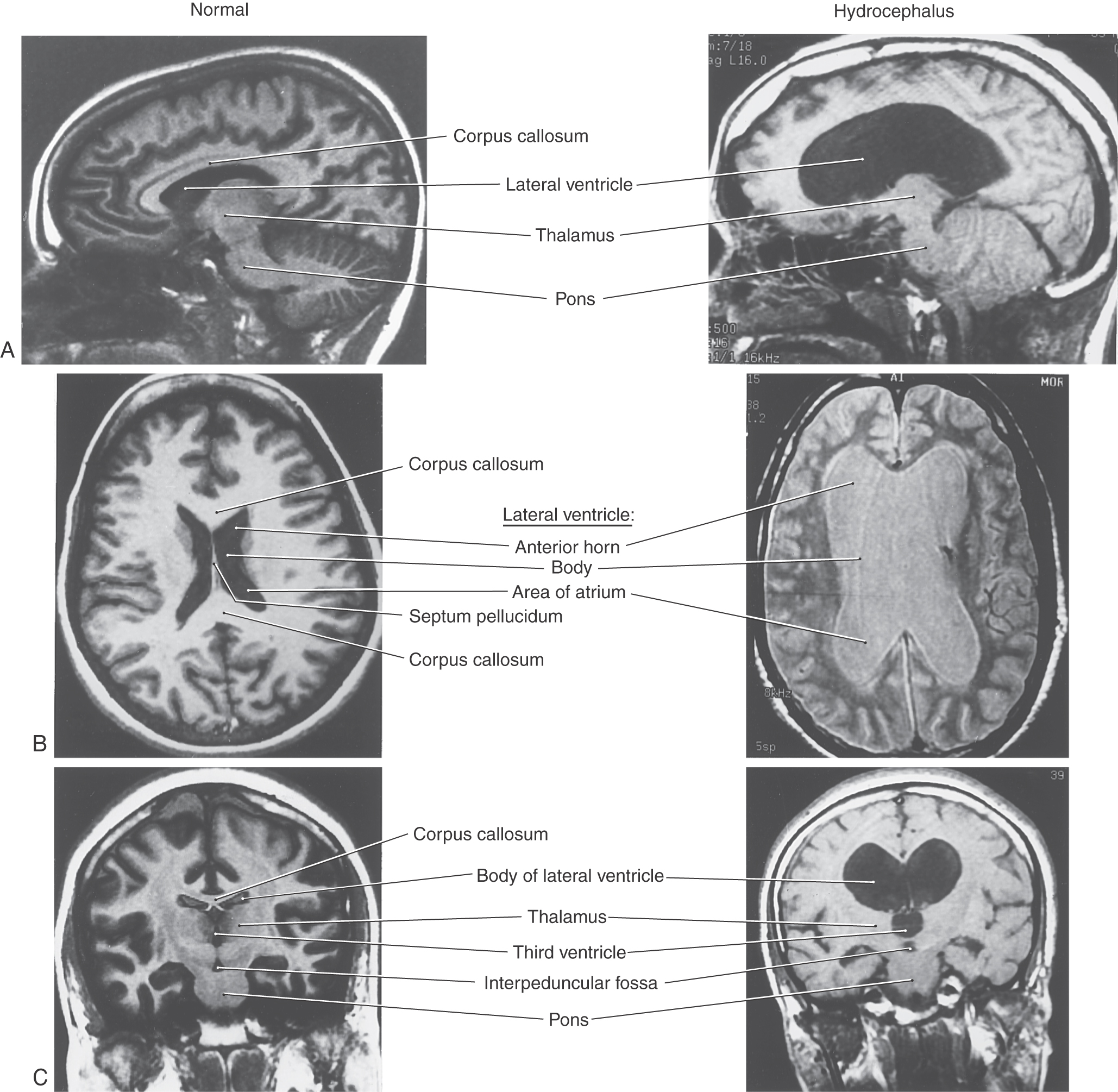
Blockage of CSF movement or a failure of the absorption mechanism will result in the accumulation of fluid in the ventricular spaces or around the brain (Fig. 6-21). The results, commonly called hydrocephalus, are characterized by an increase in CSF volume, enlargement of one or more of the ventricles, and, usually, an increase in CSF pressure. Dilation of the cerebral ventricles may result from a blockage of CSF flow through the system, as in obstructive hydrocephalus; from factors not related to impaired flow, as in communicating hydrocephalus; or from brain atrophy, as in hydrocephalus ex vacuo. Ventricular dilation may also be a sequela to trauma, meningitis, or subarachnoid hemorrhage. There is typically a moderate to severe increase in intracranial pressure in patients with hydrocephalus.
Obstructive Hydrocephalus
Obstructive hydrocephalus may result from an obstruction somewhere within the ventricular system or within the subarachnoid space. Common intraventricular sites of potential obstruction are the interventricular foramen (or foramina), cerebral aqueduct, caudal portions of the fourth ventricle, and foramen of the fourth ventricle. Extraventricular obstruction may occur at any place in the subarachnoid space but is more common around the base of the brain, at the tentorium cerebelli and tentorial notch, over the convexity of the hemisphere, and at the superior sagittal sinus.
Aqueductal Stenosis
Aqueductal stenosis may be caused by a tumor in the immediate vicinity of the midbrain (as in pineoblastoma or meningioma) that compresses the brain and occludes the cerebral aqueduct. This channel may also be occluded by the cellular debris seen after intraventricular hemorrhage, by bacterial or fungal infections, or by ependymal proliferation due to viral infections of the CNS (especially mumps). One major sequela of aqueductal blockade is enlargement of the third and both lateral ventricles (Fig. 6-21). This is sometimes called triventricular hydrocephalus because the three ventricles upstream to the blockage simultaneously enlarge as a result of one lesion or occlusion. Unilateral obstruction of one interventricular foramen, for example, by a colloid cyst in one interventricular foramen, results in enlargement of the lateral ventricle on that side. Blockage of both interventricular foramina will produce enlargement of both lateral ventricles. Obstruction of the exit channels of the fourth ventricle, the foramina of Magendie and Luschka, will result in enlargement of all parts of the ventricular system.
Communicating Hydrocephalus
In communicating hydrocephalus, the flow of CSF through the ventricular system and into the subarachnoid space is not impaired. However, movement of CSF through the subarachnoid space and into the venous system is partially or totally blocked. This block may be caused by a congenital absence (agenesis) of the arachnoid villi. Alternatively, these villi may be partially blocked by red blood cells subsequent to a subarachnoid hemorrhage. An exceedingly high level of protein in the CSF (above 500 mg/dL), as seen in patients with CNS tumors or inflammation, may also contribute to communicating hydrocephalus.
Additional causes of communicating hydrocephalus include the interruption of CSF movement through the subarachnoid space caused by either subarachnoid hemorrhage or a major CNS infection, such as leptomeningitis, and the subsequent inflammatory response. Overproduction of CSF in patients with papilloma of the choroid plexus may also be a factor. In all of these situations, there is an enlargement of all parts of the ventricular system. Although rare, hydrocephalus may also be seen in patients with impaired venous flow from the brain.
Hydrocephalus ex Vacuo
This is actually not a true hydrocephalus but rather a generalized atrophy of the brain resulting in ventricles that are relatively larger because of the loss of white matter. There is no increase in intracranial pressure, there are no neurologic deficits other than those that may be related to brain atrophy, and treatment is not indicated. Ex vacuo changes may also refer to atrophy with a change in ventricular size that may follow, by several years, an event such as a stroke.
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension (pseudotumor cerebri) is an enigmatic condition most commonly seen in obese women of childbearing age. There is an increase in intracranial pressure (>25 cm H2O), with little evidence of pressure increase on CT or magnetic resonance imaging studies, such as ventricular enlargement or effacement of sulci or cisterns. These patients usually experience headache, tinnitus due to venous turbulence (“pulsatile intracranial noise”), and visual deficits (up to blindness) due to papilledema (swelling of the optic disc). Treatment includes a program of weight loss, medication, and, if needed, shunting (lumboperitoneal) or surgical fenestration of the optic nerve sheath, which consists of making a window in the sheath to relieve pressure on the optic nerve.
Normal-Pressure Hydrocephalus
The cause of this form of hydrocephalus is unclear. The name is a misnomer because CSF pressure is elevated episodically when it is measured over time. Affected patients are usually elderly. In most cases the cause is unknown. Although intracranial pressure may initially be elevated and the ventricles enlarged, the pressure may wax and wane over time or even subside to a high-normal level; however, the effects of the increased pressure remain. The characteristic clinical triad is gait disturbance, urinary incontinence, and dementia; shunting may reverse this condition.
Patients with normal-pressure hydrocephalus experience a diagnostic triad consisting of urinary problems (frequency, urgency, or incontinence), impaired gait that is most obvious on stepping up as on a curb, and dementia. In some patients the combination of a difficult shuffling gait and dementia may mimic the clinical picture in degenerative conditions such as Alzheimer and Parkinson diseases. Treatment is a shunting procedure to reduce CSF pressure and volume. In some cases, there is general clinical improvement with lessening of all symptoms.
Sources and Additional Reading
Davson H, Welch K, Segal MB. Physiology and Pathophysiology of the Cerebrospinal Fluid. Edinburgh: Churchill Livingstone; 1987.
Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. Philadelphia: WB Saunders; 1992.
North B, Reilly P. Raised Intracranial Pressure: A Clinical Guide. Oxford: Heinemann; 1990.
Segal MB, ed. Barriers and Fluids of the Eye and Brain. Boca Raton, Fla: CRC Press; 1992.