The use of the laboratory
Specimen collection
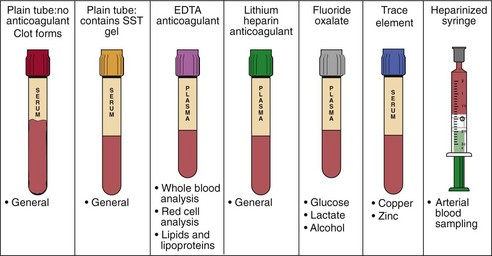
A variety of specimens are used in biochemical analysis and these are shown in Table 2.1.
Blood specimens
If blood is collected into a plain tube and allowed to clot, after centrifugation a serum specimen is obtained (Fig 2.1). For many biochemical analyses this will be the specimen recommended. In other cases, especially when the analyte in question is unstable and speed is necessary to obtain a specimen that can be frozen quickly, the blood is collected into a tube containing an anticoagulant such as heparin. When centrifuged, the supernatant is called plasma, which is almost identical to the cell-free fraction of blood but contains the anticoagulant as well.
Sampling errors
 Blood sampling technique. Difficulty in obtaining a blood specimen may lead to haemolysis with consequent release of potassium and other red cell constituents.
Blood sampling technique. Difficulty in obtaining a blood specimen may lead to haemolysis with consequent release of potassium and other red cell constituents.
 Prolonged stasis during venepuncture. Plasma water diffuses into the interstitial space and the serum or plasma sample obtained will be concentrated. Proteins and protein-bound components of plasma, such as calcium or thyroxine, will be falsely elevated.
Prolonged stasis during venepuncture. Plasma water diffuses into the interstitial space and the serum or plasma sample obtained will be concentrated. Proteins and protein-bound components of plasma, such as calcium or thyroxine, will be falsely elevated.
 Insufficient specimen. It may prove to be impossible for the laboratory to measure everything requested on a small volume.
Insufficient specimen. It may prove to be impossible for the laboratory to measure everything requested on a small volume.
 Errors in timing. The biggest source of error in the measurement of any analyte in a 24-hour urine specimen is in the collection of an accurately timed volume of urine.
Errors in timing. The biggest source of error in the measurement of any analyte in a 24-hour urine specimen is in the collection of an accurately timed volume of urine.
 Incorrect specimen container. For many analyses the blood must be collected into a container with anticoagulant and/or preservative. For example, samples for glucose should be collected into a special container containing fluoride, which inhibits glycolysis; otherwise the time taken to deliver the sample to the laboratory can affect the result. If a sample is collected into the wrong container, it should never be decanted into another type of tube. For example, blood that has been exposed, even briefly, to EDTA (an anticoagulant used in sample containers for lipids) will have a markedly reduced calcium concentration, approaching zero, along with an artefactually high potassium concentration. This is because EDTA is a chelator of calcium and is present as its potassium salt.
Incorrect specimen container. For many analyses the blood must be collected into a container with anticoagulant and/or preservative. For example, samples for glucose should be collected into a special container containing fluoride, which inhibits glycolysis; otherwise the time taken to deliver the sample to the laboratory can affect the result. If a sample is collected into the wrong container, it should never be decanted into another type of tube. For example, blood that has been exposed, even briefly, to EDTA (an anticoagulant used in sample containers for lipids) will have a markedly reduced calcium concentration, approaching zero, along with an artefactually high potassium concentration. This is because EDTA is a chelator of calcium and is present as its potassium salt.
 Inappropriate sampling site. Blood samples should not be taken ‘downstream’ from an intravenous drip. It is not unheard of for the laboratory to receive a blood glucose request on a specimen taken from the same arm into which 5% glucose is being infused. Usually the results are biochemically incredible but it is just possible that they may be acted upon with disastrous consequences for the patient.
Inappropriate sampling site. Blood samples should not be taken ‘downstream’ from an intravenous drip. It is not unheard of for the laboratory to receive a blood glucose request on a specimen taken from the same arm into which 5% glucose is being infused. Usually the results are biochemically incredible but it is just possible that they may be acted upon with disastrous consequences for the patient.
 Incorrect specimen storage. A blood sample stored overnight before being sent to the laboratory will show falsely high potassium, phosphate and red cell enzymes, such as lactate dehydrogenase, because of leakage into the extracellular fluid from the cells.
Incorrect specimen storage. A blood sample stored overnight before being sent to the laboratory will show falsely high potassium, phosphate and red cell enzymes, such as lactate dehydrogenase, because of leakage into the extracellular fluid from the cells.
Analysing the specimen
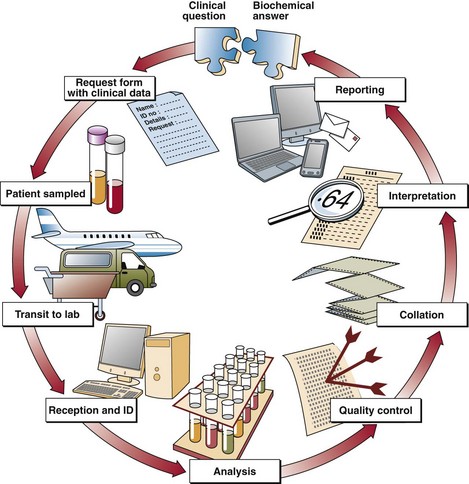
Once the form and specimen arrive at the laboratory reception, they are matched with a unique identifying number or bar-code. The average lab receives many thousands of requests and samples each day and it is important that all are clearly identified and never mixed up. Samples proceed through the laboratory as shown in Figure 2.2. All analytical procedures are quality controlled and the laboratory strives for reliability.
Once the results are available they are collated and a report is issued. Cumulative reports allow the clinician to see at a glance how the most recent result(s) compare with those tests performed previously, providing an aid to the monitoring of treatment (see p. 12).