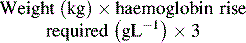11.1 The use of blood products in children
Introduction
Successful and safe transfusion practice depends on administering a quality blood component of the right type, in the right amount, in the right way, at the right time to the right patient.1
Emphasis on blood component safety, standardisation of appropriate guidelines for use of blood components and informed consent for blood component administration have led to a substantial reduction in the potential risks and complications of their use as well as increasing their appropriate usage. Informed consent with regards to the risks and benefits of blood component therapy needs to be obtained in the light of community concerns about transfusion safety, particularly the potential for infection transmission. The indication, risks, benefits, alternatives to transfusion, parental consent, response to treatment and any adverse event should be clearly documented. In non-urgent situations, parents and mature children can access publications such as ‘Children receiving a blood transfusion: A parents’ guide’2 for more information.
Clinical guidelines for use of red blood cells (RBC) in children are consensus rather than evidence based,3 with the latest National Health and Medical Research Council/Australian Society of Blood Transfusion Australian practice guidelines for blood product indication and administration available at www.nhmrc.gov.au, including haemoglobin (Hb) and platelet transfusion threshold triggers.1 It is recommended that intravenous (IV) access be 22–24G or larger for children receiving blood products through a standard blood administration set primed with normal saline or the blood component. A blood warmer is indicated at flow rates >15 ml kg−1 hr−1 in children and for exchange transfusion in infants; very slow rates are recommended in small children if rapid volume expansion is not required. Blood products should not be warmed to above 41°C.4
Whole blood
Whole blood is rarely used, being specifically used for massive blood loss requiring simultaneous restoration of oxygen-carrying capacity and blood volume, haemorrhage with uncontrolled coagulopathy and exchange transfusion. Eight ml kg−1 will increase a child’s haemoglobin concentration by 10 g/dL−1, and administration must be completed within 4 hours to avoid bacterial contamination.
Packed red blood cells (PRBC)
Indications
The child’s Hb level, patient factors, signs and symptoms of hypoxia, ongoing blood loss, risk of anaemia and risk of transfusion should be considered. Each paediatric red cell unit is 25–100 mL (mean volume 50 mL) with a haemoglobin concentration of 100–150 g L–1. PRBC is indicated if the oxygen-carrying capacity of blood is so reduced that the degree of anaemia poses a risk to the child or there is ongoing blood loss. Transfusion of PRBC in an asymptomatic child is not appropriate in most situations. PRBC transfusion is likely to be appropriate when haemoglobin is less than 70 g L–1 in critically ill children.5 The haemoglobin threshold remains uncertain in stable children with anaemia. Use of PRBC with haemoglobin in the range 70–100 g L–1 is appropriate if the child is at risk of hypoxia (cardiac, respiratory disease) and should be supported by the need to relieve clinical symptoms and signs. Criteria for PRBC in patients aged less than 4 months are different from those for older children. Infants in the former group have smaller blood volumes, decreased erythropoietin production (especially if premature) and there may be physiological anaemia of infancy.
Administration
If stored in optimal conditions, RBC have a shelf life of 35–42 days. In haemorrhagic shock, RBC infusion at an initial volume of 10–20 mL kg–1 should be considered when loss of blood volume approaches 30% (when hypotension first appears)3 and shock is refractory to non-blood fluid resuscitation. A single Hb level is not reliable in acute haemorrhage.3 If haemorrhage or haemolysis is accompanied by life-threatening hypoxia or rapid Hb decline then uncross-matched group O rhesus negative packed cells may be required. In less urgent cases, type-specific or cross-matched RBC is preferred. Rh type specific blood takes 15 minutes to cross match. If the child has no immediate need for RBC replacement and there is no ongoing bleeding or haemodynamic instability, cross-matched RBC may be administered over 4 hours. The volume required for elective top-up transfusion in mL is:
Platelets
Administration
Each paediatric unit is 40–70 mL and contains at least 5.5 × 1010 platelets.
Infusion of 5–10 mL kg–1 of platelets will lead to a rise in platelet count of 50–100 × 109 L–1.
Fresh frozen plasma (FFP)
Administration
In life-threatening exsanguination requiring massive transfusion, FFP may be required to minimise dilutional coagulopathy. One unit of FFP is required for every four units of packed cells but titrate FFP use against the coagulation profile if possible. FFP contains all coagulation factors including approximately one unit of factors VIII and V for each mL of FFP. FFP is administered at an initial dose of 10–20 mL kg–1 from a 50 mL paediatric bag. This dose raises coagulation factor level by 20% immediately.3 After thawing, FFP needs to be used immediately or stored at 2–6°C for up to 24 hours.
Cryoprecipitate
Cryoprecipitate is appropriate to use when clinical or potential bleeding (invasive procedure, trauma or DIC) is attributable to fibrinogen deficiency.3 It contains factors VIII, XIII, fibrinogen, von Willebrand’s factor and fibronectin. Approximately 200 units of each of these factors are contained in a 15 mL bag. This allows more rapid administration than FFP, whilst reducing the risk of fluid overload. In infants, a dose of 10–15 mL is sufficient to achieve haemostasis. Cryoprecipitate is not used to treat von Willebrand’s disease, haemophilia and deficiencies of factor XIII or fibronectin.
Albumin
Indications
Albumin is derived from volunteer human plasma pools and is indicated for rapid volume expansion in children with evidence of shock or poor perfusion. In paediatric emergency practice, albumin may be used in the initial fluid resuscitation for hypovolaemic shock, although it is no better than crystalloids or other colloids in this setting in adults.3 Other indications include the treatment of hypoproteinaemia, diuretic-resistant nephrotic syndrome, large volume paracentesis, severe burns after the first 24 hours and plasma exchange. There is no evidence for albumin use as a nutritional supplement or for the treatment of ascites or oedema related to portal hypertension.
Adverse effects and risks
There is evidence that albumin is associated with increased mortality and morbidity in critically-ill adults,6 but no such evidence in young children. Complications of albumin use include circulatory and sodium overload, with the relative risk of viral disease transmission being less compared with cellular blood components.
Risks of blood component use
Infections
Even though blood components are the safest they have ever been, infection transmission risk remains emotive and highly publicised, especially for human immunodeficiency virus (HIV).7 Infection acquired from a blood component is a rare occurrence when compared with non-infectious complications. The estimated risks per actual blood unit transfused are 1:200 000–1:2 000 000 for HIV, 1:30 000–1:250 000 for hepatitis B and 1:30 000–1:150 000 for hepatitis C. Disease transmission occurs primarily during the window period when a blood donor is infectious and the infection is immunologically silent and therefore undetectable on screening tests. Other infectious agents encountered include hepatitis A and G, human T lymphotropic virus 1 (HTLV I) and II, parvovirus B19, syphilis, malaria, babesiosis, Salmonella and Trypanosoma cruzi. There is no current evidence to suggest that variant Creutzfeldt–Jakob disease can be transmitted by blood transfusion. Donor selection and exclusion, donated blood screening, post-collection leucodepletion and viral inactivation help to minimise risk of infection transmission. Using pooled blood components allows contamination of the entire pool from one infectious donor and is therefore less safe than components derived from a single donor. Bacterial contamination of blood components, either from prolonged/faulty storage or acquired from the donor, is most frequently due to occult donor bacteraemia and donor skin organisms. Yersinia enterocolitica and other Gram negatives are most frequently implicated.
Transfusion-mediated immunomodulation
Allosensitisation, disturbed immunomodulation (both due to contamination by donor leucocytes) as well as infection transmission may be reduced by leucocytodepletion, psoralens and UV irradiation of blood components and using non-pooled blood components from a single donor. Immunomodulation is related to decreased cell-mediated immunity, with increased risk of reactivated viral infection, solid tumour recurrence and post-operative sepsis.8
Other transfusion-related adverse reactions
These include donor-recipient incompatibility, allergic reactions, anaphylaxis or anaphylactoid reactions, circulatory overload, haemolysis (acute/chronic, intra-/extravascular, associated fever/no fever), complications associated with massive transfusion (hypothermia, dilutional coagulopathy and thrombocytopenia) and post-transfusion thrombocytopenia.7
Controversies and future directions
 The threshold for red cell transfusion at which benefits outweigh risk in stable children and non-critically ill adults remains unclear.
The threshold for red cell transfusion at which benefits outweigh risk in stable children and non-critically ill adults remains unclear. The role and potential harm of albumin in adult resuscitation has not been investigated in paediatric resuscitation and the role of immunoglobulins in severe sepsis remains uncertain.
The role and potential harm of albumin in adult resuscitation has not been investigated in paediatric resuscitation and the role of immunoglobulins in severe sepsis remains uncertain.1 National Health and Medical Research Council/Australian Society of Blood Transfusion. Topics in Transfusion Medicine. Available from: http://www.anzsbt.org.au/publications/TTM.cfm [accessed 19.10.10]
2 National Health and Medical Research Council/Australian Society of Blood Transfusion. Children receiving a blood transfusion: A parents’ guide. 2004. Available from: www.nhmrc.gov.au. and www.anzsbt.org.au [accessed 19.10.10]
3 Robitaille N., Hume H.A. Blood products and fractionated plasma products: preparation, indications and administration. In: Arceci R.J., Hann I.M., Smith O.P., editors. Pediatric Hematology. 3rd ed. London: Blackwell Publishing Ltd; 2006:693-723.
4 National Health and Medical Research Council/Australian Society of Blood Transfusion. Guidelines for appropriate use of blood and blood components/Guidelines for the administration of blood components. 2004. Available from: www.nhmrc.gov.au;. and www.anzsbt.org.au [accessed 19.10.10]
5 Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. Transfusion strategies for patients in pediatric intensive care units. New Engl J Med. 2007;356:1609-1619.
6 SAFE Study Investigators, Finfer S., Bellomo R., McEvoy S., et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ. 2006;333(7577):1044. Epub 2006 Oct 13
7 Luban N.L.C., Wong E.C.C. Hazards of transfusion. In: Arceci R.J., Hann I.M., Smith O.P., editors. Pediatric Hematology. 3rd ed. London: Blackwell Publishing Ltd; 2006:724-744.
8 Goodnough L.T., Shander A., Brecher R.E. Transfusion medicine: Looking to the future. Lancet. 2003;361:161-169.
American Association of Blood Banks. Facts about blood and blood banking. Availalble from http://www.aabb.org, 2003. [accessed 19.10.10]
Behrman R.E., Kleigman R.M. Intercontinental childhood ITP registry. Nelson essentials of pediatrics, 4th ed.. Philadelphia:WB Saunders. 2002.
Christensen R.D. Hematologic problems of the neonate. Philadelphia: WB Saunders Company; 2000.
Goodnough L.T., Brecher M.E., Kanter M.H., AuBuchon J.P. Transfusion medicine. Blood transfusion. N Engl J Med. 1999;340:438-447.
Kruskall M.S. The perils of platelet transfusions. N Engl J Med. 1997;337:1914-1915.
Miller D.R., Bachner R.L. Blood diseases of infancy and childhood. In the tradition of Carl H Smith. Mosby-Year Book, 7th ed., St Louis, MO: Mosby, 1995.
Regan F., Taylor C. Recent developments. Blood transfusion medicine. Br Med J. 2002;325:143-147.
Sadowitz P.D., Ammanullah S., Souid A.K., et al. Hematologic emergencies in the pediatric ER. Emerg Med Clin N Am. 2002;20:177-188. vii
Smith H. Diagnosis in paediatric haematology. New York: Churchill Livingstone, 1996.
Stainsby D., Jones H., Asher D., et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20(4):273-282.