The thyroid gland
Background
Embryology, surgical anatomy and physiology
Recurrent laryngeal nerve and external branch of superior laryngeal nerve anatomy
The recurrent laryngeal nerve (RLN), also known as the inferior laryngeal nerve, is a branch of the vagus nerve. After winding around the aortic arch on the left, and the right subclavian artery, the RLNs course superomedially from the root of the neck to continue in the tracheo-oesophageal groove. The right RLN has an oblique course and the left a more vertical course, in the neck. The posteromedial aspect of the gland is therefore in close approximation to the last extralaryngeal segment of the nerve. In the last 1–2 cm of its extralaryngeal course, the RLN is juxtaposed between the lateral side of the ligament of Berry and the medial side of the TZ, plastered in place by an overlying fascia containing the tertiary branches of the inferior thyroid artery. It is here, just before entering the larynx under the cover of the cricopharyngeus muscle, that the RLN is most constant in position and also most prone to injury during thyroid surgery due to the difficulty in freeing it from the structures enveloping it. Up to 72% of RLNs divide into two or more branches before entering the larynx. The anterior-most branch carries most of the motor fibres to the laryngeal muscles, and therefore is the most important branch to preserve.1
The external branch of the superior laryngeal nerve (EBSLN), also a branch of the vagus nerve, is the motor supply of the cricothyroid muscle. In its course from the carotid sheath to the cricothyroid muscle, it comes close to the junction of the upper pole and the upper pole vessels. The Cernea Classification describes the various variations, which have relevance in surgical dissection (Fig. 2.1).
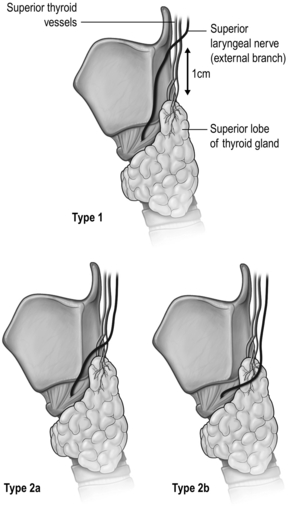
Figure 2.1 Anatomical variations of the external branch of the superior laryngeal nerve, in relation to the superior pole of the thyroid lobe. Type 1 nerve crosses the superior thyroid vessels > 1 cm above the superior pole, while type 2 nerve crosses the superior thyroid vessels < 1 cm above (type 2a) or below (type 2b) the superior pole.
Parathyroid anatomy
Parathyroid cell masses from the third and fourth pharyngeal pouches form the inferior and superior parathyroid glands respectively. They descend from their pouch origins to the final positions in close association with the developing thyroid and thymus glands. Therefore, it is not surprising that they maintain such close relationships with these glands. The majority of non-pathological superior parathyroid glands are found in the vicinity of the cricothyroid junction (77%), often closely related to the TZ and RLN, or under the capsule on the posterior surface of the superior thyroid pole (22%). The superior glands lie posterior to the RLNs. The inferior parathyroid glands, having travelled a longer distance, are more variable in their locations. They can be found on the surface of the inferior thyroid pole (42%), in the uppermost part of the thymic horn (39%), lateral to the inferior thyroid pole (15%) or in other ectopic locations (2%).2 They lie anterior to the RLNs.
Thyroid physiology
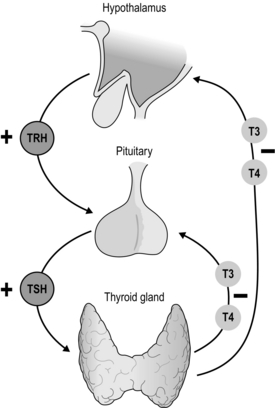
Thyroid hormone secretion in health is dependent upon a classical feedback loop. Reduced levels of T3 and T4 induce thyroid-stimulating hormone (TSH) release directly from the anterior pituitary and also indirectly through hypothalamic stimulation of thyrotropin-releasing hormone (TRH) release (which in turn induces TSH secretion). Such pathways are suppressed in periods of thyroid hormone excess. TSH exerts its effect via TSH receptors to increase iodide trapping, Tg synthesis, as well as T3/T4 production and secretion (Fig. 2.2).
Clinical history and examination
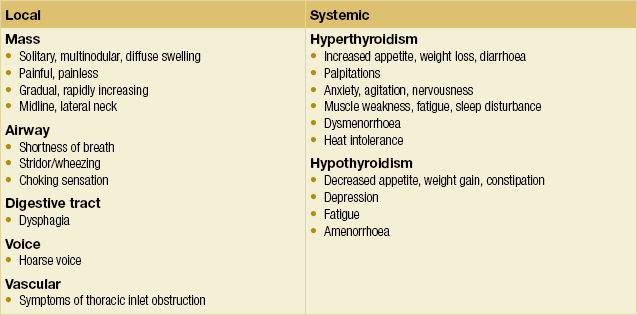
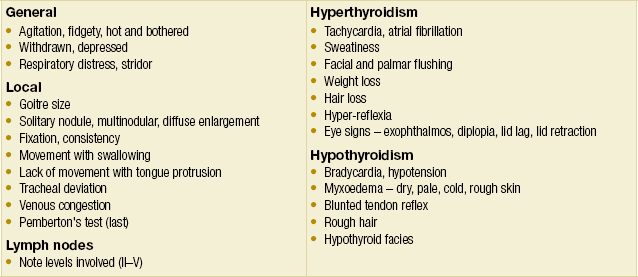
Pathologies of the endocrine organs, including the thyroid gland, give rise to a broad spectrum of symptoms and signs. The clinician must be thorough in both history-taking and physical examination, not only of the organ in question, but also other organ systems that the endocrinopathy may affect. A schema for assessing patients with thyroid complaints is suggested in Box 2.1, while Box 2.2 lists the questions that are essential to keep in mind when formulating a management plan. Some symptoms and signs of thyroid pathologies are listed in Tables 2.1 and 2.2. These are by no means exhaustive. A grading system for goitre size has been published by the World Health Organisation (Table 2.3).
Table 2.3
Simplified classification of goitre by palpation (WHO)
| Grade 0 | No palpable or visible goitre |
| Grade 1 | A goitre that is palpable but not visible when the neck is in the normal position (i.e. the thyroid is not visibly enlarged) |
| Grade 2 | A swelling in the neck that is clearly visible when the neck is in the normal position and is consistent with an enlarged thyroid when the neck is palpated |
Investigation of the thyroid
Blood tests
Thyroid function tests: Thyroid function homeostasis is dependent upon the pituitary–thyroid axis feedback loop (Fig. 2.2). Thyroid function tests (TFTs) identify hypo- or hyperfunction through quantification of not only circulating thyroid hormones (T3 and T4) but, more importantly, thyroid-stimulating hormone (TSH or thyrotropin). In keeping with the negative feedback loop, thyroid hyperfunction results in a suppressed TSH, in the presence of increased circulating T3 and T4. Conversely, reduced hormone levels lead to an increase in TSH.3 (also see section on hyperthyroidism).
Thyroglobulin antibody: Thyroglobulin antibody (TgAb) is a highly sensitive marker of Hashimoto’s disease and over 99% of patients with this condition will have elevated antibodies.4 Elevated levels may also be seen in Graves’ disease. The presence of TgAb should also be noted when monitoring Tg levels for surveillance after treatment of papillary or follicular thyroid cancer. TgAb may interfere with Tg assays and lead to spurious levels.
Thyroid peroxidase antibody: Thyroid peroxidase antibody (TPOAb) is commonly elevated in Graves’ disease but may also be seen in cases of thyroiditis. The test lacks sensitivity and specificity for Graves’ disease and is therefore only useful with a clear clinical suspicion of disease.5 Systemic autoimmune diseases may also lead to TPOAb positivity that may not be of any clinical significance.
TSH receptor antibody: TSH receptor antibodies (TRAbs) may be directly stimulatory or exert an inhibitory action on thyroid TSH receptors, leading to related changes in thyroid hormone secretion. Stimulatory antibodies are encountered in Graves’ disease and are particularly useful where the clinical diagnosis proves difficult to make. TRAbs are also identified in euthyroid Graves’ opthalmopathy, unilateral Graves’ eye disease, subclinical hyperthyroidism and thyroiditis. TRAbs can also cross the placenta and, in pregnancy, a positive test predicts for neonatal thyroid dysfunction.5
Biomarkers of malignant disease:
Thyroglobulin: Assessment of serum Tg is employed in surveillance of patients who have undergone total thyroidectomy and radioactive iodine ablation for differentiated thyroid cancers. Thyroglobulin serves as a biochemical marker of disease recurrence or progression in those with residual disease.
Calcitonin: Calcitonin is produced by the parafollicular C cells of the thyroid gland. These cells represent the cellular origin of medullary thyroid carcinoma (MTC), and calcitonin is elevated in cases of this disease. Calcitonin serves as a sensitive marker of disease recurrence and progression in MTC, and progressively higher levels at diagnosis are associated with larger tumours, regional lymph node metastases and distant metastases.6 Routine measurement of calcitonin in the work-up of thyroid nodules is recommended by the European Thyroid Cancer Taskforce; however, the American Thyroid Association (ATA) has not made a recommendation for or against this practice in their updated guidelines.7,8
Imaging studies
Ultrasonography: Ultrasound (US) is the imaging modality of choice for evaluation of the thyroid gland and associated lymph nodes. It is accessible, inexpensive, non-invasive and well tolerated. Surgeon-performed thyroid US is increasingly becoming a standard skill set and it has been shown that surgeon-performed US leads to beneficial changes in diagnosis and management.10 It also affords the clinician the added advantage of an intimate anatomical knowledge of the region to be dissected, which is of particular benefit in re-operative surgery or where selective lymph node dissection is anticipated.
US features that are suspicious for malignancy include microcalcifications, intranodular hypervascularity, hypoechoeic nodules, irregular margins and extracapsular extension (see Box 2.3).
Nuclear medicine studies: Thyroid isotope scanning employs intravenous radiolabelled iodine (131I or 123I) or technetium pertechnetate (99mTc), which are taken up by active thyroid cells and detected by gamma-ray cameras. Isotope scanning may be used in determining the cause of hyperthyroidism, identifying ectopic thyroid tissue or postoperative remnant tissue and detecting metastases of differentiated thyroid cancers. It is also used for surveillance after treatment of differentiated thyroid cancers.
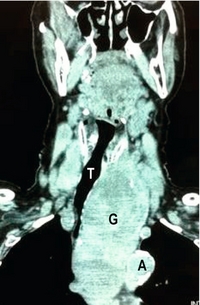
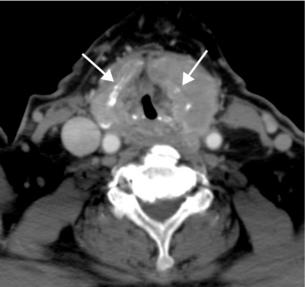
Computed tomography: Computed tomography (CT) scanning gives detailed anatomical definition of the thoracic inlet and associated structures, and is therefore of utility in the management of retrosternal disease. The degree of tracheal compression and distortion of adjacent structures by a bulky retrosternal goitre can be adequately defined (Fig. 2.3). The presence of mediastinal, pulmonary or more distant metastases in thyroid cancer can also be quantified. Often, CT scanning will dictate the surgical approach, with a large retrosternal goitre or low mediastinal metastases being indications for sternotomy. In locally aggressive cancers, CT scanning is a useful modality in assessing invasion of the aerodigestive tract and internal jugular veins (Fig. 2.4).
Tissue diagnosis
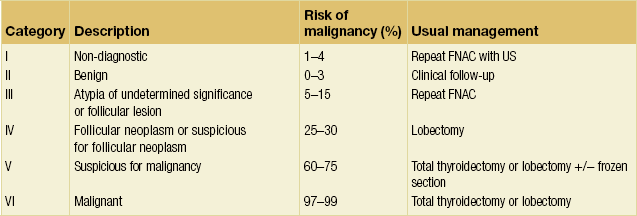
The interpretation of FNAC results has recently been standardised with the introduction of the Bethesda classification, which divides FNAC results into six categories (Table 2.4, Fig. 2.5). Each category correlates with an estimated risk of malignancy, which aids surgical decision-making (see section on management of differentiated thyroid cancers).11,12
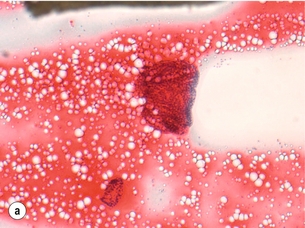
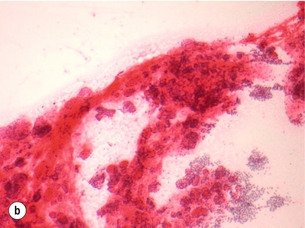
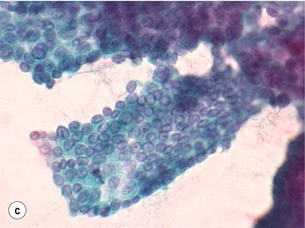
Figure 2.5 Fine-needle aspiration cytology (FNAC) showing Bethesda II, IV and VI. (a) Bethesda II – abundant colloid with some benign follicular cells arranged as microfollicles or fragments of follicles. (b) Bethesda IV – cytological preparation with high cellularity with scant colloid. The follicular cells are usually in microfollicular or trabecular arrangements. (c) Bethesda VI – nuclei showing prominent pseudo-inclusions and grooves. Images courtesy of Dr Anthony Gill.
Surgical pathologies of the thyroid
Benign goitre
Causes of multinodular goitre:
Iodine deficiency: Goitre is considered endemic when its prevalence in a region is over 10%, and iodine deficiency is the primary cause of endemic MNG. Iodine deficiency is mainly due to a low dietary intake in areas of iodine-poor soil, regardless of altitude. While some of the most severely iodine-deficient regions are high up in the mountains, such as the Pyrenees, the Himalayas and the Cordillera of the Andes, populations in coastal areas, large cities and highly developed countries can also be found to be iodine deficient. The Sydney basin on Australia’s eastern coast is one example.
Genetics: Although no single causative gene with a clear mode of inheritance has been described, the familial clusters and higher concordance rates in monozygotic twins with sporadic MNG point towards a genetic aetiology. Genes implicated in familial goitre include the thyroglobulin gene, the thyroid-stimulating hormone receptor gene, the Na+/I− symporter gene and the MNG marker 1 on chromosome 14. A defect in any of these genes can result in dyshormonogenesis, leading to compensatory goitre formation. Further studies are required for the significance of these genes to be extrapolated to the general population.13
Goitrogens: Thiocyanate is the goitrogen found in cassava and vegetables of the brassica family (e.g. cabbage, Brussels sprouts, cauliflower, mustard and turnip). Their goitrogenic effects are usually seen in areas where these food types are the staple, and especially where the iodine intake is also borderline.14
Pathogenesis: There are two stages in the development of MNG, which may be separated by a long period of time, sometimes as long as decades. The early stimulus for generalised thyroid hyperplasia is most commonly due to iodine deficiency in endemic areas, whereas in sporadic MNG, genetic predisposition or ingested goitrogens may be the stimulus. The second stage of MNG formation is due to focal somatic mutations. Although most of the mutations result in enlarged colloid follicles, focal hyperplasia, hypertrophy, adenoma or even carcinoma can all contribute to the MNG. Over time, these nodules become intersected by areas of fibrosis.15–17
Management of benign MNG: Surgery is the only effective way of treating compressive symptoms of the aerodigestive tract caused by MNG. As such, this constitutes the main indication for surgery. Other indications include MNG with nodules suspicious of malignancy on FNA, toxic MNG and retrosternal goitre.
Total thyroidectomy (TTx) has replaced subtotal thyroidectomy (STTx) as the procedure of choice for benign MNG. The major issue with STTx is recurrence, with long-term follow-up data showing eventual recurrence in up to 50% of patients.18 Furthermore, if secondary surgery is subsequently required for symptomatic recurrent goitre, the risk of complications rises. A significantly higher complication rate has been reported in patients undergoing re-operative thyroidectomy for recurrence after initial STTx, compared to those who had a primary TTx.19
Thyroid cysts
Thyroid cysts are usually benign and account for up to a third of surgically excised solitary thyroid lesions. However, up to 10% of mixed solid and cystic thyroid lesions can be malignant in nature. FNA under US guidance and targeting the solid component of a mixed solid/cystic lesion ensures optimal cellular harvest. Indications for surgery include malignant or suspicious cytology, large cyst (> 4 cm), rapid refill after aspiration, heavily bloodstained aspirate, and a history of head and neck irradiation.20
Malignant conditions
The incidence of thyroid cancer has increased exponentially over the last three or so decades according to data from countries such as Australia, the USA, Canada and France.21–24 This steep rise in incidence is due to increased diagnosis of papillary thyroid carcinoma (PTC), especially microcarcinomas, with mortality due to thyroid cancer remaining consistently low (5-year relative survival of 96%).22,25 Females are four times more likely to be diagnosed with thyroid cancer than males.
Molecular biology of thyroid cancers
The underlying molecular mechanisms that result in thyroid cancer development have gradually been elucidated in the last 20 years. A brief summary is given here.26
Papillary thyroid carcinoma: The molecular studies over the last two decades have led to the observation that PTC is characterised by genetic lesions that activate the mitogen-activated protein kinase (MAPK) signalling pathway. These genetic lesions can be produced by chromosomal rearrangements such as RET/PTC, TRK and AKAP9/BRAF oncogenes, or point mutations such as BRAF and RAS oncogenes.26
The RET proto-oncogene encodes a receptor-type tyrosine kinase. In PTC, RET is mutated by chromosomal rearrangements where the tyrosine kinase domain is fused to a variety of donor genes causing constitutive activation of the tyrosine kinase domain. RET/PTC1 and RET/PTC3 are the commonest combinations, and they account for over 90% of all RET rearrangements in PTC. Tumours with RET/PTC rearrangements are typically of the classical variant of PTC.26
The BRAF protein is the B-isoform of the intracellular Raf kinase within the MAPK signalling cascade. The BRAF gene is mutated in a variety of human cancers, and by far the commonest mutation involves a valine-to-glutamate substitution at residue 600 (BRAFV600E). This substitution results in constitutive activation of Raf kinase and subsequently up-regulation of the MAPK pathway. BRAFV600E is detected in 29–69% of PTC cases, and can be associated with the classical and tall cell variants of PTC, as well as poorly differentiated and anaplastic thyroid carcinomas. Some studies report association of BRAFV600E with more aggressive clinicopathological features; however, this view is not universal.26
Follicular thyroid carcinoma: The common genetic mutations associated with FTCs are RAS mutations, PAX8/PPAR-γ rearrangement and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway deregulation.
Oncogenic mutations may involve any of the three members of the RAS gene family. RAS mutations reportedly occur in up to 50% of FTCs, 40% of follicular adenomas, 25% of Hurthle cell carcinomas (HCCs) and 20% of follicular variant PTCs. They are also seen frequently in PDTC and ATC. The presence of RAS mutations in follicular adenomas is a clue to the adenoma–carcinoma sequence in the pathogenesis of FTCs. Significant correlation between RAS mutations to metastases and poor prognosis has been found.26
The PAX8/PPAR-γ rearrangement results in a fusion of the DNA-binding domain of PAX8 to the peroxisome proliferator-activated receptor PPAR-γ. The fusion protein stimulates proliferation of thyrocytes by an unknown mechanism. The PI3K/Akt pathway is central for many cellular events such as growth, proliferation and apoptosis. Its constitutive activation by mutations is a common feature in many cancers, including FTC.26
Differentiated thyroid cancers (PTC and FTC)
Risk factors: The most well-established environmental risk factor for thyroid cancer is exposure to ionising radiation.27 PTC is the type of thyroid cancer that is associated with radiation exposure, which induces damage to cellular DNA, commonly causing RET/PTC chromosomal rearrangements. The effect is most pronounced in children, and the latency period ranges from 5 to 30 years. In a patient with a history of radiation exposure, the overall risk of malignancy in a nodule is 30–40%; therefore, an initial TTx is recommended.28
No susceptibility gene for hereditary non-medullary thyroid cancer (HNMTC) has been identified; however, epidemiological studies have shown that they are more aggressive than sporadic disease. The risk of developing thyroid cancer is 5 to 10 times higher in patients with a first-degree relative who has thyroid cancer, when compared to the general population. Features such as early age at presentation, reversed gender distribution, large tumour size, tumour multicentricity and aggressive tumour biology are clues to suspect such a kindred. Until specific gene(s) are identified, a detailed family history is the only way to identify these at-risk families. HNMTC may also be part of another familial syndrome, such as familial adenomatous polyposis (APC), Cowden syndrome (PTEN), Carney complex type 1 (PRKAR1α), McCune–Albright syndrome (GNAS1) and Werner syndrome (WRN) (see Chapter 4 for further details).29
Papillary thyroid carcinoma: Papillary thyroid carcinoma is an epithelial malignant tumour of the thyroid gland, which still retains follicular cell differentiation and is characterised by unique nuclear features. The nuclei typically show a clear or ground-glass appearance, and irregularities of the nuclear contours can often be seen as nuclear grooves and pseudo-inclusions.30
Papillary thyroid microcarcinoma (PTMC) is defined by the World Health Organisation (WHO) as a PTC that is 1.0 cm or less in largest dimension.30 Its prevalence in autopsy series varies widely, from 6–7% in the USA to 35% in Finland.31 It is being diagnosed with increasing frequency due to the widespread use of US and FNA biopsy, as well as the improved resolution of ultrasonography.32,33 This increase has contributed significantly to the overall increase in incidence of newly diagnosed PTC.25 The prognosis for this group of patients had been shown to be excellent in a large study, despite 30% nodal involvement on presentation.34 A study from the Mayo Clinic also showed that PTMC does not affect overall survival, and that neither postoperative radioactive iodine (RAI) nor total thyroidectomy (or completion thyroidectomy) reduced recurrence rates compared to unilateral lobectomy. However, multifocal tumours and nodal positivity were predictors of recurrence.34
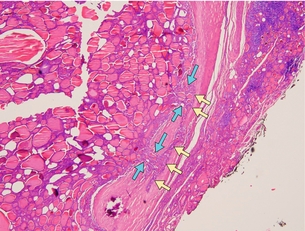
The follicular variant of PTC can be a challenge for pathologists to diagnose accurately. This variant displays follicular architecture, but retains the nuclear features of PTC (Fig. 2.6). It can be confused with follicular adenoma or carcinoma. However, it is important to distinguish the follicular variant of PTC from FTC because the prognosis of these patients is similar to that of patients with PTC rather than FTC.
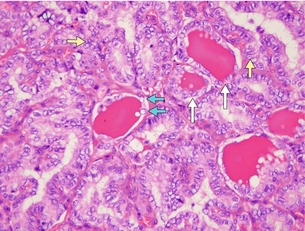
Figure 2.6 Follicular variant of PTC histology. Although the cells show follicular architecture, the nuclei features are diagnostic of PTC. Examples of nuclear clearing (‘orphan Annie eyes’) are shown by blue arrows, nuclear grooving by yellow arrows and follicular architecture by white arrows. Image courtesy of Dr Anthony Gill.
The tall cell variant is an uncommon variant that is usually found in older patients. It has a more aggressive clinical behaviour, and is often associated with necrosis, mitotic activity and extrathyroidal extension (which are all aggressive features). Columnar and diffuse sclerosing variants are two other variants associated with more aggressive disease behaviour.30
Follicular thyroid carcinoma: Like PTC, FTC is a malignancy of the epithelial follicular cells. However, unlike PTC, it lacks diagnostic nuclear features, so the diagnosis of FTC can only be made on histological examination (Fig. 2.7). Aspirates that are hypercellular, with a microfollicular pattern and scant colloid, suggest follicular neoplasm (adenoma or carcinoma). FTC can only be diagnosed by histological confirmation of capsular invasion and/or vascular invasion. The main variants are conventional or oncocytic types. The oncocytic type is also known as Hurthle cell carcinoma.
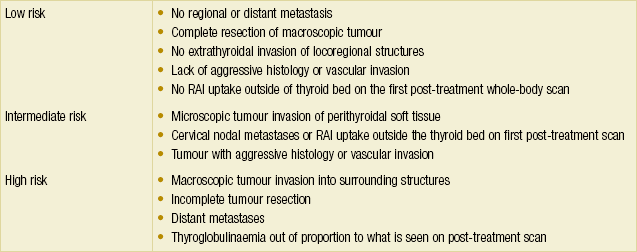
Staging: As many as 17 staging and prognostic systems for DTC have been reported in the literature since 1979.35 The sixth edition AJCC/IUCC staging system is currently one of the most commonly used systems. Other commonly used systems include AMES (Age, Metastasis, Extent, Size) from the Lahey Clinic, AGES (Age, Grade, Extent, Size) and MACIS (Metastasis, Age, Completeness of resection, Invasion, Size) from the Mayo Clinic, and EORTC staging from the European Organisation for Research and Treatment of Cancer. Some interesting observations can be made from these systems. Like all other staging systems, pathological features such as tumour size, grade, extent and presence of metastasis feature uniformly throughout the different classification systems. However, nodal status is notably absent in many of these systems apart from the AJCC system. This relates to the fact that these systems were mostly developed to predict disease-specific survival, and so far most studies do not correlate nodal status with survival, although the evidence is not conclusive at this stage. This must be borne in mind when using these prognostic systems during patient follow-up for recurrence. The ATA published a three-tier risk stratification that is useful for the purpose of surveillance (Table 2.5). Another unique factor in the AJCC staging for DTC is the inclusion of age. Patients under the age of 45 have excellent prognosis regardless of nodal status, and a small decrease in survival in the presence of metastases. As such, the highest stage for patients under 45 years of age is Stage II.
Work-up: Patients with thyroid cancer most commonly present with a neck lump. They can also be completely asymptomatic and be diagnosed incidentally on imaging or on the operative specimen where the indication for thyroidectomy was for a benign condition. A thorough history and examination, followed by appropriate investigations, is required when a patient presents with a complaint related to the thyroid. Certain clinical features should raise the suspicion of malignant thyroid disease (Box 2.3).
Management of DTC
Surgery of DTC: Various guidelines have been developed based on mostly retrospective data (Box 2.4). Due to the overall high rate of long-term survival of patients with thyroid DTC, prospective randomised data are near impossible to obtain. This has resulted in difficulties in resolving some of the controversies in the management of DTC.
It is important to recognise the various goals of initial treatment for DTC. Besides the usual goals of complete resection and accurate pathological staging, minimising treatment morbidity and facilitating long-term surveillance are more important than in other cancers due to the excellent long-term survival of patients with DTC. The management goals are listed in Table 2.6.
Table 2.6
The management goals for patients with DTC8
| Goals | Notes |
| 1. Complete resection of primary disease and involved cervical lymph nodes | Residual metastatic lymph nodes are the commonest sites of disease persistence or recurrence |
| 2. Minimising morbidities associated with treatment | Such as permanent hypocalcaemia and recurrent laryngeal nerve palsy |
| 3. Accurate staging of the disease | To facilitate initial prognostication |
| 4. Facilitating adjuvant radioactive iodine (RAI) treatment | Remnant malignant cells or metastatic deposits can only be ablated by RAI if all normal thyroid tissue is removed |
| 5. Facilitating long-term surveillance with RAI whole-body scan and serumTg | All normal thyroid tissue capable of taking up iodine and producing Tg needs to be surgically removed and ablated with RAI to enable long-term surveillance by these methods |
| 6. Minimising the risk of disease recurrence and metastasis | Adequate surgical removal of malignant tissue is the most important treatment variable influencing prognosis, evident by the inclusion of extent of surgery into some prognostic staging systems |
Extent of thyroidectomy: The choice between initial TTx and hemithyroidectomy (HTx; also called unilateral lobectomy) depends on factors such as size and number of lesions, Bethesda category, risk factors, need for RAI ablation, and contraindications to TTx. The technical aspects of thyroidectomy are discussed later in this chapter.
Diagnostic HTx is recommended when the FNA shows a follicular lesion with atypia or follicular neoplasm (Bethesda III and IV), since FNA is unable to distinguish between follicular adenoma and follicular carcinoma. If carcinoma is confirmed on histology, completion thyroidectomy is recommended. The exceptions are those with unifocal, low-risk (minimally invasive), intrathyroidal, node-negative tumours that are < 1 cm. An initial TTx is recommended for patients with Bethesda III or IV FNA results if the lesion is > 4 cm, when there is marked atypia on the FNA, or when there is a family history of thyroid carcinoma or history of radiation exposure, because of an increased risk of malignancy.8 Other reasons for recommending an initial total thyroidectomy for patients with Bethesda III or IV FNA results include bilateral nodular disease and patients wishing to avoid the possibility of requiring a completion thyroidectomy.
Contraindications to total thyroidectomy: There are very few, if any, absolute contraindications to total thyroidectomy, especially in the setting of thyroid malignancy. In situations where the administration of thyroxine after thyroidectomy cannot be assured, due either to issues of supply or compliance, less than total thyroidectomy may need to be considered as an alternative.
Lymph node dissection: The lymph node dissection for thyroid cancer is generally divided into dissections of the central and lateral compartments. The central compartment nodes include prelaryngeal (Delphian), pretracheal and paratracheal nodes. Lymph nodes in this compartment are also known as level VI nodes.37,38 On the other hand, the lateral compartment comprises levels II–V.39
Cervical lymph node metastasis occurs in up to 90% of patients with PTC at first diagnosis.40 The risk of local recurrence is higher in patients with lymph node metastases, especially if they are macroscopic, multiple or associated with extranodal extension.41 Although lymph node metastases were not shown to be an adverse factor on disease-free survival in some earlier reports, more recent, large-scale studies suggest an increase in mortality with regional lymph node metastases.42,43
Both preoperative ultrasonography and intraoperative clinical assessment of central lymph nodes are unreliable in detecting central lymph node metastasis. Therefore, the only reliable assessment of central lymph node metastasis is on histology. While central lymph node dissection (CLND) can be achieved with low morbidity in experienced hands, others have reported higher morbidity in terms of transient hypoparathyroidism with no reduction in recurrence.44,45 As a result of conflicting reports, most surgeons would agree with the ATA consensus statement recommendation of formal CLND when there are clinically apparent metastases. The role of prophylactic CLND is controversial. Secondary CLND can be performed in expert hands without added morbidity, and is recommended when proven recurrence in the central compartment is detected, if a CLND has not been performed during the initial thyroidectomy.46
Adjuvant treatments: Patients with thyroid cancer should be managed in a multidisciplinary setting, involving endocrine surgeons, endocrinologists, nuclear medicine physicians, radiologists and oncologists.
Follow-up: Initial follow-up is recommended 6–12 months after initial treatment, with Tg measurements, diagnostic whole-body nuclear medicine scan and neck US. Disease-free status is defined by absence of clinical evidence of disease, absence of imaging evidence of disease and undetectable Tg level during TSH suppression and stimulation, in the absence of Tg antibodies.
Surveillance of recurrence is also by means of Tg measurements, diagnostic nuclear medicine scan and neck US. The frequency depends on the patient’s ATA risk category (Table 2.5).
Poorly differentiated thyroid cancer (PDTC)
1. Presence of a trabecular/insular/solid growth pattern.
2. Absence of the classical PTC nuclear features.
3. Presence of convoluted nuclei, mitotic activity > 3 × 10HPF (high-power field) or tumour necrosis.47
Pathologically, most of these tumours consist of a mixture of trabecular, insular and solid growth patterns. PDTC can arise de novo or from areas of pre-existing PTC or FTC. It is important to separate PDTC from PTC and ATC because the survival pattern of patients with PDTC lies between the latter two cancers.48 Specifically, the presence of necrosis and a mitotic index of > 3 × 10HPF are negative prognostic indicators. It is also important to note that anaplastic transformation in PDTC is possible, and that even when only small foci of dedifferentiation are observed, the survival pattern follows that of ATC.
Medullary thyroid carcinoma
Medullary thyroid carcinoma was originally described by Jaquet as then ‘malignant goitre with amyloid’ in the early 1900s. Hazard and colleagues further defined the disease in 1959 and labelled it medullary (solid) carcinoma of the thyroid.49 MTC accounts for between 5% and 10% of all thyroid cancer. It is now a disease characterised by the RET gene mutation, and can be classified as hereditary (HMTC) or sporadic (SMTC). HMTC comprises 25% and SMTC 75% of the disease burden.50 Associations with the autosomal dominant multiple endocrine neoplasia (MEN) and familial non-MEN syndromes are well known (see Chapter 4 on familial endocrine diseases).
Pathology: Medullary thyroid carcinoma is a disease of the neuroendocrine-derived, calcitonin-producing, parafollicular C cells (Fig. 2.8). Whilst primarily characterised by discovery of the RET proto-oncogene, up to 50% of cases do not harbour an identifiable mutation.51 The RET mutation is thought to trigger constitutive phosphorylation of the tyrosine kinase receptor, leading to an increase in intracellular messaging and unregulated cellular proliferation. This typically leads to C-cell hyperplasia as a precursor to development of carcinoma. Characteristic histopathology is supplemented by immunohistochemistry showing calcitonin positivity. Cases of MTC are not uncommonly identified by FNAC.
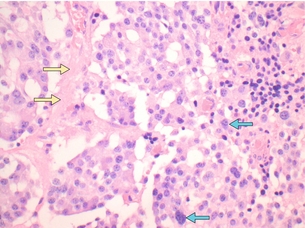
Figure 2.8 Medullary thyroid carcinoma.The histopathological appearance of MTC can be quite variable. In this figure, the cells are arranged in nests. The nuclei are oval and regular, containing coarse chromatin. Occasionally the nuclei are enlarged, pleomorphic and hyperchromatic (blue arrows). A small deposit of amyloid is seen (yellow arrows). Most MTCs show positive calcitonin staining, as well as CEA, chromogranin A and synaptophysin. Image courtesy of Dr Anthony Gill.
Clinical features: Patients undergoing genetic testing may be brought to the clinician’s attention before development of clinical features, and this is the most common mode of presentation in HMTC. Sporadic disease, by contrast, is more likely to manifest clinically with local symptoms or even problems related to metastases. Identification of a thyroid nodule or mass is the most common presenting complaint. Compressive symptoms of neck fullness, dysphagia, shortness of breath or hoarse voice may occur with more advanced disease. Systemic symptoms such as bone pain, flushing or diarrhoea may be encountered as the presenting feature in 10% of patients. Distant metastases most commonly occur in bone, liver and lung.52
Patients with SMTC typically present later than those with HMTC (47 years vs. 30 years).52 The burden of disease at diagnosis is dependent upon the mode of presentation. In patients with palpable disease at diagnosis, two-thirds will have involved ipsilateral cervical lymph nodes and one-third will have contralateral nodal involvement.53
Diagnosis: Diagnosis follows the general schema for investigation of a thyroid nodule. Imaging should include assessment of the cervical lymph nodes. If suspicious for MTC, FNAC of the thyroid nodule or cervical lymph node should also be stained for calcitonin. Identification of MEN-associated pathology such as phaeochromocytoma or hyperparathyroidism should prompt investigation to identify a related MTC.
Treatment: Management of MTC is based on the extent of disease at presentation. A preoperative diagnosis should be treated with TTx and bilateral CLND (level VI).54 Any lateral nodal disease evident clinically, radiologically or proven by biopsy should be managed with an ipsilateral compartmental LLND (levels II, III, IV and V).
During thyroidectomy, resected or devascularised parathyroid glands should be autografted but the preferred site of transplantation differs according to genetic mutation status if known, owing to the possibility of future parathyroid pathology. Autografts should be in the neck for RET-negative, MEN2B and familial MTC (FMTC) patients, and in a heterotopic site (e.g. brachioradialis muscle) for MEN2A patients.54
Medical therapy for advanced disease with tyrosine kinase inhibitors has shown promise in recent clinical trials.55 Radiotherapy is controversial and has not been shown to lead to improved survival outcomes, but may be utilised in selected cases for locoregional control of disease.54
Prophylactic thyroidectomy for RET-positive family members of the index case may need to be undertaken. This may involve operating upon paediatric patients, often under the age of 1 year, depending on the inherited mutation. TTx in these circumstances is technically challenging and should only ever be undertaken in tertiary centres by experienced surgeons. ATA guidelines provide a schema that identifies when patients should be offered a total thyroidectomy based on the inherited RET mutation.54
Prognosis: Overall 10-year survival of patients with MTC averages 75%.52 It can, however, be a heterogeneous disease, with some patients living years with bulky and persistent disease. Numerous clinical (age, gender), histopathological (tumour size, lymphovascular invasion), biochemical (calcitonin, CEA) and genetic (RET mutation) variables have been evaluated as potential markers of prognostic significance.
Only age and stage of disease at diagnosis have been found to be statistically significant predictors of survival on multivariate analyses, with 5-year survival rates ranging from 100% (Stage I) to 56% (Stage IV). The presence of cervical node metastases is associated with persistent or recurrent disease but does not appear to confer negatively upon survival and is therefore not considered an independent risk factor. Overall 5- and 10-year cause-specific mortality has been shown to be 13–33% and 22–39%, respectively.52
Follow-up: Serum calcitonin (and/or CEA) estimation at 3 months allows stratification of patients into one of two groups: those negative for disease and patients with elevated calcitonin who are considered to have ‘persistent’ disease. Persistent and recurrent cases can be considered to have ‘residual’ disease. In general, most patients can be monitored 3-, 6- and 12-monthly, with annual clinical examination and biomarker estimation. Ultrasound may be used as an adjunct to investigate clinical or biochemical suspicion of local disease recurrence or where residual disease is being monitored. Assessment of metastatic disease is typically with multimodal imaging, including US, CT, magnetic resonance imaging (MRI) and bone scan where appropriate.
Anaplastic thyroid carcinoma
Anaplastic thyroid carcinoma is an aggressive malignancy with a poor prognosis (Fig. 2.9). It typically affects elderly women, who usually present with local symptoms. Multimodal therapy with chemotherapy and radiotherapy is the mainstay of treatment for a disease that conveys a prognosis of less than 6 months in most series.56 Historically, surgical treatment was only considered as a palliative measure to prevent aerodigestive tract invasion/obstruction but is now rarely performed.
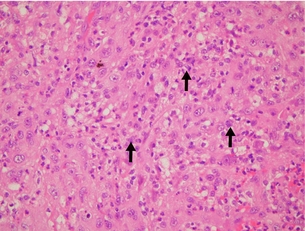
Figure 2.9 Anaplastic histology. Anaplastic carcinomas are composed of an admixture of spindle cells (not shown in this figure), pleomorphic giant cells and epithelioid cells. The cells can contain single or multiple bizarre-appearing nuclei (arrows). Thyroglobulin and TTF-1 staining are usually negative, while cytokeratin staining is often positive. Image courtesy of Dr Anthony Gill.
Other malignancies
Primary thyroid lymphoma: Primary thyroid lymphoma accounts for less than 5% of all thyroid malignancy. It is important to differentiate it from other forms of thyroid cancer, as a prompt diagnosis leads to potentially curative treatment without the need for surgery. It generally presents in older patients with a rapidly enlarging painless neck mass and associated local compressive symptoms. A history of autoimmune thyroiditis is important and this is thought to be the means by which lymphoid aggregates develop within the thyroid parenchyma, as in health there is no lymphatic tissue within the gland itself. Thus, Hashimoto’s disease is a strong risk factor. Non-Hodgkin B-cell lymphoma is the most common pathology encountered. Fine-needle biopsy yields mixed results and a biopsy dominated by lymphocytes (particularly if atypical) should arouse suspicion, but may still be difficult to distinguish from Hashimoto’s disease. Core or open surgical biopsy with histology, immunohistochemistry and flow cytometry is the preferred means of diagnosis.57
Treatment consists of chemoradiation. The role of surgery is controversial and should be considered on an individual basis primarily for palliative debulking of compressive neck disease. Prognosis is dependent upon the specific subtype of lymphoma and the associated grade and stage of disease. In general, a 60% overall 5-year survival can be expected.58
Squamous cell carcinoma: Primary squamous cell carcinoma of the thyroid is a rare and aggressive disease. When identified, distinguishing primary disease from a metastatic deposit is important relative to further investigation and management. The prognosis for primary disease is poor; palliative, supportive therapy may be the only option at diagnosis.59
Metastatic carcinoma to the thyroid: Metastatic disease to the thyroid from another primary site may occur with a range of malignancies. The use of modern PET scanning, for instance, is leading to detection of small metastatic deposits in thyroid tissue during staging for other cancers. Management is dependent on the primary disease, with the indication for thyroidectomy or debulking entirely hinging on the prognosis of the disease.
Hyperthyroidism
Causes
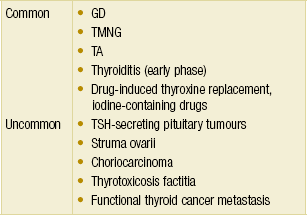
The most common causes of hyperthyroidism in western countries are Graves’ disease (GD), toxic multinodular goitre (TMNG) and toxic adenoma (TA) (Table 2.7). The ATA has recently updated the management guidelines for hyperthyroidism and other causes of thyrotoxicosis.60
Clinical features
The symptoms and signs of overt and subclinical thyrotoxicosis are similar, but differ in magnitude (Tables 2.1 and 2.2). The correlation between thyroid hormone levels and degree of symptoms and signs is only moderate.
Investigations
Diagnosis of thyrotoxicosis: The most sensitive and specific single blood test in the evaluation of thyroid function status is serum TSH, and it can be used as a screening test. This is due to the inverse log-linear relationship between TSH and free T4 (FT4) in patients with an intact pituitary–thyroid axis. That is, small changes of FT4 result in large changes in TSH. With the addition of serum FT4, the diagnostic accuracy is enhanced for patients with suspected hyperthyroidism. Typical thyroid function test (TFT) profiles are shown in Table 2.8.
Determination of aetiology: In most situations, after thorough history, examination and review of the medication list, TRAb levels and thyroid uptake scans are sufficient to establish the common underlying aetiology of thyrotoxicosis. Graves’ disease is suspected in a patient with a diffuse goitre, recent onset of ophthalmopathy, and moderate to severe hyperthyroidism (undetectable TSH and significantly elevated FT4). The diagnosis can be confirmed by elevated TRAb levels. No uptake scan is required in this situation. However, if the diagnosis is still uncertain or other causes of thyrotoxicosis are considered, a thyroid scan is indicated in the setting of suppressed TSH. The pattern of tracer uptake in the thyroid gland provides clues to the likely aetiology. However, it should always be interpreted in conjunction with the clinical presentation and other investigations. Table 2.9 and Figure 2.10 show the various patterns of tracer uptake and their likely diagnosis.
Table 2.9
Radio-tracer uptake scan patterns of hyperthyroidism
| Uptake pattern | Likely diagnosis |
| Diffusely increased uptake | GD |
| Patchy uptake (areas of intense and suppressed uptake) | TMNG |
| Focal uptake with suppression of surrounding thyroid and contralateral lobe | TA |
| Absent uptake | Thyroiditis, factitious thyrotoxicosis, recent excess iodine intake |
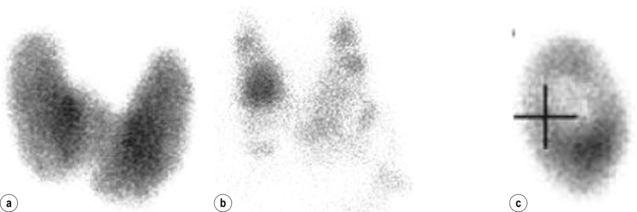
Figure 2.10 Tracer uptake patterns. Radio-tracer uptake patterns demonstrating: (a) Graves’ disease – diffusely increased uptake; (b) toxic multinodular goitre – patchy uptake; and (c) toxic adenoma – increased uptake in the lesion occupying the right thyroid lobe, with suppression of the rest of the gland. Images courtesy of Dr Paul Roach.
Ultrasonography of the thyroid gland can provide further anatomical information, in particular regarding gland nodularity, echogenicity and vascularity. In some European centres, sonography can provide enough information for the diagnosis of the aetiology of hyperthyroidism without thyroid scintigraphy. This technique is of particular value in situations where thyroid scintigraphy is not appropriate, such as during pregnancy.61
End-organ effects: Cardiac function in the elderly is especially affected by long-term hyperthyroidism, in particular due to GD. Evaluation of cardiac function may be necessary, and is essential prior to undergoing surgery. Cardiac function assessment can be achieved by way of echocardiogram, electrocardiogram, Holter monitor and/or myocardial perfusion studies.
Management
Treatment strategies can be considered as symptomatic and definitive. Medical treatment options can sometimes be symptomatic only in preparation for definitive surgical management, or it may be the definitive treatment itself.60
Symptomatic management: Beta-adrenergic blockade is the first-line treatment for patients with symptomatic thyrotoxicosis. It not only decreases heart rate, systolic blood pressure, muscle weakness and tremor, but also the degree of irritability, emotional lability and improves exercise tolerance. Commonly used beta-blockers include propranolol, atenolol and metoprolol. Alternatives or adjuncts to beta-blockers are calcium channel blockers such as verapamil and diltiazem.
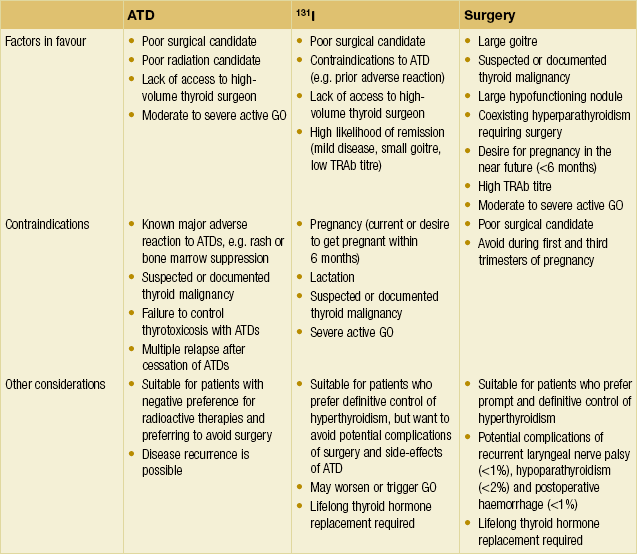
Management – Graves’ disease: Graves’ disease can be treated in one of three ways – 131I therapy, antithyroid drugs (ATDs) or TTx. There are regional differences in preference around the world. For example, in the USA, RAI is the preferred therapy, whereas in Europe and Japan there is greater preference for ATDs and/or surgery. A detailed discussion between the treating doctor and patient regarding the strengths and weaknesses of each modality is required to reach a treatment decision (Table 2.10). There is no difference in the long-term quality of life in patients with GD treated with any of the three modalities.
131I: Radioactive iodine therapy is generally well tolerated. Complications are rare in the absence of Graves’ ophthalmopathy. Thyroid storm following therapy has rarely been reported. Pre-treatment with beta-blockers and carbimazole has been employed to minimise worsening of symptoms for patients with severe toxicosis. Most patients can achieve normal levels of TFTs and resolution of symptoms within 4–8 weeks of treatment, provided sufficient radiation is administered in a single dose. Some will progress to hypothyroidism in the ensuing 6 months. Therefore, monitoring of clinical features and levels of TFTs, with appropriate thyroxine replacement, is required after 131I therapy.
ATD: Carbimazole or methimazole are the drugs of choice in treating patients with hyperthyroidism. The alternative, propylthiouracil (PTU), is used during the first trimester of pregnancy, for the treatment of thyroid storm, and in patients with major reactions to methimazole or carbimazole. The side-effects of ATDs include rash, cholestatic hepatotoxicity and agranulocytosis. Although PTU can cause fulminant hepatic necrosis and deaths have been reported, methimazole may cause embryopathy, including choanal and oesophageal atresia and aplasia cutis of the scalp. Baseline white cell counts and liver function tests are recommended prior to starting ATDs.
Management – TMNG: Although both surgery and 131I are recommended options in the ATA guidelines, a 20% risk of recurrence following 131I therapy is considered unacceptably high in some centres, hence surgery is the preferred treatment for TMNG. Recurrence after total thyroidectomy by experienced endocrine surgeons should be < 1%. Likely sites of recurrence are at the pyramid, tubercle of Zuckerkandl and retrosternal rests.62
Surgical indications
Surgical treatment in the way of thyroidectomy may be the treatment of choice or salvage in cases of failed medical therapies. The indications are listed in Box 2.5.
Operative strategy
Preoperative considerations: Patients with hyperthyroidism should be rendered euthyroid before submitting to surgery. This is best achieved with carbimazole. Beta-blockers can be used as supplementary symptomatic control, while iodine is given for 10 days before surgery for patients with GD. Treatment with iodine (in the form of Lugol’s solution or saturated solution of potassium iodide or inorganic iodine) facilitates surgery by decreasing thyroid vascularity. Iodine treatment is not necessary in patients with TMNG. Corticosteroids can also be administered in addition to iodine, especially in cases of emergency surgery requiring rapid preparation. Close monitoring of the patients after surgery is still required as thyroid storm can occur up to 24 hours after removal of the thyroid gland.
Operative considerations: Total thyroidectomy is the procedure of choice for the treatment of GD and TMNG. When performed by experienced surgeons it is associated with a very high cure rate and low morbidity. Subtotal thyroidectomy (such as Dunhill’s procedure) carries an 8% risk of persistence or recurrence, and a 50% chance of requiring thyroid hormone supplementation. Therefore, in most endocrine surgical centres, subtotal thyroidectomy for hyperthyroidism is now mostly historical.63,64 In the case of TA, a unilateral lobectomy (or hemithyroidectomy) is usually all that is required.
Postoperative considerations: Patients with severe GD may develop ‘bone hunger’ postoperatively, requiring large amounts of calcium supplementation. However, oral calcium and calcitriol supplementation is usually adequate. Routine measurements of serum corrected calcium and intact PTH (iPTH) should guide calcium supplementation for all patients after TTx. Serum magnesium level should be measured and supplemented as required if intravenous calcium is required to maintain adequate serum calcium levels in the postoperative period.
Thyroiditis
While generally treated effectively by medical management, a working knowledge of thyroiditis is useful to assist in clinical diagnostics, decision-making and to avoid surgery where necessary. Causes of thyroiditis are listed in Table 2.11.
Table 2.11
| Underlying pathology | Type of thyroiditis |
| Viral infection | De Quervain’s |
| Autoimmune | Hashimoto’s |
| Fibrosis | Riedel’s |
| Abscess-forming infections (bacterial or fungal) |
Acute suppurative |
| Pregnancy | Postpartum |
Subacute thyroiditis (de Quervain’s thyroiditis)
Subacute or de Quervain’s thyroiditis is a self-limiting, painful, inflammatory thyroiditis that is thought to be secondary to viral infection.65 Following a viral prodrome, individuals with a genetic predisposition may progress to granulomatous inflammation of the thyroid gland. The gland becomes infiltrated with mononuclear cells, lymphocytes, neutrophils and later multinucleated giant cells, consistent with granulomatous inflammation. The natural history of disease involves four phases: an acute, painful hyperthyroid phase, followed by a euthyroid phase, then a mild hypothyroid phase and finally recovery to the euthyroid state.
Surgery of the thyroid
Our own experience has led to a standardised capsular dissection technique that can be repeated by trainee, non-specialist and specialist endocrine surgeons alike.66 Vocal cord assessment by an experienced laryngologist documents their baseline function preoperatively. Preoperative laryngoscopy to assess and document the baseline function of the vocal cords is strongly recommended. This serves as a point of reference should there be any doubt about their function postoperatively.
Unilateral total thyroid lobectomy (hemithyroidectomy)
Mobilisation
Attention is then turned to the superior pole. Any adherent strap muscles are dissected off the anterolateral surfaces of the superior pole. Then, with inferolateral traction of the superior pole, the avascular cricothyroid space (of Reeve) medial to the superior pole is opened and the EBSLN identified. Always be aware of the possibility of a Cernea type 2 nerve (Fig. 2.1). Once the nerve is identified and protected, the superior thyroid vessels are defined, ligated and divided close to the gland capsule. With the release of fibrous attachments posteriorly, the superior pole is thus mobilised.
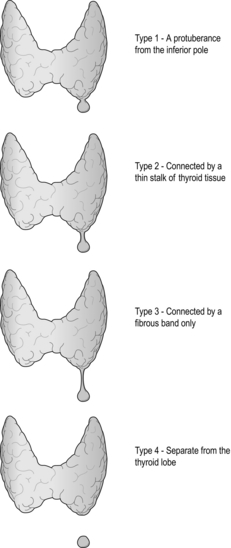
The inferior pole is next mobilised by freeing it from the overlying strap muscles, the inferior thyroid veins and the thyrothymic tract. When ligating and dividing the inferior vessels, care must be taken to avoid inadvertent division of a ‘high riding’ or ‘anterior’ RLN. The inferior parathyroid gland can often be found within the superior part of the thymic horn, which is in continuation with the fibrous thyrothymic tract, and is ideally left in situ to preserve its vascularity. Care must also be taken to ensure that no thyrothymic rest of thyroid tissue is left behind, which is another common cause of goitre recurrence (Fig. 2.11).
RLN and parathyroid glands
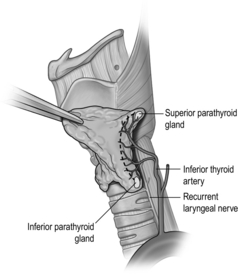
The pretracheal fascia enveloping the thyroid lobe is incised along the posterior surface, in the craniocaudal direction, anterior to the parathyroid glands (Fig. 2.12). The dissection continues on a broad front towards the tracheo-oesophageal groove. In doing so, if the parathyroid glands are on the surface of the thyroid gland, they and their blood supply are safely dissected off. The TZ is then rotated anteromedially to reveal the underlying RLN. However, it must be noted that, on rare occasions, the RLN may be lateral to the tubercle. It is important to trace the RLN inferiorly for a couple of centimetres to ensure that there is not a more anterior motor branch. With the anteromedial rotation of the tubercle, the nerve is often lifted out of the tracheo-oesophageal groove into an artificial ‘genu’, being held up by an overlying fascia containing the tertiary branches of the inferior thyroid artery. Once this overlying fascial layer is divided, the RLN is fully exposed and can be gently pushed away from the ligament of Berry. With the RLN clearly displayed, remaining branches of the inferior thyroid artery and other attachments of the gland to the trachea can be divided. Finally, the division of the ligament of Berry completes the lobectomy.
Total thyroidectomy
Total thyroidectomy follows the same steps as above, repeating for the contralateral lobe.
Neuromonitoring
Permanent RLN injury is the most feared complication of thyroid surgery. Despite advances in surgical technique, a rate of 1–2% is still reported, with higher rates for re-operative surgery.67,68 It is the commonest reason for medicolegal claims in relation to thyroid surgery. The symptoms range from minor voice change in well-compensated cases, to severe functional effects requiring tracheostomy or cordectomy in cases of bilateral palsy.69 Social, psychological and occupational sequelae are also significant.
Intraoperative neuromonitoring (IONM) is a technique that has been available over the last few decades, and has recently been gaining popularity. It aims to prevent RLN injury during thyroidectomy. However, there are no convincing data to suggest improved outcome with the introduction of IONM into routine thyroidectomy.70 A randomised controlled trial comparing visualisation versus neuromonitoring of RLN during thyroidectomy showed that IONM decreases the rate of transient but not permanent RLN palsies.71 However, for a study to be adequately powered to demonstrate a significant improvement in permanent RLN palsy rate, over 2500 patients are required in each arm. A meta-analysis also did not show any advantage of IONM in either primary or revision thyroid surgery.72
Sutureless thyroidectomy
Sutureless thyroidectomy refers to the replacement of ligatures with vessel-sealing devices. This change signifies the biggest advancement in thyroidectomy technique since the times of Kocher, Dunhill and Mayo.73 Some units, such as the Sydney University Endocrine Surgical Unit, have adopted this technique since 2006, and have performed thousands of thyroidectomies with no difference in complication rates when compared to the traditional technique, but shorter operating time. Most endocrine surgery units around the world have at least adopted these vessel-sealing devices and perform part of the operation with them. A meta-analysis published in 2009 confirmed the safety of one such device.74
Minimally invasive and robotic surgery
The term minimally invasive thyroid surgery encompasses a host of different techniques, which can be regarded as small incision open surgery, endoscopic surgery and robotic surgery. Various endoscopic approaches have been described, either via a lateral or central neck incision. Evidence suggests that, in the hands of experienced endoscopic endocrine surgeons, their safety outcome is comparable to open surgery, and there may be some cosmetic advantage. However, widespread adoption of these techniques cannot be recommended based on evidence.75–77 More recently, robotic thyroid surgery via axillary or mammary incisions avoids placement of cervical incisions all together. However, specific indications and safety are still being evaluated.78,79
Complications of thyroidectomy
A thorough knowledge and application of general surgical discipline and increasing experience within the context of thyroidectomy will ensure complications are kept to a minimum. Complications of significance during and after thyroidectomy are listed in Box 2.6.
Hypoparathyroidism
Parathyroid injury may lead to transient or permanent hypoparathyroidism, and occurs in up to 10% and 3% of total thyroidectomy cases, respectively.80 Associated acute postoperative hypocalcaemia can be a dangerous phenomenon. Serum PTH, corrected calcium and clinical symptoms should be monitored closely in the postoperative period. Permanent hypoparathyroidism is not a trivial complication and patients can be significantly compromised despite supplemental calcium and vitamin D analogues. Identification and preservation of parathyroid tissue and autografting where appropriate will aid in avoidance of this complication.
Miscellaneous
Tracheomalacia was previously a feared postoperative complication of thyroidectomy for goitre with tracheal compression. Recent series have failed to justify such concern in the modern era of thyroid surgery. However, it is a well-known phenomenon in third world countries with large neglected goitres.81
Tracheal perforation may occur during thyroidectomy and most commonly during re-operative surgery where tissue planes have become obliterated. The anaesthetist should be informed immediately to ensure that the endotracheal tube cuff has not been damaged and the airway is secure. Inadvertent perforation may be managed with primary suture or muscle flap repair.82
References
1. Serpell, J.W., Yeung, M.J., Grodski, S., The motor fibers of the recurrent laryngeal nerve are located in the anterior extralaryngeal branch. Ann Surg. 2009;249(4):648–652. 19300223
2. Wang, C., The anatomic basis of parathyroid surgery. Ann Surg. 1976;183(3):271–275. 1259483
3. de los Santos, E.T., Starich, G.H., Mazzaferri, E.L., Sensitivity, specificity, and cost-effectiveness of the sensitive thyrotropin assay in the diagnosis of thyroid disease in ambulatory patients. Arch Intern Med. 1989;149(3):526–532. 2493228
4. Saravanan, P., Dayan, C.M., Thyroid autoantibodies. Endocrinol Metab Clin North Am. 2001;30(2):315–337. 11444165
5. Matthews, D., Syed, A., The role of TSH receptor antibodies in the management of Graves’ disease. Eur J Intern Med. 2011;22(3):213–216. 21570635
6. Machens, A., Dralle, H., Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95(6):2655–2663. 20339026 This paper elegantly correlated the disease burden to the pre-treatment level of serum calcitonin in patients with a new diagnosis of MTC.
7. Pacini, F., European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787–803. 16728537
8. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper, D.S., Doherty, G.M., Haugen, B.R., et al, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. 19860577 Revised in 2009, these guidelines form an important reference for clinicians managing patients with thyroid nodules and differentiated thyroid cancers.
9. Abraham, D., Delbridge, L., Clifton-Bligh, R., et al, Medullary thyroid carcinoma presenting with an initial CEA elevation. Aust N Z J Surg. 2010;80(11):831–833. 20969693
10. Milas, M., Stephen, A., Berber, E., et al, Ultrasonography for the endocrine surgeon: a valuable clinical tool that enhances diagnostic and therapeutic outcomes. Surgery. 2005;138(6):1193–1201. 16360408
11. Cibas, E.S., Ali, S.Z., The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132(5):658–665. 19846805 The conclusions of the National Cancer Institute Thyroid FNA State of the Science Conference held in Bethesda, 2007, led to the Bethesda System for Reporting Thyroid Cytopathology. The system aims to clarify thyroid cytopathology terminology to facilitate communication among different specialties involved in managing patients with thyroid diseases. It also allows for reliable sharing of data from different laboratories for national and international collaborative studies.
12. Pitman, M.B., Abele, J., Ali, S.Z., et al, Techniques for thyroid FNA: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36(6):407–424. 18478608
13. Brix, T.H., Kyvik, K.O., Hegedus, L., Major role of genes in the etiology of simple goiter in females: a population-based twin study. J Clin Endocrinol Metab. 1999;84(9):3071–3075. 10487667
14. Knudsen, N., Bülow, I., Jorgensen, T., et al, Goitre prevalence and thyroid abnormalities at ultrasonography: a comparative epidemiological study in two regions with slightly different iodine status. Clin Endocrinol (Oxf). 2000;53(4):479–485. 11012573
15. Krohn, K., Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev. 2004;26(4):504–524. 15615818
16. Ramelli, F., Studer, H., Bruggisser, D., Pathogenesis of thyroid nodules in multinodulargoiter. Am J Pathol. 1982;109(11):215–223. 7137321
17. Studer, H., Peter, H.J., Gerber, H., Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goiter growth. Endocr Rev. 1989;10(2):125–135. 2666115
18. Agarwal, G., Aggarwal, V., Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J Surg. 2008;32(7):1313–1324. 18449595 A systematic review that reaffirmed that total thyroidectomy is the procedure of choice for surgical management of benign MNG. Grade B recommendation was made to avoid subtotal thyroidectomy due to significant recurrence rates, inadequately treating incidental thyroid cancers, and providing minimal safety advantage over total thyroidectomy. Grade C recommendation was made regarding total thyroidectomy being a safe and effective procedure for benign MNG in expert hands.
19. Ríos, A., Rodríguez, J.M., Canteras, M., et al, Surgical management of multinodular goiter with compression symptoms. Arch Surg. 2005;140(1):49–53. 15655205
20. Koo, J.H., Shin, J.H., Han, B.-K., et al, Cystic thyroid nodules after aspiration mimicking malignancy: sonographic characteristics. J Ultrasound Med. 2010;29(10):1415–1421. 20876894
21. NSW Cancer Institute. Cancer in NSW: incidence and mortality report 2008. NSW Cancer Institute; 2010. [p. 1–176].
22. Enewold, L., Zhu, K., Ron, E., et al, Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–791. 19240234
23. Liu, S., Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age–period–cohort effects. Br J Cancer. 2001;85(9):1335–1339. 11720471
24. Leenhardt, L., Grosclaude, P., Chérié-Challine, L., Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14(12):1056–1060. 15650358
25. Grodski, S., Brown, T., Sidhu, S., et al, Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144(6):1038–1043. 19041015
26. Greco, A., Borrello, M.G., Miranda, C., Molecular pathology of differentiated thyroid cancer. Q J Nucl Med Mol Imaging 2009; 53:440–454. 19910897
27. Nikiforova, M.N., Gandi, M., Kelly, L., et al, MicroRNA dysregulation in human thyroid cells following exposure to ionizing radiation. Thyroid. 2011;21(3):261–266. 21323591
28. Suliburk, J., Delbridge, L., Surgical management of well-differentiated thyroid cancer: state of the art. Surg Clin North Am. 2009;89(5):1171–1191. 19836491
29. Kebebew, E., Hereditary non-medullary thyroid cancer. World J Surg. 2007;32(5):678–682. 18058169
30. DeLellis, R.A. Pathology and genetics of tumours of endocrine organs. World Health Organisation; 2004.
31. Hedinger, C., Williams, E.D., The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer 1989; 63:908–911. 2914297
32. Noguchi, S., Yamashita, H., Uchino, S., et al, Papillary microcarcinoma. World J Surg. 2008;32(5):747–753. 18264828
33. Grodski, S., Delbridge, L., An update on papillary microcarcinoma. Curr Opin Oncol. 2009;21(1):1–4. 19125011
34. Hay, I.D., Hutchinson, M.E., Gonzalez-Losada, T., et al, Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144(6):980–988. 19041007 This large series from the Mayo Clinic highlighted the low-risk nature of papillary thyroid microcarcinoma, and did not recommend the use of RAI ablation after surgery for these patients.
35. Lang, B.H.-H., Lo, C.-Y., Chan, W.-F., et al, Staging systems for papillary thyroid carcinoma. Ann Surg. 2007;245(3):366–378. 17435543
36. Hegedus, L., Bonnema, S.J., Bennedbaek, F.N., Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24(1):102–132. 12588812
37. Carty, S.E., Cooper, D.S., Doherty, G.M., et al, Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19(11):1153–1158. 19860578 This statement published by the ATA Surgery Working Group discusses the pattern of central lymph node involvement in thyroid cancer, reviews the surgical anatomy and defines a consistent terminology to CLND.
38. Tufano, R.P., Kandil, E., Considerations for personalized surgery in patients with papillary thyroid cancer. Thyroid. 2010;20(7):771–776. 20604686
39. Robbins, K., Shaha, A., Medina, J., Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134(5):536–538. 18490577
40. Grebe, S.K., Hay, I.D., Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):43–63. 8789493
41. Leboulleux, S., Rubino, C., Baudin, E., Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90(10):5723–5729. 16030160 This study highlighted the excellent survival rate of PTC patients even with lymph node metastases and/or minimal extrathyroidal extension. This in turn stresses the importance of finding the balance between achieving disease-free survival and treatment morbidities.
42. Lundgren, C.I., Hall, P., Dickman, P.W., et al, Clinically significant prognostic factors for differentiated thyroid carcinoma. Cancer. 2006;106(3):524–531. 16369995
43. Podnos, Y.D., Smith, D., Wagman, L.D., et al, The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71(9):731–734. 16468507
44. Grodski, S., Cornford, L., Sywak, M., et al, Routine level VI lymph node dissection for papillary thyroid cancer: surgical technique. Aust N Z J Surg. 2007;77(4):203–208. 17388820 A great description of central lymph node dissection technique.
45. Lee, Y.S., Kim, S.W., Kim, S.W., et al, Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg. 2007;31(10):1954–1959. 17687598
46. Alvarado, R., Sywak, M.S., Delbridge, L., et al, Central lymph node dissection as a secondary procedure for papillary thyroid cancer: is there added morbidity? Surgery. 2009;145(5):514–518. 19375610
47. Volante, M., Collini, P., Nikiforov, Y.E., et al, Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31(8):1256–1264. 17667551
48. Volante, M., Landolfi, S., Chiusa, L., et al, Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004;100(5):950–957. 14983490
49. Williams, E.D., Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19(2):114–118. 5948665
50. Pelizzo, M.R., Boschin, I.M., Bernante, P., et al, Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol. 2007;33(4):493–497. 17125960
51. Cerrato, A., De Falco, V., Santoro, M., Molecular genetics of medullary thyroid carcinoma: the quest for novel therapeutic targets. J Mol Endocrinol. 2009;43(4):143–155. 19383830
52. Kebebew, E., Ituarte, P.H., Siperstein, A.E., et al, Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88(5):1139–1148. 10699905
53. Moley, J.F., DeBenedetti, M.K., Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229(6):880–888. 10363903
54. American Thyroid Association Guidelines Task ForceKloos, R.T., Eng, C., Evans, D.B., et al, Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. 19469690 Up-to-date and evidence-based recommendations in the management of MTC, published by the ATA.
55. Gild, M.L., Bullock, M., Robinson, B.G., et al, Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat Rev Endocrinol. 2011;7(10):617–624. 21862995
56. Siironen, P., Hagström, J., Mäenpää, H.O., et al, Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategies and treatment outcome of 52 consecutive patients. Oncology. 2010;79(5–6):400–408. 21455012
57. Sakorafas, G.H., Historical evolution of thyroid surgery: from the ancient times to the dawn of the 21st century. World J Surg. 2010;34(8):1793–1804. 20401481
58. Meyer-Rochow, G.Y., Sywak, M.S., Reeve, T.S., et al, Surgical trends in the management of thyroid lymphoma. Eur J Surg Oncol. 2008;34(5):576–580. 17604588
59. Syed, M.I., Stewart, M., Syed, S., et al, Squamous cell carcinoma of the thyroid gland: primary or secondary disease? J Laryngol Otol. 2011;125(1):3–9. 20950510
60. Bahn Chair, R.S., Burch, H.B., Cooper, D.S., et al, Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21(6):593–646. 21510801 Another useful set of guidelines published by the ATA, in the management of hyperthyroidism.
61. Kahaly, G.J., Bartalena, L., Hegedüs, L., The American Thyroid Association/American Association of Clinical Endocrinologists guidelines for hyperthyroidism and other causes of thyrotoxicosis: a European perspective. Thyroid. 2011;21(6):585–591. 21663420
62. Snook, K.L., Stalberg, P.L.H., Sidhu, S.B., et al, Recurrence after total thyroidectomy for benign multinodular goiter. World J Surg. 2007;31(3):593–598. 17308855
63. Barakate, M., Agarwal, G., Reeve, T., Total thyroidectomy is now the preferred option for the surgical management of Graves’ disease. Aust N Z J Surg 2002; 72:321–324. 12028087
64. Lal, G., Ituarte, P., Kebebew, E., et al, Should total thyroidectomy become the preferred procedure for surgical management of Graves’ disease? Thyroid. 2005;15(6):569–574. 16029123
65. Engkakul, P., Mahachoklertwattana, P., Poomthavorn, P., Eponym. Eur J Pediatr. 2010;170(4):427–431. 20886353
66. Gauger, P., Delbridge, L., Surgeon’s approach to the thyroid gland: surgical anatomy and the importance of technique. World J Surg. 2000;24(8):891–897. 10865032
67. Veyseller, B., Aksoy, F., Yildirim, Y.S., et al, Effect of recurrent laryngeal nerve identification technique in thyroidectomy on recurrent laryngeal nerve paralysis and hypoparathyroidism. Arch Otolaryngol Head Neck Surg. 2011;137(9):897–900. 21844405
68. Chiang, F.-Y., Wang, L.-F., Huang, Y.-F., et al, Recurrent laryngeal nerve palsy after thyroidectomy with routine identification of the recurrent laryngeal nerve. Surgery. 2005;137(3):342–347. 15746790
69. Dispenza, F., Dispenza, C., Marchese, D., et al, Treatment of bilateral vocal cord paralysis following permanent recurrent laryngeal nerve injury. Am J Otolaryngol. 2012;33(3):285–288. 21924522
70. Chan, W., Lang, B., Lo, C., The role of intraoperative neuromonitoring of recurrent laryngeal nerve during thyroidectomy: a comparative study on 1000 nerves at risk. Surgery. 2006;140(6):866–873. 17188132 Studies such as this add to the current debate on the utility of intraoperative neuromonitoring as a tool for reducing injury to the RLN.
71. Barczynski, M., Konturek, A., Cichon, S., Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg. 2009;96(3):240–246. 19177420
72. Higgins, T.S., Gupta, R., Ketcham, A.S., et al, Recurrent laryngeal nerve monitoring versus identification alone on post-thyroidectomy true vocal fold palsy: a meta-analysis. Laryngoscope. 2011;121(5):1009–1017. 21520117
73. Delbridge, L.W., Sutureless thyroidectomy – technological advance or toy? Arch Surg. 2009;144(12):1174–1175. 20030037
74. Yao, H.S., Wang, Q., Wang, W.J., et al, Prospective clinical trials of thyroidectomy with LigaSurevs conventional vessel ligation: a systematic review and meta-analysis. Arch Surg. 2009;144(12):1167–1174. 20026837
75. Alvarado, R., McMullen, T., Sidhu, S.B., et al, Minimally invasive thyroid surgery for single nodules: an evidence-based review of the lateral mini-incision technique. World J Surg. 2008;32(7):1341–1348. 18373119
76. Lombardi, C.P., Raffaelli, M., De Crea, C., et al, Video-assisted thyroidectomy: lessons learned after more than one decade. Acta Otorhinolaryngol Ital. 2009;29(6):317–320. 20463836
77. Slotema, E.T., Sebag, F., Henry, J.F., What is the evidence for endoscopic thyroidectomy in the management of benign thyroid disease? World J Surg. 2008;32(7):1325–1332. 18327525
78. Lee, J., Kang, S.W., Jung, J.J., et al, Multicenter study of robotic thyroidectomy: short-term postoperative outcomes and surgeon ergonomic considerations. Ann Surg Oncol. 2011;18(9):2538–2547. 21373954
79. Luginbuhl, A., Schwartz, D.M., Sestokas, A.K., et al, Detection of evolving injury to the brachial plexus during transaxillary robotic thyroidectomy. Laryngoscope. 2012;122(1):110–115. 22095913
80. Khan, M.I., Waguespack, S.G., Hu, M.I., Medical management of postsurgical hypoparathyroidism. Endocr Pract. 2011;17(Suppl. 1):18–25. 21134871
81. Agarwal, A., Mishra, A.K., Gupta, S.K., et al, High incidence of tracheomalacia in longstanding goiters: experience from an endemic goiter region. World J Surg. 2007;31(4):832–837.
82. Gosnell, J.E., Campbell, P., Sidhu, S., et al, Inadvertent tracheal perforation during thyroidectomy. Br J Surg. 2006;93(1):55–56. 16278924