Chapter 16
The Telencephalon
Vasculature of the Cerebral Cortex
White Matter of the Cerebral Hemisphere
Commissural Fibers: The Corpus Callosum
Projection Fibers: The Internal Capsule
Vasculature of the Internal Capsule
Nucleus Accumbens and Substantia Innominata
Subthalamic Nucleus and Substantia Nigra
Major Connections of the Basal Nuclei
The telencephalon is the largest part of the human brain, constituting about 85% of total brain weight, and is that portion in which all modalities are represented. Various sensory inputs (such as vision and hearing) are localized in some areas, whereas motor functions are represented in other regions and are modulated by subcortical nuclei. The telencephalon contains circuits that interrelate regions that have specific functions, such as motor or visual, with other regions called association areas. Seeing a familiar image may precipitate a cascade of neural events having olfactory, emotional, sensory, and motor components. Damage to association areas results in complex neurologic deficits. The patient may not be blind or paralyzed but may be unable to recognize sensory input (agnosia), express ideas or thoughts (aphasia), or perform complex goal-directed movements (apraxia).
OVERVIEW
The telencephalon consists of large hemispheres separated from each other by a deep longitudinal cerebral fissure. Each hemisphere has an outer surface, the cerebral cortex, which is composed of layers of cells. The cortex is thrown into elevations called gyri (singular, gyrus) that are separated by grooves called sulci (singular, sulcus). Internal to the cortex are large amounts of subcortical white matter along with aggregates of gray matter that form the basal nuclei and the amygdala. Although not parts of either the telencephalon or the basal nuclei, the subthalamic nucleus (of the diencephalon) and the substantia nigra (of the mesencephalon) have important connections that functionally link them with the basal nuclei.
Information passing into or out of the cerebral cortex must traverse the subcortical white matter. The myelinated fibers forming the white matter are organized into (1) association bundles that connect adjacent or distant gyri in one hemisphere; (2) commissural bundles that connect the hemispheres, the largest of these being the corpus callosum; and (3) the internal capsule. The internal capsule contains axons projecting to numerous downstream nuclei (corticofugal fibers) and axons conveying information to the cerebral cortex (corticopetal fibers). The terms corticofugal and corticopetal are umbrella terms that include all efferent and all afferent fibers, respectively, of the cerebral cortex. Specific cortical efferent fibers (such as corticospinal or corticostriatal fibers) or afferent fibers (such as thalamocortical fibers) are discussed in other chapters.
The hippocampal complex and the amygdala are located in the walls of the temporal horn of the lateral ventricle. The axons of cells in these structures coalesce to form the fornix, stria terminalis, and amygdalofugal pathway.
DEVELOPMENT
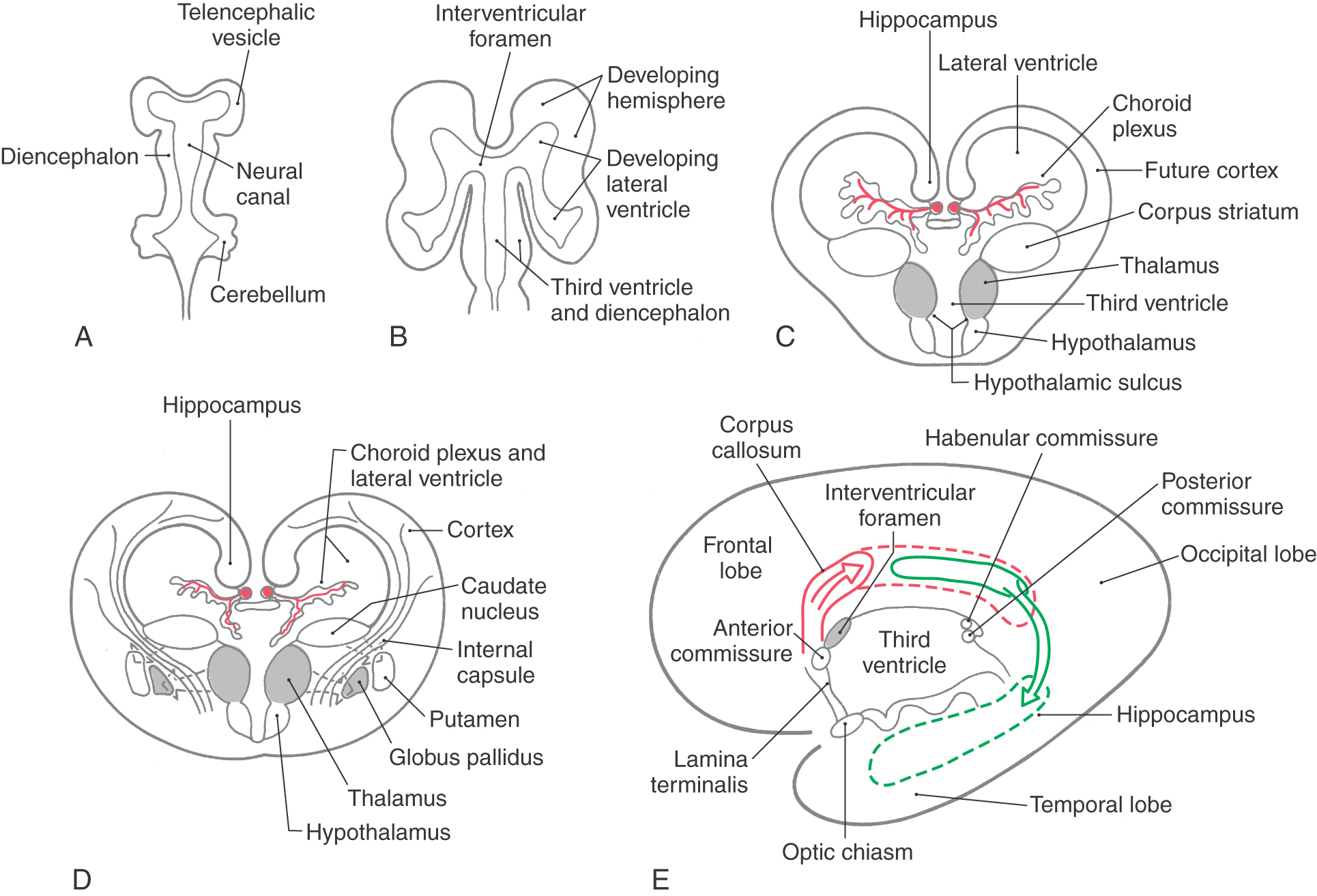
Enlargements of the prosencephalon, the telencephalic (cerebral) vesicles, appear at about 5 weeks of gestation. As the cerebral vesicles enlarge in all directions, they pull along portions of the neural canal that will form the cavities of the telencephalon, the lateral ventricles (Fig. 16-1A, B). The primitive lateral ventricles extend into frontal, parietal, temporal, and occipital areas as they develop and form that portion of the ventricle found in each of these lobes in the adult. The interventricular foramina, which connect each lateral ventricle to the midline third ventricle (cavity of the diencephalon), are initially large but become smaller as development progresses. In the adult brain, each interventricular foramen is bordered rostromedially by the column of the fornix and caudolaterally by the anterior tubercle of the dorsal thalamus (see Fig. 15-12).
Cells forming the corpus striatum appear in the floor of the developing lateral ventricle at the time when primordial cell groups in the wall of the third ventricle are giving rise to diencephalic structures (Fig. 16-1C, D). As development progresses, the corpus striatum is bisected by axons growing to and from the cerebral cortex. These axons form the internal capsule of the adult and divide the corpus striatum into a medially located caudate nucleus and a laterally located putamen. As the diencephalon enlarges, it gives rise to the thalamus and hypothalamus and to cells that migrate across the developing internal capsule to assume a position medial to the putamen (Fig. 16-1D). These cells become the globus pallidus of the adult and, in combination with the putamen, form the lenticular nucleus.
The initial development of the major commissural bundles and of the hippocampus takes place along the medial aspect of the hemisphere (Fig. 16-1C-E). In the adult brain, there are three major interhemispheric commissures: the anterior commissure, the hippocampal commissure, and the corpus callosum. The first of these to appear, the anterior commissure, arises within the lamina terminalis, a membrane-like structure that extends from the anterior commissure anteriorly (ventrally) to the rostral edge of the optic chiasm (see Fig. 15-4). The second to form, the hippocampal commissure, develops along with the hippocampal primordium. As growth occurs, the hippocampus, which originates in the posteromedial part of the hemisphere, is displaced into the temporal lobe, where it assumes a position characteristic of the adult (Fig. 16-1E). In the process, fibers from one side cross to the other side, as the hippocampal commissure, just inferior to the area that will be occupied by the corpus callosum. The third commissure to develop, the corpus callosum, originates from the area of the lamina terminalis as a structure initially composed of astrocytic processes. Axons from developing neurons in each hemisphere traverse this glial structure to access the contralateral side. As this takes place, the corpus callosum enlarges in a caudal direction to form the prominent structure found in the adult (Figs. 16-1E and 16-2A).
Developmental Defects
There are numerous developmental events that may cause defects in the configuration of the telencephalon; many of these are described in Chapter 5 and will be only briefly mentioned here. One of the developmental failures that will result in aberrant development of the telencephalon is the improper migration of maturing neurons on radial glia. This failure results in structural and in some cases corresponding functional defects in the arrangement of the cerebral cortex. Some examples include lissencephaly (a lack of gyri and sulci, a smooth brain), pachygyria (abnormally large gyri that are few in number), and microgyria (abnormally small gyri that are greater in number).
Holoprosencephaly is a preneurulation defect that is represented by three general forms. Alobar holoprosencephaly, the most severe form, consists of a midline ventricle, no hemispheres or corpus callosum, and severe retardation. Semilobar holoprosencephaly consists of a partial formation of lobes with the ventricles formed; the frontal lobes may be fused, and the occipital lobes may be separated by an incomplete longitudinal fissure. Although the ventricles are formed, midline structures such as the septum pellucidum are missing. In lobar holoprosencephaly, the least severe form, the longitudinal fissure is largely complete, hemispheres exist, generally normal patterns of sulci and gyri are seen, but there is a fusion of the hemispheres at the frontal pole or at the orbital surface of the frontal lobe.
Anencephaly is a severe developmental failure in which the telencephalon and the surrounding skull are largely absent. This defect is catastrophic and not compatible with life. Anencephaly is generally associated with a failure of the anterior neuropore to close. The lamina terminalis represents the adult position of the anterior neuropore.
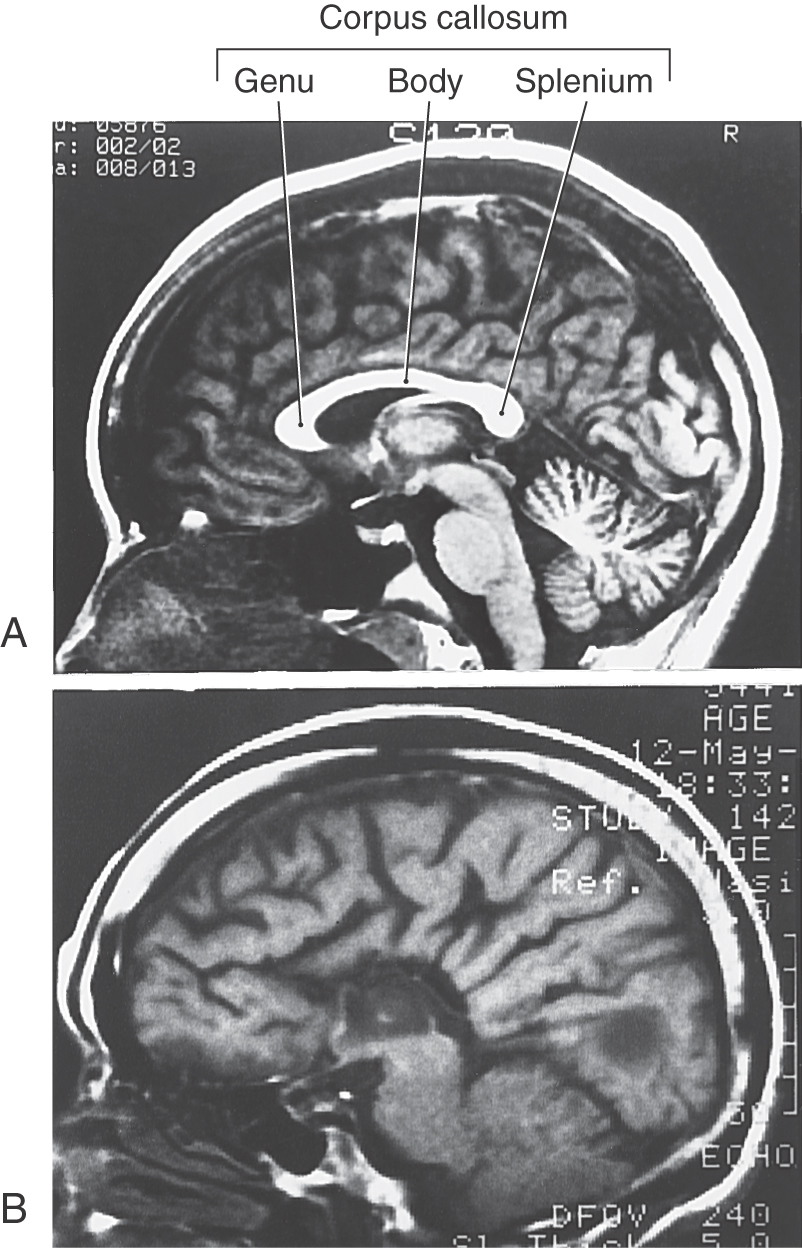
Failure of the corpus callosum to develop (agenesis of the corpus callosum) may be accompanied by an absence of the anterior and hippocampal commissures (Fig. 16-2A, B). Although some patients with this condition may experience focal seizures and have mental retardation, others live for many years with few or no obvious neurologic deficits. These individuals frequently have developmental abnormalities in other parts of the nervous system.
LOBES OF THE CEREBRAL CORTEX
On the basis of the arrangement of major sulci, the cerebral cortex is divided into six lobes, five of which are exposed on the surface of the cerebral hemisphere and one (the insular lobe) is located internal to the lateral sulcus. Four of these lobes are named according to the overlying bones of the skull.
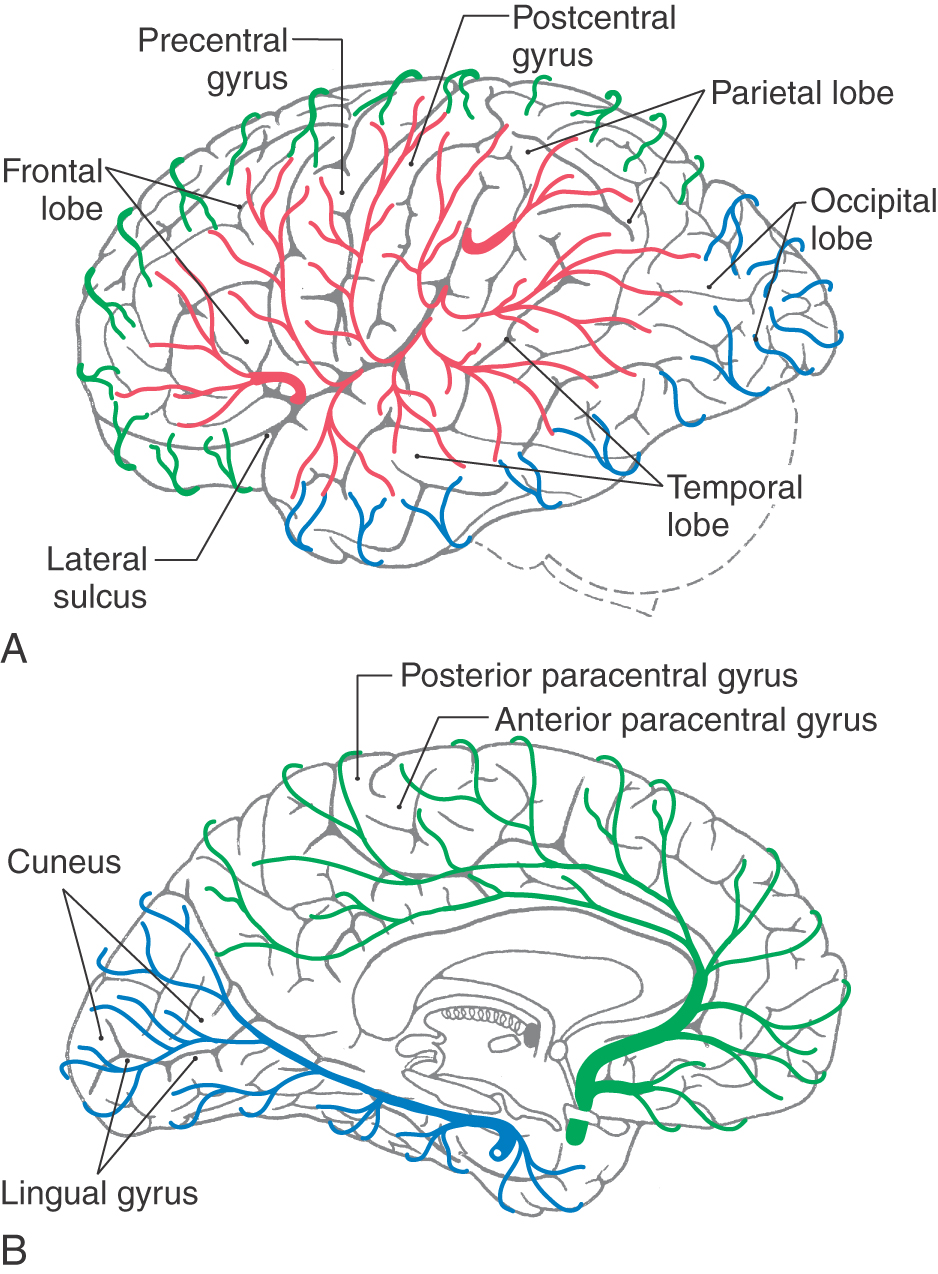
On the lateral surface of the hemisphere, the major sulci are the central sulcus, the lateral (sylvian) sulcus, and a small lateral end of the parietooccipital sulcus (Fig. 16-3; see also Fig. 16-5). The preoccipital notch is a small but distinct indentation along the lateral margin of the hemisphere. An imaginary line connecting the terminus of the parietooccipital sulcus with the preoccipital notch intersects with another line drawn caudally from the lateral sulcus. These lines, along with the central and lateral sulci, divide the lateral surface into frontal, parietal, temporal, and occipital lobes (Fig. 16-3).
Figure 16-3. Lateral aspect of the left cerebral hemisphere showing lobes and their associated gyri and sulci.
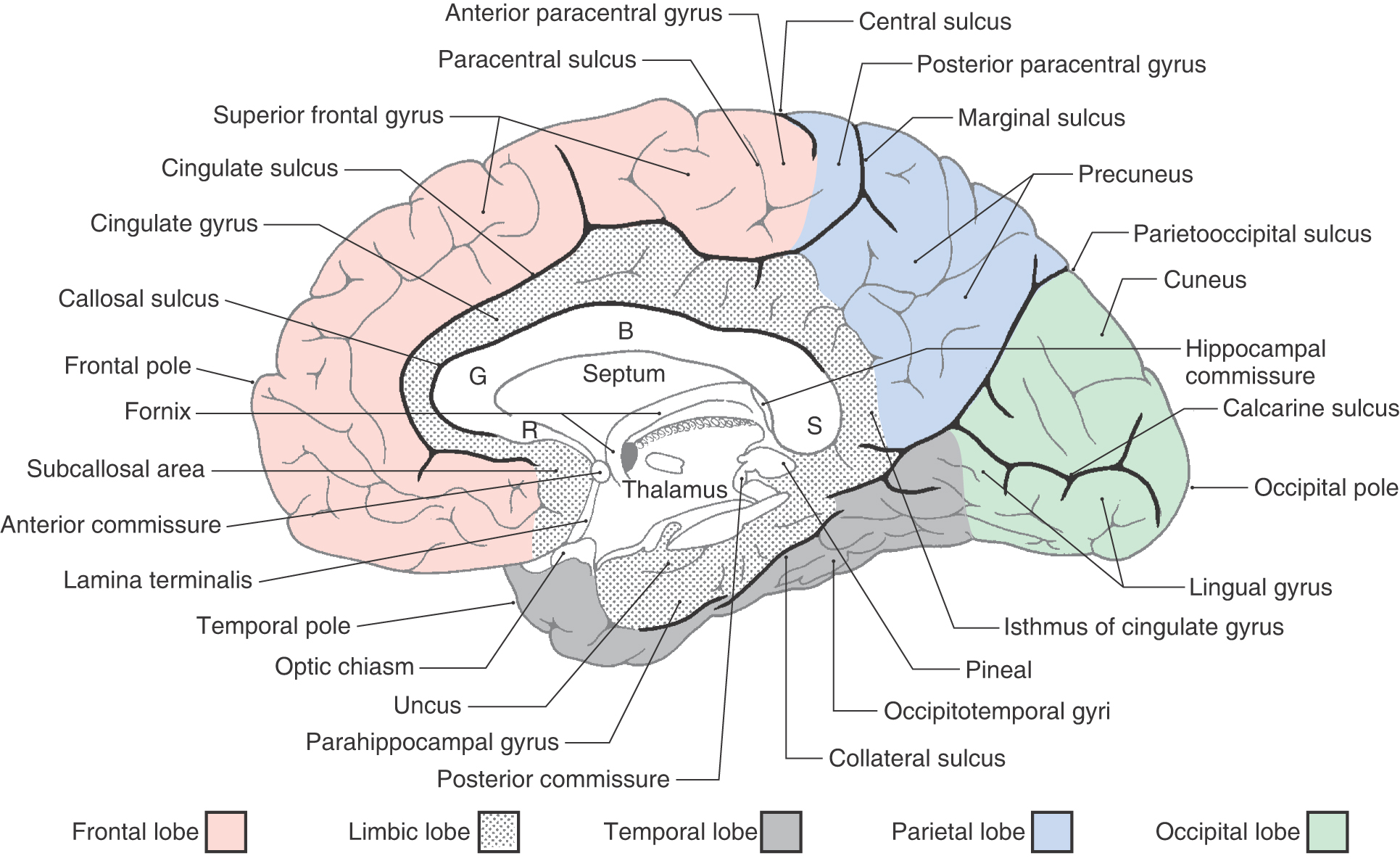
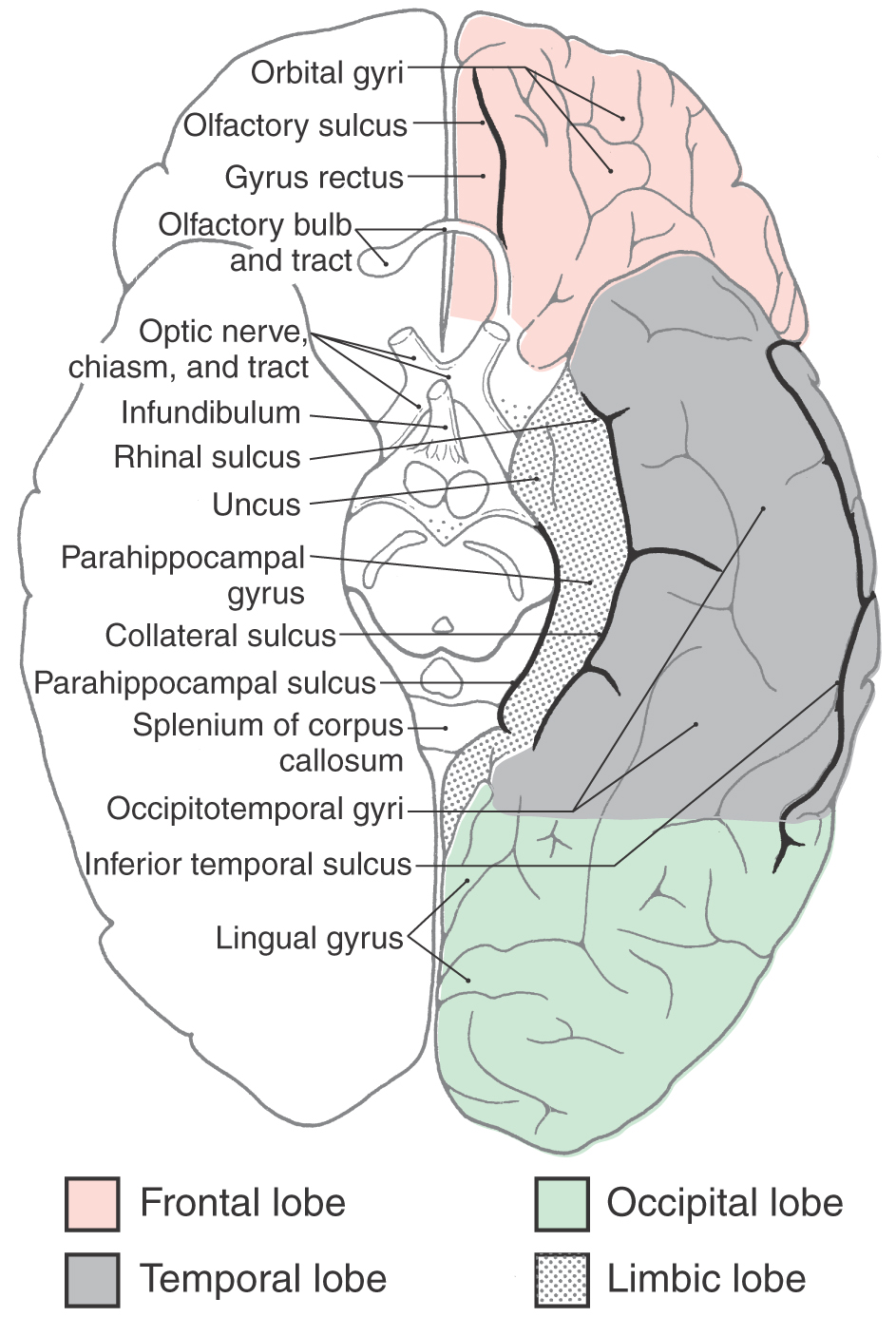
On the medial surface of the hemisphere, the major sulci separating lobes are the cingulate, parietooccipital, and collateral (Figs. 16-4 and 16-5). Two imaginary lines also separate lobes on the medial surface. One connects the medial end of the central sulcus with the cingulate sulcus; the other joins the parietooccipital sulcus with the preoccipital notch. This combination of sulci and lines separates the four lobes noted previously, plus the limbic lobe, on the medial surface of the hemisphere (Fig. 16-4).
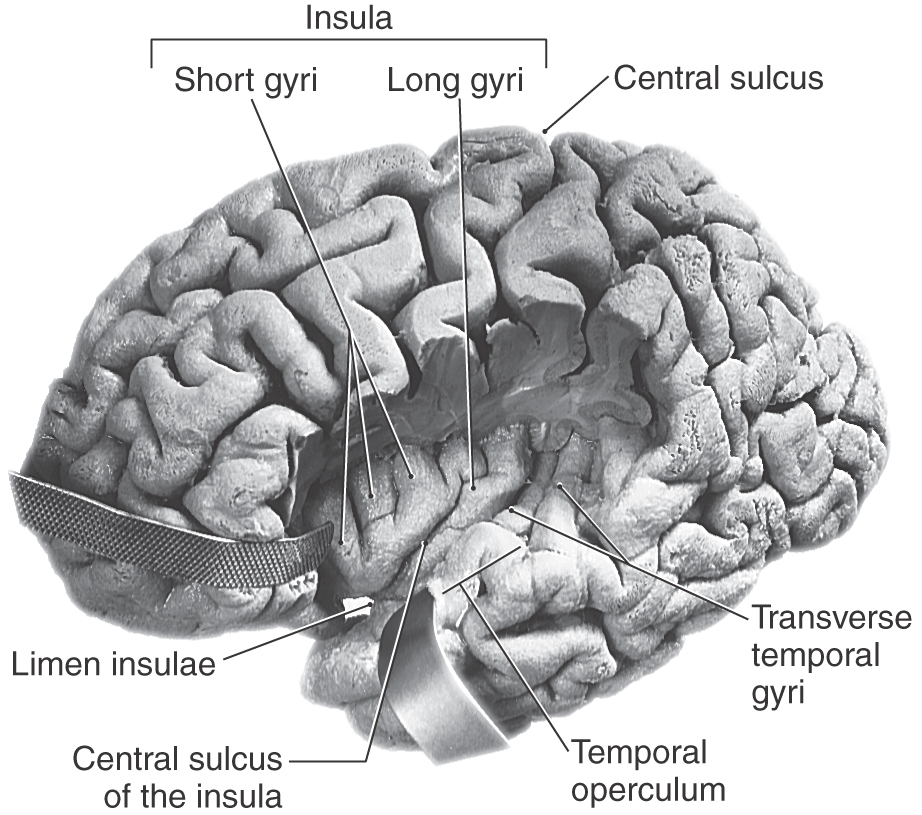
Deep to the lateral (sylvian) sulcus is an infolded region of cortex called the insula (see Fig. 16-10). This region is separated from the opercula of the frontal, parietal, and temporal lobes by the circular sulcus of the insula. Consequently, this region satisfies the definition of a lobe (insular lobe) in that it is separated from the adjoining cortical structures by a named sulcus.
Frontal Lobe
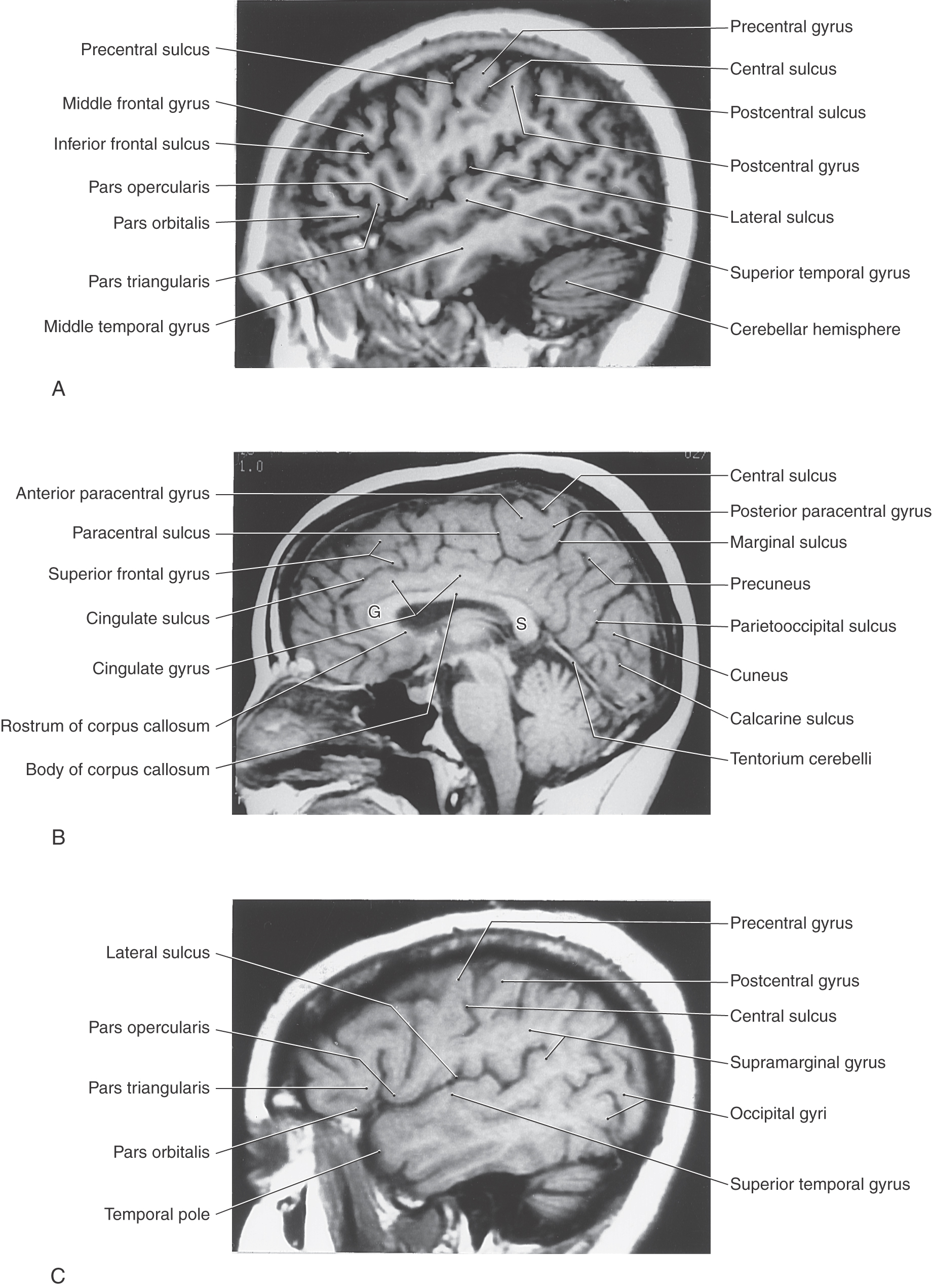
The lateral surface of the frontal lobe is divided by inferior and superior frontal sulci into inferior, middle, and superior frontal gyri (Fig. 16-3), the last folding onto the medial aspect of the hemisphere (Figs. 16-4 and 16-5B). The inferior frontal gyrus is divided into a pars opercularis, pars triangularis, and pars orbitalis (Fig. 16-5A). The anterior (orbital) surface of the frontal lobe is composed of the gyrus rectus, the olfactory sulcus, and a series of orbital gyri (Fig. 16-6). The most rostral point of this lobe is the frontal pole of the brain.
Figure 16-6. Anterior (ventral) aspect of the left cerebral hemisphere showing lobes and their associated gyri and sulci.
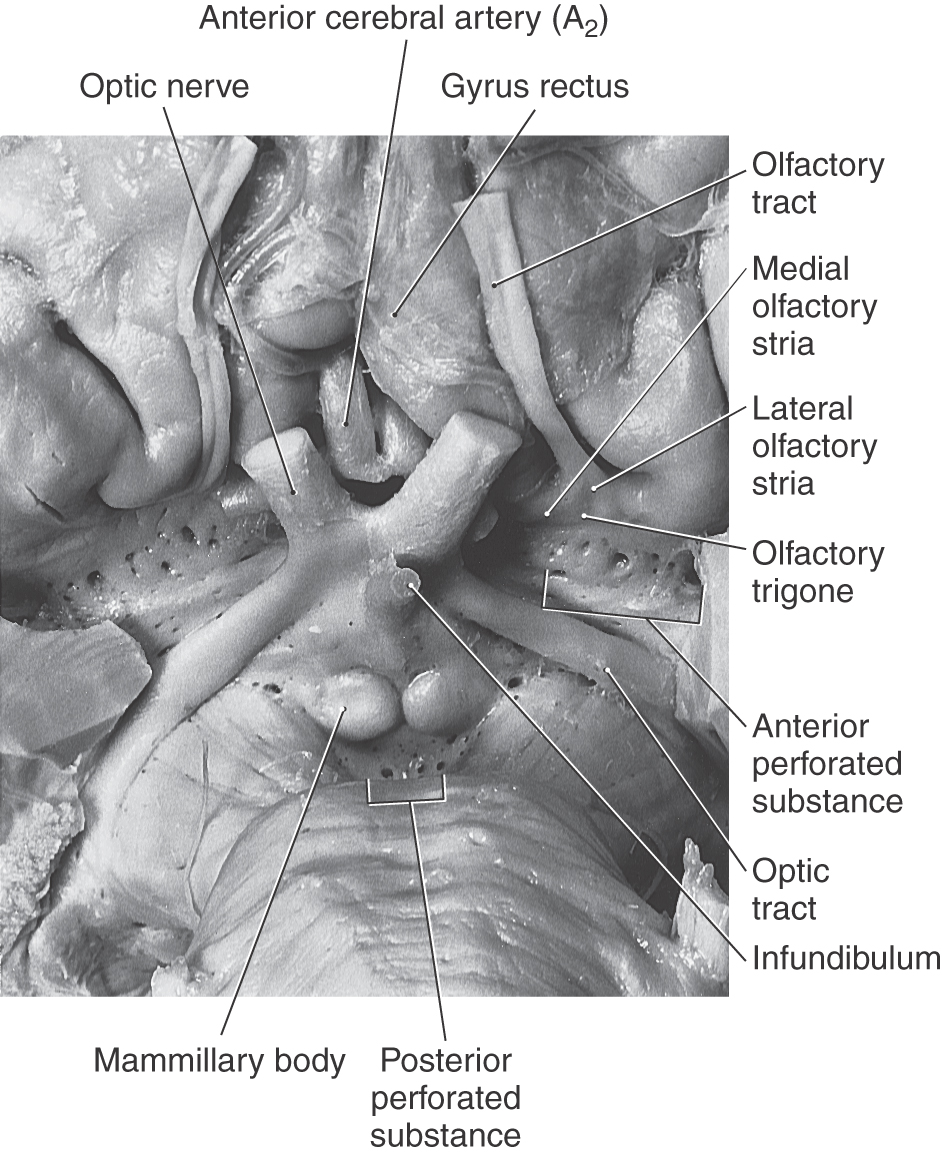
The olfactory bulb and tract, which relay sensory information, lie on the anterior surface of the frontal lobe in the olfactory sulcus (Fig. 16-6). At the point where the olfactory tract attaches to the hemisphere, it bifurcates into medial and lateral striae (Fig. 16-7). The triangle formed by this bifurcation is called the olfactory trigone. Immediately caudal to this trigone, the surface of the hemisphere is characterized by numerous small holes formed by vessels (lenticulostriate arteries) as they enter the brain; this is the anterior perforated substance (Fig. 16-7). The olfactory tract and striae and the cell groups associated with the anterior perforated substance are functionally related to the limbic system.
Figure 16-7. Anterior (ventral) aspect of the hemisphere in the area of the anterior perforated substance.
The precentral gyrus is continuous on the medial surface of the hemisphere with the anterior paracentral gyrus; the latter is separated from the superior frontal gyrus by the paracentral sulcus (Fig. 16-4). These two gyri collectively form the primary somatomotor cortex.
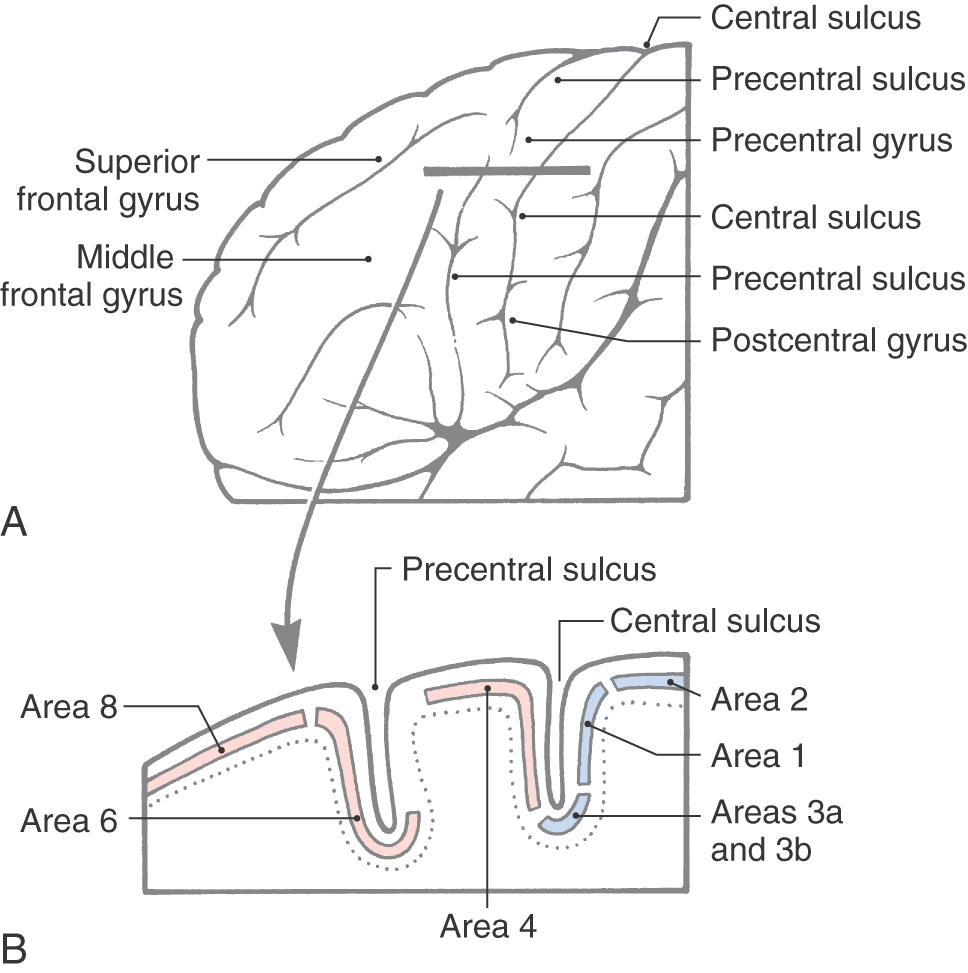
Specific functions are associated with some of the gyri of the frontal lobe, and lesions of these areas result in characteristic deficits. The body is somatotopically represented in the precentral and anterior paracentral gyri; these gyri collectively form the primary somatomotor cortex (Brodmann area 4). Beginning at the lateral sulcus (Fig. 16-8), the face is represented in about the lateral third of the precentral gyrus, the hand and upper extremity in about the middle third (emphasis on the hand area), and the trunk in about the medial third of the precentral gyrus. The hip is represented at about the point where the precentral gyrus turns over the edge of the hemisphere to become the anterior paracentral gyrus, and the lower extremity and foot are represented in the anterior paracentral gyrus (Fig. 16-8). Lesions of these areas of the motor cortex may result in weakness or paralysis of the corresponding part of the body on the contralateral side (see Chapters 24 and 25).
The frontal eye field in humans is located in the depths of the precentral sulcus, in the cortex forming the rostral bank of the precentral sulcus, and extending onto the surface of the middle frontal gyrus (Fig. 16-9). This cortical area is largely coextensive with Brodmann area 6 and extends to the transitional area between areas 6 and 8 in the most caudal portion of the middle frontal gyrus (Fig. 16-9). The current best evidence in humans supports the view that the frontal eye field is located primarily in area 6. This cortical area projects to nuclei in the midbrain and pons that in turn project to the oculomotor, trochlear, and abducens nuclei that control eye movement. Irritative cortical lesions of the frontal eye field result in conjugate deviation of the eyes away from the side of the lesion, whereas destructive lesions result in conjugate deviation of the eyes toward the side of the lesion. An easy way to remember this is “the patient looks away from the irritation but toward the destruction.”
The inferior frontal gyrus in the left (dominant) hemisphere is sometimes called the Broca convolution because lesions in this area (especially in the pars opercularis, Brodmann area 44, and extending into the pars triangularis) result in Broca aphasia. These patients do not have paralysis of the speech apparatus but have great difficulty translating thoughts and concepts into coherent sentences. Broca aphasia is also called expressive aphasia.
Parietal Lobe
The parietal lobe consists of the postcentral gyrus, located between the central and postcentral sulci, and the superior and inferior parietal lobules, which are separated by the intraparietal sulcus (Fig. 16-3). Gyri forming the superior parietal lobule extend onto the medial surface of the hemisphere as the precuneus, whereas the inferior parietal lobule is made up of the angular and supramarginal gyri. The latter is a crescent-shaped ridge of cortex around the caudal terminus of the lateral sulcus.
As the postcentral gyrus extends onto the medial surface of the hemisphere, it is continuous with the posterior paracentral gyrus (Figs. 16-4 and 16-5). This cortical area is bordered rostrally by an imaginary line that connects the central sulcus to the cingulate sulcus and caudally by the marginal ramus of the cingulate sulcus. The latter is frequently called the marginal sulcus. Taken together, the postcentral gyrus and posterior paracentral lobule constitute the primary somatosensory cortex.
Specific functional areas in the parietal lobe include the primary somatosensory cortex (Brodmann areas 3, 1, 2) and the gyri that are part of the Wernicke area (supramarginal gyrus—Brodmann area 40 and the angular gyrus—Brodmann area 39). Clinically, the Wernicke area is believed to extend into the temporal lobe and to encompass portions of Brodmann area 22 and some of area 21. The body is somatotopically organized in the postcentral and posterior paracentral gyri in a pattern generally similar to that seen in the precentral gyrus (Fig. 16-8). Within the postcentral gyrus, the face is represented in the lateral third, the upper extremity (with emphasis on the fingers) in the middle third, and the trunk, hip, and thigh in about the medial third; the leg, foot, and genitalia are represented in the posterior paracentral lobule. Damage to the primary somatosensory cortex results in an alteration of sensory (pain, thermal, and proprioception) perception.
The angular gyrus (Brodmann area 39) and the supramarginal gyrus (Brodmann area 40) collectively form a portion of the Wernicke area; these two gyri also comprise the inferior parietal lobule. Lesions of the Wernicke area result in a constellation of deficits called Wernicke aphasia (or receptive aphasia). These patients cannot understand what they hear, cannot read or write, and speak in a jumble of words that makes no sense. Theirs is a receptive problem; information is received but it cannot be understood or used to express coherent thought.
Temporal Lobe
The gyri that form the temporal lobe are found on lateral and inferior aspects of the hemisphere between the lateral sulcus and the collateral sulcus (Figs. 16-3 to 16-6). These gyri are, beginning at the lateral sulcus, the superior, middle, and inferior temporal gyri and a broad area of cortex, the occipitotemporal gyri, extending from the temporal pole to the occipital lobe. The superior temporal sulcus ends in the loop of cortex forming the angular gyrus of the inferior parietal lobule. An inferior temporal sulcus may be found between the inferior temporal and occipitotemporal gyri, or it may be absent, in which case these gyri blend around the inferior margin of the hemisphere.
On the upper margin of the temporal lobe and extending into the depths of the lateral fissure are the transverse temporal gyri (of Heschl). These gyri (Fig. 16-10) form the primary auditory cortex (Brodmann area 41). Lesions of the auditory cortex may result in difficulty in interpreting a sound or localizing a sound in space, but they do not lead to deafness in one ear. In the case of large cortical lesions, these somewhat subtle auditory deficits may be masked by other more obvious signs or symptoms.
Insular Lobe
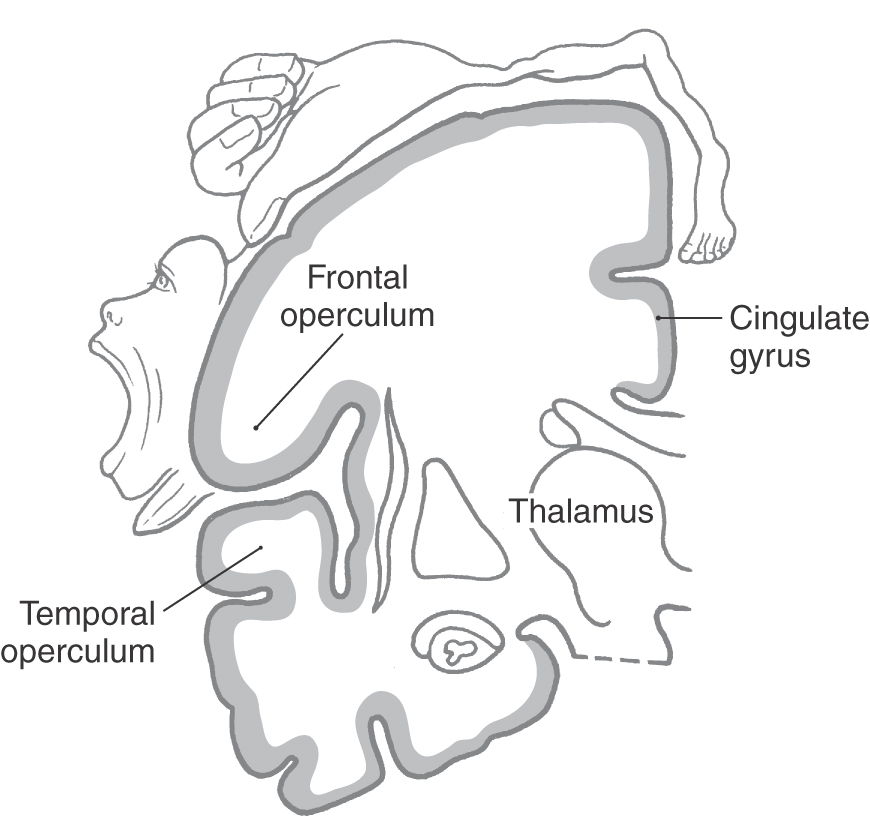
The oval region of cortex located in the depths of the lateral fissure is the insular lobe (Figs. 16-5C and 16-10). This area is characterized by a set of long gyri in its caudal part (the gyri longi) and a set of short gyri in its rostral part (the gyri breves). These gyri are separated from each other by the central sulcus of the insula. The insular cortex is continuous, at the circular sulcus of the insula, with that of the adjacent frontal, parietal, and temporal lobes. This continuity forms lips on each lobe that overlie the insula to form the frontal, parietal, and temporal opercula (Fig. 16-10; see also Fig. 16-12). The limen insulae (threshold to the insula) is the area in which the inferior surface of the hemisphere is continuous with the insular cortex (Fig. 16-10). Although the function of the insula is still somewhat unclear, it is known that the insular cortex receives nociceptive and viscerosensory input. However, it is known that spontaneous lesions of the insular cortex may result in the diminution or complete loss of the desire to continue addictive behavior, such as smoking.
Occipital Lobe
The occipital lobe forms the caudal portion of the hemisphere; its caudal extreme is the occipital pole of the brain (Figs. 16-3 to 16-5B). The irregular collection of gyri on the lateral surface of the occipital lobe constitutes the occipital gyri. On the medial surface of the hemisphere, the parietooccipital sulcus separates the cuneus, an occipital lobe structure, from the precuneus, a parietal lobe structure.
An important landmark on the medial aspect of the occipital lobe is the calcarine sulcus. This sulcus separates the cuneus, which is superior to it, from the lingual gyrus, which is inferiorly located. The primary visual cortex (Brodmann area 17) is located in the portions of these gyri that are found directly superior and inferior to the calcarine sulcus. A lesion of the primary visual cortex of one occipital lobe results in a loss of visual input from the contralateral half of the visual field of each eye. This is called homonymous hemianopia and it may be further designated right or left, depending on whether the deficit involves the right or left half of each visual field.
Limbic Lobe
The limbic lobe is part of a more complex entity commonly called the limbic system. As discussed in Chapter 31, the limbic system includes this lobe plus its afferent and efferent connections with other telencephalic, diencephalic, and brainstem nuclei.
The limbic lobe is a ring of cortex that makes up the most medial rim of the hemisphere. Although not specified as a separate lobe in some texts, it is designated here as such because of its unique functional and structural characteristics. Beginning anteriorly (and just inferior to the rostrum of the corpus callosum), this ring of cortex consists of the subcallosal area, cingulate gyrus, isthmus of the cingulate gyrus, parahippocampal gyrus, and uncus (Figs. 16-4 and 16-6). The limbic cortex is separated from adjacent cortical areas by the cingulate sulcus and the collateral sulcus and from the corpus callosum by the callosal sulcus. On the inferior surface of the temporal lobe, the rhinal sulcus is located between the rostral extreme of the parahippocampal gyrus and the laterally adjacent occipitotemporal gyri (Fig. 16-6).
The function of the limbic lobe is complex and cannot be designated, for example, as primarily sensory or motor. Rather, it is linked to circuits that influence complex functions such as memory, learning, and behavior.
Vasculature of the Cerebral Cortex
The lobes comprising the cerebral cortex receive their blood supply via terminal branches of the anterior, middle, and posterior cerebral arteries. Details of individual branches are given in Chapter 8; only general points are reviewed here.
The anterior cerebral artery, the smaller of the terminal branches of the internal carotid artery, passes rostromedially and is joined to its counterpart by the anterior communicating artery. The anterior cerebral artery distal to the anterior communicating artery arches superiorly around the genu of the corpus callosum and courses caudally to serve the medial surface of the hemisphere to about the level of the parietooccipital sulcus (Fig. 16-11). The part of the anterior cerebral artery between the internal carotid and the anterior communicator is the A1 segment. Branches of A1 serve structures in the immediate vicinity, including the optic chiasm and anterior parts of the hypothalamus. The branches of the anterior cerebral distal to the anterior communicator collectively form segments A2 to A5. These segments serve the medial surface of the frontal and parietal lobes, including the anterior (lower extremity part of motor cortex) and posterior (lower extremity part of somatosensory cortex) paracentral gyri.
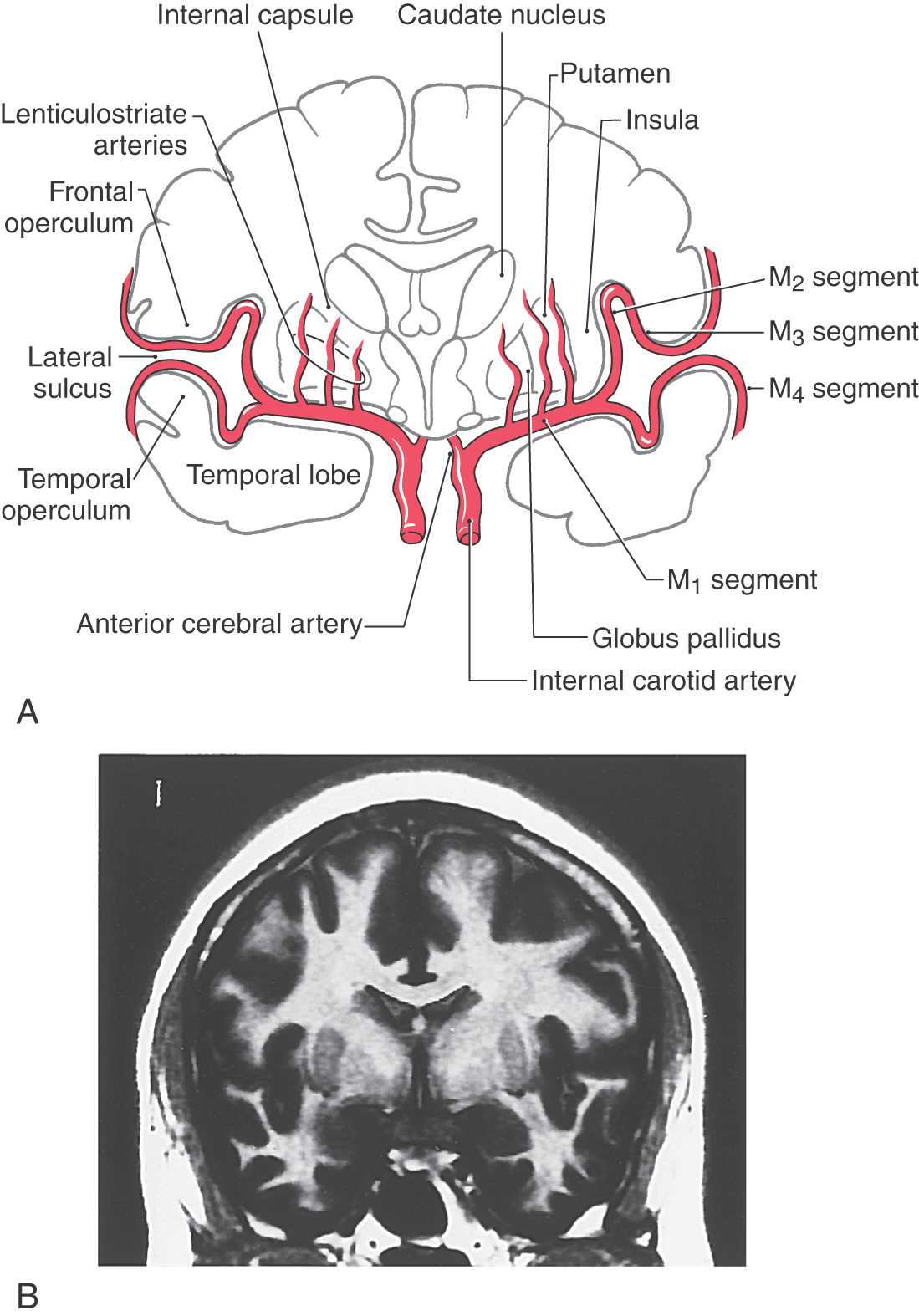
The middle cerebral artery, the larger of the terminal branches of the internal carotid artery, passes laterally to the limen insulae, where it generally branches into superior and inferior trunks (Figs. 16-11 and 16-12). This initial part of the middle cerebral, the M1 segment, gives rise to the lenticulostriate (lateral striate) arteries. The superior and inferior trunks and their distal branches collectively form the M2, M3, and M4 segments. The insular lobe itself is served by branches of M2. As the distal branches of the superior and inferior trunks pass onto the inner surface of the opercula, they become the M3 segment; and on exiting the lateral sulcus, they become the M4 segment (Fig. 16-12A). Important cortical regions served by the M4 segment include the trunk, upper extremity, and head areas of the motor cortex (precentral gyrus), somatosensory cortex (postcentral gyrus), auditory cortex (transverse temporal gyri), parietal association cortex (parietal lobules), and large parts of the superior and lateral surface of the frontal lobe.
The posterior cerebral artery begins at the basilar bifurcation (Fig. 16-11). The first and second parts of this vessel (P1 and P2 segments) are located, respectively, between the basilar bifurcation and the posterior communicating artery and just distal to the latter vessel. Midbrain and diencephalic structures are the main targets of branches of P1 and P2 segments. The P3 segment of the posterior cerebral artery is the part from which the temporal branches originate, and P4 is the segment that gives rise to parietooccipital and calcarine arteries. The calcarine artery is the source of blood to the primary visual cortex, which borders on the calcarine sulcus. Other important telencephalic structures in the domains of P3 and P4 include the parahippocampal gyrus and the precuneus.
WHITE MATTER OF THE CEREBRAL HEMISPHERE
All information entering or leaving the cerebral cortex or connecting one part of the cortex with another must pass through the subcortical white matter. In general, the white matter core of the hemisphere contains association fibers, commissural fibers, and projection fibers.
Association Fibers
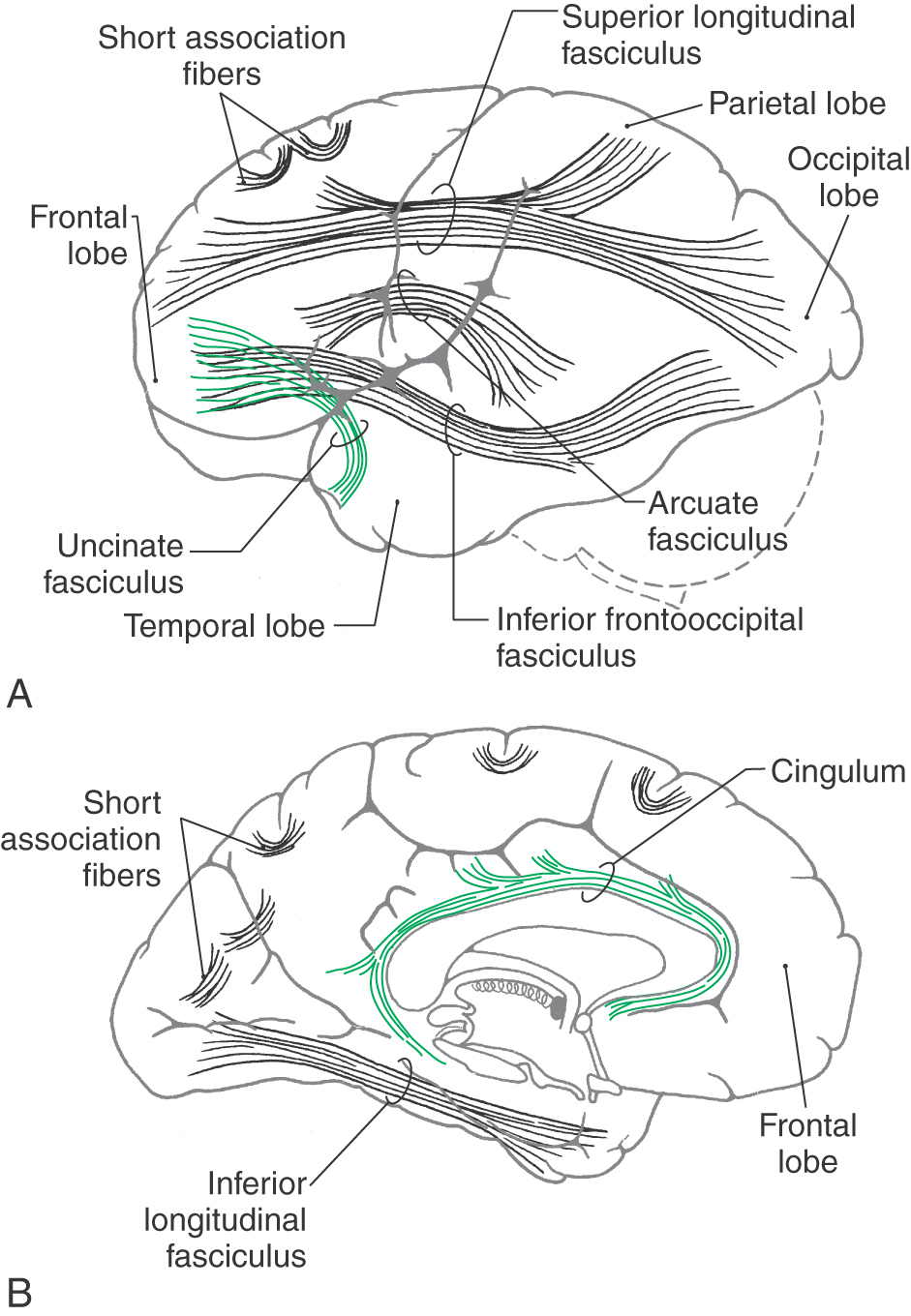
The association fibers interconnect various areas of cortex within the same hemisphere. These may be short association fibers that connect the cortices of adjacent gyri or long association fibers that interconnect more distant areas of cortex (Fig. 16-13). Important examples of the latter are the cingulum located internal to the cingulate gyrus and continuing into the parahippocampal gyrus, the inferior longitudinal fasciculus (temporal-occipital interconnections), and the uncinate fasciculus (frontal-temporal interconnections). The superior longitudinal fasciculus, located in the core of the hemisphere, interconnects frontal, parietal, and occipital cortices, whereas the arcuate fasciculus interconnects frontal and temporal lobes (Fig. 16-13). In the white matter of the temporal lobe, fibers passing between the frontal and occipital areas make up the inferior frontooccipital fasciculus.
Figure 16-13. Main association bundles as visualized from lateral (A) and medial (B) aspects of the left hemisphere.
The claustrum, a thin layer of neuron cell bodies located internal to the insular cortex, is sandwiched between two small association bundles (Fig. 16-12). The external capsule is insinuated between the claustrum and putamen, and the extreme capsule is located between the claustrum and the insular cortex.
Commissural Fibers: The Corpus Callosum
In general, commissural fibers interconnect corresponding structures on either side of the neuraxis. The largest bundle of commissural fibers is the corpus callosum (Figs. 16-2 and 16-4). This huge bundle is located superior to the diencephalon and forms the roof of much of the lateral ventricles. The corpus callosum consists of, from rostral to caudal, a rostrum, genu, body (also called trunk), and splenium (Figs. 16-4 and 16-5B). Many of the fibers passing through the genu arch rostrally to interconnect the frontal lobes; these form the minor (or frontal) forceps. The fibers interconnecting the occipital lobes loop through the splenium of the corpus callosum, forming the major (or occipital) forceps. The tapetum, which is located in the lateral wall of the atrium and posterior horn of the lateral ventricle, is also composed of fiber bundles that cross in the splenium.
Lesions of the corpus callosum, whether the result of surgery or spontaneous, disconnect one hemisphere from the other. About 80% of patients who have severe generalized seizures experience significant relief after section of the anterior 75% of the corpus callosum, largely sparing the splenium. Spontaneous lesions of the corpus callosum may result from vascular infarct, tumor (such as oligodendroglioma), or necrosis or demyelination (as in Marchiafava-Bignami disease). It is recognized that some of these patients may have a disconnection syndrome.
Smaller commissural bundles include the anterior commissure and the hippocampal commissure (Fig. 16-4). In sagittal views, the anterior commissure is located caudal to the rostrum of the corpus callosum but rostral to the main part of the fornix. This bundle interconnects various parts of the frontal and temporal lobes. The hippocampal commissure is formed by fibers that originate in the hippocampal formations and cross the midline as a thin layer inferior to the splenium of the corpus callosum.
The posterior commissure and the habenular commissure are small fiber bundles traversing the midline that connect caudal parts of the diencephalon (Figs. 16-1 and 16-4). The former crosses the midline at the base of the pineal gland and just posterior (dorsal) to the cerebral aqueduct. The latter is a small fascicle running along the upper aspect of the posterior commissure and interconnecting the habenular nuclei.
Projection Fibers: The Internal Capsule
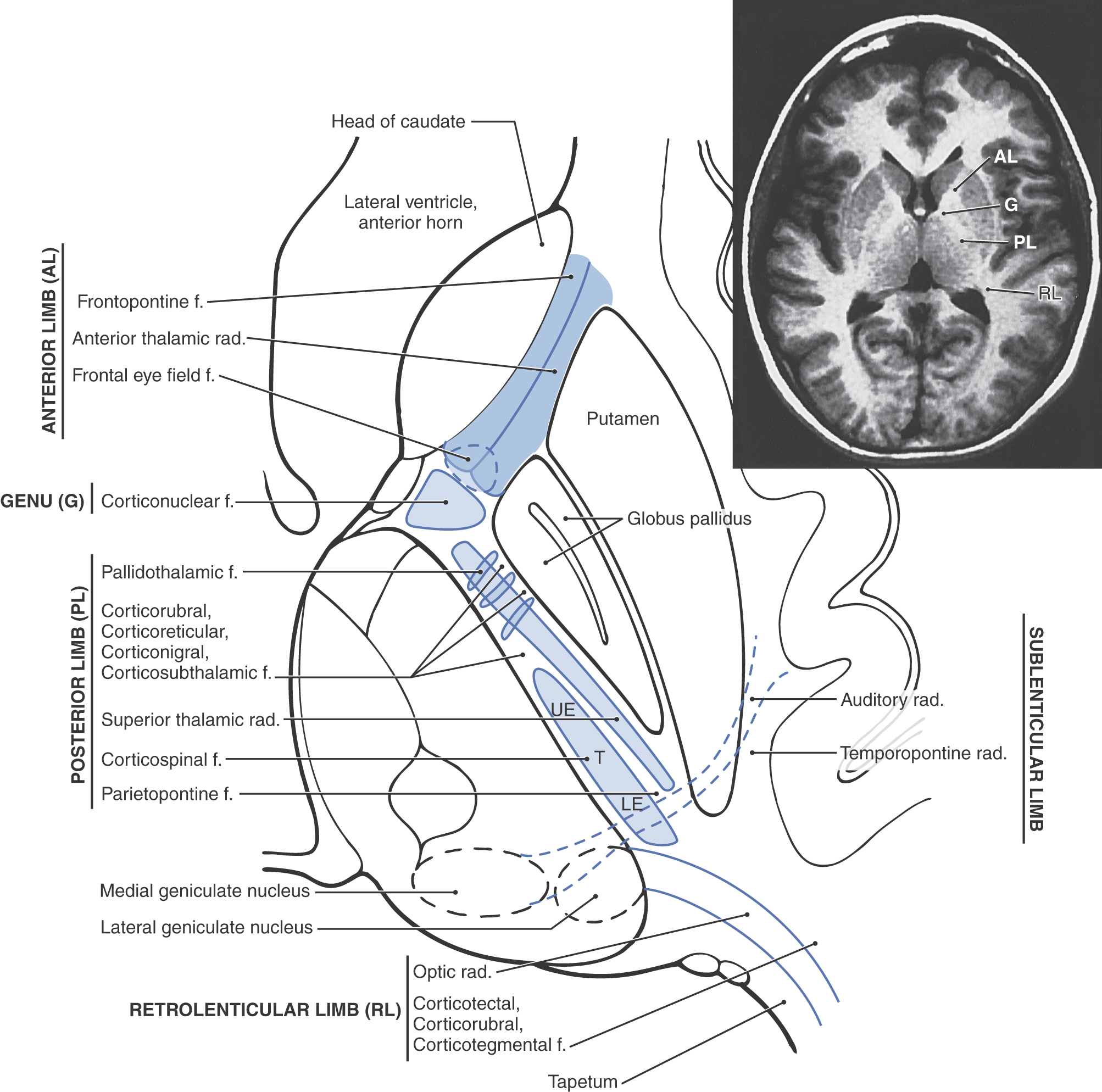
The projection fibers of the hemispheres include both the axons that originate outside the telencephalon and project to the cerebral cortex (corticopetal) and the axons that arise from cerebral cortical cells and project to downstream targets (corticofugal). A prime example of the former are projections from the thalamus to the cerebral cortex (thalamocortical fibers); examples of the latter are corticospinal, corticopontine, and corticothalamic fibers. Projection fibers are organized into a large, compact bundle called the internal capsule (Fig. 16-14), which has intimate structural associations with the diencephalon and basal nuclei. Consequently, to divide the internal capsule into its constituent parts, reference must be made to these adjacent cell groups.
In an axial plane through the hemisphere, the internal capsule appears as a prominent V-shaped structure with the V pointing medially (Figs. 16-14 and 16-15). It is divided into three parts: (1) an anterior limb insinuated between the head of the caudate nucleus and the lenticular nucleus, (2) a posterior limb located between the dorsal thalamus and the lenticular nucleus, and (3) a genu located at the intersection of the anterior and posterior limbs, which is located approximately at the level of the interventricular foramen (Fig. 16-14).
Figure 16-15. Blood supply to the basal nuclei, internal capsule, and thalamus shown in the axial plane.
The anterior limb of the internal capsule contains thalamocortical-corticothalamic fibers (collectively called the anterior thalamic radiations) that interconnect the dorsomedial and anterior thalamic nuclei with areas of the frontal lobe and the cingulate gyrus. Frontopontine fibers, especially those from the prefrontal areas, also pass through this structure.
The genu of the internal capsule contains corticonuclear fibers that arise in the frontal cortex just rostral to the precentral sulcus and from the precentral gyrus (primary motor cortex) and project to the motor nuclei of cranial nerves. Lesions of these fibers give rise to motor deficits of cranial nerves, most notably deficits related to the facial and hypoglossal nerves.
The posterior limb of the internal capsule is larger and more complex (Fig. 16-14). It is sometimes divided into a thalamolenticular part (located between the thalamus and the lenticular nucleus), a sublenticular part (fibers passing ventral to the lenticular nucleus), and a retrolenticular part (fibers located caudal to the lenticular nucleus). However, contemporary terminology and common usage refer to the thalamolenticular part as the posterior limb, the sublenticular part as the sublenticular limb, and the retrolenticular part as the retrolenticular limb. This terminology is considerably less cumbersome and much easier to remember and is the convention followed here (Figs. 16-14 and 16-15). By this scheme, the internal capsule consists of five parts: anterior limb, genu, posterior limb, sublenticular limb, and retrolenticular limb.
The major fiber populations passing through the posterior, sublenticular, and retrolenticular limbs of the internal capsule are summarized in Figure 16-14. Included in the posterior limb are corticospinal fibers arising from the motor cortex and projecting to the contralateral spinal cord and thalamocortical-corticothalamic fibers (as part of the central thalamic radiations) that interconnect nuclei of the dorsal thalamus with the overlying cortex. Studies in humans have revealed that corticospinal fibers are somatotopically arranged in about the caudal half of the posterior limb. Geniculotemporal radiations (auditory radiations) convey auditory information from the medial geniculate nucleus to the transverse temporal gyri through the sublenticular limb. Visual input from the lateral geniculate body to the occipital cortex is conveyed via geniculocalcarine radiations (optic radiations) through the retrolenticular limb (Fig. 16-14). Optic radiations form a distinct lamina of fibers immediately lateral to the tapetum as they course caudally into the occipital lobe.
Fibers of the internal capsule flare out into the hemisphere as they pass distal to the caudate and putamen. This abrupt divergence of internal capsule fibers forms the corona radiata (“radiating crown”), which contains converging corticofugal fibers as well as diverging corticopetal fibers (see Fig. 16-20).
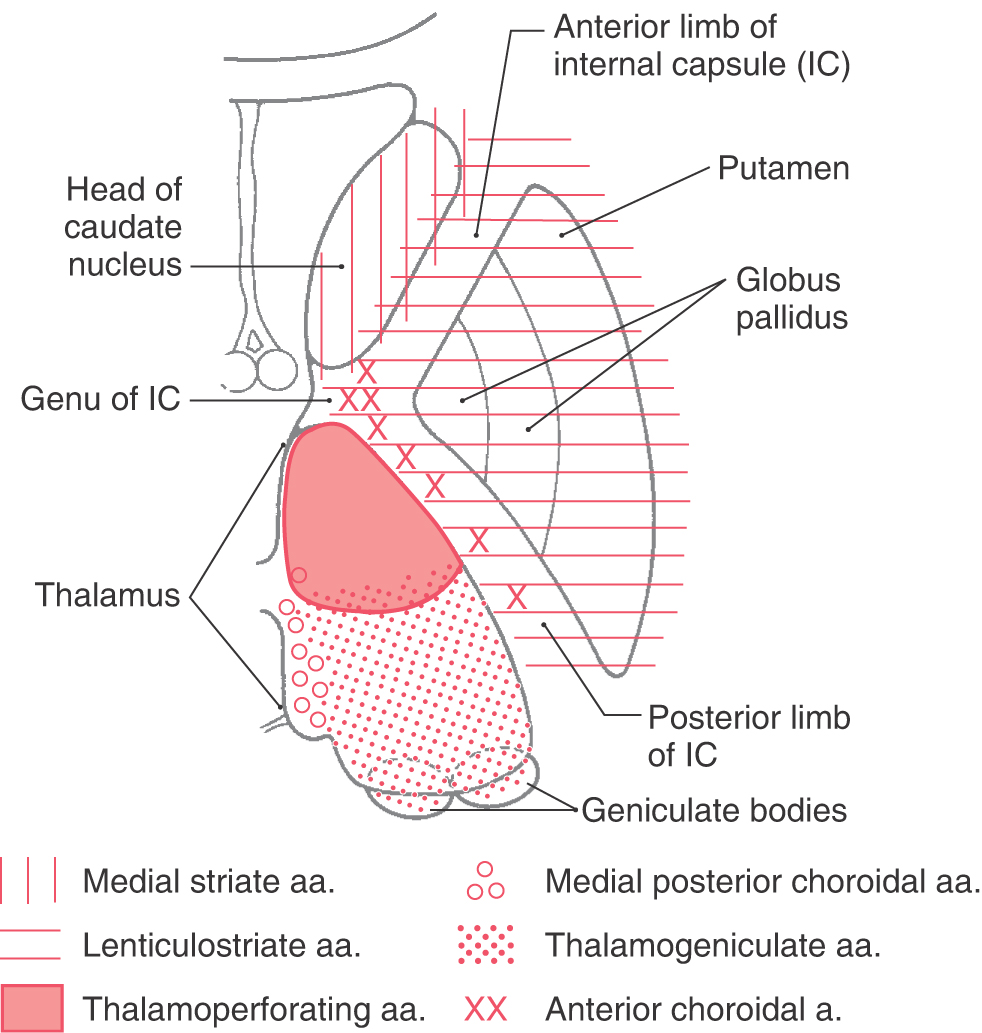
Vasculature of the Internal Capsule
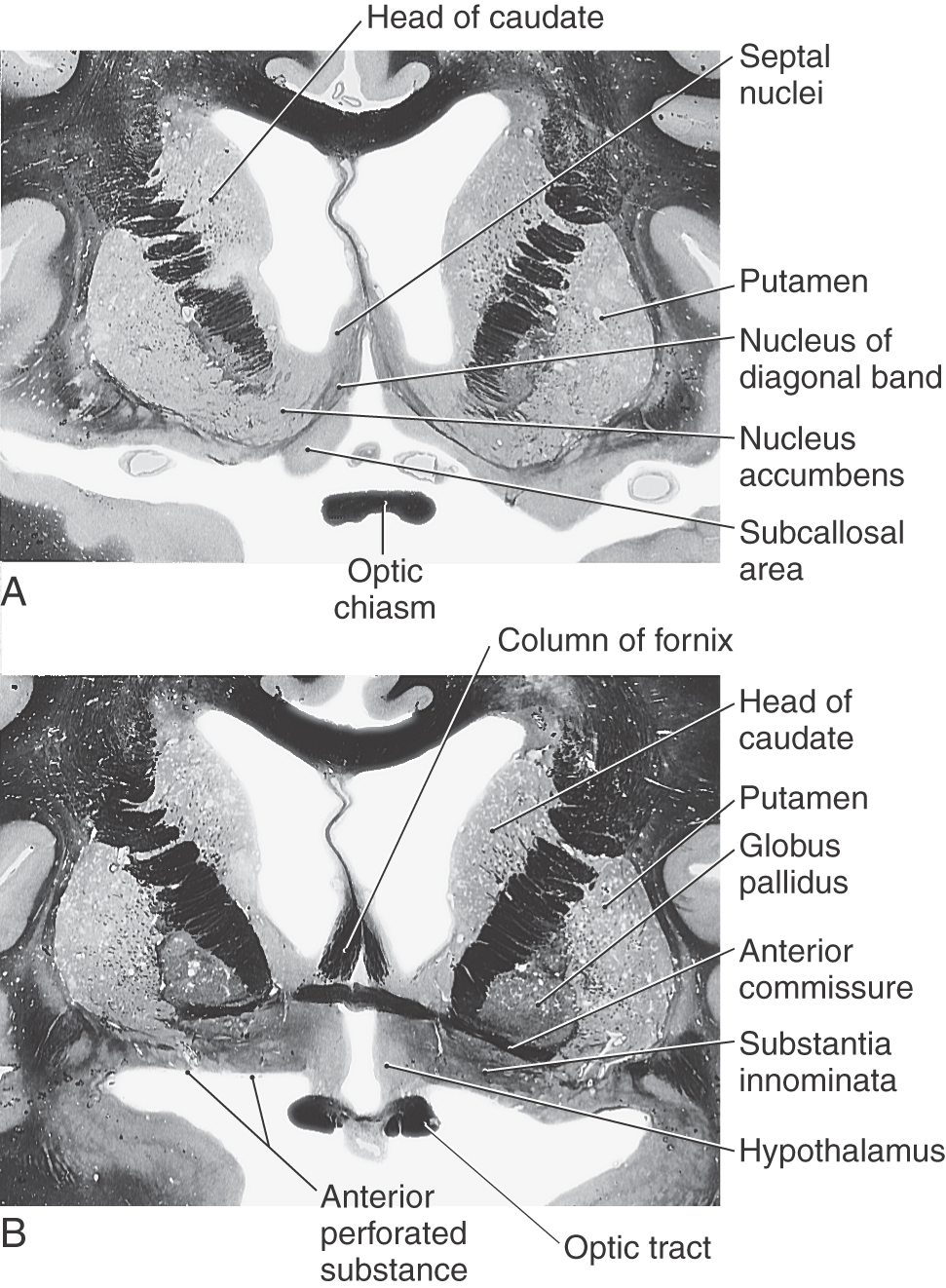
The blood supply to the genu and most of the posterior limb of the internal capsule is via the lenticulostriate arteries; these are branches of the M1 segment (Figs. 16-12 and 16-15; see also Fig. 15-18). Branches of the anterior choroidal artery supply the inferior region of the posterior limb, optic tract, portions of the optic radiation (the Meyer loop), inferior parts of the basal nuclei, hippocampus, amygdala, choroid plexus in the temporal horns, and immediately adjacent retrolenticular limb. An occlusion of this vessel gives rise to a constellation of deficits, called the anterior choroidal artery syndrome, reflecting damage to these structures.
The anterior limb receives somewhat of a dual blood supply in that lenticulostriate arteries and branches of the medial striate artery (usually a branch of A2) serve this area.
Whereas a variety of clinical events may damage the fibers of the internal capsule, the vast majority (about 95%) that produce rapid-onset deficits are vascular related. Hemorrhagic stroke (about 15% of cases: rupture of or bleeding from a vessel serving the capsule) and occlusive stroke (about 85% of cases: occlusion of a vessel serving the capsule) are the most common causes. Lesions of the posterior limb may result in a combination of motor (corticospinal tract involvement) and sensory (thalamocortical fiber involvement) deficits that are seen on the side of the body contralateral to the lesion. Indeed, a characteristic feature of hemisphere lesions is the appearance of motor and sensory deficits on the same side of the body. Lesions of the retrolenticular limb result in visual deficits (optic radiation fiber involvement) that may, depending on the extent of the damage, involve the contralateral hemifield (one half of each visual field, a hemianopia) or a contralateral quadrant (about one fourth of each visual field, a quadrantanopia) of the visual field of each eye. Damage to the sublenticular limb may result in tinnitus and difficulty localizing sound.
BASAL NUCLEI
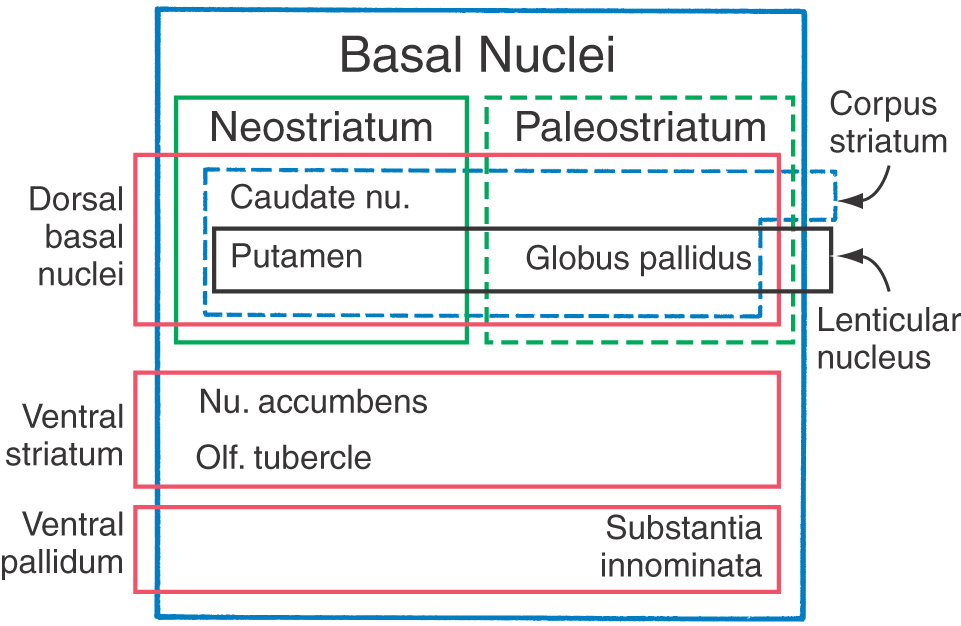
The term basal ganglia is a misnomer. The cells forming these structures are not “ganglia”—a term reserved to describe aggregations of neuronal somata in the peripheral nervous system—but “nuclei” in the central nervous system. In addition, the definition of cell groups comprising the basal nuclei has been revised over the years, with contemporary views focusing on the functional characteristics of these nuclei. In this respect, the basal nuclei consist of (1) the caudate and lenticular nuclei (together forming the dorsal basal nuclei), (2) the nucleus accumbens plus parts of the adjacent olfactory tubercle (the ventral striatum), and (3) the substantia innominata (ventral pallidum) (Fig. 16-16). As described previously, the subthalamic nucleus and the substantia nigra are not components of the basal nuclei, but they are included in this discussion because of their important structural and functional relationship with the caudate and lenticular nuclei.
Figure 16-16. A series of stacked boxes showing which nuclei form the various parts of the basal nuclei and the terms used to describe them.
The basal nuclei function primarily in the motor sphere. Damage to these nuclei, as in vascular lesions, degenerative genetic disorders, or problems of unknown etiology, results in a variety of motor deficits, some of which are recognized as characteristic involuntary movements.
Caudate and Lenticular Nuclei
The caudate and lenticular nuclei collectively form the corpus striatum. The corpus striatum, in turn, is divided into the neostriatum, consisting of the caudate nucleus and the putamen, and the paleostriatum, or globus pallidus (Fig. 16-16). The globus pallidus and putamen collectively form the lenticular nucleus.
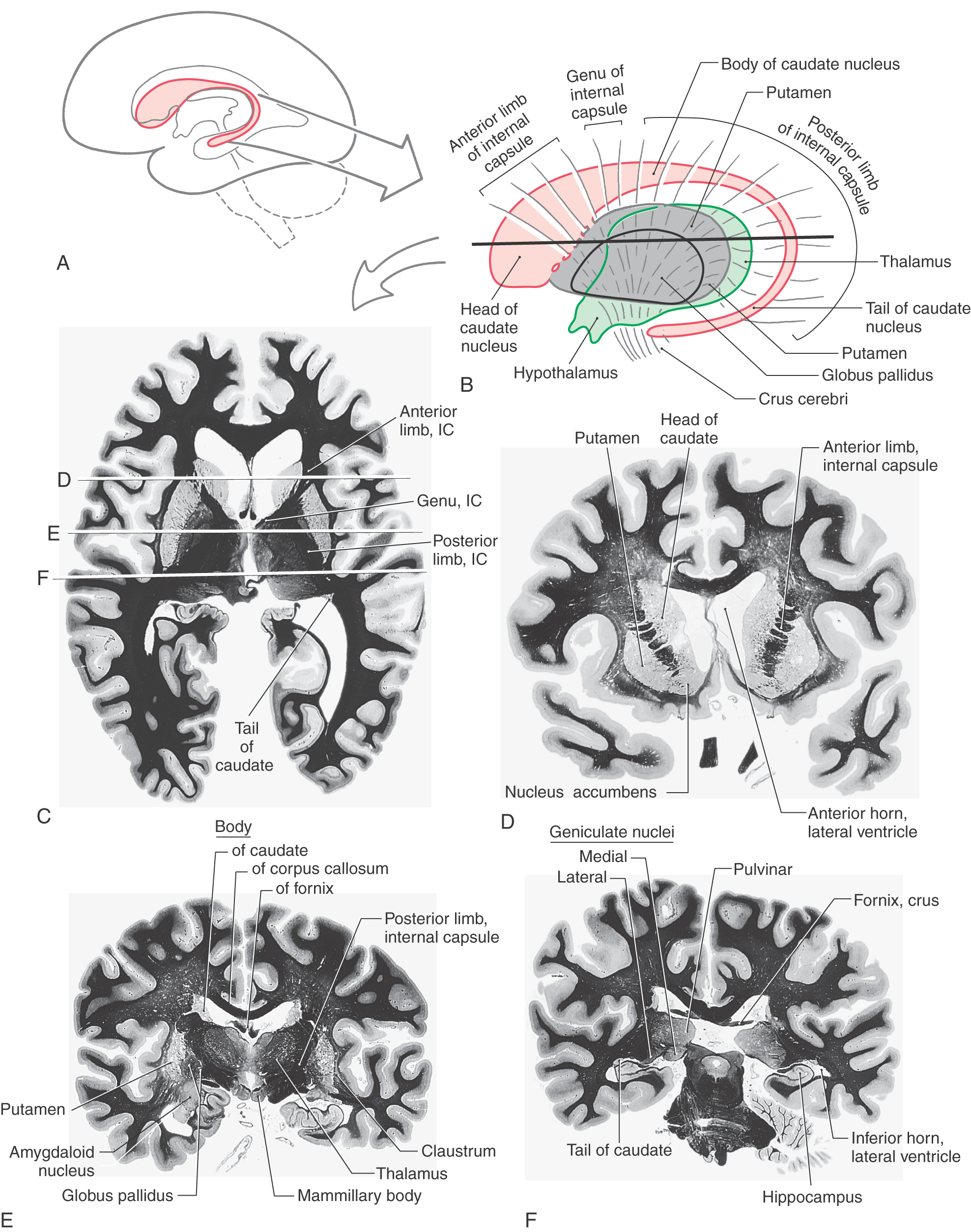
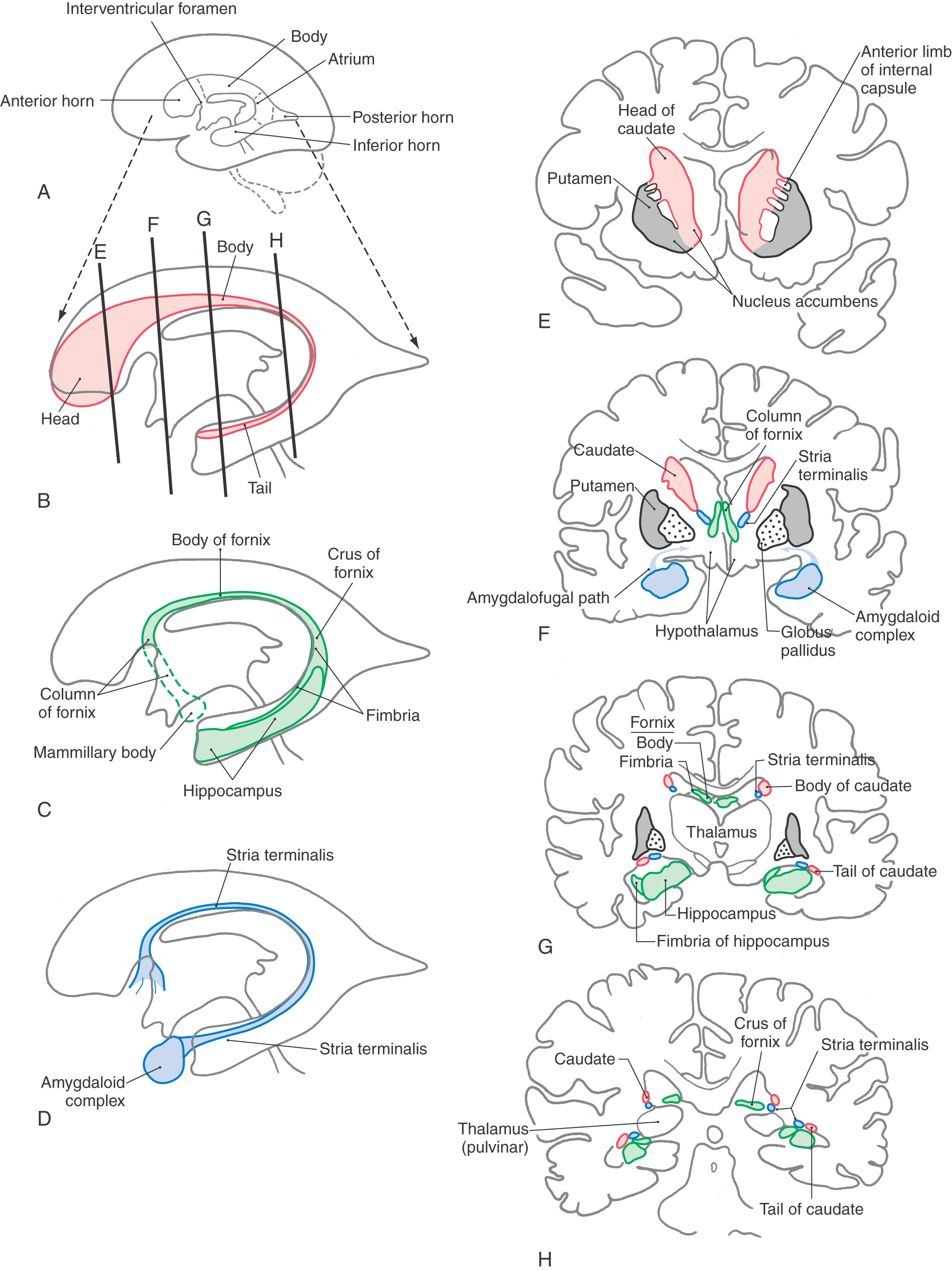
The caudate nucleus is characteristically located in the lateral wall of the lateral ventricle and consists of three parts: head, body, and tail (Figs. 16-17 and 16-18). The head of the caudate nucleus forms a prominent bulge in the anterior horn of the lateral ventricle. In Huntington disease (also called Huntington chorea), an inherited neurodegenerative disease, the head of the caudate nucleus is characteristically diminished in size or absent in magnetic resonance imaging or computed tomography. At about the level of the interventricular foramen, the caudate diminishes in size but continues caudally as the body of the caudate nucleus in the lateral wall of the body of the lateral ventricle. In the lateral wall of the atrium of the lateral ventricle, the body of the caudate nucleus turns inferiorly and rostrally to continue as the tail of the caudate nucleus in the posterolateral (dorsolateral) wall of the temporal horn of the lateral ventricle. Thus the C shape of the caudate nucleus faithfully follows the C shape of the lateral ventricle (excluding the posterior horn) (Figs. 16-17A, B and 16-18A, B, and E-H).
The lenticular nucleus is located within the base of the hemisphere and is surrounded by white matter (Figs. 16-17 and 16-18). The internal capsule borders the lenticular nucleus medially, and the external capsule separates it from the claustrum laterally. The lenticular nucleus consists of a larger lateral part, the putamen, and a small medial portion, the globus pallidus (or pallidum). The putamen extends more superior, more rostral, and more caudal compared with the globus pallidus and is clearly the larger part of the lenticular nucleus when viewed in axial or coronal planes (Fig. 16-17B-E). The globus pallidus is located internal to the putamen and is smaller in all dimensions (Figs. 16-17 to 16-19). It is divided into medial (internal) and lateral (external) parts by thin sheets of vertically oriented white matter. The globus pallidus is also separated from the putamen by a thin lamina of white matter.
Nucleus Accumbens and Substantia Innominata
The nucleus accumbens is located rostrally in the hemisphere where the putamen is continuous with the head of the caudate nucleus (Fig. 16-19). This cell group is also closely apposed to the septal nuclei and the nucleus of the diagonal band, both of which extend into the base of the septum pellucidum. At a slightly more caudal level, the substantia innominata (basal nucleus of Meynert) is located internal to the anterior perforated substance in the area inferior to the anterior commissure (Fig. 16-19). Although many areas of the nervous system are affected in Alzheimer disease, there is an especially noticeable loss of larger neurons in the substantia innominata.
Subthalamic Nucleus and Substantia Nigra
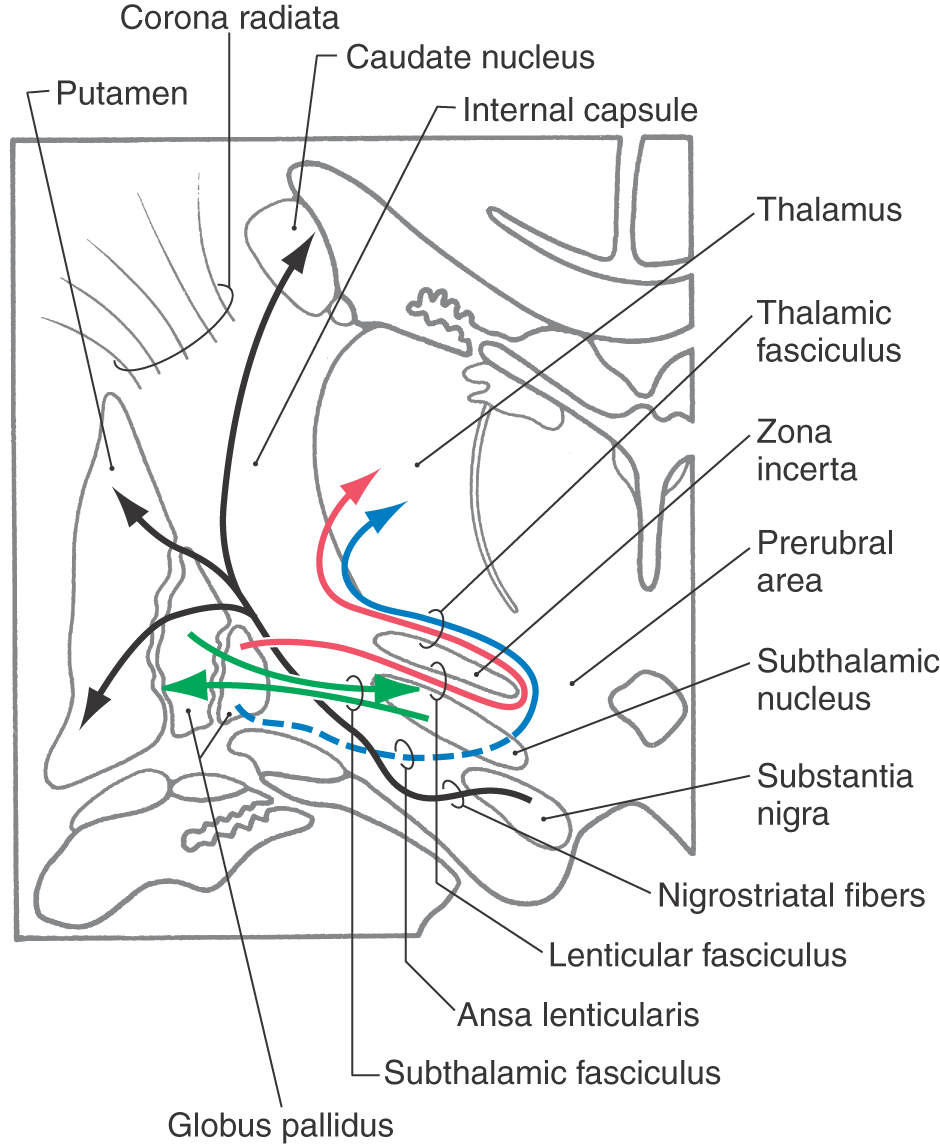
Although not a part of the telencephalon either developmentally or geographically, the subthalamic nucleus and the substantia nigra are intimately allied with the basal nuclei on the basis of their connections (Fig. 16-20). The subthalamic nucleus, a component of the diencephalon, is a flattened, lens-shaped cell group located rostral to the substantia nigra. It is medial to the internal capsule and is capped by a thin sheet of fibers called the lenticular fasciculus. Lesions of the subthalamic nucleus, which are commonly hemorrhagic in origin, result in a characteristic motor deficit of the contralateral extremities (primarily the upper) called hemiballismus.
Figure 16-20. Major fiber bundles associated with the basal nuclei and with the subthalamic nucleus and substantia nigra.
The substantia nigra, a part of the midbrain, is found internal to the crus cerebri and immediately caudal to the subthalamic nucleus. It is divided into a reticulated part (pars reticulata) and a compact part (pars compacta); the latter is characterized by numerous melanin-containing neuron cell bodies. These melanin-containing cells of the pars compacta use dopamine as their neurotransmitter. The progressive loss of these cells, due largely to unknown causes, gives rise to the characteristic motor deficits seen in Parkinson disease.
Major Connections of the Basal Nuclei
The connections of the basal nuclei are discussed in their entirety in Chapter 26. It is appropriate, however, to briefly review these relationships here, with particular emphasis on the major fiber bundles of the basal nuclei (Fig. 16-20).
The two largest bundles of efferent fibers exiting the basal nuclei are the lenticular fasciculus and the ansa lenticularis. The lenticular fasciculus leaves the globus pallidus, passes through the posterior limb of the internal capsule at right angles to most fibers in the posterior limb, and forms a thin sheet of fibers insinuated between the subthalamic nucleus and the zona incerta. These fibers loop around the medial aspect of the zona incerta and pass laterally (and superiorly) as the thalamic fasciculus (Fig. 16-20). The fibers of the ansa lenticularis originate at a slightly more rostral level, arch around the inferomedial aspect of the internal capsule, and pass caudally to join with the fibers of the lenticular fasciculus as they enter the thalamic fasciculus (Fig. 16-20).
Two smaller but equally important bundles are the subthalamic fasciculus and connections between the substantia nigra and neostriatum (Fig. 16-20). The subthalamic fasciculus is composed of bidirectional connections between the globus pallidus and the subthalamic nucleus; these fibers also traverse the posterior limb of the internal capsule similar to those of the lenticular fasciculus. The connections between the substantia nigra and the neostriatum are named according to the origin and termination of the fiber. Axons that project from nigral cells to the neostriatum are nigrostriatal projections, and fibers that project from striatal cells to the substantia nigra are striatonigral fibers. These bidirectional connections between the neostriatum and the substantia nigra course through the lateral aspect of the midbrain-diencephalic junction at the interface between the crus cerebri and the substantia nigra (Fig. 16-20).
Vasculature of the Basal Nuclei and Related Structures
The blood supply to the caudate and putamen is provided by branches of the medial striate artery, lenticulostriate branches of the M1 segment, and the anterior choroidal artery (Figs. 16-12 and 16-15). The medial striate artery, usually a branch of proximal A2, or the junction of the anterior communicating with the anterior cerebral arteries (also called the ACA-ACom corner), serves much of the head of the caudate nucleus. Most of the lenticular nucleus and the surrounding internal and external capsules are supplied by the lenticulostriate branches of M1. Inferior (ventral) and medial portions of the head of the caudate as well as the body of the caudate are also served by these arteries. The tail of the caudate, adjacent portions of the lenticular nucleus, and adjacent temporal lobe structures (hippocampus, choroid plexus) receive their blood supply via the anterior choroidal artery, a branch of the internal carotid artery. The anterior choroidal artery also serves the optic tract and inferior regions of the posterior limb of the internal capsule.
The blood supply to the subthalamic nucleus and the substantia nigra arises from the posteromedial branches of the P1 segment and branches of the posterior communicating artery. These vessels pass through the posterior perforated substance at the midbrain-diencephalon junction.
HIPPOCAMPUS AND AMYGDALA
The hippocampal formation and the amygdaloid complex are located in the temporal lobe. The former lies in the inferomedial floor of the temporal horn of the lateral ventricle and the latter in the rostral end of this space. Through a variety of pathways, these structures interconnect with numerous telencephalic and diencephalic centers.
Developmentally, the hippocampus is formed by an invagination of primitive cortex to form the curved, multilayered structure characteristic of the adult brain (Fig. 16-17F). The hippocampal formation is found internal to the parahippocampal gyrus and is composed of the subiculum, the hippocampus proper (also called Ammon horn), and the dentate gyrus. The cortex of the parahippocampal gyrus is continuous with the subiculum, which in turn is continuous with the hippocampus proper. The dentate gyrus forms a reverse loop adjacent to the hippocampus and, in doing so, presents a serrated surface that is medially exposed to the subarachnoid space. Details of the internal structure of the hippocampal formation are discussed in Chapter 31.
Axons of hippocampal neurons converge to form a prominent bundle that arches around caudal, superior, and rostral aspects of the thalamus. This bundle, the fornix, is a major efferent path of the hippocampal formation (Fig. 16-18A-C and F-H). It is composed of a flattened caudal part, the crus; a compact superior portion, the body; and a part that arches around the rostral part of the thalamus and passes through the hypothalamus to terminate in the mammillary body—this is the column of the fornix. Located along the edge of the dentate gyrus and continuing on the lateral edge of the crus and body of the fornix is a thin fringe of fibers called the fimbria.
The amygdaloid nuclear complex (commonly called the amygdala) is located internal to the cortex of the uncus (Figs. 16-4 and 16-18D-F). It is composed of several cell groups, including caudomedial, basolateral, and central subdivisions. Two major efferent bundles are related to the amygdala. First, the stria terminalis follows a looping trajectory that shadows, in a reverse direction, the orientation of the caudate nucleus (Fig. 16-18D). In the temporal horn, the stria terminalis is located just medial to the tail of the caudate nucleus. As the stria terminalis arches superiorly and rostrally, it assumes a position in the shallow groove between the caudate nucleus and the dorsal thalamus (Fig. 16-18G, H), where it is accompanied by the terminal vein (superior thalamostriate vein). At about the level of the interventricular foramen, the fibers of the stria terminalis fan out to enter and terminate in the hypothalamus, the septal area, and the neostriatum.
The second major efferent bundle of the amygdala is the diffusely arranged ventral amygdalofugal pathway. These fibers leave the amygdaloid complex, pass medially through the substantia innominata, and continue medially to enter hypothalamic and septal nuclei or turn caudally and distribute to the brainstem (Fig. 16-18F).
Cell groups located internal to the subcallosal area collectively form the septal nuclei (Fig. 16-19). Consequently, the subcallosal area, together with a small strip of cortex located adjacent to the lamina terminalis, the paraterminal gyrus, is commonly called the septal area. The septal nuclei are medially adjacent to the nucleus accumbens and continuous with sheets of neuronal cell bodies that extend into the septum pellucidum. The latter structure extends, in general, from the fornix to the inner surface of the corpus callosum. It forms the medial wall of the anterior horns and a small part of the bodies of the lateral ventricles (Figs. 16-14 and 16-17). In general, the septal nuclei have complex interconnections with hippocampal, amygdaloid, and other limbic structures.
Temporal Lobe Lesions
Injury to the temporal lobe, especially bilateral damage, almost always involves the hippocampus and amygdala. Deficits most directly linked to trauma to these structures include profound changes in eating and sexual behavior, a decrease in aggression levels, and deficits in memory function. Regarding memory deficits, the patient may demonstrate a loss of recent memory or show an inability to acquire new memory (learn new tasks) while memory of events that took place in the distant past remains intact.
Vasculature of the Hippocampus and Amygdala
The blood supply to the hippocampal formation and amygdaloid complex is primarily via the anterior choroidal artery. This vessel arises from the internal carotid, passes along the medial edge of the temporal horn, and sends branches into the hippocampus and amygdala. It also serves the tail of the caudate, the choroid plexus of the temporal horn, the inferior regions of the lenticular nucleus, and the optic tract. The cortex of the uncus and that of the parahippocampal gyrus are served by superficial branches of the middle cerebral and posterior cerebral arteries, respectively.
Sources and Additional Reading
Bailey P, von Bonin G. The Isocortex of Man. Urbana, Ill: University of Illinois Press; 1951.
Parent A. Carpenter’s Human Neuroanatomy. ed 9 Baltimore: Williams & Wilkins; 1996.
Paxinos G, ed. The Human Nervous System. San Diego: Academic Press; 1990. 439–755
Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563.